Abstract
Human behavioral studies demonstrate that healthy aging is often accompanied by increases in memory distortions or errors. Here we used event-related functional MRI to examine the neural basis of age-related memory distortions. We utilized the memory conjunction error paradigm, a laboratory procedure known to elicit high levels of memory errors. For older adults, right parahippocampal gyrus showed significantly greater activity during false than during accurate retrieval. We observed no regions in which activity was greater during false than during accurate retrieval for young adults. Young adults, however, showed significantly greater activity than old adults during accurate retrieval in right hippocampus. By contrast, older adults demonstrated greater activity than young adults during accurate retrieval in right inferior and middle prefrontal cortex. These data are consistent with the notion that age-related memory conjunction errors arise from dysfunction of hippocampal system mechanisms, rather than impairments in frontally-mediated monitoring processes.
INTRODUCTION
Memory is not an exact replay of the past. Instead, memory depends on constructive processes that are occasionally susceptible to illusions, errors, or distortions (Roediger and McDermott, 2000; Schacter, 2001; Schacter and Slotnick, 2004). Importantly, a growing number of studies have documented that older adults can be more susceptible to various kinds of memory distortions than are younger adults (Dodson, Koutstaal, and Schacter, 2001; Jacoby and Rhodes, 2006). For example, studies of false recognition, where people incorrectly claim that they have recently encountered a novel item or event, and express high confidence in these false claims (Underwood, 1965), have revealed striking age-related increases in memory errors (e.g., Dodson & Schacter, 2002; Koutstaal & Schacter, 1997; Jennings & Jaocby, 1997; Norman & Schacter, 1997; Tun et al., 1998).
A type of false recognition procedure known as the memory conjunction error paradigm, developed initially by Underwood and Zimmerman (1973), has also revealed clear evidence for aging effects. Memory conjunction errors represent a form of memory illusion in which participants falsely claim to recognize an item because all of its constituent components were contained across several previously presented items. For example, following presentation of study words such as “blackmail”, “jailbird”, “shoestring”, participants may subsequently claim to recognize conjunction lures (e.g., “blackbird” – in which both parts are studied, yet recombined) and feature lures (e.g., “drawstring” – in which one part is studied and one part is novel). In this paradigm, memory failures lead participants to endorse as “old” conjunction and feature lures because such stimuli consist of studied elements - though not the specific combination of elements seen at study. Conjunction errors have been observed across of a wide set of stimulus materials including compound and noncompound words, nonsense words, word pairs, sentences, pictures, and faces (Dorfman, 1994; Jha, Kroll, Baynes, and Gazzaniga, 1997; Jones and Jacoby, 2005; Kroll, Knight, Metcalfe, Wolf, Tulving, 1996; Reinitz, Lammers, Cochran, 1992; Reinitz, Verfaellie, Milberg, 1996; Rubin, Van Patten, Glisky, and Newberg, 1999; Underwood, Kapelak, and Malmi, 1976) and in a variety of participant groups including healthy young adults and older adults (Jones and Jacoby, 2005; Kroll, Knight, Metcalfe, Wolf, Tulving, 1996; Rubin, Van Patten, Glisky, and Newberg, 1999), as well as several neuropsychological patient populations (Jha, Kroll, Baynes, and Gazzaniga, 1997; Kroll, Knight, Metcalfe, Wolf, Tulving, 1996; Rubin, Van Patten, Glisky, and Newberg, 1999).
To date, the mnemonic processes underlying age-related conjunction errors remain unclear. One proposal is that these errors reflect a binding failure during encoding, such that elements of an initial learning event are inappropriately recombined into episodes that did not actually take place (Kroll, Knight, Metcalfe, Wolf, Tulving, 1996). Binding failures may arise from weak and inefficient binding of stimulus elements, perhaps reflecting a failure to encode relational information. This proposal was suggested by Kroll and colleagues (1996), who conducted two experiments with undergraduate students, older adults, and patients with severe memory impairment (following damage to the hippocampal system). Results from both experiments revealed that older adults, and to an even greater extent memory impaired patients, showed a lower rate of correctly endorsing stimuli presented at study (i.e., hit rates), as well as higher rates of memory conjunctions errors (i.e., conjunction lures) compared to young adults. The results were taken as evidence that older adults and hippocampal patients may encode and store the components of stimuli, but not the relationship among these components (i.e., a binding deficit at encoding).
Such an account proposes two types of representations: features and conjunctions (Jones, Jacoby, & Gellis, 2001). Whereas features are the basic components or representations of stimuli, configural representations arise when two or more features are bound together. For example, the word “shoestring” consists of two separate features, “shoe” and “string”, as well as the configural presentation of “shoestring”. According to this representational account, age-related conjunction errors arise either from feature representations in the absence of configural representations or inaccurate configuration representations formed at encoding (Kroll, Knight, Metcalfe, Wolf, Tulving, 1996).
A contrasting proposal is that age-related conjunction errors arise from faulty monitoring processes at retrieval. Proponents of this processing account suggest that individuals mistake the ease in processing of studied elements (i.e., processing fluency) with familiarity of the whole word, and as a consequence endorse as “old” conjunction and feature lures (Rubin, Van Patten, Glisky, and Newberg, 1999). Because conjunction and feature lures engender more familiarity than new words, such lures are identified as “old” more often than baseline. Rubin and colleagues (1999) examined memory conjunction errors in young and older adults who were characterized according to their neuropsychological status. The results indicated that neuropsychological tasks sensitive to executive functions (i.e., frontal lobe function) predicted conjunction error rates, but not correct responses. An additional electrophysiological study of younger adults using event related potential (ERPs) provided evidence that memory conjunction errors are retrieved differently than correct responses. Based on these collective findings, the authors suggested that lures to conjunction lures are products of faulty, frontally-mediated monitoring processes during retrieval.
More recently, Jones and Jacoby (2005) extended the processing view by suggesting that in some instances, individuals may avoid a conjunction error at test by remembering that a different word was presented at study. They cast this proposal within the context of a dual process theory of recognition which postulates two bases for recognition judgments: recollection of the study episode and familiarity with the test probe. Although familiarity may typically lead to conjunction errors, if individuals can recollect the studied words, that recollection should counteract the effects of fluency.
Jones and Jacoby (2005) explored the hypothesis that older adults’ inability to recollect studied items may lead to their increase in familiarity-based conjunction errors. To alter the likelihood that recollection would occur, study repetition was manipulated such that some compound words occurred once, while others occurred three times (with increased repetitions enhancing the likelihood of recollection). In addition, in Experiment 2, they used a modality manipulation (i.e., words seen or heard) to provide participants with an additional source of information that they could recollect to avoid conjunction errors. Age-related differences were observed for hit rates, but not false alarms rates. All participants showed the same pattern of “old” responses across item types (old>conjunction lure> feature lure> new). Age-related differences emerged, however, when retrieval of modality information provided a way to avoid conjunction errors. The results suggest that older adults’ inability to recollect study stimuli (i.e., a retrieval-based impairment) lead to increased memory conjunction errors.
