Abstract
When the allele of a wild species at a quantitative trait locus (QTL) conferring a desirable trait is introduced into cultivated species, undesirable effects on other traits may occur. These negative phenotypic effects may result from the presence of wild alleles at other closely linked loci that are transferred along with the desired QTL allele (i.e., linkage drag) and/or from pleiotropic effects of the desired allele. Previously, a QTL for resistance to Phytophthora infestans on chromosome 5 of Solanum habrochaites was mapped and introgressed into cultivated tomato (S. lycopersicum). Near-isogenic lines (NILs) were generated and used for fine-mapping of this resistance QTL, which revealed coincident or linked QTL with undesirable effects on yield, maturity, fruit size, and plant architecture traits. Subsequent higher-resolution mapping with chromosome 5 sub-NILs revealed the presence of multiple P. infestans resistance QTL within this 12.3 cM region. In our present study, these sub-NILs were also evaluated for 17 horticultural traits, including yield, maturity, fruit size and shape, fruit quality, and plant architecture traits in replicated field experiments over the course of two years. Each previously detected single horticultural trait QTL fractionated into two or more QTL. A total of 41 QTL were detected across all traits, with ∼30% exhibiting significant QTL × environment interactions. Colocation of QTL for multiple traits suggests either pleiotropy or tightly linked genes control these traits. The complex genetic architecture of horticultural and P. infestans resistance trait QTL within this S. habrochaites region of chromosome 5 presents challenges and opportunities for breeding efforts in cultivated tomato.
Keywords: tomato, Solanum lycopersicum, introgression, QTL mapping, linkage drag
The value of wild species as sources of genetic diversity for breeding their crop species relatives has long been appreciated, and there are many examples of their use in crop improvement (Labate and Robertson 2012). In modern breeding, introgression of genes controlling useful traits from wild into cultivated species is often facilitated by first associating molecular marker genotypes with trait phenotypes, a process known as gene mapping or quantitative trait locus (QTL) mapping (Collard et al. 2005). Once the chromosomal locations of QTL controlling a particular trait are known, marker-assisted selection (MAS) can be used to transfer wild alleles with favorable trait effects into a crop species while simultaneously selecting against wild alleles at other loci to create improved varieties (Xu and Crouch 2008). MAS breeding can be particularly effective in the improvement of quantitative traits, traits that are controlled by a few to many genes that may interact with each other and with the environment (St. Clair 2010). However, effective use of QTL alleles from wild species can be complicated by linkages among desirable and undesirable trait loci and/or by interactions of QTL with the environment (Bernardo 2008). Genes controlling a desirable trait may also affect one or more other traits, a phenomenon known as pleiotropy (Chen and Luebberstedt 2010).
Tomato is an important crop worldwide and is the second most valuable vegetable in United States production (National Agriculture Statistics Service 2011). Cultivated tomato (Solanum lycopersicum) has limited genetic diversity in comparison with its diverse wild tomato species relatives, primarily because of genetic bottlenecks during domestication (Miller and Tanksley 1990; Park et al. 2004). Wild Solanum habrochaites is an important source of genetic diversity for tomato improvement. Among the traits that can be improved via introgressions from S. habrochaites are horticultural traits, such as yield, fruit size, and fruit quality (Bernacchi et al. 1998b; Monforte and Tanksley 2000; Yates et al. 2004; Ben Chaim et al. 2006; Mathieu et al. 2009), and resistance to diseases, such as late blight, bacterial canker, gray mold, and early blight (Foolad et al. 2002; Zhang et al. 2003; Brouwer et al. 2004; Brouwer and St. Clair 2004; Coaker and Francis 2004; Finkers et al. 2007). Most of these horticultural and resistance traits are quantitatively inherited.
A very desirable trait of S. habrochaites is its resistance to Phytophthora infestans, the oomycete pathogen responsible for late blight disease of tomato and its close relative, potato (Brouwer et al. 2004; Foolad et al. 2008). Late blight disease causes significant losses in tomato, resulting in approximately $5 billion in crop losses and chemical control costs annually (Judelson and Blanco 2005). Brouwer et al. (2004) mapped QTL for quantitative resistance to P. infestans from S. habrochaites on each of the 12 tomato chromosomes. Brouwer and St. Clair (2004) further refined the locations of three of the QTL (on chromosomes 4, 5, and 11) by fine-mapping with near-isogenic lines (NILs). These three resistance QTL were also found to be colocated and/or linked with some QTL having negative effects on horticultural traits, such as plant height, plant shape, maturity, yield, and fruit size, suggesting linkage drag.
Our interests in understanding the genetic basis of QTL controlling horticultural traits and their linkage relationships with QTL for resistance to P. infestans, as well as in developing material useful for breeding improved tomato varieties, led us to conduct further investigations regarding the introgressed chromosome 5 region from S. habrochaites. In a companion study (Johnson et al. 2012), we mapped the chromosome 5 resistance QTL reported by Brouwer and St. Clair (2004) at higher resolution with sub-NILs. We found that the resistance QTL located in a 12.3 cM region subsequently fractionated into two and three QTL groups for foliar and stem resistance, respectively. In the present study we used the same set of chromosome 5 sub-NILs as Johnson et al. (2012), focusing on mapping loci associated with horticultural traits and determining linkage relationships among these loci and the late blight resistance QTL. Our goals were as follows: (1) to determine the genetic architecture and environmental stability of QTL controlling horticultural traits within the chromosome 5 introgressed region from S. habrochaites; (2) to determine the linkage relationships among loci controlling horticultural and P. infestans resistance traits; and (3) to assess the implications of trait genetic architecture, QTL environmental stability, and linkage relationships among QTL for the potential improvement of cultivated tomato via breeding.
Materials and Methods
Plant materials, genotyping, and marker-assisted selection
We developed a set of sub-NILs in S. lycopersicum for a chromosome 5 introgression from a P. infestans–resistant donor parent, wild tomato S. habrochaites accession LA 2099, via marker-assisted selection during backcrossing and selfing generations, as described by Johnson et al. (2012). Genomic DNA extractions, genotyping with chromosome 5 PCR-based markers (SCAR, CAPS, and SSR), primer sequences, enzymatic reaction conditions, and restriction enzymes used for each marker have been described by Johnson et al. (2012).
We genotyped 1589 BC6S1 progeny to identify recombinant sub-NIL progeny; a subset of 652 progeny (150 recombinant plus 502 nonrecombinant) was used to construct a linkage map for the introgressed region (see Linkage and QTL mapping section). Heterozygous recombinant BC6S1 individuals underwent self-pollination and progeny were marker-selected to obtain homozygous recombinant BC6S2 sub-NILs. These plants underwent self-pollination to obtain ample BC6S3 sub-NIL seeds for replicated field experiments. We evaluated 58 BC6S3 sub-NILs in the 2009 field experiments. In the 2010 field experiments, a subset of 41 of the 58 sub-NILs was evaluated to allow increased replication per location while reducing genetic redundancy, as explained by Johnson et al. (2012). Graphical marker genotypes for the 58 selected BC6S3 sub-NILs are presented in Supporting Information, Table S1.
Field experimental design and procedures
BC6S3 sub-NILs and the parental NIL from which they were derived (subsequently referred to as NIL5) were evaluated in replicated experiments at field locations in Salinas, California (designated as locations 1 and 2) and in Davis, California (locations 3 and 4) over the course of 2 years. Summer in Salinas is generally cool and humid, which is conducive to late blight disease development, whereas Davis summers are warm and dry, typical of processing tomato production areas in California’s Central Valley.
Seedlings were grown in a greenhouse for 6 wk and then transplanted into the field locations. Sixty-one genotypes (NIL5, 58 sub-NILs for chromosome 5, and two commercial processing cultivars, E6203 and Hypeel 45) and 44 genotypes (NIL5, 41 sub-NILs, Hypeel 45, and E6203) were included in the 2009 and 2010 experiments, respectively. Experiments were arranged in a randomized complete block design. For both years, one plot per genotype per block was included, except for controls, for which there were two plots per block. In 2009, three blocks per location were used. In 2010, use of a reduced number of 41 sub-NILs enabled replication to be increased to five blocks in each of locations 1 and 2 and to four blocks in each of locations 3 and 4. At each of the four locations, each plot consisted of five plants spaced 0.30 m apart in rows separated by 1.02 m in locations 1 and 2, and by 1.52 m in locations 3 and 4. Border rows and plots with the cultivar E6203 surrounded each experiment at each location. Standard horticultural field practices for processing tomato were used at all locations. Locations 1 and 2 were sprinkler-irrigated, whereas locations 3 and 4 were furrow-irrigated, as needed.
Phenotypic trait evaluations
All traits (Table 1) were evaluated on a per-plot basis. Vegetative horticultural traits were evaluated at all four locations. Late blight disease was only evaluated in Salinas (locations 1 and 2) because this disease did not occur in Davis, as expected, because of typical warm, dry summers. Reproductive traits were only evaluated at Davis (locations 3 and 4) because of logistics of timely sampling of ripe fruit. Vegetative horticultural traits evaluated were plant height (H) and width (W) in cm, canopy density (CD; visual rating scale: 1 = very sparse to 5 = very dense), and plant habit (HAB; visual rating scale: 1 = prostrate to 5 = very upright). H, W, CD, and HAB were assessed at locations 1 and 2 at 71 and 46 days after planting (DAP) in 2009 and 2010, respectively. At locations 3 and 4, these traits were evaluated at 80 DAP in 2009 and at 68 (H and W) and 73 (CD and HAB) DAP in 2010. From plant height and width, two secondary traits were derived, plant size (SZ; product of height × width) and shape (SH; ratio of height to width). The reproductive horticultural traits measured or scored were as follows. DAP to maturity was evaluated at two stages of maturity: when each plant in the plot had its first ripe fruit (DAP1st) and when 50% of fruit in a plot were ripe (DAP50). The weight of 30 ripe fruits was evaluated when 50% of fruit in a plot were ripe (30Wt). Yield in kg (YLD) was evaluated when 95% of the fruit in a plot were ripe. Ripe fruit were used to obtain the weight of 100 seeds (SW), which was measured only in 2009 because of labor limitations. The ripe fruit quality traits pH and Brix (i.e., sugar content or soluble solids) were measured using a pureed sample of 10 whole fruits obtained from plots with 50% ripe fruit using an Oakton pH Testr2 (Oakton Instruments, Vernon Hills, IL) and a Reichert AR200 digital refractometer (Reichert Technologies, Buffalo, NY), respectively. Size traits obtained for ripe fruit were fruit perimeter (FP), fruit width (FW; width at mid height), and fruit height (FH; height at mid width). These traits were measured on flatbed scanner images of eight longitudinally sliced fruit per plot using Tomato Analyzer software (Brewer et al. 2006), which refers to fruit length as height and to fruit longitudinal circumference as perimeter. From FH and FW, the secondary variable, fruit shape (FS; ratio of FH to FW), was obtained. Trait names, abbreviations, and brief descriptions are given in Table 1.
Table 1. Abbreviations for traits evaluated in this study.
| Trait Type | Abbreviation | Description |
|---|---|---|
| Late blight | LEAF | AUDPC for foliar symptoms |
| STEM | AUDPC for stem symptoms | |
| Maturity | DAP1st | Time after planting to first ripe fruit (d) |
| DAP50 | Time after planting to 50% ripe fruit (d) | |
| Yield | YLD | Fruit yield (kg) |
| Fruit size/shape | FH | Fruit height (mm) |
| FW | Fruit width (mm) | |
| FS | Fruit shape (FH × FW; mm2) | |
| FP | Fruit perimeter (mm) | |
| 30Wt | Weight of 30 fruits (g) | |
| Fruit quality | Brix | Brix (soluble solids content) |
| pH | Fruit acidity | |
| Plant | CD | Canopy density (visual rating: 1 = very sparse to 5 = very dense) |
| architecture | HAB | Plant habit (visual rating: 1 = prostrate to 5 = very upright) |
| H | Plant height (cm) | |
| W | Plant width (cm) | |
| SH | Plant shape (H:W; cm2) | |
| SZ | Plant size (H × W; cm2) | |
| SW | Weight of 100 seeds (g) |
AUDPC, area under the disease progress curve.
On September 15, 2009, Salinas locations 1 and 2 were inoculated with a local P. infestans isolate per Johnson et al. (2012). In 2010 in mid September, a natural P. infestans infection occurred in both locations, precluding the need for inoculation. As described by Johnson et al. (2012), phenotypic scoring of late blight disease symptoms was performed visually and symptom data were used to calculate area under the disease pressure curve (AUDPC) for foliar and stem disease symptom progression (referred to as LEAF and STEM, respectively). Lower AUDPC values indicate less disease symptom progress and are therefore indicative of increased disease resistance.
