Abstract
Background
Bosutinib is a third-generation dual tyrosine kinase inhibitor (TKI) inhibiting Abl and Src kinases. It was developed to act on up-regulated tyrosine kinases (TKs) like BCR-ABL in Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML) when resistance to first- and second-generation TKIs developed. However, first- and second-generation TKIs show off-target effects on bone metabolism, whereas studies on skeletal adverse effects of bosutinib are still lacking. Therefore, it was the aim of this study to continuously expose juvenile rats to bosutinib and to analyze its influence on the growing bone.
Material/Methods
Starting after weaning, 4-week-old Wistar rats were chronically exposed over a 28-day period to varying concentrations of bosutinib, which were continuously administered subcutaneously via implanted Alzet® micro-osmotic pumps. After necropsy, the length of the femora and tibiae were analyzed.
Results
Continuous administration of bosutinib by micro-osmotic pumps led to serum drug levels in the lower therapeutic range, was well tolerated, and exhibited only minor adverse effects on the growing skeleton.
Conclusions
Micro-osmotic pumps represent a convenient system for continuous TKI release in young growing rats. Compared to first- and second-generation TKIs, bosutinib seems to exert fewer adverse effects on the growing bone.
Keywords: bosutinib, TKI (tyrosine kinase inhibitor), micro-osmotic pump, bone
Background
Protein tyrosine kinases (TKs) play a crucial role in signal transduction pathways regulating numerous cellular functions, including differentiation and proliferation. Dysregulation may lead to increased cellular proliferation and differentiation. Chronic myeloid leukemia (CML) is caused by the constitutively up-regulated TK BCR-ABL1 resulting from the reciprocal balanced chromosomal translocation t(9;22), the so-called Philadelphia chromosome (Ph+) [1]. Targeting BCR-ABL1 for treatment of CML has led to the development of the specific TK inhibitor (TKI) imatinib (Gleevec®, Novartis, Basel, Switzerland), which remarkably improved therapeutic response of Ph+ CML in adults and children [1,2]. However, development of imatinib resistance or intolerance promoted further development of second- and also third-generation TKIs like bosutinib (SKI-606, Pfizer, New York, USA). Bosutinib functions as a dual inhibitor of the TKs Src and Abl1 and has demonstrated promising results in CML patients with resistance or intolerance to imatinib in clinical trials [3–5].
During recent years, a growing number of reports have shown disturbances in bone metabolism as an adverse effect of imatinib treatment [6,7]. Pediatric CML patients under imatinib treatment experienced growth retardation [8–11] and studies on adverse effects of bosutinib in vivo and in vitro on the growing skeleton have not yet been performed. Therefore, we analyzed the influence of bosutinib on bone growth and structure in a juvenile rodent model. The drug was continuously released subcutaneously via micro-osmotic pumps.
Material and Methods
Animals and experimental design
Over a period of 28 days, 2 groups of 4-week-old juvenile male Wistar rats (Elevage Janvier, Le Genest St. Isle, France) were chronically exposed to targeted bosutinib mean doses of 2.5 mg/kg/day or 5.0 mg/kg/day (each group, n=4 animals) administered continuously by the use of subcutaneously implanted Alzet® micro-osmotic pumps (200 μL total volume, model 2002, Charles River Laboratories, Sulzfeld, Germany). Controls received vehicle, either 100% DMSO or 0.9% sterile saline (each group, n=4). Filling and preparation of micro-osmotic pumps for implantation was done as described by the manufacturer. Pumping rate was 0.5 μl/h and pumping duration was 14 days. To ensure continuous exposure during the 28-day period, 2 micro-osmotic pumps were consecutively implanted: the first pump on the right and after 14 days the second micro-osmotic pump on the left dorsal skin of an individual rat under general anesthesia. Following implantations, a single dose of prophylactic antibiotic treatment (Duphamox® L.A., Fort Dodge Animal Health Ltd., Würselen, Germany, 15 mg/kg body weight) was administered subcutaneously. Due to physiological rapid body weight gain during the experiment, the drug concentration within the pumps was adjusted: to achieve a mean targeted concentration of 5.0 mg/kg/day, bosutinib was dissolved in DMSO at a concentration of 60 μg/μl for the first micro-osmotic pump implantation and at a concentration of 88 μg/μl for the second pump implantation. To achieve the targeted bosutinib concentration of close to 2.5 mg/kg/day, these solutions were diluted 1:1 with DMSO. Juvenile rats were kept under standardized conditions at 21°C room temperature and 12 h/day light (06:00–18:00) with free access to food and water until the end of the experiment (age 8 weeks) when the animals were humanely killed. During exposure, the animals’ behavior and weight gain were monitored Monday through Friday. All experiments were carried out in accordance with the Institutional Animal Care and Use Guidelines and were approved by the authorities of the Government of Saxony (permit number 24-9168.11-1/2009-16).
