Abstract
Glucose deprivation has been hypothesized to cause cytotoxicity by inducing metabolic oxidative stress in human cancer cells. The current work tests the hypothesis that 2-deoxy-d-glucose (2DG) combined with cisplatin [cis-diamminedichloroplatinum(II)] can enhance cytotoxicity in human head and neck cancer cells (FaDu) by mechanisms involving oxidative stress. Exposure of FaDu cells to the combination of 2DG and cisplatin resulted in a significant decrease in cell survival when compared with 2DG or cisplatin alone. Treatment with 2DG and cisplatin also caused perturbations in parameters indicative of oxidative stress, including decreased intracellular total glutathione and increased percentage of glutathione disulfide. Simultaneous treatment with the thiol antioxidant N-acetylcysteine (NAC) inhibited parameters indicative of oxidative stress, as well as protected FaDu cells from the cytotoxic effects of cisplatin alone and the combination of 2DG and cisplatin. In addition, polyethylene glycol–conjugated antioxidant enzymes (PEG-superoxide dismutase and PEG-catalase) also protected FaDu cells from 2DG toxicity. An inhibitor of glutathione synthesis, l-buthionine-[S,R]-sulfoximine (BSO), sensitized FaDu cells to the cytotoxic effects of 2DG and cisplatin, and these effects were inhibited by NAC. Furthermore, the combination of 2DG, cisplatin, and BSO significantly increased the percentage of glutathione disulfide, which was also inhibited by NAC. These results support the hypothesis that exposure of human head and neck cancer cells to 2DG combined with cisplatin enhances cytotoxicity via metabolic oxidative stress. These findings provide a strong biochemical rationale for evaluating inhibitors of glucose and hydroperoxide metabolism in combination with cisplatin for the treatment of head and neck cancer.
Introduction
Neoplastic transformed cells (cancer cells) show altered metabolism when compared with untransformed (normal) cells (1–4), and the metabolic disruptions seem to involve increased metabolism of glucose and the loss of regulation between glycolytic metabolism and respiration (1–4). In general, cancer cells have increased rates of glycolysis as well as pentose phosphate cycle activity, and slightly reduced rates of respiration (1–4). Accordingly, cancer cells have been hypothesized to compensate for a proposed “defect” in respiration by increasing glycolysis (1) but the mechanisms responsible for these metabolic perturbations remain obscure.
Several studies have shown that glucose deprivation induces oxidative stress in human cancer cells and this effect seems to be more pronounced in transformed versus normal cells (5–7). In addition, increased pro-oxidant production and profound disruptions in thiol metabolism consistent with metabolic oxidative stress were also noted in cancer cells during glucose deprivation or when treated with the glucose analogue 2-deoxy-d-glucose (2DG; refs. 5–10). Glucose deprivation or 2DG-induced cytotoxicity and increases in parameters indicative of oxidative stress have also been shown to be inhibited by the thiol antioxidant N-acetylcysteine (NAC; refs. 5, 6, 10), as well as overexpression of enzymes that scavenge reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (9). These results have led to the hypothesis that metabolic oxidative stress caused by ROS is causally related to the effects of glucose deprivation in transformed cells.
Cisplatin [cis-diamminedichloroplatinum(II)] is an effective antitumor agent and is one of the most widely used drugs either alone or in combination with other chemotherapeutic agents or with radiotherapy in the management of locally advanced or recurrent squamous cell carcinomas of the head and neck (11). DNA is believed to be the major critical target of cisplatin-induced toxicity, and efficacy is believed to be a function of cisplatin-DNA adducts inhibiting DNA replication and transcription, ultimately resulting in cell death (12). Several recent studies have shown that the cytotoxicity of cisplatin may also be related to the inhibition of thioredoxin reductase activity (13–15), which participates in important cellular defense systems that protect against oxidative stress induced by hydroperoxides in cancer cells (16, 17). In addition, the reduced efficacy of cisplatin is often observed in cells with increased glutathione levels, which represents another major thiol antioxidant defense (18). Modulation of intracellular thiol levels has been shown to influence cisplatin cytotoxicity in numerous studies, and cisplatin has been hypothesized to cause oxidative stress (19, 20).
Because the glucose analogue 2DG is a clinically relevant inhibitor of glucose metabolism believed to mimic the effects of glucose deprivation (21, 22), we hypothesized that 2DG in combination with cisplatin may act to inhibit critical aspects of thiol-mediated hydroperoxide metabolism leading to increased steady-state levels of ROS and enhanced tumor cell killing via metabolic oxidative stress. The current experiments were designed to test the aforementioned hypothesis using human head and neck squamous cell carcinoma cells. We predicted that the combination of 2DG and cisplatin would have an additive and possibly synergistic effect on clonogenic cell killing in FaDu human head and neck squamous carcinoma cells by enhancing metabolic oxidative stress.
The results indicate that treatment of human head and neck carcinoma cells (FaDu) with 2DG and cisplatin causes a decrease in total glutathione content = [reduced glutathione (GSH) + glutathione disulfide (GSSG)], an increase in percentage of GSSG, as well as enhanced cytotoxicity. Both the disruptions in thiol metabolism and the cytotoxicity induced by exposure to the combination of 2DG and cisplatin were reversed by treatment with the thiol antioxidant NAC. Finally, the cytotoxicity and oxidative stress induced by the combination of 2DG and cisplatin were significantly enhanced by the GSH-depleting agent l-buthionine-[S,R]-sulfoximine (BSO), and this effect was also inhibited by NAC. These results provide strong support for the hypothesis that the combination of 2DG and cisplatin enhances cytotoxicity in human head and neck cancer cells via metabolic oxidative stress. These results also suggest a clear biochemical rationale for combining inhibitors of glucose and hydroperoxide metabolism for the enhancement of the anticancer effects of cisplatin in human head and neck cancer cells.
Materials and Methods
Cells and culture conditions
FaDu human head and neck squamous cell carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in DMEM containing 4 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 1.5 g/L sodium bicarbonate, and 4.5 g/L glucose with 10% fetal bovine serum (FBS; Hyclone, Logan, UT). Cultures were maintained in 5% CO2 and humidified in a 37°C incubator.