Statistical data analysis
Data for each trait (Table S2) were tested for normality using the Shapiro/Wilk W statistic in PROC UNIVARIATE in SAS (SAS Institute, Cary, NC) and for homogeneity of variance using the Levene's test. Data for heteroscedastic traits were weighted by the reciprocal of the variance for those terms with significant departure from the assumption of equal variance. ANOVA for each trait was performed using PROC GLM in SAS with the following linear additive model for a randomized complete block design and multiple locations:
where Trait was a given phenotypic trait, Loc was the effect of location, Genotype was the individual sub-NIL or control (NIL5, E6203, Hypeel 45), * indicated an interaction, and parentheses indicated a nested variable. Block(Loc) was considered a random variable. Significant genotype × location interactions were detected in 2009 for traits LEAF, DAP50, YLD, FH, FS, FP, 30Wt, Brix, pH, CD, H, W, SH, and SZ, and in 2010 for STEM, DAP1st, DAP50, FH, FW, FS, FP, 30Wt, Brix, pH, height, width, SH, and SZ. For these traits, separate analyses were conducted for each location. PROC MIXED in SAS was used to estimate least squares means, because of missing data for some traits, and to perform means separation with Tukey's HSD.
Pearson correlation coefficients (r) were calculated for pairwise combinations of all trait genotypic means in 2009 and in 2010 using Proc CORR in SAS. Only significant (P ≤ 0.05) correlations ≥0.4 are reported.
Linkage and QTL mapping
A linkage map for the introgressed region was constructed using DNA marker genotype data across 17 loci for 652 BC6S1 progeny (150 recombinants plus 502 nonrecombinants). The map was constructed with JoinMap 3.0 (van Ooijen and Voorips 2001) using the Kosambi mapping function and a 3-LOD significance threshold. After the release of the tomato genome sequence (Sato et al. 2012), we developed additional markers using the SL2.40 genome build (http://solgenomics.net) to help define the physical extent of the chromosome 5 S. habrochaites introgression within NIL5 (File S1) .
The Composite Interval Mapping (CIM) module in WinQTLCartographer2.5 (Wang et al. 2011) was used for detection of QTL using sub-NIL means obtained from ANOVA for each trait. QTL mapping was performed using CIM Model 6 (standard model) and the forward and backward regression method with a walk speed of 1 cM and a window size of 2 cM. Trait-specific permuted LOD thresholds (P = 0.05) were empirically established for each trait using 1000 permutations (Churchill and Doerge 1994) in WinQTLCartographer.
A QTL for a trait was considered significant at P ≤ 0.05 if the peak LOD value exceeded the trait-specific permuted threshold. Multiple QTL were declared for a single trait when the LOD values between significant (P ≤ 0.05) peaks within the introgressed region decreased below the significance threshold for at least two contiguous markers. Each significant QTL was denoted by trait name, location, and year. For example, DAP1st34_2009 is a QTL detected in the analysis of DAP1st trait data from locations 3 and 4 in 2009.
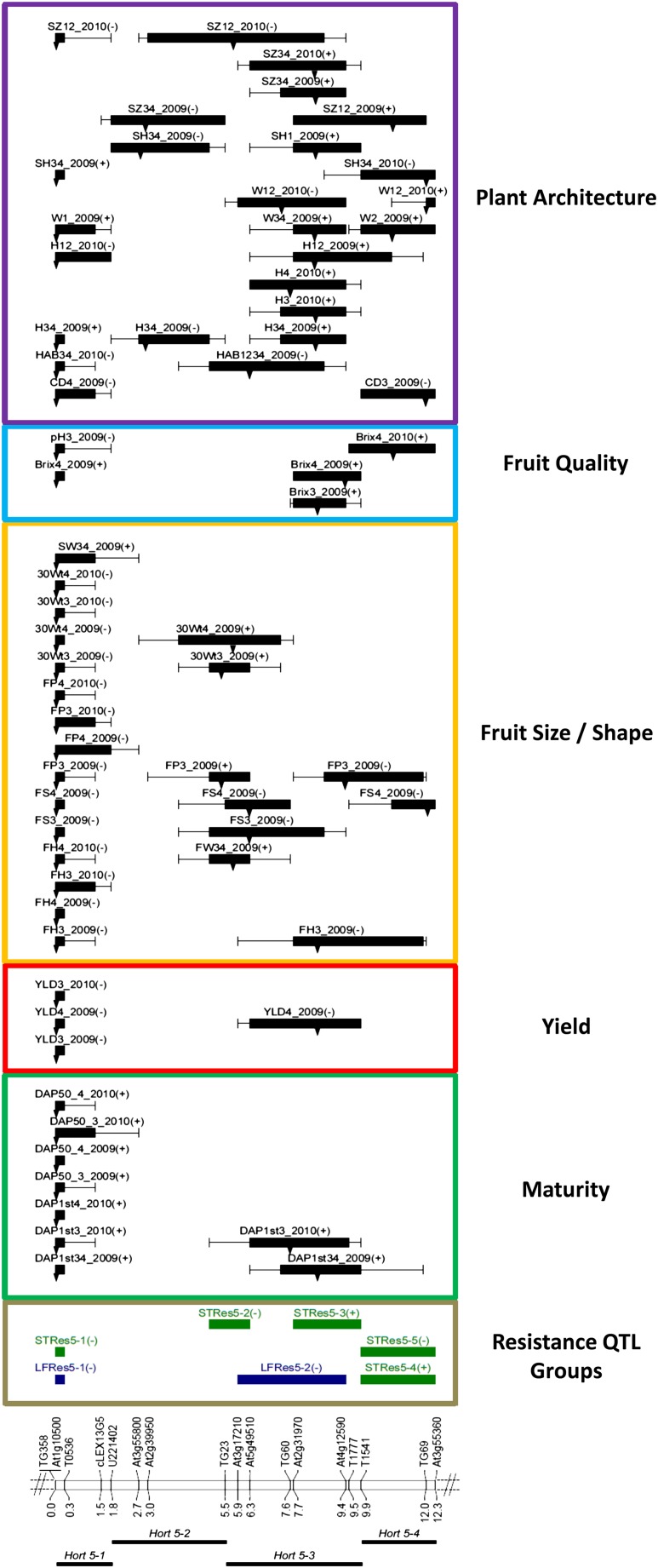
A linkage map showing locations of significant QTL was constructed using MapChart2.1 (Voorips 2002). QTL locations were indicated as 1-LOD bars and 2-LOD whiskers (Figure 1). For purposes of discussion, QTL were assigned to QTL trait groups (delineated as Hort 5-1 through Hort 5-4) based on coincidence of their 1-LOD support intervals. Although a few of the QTL have 1-LOD support intervals that extended beyond the boundary of their assigned group, their peak locations supported their placement in their respective groups.
Figure 1.
QTL mapped to a chromosome 5 region introgressed from Solanum habrochaites to S. lycopersicum. Horticultural trait QTL and Phytophthora infestans resistance QTL groups detected in chromosome 5 sub-NILs evaluated in 2009 and 2010 field experiments, sorted by trait class. Below the linkage map are horticultural trait QTL group names, locations, and distances in cM; above the linkage map are P. infestans resistance trait QTL groups (LfRes and StRes refer to LEAF and STEM resistance, respectively) (Johnson et al. 2012) and QTL detected for horticultural traits, sorted by trait class. Boxes and whiskers show 1-LOD and 2-LOD intervals, respectively. Arrows on QTL bars indicate LOD peak locations. QTL names are given by trait, location(s), and year evaluated (see Materials and Methods section). The effect of the S. habrochaites allele at a QTL is indicated after the QTL name: (−) indicates a decrease in that trait value.
Comparisons were made among QTL for P. infestans resistance traits (LEAF and STEM) (Johnson et al. 2012) and horticultural traits for QTL coincidence by visual inspection of their chromosomal locations on the linkage map. A statistical test based on the hypergeometric probability distribution (Lin et al. 1995) was used to calculate QTL correspondence, the probability of obtaining the observed number of matching QTL by chance. A QTL match was declared when the 1-LOD support intervals overlapped. The number of comparison intervals was seven, based on the average size of our resistance and horticultural trait QTL (1.9 cM) and the overall map distance of the S. habrochaites introgression (12.3 cM).
Our QTL locations were also compared to those previously reported for both disease resistance and horticultural trait QTL on chromosome 5 in tomato and in potato, based on common markers as well as genomic sequence data for both crop species. Sources used for QTL location comparisons included the following: tomato (Eshed and Zamir 1996; Bernacchi et al. 1998b; Zhang et al. 2002; Causse et al. 2004; Coaker and Francis 2004; Semel et al. 2006; Brewer et al. 2007; Jones et al. 2007); potato (Collins et al. 1999; Visker et al. 2003; Visker et al. 2005; Sliwka et al. 2007; Achenbach et al. 2009; Danan et al. 2011); and genomic sequences (http://solgenomics.net; Xu et al. 2011; Sato et al. 2012). When common markers were not available, the Tomato-Expen 2000 map (Fulton et al. 2002b) available on the Sol Genomics Network (http://solgenomics.net; Bombarely et al. 2011) was used to facilitate map alignment.
Selection of sub-NIL breeding lines
Truncation selection was applied sequentially for traits LEAF, YLD, FP, 30Wt, and DAP1st to identify breeding lines potentially useful for development of tomato varieties with improved resistance to P. infestans. Out of 41 sub-NILs evaluated in both years, the first round of truncation removed seven lines with LEAF values less resistant than control cultivar E6203 in 2 years or locations. The second round removed eight lines with YLD <66% of E6203 in 2 years or locations, whereas the third round removed six lines with FP <92% of E6203 in 2 years or locations. The fourth round removed six lines with 30Wt <80% of E6203 in 2 years or locations. The final round removed three lines with DAP1st more than 10 days later than E6203 in 2 years or locations; however, five lines that would have been removed based on this criterion were kept because of their relatively higher levels of foliar resistance to P. infestans (i.e., lower LEAF values). At the end of the selection process, 11 lines were chosen.
Results
ANOVA
In 2009, 61 genotypes (sub-NILs and controls) were evaluated for late blight disease symptom traits (Johnson et al. 2012) and horticultural traits (Table 1). For all traits, genotypes were significantly different (P ≤ 0.05) (Table 2). Significant genotype × location interactions were detected in 2009 for LEAF, DAP50, YLD, FH, FS, FP, 30Wt, Brix, pH, CD, H, W, SH, and SZ. As a result, these traits were analyzed separately by location. R2 values per trait ranged from 0.45 to 0.91.
Table 2. Summary of ANOVA performed on trait data.