Determination of bosutinib serum levels
At 8 weeks of age, animals were sacrificed under general anesthesia and serum was collected via cardiocentesis. Drug serum levels were measured by a commercial supplier (PharmaNet, Princeton, NJ, USA).
Osseous specimen collection and measurement of bone length
Femora and tibiae were excised, defleshed and fixed in 70% ethanol for analysis. Length of femur and tibiae were determined with a Merox® digital caliper (precision of 0.01 mm, Warenimport und Handels GmbH, Vienna, Austria).
Results
Consequences of micro-osmotic pump implantation
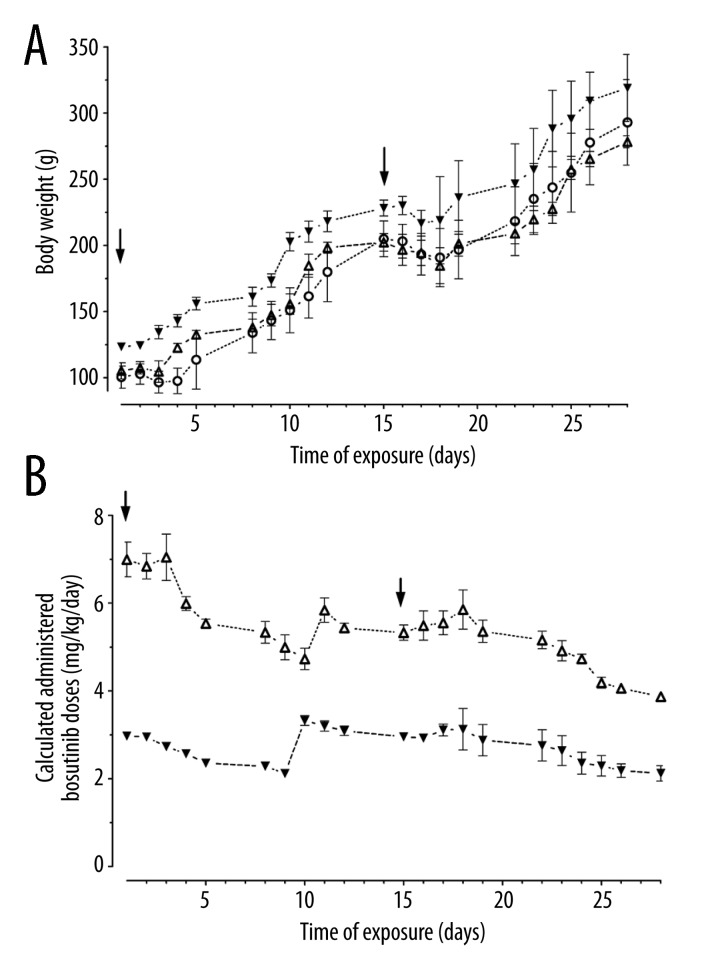
Following the surgical procedure of pump implantation, a temporary delay in body weight gain was observed. Figure 1A illustrates the time points of Alzet® micro-osmotic pump implantation (black arrows) and weight gain of controls and drug-exposed rats. Following the first implantation, physiological body weight increase stopped for 2 days. After the second implantation all animals exhibited a loss of weight of up to 8.0±0.7% but regained the lost weight by 3 days post-implantation. However, during this experiment, 4 out of the total cohort of 16 animals died from peritonitis, despite prophylactic and repeated antibiotic treatment. These infections happened in 2 control animals treated with vehicle (0.9% saline) at Day 7 and Day 14 after pump implantation and in another 2 animals who died at Day 2 and Day 24 after pump implantation filled with bosutinib. These infections occurred despite precautions to minimize the infectious risk by performing all steps (preparation of bosutinib stock solution, filling/precalibration of Alzet® micro-osmotic pumps, and the surgical procedure) under sterile conditions. Aside from these 4 premature losses of animals, no additional adverse effects were observed in the remaining 12 rats, and the pumps as well as the drug and vehicles were well tolerated.
Figure 1.