Drug treatment
2DG, NAC, cisplatin, BSO, polyethylene glycol (PEG)-CuZn superoxide dismutase (SOD), and PEG-catalase were obtained from Sigma Chemical Co. (St. Louis, MO). PEG was obtained from Fisher Scientific (Fair Lawn, NJ). All drugs were used without further purification. Drugs were added to cells at a final concentration of 20 mmol/L 2DG, 0.5 μmol/L cisplatin, 15 mmol/L NAC, 1.0 mmol/L BSO, 18 μmol/L PEG, 100 units/mL PEG-SOD, 100 units/mL PEG-catalase, or 50 units/mL PEG-SOD + PEG-catalase. All stock solutions were dissolved in PBS except NAC was dissolved in 1 mol/L sodium bicarbonate (pH 7.0), and the required volume was added directly to complete cell culture media on cells to achieve the desired final concentrations. All cells were placed in a 37°C incubator and harvested at the time points indicated.
Cell pellet collection
Following treatment, medium was collected and centrifuged to harvest floating cells. Attached cells were scrape harvested in ice-cold PBS and centrifuged at 4°C, the supernatant was discarded, and the cell pellets were transferred to 1.5-mL tubes and frozen at 20°C until biochemical analysis was done.
Glutathione assay
Cell pellets were thawed and homogenized in 50 mmol/L potassium phosphate buffer (pH 7.8) containing 1.34 mmol/L diethylenetriaminepentaacetic acid buffer. Total glutathione content was determined by the method of Anderson (23). GSH and GSSG were distinguished by addition of 2 μL of a 1:1 mixture of 2-vinylpyridine and ethanol per 30 μL of sample followed by incubation for 1 h and assayed as previously described (24). All glutathione determinations were normalized to the protein content of whole homogenates using the method of Lowry et al. (25).
Clonogenic cell survival experiments
Floating cells in medium from the experimental dishes were collected and combined with the attached cells from the same dish that were trypsinized with 1-mL trypsin-EDTA (CellGro, Herndon, VA) and inactivated with DMEM containing 10% FBS (Hyclone). The cells were diluted and counted using a Coulter counter. Cells were plated at low density (300–1,000 per plate) and clones were allowed to grow in a humidified 5% CO2, 37°C environment for 14 days in complete medium in the presence of 0.1% gentamicin. Cells were fixed with 70% ethanol and stained with Coomassie blue for analysis of clonogenic cell survival as previously described (26). Individual assays were done with multiple dilutions with at least four cloning dishes per data point, repeated in at least three separate experiments.
Statistical analysis
Statistical analysis was done using GraphPad Prism version 4 for Windows (GraphPad Software San Diego, CA). To determine differences between three or more means, one-way ANOVA with Bonferroni post-tests was done. Two-way ANOVA was used to determine differences over different time points and treatment groups. Error bars represent ±1 SD. All statistical analyses were done at the P < 0.05 level of significance.
Results
Inhibition of cell growth with 2DG and cisplatin
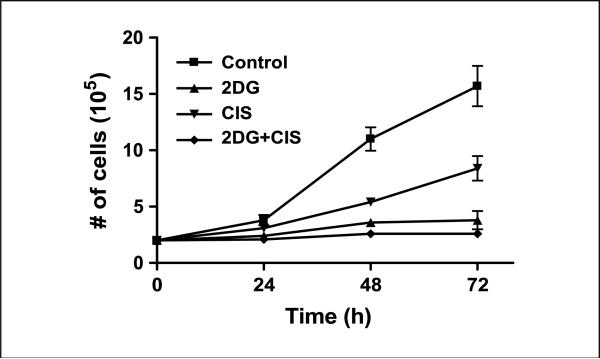
2DG at 20 mmol/L was chosen for the current studies to ensure that a physiologically relevant ratio of 2DG/glucose (ratio, 0.8) was used to inhibit glucose metabolism in the FaDu cells grown in DMEM that contained 25 mmol/L glucose (27). In addition, 0.5 μmol/L cisplatin was chosen because it represents a clinically relevant dose that is within the achievable plasma levels in head and neck patients during cisplatin therapy (28). The cell growth curves shown in Fig. 1 show the growth delay of FaDu cells treated with 20 mmol/L 2DG and/or 0.5 μmol/L cisplatin over the 72-h exposure period. Cells treated with 0.5 μmol/L cisplatin caused a significant growth delay compared with the control cells at 48 and 72 h (Fig. 1). Treatment of cells with 2DG inhibited cell growth and was significantly different from control and cisplatin-treated cells at 48 and 72 h (Fig. 1). The combination of 20 mmol/L 2DG and 0.5 μmol/L cisplatin inhibited cell growth similar to 2DG alone (Fig. 1).
Figure 1.
Effect of 2DG and cisplatin alone and in combination on growth of FaDu cells. The cells treated with 20 mmol/L 2DG and the combination of 20 mmol/L 2DG + 0.5 μmol/L cisplatin (CIS) for 24 h showed a significant growth delay compared with control (P < 0.001) and cisplatin (P < 0.05). Points, average cell counts from three treatment dishes at each time point; bars, SD.