While findings from these behavioral studies show clear age differences in memory conjunction errors, they are ambiguous as to whether age-related memory distortions reflect failures to appropriately bind stimulus elements at study or retrieve the links/associations between stimulus components at test (i.e., representational account) or result from errors regarding the ease in processing of studied elements or familiarity in the absence of recollection (i.e., processing account). Importantly, although the existing literature predominantly casts the representational and processing accounts as mutually exclusive, it is possible that there are circumstances under which individuals may rely more on representations (e.g., when an item is encoded strongly) and other circumstances under which familiarity is important (e.g., weak memory traces). Moreover, even within the same subject, both of these processes could be operating, depending on the strength of the memory representation. As such, age-related memory conjunction errors may arise from some combination of faulty representational and processing mechanisms.
Finally, regarding brain circuitry, the neural mechanisms underlying age-related memory conjunction errors have been inferred based mainly upon indirect findings from neuropsychological data. Elucidating the neural bases of such errors may provide valuable insight into the core mnemonic processes. For example, two recent neuroimaging studies have explored age-related brain changes during true and false memory formation (Dennis, Kim, and Cabeza, 2007) and retrieval (Dennis, Kim, and Cabeza, 2008) using a modified version of the Deese-Roediger-McDermott (DRM) paradigm. In this paradigm, participants study lists of words that are semantically related to a word that is not presented (related lure) and, at test, show a strong tendency to incorrectly recognize that related lure (Roediger and McDermott, 1995). Both neuroimaging studies revealed age-related increases in left middle temporal gyrus for processing of false memories, as well as age-related reductions in the medial temporal lobe (hippocampus) for processing of true memories. This latter finding is consistent with a prior study linking reductions in hippocampal activity with older adults’ recollection deficits (Deselaar et al., 2006). Importantly, these results indicate that the nature of the memory distortion (e.g., gist-based false recognition, memory conjunction error), as well as the paradigm used to elicit the memory errors, may determine the locus of age-related differences in neural activity during false memories.
In the current study we examined directly the impact of age on the neural basis of memory conjunction error retrieval. To do so, we conducted an event-related functional magnetic resonance imaging (fMRI) study in young and healthy old adults during retrieval of intact (i.e. direction repetitions), conjunction, feature, and new compound words. To equate overall recognition performance between the young adults and older adults, we inserted a two-hour delay between the study and test sessions for young participants.
In light of the aforementioned evidence and theoretical views of memory conjunction errors, we predicted that compared with young adults, older adults would show reductions in hippocampal activity during retrieval of true memories (i.e., hits>conjunction errors). Additionally, we predicted that older adults would exhibit increased recruitment of other brain regions, such as the frontal lobes, during retrieval of true memories, consistent with prior reports demonstrating a shift from hippcampal- to frontal-based processing in healthy older adults during encoding (e.g., Gutchess, Welsh, Hedden, Bangert, Minear, Liu, and Park, 2005) and retrieval (e.g., Grady, McIntosh, and Craik, 2005) of veridical memories. Finally, we investigated the possibility that regions within the parahippocampal gyrus, a medial temporal lobe region, can be associated with processing of stimulus familiarity (e.g., Daselaar, Fleck, and Cabeza, 2006) and may play a role in age-related conjunction errors (conjunction false alarms and feature false alarms > hits).
METHODS
Participant Demographics
Fifteen young adults between the ages of 19 and 28 years (M = 22.3, SD = 2.9; 10 female) and fifteen older adults between the ages of 66 and 80 years (M = 72.3, SD = 4.7; 9 female) were paid for their participation1. Young adults were recruited from flyers posted on the Harvard University campus and older adults were recruited from Cambridge, Massachusetts and the surrounding communities. All participants were screened to ensure that they were healthy, right-handed, had no contra-indications for functional magnetic resonance imaging (fMRI), and were not taking psychotropic medication. Informed consent was obtained from all participants.
Neuropsychological Assessment
In addition, older adult participants were given a battery of neuropsychological tests to assess their mental functioning and to ensure that they were not demented. The neuropsychological battery consisted of the Mini-Mental State Exam, subtests from the Wechsler Adult Intelligence Scale-III (Mental Arithmetic, Visual Paired Associates, Verbal Paired Associates), subtests from the Wechsler Adult Memory Tests – III (Logical Memories, Mental Control, Digit Span Backward), the California Learning Test, the modified Wisconsin Card Sorting Test, and the Controlled Oral Word Association Test. Participants whose performance was greater than one standard deviation below the mean were excluded from participation.
Stimuli and Experimental Cognitive Task
Stimuli were 315 compound words and consisted of 105 triplet sets. Each triplet consisted of 2 compound words (e.g., courtship, birdhouse) and a third compound word that was a recombination of the other two stimuli (e.g., courthouse).
All participants began with a practice session. Stimuli in the practice session were non-compound words. We chose non-compound words for the practice session to instruct subjects to endorse as “old” only those stimuli that were an exact match to a studied word, while reserving the valuable compound word triplets for the actual task.
Both the practice session and the study phase were conducted outside of the scanner. At study, participants viewed one compound word at a time on the computer screen and were asked to decide whether or not they found the compound word to be pleasant or unpleasant. Participants were told that there was no correct answer and that their judgment should reflect their opinion. The study listed consisted of 105 compound stimuli: 21 “old” compound stimuli (e.g., snowman), 42 compound stimuli (e.g., checklist, needlepoint) whose components contributed to the 21 “conjunction lures” in the test phase (e.g., checkpoint), and 42 stimuli (e.g., shoestring) whose components contributed to the 42 “features lures” (e.g., drawstring) in the test phase. Participants were instructed to press “1” to indicate that the word was pleasant and to press the number “2” to indicate that the word was unpleasant. For older participants, there was approximately a 15 minute delay period between the study and test phases during which the participants were placed into the scanner. For young participants, there was a two hour delay between the study and test phases. The delay interval, the duration of which was determined based on pilot data, was utilized to equate recognition accuracy performance between the young and old groups.
During the scanned test phase, participants viewed one compound word at a time and were asked to decide whether or not each word appeared previously. The test list consisted of 105 stimuli, 21 “intact” stimuli (e.g., snowman, direct repetitions from the study list), 21 “conjunction lures” (e.g., checkpoint, recombinations of studied stimulus components), 42 “features lures” (e.g., drawstring, one component taken from a studied word and one component novel) and 21 “new” (e.g., yardstick, completely novel) stimuli. Participants were instructed to press “1” to indicate that the word had appeared previously (“old”) and to press the number “2” to indicate that the word had not appeared previously (“new”).
Imaging Session
The imaging session lasted 30 minutes and included two high resolution T1-weighted (MP-RAGE) structural scans and one functional run, 10 minutes 30 seconds in length. The fMRI run contained 21 intact words, 21 recombined lure words, 42 feature lures, 21 new words. Presentation of these words was jittered with presentation of baseline events, during which participants viewed a fixation cross. Compound words occurred for exactly 4 seconds, while baseline trials occurred for either 4, 8, or 16 seconds. The task was presented using MacStim software (CogState Ltd, Melbourne, Australia).