(A) Body weight gain of juvenile rats and (B) calculated daily administered bosutinib doses during chronic exposure via subcutaneously implanted micro-osmotic pumps (Mean ± standard deviation). Black arrows indicate the points of time when the first and second pump of 2 consecutively implanted pumps were implanted. Bosutinib doses were calculated based on the fixed concentrations of bosutinib dissolved in DMSO in the micro-osmotic pumps, the fixed constant pumping rate, and the measured body weights of the growing animals. (pooled controls: ○; bosutinib target concentration: ▼ 2.5 mg/kg/day; △ 5.0 mg/kg/day)
Bosutinib serum levels
Based on the fixed pump rate of 0.5 μl/h and the fixed bosutinib concentration in the micro-osmotic pumps, a calculation of the daily drug dose per kg body weight applied was performed for the growing animals. Results plotted against time are shown in Figure 1B. At the end of the exposure time, the micro-osmotic pumps were completely emptied, revealing total release of the drug. The plasma elimination half-life of bosutinib in rats is reported to be in the range of 3.0–3.7 h after oral or intravenous administration [17].
Thus, when the animals were killed during the late morning hours of Day 29 (when probably 3–4 half-life times had passed after the pumps were exhausted), the blood still had measurable drug levels. Animals receiving target bosutinib doses of 2.5 mg/kg/day and 5.0 mg/kg/day exhibited mean bosutinib serum levels of 1.37±0.32 ng/ml and 2.79±0.78 ng/ml, respectively.
Bone length
No differences in bone lengths could be observed in controls receiving either 100% DMSO or 0.9% sterile saline; therefore, these data were pooled for statistical analysis using Prism software for Windows, version 5.04 (GraphPad Software, Inc., La Jolla, CA, USA). Due to the small number of animals, bone lengths were analyzed using the Kruskal-Wallis test to determine significance between bosutinib-treated groups and pooled control groups.
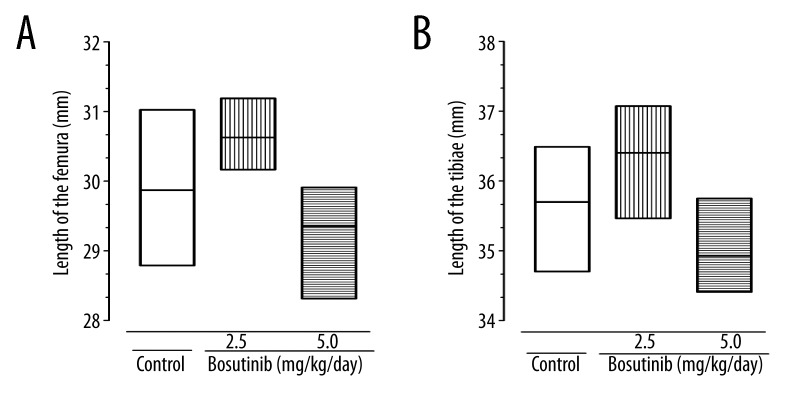
Bone length was not affected in animals receiving the lower dose of bosutinib and even showed a tendency to be increased (Figure 2A, 2B). The higher targeted bosutinib dose of 5.0 mg/kg/day resulted in a non-significant tendency of reduced femoral and tibial bone length (p=0.09).
Figure 2.
Length of the (A) femur, and (B) tibia. Bone length was determined using Merox® digital caliper with a precision of 0.01 mm. Measured data are presented as floating bars showing the minimum, maximum, and mean.
Discussion
To maintain its quality, bone is continuously remodeled during the lifetime. The long-term consequences of TKI treatment in growing humans on bone metabolism are still unclear. Previously, in a juvenile growing rat model, we demonstrated that TKIs, like imatinib and dasatinib, reduce bone length and trabecular bone mineral density [18]. Contrasting these observations with first- and second-generation TKIs, we here show that the third-generation TKI, bosutinib, exerts only minor effects on growing bone. Until now, in vivo data on the influence of bosutinib on the growing bones in children has not been available. Clinical phase III trials are focusing on the efficacy and safety of bosutinib in comparison to imatinib in newly diagnosed adult patients with Ph+ leukemia [19].