Enhanced cytotoxicity seen with the combination of 2DG and cisplatin is inhibited by NAC
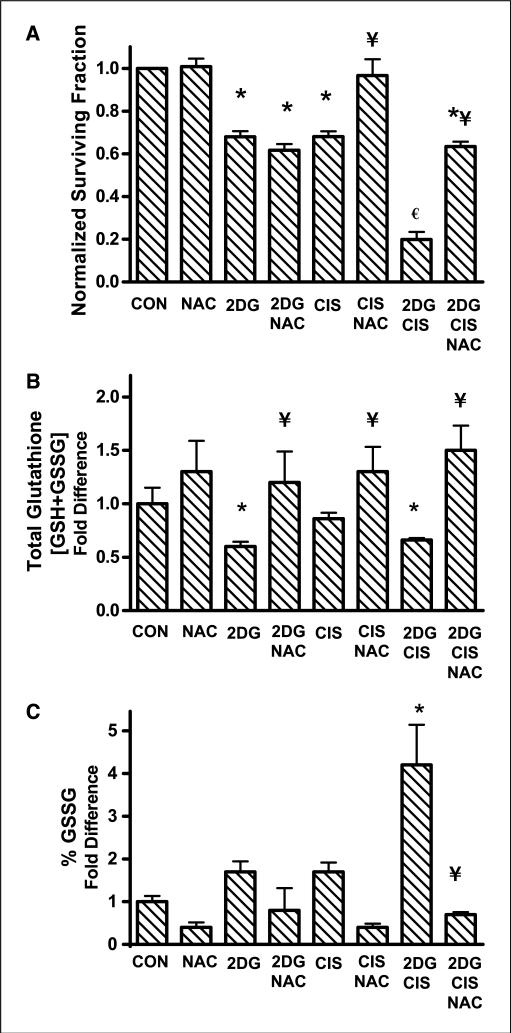
To determine if 2DG in combination with cisplatin would increase clonogenic cell killing compared with either drug alone, clonogenic assays were done following treatment with 20 mmol/L 2DG and/or 0.5 μmol/L cisplatin for 24 h. Treatment with 2DG or cisplatin alone caused 32% cell killing relative to untreated control cells (Fig. 2A). The combination of 2DG and cisplatin caused a significant increase to 80% cell killing, suggesting at least an additive and possibly a more than additive effect of 2DG and cisplatin when compared with 2DG and cisplatin alone (Fig. 2A). To probe the possible involvement of oxidative stress in the mechanism responsible for 2DG and cisplatin–induced cytotoxicity, the thiol antioxidant NAC was investigated for its ability to alter the observed cytotoxicity. Cells were treated with 15 mmol/L NAC for 1 h before and during exposure to 2DG and cisplatin and analyzed for clonogenic survival. NAC inhibited the cytotoxicity induced by treatment with cisplatin alone and partially but significantly inhibited the combination of 2DG and cisplatin but not 2DG alone (Fig. 2A). To assess the ability of NAC to rescue the cells from 2DG in combination with cisplatin after the drugs were allowed to interact with the cells, treatment with NAC was given either 1 h before or 1 h after the addition of 2DG and cisplatin. Consequently, we found that there was no statistically significant difference in the ability of NAC to rescue cells from the toxicity seen when NAC was added 1 h after 2DG and cisplatin, relative to when NAC was added 1 h before 2DG and cisplatin (data not shown). These results support the hypothesis that the direct reaction of cisplatin with NAC does not seem to completely account for the protective effects of NAC and that some other mechanism (which could include inhibition of oxidative stress) seems to play a role in the toxicity seen with the combination of 2DG + cisplatin. Taken together, these results suggest that treatment with a thiol antioxidant is able to significantly inhibit the cytotoxicity induced by 2DG in combination with cisplatin in human head and neck cancer cells.
Figure 2.
Effect of 2DG, cisplatin, and NAC on cytotoxicity (A), total glutathione (B), and percentage of oxidized glutathione (%GSSG) levels (C) in FaDu cells. A, cells were treated with 20 mmol/L 2DG and/or 0.5 μmol/L cisplatin for 24 h with or without treatment with 15 mmol/L NAC for 1 h before and during 2DG and cisplatin exposure. Clonogenic cell survival data were normalized to control (CON). Columns, mean of N = 3 experiments done on different days with at least four cloning dishes taken from one treatment dish; bars, SD. B and C, cells were treated as stated above and harvested for glutathione analysis using the spectrophotometric recycling assay. Columns, mean of N = 4 experiments; bars, SD. *, P < 0.001, versus control; ¥, P < 0.001, versus respective treatment without NAC; €, P < 0.001, versus 2DG and cisplatin alone.
2DG and cisplatin–induced disruptions in glutathione metabolism indicative of oxidative stress are inhibited by NAC
Glutathione is a major intracellular redox buffer such that the ratio of GSH to GSSG can be used as a reflection of intracellular redox status (29). Because glucose deprivation has previously been shown to alter GSH/GSSG levels (5–10) consistent with causing oxidative stress, thiol analysis was done to determine if NAC caused any effects on intracellular GSH/GSSG in cells treated with 2DG and cisplatin. Exposure of cells to 2DG and the combination of 2DG + cisplatin caused a 30% to 40% decrease in total glutathione content whereas cisplatin treatment alone did not seem to significantly alter total glutathione levels (Fig. 2B). Coincubation with 15 mmol/L NAC inhibited the effects of 2DG and 2DG + cisplatin on total glutathione compared with the same treatment groups without NAC (Fig. 2B). The combination of 2DG and cisplatin caused the percentage of GSSG to increase >3-fold compared with control (Fig. 2C), indicating that this drug combination was causing oxidative stress. When the cells were treated with the combination of 2DG and cisplatin in the presence of NAC, percentage of GSSG decreased to control levels (Fig. 2C). Taken together, the data in Fig. 2 suggest that the cytotoxic effects of 2DG in combination with cisplatin were mediated by disruptions in thiol metabolism consistent with oxidative stress, which was reversed by the thiol antioxidant NAC.