Imaging Acquisition and Analysis
Acquisition
Whole-brain gradient-echo, echo-planar images were collected (twenty-six 3.2-mm slices, TR = 2, TE = 40) using a 1.5T Siemens Avanto scanner while the participants performed the test phase of the cognitive task. Slices were oriented along the long axis of the hippocampus with a resolution of 3.125mm × 3.125mm × 3mm. High resolution T1-weighted (MP-RAGE) structural images were collected for anatomic visualization. Stimuli were back-projected onto a screen and viewed in a mirror mounted above the participant’s head. Responses were recorded using an MR-compatible response box. Head motion was restricted using a pillow and foam inserts.
Analysis
All preprocessing and data analysis were conducted using SPM 99 (Statistical Parametric Mapping; Wellcome Department of Neurology, UK). Slice acquisition timing was corrected by resampling all slices in time relative to the first slice, followed by rigid body motion correction. The functional data were then normalized spatially to the standard T1 Montreal Neurological Institute template. Images were resampled into 3-mm cubic voxels and smoothed spatially with an 8-mm full-width half-maximum isotropic Gaussian kernel.
For each participant, on a voxel-by-voxel basis, an event-related analysis was first conducted in which all instances of a particular event type were modeled through the convolution with a canonical hemodynamic response function. All participants had at least 6 instances of every modeled event type (see Chua et al., 2006 for an example of modeling less than 10 instances per event type). Effects for each event type were estimated using a subject-specific, fixed effects model. These data were then entered into a second order, random-effects analysis. Analyses contrasted activation as a function of memory performance (comparing accurate retrieval to false retrieval) using the three trial types (intact, conjunction lure, feature lure). Regions consisting of at least five contiguous voxels that exceeded the threshold of p< 0.001 were considered reliable.
Conjunction analyses (using the masking function in SPM99) then examined what neural regions were: (1) commonly activated by young and older participants during accurate retrieval and (2) differential activated by young or older participants during accurate and false retrieval. For conjunction analyses examining commonalities between groups, the threshold for each contrast entered in to a conjunction analysis was set at p < .01 (such that the conjoint probability of the conjunction analysis, using Fisher’s estimate (Fisher, 1950; Lazar, Luna, Sweeney, and Eddy, 2002) was p < .001). For conjunction analyses examining differences between groups, the threshold for the first contrast entered in to a conjunction analysis was set at p < .01, while the threshold for the second contrast entered into the conjunction analysis was set at p<.001 (such that the conjoint probability of the conjunction analysis, using Fisher’s estimate was p<.001).
All activations are presented in neurological coordinates (i.e., activity in the right hemisphere is presented on the right side of the brain image). Voxel coordinates are reported in Montreal Neurological Institute (MNI) coordinates and reflect the most significant voxel within the cluster. For young adults, activity is projected onto a composite image created by averaging the structural images of all young adult participants. For old adults, activity is projected onto a composite image created by averaging the structural images of all old adult participants.
RESULTS
Behavioral Data
The proportion of studied and unstudied stimuli endorsed as “old” are shown in Table 1. An analysis of variance (ANOVA) with response type (hit, conjunction false alarm, feature false alarm, novel false alarm) as a within-subject factor and group (young, old) as a between-subject factor revealed a main effect of response type (F(1, 3) = 219.20, p<.0001). The main effect of response type stemmed from the fact that intact words were judged “old” at a rate higher than that of conjunction lures, which were identified “old” at a rate higher than that of feature lures, which in turn, were identified “old” at a rate higher than that for new words (i.e., old > conjunction > feature > new), all p’s < .05. There was no main effect of group (F(1,28) < 1) and no interaction between group and response type (F(1,3) = 1.01, p > .3). These findings indicate that the matching procedure successfully equated performance between young and old groups. As such, any age differences observed in the functional imaging analysis cannot be attributable to accuracy differences between the two groups.
Table 1.
| A. Proportion of studied and unstudied stimuli endorsed as “old” as a function of age. Standard deviations are shown in parentheses.
| ||
|---|---|---|
| Stimulus Type | Young | Old |
| Intact | .82 (.12) | .78 (.08) |
| Conjunction | .39 (.14) | .41 (.11) |
| Feature | .25 (.12) | .29 (.16) |
| New | .18 (.11) | .17 (.10) |
| B. Mean number of trials in each condition for each age Group. Standard deviations are shown in parentheses.
| ||
|---|---|---|
| Stimulus Type | Young | Old |
| Intact | 17.3 (2.6) | 16.4 (1.7) |
| Conjunction | 9.2 (2.9) | 8.5 (2.3) |
| Feature | 10.2 (4.1) | 12.0 (5.8) |
| New | 3.7 (2.4) | 3.6 (2.1) |
An ANOVA conducted on the response latencies associated with making the recognition judgments revealed a main effect of response type (F(1, 3) = 5.17, p <.005). Specifically, response latencies for hits were significantly faster than response latencies to each type of false alarm, all p’s < .05. There was no main effect of group (F(1,28) < 1) and no interaction between group and response type (F(1,3) < 1). Thus, any age differences observed in the functional imaging analysis cannot be attributed to response time differences between the two groups.
Imaging Data
Neural regions associated with accurate and distorted memories in young adults
We assessed regions activated by young adults for accurate (i.e., hits) and distorted (i.e., conjunction lures and features lures) memories2. First, we contrasted activity for hits compared to conjunction lures (see Table 2). This contrast showed bilateral activations in parietal (BA 39/40) and frontopolar regions (BA10), as well as activity in left middle temporal gyrus (BA 21), right superior temporal gyrus (BA 22), left anterior cingulate (BA 32), right posterior cingulate (BA 31), and right orbitofrontal cortex/middle frontal gyrus (BA 9/46). Second, we contrasted activity for hits compared to feature lures (see Table 2). This contrast revealed bilateral activity in superior frontal gyrus (BA 10) and anterior cingulate (BA 24/32), as well as activity in right precuneus (BA 7), right middle temporal gyrus (BA 21), left inferior parietal lobule (BA 40), left posterior cingulate (BA 23), and left parahippocampal gyrus/hippocampus. Finally, we performed a conjunction analysis and contrasted activity for hits compared to conjunction lures and hits compared to feature lures (hit>conjunction lure AND hit> feature lure). This analysis revealed bilateral activity in parietal regions (BA 39/40), frontopolar cortex (BA 10), and posterior cingulate (BA 23), as well as activity in right middle temporal gyrus (BA 21) and parahippocampal gyrus/hippocampus3. Of note, no regions were significantly activated for the reverse subtractions/analysis (i.e., conjunction lures > hits or feature lures>hits) at the specified thresholds.
Table 2.