| F Values | |||||||
|---|---|---|---|---|---|---|---|
| Trait Class | Trait Code | Trait | Year | Location | Genotype | Location | R2 |
| Late blight | LEAF | Leaf AUDPC | 2009 | 1 | 1.69‡ | — | 0.47 |
| resistance | 2 | 2.77‡ | — | 0.77 | |||
| 2010 | 1 & 2 | 7.02‡ | 68.70‡ | 0.77 | |||
| STEM | Stem AUDPC | 2009 | 1 & 2 | 5.03‡ | 0.01 ns | 0.64 | |
| 2010 | 1 | 5.63‡ | — | 0.59 | |||
| 2 | 5.78‡ | — | 0.66 | ||||
| Maturity | DAP1st | Time to first ripe fruit (d) | 2009 | 3 & 4 | 11.01‡ | 44.19† | 0.78 |
| 2010 | 3 | 14.94‡ | — | 0.83 | |||
| 4 | 7.13‡ | — | 0.71 | ||||
| DAP50 | Time to 50% ripe fruit (d) | 2009 | 3 | 12.82‡ | — | 0.86 | |
| 4 | 6.88‡ | — | 0.77 | ||||
| 2010 | 3 | 34.47‡ | — | 0.92 | |||
| 4 | 39.79‡ | — | 0.86 | ||||
| Yield | YLD | Yield | 2009 | 3 | 6.25‡ | — | 0.76 |
| 4 | 2.74‡ | — | 0.60 | ||||
| 2010 | 3 | 3.89‡ | — | 0.56 | |||
| Fruit size/shape | FH | Fruit height | 2009 | 3 | 6.37‡ | — | 0.77 |
| 4 | 10.13‡ | — | 0.83 | ||||
| 2010 | 3 | 19.30‡ | — | 0.86 | |||
| 4 | 14.53‡ | — | 0.83 | ||||
| FW | Fruit width | 2009 | 3 & 4 | 5.07‡ | 26.66† | 0.68 | |
| 2010 | 3 | 5.89‡ | — | 0.66 | |||
| 4 | 7.35‡ | — | 0.71 | ||||
| FS | Fruit shape | 2009 | 3 | 18.79‡ | — | 0.91 | |
| 4 | 18.26‡ | — | 0.90 | ||||
| 2010 | 3 | 23.99‡ | — | 0.89 | |||
| 4 | 16.88‡ | — | 0.85 | ||||
| FP | Fruit size | 2009 | 3 | 3.14‡ | — | 0.63 | |
| 4 | 3.73‡ | — | 0.65 | ||||
| 2010 | 3 | 8.44‡ | — | 0.74 | |||
| 4 | 9.35‡ | — | 0.76 | ||||
| 30Wt | Fruit weight | 2009 | 3 | 11.03‡ | — | 0.84 | |
| 4 | 6.97‡ | — | 0.77 | ||||
| 2010 | 3 | 14.84‡ | — | 0.83 | |||
| 4 | 14.65‡ | — | 0.83 | ||||
| SW | Seed weight | 2009 | 3 & 4 | 7.45‡ | 27.11‡ | 0.71 | |
| Fruit quality | Brix | Brix | 2009 | 3 | 4.50‡ | — | 0.69 |
| 4 | 4.44‡ | — | 0.71 | ||||
| 2010 | 3 | 10.79‡ | — | 0.78 | |||
| 4 | 6.39‡ | — | 0.69 | ||||
| pH | pH | 2009 | 3 | 2.21‡ | — | 0.51 | |
| 4 | 2.71‡ | — | 0.58 | ||||
| 2010 | 3 | 2.83‡ | — | 0.49 | |||
| 4 | 2.44‡ | — | 0.45 | ||||
| Plant architecture | CD | Canopy density | 2009 | 1 & 2 | 4.97‡ | 0.32 ns | 0.59 |
| 3 | 2.78‡ | — | 0.59 | ||||
| 4 | 5.48‡ | — | 0.72 | ||||
| 2010 | 3 & 4 | 4.66‡ | 44.15‡ | 0.58 | |||
| H | Plant height | 2009 | 1 & 2 | 8.34‡ | 0.30 ns | 0.69 | |
| 3 & 4 | 9.75‡ | 60.27† | 0.76 | ||||
| 2010 | 1 & 2 | 5.75‡ | 426.88‡ | 0.67 | |||
| 3 | 5.95‡ | — | 0.66 | ||||
| 4 | 7.55‡ | — | 0.52 | ||||
| W | Plant width | 2009 | 1 | 3.85‡ | — | 0.71 | |
| 2 | 2.54‡ | — | 0.55 | ||||
| 3 & 4 | 6.27‡ | 252.89 | 0.72 | ||||
| 2010 | 1 & 2 | 4.71‡ | 75.32‡ | 0.54 | |||
| 3 & 4 | 15.92‡ | 26.86† | 0.75 | ||||
| Plant architecture | SH | Plant shape (H:W) | 2009 | 1 | 1.60* | — | 0.54 |
| 2 | 1.75† | — | 0.48 | ||||
| 3 & 4 | 3.91‡ | 3.19 ns | 0.55 | ||||
| 2010 | 1 & 2 | 3.81‡ | 106.51‡ | 0.43 | |||
| 3 & 4 | 7.29‡ | 81.65‡ | 0.61 | ||||
| SZ | Plant size | 2009 | 1 & 2 | 9.06‡ | 0.31 ns | 0.72 | |
| 3 & 4 | 11.24‡ | 193.22‡ | 0.80 | ||||
| 2010 | 1 & 2 | 5.60‡ | 192.19‡ | 0.66 | |||
| 3 & 4 | 17.28‡ | 4.01 ns | 0.75 | ||||
| HAB | Plant habit | 2009 | 1, 2, 3, 4 | 2.83‡ | 38.50‡ | 0.45 | |
| 2010 | 3 & 4 | 7.57‡ | 11.42* | 0.59 | |||
F test values and R2 values are presented for each analysis by trait, year, and location or combination of locations (see Materials and Methods section). R2 indicates the fit of the data to the linear additive model for each analysis. Late blight resistance results are from Johnson et al. (2012). —, not included in model; AUDPC, area under the disease progress curve; ns, not significant.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
In 2010, 41 genotypes were evaluated for disease symptom traits and horticultural traits (Table 1). For all traits, genotypes were significantly different (P ≤ 0.05) (Table 2). Significant genotype × location interactions were detected in 2010 for STEM, DAP1st, DAP50, FH, FW, FS, FP, 30Wt, Brix, pH, H, W, SH, and SZ. Therefore, these traits were analyzed separately by location. R2 values per trait ranged from 0.43 to 0.89. In general, foliar resistance to P. infestans (LEAF) exhibited higher R2 values than stem resistance (STEM), with the exception of location 1 in 2009. Horticultural traits involved with fruit size measurements had higher R2 values than those associated with fruit quality or plant architecture.
Means separation
There were significant (P ≤ 0.05) differences among genotypic means for all traits, except for CD in location 3 in 2009 (Table S1). In general, sub-NILs with S. habrochaites alleles at marker loci TG358 and At1g10500 matured significantly later (DAP1st and/or DAP50) than control cultivar E6203 in at least one trait and year or location combination, and most of these lines also had significantly reduced YLD and 30Wt. Relative to E6203, NIL5 exhibited significantly (P ≤ 0.05) greater foliar resistance to P. infestans (i.e., lower LEAF values) and increased Brix, but also had later maturity (DAP1st and DAP50) and larger plant size (H, W, SZ). Sub-NILs 08GH5516, 08GH5616, and 08GH5861 also displayed significantly (P ≤ 0.05) improved foliar resistance (LEAF) and larger plant size, with the latter two also having significantly higher Brix.
Correlations
Pearson correlation coefficients (r) were obtained for P. infestans resistance trait means with horticultural trait means within each year (Table 3). Only significant correlations (P ≤ 0.05) ≥0.4 are discussed. CD was weakly negatively correlated with LEAF and STEM (r = −0.41; range, −0.42 to −0.46, respectively). HAB was positively correlated with LEAF and STEM (r = 0.41; range, 0.57–0.64, respectively), with upright plants having higher AUDPC values (i.e., more susceptible). Maturity traits were negatively correlated with LEAF and STEM only in 2010, although this was influenced by location and time of maturity trait evaluation (DAP1st vs. DAP50). Significant (P ≤ 0.05) correlations were also found between pairs of horticultural traits (Table S3). Of particular note, YLD was negatively correlated with maturity traits DAP1st and DAP50 in both years (range, −0.50 to −0.78), and CD was significantly positively correlated with the maturity traits in both years (range, 0.53–0.83).
Table 3. Trait correlations.
| 2009 | LEAF1 | LEAF2 | |
|---|---|---|---|
| CD4 | −0.41† | ||
| HAB1234 | 0.41† | ||
| 2010 | LEAF12 | STEM1 | STEM2 |
| DAP1st3 | −0.53‡ | −0.44† | −0.45† |
| DAP1st4 | −0.53‡ | ||
| DAP50_3 | −0.47† | ||
| DAP50_4 | −0.47† | −0.46† | |
| YLD3 | 0.54‡ | 0.46† | |
| FH3 | 0.43† | ||
| FH4 | 0.42† | ||
| FW4 | 0.45† | ||
| FP3 | 0.43† | ||
| FP4 | 0.43† | ||
| 30Wt3 | 0.49† | ||
| 30Wt4 | 0.50† | ||
| Brix4 | 0.45† | 0.54‡ | |
| CD34 | −0.42† | −0.46† | |
| HAB34 | 0.57‡ | 0.64‡ | |
| H12 | 0.70‡ | 0.71‡ | |
| H3 | −0.65‡ | ||
| H4 | −0.61‡ | ||
| W12 | 0.48‡ | ||
| W34 | 0.67‡ | 0.77‡ |
Pearson correlation coefficients (r) among Phytophthora infestans resistance traits (LEAF and STEM) (data from Johnson et al. 2012) and horticultural traits were performed using genotype means. Only significant correlations ≥0.4 are presented. Trait names are given by year according to trait and location(s) (see Materials and Methods section).
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
Linkage mapping
The linkage map for the chromosome 5 introgressed region from S. habrochaites was 12.3 cM and spanned markers TG358 to At3g55360 (Figure 1). The average marker spacing was 0.7 cM and the largest gap was 2.5 cM between markers At2g39950 and TG23. Using additional markers developed from the SL2.40 S. lycopersicum tomato genome sequence build (http://solgenomics.net), we determined that the introgression in NIL5 extended north of TG358 to at least TG318, and south of At3g55360 to at least CT130. Comparison of our genetic map with the SL2.40 S. lycopersicum genome sequence also indicated that the order of markers TG69 and At3g55360 was reversed, suggesting an inversion or possibly errors in the reference genome sequence.
The 12.3 cM region from TG358 to At3g55360 corresponds to a physical distance of 2.35 Mbp in the SL2.40 S. lycopersicum genome sequence build, whereas the 12.0 cM region from TG358 to TG69 corresponds to a S. lycopersicum physical distance of 2.7 Mbp. However, the physical distances based on S. lycopersicum are approximate only because the introgressed region is from S. habrochaites, which may have a different physical distance. Based on the physical distance between markers in the S. lycopersicum genome sequence, the physical extent of the S. habrochaites introgression beyond the boundaries of our linkage map likely includes at least another 896 kb north of TG358 and at least another 680 kb south of TG69.
Mapped QTL
Within the introgressed chromosome 5 region containing resistance QTL lb5b (Brouwer and St. Clair 2004), 67 significant (P ≤ 0.05) QTL were detected for 17 horticultural traits (Figure 1 and Table 4). In 2009, 45 QTL were detected; 22 QTL were detected in 2010. If we consider multiple coincident QTL for the same trait as a single, unique QTL, then a total of 41 unique QTL were mapped across the 17 traits.
Table 4. Summary of significant QTL for horticultural traits.
| Trait Class | Trait Code | Trait | Group | Year | Location(s) | Peak Marker or Interval | Peak LOD/Threshold LOD | R2 |
|---|---|---|---|---|---|---|---|---|
| Maturity | DAP1st | Time to first ripe fruit (d) | Hort5-1 | 2009 | 3 & 4 | TG358 | 7.24/1.60 | 0.42 |
| 2010 | 3 | TG358 | 7.06/1.68 | 0.51 | ||||
| 2010 | 4 | TG358 | 4.82/1.70 | 0.41 | ||||
| Hort5-3 | 2009 | 3 & 4 | At2g31970–At4g12590 | 2.88/1.60 | 0.16 | |||
| 2010 | 3 | At2g31970 | 2.06/1.68 | 0.11 | ||||
| DAP50 | Time to 50% ripe fruit (d) | Hort5-1 | 2009 | 3 | TG358 | 4.42/1.71 | 0.29 | |
| 2009 | 4 | TG358 | 4.90/1.67 | 0.30 | ||||
| 2010 | 3 | TG358 | 3.26/1.63 | 0.30 | ||||
| 2010 | 4 | TG358 | 6.19/1.79 | 0.49 | ||||
| Yield | YLD | Yield | Hort5-1 | 2009 | 3 | TG358 | 13.42/1.63 | 0.65 |
| 2009 | 4 | TG358 | 10.09/1.75 | 0.54 | ||||
| 2010 | 3 | TG358 | 6.51/1.71 | 0.49 | ||||
| Hort5-3 | 2009 | 4 | At2g31970–At4g12590 | 3.40/1.75 | 0.16 | |||
| Fruit size/shape | FH | Fruit height | Hort5-1 | 2009 | 3 | TG358 | 10.20/1.79 | 0.42 |
| 2009 | 4 | TG358 | 5.14/1.68 | 0.24 | ||||
| 2010 | 3 | TG358 | 4.49/1.63 | 0.39 | ||||
| 2010 | 4 | TG358 | 4.77/1.76 | 0.41 | ||||
| Hort5-3 | 2009 | 3 | At2g31970–At4g12590 | 2.52/1.79 | 0.07 | |||