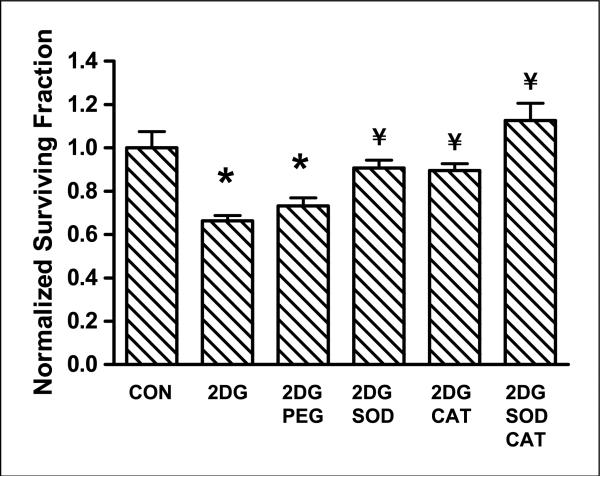
Inhibition of 2DG cytotoxicity with PEG-SOD and PEG-catalase
To investigate the role of superoxide and hydrogen peroxide in the biological effects of 2DG, FaDu cells were pretreated with 100 units/mL PEG-SOD or PEG-catalase or 50 units/mL PEG-SOD + 50 units/mL PEG-catalase for 1 h before and during treatment with 2DG for 24 h. The survival data in Fig. 3 show that PEG-SOD and PEG-catalase significantly protected the cells from 2DG-induced cytotoxicity (P < 0.01). The combination of PEG-SOD and PEG-catalase seemed to further increase the protection from 2DG toxicity induced by PEG-SOD and PEG-catalase alone, but these differences did not reach statistical significance when compared with either agent alone (Fig. 3). Exposure of cells to PEG, PEG-SOD, and PEG-catalase in the absence of 2DG had no effect on survival (data not shown). Cells treated with 2DG + PEG showed no inhibition of toxicity showing that the protection exhibited by PEG-SOD and PEG-catalase was due to the antioxidant enzymes and not due to PEG (Fig. 3). These results strongly suggest that increases in ROS (i.e., superoxide and hydrogen peroxide) contribute to the toxicity induced by 2DG.
Figure 3.
Effect of PEG-SOD and PEG-catalase on 2DG toxicity in FaDu cells. Cells were treated with 18 μmol/L PEG, 100 units/mL PEG-SOD, 100 units/mL PEG-catalase (PEG-CAT), or 50 units/mL PEG-SOD + 50 units/mL PEG-catalase for 1 h before and during treatment with 20 mmol/L 2DG for 24 h. Clonogenic cell survival data were normalized to control. Columns, mean of N = 3 experiments done on different days with at least four cloning dishes taken from one treatment dish; bars, SD. *, P < 0.01, versus control; ¥, P < 0.01, versus 2DG.
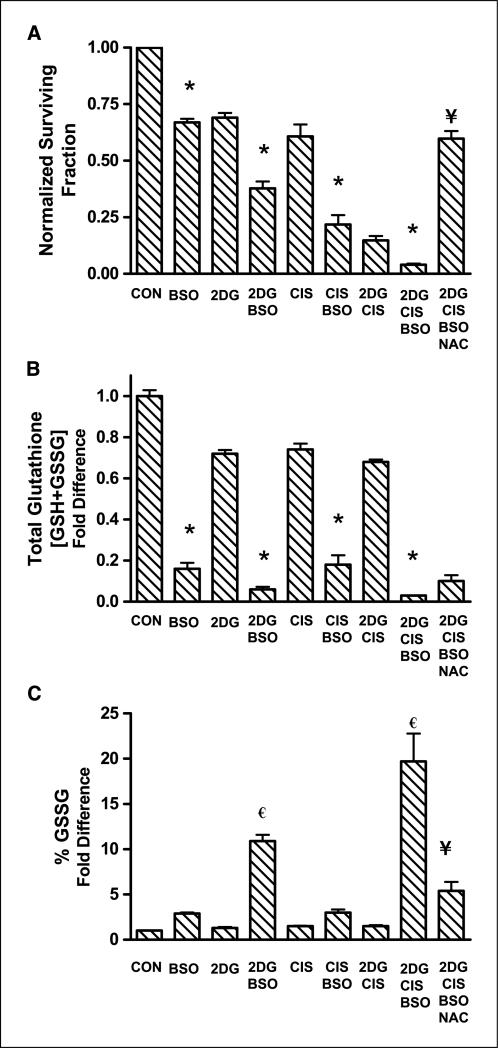
2DG and cisplatin–induced cytotoxicity is enhanced by BSO
To determine if GSH depletion would enhance the toxicity and oxidative stress induced by treatment with 2DG and cisplatin, FaDu cells were treated with 1 mmol/L BSO for 1 h before and during treatment with 2DG and cisplatin for 24 h. The results indicate that treatment with the combination of 2DG and BSO enhanced cell killing compared with 2DG alone (30% versus 60% cell killing, respectively), whereas the combination of cisplatin and BSO also enhanced cell killing compared with cisplatin alone (40% versus 78%, respectively; Fig. 4A). Additionally, Fig. 4A shows that BSO further sensitized cells to the cytotoxicity of the combination of 2DG and cisplatin (2DG + cisplatin + BSO, >95% killing, versus 2DG + cisplatin, 85% killing). Furthermore, NAC partially but significantly protected against the cytotoxicity of 2DG + cisplatin + BSO (Fig. 4A). The control of BSO + NAC was not different from BSO alone in our cell model system (data not shown). These data support the hypothesis that inhibition of glutathione (GSH) synthesis with BSO significantly enhanced the cytotoxicity observed with 2DG and cisplatin in FaDu cells.
Figure 4.
Effect of 2DG, cisplatin, and BSO on cytotoxicity (A), total glutathione (B), and percentage oxidized glutathione levels (C) in FaDu cells. A, cells were treated with 20 mmol/L 2DG and/or 0.5 μmol/L cisplatin for 24 h with or without treatment with 1 mmol/L BSO for 1 h before and during 2DG and cisplatin exposure. Clonogenic cell survival data were normalized to control. Columns, mean of N = 3 experiments done on different days with at least four cloning dishes taken from one treatment dish; bars, SD. B and C, cells were treated as stated above and harvested for glutathione analysis using the spectrophotometric recycling assay. *, P < 0.001, versus respective treatment without BSO; €, P < 0.001, versus control; ¥, P < 0.001, versus 2DG + cisplatin + BSO.