Regions of Significant Activation in Young Adults
| MNI Coordinates
|
|||||
|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | x | y | z |
| Hit > Conjunction FA | |||||
| Inferior parietal lobule | L | 40 | −48 | −42 | 51 |
| −42 | −51 | 57 | |||
| Parietal lobe, angular gyrus | R | 39 | 42 | −60 | 24 |
| Middle temporal gyrus | L | 21 | −54 | −57 | 21 |
| Parietal lobe, angular gyrus | L | 39 | −48 | −54 | 27 |
| −51 | −57 | 39 | |||
| −45 | −60 | 45 | |||
| Parietal lobe, angular gyrus | R | 39 | 48 | −51 | 36 |
| Posterior Cingulate | R | 31 | 3 | −42 | 27 |
| Frontopolar, middle frontal gyrus | L | 10 | −3 | 57 | 0 |
| Frontopolar, superior frontal gyrus | R | 10 | 6 | 60 | 3 |
| Superior temporal gyrus | R | 22 | 51 | −9 | 3 |
| Anterior cingulate | L | 32 | −3 | 42 | −6 |
| Parietal lobe, precuneus | R | 7 | 3 | −60 | 36 |
| Orbitofrontal cortex/middle frontal gyrus | R | 9/46 | 27 | 36 | 33 |
| Insular cortex | R | 13 | 45 | −9 | −3 |
| 48 | 0 | −3 | |||
| Hit > Feature FA | |||||
| Frontopolar, superior frontal gyrus | L | 10 | −3 | −54 | 6 |
| Parietal lobe, precuneus | R | 7 | 6 | −54 | 33 |
| Middle temporal gyrus | R | 21 | 42 | −69 | 21 |
| Parietal lobe, precuneus | R | 7 | 21 | −54 | 33 |
| Superior temporal | R | 22 | 48 | −6 | −9 |
| Parahippocampal gyrus/Hippocampus | L | 35/na | −18 | −18 | −21 |
| Anterior cingulate | R | 24 | 12 | −18 | 45 |
| Anterior cingulate | L | 32 | −3 | 45 | −3 |
| Frontopolar, superior frontal gyrus | R | 10 | 15 | 63 | 6 |
| Inferior parietal lobule | R | 40 | 51 | −45 | 45 |
| Middle Occipital gyrus | L | 19 | −39 | −75 | 30 |
| Posterior cingulate | L | 23 | −3 | −39 | 24 |
| Hit > Conjunction FA AND Hit > Feature FA | |||||
| Parietal, angular gyrus | R | 39 | 42 | −60 | 24 |
| 39 | −66 | 30 | |||
| 48 | −69 | 33 | |||
| Inferior parietal lobule | R | 40 | 48 | −54 | 39 |
| 54 | −57 | 45 | |||
| Posterior cingulate | R | 23 | 3 | −42 | 27 |
| 3 | −60 | 36 | |||
| Posterior cingulate | L | 23 | −6 | −48 | 33 |
| Parietal, angular gyrus | L | 39 | −48 | −54 | 27 |
| −39 | −75 | 30 | |||
| −45 | −69 | 30 | |||
| Frontopolar, Middle frontal gyrus | L | 10 | −3 | 57 | 0 |
| −3 | 42 | −6 | |||
| Frontopolar, Superior frontal gyrus | R | 10 | 6 | 60 | 3 |
| Insular cortex | R | 13 | 45 | −9 | −3 |
| Inferior parietal lobule | L | 40 | −48 | −48 | 45 |
| −48 | −57 | 42 | |||
| Anterior cingulate | R | 24 | 6 | −15 | 39 |
| Inferior parietal lobule | R | 40 | 39 | −45 | 51 |
| Middle temporal gyrus | R | 21 | 60 | −54 | 18 |
| Parahippocampal gyrus/Hippocampus | R | 21 | −3 | −21 | |
Regions significant at uncorrected p <.001 with an extent > 5.
BA = Brodmann area (approximate)
Neural regions associated with accurate and distorted memories in older adults
Next, we assessed regions activated by older adults for accurate and distorted memories. First, the contrast of hits compared to conjunction lures revealed activations in right precental gyrus (BA 6) and right orbitofrontal cortex (BA 11) (Table 3). Second, the contrast of hits compared to feature lures revealed activity in left inferior frontal gyrus (BA 45). Third, the conjunction analysis contrasting activity for hits compared to conjunction lures and hits compared to feature lures (hit>conjunction lure AND hit> feature lure) revealed activity in middle temporal gyrus (BA 21). We then assessed activity for the reverse subtractions/analysis to examine neural regions associated with distorted memories. We observed no significant activations for the contrast of conjunctions lures compared to hits. The contrast of activity for feature lures compared to hits revealed activity in right parahippocampal cortex. Likewise, the conjunction analysis for conjunction lures compared to hits and feature lures compared to hits (conjunction lure>hit AND feature lure>hit) revealed activity in parahippocampal gyrus.
Table 3.
Regions of Significant Activation in Older Adults
| MNI Coordinates
|
|||||
|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | x | y | z |
| Hit > Conjunction FA | |||||
| Precentral gyrus | R | 6 | 39 | 0 | 48 |
| Orbitofrontal cortex | R | 11 | 18 | 42 | −15 |
| Hit > Feature FA | |||||
| Inferior frontal gyrus | L | 45 | −42 | 36 | 6 |
| Hit > Conjunction FA AND Hit > Feature FA | |||||
| Middle temporal gryus | R | 21 | 45 | −57 | 15 |
| Conjunction FA > Hit | |||||
| no significant activations | |||||
| Feature FA > Hit | |||||
| Limbic lobe, isthmus | R | 27 | 16 | −27 | −12 |
| Conjunction FA > Hit and Feature FA > Hit | |||||
| Limbic lobe, isthmus | R | 27 | 16 | −27 | −12 |
| Parahippocampal gyrus | R | 35 | 33 | −21 | −24 |
Regions significant at uncorrected p <.001 with an extent > 5.
BA = Brodmann area (approximate)
Neural regions commonly associated with young and old during accurate retrieval
We examined shared regions of activation across young and old participants during retrieval of accurate, as compared to distorted, memories (see Table 4). To do so, we first conducted a conjunction analysis to identify regions that were more active during the “intact” (hits) than during the “conjunction lure” (false alarms) for both young and old groups. This analysis revealed activity in middle temporal gyrus (BA 21) and precental gyrus (BA 4). Next, we conducted the same type of conjunction analysis with hits and feature lures (false alarms). This analysis showed activity in right precuneus, right middle temporal gyrus and left middle frontal gyrus (BA 10). Finally, we conducted a conjunction analysis to identify regions that were more active during the “intact” condition than during the “conjunction lure” and “feature lure” condition for both young and old groups (hit>conjunction lure AND hit>feature lure). This analysis showed activity in right precuneus and right middle temporal gyrus.
Table 4.