| FW | Fruit width | Hort5-2 | 2009 | 3 & 4 | At3g17210 | 4.11/1.64 | 0.27 | |
| FS | Fruit shape (H:W) | Hort5-1 | 2009 | 3 | TG358 | 4.97/1.69 | 0.16 | |
| 2009 | 4 | TG358 | 2.04/1.53 | 0.05 | ||||
| Hort5-2 | 2009 | 3 | At5g49510 | 3.86/1.69 | 0.12 | |||
| 2009 | 4 | At5g49510 | 4.24/1.53 | 0.12 | ||||
| Hort5-4 | 2009 | 4 | TG69 | 2.55/1.53 | 0.07 | |||
| FP | Fruit size | Hort5-1 | 2009 | 3 | TG358 | 6.83/1.69 | 0.38 | |
| 2009 | 4 | TG358 | 1.71/1.64 | 0.13 | ||||
| 2010 | 3 | TG358 | 2.85/1.74 | 0.27 | ||||
| 2010 | 4 | TG358 | 2.25/1.87 | 0.16 | ||||
| Hort5-2 | 2009 | 3 | TG23 | 2.74/1.69 | 0.12 | |||
| Hort5-4 | 2009 | 3 | At4g12590 | 2.15/1.69 | 0.09 | |||
| 30Wt | Fruit weight | Hort5-1 | 2009 | 3 | TG358 | 7.03/1.79 | 0.34 | |
| 2009 | 4 | TG358 | 5.09/1.58 | 0.29 | ||||
| 2010 | 3 | TG358 | 4.99/1.56 | 0.36 | ||||
| 2010 | 4 | TG358 | 4.72/1.80 | 0.39 | ||||
| Hort5-2 | 2009 | 3 | TG23 | 3.85/1.79 | 0.15 | |||
| 2009 | 4 | At3g17210 | 2.07/1.58 | 0.10 | ||||
| SW | Seed weight | Hort5-1 | 2009 | 3 & 4 | T0536 | 2.08/1.60 | 0.06 | |
| Fruit quality | Brix | Brix | Hort5-1 | 2009 | 4 | TG358 | 4.44/1.60 | 0.21 |
| Hort5-3 | 2009 | 3 | At2g31970–At4g12590 | 7.18/1.74 | 0.45 | |||
| 2009 | 4 | At4g12590 | 6.66/1.60 | 0.39 | ||||
| Hort5-4 | 2010 | 4 | T1541–TG69 | 4.73/1.65 | 0.41 | |||
| pH | pH | Hort5-1 | 2009 | 3 | TG358 | 2.54/1.68 | 0.18 | |
| Plant architecture | CD | Canopy density | Hort5-1 | 2009 | 4 | TG358 | 2.31/1.72 | 0.17 |
| Hort5-4 | 2009 | 3 | TG69 | 3.92/1.55 | 0.27 | |||
| HAB | Plant habit | Hort5-1 | 2010 | 3 & 4 | TG358 | 4.36/1.75 | 0.38 | |
| Hort5-2 | 2009 | 1, 2, 3, 4 | At5g49510 | 3.65/1.69 | 0.25 | |||
| H | Plant height | Hort5-1 | 2009 | 3 & 4 | TG358 | 5.81/1.73 | 0.32 | |
| 2010 | 1 & 2 | TG358 | 3.68/1.74 | 0.33 | ||||
| Hort5-2 | 2009 | 3 & 4 | At2g39950 | 3.53/1.73 | 0.19 | |||
| Hort5-3 | 2009 | 1 & 2 | At2g31970–At4g12590 | 4.05/1.73 | 0.27 | |||
| 2009 | 3 & 4 | At2g31970–At4g12590 | 5.32/1.73 | 0.35 | ||||
| 2010 | 3 | At2g31970 | 2.96/1.80 | 0.28 | ||||
| 2010 | 4 | At2g31970 | 3.78/1.73 | 0.32 | ||||
| W | Plant width | Hort5-1 | 2009 | 1 | TG358 | 3.05/1.65 | 0.22 | |
| Hort5-3 | 2009 | 3 & 4 | At2g31970–At4g12590 | 9.28/1.67 | 0.55 | |||
| 2010 | 1 & 2 | At2g31970–At4g12590 | 3.32/1.66 | 0.30 | ||||
| Hort5-4 | 2009 | 2 | T1541–TG69 | 2.17/1.65 | 0.18 | |||
| 2010 | 1 & 2 | TG69 | 3.07/1.66 | 0.20 | ||||
| SH | Plant shape (H:W) | Hort5-1 | 2009 | 3 & 4 | TG358 | 11.19/1.63 | 0.58 | |
| Hort5-2 | 2009 | 3 & 4 | At3g55800 | 2.34/1.63 | 0.08 | |||
| Hort5-3 | 2009 | 1 | At4g12590 | 3.23/1.48 | 0.22 | |||
| Hort5-4 | 2010 | 3 & 4 | TG69 | 2.11/1.65 | 0.21 | |||
| SZ | Plant size | Hort5-1 | 2009 | 1 & 2 | TG358 | 3.54/1.73 | 0.24 | |
| 2010 | 1 & 2 | TG358 | 3.61/1.80 | 0.27 | ||||
| Hort5-2 | 2009 | 3 & 4 | At2g39950 | 2.70/1.62 | 0.14 | |||
| 2010 | 1 & 2 | At3g17210 | 1.88/1.80 | 0.13 | ||||
| Hort5-3 | 2009 | 3 & 4 | At2g31970–At4g12590 | 5.29/1.62 | 0.35 | |||
| 2010 | 3 & 4 | At2g31970–At4g12590 | 2.69/1.67 | 0.32 | ||||
| Trait Class | Trait Code | Trait | Group | Year | Location(s) | Allele Direction | 1-LOD Support Interval | Flanking Markers |
| Maturity | DAP1st | Time to first ripe fruit (d) | Hort5-1 | 2009 | 3 & 4 | (+) | 0.0–0.3 | TG358-T0536 |
| 2010 | 3 | (+) | 0.0–0.3 | TG358-T0536 | ||||
| 2010 | 4 | (+) | 0.0–0.3 | TG358-T0536 | ||||
| Hort5-3 | 2009 | 3 & 4 | (+) | 7.3–9.9 | At5g49510-T1541 | |||
| 2010 | 3 | (+) | 6.3–9.5 | At5g49510-T1777 | ||||
| DAP50 | Time to 50% ripe fruit (d) | Hort5-1 | 2009 | 3 | (+) | 0.0–0.3 | TG358-T0536 | |
| 2009 | 4 | (+) | 0.0–0.3 | TG358-T0536 | ||||
| 2010 | 3 | (+) | 0.0–0.3 | TG358-T0536 | ||||
| 2010 | 4 | (+) | 0.0–0.3 | TG358-T0536 | ||||
| Yield | YLD | Yield | Hort5-1 | 2009 | 3 | (−) | 0.0–0.3 | TG358-T0536 |
| 2009 | 4 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| 2010 | 3 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| Hort5-3 | 2009 | 4 | (−) | 6.3–9.9 | At5g49510-T1541 | |||
| Fruit size/shape | FH | Fruit height | Hort5-1 | 2009 | 3 | (−) | 0.0–0.3 | TG358-T0536 |
| 2009 | 4 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| 2010 | 3 | (−) | 0.0–1.3 | TG358-cLEX13G5 | ||||
| 2010 | 4 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| Hort5-3 | 2009 | 3 | (−) | 7.7–11.9 | At2g31970-TG69 | |||
| FW | Fruit width | Hort5-2 | 2009 | 3 & 4 | (+) | 5.0–6.3 | At2g39950-At5g49510 | |
| FS | Fruit shape (H:W) | Hort5-1 | 2009 | 3 | (−) | 0.0–0.3 | TG358-T0536 | |
| 2009 | 4 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| Hort5-2 | 2009 | 3 | (−) | 5.0–8.7 | At2g39950-At4g12590 | |||
| 2009 | 4 | (−) | 5.5–7.6 | At2g39950-TG60 | ||||
| Hort5-4 | 2009 | 4 | (−) | 10.9–12.3 | T1541-At3g55360 | |||
| FP | Fruit size | Hort5-1 | 2009 | 3 | (−) | 0.0–0.3 | TG358-T0536 | |
| 2009 | 4 | (−) | 0.0–1.8 | TG358-U221402 | ||||
| 2010 | 3 | (−) | 0.0–1.3 | TG358-cLEX13G5 | ||||
| 2010 | 4 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| Hort5-2 | 2009 | 3 | (+) | 5.0–6.3 | At2g39950-At5g49510 | |||
| Hort5-4 | 2009 | 3 | (−) | 8.7–11.9 | At2g31970-TG69 | |||
| 30Wt | Fruit weight | Hort5-1 | 2009 | 3 | (−) | 0.0–0.3 | TG358-T0536 | |
| 2009 | 4 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| 2010 | 3 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| 2010 | 4 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| Hort5-2 | 2009 | 3 | (+) | 5.0–6.3 | At2g39950-At5g49510 | |||
| 2009 | 4 | (+) | 4.0–7.3 | At2g39950-TG60 | ||||
| SW | Seed weight | Hort5-1 | 2009 | 3 & 4 | (+) | 0.0–1.3 | TG358-cLEX13G5 | |
| Fruit quality | Brix | Brix | Hort5-1 | 2009 | 4 | (+) | 0.0–0.3 | TG358-T0536 |
| Hort5-3 | 2009 | 3 | (+) | 7.7–9.4 | At2g31970-At4g12590 | |||
| 2009 | 4 | (+) | 7.7–9.9 | At2g31970-T1541 | ||||
| Hort5-4 | 2010 | 4 | (+) | 9.5–12.3 | T1777-At3g55360 | |||
| pH | pH | Hort5-1 | 2009 | 3 | (−) | 0.0–0.3 | TG358-T0536 | |
| Plant architecture | CD | Canopy density | Hort5-1 | 2009 | 4 | (+) | 0.0–1.3 | TG358-cLEX13G5 |
| Hort5-4 | 2009 | 3 | (−) | 9.9–12.3 | T1777-At3g55360 | |||
| HAB | Plant habit | Hort5-1 | 2010 | 3 & 4 | (−) | 0.0–0.3 | TG358-T0536 | |
| H | Plant height | Hort5-1 | 2009 | 3 & 4 | (+) | 0.0–0.3 | TG358-T0536 | |
| 2010 | 1 & 2 | (−) | 0.0–1.8 | TG358-U221402 | ||||
| Hort5-2 | 2009 | 3 & 4 | (−) | 2.7–5.0 | At3g55800-TG23 | |||
| Hort5-3 | 2009 | 1 & 2 | (+) | 7.7–10.9 | At2g31970-TG69 | |||
| 2009 | 3 & 4 | (+) | 7.3–9.4 | At5g49510-At4g12590 | ||||
| 2010 | 3 | (+) | 7.3–9.4 | At5g49510-At4g12590 | ||||
| 2010 | 4 | (+) | 6.3–9.4 | At5g49510-At4g12590 | ||||
| W | Plant width | Hort5-1 | 2009 | 1 | (−) | 0.0–1.3 | TG358-T0536 | |
| Hort5-3 | 2009 | 3 & 4 | (+) | 7.7–9.4 | At2g31970-At4g12590 | |||
| 2010 | 1 & 2 | (−) | 5.9–9.4 | At3g17210-At4g12590 | ||||
| Hort5-4 | 2009 | 2 | (+) | 9.9–12.3 | T1777-At3g55360 | |||
| 2010 | 1 & 2 | (+) | 12.0–12.3 | TG69-At3g55360 | ||||
| SH | Plant shape (H:W) | Hort5-1 | 2009 | 3 & 4 | (+) | 0.0–0.3 | TG358-T0536 | |
| Hort5-2 | 2009 | 3 & 4 | (−) | 1.8–5.0 | U221402-TG23 | |||
| Hort5-3 | 2009 | 1 | (+) | 7.7–9.9 | At2g31970-T1541 | |||
| Hort5-4 | 2010 | 3 & 4 | (−) | 9.9–12.3 | T1777-At3g55360 | |||
| Plant architecture | SZ | Plant size | Hort5-1 | 2009 | 1 & 2 | (−) | 0.0–0.3 | TG358-T0536 |
| 2010 | 1 & 2 | (−) | 0.0–0.3 | TG358-T0536 | ||||
| Hort5-2 | 2009 | 3 & 4 | (−) | 1.8–5.5 | U221402-TG23 | |||
| 2010 | 1 & 2 | (−) | 3.0–8.7 | At2g39950-At4g12590 | ||||
| Hort5-3 | 2009 | 3 & 4 | (+) | 7.3–9.4 | At5g49510-At4g12590 | |||
| 2010 | 3 & 4 | (+) | 6.3–9.4 | At5g49510-At4g12590 |
Group indicates coincident QTL, as defined by colocation of the 1-LOD intervals. R2 values are the proportion of phenotypic variation explained by the marker–trait association. Allele direction is the direction of the effect of the S. habrochaites allele at that QTL, in terms of the trait being measured. The 1-LOD support interval positions refer to the cM distances on the linkage map for the introgressed region from S. habrochaites. See Johnson et al. (2012) for LEAF and STEM QTL results.
Horticultural trait QTL groups
Based on their location on the linkage map, four major horticultural trait QTL groups (Hort5-1 through Hort5-4) were delineated (Figure 1 and Table 4) as described in the Materials and Methods section. Hort5-1 contained QTL for every trait evaluated with the sole exception of fruit width (Figure 1 and Table 4). The QTL controlling reproductive traits within this group tended to explain a higher %PV than those of the other groups. This group includes QTL for days to first ripe fruit, days to 50% ripe fruit, yield, fruit height, fruit size, and fruit weight. The only QTL detected for pH and seed weight were also located within this group. QTL in Hort5-1 also controlled the plant architecture traits canopy density, plant habit, plant height, plant width, plant shape, and plant size (Table 4). There was evidence of genotype × environment interaction (G × E) for plant architecture, because the presence of the S. habrochaites allele at these QTL produced denser, taller plants in 2009, whereas it resulted in a smaller, shorter, more prostrate phenotype in 2010. Plants with the wild allele at these QTL also had delayed maturity, reduced fruit size and weight, but slightly higher seed weight and Brix.
The Hort5-2 QTL group contained QTL for 8 of the 17 horticultural traits evaluated and included primarily QTL for traits involving fruit size, fruit shape, and plant architecture traits (Table 4). The S. habrochaites allele at the Hort5-2 QTL group resulted in a shorter, more prostrate plant architectural phenotype. The wild allele also increased fruit size and weight, primarily as a result of increased fruit width. The Hort5-3 QTL group contained QTL for 8 of the 17 traits evaluated (Table 4). In contrast to the Hort5-2 effect on plant size, the S. habrochaites allele at the Hort5-3 QTL group increased plant size (both height and width). Maturity was slightly delayed by the presence of the wild allele in both years, but only in a single location in 2010. The Hort5-4 QTL group contained QTL for the following 6 of the 17 traits evaluated: fruit shape; fruit size; plant width; plant shape; Brix; and canopy density (Table 4). The S. habrochaites allele at the Hort5-4 QTL group was associated with a wider, more prostrate, and less dense plant architecture and decreased fruit size.