2DG and cisplatin–induced oxidative stress is enhanced by BSO
To determine if oxidative stress contributed to the cytotoxic effect of 2DG, cisplatin, and BSO, thiol analysis was done on FaDu cells treated with the three drugs alone and in combination (Fig. 4B and C). BSO caused significant GSH depletion compared with treatments without BSO (Fig. 4B). In addition, cells treated with 2DG + BSO and 2DG + cisplatin + BSO showed significant increases in percentage of GSSG (11- and 20-fold, respectively) compared with the other treatments (Fig. 4C). Furthermore, exposure to NAC significantly decreased the percentage of GSSG induced by treatment with 2DG + cisplatin + BSO (Fig. 4C). These results indicate that BSO effectively decreased total GSH and increased a parameter indicative of oxidative stress (percentage of GSSG) when coincubated with 2DG and the combination of 2DG and cisplatin. Furthermore, the fact that NAC suppressed the increase in percentage of GSSG in 2DG + cisplatin + BSO treated cells (Fig. 4C), as well as suppressed the cytotoxicity of 2DG + cisplatin + BSO (Fig. 4A), supports the hypothesis that oxidative stress was causally related to the enhanced toxicity seen with these three drugs.
Discussion
Cancer cells have been suggested to have a fundamental defect in their respiratory mechanism, which has been suggested to lead to increased generation of pro-oxidants (i.e., superoxide, hydrogen peroxide, etc.) by mitochondria (5–10). In addition, increased glucose metabolism and pentose phosphate cycle activity have been observed in cancer cells compared with untransformed (normal) cells (1–4). Because the products of glucose metabolism, pyruvate (from glycolysis) and NADPH (from the pentose cycle), are believed to function in hydroperoxide detoxification (30, 31), it is hypothesized that the up-regulation in glucose metabolism in cancer cells is necessary to produce more pyruvate and NADPH to compensate for the increase in intracellular pro-oxidant production.
The increased dependency of cancer cells on glucose metabolism for hydroperoxide detoxification is an attractive target that may be exploited to gain a therapeutic advantage when trying to kill cancer cells while sparing normal tissues. Accordingly, glucose deprivation and treatment with 2DG have been shown to induce cytotoxicity, significant increases in pro-oxidant production, and profound disruptions in thiol metabolism in colon, breast, cervical, and prostate cancer cells, suggesting that oxidative stress was involved with the mechanism of action (5–10). In the current studies, 2DG was used to mimic the effects of glucose deprivation in head and neck squamous cell carcinoma cells. 2DG is a clinically relevant analogue of glucose that competes with glucose for uptake and entry into glucose metabolic pathways (22, 32–35). 2DG can therefore create a drug-induced state of glucose deprivation, although it does not completely inhibit the regeneration of NADPH from NADP+ because it is a substrate for glucose-6-phosphate dehydrogenase (35). Inhibition of glucose metabolism was observed in animals administered 2DG without toxicity until high levels (>2 g/kg body weight) were achieved (32) and 2DG has been shown to be tolerable in humans when administered up to 200 mg/kg (27).
In the current study, we have shown that 2DG completely inhibited FaDu cell growth over the 72-h exposure period (Fig. 1) while causing 32% clonogenic inactivation after a 24-h exposure period (Fig. 2A). These findings support previous studies from our laboratory, which have shown 2DG-induced growth inhibition and cytotoxicity in MDA-MB231 cells (8), which was accompanied by increases in parameters indicative of oxidative stress and enhanced with BSO treatment. Interestingly, other researchers have shown that 2DG increased the efficacy of Adriamycin and paclitaxel in human osteosarcoma and non–small-cell lung cancers in vivo (36). This suggests that 2DG may potentially increase the efficacy of standard chemotherapeutic drugs. Based on these previous studies, we hypothesized that 2DG combined with cisplatin would increase toxicity in FaDu head and neck cancer cells by mechanisms involving oxidative stress, which could be enhanced with BSO.
Cisplatin has been successfully used as a chemotherapeutic agent against malignant solid tumors in the head and neck region (11). However, there have been barriers to the use of cisplatin in the clinical setting of head and neck cancer including nephrotoxicity (37) and cisplatin resistance (18, 38). By combining relatively nontoxic drugs such as 2DG and BSO with cisplatin, it is possible that tumor cell killing could be enhanced at lower doses, therefore minimizing the side effects of cisplatin as well as potentially helping to overcome cisplatin resistance. In the current study, we found that the combination of 2DG and cisplatin showed at least additive (and possibly more than additive) cell killing in FaDu cells compared with 2DG or cisplatin alone (Fig. 2A).
The increase in percentage of GSSG induced by 2DG + cisplatin (Fig. 2C) suggests that oxidative stress is involved. We believe that the combination of 2DG + cisplatin causes an increase in steady state levels of hydroperoxides and this increase exceeds the metabolic capabilities of the glutathione peroxidase and glutathione reductase system to maintain glutathione in the reduced form. To further support this idea, the thiol antioxidant NAC was able to inhibit the increase in percentage of GSSG (Fig. 2C) and inhibit the cytotoxicity induced by 2DG + cisplatin (Fig. 2A). Because NAC caused significant increases in total glutathione in 2DG- and/or cisplatin-treated cells (Fig. 2B), NAC may function by increasing intracellular thiol pools necessary for counteracting the effects of 2DG and cisplatin.
Key reactive oxygen species (ROS)–detoxifying enzymes in the cell include SOD, which removes superoxide anion, and catalase, which degrades hydrogen peroxide (9, 39). Because the cytotoxicity induced by 2DG was not significantly inhibited with NAC (Fig. 2A), we examined the possible role of ROS in the effects of 2DG using specific scavengers of superoxide and hydrogen peroxide. Treatment of the cells with the combination of the PEGylated forms of SOD and catalase (PEG-SOD + PEG-catalase) significantly inhibited the cytotoxicity induced by 2DG exposure (Fig. 3). These results suggest that the biological effects of 2DG do involve superoxide and H2O2. NAC may have not been able to inhibit the cytotoxicity induced by 2DG because the subsequent increase in thiols induced by NAC (Fig. 2B) was not sufficient to significantly enhance the detoxification of the ROS produced from 2DG treatment. This observation remains to be explored.