Regions of Significant Activation Common to Young and Older Adults
| MNI Coordinates
|
|||||
|---|---|---|---|---|---|
| Region of Activation | Hemisphere | BA | x | y | z |
| Hit > Conjunction FA | |||||
| Middle temporal gyrus | R | 21 | 42 | −57 | 18 |
| Precentral gyrus | R | 4 | 42 | −15 | 60 |
| Hit > Feature FA | |||||
| Parietal lobe, precuneus | R | 7 | 6 | −51 | 33 |
| 9 | −60 | 33 | |||
| 15 | −45 | 30 | |||
| Middle temporal gyrus | R | 21 | 45 | −60 | 15 |
| Middle frontal gyrus | L | 10 | −3 | 45 | −3 |
| Hit > Conjunction FA AND Hit > Feature FA | |||||
| Parietal lobe, precuneus | R | 7 | 9 | −57 | 33 |
| Middle temporal gyrus | R | 21 | 45 | −57 | 15 |
Regions significant at uncorrected p <.001 with an extent > 5.
BA = Brodmann area (approximate)
Neural regions commonly associated with young and old during inaccurate retrieval
No regions were commonly associated with young and old during retrieval of distorted memories as compared to true memories. This result was anticipated because no regions were found to be activated in any of the distorted memory compared to accurate memory contrasts in young adults.
Neural regions associated with retrieval of accurate memories as a function of age
We assessed regions uniquely activated by young (i.e., young> old) or older (i.e., old> young) adults for retrieval of accurate, as compared to distorted, memories (threshold set at p<.001, see page 9). To do so, we contrasted activity during “intact” (i.e., accurate retrieval) versus “conjunction lure” (i.e., false retrieval) conditions, as well as activity during “intact” (i.e., accurate retrieval) versus “feature lure” (i.e., false retrieval) conditions. The comparison of activity during “intact” versus “conjunction lure” conditions showed that young adults (versus older adults) activated right amygdala/hippocampus and left caudate during accurate retrieval compared with distorted retrieval (see Figure 2A). Previous studies in young adults have documented hippocampal and/or thalamic activity during the successful recollection of studied stimuli (e.g., Eldridge, Knowlton, Furmanksi, Bookheimer, and Engel, 2000; Henson, Rugg, Shallice, Josephs, and Dolan, 1999). The comparison of activity during “intact” versus “feature lure” conditions demonstrated that young adults (versus older adults) activated right hippocampus and right thalamus during accurate retrieval (see Figure 2B). Of note, the peak of the activity for the “intact” versus “feature lure” contrast was located at a more posterior location on the long axis of the hippocampus than was the peak for the “intact” versus “conjunction lure” comparison. Taken together, these results demonstrate that young adults, as compared to older adults, activated right anterior hippocampus and amygdala, as well as bilateral thalamus during veridical retrieval compared with distorted retrieval.
Figure 2.
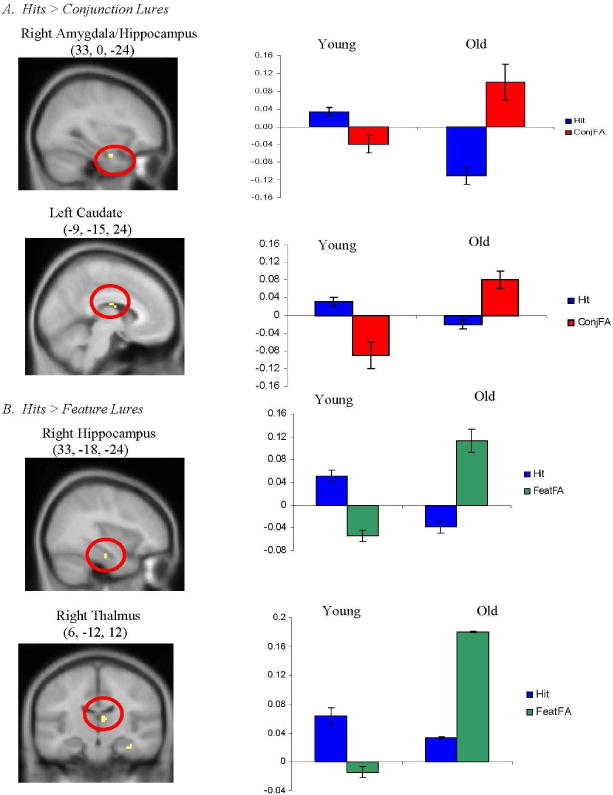
Regions uniquely activated by young adults during retrieval of accurate (“hits”), as compared to distorted (A. “conjunction lures” and B. “feature lures”), memories. Coordinates are given in standard Montreal Neurological Institute (MNI) space. Activity is projected onto a composite structural image created by averaging the structural images of all young adult participants.
By contrast, the comparison of activity during “intact” versus “conjunction lure” conditions for older adults (versus young adults) showed that the elderly activated right inferior frontal, right posterior cingulate and left insula during accurate retrieval (see Figure 3A). Similarly, the comparison of activity during “intact” versus “feature lure” conditions demonstrated that older adults (versus young adults) activated right middle frontal during accurate retrieval (see Figure 3B). Thus, older adults, as compared to young adults, activated right inferior and middle frontal regions during veridical retrieval as compared with distorted retrieval.
Figure 3.
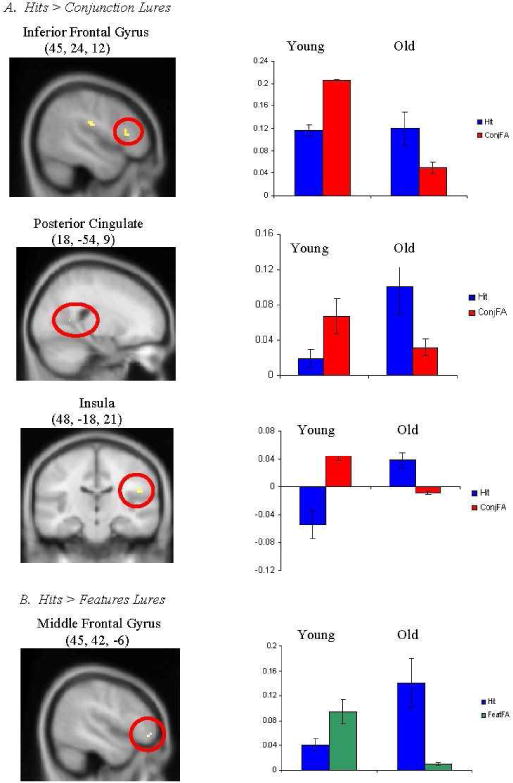
Regions uniquely activated by old adults during retrieval of accurate (“hits”), as compared to distorted (A. “conjunction lures” and B. “feature lures”), memories. Coordinates are given in standard Montreal Neurological Institute (MNI) space. Activity is projected onto a composite structural image created by averaging the structural images of all older adult participants.