Plant architecture was influenced by the presence of the S. habrochaites introgression at each of the four QTL groups, with Hort5-2 causing a reduction in plant size, Hort5-3 and Hort5-4 causing an increase in plant size, and Hort5-1 having an environmentally dependent effect. Fruit size was impacted by Hort5-1 and Hort5-4, both reducing fruit size, and by Hort5-2, which was associated with larger fruit. Delayed maturity and increased Brix were caused by Hort5-1 and Hort5-3, whereas the other two groups had no significant effect on these important traits.
Horticulture trait QTL and linkage with P. infestans resistance QTL
In our companion study (Johnson et al. 2012), we detected two and five QTL groups within the introgressed chromosome 5 region controlling foliar (LEAF) and stem (STEM) resistance to P. infestans, respectively, with the QTL groups designated as LFRes5-1, LFRes5-2, and STRes5-1 through STRes5-5 (Figure 1). We used markers in common to align the QTL groups and visual inspection. LFRes5-1 and STRes5-1 were colocated with Hort5-1 (Figure 1). STRes5-2 slightly overlapped Hort5-2. LFRes5-2, STRes5-2, and STRes5-3 were colocated with Hort5-3. STRes5-4 and STRes5-5 were colocated with Hort5-4.
The Hort5-1 QTL group included QTL for 16 of the 17 horticultural traits measured. Most of the horticultural trait QTL within this group mapped within marker interval TG358–T0536, and all shared a peak LOD at TG358 (Figure 1 and Table 4). LFRes5-1 and STRes5-1 each contained only a single QTL spanning the same interval TG358–T0536, also with peak LOD at TG358, thus the horticultural trait QTL and these resistance QTL were colocated.
QTL were detected in the Hort5-2 QTL group for 8 of the 17 horticultural traits measured (Figure 1 and Table 4). The 1-LOD support intervals for the QTL within Hort5-2 and STRes5-2 overlapped slightly and the peaks for plant height, plant shape, and plant size QTL comprising this group were at At2g39950 and At3g55800. However, STRes5-2 consisted of only a single QTL with a peak approximately 2.5 cM away at TG23, suggesting that Hort5-2 is only linked to, rather than colocated with, STRes5-2.
The Hort5-3 QTL group included QTL for 8 of the 17 horticultural traits evaluated, with each QTL having peak LOD either at At2g31970 or in the interval At2g31970–At4g12590 (Figure 1 and Table 4). In contrast, LFRes5-2 was composed of three overlapping resistance QTL with the common lower boundary marker At4g12590 but with different upper boundaries of markers At3g17210, At2g31970, and At5g49510 (see Table 4 in Johnson et al. 2012). The uppermost of these three resistance QTL had peak LOD at At5g49510, whereas the lower two QTL had peaks in the interval At2g31970–At4g12590. Their peak locations suggest a closer linkage of the QTL in Hort5-3 with the lower two resistance QTL in LFRes5-2 than with the uppermost QTL. The two QTL for DAP1st and the yield QTL in this group were colocated with LFRes5-2, each spanning the interval At5g49510-T1541.
The Hort 5-4 QTL group included QTL for 6 of the 17 horticultural traits measured, but only for traits related to fruit size, fruit shape, fruit quality, and plant architecture (Figure 1 and Table 4). The colocated resistance QTL groups STRes5-2 and STRes5-3 were composed of three QTL spanning interval T1541-At3g55360: one QTL for resistance from the S. habrochaites allele with its peak at TG69 and two QTL with opposite allelic effect, with peaks at T1541 and TG69 (see Table 4 in Johnson et al. 2012). The horticultural trait QTL and the resistance QTL in this interval exhibited unstable expression across experimental environments, which precluded a more precise determination of the colocalization of these QTL.
QTL stability and QTL × environment interaction
QTL for yield, fruit size traits (FH, FP, and 30Wt), and maturity traits (DAP1st and DAP50) at Hort5-1 were detected repeatedly over years and locations, indicating stability of QTL expression (Figure 1 and Table 4). In contrast, the fruit shape QTL in this Hort5-1 group were detected in two locations in 2009 but not in 2010, suggesting environmental influence on QTL expression. Hort5-1 also contained QTL for plant height, but depending on the year and location, the wild allele had opposite effects on plant height. In 2009, in Davis, the presence of the wild allele increased height. In 2010, in Salinas, it decreased height. This difference in allelic effect over years was likely attributable to the large environmental differences between the locations and years. Within the region represented by this QTL group, Brix, pH, canopy density, and plant width were only detected in a single location in a single year, indicating QTL × environment (QTL × E) interactions.
The significant QTL at Hort5-2 were detected only in 2009 in both Davis locations, with the exception of plant size. Therefore, all the QTL in this group exhibited QTL × E interactions.
In general, QTL for architectural traits (plant habit, plant height, and plant size) at Hort5-3 were stable because they were detected over years and locations. However, differences in allele directionality for plant width indicated QTL × E effects, possibly as a result of different row spacing used in Salinas compared with Davis. Also, a QTL for plant shape was detected only in one location in 2009. The QTL for Brix in this group were detected in both Davis locations, but only during a single year, also suggesting QTL × E interactions.
In group Hort5-4, QTL for plant width were detected in one location in 2009 and in two locations in 2010. The other QTL in this region were only detected during a single year (for plant shape and plant size) or in a single location (for fruit shape, fruit perimeter, Brix, and canopy density), again indicating QTL × E interactions and QTL instability.
Coincidence of QTL between horticultural and resistance traits
The hypergeometric probability distribution was used to test the significance of correspondence of QTL for P. infestans resistance (Johnson et al. 2012) with those for horticultural traits. The correspondence of LEAF QTL with both DAP1st and yield QTL was significantly different from chance (P = 0.047 for each comparison). No other QTL coincidences between P. infestans resistance QTL and horticultural trait QTL were significant.
Selection of sub-NIL breeding lines
The following nine sub-NILs were selected as being potentially useful as breeding lines for development of tomato varieties with improved resistance to P. infestans: 08GH5516; 08GH5616; 08GH5861; 08GH6042; 08GH6261; 08GH6288; 08GH6321; 08GH6345; and 08GH6805 (Table S1). We compared these lines with cultivar E6203 to evaluate their potential in breeding. Three lines (08GH5516, 08GH5616, and 08GH5861) had significantly improved foliar resistance (i.e., lower LEAF values) at both Salinas locations in 2010 and had generally lower LEAF values (although not significantly different) in both locations in 2009. Lines 08GH5616 and 08GH5861 had significantly higher Brix in all experiments. Lines 08GH5516, 08GH6288, 08GH6321, and 08GH6805 did not have significantly later maturity and none of the selected lines was significantly taller, except 08GH5616.
Discussion
Genetic architecture of horticultural traits
Our higher-resolution mapping of horticultural trait QTL within an introgressed chromosome 5 region from S. habrochaites revealed a complex genetic architecture for various traits, including maturity, yield, fruit size, fruit shape, and fruit weight, Brix, canopy density, plant size, and plant shape (Figure 1 and Table 4). Factors contributing to this genetic complexity include QTL previously detected as single QTL fractionating into multiple QTL for a given trait, tight linkages among QTL for multiple traits, the presence of previously unmapped horticultural trait QTL, and QTL with opposite directionality of allelic effects in different environments.
Brouwer and St. Clair (2004) used NILs for the chromosome 5 region introgressed from S. habrochaites to map late blight disease resistance and horticultural trait QTL for plant height, plant shape (referred to here as plant habit), maturity, yield, and fruit size. In our present study, the single QTL mapped for each trait in Brouwer and St. Clair (2004) each fractionated into multiple QTL after higher-resolution mapping, a phenomenon reported previously in other mapping studies (Chen and Tanksley 2004; Lecomte et al. 2004; Edwards and Mackay 2009; Studer and Doebley 2011; Johnson et al. 2012). Studies of tomato interspecific populations have found similarly complex genetic architectures for traits such as fruit size and fruit quality when mapping within defined chromosomal regions (Fridman et al. 2002; Yates et al. 2004). In contrast, some high-resolution mapping studies reported only single QTL for traits such as Brix (Fridman et al. 2000; Fridman et al. 2004), fruit shape (Ku et al. 2001), and fruit weight (Alpert and Tanksley 1996; Frary et al. 2000).
Tight linkages were identified among multiple QTL controlling horticultural traits in our study. The most complex group, Hort5-1, contained QTL for 16 of the 17 traits evaluated and all were mapped within a single 1.8 cM interval. Coincident QTL for multiple traits have also been detected within similarly small genetic map distances in other studies of wild species introgressions in cultivated tomato (Frary et al. 2003; Yates et al. 2004; Gur et al. 2010).
Contributing to the genetic complexity of this chromosome 5 region are the presence of QTL for canopy density and plant size, which remained undetected in the previous study by Brouwer and St. Clair (2004). This result may be explained by the presence of closely linked QTL in repulsion with opposite allelic effects on these traits (Figure 1 and Table 4). The individual effects of these QTL can only be separated by mapping with additional recombinants to provide increased resolution (Mackay et al. 2009). Higher-resolution mapping has also allowed detection of QTL controlling stem resistance to P. infestans (STEM) (Johnson et al. 2012) that were previously undetected at lower resolution by Brouwer and St. Clair (2004).
The traits mapped within this region of chromosome 5 from S. habrochaites showed diverse genetic complexity, each varying in number of QTL and direction of allelic effects (Figure 1 and Table 4). Yield, maturity (DAP1st), fruit height, and plant habit were each controlled by two QTL with the same direction of allelic effect, whereas fruit width and canopy density were each controlled by two QTL of opposite allelic effect. Fruit shape and Brix were each controlled by three QTL with the same direction of allelic effect, whereas fruit perimeter, plant height, plant width, and plant size were each controlled by three QTL of varying direction of allelic effect. Plant shape had the most complex genetic architecture, being controlled by four QTL with alternating direction of allelic effect. Similar genetic complexity, including multiple closely linked QTL with varying direction of allelic effects, has previously been reported for higher-resolution mapping of QTL in maize (Graham et al. 1997), Drosophila melanogaster (Pasyukova et al. 2000; De Luca et al. 2003), mice (Mollah and Ishikawa 2011), and rat (Granhall et al. 2006).
Brouwer and St. Clair (2004) originally reported the linkage of P. infestans resistance QTL lb5b from S. habrochaites with QTL controlling plant shape and some reproductive traits. Subsequently, Johnson et al. (2012) described fractionation of this single resistance QTL into multiple QTL. In the present study, we used the same set of sub-NILs for chromosome 5 as Johnson et al. (2012) and mapped horticultural trait QTL linked to P. infestans resistance QTL. Other studies have reported linkage of horticultural trait QTL with disease and pest resistance genes or QTL introgressed from wild tomato species (Tanksley et al. 1998; Robert et al. 2001). Close linkage of QTL for disease resistance and horticultural traits has been reported for interspecific populations in other crop species, including potato (Visker et al. 2003; Danan et al. 2011). Similar results have also been observed in intraspecific populations in potato (Bradshaw et al. 2004), pepper (Ben Chaim et al. 2001; Barchi et al. 2007), bean (Miklas et al. 2003; Ender and Kelly 2005; Mkwaila et al. 2011), and cacao (Brown et al. 2007). Horticultural traits associated with resistance QTL may be related causally to the resistance, for example, traits such as plant height, lodging resistance, and canopy density that affect a plant’s ability to avoid environmental conditions conducive to infection. Alternately, the cosegregation of QTL for horticultural traits with resistance traits may be attributable to repressed recombination between loci controlling the two traits, particularly in introgressions from wild species.
Tight linkage and pleiotropy
Colocation of QTL controlling multiple horticultural traits with each P. infestans resistance QTL group (Figure 1) may be attributable either to tight linkage or to pleiotropy (Brown 2002; Chen and Luebberstedt 2010). Our results suggest tight linkage between QTL groups controlling foliar resistance to P. infestans (LEAF, designated as LFRes) (Johnson et al. 2012) and maturity (Figure 1). This linkage is particularly interesting because of previously reported correlations between these traits and coincidence of maturity and resistance QTL in potato (see P. infestans Resistance and Plant Maturity section). Each of the three horticultural QTL groups coincident with P. infestans resistance QTL groups (Hort5-1, Hort5-3, and Hort5-4) also contain QTL controlling other (nonmaturity) traits, suggesting tight linkage to resistance QTL (Figure 1). Alternatively, QTL coincidence may be attributable to pleiotropy in some cases, but we cannot determine this from our current data because it would require additional studies involving thousands of segregating progeny (Mackay et al. 2009; Chen and Luebberstedt 2010).