Micro-osmotic pumps have the advantage of continuous release of a drug due to the constant pumping rate, but the disadvantage of continuous decline in the daily drug dose exposure ratio (expressed as dose per kg body weight per day) in rapidly growing, body weight gaining juvenile animals. During the 28-day duration of the experiment, the average body weight of a rat increased more than 3-fold (from 100–150 g at age 4 weeks to 250–310 g at age 8 weeks, see Figure 1A). With regard to the expected increasing body weight, we calculated a daily bosutinib dose in such a way that at the start of the experiment approximately 150% of the targeted daily dose was administered. This targeted daily dose was calculated on a body weight of 130 g (at 5 weeks of age) for the drug’s concentration in the first pump and 220 g (at 7 weeks of age) in the second pump. Because the pumps delivered a constant dose over time, the resulting dose per kg body weight continuously declined in such a way that the targeted daily dose was achieved at the middle of the implantation period after 1 week, but only 50% was administered by the end of the implantation in the second week (Figure 1B). However, drug administration via micro-osmotic pumps depends on the concentration of the drug solution within the pump, which is only limited by its solubility in the vehicle and was uncomplicated because the solubility of bosutinib is >50 mg/ml in 100% DMSO. Furthermore, contrasting the manufacturer’s instructions recommending a maximum concentration of 50% DMSO for vehicle in Alzet micro-osmotic pumps, we could show that these pumps also tolerate and work reliably with 100% DMSO as vehicle.
Long-term oral administration of a fixed drug dose based on body weight is a specific problem in pediatrics during rapid growth of neonates and infants. Liquid formulations like syrups allow correct dosing if available, and tablets may be divided and the dose is adjusted to the nearest size of the smallest tablet. For a body weight adjusted and continuous drug exposure over prolonged period in juvenile rats, daily subcutaneous or intraperitoneal injections would be the most exact method; however, repeated injection may be associated with increased losses of the animals because of associated injures or infections. Also these procedures would require working hours of research staff members on weekends. The same holds true for daily oral drug application by gavage in young animals. Young, still-growing rats are very sensitive to stress and to pharyngeal or esophageal injuries caused by gavage. It remains to be shown whether this risk can be compensated for by experienced staff who are specially trained on performing gavage in young animals.
Subcutaneous implantation of micro-osmotic pumps is expensive and requires skilful technique for implantation of the device under general anesthesia, but it circumvents the problem of manipulating the animals daily. The fact that 4 animals died from peritoneal infections (none of which were localized subcutaneously where the pump was implanted) points to the necessary sterile requirements, but also stresses that young animals are especially prone to infectious complications. However, micro-osmotic pumps represent an attractive alternative for continuous release of the drug in still-growing animals.
Bosutinib serum levels determined 30 min after a single-bolus intravenous administration of 2 mg/kg and 5 mg/kg bosutinib are reported to be in the range of 330±57 ng/mL and 554±62 ng/mL, respectively [25,26]. In contrast, the serum levels we achieved after continuous subcutaneous were almost 200 times lower, but turned out to be 2 times higher than after oral administration of 10 mg/kg/day bosutinib, reported to be maximally 1.23 ng/mL [26]. As discussed above, juvenile rats triple their body weight during the time period of micro-osmotic exposure, leading to a relative TKI overdose during the first week after pump implantation, followed by relative underdosage. Because the serum was collected at the end of the experiment after the period of underdosage of bosutinib and when the pumps were empty, the levels represent just the serum concentration during the wash-out period after 3–4 half-life times had passed.
Conclusions
Until now, therapy with TKI in humans has usually been lifelong therapy, leading to different judgments on long-term adverse effects from the viewpoints of either a pediatric or older adult patient with CML. Since pediatric patients with CML may experience growth retardation under imatinib treatment, bosutinib may offer a new therapeutic option, avoiding this adverse effect on the growing bone. However, the chosen exposure time frame of 28 days in this juvenile rat model, spanning the period from end of weaning until late puberty, does not reflect a lifelong TKI therapy scheme as envisaged in pediatric patients. Clearly, further research is needed to assess the influence of bosutinib on growing bone during prolonged exposure by more precisely evaluating this adverse effect for pediatric patients in the clinical setting.