Neural regions associated with retrieval of distorted memories as a function of age
To examine the regions that were uniquely activated by young (i.e., young> old) or older (i.e., old> young) adults during retrieval of distorted memories as compared to true memories, we looked for regions in which activity was greater during “conjunction lure” and “feature lure” conditions than during the “intact” condition. For young adults, we observed no regions in which activity was greater during “conjunction lure” and “feature lure” conditions than during the “intact” condition. For older adults, however, right parahippocampal gyrus showed significant activity during “conjunction lure” and “feature lure” conditions than during the “intact” condition (see Figure 4). It should be noted that such activity in right parahippocampal gyrus may have been driven primarily by the feature lure condition, as this condition also elicited parahippocampal activity in the comparison of distorted versus accurate retrieval (i.e., feature false alarm versus hits) in older adults.
Figure 4.
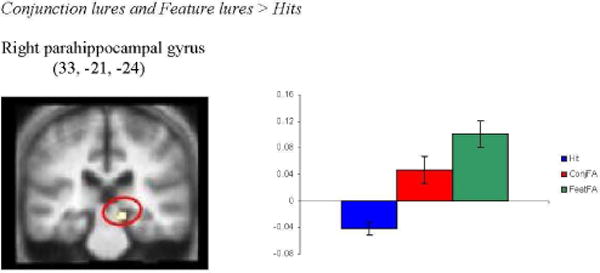
Regions uniquely activated by old adults during retrieval of distorted, as compared to accurate, memories. Coordinate is given in standard Montreal Neurological Institute (MNI) space. Activity is projected onto a composite structural image created by averaging the structural images of all older adult participants.
DISCUSSION
The current study utilized the memory conjunction error paradigm and event-related fMRI to examine the cognitive and neural basis of age-related memory distortions. There were three main findings. First, young adults showed significantly greater activity than older adults in right hippocampus during retrieval of accurate memories. Second, older adults demonstrated greater activity than young adults in right inferior and middle prefrontal cortex during accurate retrieval. Finally, the right parahippocampal gyrus showed a stronger relation to false retrieval in older adults than in young adults. We observed no regions in which activity showed a stronger relation to false retrieval in young adults. Below we consider each of these findings.
Age-related neural differences during veridical retrieval
In regard to accurate retrieval, young adults showed significantly greater activity than older adults in right hippocampus (figure 2). Activity in the hippocampus has been linked to the conscious recollection of learned episodes (e.g., Eldridge, Knowlton, Furmanski, Bookheimer, and Engel, 2000; Schacter, Alpert, Savage Rauch, and Albert, 1996) or to retrieval of associations between components of an event (e.g., Giovanello, Schnyer, and Verfaellie, 2004; Giovanello, Schnyer, and Verfaellie, 2008; Preston, Shrager, Dudukovic, Gabrieli, 2002). As such, the age-related decreases in hippocampal activity observed in the current study may reflect declines in recollection processes or decrements in associative memory. Indeed, age-related reductions in recollection (Bastin and Van der Linden, 2003; Davidson and Glisky, 2002, Parkin and Walter, 1992) and associative memory (see Old and Naveh-Benjamin, 2008 for a review) have been reported by several investigators, and more recently, older adults’ recollection deficits have been linked to reductions in hippocampal activity (Daselaar et al., 2006).
Interestingly, the hippocampal activity observed in the current study was located in an anterior region of the hippocampus. Such anterior hippocampal activity has been observed during successful encoding (Chua et al., 2007) and retrieval (Giovanello et al, 2004; 2008) of associations, as well as when participants imagine future events by recombining elements of past experiences (Addis and Schacter, 2008). Behaviorally, older adults show deficits in memory specificity when retrieving past events and imagining future events (Addis, Wong, and Schacter, 2008). Taken together, these findings suggest a relationship between anterior hippocampal activity and the operation of adaptive, constructive mnemonic processes (Schacter and Addis, 2009).
In contrast to the young adult findings, older adults demonstrated greater activity than young adults during accurate retrieval in right inferior and middle prefrontal cortex. Such a shift from hippocampal- to frontal-based processing in healthy older adults has been documented previously during episodic encoding (e.g., Gutchess, Welsh, Hedden, Bangert, Minear, Liu, and Park, 2005) and veridical episodic retrieval (e.g., Grady, McIntosh, and Craik, 2005). In these studies, significant correlations were obtained between decreased hippocampal activity and increased prefrontal activity during accurate performance. These results have been interpreted to reflect compensatory processes whereby prefrontal regions are recruited to counteract neurocognitive decline in the context of deficient hippocampal system function.
Indeed, several laboratories have reported that recruitment of prefrontal regions is associated with successful memory in older adults (e.g., Cabeza, Anderson, Locantore, and McIntosh 2002; Grady, McIntosh, and Craik, 2005; Morcom, Good, Frackowiak, and Rugg, 2003; Rosen et al., 2002), leading to the suggestion that age-related increased recruitment of unique brain regions may reflect a compensatory process. Of note, the increased prefrontal activity (in the context of decreased hippocampal activity) reported by Grady, McIntosh, and Craik (2005) occurred under conditions in which performance was not matched between young and older adults. In the current study, however, we utilized a matching procedure to equate performance between the young and older groups, yet observed increased activity in right inferior and middle prefrontal cortex in an analysis contrasting hits and errors. This finding suggests that group differences in behavioral performance are not a pre-requisite for observing age-related recruitment of prefrontal regions during accurate retrieval.
Finally, two regions were commonly associated with young and old during the retrieval of accurate memories: right precuneus and right superior temporal gyrus. Activity in both right precuneus and right superior temporal gyrus has been observed in previous studies of veridical retrieval (Cabeza, Mangels, Nyberg, Habib, Houle, McIntosh, and Tulving, 1997; Fletcher, Shallice, Frith, Frackowiak, and Dolan, 1996). In the current study, engagement of right superior temporal gyrus, an important region for the processing of speech, may reflect participants’ covert generation of the sounds of studied stimuli, while activity in right precuneus may have arisen if participants were mentally picturing the studied word form. In both cases, such strategies may help to guide veridical retrieval of studied items.
Age-related neural differences during false retrieval
Behaviorally, both young and older adults showed the identical pattern of “old” responses to items (intact > conjunction lure > feature lure> new). This pattern of performance has been observed previously (Jones and Jacoby, 2005; Rubin, Van Patten, Glisky, and Newberg, 1999) and likely reflects participants’ sensitivity to the familiarity of studied stimulus elements. That is, participants are more likely to respond “old” at recognition to conjunction lures which consist of two studied components, than to feature lures which consistent of one studied component, than to novel items which consist of no studied components.
In the current study, behavioral performance was equated between young and older adults by testing older adults immediately following encoding and testing young adults following a two-hour delay. Such a matching procedure may have qualitatively changed the memory process for young adults, requiring them to rely somewhat more on familiarity than they otherwise would have done (i.e., had they been tested immediately). It would be of interest for future investigations to examine this issue.