Increased mapping resolution in our study revealed that some of the previously identified coincidences among QTL for horticultural and resistance traits in Brouwer and St. Clair (2004) were most likely attributable to tight linkage, rather than to pleiotropy. QTL within Hort5-2 and Hort5-4 had 1-LOD support intervals that did not overlap with the two foliar resistance QTL groups LFRes5-1 and LFRes5-2 and their coincident stem resistance QTL groups STRes5-1 and STRes5-2. This result suggests that the Hort5-2 QTL for plant height, plant size, and plant shape are only linked, not pleiotropic, to LFRes5-1 and STRes5-1. Similarly, the Hort5-2 QTL and the Hort5-4 QTL for fruit shape, Brix, canopy density, plant width, and plant shape are likely to be linked, not pleiotropic, to LFRes5-2 and STRes5-2. Other studies of coincident QTL controlling different traits in tomato have also found the QTL to be tightly linked, rather than pleiotropic, when mapped at higher resolution using sub-NILs (Monforte et al. 2001; Lecomte et al. 2004).
Stability of QTL and QTL × E interaction
The majority of QTL mapped in our study was stably expressed over environments and detected with coincident or overlapping 1-LOD support intervals over multiple years and/or locations. Our inferences regarding QTL stability are limited to two locations across 2 years for all other traits because only the plant architecture traits were evaluated in all four locations (Salinas and Davis). Of the 41 horticultural trait QTL mapped, 29 were stably expressed over multiple environments, including QTL for maturity, yield, fruit size, fruit shape, Brix, plant size and plant shape. In all years and locations, in Hort5-1 QTL were detected for maturity, yield, fruit height, fruit size, and fruit weight, and in Hort5-3 QTL were detected for plant height. The remaining 12 horticultural trait QTL were only mapped for a single location within a single year.
Inconsistent detection of QTL across environments (years, locations, and other factors) may be attributable to interaction between expression of the QTL and the environments in which it is evaluated, described as QTL × E (Bernardo 2008; Xu and Crouch 2008; Mackay et al. 2009). Such interactions may reduce the efficiency of marker-assisted selection and may ultimately limit the utility of beneficial QTL alleles, depending on the target environments (Xu and Crouch 2008). Traits exhibiting QTL × E in our study included yield, fruit height, fruit shape, fruit size, Brix, pH, canopy density, and plant shape. Whereas many mapping studies conducted across multiple environments report QTL that are stable, QTL instability (i.e., QTL × E) is also common. For example, studies of tomato (Bernacchi et al. 1998b; Frary et al. 2004), potato (Constanzo et al. 2005), and maize (Peng et al. 2011) all identified QTL that were stable across multiple environments and others that exhibited QTL × E interactions.
Comparison of our results to those of other QTL mapping studies of tomato was limited because some studies were conducted in only a single environment (Monforte and Tanksley 2000; van der Knaap et al. 2002; Semel et al. 2006) or did not test for or report on genotype × environment interactions (Causse et al. 2007; Mathieu et al. 2009). Consequently, we focused our comparisons to studies that mapped QTL in tomato populations evaluated across multiple locations and/or years and that tested for environmental interactions. In these studies, detection of QTL in a single environment was observed for 25–50% (Frary et al. 2004; Johnson et al. 2012), 10–25% (Bernacchi et al. 1998b), and <10% of mapped QTL (Eshed et al. 1996; Eshed and Zamir 1996; Tanksley et al. 1996; Brouwer and St. Clair 2004; Do et al. 2010). Our results (29% of QTL detected in only a single environment) are within the upper range of these studies.
QTL × E may also explain differences in the direction of allelic effects at QTL in some locations compared with others, as exhibited by QTL controlling plant architecture in our study. A QTL in Hort5-1 increased plant height in both Davis locations in 2009 but decreased plant height in both Salinas locations in 2010, and a similar pattern was observed for plant width QTL in Hort5-3 (Table 4). Other studies have reported changes in the direction of allelic effect depending on environment, for example, QTL controlling yield, fruit color, and Brix in tomato (Bernacchi et al. 1998a), yield in maize (Bouchez et al. 2002; Moreau et al. 2004), and plant height and kernel weight in wheat (Campbell et al. 2003).
QTL comparisons to previously mapped QTL in tomato and potato
The map resolution of most previously reported QTL are not sufficient for determining precise locations of QTL. Nonetheless, comparisons based on common markers and informed by genomic sequence data suggested correspondence of some of our QTL with previously reported chromosome 5 QTL for disease resistances and horticultural traits in interspecific tomato populations. Bernacchi et al. (1998b) reported QTL for yield, soluble solids (Brix), and maturity associated with marker TG69 in lines derived from S. lycopersicum × S. habrochaites. These QTL may correspond to our Hort5-3 group QTL for yield, Brix, and days to first ripe fruit, although the low resolution of their map and single marker regression analysis precluded more precise QTL localization. In lines derived from S. lycopersicum × S. pimpinellifolium, Brewer et al. (2007) reported a QTL for heart-shape fruit in the interval TOM152–TG60 that may correspond to QTL we detected for fruit shape in Hort5-1, Hort5-2, or Hort5-3. They also mapped a QTL for distal blockiness (a trait defined by Tomato Analyzer software) in the interval TG60–TG185 that may be coincident to QTL we detected for fruit shape in Hort5-3 or Hort5-4. Using S. pennellii introgression lines (ILs), Causse et al. (2004) identified a QTL for pH in IL5-3 that may correspond to our Hort5-1 group QTL for pH and a QTL in IL5-4 for Brix and other sugar-related traits that may be coincident to our Hort5-3 and/or Hort5-4 QTL for Brix. With S. pennellii ILs, Eshed and Zamir (1996) detected QTL in IL5-4 for plant weight, Brix, and yield that may be coincident to our Hort5-3 QTL for plant size, Brix, and yield or to our Hort5-4 QTL for plant size and Brix. Jones et al. (2007) used S. pennellii ILs to map SP5G, a paralog of the self-pruning (sp) gene for determinant growth, to interval TG351–TG60, which is coincident to the upper portion of our Hort5-3 group and includes QTL for plant height, plant width, and plant size. The wild allele from S. pennellii at the SP5G locus delayed the expression of determinacy, which resulted in increased plant height. We observed similarly increased plant height in sub-NILs containing the S. habrochaites allele compared to those with the cultivated allele at TG60, which is closely linked to SP5G. Semel et al. (2006) detected QTL in S. pennellii IL5-3 for biomass, Brix, seed weight, and yield that may by coincident with our Hort5-1, Hort5-2, and/or Hort5-3 QTL for plant size, plant height, plant width, Brix, seed weight, and yield.
Previous reports of resistance QTL corresponding to the lb5b introgressed region are detailed in Johnson et al. (2012). Several studies have described QTL for resistance to P. infestans on chromosome 5 of potato, a close relative of tomato. The majority of these studies report a resistance QTL located between potato markers GP21 and GP179 (Collins et al. 1999; Visker et al. 2003; Visker et al. 2005; Achenbach et al. 2009; Danan et al. 2011). This interval coincides with the upper extent of the S. habrochaites introgression in NIL5, which suggests that the potato QTL at that interval may correspond to LFRes5-1 and STRes5-1. Some of these studies also reported linkage between the QTL for resistance and QTL for delayed maturity (Collins et al. 1999; Visker et al. 2003; Visker et al. 2005). This maturity QTL in potato may correspond to our maturity QTL at Hort5-1.
P. infestans resistance and plant maturity
P. infestans resistance (i.e., lower STEM or LEAF values) was significantly negatively correlated with earlier maturity in the sub-NILs (Table 3), indicating that later maturity was associated with increased resistance. As previously noted, resistance QTL groups were also colocated with QTL for maturity traits (Figure 1). The observed QTL correspondence was found to be unlikely because of chance alone, according to a statistical test based on the hypergeometric probability function. A number of studies of the potato have reported a significant positive correlation of increased resistance to P. infestans with late maturity (Toxopeus 1958; Collins et al. 1999; Bradshaw et al. 2004; Danan et al. 2009). Linkage of resistance and maturity QTL in potato was noted on chromosome 5, which is syntenic to tomato chromosome 5 (Collins et al. 1999; Oberhagemann et al. 1999; Visker et al. 2003; Bradshaw et al. 2004; Visker et al. 2005). Overall, our findings agree with the observations of others regarding the correlation of P. infestans resistance with maturity and linkage of QTL controlling these traits on chromosome 5.
Alignment of our tomato chromosome 5 map with the potato MetaQTL map (Danan et al. 2011) using common markers, facilitated by the Tomato-Expen 2000 map (Fulton et al. 2002b) on SGN (http://solgenomics.net), suggests that the potato QTL for resistance and maturity that mapped to marker interval GP21–GP179 likely corresponds to our LFRes5-1, STRes5-1, and Hort5-1 QTL groups, respectively. This QTL coincidence suggests conservation of gene function and synteny between these closely related genera for genes controlling these two traits within this region of chromosome 5. Genome-wide conservation of gene function and order between these genera are supported by evolutionary studies of the Solanaceae (Grube et al. 2000; Fulton et al. 2002a; Wu and Tanksley 2010) and by direct comparisons of the tomato and potato genome sequences (Sato et al. 2012).
Late plant maturity may contribute to increased resistance to P. infestans because of temporal variation in inoculum production and/or increased susceptibility of plants during the seedling and reproductive growth phases (Collins et al. 1999). These factors have primarily been investigated in potato. During an epidemic, P. infestans inoculum production increases and then decreases as uninfected host tissue becomes scarce and/or the environment becomes less favorable (Ferrandino 2012). Young plants tend to be more susceptible but exhibit increased resistance during vigorous vegetative growth before becoming more susceptible again during their reproductive phase (Collins et al. 1999). In our study, earlier maturing lines may have exhibited greater susceptibility because of the coincidence of favorable environmental conditions for increased P. infestans inoculum production with their more susceptible reproductive growth phase. This explanation was supported by our observations that, in both years in the field, symptoms of P. infestans infection were first observed while the earlier lines were flowering and setting fruit but the later maturing lines were still growing vigorously. Additional experiments would be required to determine whether the observed correspondence between maturity and resistance had a physiological basis or was merely a coincidence of the planting dates and onset of environmental conditions favorable to pathogenesis.
Implications for tomato breeding
Our study revealed a complex genetic architecture of QTL for horticultural traits and P. infestans resistance within an introgressed region of chromosome 5 from S. habrochaites, primarily because of linkage and/or pleiotropy between resistance QTL and horticultural trait QTL and the presence of QTL × E for some traits. This complexity presents challenges for use of wild species QTL alleles in breeding. The beneficial QTL alleles can be useful for tomato breeding if progeny without unfavorable repulsion phase linkages are obtained via recombination. In addition, suitable environments can be targeted for deployment of alleles exhibiting environmental interactions and complementary genetic backgrounds for expression of these alleles can be identified.
We identified favorable progeny sub-NILs that resulted from recombination between linked QTL with alleles in repulsion, for example, at the Hort5-3 QTL for Brix (39–45% PV) and the Hort5-2 QTL for fruit weight (10–15% PV) (Table 4). Based on the genotypes and trait phenotypes of the sub-NILs, the beneficial alleles from S. habrochaites at these two horticultural trait QTL and at the P. infestans resistance QTL group of largest phenotypic effect (LFRes5-2, 18–47% PV) (Johnson et al. 2012) are linked in coupling and not pleiotropic to the QTL for which the wild allele has the largest negative effects (%PV) on maturity, yield, fruit height, fruit size, and fruit weight in Hort5-1 (Figure 1 and Table 4). Colocation of these beneficial QTL alleles linked in coupling makes the marker interval TG23–T1541 a particularly desirable target for marker-assisted breeding. It would be helpful to identify other recombinants with additional favorable allelic combinations at linked QTL, particularly if the resistance QTL LFRes5-1 and STRes5-2 are to be used to develop cultivars with improved resistance as well as acceptable horticultural trait phenotypes.
Slightly negative phenotypic effects of linkage drag need not preclude the use of QTL in certain breeding situations. For example, the Fhb1 QTL for resistance to Fusarium head blight (F. graminearum) in wheat has been used in MAS breeding, despite the linkage of this QTL to loci causing a minor delay in heading date (Haeberle et al. 2009). Sub-NILs possessing the wild allele at Hort5-3, LFRes5-2, and STRes5-2 QTL but lacking the wild parent allele at Hort5-1, Hort5-2, LFRes5-1, and STRes5-1 QTL, such as sub-NIL 08GH5516 or 08GH6261, may be more immediately useful for breeding improved tomato cultivars because they possess more favorable horticultural trait phenotypes. MAS breeding for target wild alleles at Hort5-3 simultaneously with background selection against wild alleles at Hort5-4 in segregating progeny populations from crosses between these donor lines and other cultivated tomato lines would eliminate the negative effects of wild alleles at Hort5-4, producing more useful breeding lines.
Breeders prefer to deploy QTL alleles exhibiting stable expression across a wide range of production environments to maximize applicability for crop improvement. However, the presence of QTL × E does not necessarily impede the use of a QTL in breeding for improved cultivars. QTL for specific environments may be useful if those environments happen to be the major target environments for production of that crop (Paterson et al. 1991; Asins 2002; Paterson et al. 2003). By integrating crop modeling and MAS, QTL with environmental interactions can be exploited in breeding crop ideotypes for particular environments (Yin et al. 2003; Cooper et al. 2009). The favorable QTL identified in our experiments for Brix and fruit weight in Hort5-3 were detected in both locations in which they were evaluated, but only during 1 of 2 years. Further assessment of the stability of these QTL effects in target environments for processing tomato production would determine their value for cultivar improvement.