Footnotes
Source of support: This work was supported by grants DFG SU122/3-1 to MS, HO1875/10-1 to LCH, Austrian Science Fund FWF I 734-B13 to RGE, and by Peter Escher Foundation, Leipzig
References
- 1.Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. Mayo Clin Proc. 2006;81:973–88. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Lydon B. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest. 2000;105:3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenone S, Zanoli S, Brullo C, et al. Current Advances in the Development of Anticancer Drugs Targeting Tyrosine Kinases of the Src Family. Current Drug Therapy. 2008;3:158–76. [Google Scholar]
- 4.Remsing Rix LL, Rix U, Colinge J, et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia. 2009;23:477–85. doi: 10.1038/leu.2008.334. [DOI] [PubMed] [Google Scholar]
- 5.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–76. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman E, Nicolaides M, Maki RG, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354:2006–13. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- 7.Fitter S, Dewar AL, Kostakis P, et al. Long-term imatinib therapy promotes bone formation in CML patients. Blood. 2008;111:2538–47. doi: 10.1182/blood-2007-07-104281. [DOI] [PubMed] [Google Scholar]
- 8.Mariani S, Giona F, Basciani S, et al. Low bone density and decreased inhibin-B/FSH ratio in a boy treated with imatinib during puberty. Lancet. 2008;372:111–12. doi: 10.1016/S0140-6736(08)61023-5. [DOI] [PubMed] [Google Scholar]
- 9.Schmid H, Jäger B, Lohse J, Suttorp M. Longitudinal growth retardation in a prepupertal girl with chronic myeloid leukemia on long-term imatinib treatment. Haematologica. 2009;94:1177–79. doi: 10.3324/haematol.2009.008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimoto T, Inoue M, Kawa K. Growth deceleration in a girl treated with imatinib. Int J Hematol. 2009;89:251–52. doi: 10.1007/s12185-008-0251-8. [DOI] [PubMed] [Google Scholar]
- 11.Millot F, Baruchel A, Guilhot J, et al. Imatinib is efficient but has a negative impact on growth in children with previously untreated chronic myelogenous leukaemia (CML) in early chronic phase (CP): results of the French national phase IV trial. (abstract) Blood. 2009;114:356. [Google Scholar]
- 12.Dewar AL, Cambareri AC, Zannettino AC, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–32. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 13.Knight B, Tirnitz-Parker JE, Olynyk JK. C-kit inhibition by imatinib mesylate attenuates progenitor cell expansion and inhibits liver tumor formation in mice. Gastroenterology. 2008;135:969–79. doi: 10.1053/j.gastro.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 14.Tibullo D, Barbagallo I, Giallongo C, et al. Effects of second-generation tyrosine kinase inhibitors towards osteogenic differentiation of human mesenchymal cells of healthy donors. Hematol Oncol. 2012;30:27–33. doi: 10.1002/hon.988. [DOI] [PubMed] [Google Scholar]
- 15.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–22. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 16.Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6:834–48. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 17.Pfizer Laboratories. Pharmacology/Toxicology NDA Review and Evaluation. Food and Drug Administration (FDA) Center for Drug Evaluation and Research; NDA 2011 ID: 203341 Available from: URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203341Orig1s000PharmR.pdf. [Google Scholar]
- 18.Tauer JT, König S, Hofbauer LC, Suttorp M. A rat model to predict alterations in bone growth and metabolism in children with CML on imatinib. Haematologica. 2011;96:28. (abstract) [Google Scholar]
- 19.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486–92. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibullo D, Giallongo C, La CP, et al. Effects of imatinib mesylate in osteoblastogenesis. Exp Hematol. 2009;37:461–68. doi: 10.1016/j.exphem.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Id BH, Lagneaux L, Najar M, Piccart M, et al. The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer. 2010;10:298. doi: 10.1186/1471-2407-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandyke K, Dewar AL, Diamond P, et al. The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J Bone Miner Res. 2010;25:1759–70. doi: 10.1002/jbmr.85. [DOI] [PubMed] [Google Scholar]
- 23.O’Sullivan S, Lin JM, Watson M, et al. The skeletal effects of the tyrosine kinase inhibitor nilotinib. Bone. 2011;49:281–89. doi: 10.1016/j.bone.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Tokunaga A, Oya T, Ishii Y, et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23:1519–28. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 25.Liang S, Pong K, Gonzales C, et al. Neuroprotective profile of novel SRC kinase inhibitors in rodent models of cerebral ischemia. J Pharmacol Exp Ther. 2009;33:827–35. doi: 10.1124/jpet.109.156562. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Luan X, Liu H, et al. Pharmacokinetics and metabolite identification of a novel VEGFR-2 and Src dual inhibitor 6-chloro-2-methoxy-N-(2-methoxybenzyl) acridin-9-amine in rats by liquid chromatography tandem mass spectrometry. Talanta. 2012;89:70–76. doi: 10.1016/j.talanta.2011.11.061. [DOI] [PubMed] [Google Scholar]