Though there have been extensive discussions about the role of the parahippocampal gyrus in episodic retrieval (see Diana, Yonelinas, and Ranganath, 2007 for recent review), our results are consistent with evidence suggesting that parahippocampal activity can be associated with processing episodic familiarity signals (Daselaar, Fleck, and Cabeza, 2006), and that reliance on familiarity signals within the medial temporal lobe may be increased with age (Daselaar, Fleck, Dobbins et al., 2006). Daselaar, Fleck, and Cabeza, 2006 (2006) reported a triple dissociation in the medial temporal lobes in which the anterior hippocampal region and the rhinal cortex exhibited novelty related activity, the posterior half of the hippocampus demonstrated recollection-related activity, and the parahippocampal cortex showed familiarity-related activity. More specifically, activity in posterior parahippocampal gryus exhibited a continuous increase in activity with increasing levels of stimulus oldness. The finding that posterior parahippocampal activity is linked to stimulus familiarity is consistent with prior fMRI studies demonstrating parahippocampal activity during item-based perceptual retrieval processes (Cabeza et al., 2001; Goh et al., 2004). In their discussion, Daselaar and colleagues (2006) noted that the posterior parahippocampal gyrus receives direct input from several unimodal and polymodal cortical regions (visual, parietal, and temporal cortices) and provides approximately one-third of the sensory input into the hippocampus (Suzuki and Amaral, 1994). This fact, coupled with the idea that familiarity depends more heavily on perceptual processing than on recollection (Yonelinas, 2002), suggests that increases in posterior PHG with oldness may reflect an increased reliance on perceptual fluency during familiarity-based recognition.
In the current study, we observed increased activity in parahippocampal gyrus in older adults for distorted retrieval than accurate retrieval. Specifically, the comparison of feature lures greater than hits, as well as the conjunction analysis (feature and conjunction lures greater than hits) revealed activity in right parahippocampal gyrus. The engagement of parahippocampal gyrus suggests that older adults may have over relied on the perceptual familiarity of stimulus elements, which led to incorrect endorsement of conjunction and features lures. Such findings indicate that older adults may retrieve information that is less relational (or more familiarity-based) than the information young adults retrieve. Nonetheless, older adults can achieve the same level of performance as young adults through successful monitoring of retrieved information via recruitment of prefrontal regions.
It should be noted that even young adults made about as many false alarms as did older adults, we did not find any neural activity for young adults in the false greater than true memory comparisons at our specified significance threshold. At lower thresholds, however, several regions emerged, including bilateral insula and inferior prefrontal cortex, as well as medial prefrontal cortex. Future research will be required to determine whether these findings are meaningful or simply the result of chance. Perhaps stronger effects are observed in older adults because there was a dominant processes driving their performance (i.e., reliance on parahippocampal familiarity signals), whereas false memories in younger adults might reflect the influence of a number of processes, including familiarity, mis-recollection, and monitoring failures.
The current study focused on one form of memory distortion (i.e., conjunction errors). However, memory distortions can take several forms. Individuals can incorrectly endorse an entirely novel stimulus, or a stimulus composed of previously presented components as “old” (i.e., memory conjunction error); incorrectly accept a thematically-related, yet non-studied stimulus as “old” (i.e., gist-based false recognition; Koutstaal & Schacter, 1997); or indicate that an imagined event was perceived or performed (i.e., reality monitoring error; Johnson and Raye, 1981).
As described in the Introduction, two recent neuroimaging studies have examined age-related brain changes during true and false memory formation (Dennis, Kim, and Cabeza, 2007) and retrieval (Dennis, Kim, and Cabeza, 2008) using a modified version of the Deese-Roediger-McDermott (DRM) paradigm (i.e., gist-based false recognition). Both neuroimaging studies reported age-related reductions in the medial temporal lobe (hippocampus) for processing of true memories. This finding is consistent with the age-related differences in hippocampal activity observed in the current study. The prior reports, however, documented age-related increases in left middle temporal gyrus for processing of false memories. This activity was interpreted as an increased reliance on semantic gist by older adults. In the current study, we observed age-related increases in right parahippocampal gyrus during false retrieval, possibly reflecting an over reliance on stimulus familiarity by older adults. Taken together, these results suggest that the nature of the memory distortion (i.e., false recognition, memory conjunction error, gist-based false recognition, or reality monitoring error), as well as the paradigm used to elicit the memory errors, may determine the locus of age-related differences in neural activity during false memories. Future studies aimed at directly comparing neural activity elicited for different forms of memory distortions will serve to further clarify the nature of age-related increases in memory errors.
Conclusion
We used the memory conjunction error paradigm to examine age-related neural differences during retrieval of true and false memories. Young adults compared to older adults recruited the hippocampus during true memory retrieval, consistent with a role for this structure in the retrieval of recollected or associative information. Older adults compared to young adults showed increased recruitment of prefrontal regions during veridical retrieval. Such increased prefrontal activity in older adults has been observed previously and may reflect a compensatory mechanism. Performance in the current study was matched between young and older adults. The findings indicate that a difference in behavioral performance between age groups is not a pre-requisite for observing age-related increase in prefrontal activity during retrieval.
Although we observed no regions in which activity was greater during false than during accurate retrieval for young adults, older adults showed significantly greater activity during false than during accurate retrieval in the right parahippocampal gyrus. This result likely reflects an over reliance by older adults on the familiarity of stimulus elements and points to the importance of the nature of memory distortions as a contributor to the locus of age-related differences in neural activity during false memories. Finally, our findings suggest that older adults’ memory conjunction errors can be caused by faulty hippocampal retrieval processes.
Figure 1.
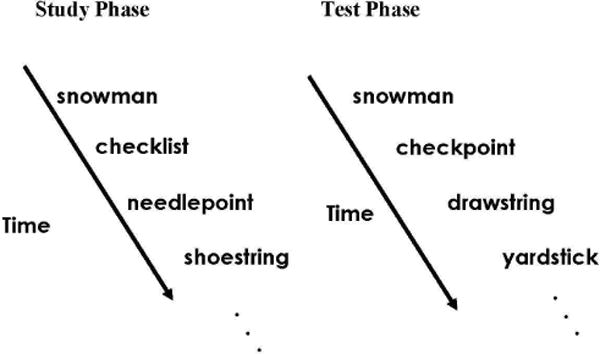
Depiction of experimental protocol (see Methods for details). At study, participants were shown compound words and instructed to judge whether the word was “pleasant” or “unpleasant”. At test, four types of compound words were presented: (i) “intact” words - direct repetitions from the study list, (ii) “conjunction lures” -recombinations of studied stimuli, (iii) “feature lures” – one component taken from a studied word and one component novel, and (iv) “new” words – completely novel stimuli. Participants were asked to decide if each compound word was “old” (i.e., appeared during the study phase) or “new” (i.e., novel). Accurate retrieval was defined as an “old” response to an “intact word” (hit), while false retrieval was defined as an “old” response to either a “conjunction lure” (conjunction false alarm) or a “feature lure” (feature false alarm).
Acknowledgments
We thank Chris Moore for assistance in identifying stimulus materials and an anonymous reviewer for helpful comments. This work was supported by grants AG023439 from the National Institute on Aging to KSG, MH070199 from the National Institute of Mental Health to EAK, and AG08441from the National Institute on Aging to DLS.