Truncation selection for multiple traits resulted in the selection of the following nine sub-NILs that had improved resistance relative to processing tomato cultivar E6203: 08GH5516; 08GH5616; 08GH5861; 08GH6042; 08GH6261; 08GH6288; 08GH6321; 08GH6345; and 08GH6805 (Table S1). In addition, two (08GH5616 and 08GH5861) had significantly higher Brix than their cultivated parent, E6203, in every environment in which they were evaluated. The selected sub-NILs can be used directly as donor lines for MAS breeding to improve fruit weight, Brix, and P. infestans resistance in tomato cultivars (Collard et al. 2005; Foolad and Panthee 2012). Additionally, they can be exploited as parents in crosses with other trait QTL donor lines to combine the beneficial effects of QTL alleles for desirable traits via MAS breeding, a technique known as QTL pyramiding (Collard and Mackill 2008; St. Clair 2010; Wang et al. 2012). Our most P. infestans–resistant sub-NILs with the most acceptable horticultural phenotypes (e.g., 08GH5516 or 08GH6261) could be intercrossed with selected sub-NILs containing late blight disease resistance QTL from chromosome 11 (Johnson et al. 2012; J.E. Haggard, E.B. Johnson, and D.A. St. Clair, unpublished results) to breed for improved resistance. Similar efforts could also be pursued for use of beneficial wild alleles at QTL for Brix and fruit weight in breeding for improved horticultural traits in processing tomato.
Supplementary Material
Acknowledgments
We thank Dr. Neil Willits for statistical consultation, Sharon Benzen and the USDA-ARS Station in Salinas, California, for field experiment operations, Dr. Marilyn West for technical assistance, and the St. Clair laboratory group members for assistance with field, greenhouse, and laboratory research. This research was supported by USDA-NIFA grant 2007-35300-18356 (to D.A.S.).
Footnotes
Communicating editor: N. D. Young
Literature Cited
- Achenbach U., Paulo J., Ilarionova E., Lubeck J., Strahwald J., et al. , 2009. Using SNP markers to dissect linkage disequilibrium at a major quantitative trait locus for resistance to the potato cyst nematode Globodera pallida on potato chromosome V. Theor. Appl. Genet. 118: 619–629. [DOI] [PubMed] [Google Scholar]
- Alpert K. B., Tanksley S. D., 1996. High-resolution mapping and isolation of a yeast artificial chromosome contig containing fw2.2: A major fruit weight quantitative trait locus in tomato. Proc. Natl. Acad. Sci. USA 93: 15503–15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asins M., 2002. Present and future of quantitative trait locus analysis in plant breeding. Plant Breed. 121: 281–291. [Google Scholar]
- Barchi, L., V. Lefebvre, P. Signoret, A. M. Sage-Palloix, S. Lanteri et al., 2007 Horticultural traits mapping and their association with resistance traits in pepper genome. Consequences for multi-trait breeding using marker assisted selection, pp. 169–180 in Progress in research on capsicum and eggplant. Proceedings of the XIIIth EUCARPIA Meeting. Warsaw University of Life Sciences, Warsaw, Poland. [Google Scholar]
- Ben Chaim A., Grube R. C., Lapidot M., Jahn M., Paran I., 2001. Identification of quantitative trait loci associated with resistance to cucumber mosaic virus in Capsicum annuum. Theor. Appl. Genet. 102: 1213–1220. [Google Scholar]
- Ben Chaim A., Borovsky Y., Rao G., Gur A., Zamir D., et al. , 2006. Comparative QTL mapping of fruit size and shape in tomato and pepper. Isr. J. Plant Sci. 54: 191–203. [Google Scholar]
- Bernacchi D., Beck-Bunn T., Emmatty D., Eshed Y., Inai S., et al. , 1998a Advanced backcross QTL analysis of tomato. II. Evaluation of near-isogenic lines carrying single-donor introgressions for desirable wild QTL-alleles derived from Lycopersicon hirsutum and L. pimpinellifolium. Theor. Appl. Genet. 97: 170–180. [Google Scholar]
- Bernacchi D., Beck-Bunn T., Eshed Y., Lopez Y., Petiard V., et al. , 1998b Advanced backcross QTL analysis in tomato. I. Identification of QTLS for traits of agronomic importance from Lycopersicon hirsutum. Theor. Appl. Genet. 97: 381–397. [Google Scholar]
- Bernardo R., 2008. Molecular markers and selection for complex traits in plants: Learning from the last 20 years. Crop Sci. 48: 1649–1664. [Google Scholar]
- Bombarely A., Menda N., Tecle I. Y., Buels R. M., Strickler S., et al. , 2011. The Sol Genomics Network (solgenomics.net): growing tomatoes using Perl. Nucleic Acids Res. 39: D1149–D1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchez A., Hospital F., Causse M., Gallais A., Charcosset A., 2002. Marker-assisted introgression of favorable alleles at quantitative trait loci between maize elite lines. Genetics 162: 1945–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J. E., Pande B., Bryan G. J., Hackett C. A., McLean K., et al. , 2004. Interval mapping of quantitative trait loci for resistance to late blight (Phytophthora infestans (Mont.) de Bary), height and maturity in a tetraploid population of potato (Solanum tuberosum subsp. tuberosum). Genetics 168: 983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer M. T., Lang L., Fujimura K., Dujmovic N., Gray S., et al. , 2006. Development of a controlled vocabulary and software application to analyze fruit shape variation in tomato and other plant species. Plant Physiol. 141: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer M. T., Moyseenko J. B., Monforte A. J., van der Knaap E., 2007. Morphological variation in tomato: a comprehensive study of quantitative trait loci controlling fruit shape and development. J. Exp. Bot. 58: 1339–1349. [DOI] [PubMed] [Google Scholar]
- Brouwer D. J., St. Clair D. A., 2004. Fine mapping of three quantitative trait loci for late blight resistance in tomato using near isogenic lines (NILs) and sub-NILs. Theor. Appl. Genet. 108: 628–638. [DOI] [PubMed] [Google Scholar]
- Brouwer D. J., Jones E. S., St. Clair D. A., 2004. QTL analysis of quantitative resistance to Phytophthora infestans (late blight) in tomato and comparisons with potato. Genome 47: 475–492. [DOI] [PubMed] [Google Scholar]
- Brown J. K., 2002. Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 5: 339–344. [DOI] [PubMed] [Google Scholar]
- Brown J. S., Phillips-Mora W., Power E. J., Krol C., Cervantes-Martinez C., et al. , 2007. Mapping QTLs for resistance to frosty pod and black pod diseases and horticultural traits in Theobroma cacao L. Crop Sci. 47: 1851–1858. [Google Scholar]
- Campbell B. T., Baenziger P. S., Gill K. S., Eskridge K. M., Budak H., et al. , 2003. Identification of QTLs and environmental interactions associated with agronomic traits on chromosome 3A of wheat. Crop Sci. 43: 1493–1505. [Google Scholar]
- Causse M., Duffe P., Gomez M. C., Buret M., Damidaux R., et al. , 2004. A genetic map of candidate genes and QTLs involved in tomato fruit size and composition. J. Exp. Bot. 55: 1671–1685. [DOI] [PubMed] [Google Scholar]
- Causse M., Chaib J., Lecomte L., Buret M., Hospital F., 2007. Both additivity and epistasis control the genetic variation for fruit quality traits in tomato. Theor. Appl. Genet. 115: 429–442. [DOI] [PubMed] [Google Scholar]
- Chen K.-Y., Tanksley S. D., 2004. High-resolution mapping and functional analysis of se2.1: A major stigma exsertion quantitative trait locus associated with the evolution from allogamy to autogamy in the genus Lycopersicon. Genetics 168: 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Luebberstedt T., 2010. Molecular basis of trait correlations. Trends Plant Sci. 15: 454–461. [DOI] [PubMed] [Google Scholar]
- Churchill G. A., Doerge R. W., 1994. Empirical threshold value for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coaker G. L., Francis D. M., 2004. Mapping, genetic effects, and epistatic interaction of two bacterial canker resistance QTLs from Lycopersicon hirsutum. Theor. Appl. Genet. 108: 1047–1055. [DOI] [PubMed] [Google Scholar]
- Collard B. C. Y., Mackill D. J., 2008. Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363: 557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard B. C. Y., Mackill D. J., 2008. Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363: 557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard B. C. Y., Jahufer M. Z. Z., Brouwer J. B., Pang E. C. K., 2005. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 142: 169–196. [Google Scholar]
- Collins A., Milbourne D., Ramsay L., Meyer R., Chatot-Balandras C., et al. , 1999. QTL for field resistance to late blight in potato are strongly correlated with maturity and vigour. Mol. Breed. 5: 387–398. [Google Scholar]
- Constanzo S., Simko I., Christ B. J., Haynes K. G., 2005. QTL analysis of late blight resistance in a diploid potato family of Solanum phureja x S. stenotomum. Theor. Appl. Genet. 111: 609–617. [DOI] [PubMed] [Google Scholar]
- Cooper M., van Eeuwijk F. A., Hammer G. L., Podlich D. W., Messina C., 2009. Modeling QTL for complex traits: detection and context for plant breeding. Curr. Opin. Plant Biol. 12: 231–240. [DOI] [PubMed] [Google Scholar]
- Danan S., Chauvin J.-E., Caromel B., Moal J.-D., Pellé R., et al. , 2009. Major-effect QTLs for stem and foliage resistance to late blight in the wild potato relatives Solanum sparsipilum and S. spegazzinii are mapped to chromosome X. Theor. Appl. Genet. 119: 705–719. [DOI] [PubMed] [Google Scholar]
- Danan S., Veyrieras J.-B., Lefebvre V., 2011. Construction of a potato consensus map and QTL meta-analysis offer new insights into the genetic architecture of late blight resistance and plant maturity traits. BMC Plant Biol. 11: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M., Roshina N. V., Geiger-Thornsberry G. L., Lyman R. F., Pasyukova E. G., et al. , 2003. Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nat. Genet. 34: 429–433. [DOI] [PubMed] [Google Scholar]
- Do P. T., Prudent M., Sulpice R., Causse M., Fernie A. R., 2010. The influence of fruit load on the tomato pericarp metabolome in a Solanum chmielewskii introgression line population. Plant Physiol. 154: 1128–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. C., Mackay T. F. C., 2009. Quantitative trait loci for aggressive behavior in Drosophila melanogaster. Genetics 182: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ender M., Kelly J. D., 2005. Identification of QTL associated with white mold resistance in common bean. Crop Sci. 45: 2482–2490. [Google Scholar]
- Eshed Y., Zamir D., 1996. Less-than-additive epistatic interactions of quantitative trait loci in tomato. Genetics 143: 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y., Gera G., Zamir D., 1996. A genome-wide search for wild-species alleles that increase horticultural yield of processing tomatoes. Theor. Appl. Genet. 93: 877–886. [DOI] [PubMed] [Google Scholar]
- Ferrandino F. J., 2012. Time scales of inoculum production and the dynamics of the epidemic. Phytopathology 102: 728–732. [DOI] [PubMed] [Google Scholar]
- Finkers R., van den Berg P., van Berloo R., ten Have A., van Heusden A. W., et al. , 2007. Three QTLs for Botrytis cinerea resistance in tomato. Theor. Appl. Genet. 114: 585–593. [DOI] [PubMed] [Google Scholar]
- Foolad M. R., Panthee D. R., 2012. Marker-assisted selection in tomato breeding. Crit. Rev. Plant Sci. 31: 93–123. [Google Scholar]
- Foolad M. R., Zhang L. P., Khan A. A., Nino-Liu D., Lin G. Y., 2002. Identification of QTLs for early blight (Alternaria solani) resistance in tomato using backcross populations of a Lycopersicon esculentum X L. hirsutum cross. Theor. Appl. Genet. 104: 945–958. [DOI] [PubMed] [Google Scholar]
- Foolad M. R., Merk H. L., Ashrafi H., 2008. Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit. Rev. Plant Sci. 27: 75–107. [Google Scholar]
- Frary A., Nesbitt T. C., Frary A., Grandillo S., van der Knaap E., et al. , 2000. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 289: 85–88. [DOI] [PubMed] [Google Scholar]
- Frary A., Doganlar S., Frampton A., Fulton T., Uhlig J., et al. , 2003. Fine mapping of quantitative trait loci for improved fruit characteristics from Lycopersicon chmielewskii chromosome 1. Genome 46: 235–243. [DOI] [PubMed] [Google Scholar]
- Frary A., Fulton T. M., Zamir D., Tanksley S. D., 2004. Advanced backcross QTL analysis of a Lycopersicon esculentum X L. pennellii cross and identification of possible orthologs in the Solanaceae. Theor. Appl. Genet. 108: 485–496. [DOI] [PubMed] [Google Scholar]