Although our data suggest that disruptions in thiol metabolism are involved with the toxicity of cisplatin alone, this mechanism may not be the underlying mechanism of cytotoxicity in cells treated with 2DG alone in this model system. However, the data shown in Fig. 3 suggest that and H2O2 contribute to the mechanism of 2DG-induced cytotoxicity as evidenced by the ability of PEG-SOD + PEG-catalase to protect against 2DG-induced toxicity (Fig. 3). Therefore, when 2DG is combined with cisplatin, increases in steady-state levels of ROS caused by treatment with 2DG may enhance the disruption in thiol metabolism caused by cisplatin, leading to increased oxidative stress (as evidenced by increased percentage of GSSG) and increased cell killing. Moreover, in support of this interpretation, NAC is able to significantly inhibit cytotoxicity induced by 2DG + cisplatin (Fig. 2A) as well as decrease oxidative stress as measured by a decrease in percentage of GSSG (Fig. 2C).
Recently, we have shown that inhibition of GSH synthesis with BSO increased parameters indicative of oxidative stress and sensitized breast cancer cells to 2DG-induced cytotoxicity (8). BSO is an inhibitor of glutamate cysteine ligase, which is believed to be the rate-limiting enzyme in the synthesis of GSH (40, 41). BSO has also been used in clinical trials for cancer therapy to enhance the cytotoxicity of chemotherapeutic agents (42). In the current study, BSO was found to sensitize FaDu cells to 2DG and cisplatin as well as significantly increase the cytotoxicity induced by 2DG + cisplatin (Fig. 4A). As expected, BSO significantly decreased total GSH levels when combined with 2DG and/or cisplatin (Fig. 4B). When BSO was combined with 2DG, oxidative stress was enhanced, as evidenced by increases in percentage of GSSG (Fig. 4C), which is comparable to previous results in our laboratory in MDA-MB231 human breast cancer cells (8). This would be expected given that superoxide and hydrogen peroxide seem to contribute to 2DG-induced cytotoxicity (Fig. 3) and glutathione is integrally related to hydrogen peroxide detoxification through the action of glutathione peroxidase. When BSO was combined with 2DG + cisplatin, percentage of GSSG further increased, suggesting that the depletion of GSH with BSO further enhanced the oxidative stress induced by 2DG + cisplatin (Fig. 4C). Furthermore, NAC significantly inhibited the cytotoxicity and the increase in oxidative stress (as evidenced by decreased percentage of GSSG) induced by 2DG + cisplatin + BSO without reversing the effects of BSO on GSH depletion (Fig. 4A and C). These results support the hypothesis that NAC inhibits the oxidative stress associated with 2DG + cisplatin + BSO independent of increased GSH, suggesting that NAC acts to augment intracellular thiol antioxidants and inhibit thiol oxidation reactions that do not depend entirely on GSH synthesis. Additionally, these results support the hypothesis that metabolic oxidative stress significantly contributes to the interaction of 2DG + cisplatin ± BSO in killing FaDu cells in this cell culture model.
Manipulations of glutathione have long been known to affect cellular sensitivity to cisplatin (43–47), with levels of GSH and cisplatin resistance being directly proportional. However, the mechanism leading to cisplatin resistance under conditions of increased GSH is unclear. Many types of drugs are detoxified by conjugation to glutathione and subsequent export or degradation. Although this may occur to some degree, cisplatin is not a substrate for any glutathione S-transferase known to detoxify other drugs (44). In addition, Ikeda et al. (45) found that cell lines with elevated levels of GSH were resistant to cisplatin but cell lines with elevated GSH contained higher levels of platinum. Other possibilities include alterations in signal transduction via c-jun NH2-terminal kinase signaling (46), alterations in DNA repair via DNA-dependent protein kinase (47), and/or alterations in the function of glutathione peroxidase. Both of the former two processes have been shown to be affected by glutathione metabolism and to affect the cellular responses to cisplatin. The data provided here suggest that increased intracellular oxidative stress contributes to the toxicity of 2DG + cisplatin, and manipulating intracellular redox levels can affect cellular responses to 2DG + cisplatin. These findings may also suggest ways to improve the efficacy of 2DG + cisplatin as a sensitizer to the oxidative stress induced by exposure to ionizing radiation.
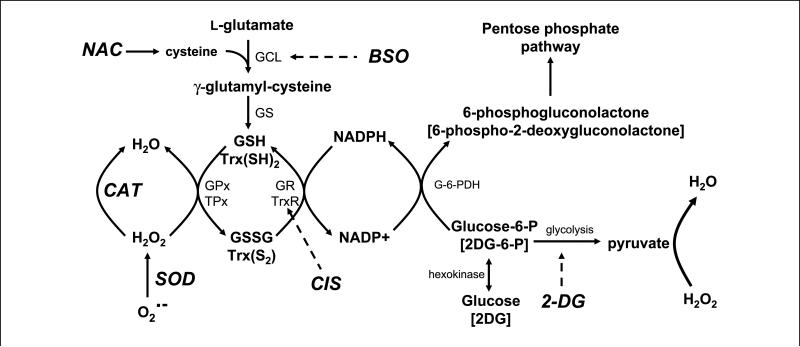
The scheme shown in Fig. 5 illustrates some of the hypothetical relationships between glucose metabolism, ROS metabolism, chemotherapeutic agents, and antioxidants suggested by the results of the current study. Inhibiting glucose metabolism with 2DG in cancer cells is hypothesized to limit the production of pyruvate and the regeneration of NADPH leading to increased steady-state levels of and H2O2 from metabolic sources resulting in cytotoxicity, which was inhibited by the antioxidant enzymes SOD and catalase. Combining 2DG and cisplatin is believed to enhance oxidative stress because cisplatin is known to be both a DNA-damaging agent and a potential inhibitor of the thioredoxin system, which is also involved with thiol homeostasis as well as the detoxification of hydroperoxides. BSO is thought to further enhance the toxicity of 2DG and cisplatin by inhibiting the synthesis of GSH that is required for GSH peroxidases and GSH transferases, both of which are believed to protect against oxidative stress. Finally, the thiol antioxidant NAC is able to protect against the combination of 2DG + cisplatin by acting to augment small molecular weight intracellular thiols that are capable of scavenging toxic species as well as protecting critical biomolecules from oxidation as well as alkylation. Overall, the results of this study support the hypothesis that 2DG combined with cisplatin enhances cytotoxicity in FaDu human head and neck cancer cells by a mechanism involving oxidative stress and disruptions in thiol metabolism. These data also strongly support the potential therapeutic use of 2DG in combination with cisplatin, as well as the new biochemical rationale shown in Fig. 5 for combining inhibitors of glucose and hydroperoxide metabolism for enhancing the cytotoxicity of anticancer agents thought to cause injury via oxidative stress.