Footnotes
An additional seven young adults (M = 24.7 years, SD = 5.1; 6 female) and six older adults (M = 71.0 years, SD = 3.2; 3 female) participated in the experiment. Data from these individuals could not be analyzed because these participants did not commit the minimum number of false alarms per condition (i.e., greater than or equal to 6).
The term “lure” in these analyses is shorthand for false alarms to the particular type of lure indicated.
The conjunction analyses were conducted by thresholding each of the two individual contrasts (e.g., hits > conjunctions, hits > feature) at p<.01, resulting in a joint probability of p<.001 (using Fisher’s estimate, as is standard for this type of conjunction analysis). Because the original contrasts (e.g., hits > conjunctions) were reported at a threshold of p<.001, there can be regions (such as the MTL) which appear in the conjunction but not in the individual contrasts. This difference in the regions revealed is due to the thresholding differences; for the hits > conjunctions contrast, the MTL is not revealed at a threshold of p<.001 but is at a threshold of p<.01.
References
- 1.Addis DR, Schacter DL. Constructive episodic simulation: Temporal distance and detail of past and future events modulate hippocampal activity. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- 2.Addis DR, Wong A, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;18:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- 3.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 4.Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, Tulving E. Brain regions differentially involved in remembering what and when. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proceeding of the Academy of Science. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. NeuroImage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Chua E, Schacter DL, Rand-Giovennetti E, Sperling RA. Evidence for a specific role of the anteior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- 8.Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- 9.Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis NA, Kim H, Cabeza R. Effects of aging on true and false memory formation: An fMRI study. Neuropsychologia. 2007;45:3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Dennis NA, Kim H, Cabeza R. Age-related differences in brain activity during true and false memory retrieval. Journal of Cognitive Neuroscience. 2008;20:1390–1402. doi: 10.1162/jocn.2008.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodson CS, Koutstaal W, Schacter DL. Escape from illusion: Reducing false memories. Trends in Cognitive Science. 2001;4:391–397. doi: 10.1016/s1364-6613(00)01534-5. [DOI] [PubMed] [Google Scholar]
- 13.Dorfman J. Sublexical components in implicit memory for novel words. Journal of Experimental Psychology. 1994;20:1108–1125. doi: 10.1037//0278-7393.20.5.1108. [DOI] [PubMed] [Google Scholar]
- 14.Eldridge LL, Knowlton BJ, Furmanksi CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 15.Fisher RA. Statistical methods for research workers. London: Oliver and Boyd; 1950. [Google Scholar]
- 16.Fletcher PC, Shallice T, Frith CD, Frackowiak RSJ, Dolan RJ. Brain activity during memory retrieval: The influence of imagery and semantic cuing. Brain. 1996;119:1587–1596. doi: 10.1093/brain/119.5.1587. [DOI] [PubMed] [Google Scholar]
- 17.Goh JO, Siong SC, Park D, Gutchess A, Hebrank A, Chee MW. Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation. Journal of Neuroscience. 2004;24:10223–10228. doi: 10.1523/JNEUROSCI.3373-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovanello KS, Schnyer D, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- 19.Giovanello KS, Schnyer D, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2008 doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grady C, McIntosh AR, Craik FIM. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Gutchess A, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased-medial temporal lobe activity. Journal of Cognitive Neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- 22.Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proceedings of the National Academy of Science USA. 1999;11:5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–72. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacoby LL, Rhodes MG. False remembering in the aged. Current Directions in Psychological Science. 2006;15:49–53. [Google Scholar]
- 25.Jha AP, Kroll NEA, Baynes K, Gazzaniga MS. Memory encoding following complete callosotomy. Journal of Cognitive Neuroscience. 1997;9:143–159. doi: 10.1162/jocn.1997.9.1.143. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MK, Raye CL. Reality monitoring. Psychological Review. 1981;88:67–85. [Google Scholar]
- 27.Jones TC, Jacoby LL. Conjunction errors in recognition memory: Modality free-errors for older but not for you adults. Acta Psychologica. 2005;120:55–73. doi: 10.1016/j.actpsy.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Jones TC, Jacoby LL, Gellis LA. Cross-modal feature and conjunction errors in recognition memory. Journal of Memory and Language. 2001;44:131–152. [Google Scholar]
- 29.Koutstaal W, Schacter DL. Memory Distortion and aging. In: Naveh Benjamin M, Moscovitch M, Roediger HL, editors. Perspectives on human memory and cognitive aging: Essays in honour of Fergus Craik. New York, NY: Psychology Press; 2001. [Google Scholar]
- 30.Kroll NEA, Knight RT, Metcalfe L, Wolf ES, Tulving E. Cohesion failure as a source of memory illusions. Journal of Memory and Language. 1996;35:176–196. [Google Scholar]
- 31.Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: A survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- 32.Morcom AM, Good CD, Good RS, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- 33.Old SR, Naveh-Benjamin M. Differential age effects on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23:104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- 34.Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. Hippocampal contribution to novel use of relational information in declarative memory. Hippocampus. 2002;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- 35.Reinitz MT, Lammers WJ, Cochran BP. Memory-conjunction errors: Miscombinations of stored stimulus features can produce illusions of memory. Memory and Cognition. 1992;20:1–11. doi: 10.3758/bf03208247. [DOI] [PubMed] [Google Scholar]
- 36.Reinitz MT, Verfaellie M, Milberg WP. Memory conjunction errors in normal and amnesic subjects. Journal of Memory and Language. 1996;35:286–289. [Google Scholar]
- 37.Roediger HL, McDermott KB. Creating false memories: remembering words not present in lists. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:803–814. [Google Scholar]
- 38.Roediger HL, McDermott KB. Tricks of memory. Current Directions in Psychological Science. 2000;9:123–127. [Google Scholar]
- 39.Rosen AC, Prull MW, O’Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- 40.Rubin R, Van Patten C, Glisky EL, Newberg WM. Memory conjunction errors in young and older adults: Event-related potential and neuropsychological data. Cognitive Neuropsychology. 1999;16:459–488. [Google Scholar]
- 41.Schacter DL. The seven sins of memory: how the mind forgets and remembers. New York: Houghton Mifflin Company; 2001. [Google Scholar]
- 42.Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical Transactions of the Royal Society (B) 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert M. Conscious recollection and the human hippocampal formation: Evidence from positron omission tomography. Proceedings of the National Academy of Science. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 46.Underwood BG. False recognition produced by implicit verbal responses. Journal of Experimental Psychology. 1965;70:122–129. doi: 10.1037/h0022014. [DOI] [PubMed] [Google Scholar]
- 47.Underwood BJ, Kapelak SM, Malmi RA. Discrete verbal units in recognition memory. Journal of Experimental Psychology: Human Learning and Memory. 1976;2:293–300. [Google Scholar]
- 48.Underwood BJ, Zimmerman J. The syllable as a source of error in multisyllable word recognition. Journal of Verbal Learning and Verbal Behavior. 1973;12:338–344. [Google Scholar]
- 49.Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]