- Fridman E., Pleban T., Zamir D., 2000. A recombination hotspot delimits a wild-species quantitative trait locus for tomato sugar content to 484 bp within an invertase gene. Proc. Natl. Acad. Sci. USA 97: 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E., Liu Y. S., Carmel-Goren L., Gur A., Shoresh M., et al. , 2002. Two tightly linked QTLs modify tomato sugar content via different physiological pathways. MGG Molecular Genetics and Genomics 266: 821–826. [DOI] [PubMed] [Google Scholar]
- Fridman E., Carrari F., Liu Y.-S., Fernie A. R., Zamir D., 2004. Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305: 1786–1789. [DOI] [PubMed] [Google Scholar]
- Fulton T. M., Bucheli P., Voirol E., Lopez J., Petiard V., et al. , 2002a Quantitative trait loci (QTL) affecting sugars, organic acids and other biochemical properties possibly contributing to flavor, identified in four advanced backcross populations of tomato. Euphytica 127: 163–177. [Google Scholar]
- Fulton T. M., Van der Hoeven R., Eannetta N. T., Tanksley S. D., 2002b Identification, analysis, and utilization of conserved ortholog set markers for comparative genomics in higher plants. Plant Cell 14: 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham G. I., Wolff D. W., Stuber C. W., 1997. Characterization of a yield quantitative trait locus on chromosome five of maize by fine mapping. Crop Sci. 37: 1601–1610. [Google Scholar]
- Granhall C., Park H.-B., Fakhrai-Rad H., Luthman H., 2006. High-resolution quantitative trait locus analysis reveals multiple diabetes susceptibility loci mapped to intervals < 800 kb in the species-conserved Niddm1i of the GK rat. Genetics 174: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube R. C., Radwanski E. R., Jahn M., 2000. Comparative genetics of disease resistance within the solanaceae. Genetics 155: 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur A., Osorio S., Fridman E., Fernie A. R., Zamir D., 2010. hi2–1, A QTL which improves harvest index, earliness and alters metabolite accumulation of processing tomatoes. Theor. Appl. Genet. 121: 1587–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle J., Holzapfel J., Schweizer G., Hartl L., 2009. A major QTL for resistance against Fusarium head blight in European winter wheat. Theor. Appl. Genet. 119: 325–332. [DOI] [PubMed] [Google Scholar]
- Johnson E. B., Haggard J. E., St. Clair D. A., 2012. Fractionation, stability, and isolate-specificity of QTL for resistance to Phytophthora infestans in cultivated tomato (Solanum lycopersicum). G3 (Bethesda) 2: 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Rick C. M., Adams D., Jernstedt J., Chetelat R. T., 2007. Genealogy and fine mapping of Obscuravenosa, a gene affecting the distribution of chloroplasts in leaf veins and evidence of selection during breeding of tomatoes (Lycopersicon esculentum; Solanaceae). Am. J. Bot. 94: 935–947. [DOI] [PubMed] [Google Scholar]
- Judelson H. S., Blanco F. A., 2005. The spores of Phytophthora: Weapons of the plant destroyer. Nat. Rev. Microbiol. 3: 47–58. [DOI] [PubMed] [Google Scholar]
- Ku H.-M., Liu J., Doganlar S., Tanksley S. D., 2001. Exploitation of Arabidopsis-tomato synteny to construct a high-resolution map of the ovate-containing region in tomato chromosome 2. Genome 44: 470–475. [DOI] [PubMed] [Google Scholar]
- Labate J. A., Robertson L. D., 2012. Evidence of cryptic introgression in tomato (Solanum lycopersicum L.) based on wild tomato species alleles. BMC Plant Biol. 12: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte L., Saliba-Colombani V., Gautier A., Gomez-Jimenez M. C., Duffe P., et al. , 2004. Fine mapping of QTLs of chromosome 2 affecting the fruit architecture and composition of tomato. Mol. Breed. 13: 1–14. [Google Scholar]
- Lin Y.-R., Schertz K. F., Paterson A. H., 1995. Comparative analysis of QTLs affecting plant height and maturity across the Poaceae, in reference to an interspecific sorghum population. Genetics 141: 391–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T. F. C., Stone E. A., Ayroles J. F., 2009. The genetics of quantitative traits: challenges and prospects. Nat. Rev. Genet. 10: 565–577. [DOI] [PubMed] [Google Scholar]
- Mathieu S., Cin V. D., Fei Z., Li H., Bliss P., et al. , 2009. Flavour compounds in tomato fruits: identification of loci and potential pathways affecting volatile composition. J. Exp. Bot. 60: 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklas P. N., Delorme R., Riley R., 2003. Identification of QTL conditioning resistance to white mold in snap bean. J. Am. Soc. Hortic. Sci. 128: 564–570. [Google Scholar]
- Miller J. C., Tanksley S. D., 1990. RFLP analysis of phylogenetic relationships and genetic variation in the genus Lycopersicon. Theor. Appl. Genet. 80: 437–448. [DOI] [PubMed] [Google Scholar]
- Mkwaila W., Terpstra K. A., Ender M., Kelly J. D., 2011. Identification of QTL for agronomic traits and resistance to white mold in wild and landrace germplasm of common bean. Plant Breed. 130: 665–672. [Google Scholar]
- Mollah M. B. R., Ishikawa A., 2011. Intersubspecific subcongenic mouse strain analysis reveals closely linked QTLs with opposite effects on body weight. Mamm. Genome 22: 282–289. [DOI] [PubMed] [Google Scholar]
- Monforte A., Friedman E., Zamir D., Tanksley S., 2001. Comparison of a set of allelic QTL-NILs for chromosome 4 of tomato: deductions about natural variation and implications for germplasm utilization. TAG Theoretical and Applied Genetics 102: 572–590. [Google Scholar]
- Monforte A. J., Tanksley S. D., 2000. Fine mapping of a quantitative trait locus (QTL) from Lycopersicon hirsutum chromosome 1 affecting fruit characteristics and agronomic traits: Breaking linkage among QTLs affecting different traits and dissection of heterosis for yield. Theor. Appl. Genet. 100: 471–479. [Google Scholar]
- Moreau L., Charcosset A., Gallais A., 2004. Use of trial clustering to study QTL x environment effects for grain yield and related traits in maize. Theor. Appl. Genet. 110: 92–105. [DOI] [PubMed] [Google Scholar]
- National Agriculture Statistics Service, 2011 Vegetables 2010 Summary. US Department of Agriculture, National Agriculture Statistics Service. Available at: http://usda01.library.cornell.edu/usda/nass/VegeSumm//2010s/2011/VegeSumm-01–27–2011_new_format.pdf. Accessed: July 2012.
- Oberhagemann P., Chatot-Balandras C., Schaefer-Pregl R., Wegener D., Palomino C., et al. , 1999. A genetic analysis of quantitative resistance to late blight in potato: Towards marker-assisted selection. Mol. Breed. 5: 399–415. [Google Scholar]
- Park Y. H., West M. A. L., St.Clair D. A., 2004. Evaluation of AFLPs for germplasm fingerprinting and assessment of genetic diversity in cultivars of tomato (Lycopersicon esculentum L.). Genome 47: 510–518. [DOI] [PubMed] [Google Scholar]
- Pasyukova E. G., Vieira C., Mackay T. F. C., 2000. Deficiency mapping of quantitative trait loci affecting longevity in Drosophila melanogaster. Genetics 156: 1129–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. H., Damon S., Hewitt J. D., Zamir D., Rabinowitch H. D., et al. , 1991. Mendelian factors underlying quantitative traits in tomato comparison across species generations and environments. Genetics 127: 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. H., Saranga Y., Menz M., Jiang C. X., Wright R., 2003. QTL analysis of genotype × environment interactions affecting cotton fiber quality. TAG Theoretical and Applied Genetics 106: 384–396. [DOI] [PubMed] [Google Scholar]
- Peng B., Li Y., Wang Y., Liu C., Liu Z., et al. , 2011. QTL analysis for yield components and kernel-related traits in maize across multi-environments. Theor. Appl. Genet. 122: 1305–1320. [DOI] [PubMed] [Google Scholar]
- Robert V. J. M., West M. A. L., Inai S., Caines A., Arntzen L., et al. , 2001. Marker-assisted introgression of blackmold resistance QTL alleles from wild Lycopersicon cheesmanii to cultivated tomato (L. esculentum) and evaluation of QTL phenotypic effects. Mol. Breed. 8: 217–233. [Google Scholar]
- Sato S., Tabata S., Hirakawa H., Asamizu E., Shirasawa K., et al. , 2012. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel Y., Nissenbaum J., Menda N., Zinder M., Krieger U., et al. , 2006. Overdominant quantitative trait loci for yield and fitness in tomato. Proc. Natl. Acad. Sci. USA 103: 12981–12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwka J., Jakuczun H., Lebecka R., Marczewski W., Gebhardt C., et al. , 2007. Tagging QTLs for late blight resistance and plant maturity from diploid wild relatives in a cultivated potato (Solanum tuberosum) background. Theor. Appl. Genet. 115: 101–112. [DOI] [PubMed] [Google Scholar]
- St. Clair D. A., 2010. Quantitative disease resistance and quantitative resistance loci in breeding. Annu. Rev. Phytopathol. 48: 247–268. [DOI] [PubMed] [Google Scholar]
- Studer A. J., Doebley J. F., 2011. Do large effect QTL fractionate? A case study at the maize domestication QTL teosinte branched1. Genetics 188: 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley S. D., Grandillo S., Fulton T. M., Zamir D., Eshed Y., et al. , 1996. Advanced backcross QTL analysis in a cross between an elite processing line of tomato and its wild relative L. pimpinellifolium. Theor. Appl. Genet. 92: 213–224. [DOI] [PubMed] [Google Scholar]
- Tanksley S. D., Bernachi D., Beck-Bunn T., Emmatty D., Eshed Y., et al. , 1998. Yield and quality evaluations on a pair of processing tomato lines nearly isogenic for the Tm2a gene for resistance to the tobacco mosaic virus. Euphytica 99: 77–83. [Google Scholar]
- Toxopeus H. J., 1958. Some notes on the relations between field resistance to Phytophthora infestans in leaves and tubers and ripening time in Solanum tuberosum subsp. tuberosum. Euphytica 7: 123–130. [Google Scholar]
- van der Knaap E., Lippman Z. B., Tanksley S. D., 2002. Extremely elongated tomato fruit controlled by four quantitative trait loci with epistatic interactions. Theor. Appl. Genet. 104: 241–247. [DOI] [PubMed] [Google Scholar]
- van Ooijen J. W., Voorips R. E., 2001. JoinMap version 3.0: Software for the Calculation of Genetic Linkage Maps. Plant Research International, Wageningen. [Google Scholar]
- Visker M. H. P. W., Keizer L. C. P., van Eck H. J., Jacobsen E., Colon L. T., et al. , 2003. Can the QTL for late blight resistance on potato chromosome 5 be attributed to foliage maturity type? Theor. Appl. Genet. 106: 317–325. [DOI] [PubMed] [Google Scholar]
- Visker M. H. P. W., Heilersig H. J. B., Kodde L. P., Van de Weg W. E., Voorrips R. E., et al. , 2005. Genetic linkage of QTLs for late blight resistance and foliage maturity type in six related potato progenies. Euphytica 143: 189–199. [Google Scholar]
- Voorips R. E., 2002. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 93: 77–78. [DOI] [PubMed] [Google Scholar]
- Wang P., Xing Y., Li Z., Yu S., 2012. Improving rice yield and quality by QTL pyramiding. Mol. Breed. 29: 903–913. [Google Scholar]
- Wang, S., C. J. Basten, and Z.-B. Zeng, 2011 Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. Available at: http://statgen.ncsu.edu/qtlcart/WQTLCart.htm. Accessed: June 2011.
- Wu F., Tanksley S. D., 2010. Chromosomal evolution in the plant family Solanaceae. BMC Genomics 11: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Pan S., Cheng S., Zhang B., Mu D., et al. , 2011. Genome sequence and analysis of the tuber crop potato. Nature 475: 189. [DOI] [PubMed] [Google Scholar]
- Xu Y., Crouch J. H., 2008. Marker-assisted selection in plant breeding: From publications to practice. Crop Sci. 48: 391–407. [Google Scholar]
- Yates H. E., Frary A., Doganlar S., Frampton A., Eannetta N. T., et al. , 2004. Comparative fine mapping of fruit quality QTLs on chromosome 4 introgressions derived from two wild tomato species. Euphytica 135: 283–296. [Google Scholar]
- Yin X., Stam P., Kropff M. J., Schapendonk A. H. C. M., 2003. Crop modeling, QTL mapping, and their complementary role in plant breeding. Agron. J. 95: 90–98. [Google Scholar]
- Zhang L. P., Khan A., Nino-Liu D., Foolad M. R., 2002. A molecular linkage map of tomato displaying chromosomal locations of resistance gene analogs based on a Lycopersicon esculentum x Lycopersicon hirsutum cross. Genome 45: 133–146. [DOI] [PubMed] [Google Scholar]
- Zhang L. P., Lin G. Y., Nino-Liu D., Foolad M. R., 2003. Mapping QTLs conferring early blight (Alternaria solani) resistance in a Lycopersicon esculentum x L. hirsutum cross by selective genotyping. Mol. Breed. 12: 3–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.