Figure 5.
Hypothetical biochemical rationale to explain the interaction between 2DG and cisplatin. 2DG competes with glucose for uptake into the cell and phosphorylation by hexokinase into 2-deoxy-d-glucose-6-phosphate (2DG-6-P) and glucose-6-phosphate (Glucose-6-P). Glucose-6-phosphate continues into glycolysis to form pyruvate, a known scavenger of hydrogen peroxide (H2O2), whereas 2-deoxy-d-glucose-6-phosphate is unable to continue glycolysis. Glucose-6-phosphate and 2-deoxy-d-glucose-6-phosphate proceed through the first step in the pentose phosphate cycle via glucose-6-phosphate dehydrogenase (G-6-PDH) to 6-phosphogluconolactone and 6-phospho-2-deoxygluconolactone, respectively, leading to the generation of NADPH from NADP+. However, 6-phospho-2-deoxygluconolactone cannot go further in the pentose phosphate cycle. NADPH is a source of reducing equivalents for the glutathione system consisting of GSH, GSSG, glutathione peroxidase (GPx), and glutathione reductase (GR) and the thioredoxin system consisting of reduced thioredoxin [Trx(SH)2], thioredoxin disulfide [Trx(S2)], thioredoxin peroxidase (TPx), and thioredoxin reductase (TrxR; inhibited by cisplatin). The glutathione and thioredoxin systems participate in the detoxification of H2O2 and organic hydroperoxides. Superoxide dismutase (SOD) converts superoxide () to H2O2, which is converted to H2O by catalase (CAT) or glutathione peroxidase. NAC provides cysteine, which reacts with l-glutamate catalyzed by glutamate cysteine ligase (GCL; inhibited by BSO) to form γ-glutamyl-cysteine. Glutathione synthetase (GS) converts γ-glutamyl-cysteine into GSH. Dashed arrows, inhibition processes.
Acknowledgments
Grant support: Department of Radiation Oncology, University of Iowa (D.M. Mattson and K.J. Dornfeld), and NIH grants RO1-CA100045 (D.R. Spitz and I.M. Ahmad), P01-CA66081 (A.L. Simons), and P30-CA086862 (D.R. Spitz).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Ling Li, Mitchell Coleman, and Dr. Nükhet Aykin-Burns of the Radiation and Free Radical Research Core Lab at the Holden Comprehensive Cancer Center, University of Iowa, for technical assistance and advice.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;132:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Weber G. Enzymology of cancer cells (first of two parts). New Eng J Med. 1977;296:486–92. doi: 10.1056/NEJM197703032960905. [DOI] [PubMed] [Google Scholar]
- 3.Weber G. Enzymology of cancer cells (second of two parts). N Engl J Med. 1977;296:541–51. doi: 10.1056/NEJM197703102961005. [DOI] [PubMed] [Google Scholar]
- 4.Lehninger AL. Biochemistry. Worth Publishers, Inc.; New York: 1976. pp. 245–441.pp. 467–71.pp. 849–50. [Google Scholar]
- 5.Lee YJ, Galoforo SS, Berns CM, et al. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem. 1998;273:5294–9. doi: 10.1074/jbc.273.9.5294. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn RV, Spitz DR, Liu X, et al. Metabolic oxidative stress activates signal transduction and gene expression during glucose deprivation in human tumor cells. Free Radic Biol Med. 1999;26:419–30. doi: 10.1016/s0891-5849(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 7.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells: a fundamental defect in metabolism? Ann N Y Acad Sci. 2000;899:349–62. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 8.Andringa KK, Coleman MC, Aykin-Burns N, et al. Inhibition of glutamate cysteine ligase (GCL) activity sensitizes human breast cancer cells to the toxicity of 2-deoxy-d-glucose. Cancer Res. 2006;66:1605–10. doi: 10.1158/0008-5472.CAN-05-3462. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad IM, Aykin-Burns N, Sim JE, et al. Mitochondrial and H2O2 Mediate glucose deprivation-induced cytotoxicity and oxidative stress in human cancer cells. J Biol Chem. 2005;280:4254–63. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Zhang F, Bradbury CM, et al. 2-Deoxy-d-Glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63:3413–17. [PubMed] [Google Scholar]
- 11.Forastiere AA. Overview of platinum chemotherapy in head and neck cancer. Semin Oncol. 1994;21:20–7. [PubMed] [Google Scholar]
- 12.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 13.Witte AB, Anestal K, Jerremalm E, Ehrsson H, Arner ES. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med. 2005;39:696–703. doi: 10.1016/j.freeradbiomed.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Arner ES, Nakamura H, Sasada T, Yodoi J, Holmgren A, Spyrou G. Analysis of the inhibition of mammalian thioredoxin, thioredoxin reductase, and glutaredoxin by cis-diamminedichloroplatinum (II) and its major metabolite, the glutathione-platinum complex. Free Radic Biol Med. 2001;3:1170–8. doi: 10.1016/s0891-5849(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 15.Biaglow JE, Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther. 2005;4:6–13. doi: 10.4161/cbt.4.1.1434. [DOI] [PubMed] [Google Scholar]
- 16.Smart DK, Ortiz KL, Mattson D, et al. Thioredoxin reductase as potential molecular target for anticancer agents that induce oxidative stress. Cancer Res. 2004;64:6716–24. doi: 10.1158/0008-5472.CAN-03-3990. [DOI] [PubMed] [Google Scholar]
- 17.Kang H-J, Hong S-M, Kim B-C, Park E-H, Kisup A, Lim C-J. Effects of heterologous expression of thioredoxin reductase on the level of reactive oxygen species in COS-7 cells. Mol Cells. 2006;22:113–8. [PubMed] [Google Scholar]
- 18.Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992;89:3070–4. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend DM, Findlay VL, Tew KD. Gluthione transferases γ-glutamyl. Transpeptidases. 2005;401:287–307. doi: 10.1016/S0076-6879(05)01019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitz DR, Phillips JW, Adams DT, Sherman CM, Deen DF, Li GC. Cellular resistance to oxidative stress is accompanied by resistance to cisplatin: the significance of increased catalase activity and total glutathione in hydrogen peroxide-resistant fibroblasts. J Cell Physiol. 1993;156:72–9. doi: 10.1002/jcp.1041560111. [DOI] [PubMed] [Google Scholar]
- 21.Laszlo J, Humphreys SR, Goldin A. Effects of glucose analogues (2-deoxy-d-glucose, 2-deoxy-d-galactose) on experimental tumors. J Natl Cancer Inst. 1960;24:267–80. [PubMed] [Google Scholar]
- 22.Shenoy MA, Singh BB. Non-nitro radiation sensitizers. Int J Radiat Biol. 1985;48:315–26. doi: 10.1080/09553008514551311. [DOI] [PubMed] [Google Scholar]
- 23.Anderson ME. Handbook of methods for oxygen radical research. CRC Press Inc; Florida: 1985. pp. 317–23. [Google Scholar]
- 24.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 26.Spitz DR, Malcolm RR, Robert RJ. Cytotoxicity and metabolism of 4-hydroxy-2-nonenol and 2-nonenol in H2O2-resistant cell lines. Do aldehydic by-products of lipid peroxidation contribute to oxidative stress? Biochem J. 1990;267:453–9. doi: 10.1042/bj2670453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanti BK, Rath GK, Anantha N, et al. Improving cancer radiotherapy with 2-deoxy-d-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35:103–11. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 28.Sunderman FW, Jr., Sporn J, Hopfer SM, Sweeney KR, Chakraborty NG, Greenberg B. Platinum in blood mononuclear cells from patients after cisplatin therapy. Ann Clin Lab Sci. 1990;20:379–84. [PubMed] [Google Scholar]
- 29.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 30.Tuttle SW, Varnes ME, Mitchell JB, Biaglow JE. Sensitivity to chemical oxidants and radiation in CHO cell lines deficient in oxidative pentose cycle activity. Int J Radiat Oncol Biol Phys. 1992;22:671–5. doi: 10.1016/0360-3016(92)90500-h. [DOI] [PubMed] [Google Scholar]
- 31.Averill-Bates DA, Przybytkowski E. The role of glucose in cellular defences against cytotoxicity of hydrogen peroxide in Chinese hamster ovary cells. Arch Biochem Biophys. 1994;312:52–8. doi: 10.1006/abbi.1994.1279. [DOI] [PubMed] [Google Scholar]
- 32.Landau BR, Lubs HA. Animal responses to 2-deoxyglucose administration. Proc Soc Exp Biol Med. 1958;99:124–7. doi: 10.3181/00379727-99-24268. [DOI] [PubMed] [Google Scholar]
- 33.Laszlo J, Humphreys SR, Goldin A. Effects of glucose analogues (2-deoxy-d-glucose, 2-deoxy-d-galactose) on experimental tumors. J Natl Cancer Inst. 1960;24:267–81. [PubMed] [Google Scholar]
- 34.Dwarkanath BS, Zolzer F, Chandana S, et al. Heterogeneity in 2-deoxy-d-glucose-induced modifications in energetics and radiation responses of human tumor cell lines. Int J Radiat Oncol Biol Phys. 2001;50:1051–61. doi: 10.1016/s0360-3016(01)01534-6. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, O'Dea JD, Suzuki T, Agar NS. 2-Deoxyglucose as a substrate for glutathione regeneration in human and ruminant red blood cells. Comp Biochem Physiol B. 1993;75:195–97. doi: 10.1016/0305-0491(83)90312-7. [DOI] [PubMed] [Google Scholar]
- 36.Maschek G, Savaraj N, Priebe W, et al. 2-deoxy-d-glucose increases the efficacy of Adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–4. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- 38.Dempke W, Voigt W, Grothey A, Hill BT, Schmoll HJ. Cisplatin resistance and oncogenes—a review. Anticancer Drugs. 2000;11:225–36. doi: 10.1097/00001813-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Veronese FM, Caliceti P, Schiavon O, Sergi M. Polyethylene glycol-superoxide dismutase, a conjugate in search of exploitation. Adv Drug Deliv Rev. 2002;54:587–606. doi: 10.1016/s0169-409x(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 40.Spitz DR, Kinter MT, Roberts RJ. The contribution of increased glutathione content to mechanisms of oxidative stress resistance in hydrogen peroxide resistant hamster fibroblasts. J Cell Physiol. 1995;165:600–9. doi: 10.1002/jcp.1041650318. [DOI] [PubMed] [Google Scholar]
- 41.Arrick BA, Griffith OW, Cerami A. Inhibition of glutathione synthesis as a chemotherapeutic strategy for trypanosomiasis. J Exp Med. 1981;153:720–25. doi: 10.1084/jem.153.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey HH. L-S,R-buthionine sulfoximine: historical development and clinical issues. Chem Biol Interact. 1998;111:239–54. doi: 10.1016/s0009-2797(97)00164-6. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton TC, Winker MA, Louie KG, et al. Augmentation of Adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985;34:2583–86. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- 44.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–20. [PubMed] [Google Scholar]
- 45.Ikeda K, Miura K, Seiichiro H, Nobumasa I, Naganuma A. Glutathione content is correlated with the sensitivity of lines of PC12 cells to cisplatin without a corresponding change in the accumulation of platinum. Mol Cell Biochem. 2001;219:51–6. doi: 10.1023/a:1011083429704. [DOI] [PubMed] [Google Scholar]
- 46.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–75. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Townsend DM, Shen H, Staros AL, Gate L, Tew KD. Efficacy of a glutathione S-transferase π-activated prodrug in platinum-resistant ovarian cancer cells. Mol Cancer Ther. 2002;1:1089–95. [PMC free article] [PubMed] [Google Scholar]