Abstract
Large doses of bone morphogenetic protein 2 (BMP2) are used clinically to induce bone formation in challenging bone defects. However, complications after treatment include swelling, ectopic bone formation, and adjacent bone resorption. While BMP2 can be effective, it is important to characterize the mechanism of the deleterious effects to optimize its use. The aim of this study was to determine the effect of BMP2 on apoptosis in osteoblast lineage cells and to determine the role of the BMP inhibitor Noggin in this process. Human mesenchymal stem cells (MSCs), immature osteoblast-like MG63 cells, and mature normal human osteoblasts (NHOst) were treated with BMP2. A model system of increased endogenous BMP signaling was created by silencing Noggin (shNOG-MG63). Finally, the BMP pathway regulating apoptosis in NHOst was examined using BMP signaling inhibitors (5Z-7-oxozeaenol, dorsomorphin, H-8). Apoptosis was characterized by caspase-3, BAX/BCL2, p53, and DNA fragmentation. BMP2 induced apoptosis in a cell-type dependent manner. While the effect was minor in MSCs, MG63 cells had modest increases and NHOst cells had robust increases apoptosis after BMP2 treatment. Apoptosis was significantly higher in shNOG-MG63 than MG63 cells. 5Z-7-oxozeaenol and dorsomorphin eliminated the BMP2-induced increase in DNA fragmentation in NHOst, suggesting roles for TAB/TAK1 and Smad signaling. These results indicate that the apoptotic effect of BMP2 is dependent on cell maturation state, inducing apoptosis in committed osteoblasts through Smad and TAB/TAK1 signaling, and is regulated by Noggin. Dose and delivery must be optimized in therapeutic applications of BMP2 to minimize complications.
Keywords: Human osteoblasts, BMP (bone morphogenetic protein), Apoptosis, Noggin silencing, Human mesenchymal stem cells
INTRODUCTION
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGF-β) super family [Miyazono et al., 2005], and a subset of BMPs possess the ability to induce bone and cartilage formation and enhance osteogenesis [Reddi and Huggins, 1972; Urist, 1965]. BMP2 is used clinically to induce bone formation in challenging done defects in orthopaedics and dentistry [Vukicevic and Sampath, 2004]. However, incidences of complications have been reported in orthopaedic applications including soft tissue swelling, ectopic bone formation, and even resorption of adjacent vertebral bodies [Lewandrowski et al., 2007; Smoljanovic et al., 2009].
BMP2 must form homodimers or heterodimers to activate the BMP signaling pathway [Guo and Wu, 2012]. The dimer then binds to a complex of four transmembrane receptors, consisting of two BMP type I receptors and two BMP type II receptors [Miyazono et al., 2005], and one of three signaling pathways is initiated depending on the conformation of receptors upon ligand binding [Nohe et al., 2002]. In the first, BMP type I receptors phosphorylate Smad1, Smad5, or Smad8. This Smad then associates with Smad4 and the complex translocates to the nucleus and activates transcription of downstream genes [Miyazono et al., 2010]. Alternatively, p38 or JNK mitogen-activated protein kinase (MAPK) signaling can be initiated by TGF-β1 activated kinase-1 (TAK-1) and TAK1 binding protein (TAB) [Shibuya et al., 1996]. Finally, protein kinase A (PKA) can be activated [Lee and Chuong, 1997; Zhao et al., 2006], potentially leading to ERK1/2 MAPK activation. BMP signaling is regulated extracellularly by soluble inhibitors such as Noggin, Gremlin, Cerberus, and Chordin, which physically bind to BMPs, preventing their dimerization or receptor binding, inhibiting transduction of downstream signaling [Walsh et al., 2010].
BMPs induce cartilage and bone formation both during embryonic development and post-natally [Cao and Chen, 2005; Nie et al., 2006] and they regulate other developmental and adult processes including cell growth and differentiation [Hogan, 1996; Reddi, 2005], as well as apoptosis [Hay et al., 2001; Kawamura et al., 2002; Marazzi et al., 1997; Zhang et al., 2012]. Control of apoptosis during development ensures proper tissue morphogenesis. For example, BMP2 regulates fibroblast growth factor signaling to induce apoptosis in the apical ectodermal ridge during limb formation [Pajni-Underwood et al., 2007]. In the chick limb bud, both BMP2 and BMP4 are expressed in the anterior, posterior, and interdigital necrotic zones prior to apoptosis [Yokouchi et al., 1996]. However, there is limited evidence about the effects of BMP2 on apoptosis in adult osteoprogenitor cells or in committed osteoblasts.
Given the effects seen following the clinical use, the aim of this study was to characterize the effect of BMP2 on apoptosis of osteoprogenitor cells and osteoblasts, and the role of soluble BMP antagonist Noggin on this process, and lastly the BMP pathway used to induce apoptosis.
MATERIALS AND METHODS
Cell Culture
The effect of BMP2 on apoptosis was examined in osteoprogenitor cells (MSCs), immature osteoblasts (MG63 cells), and mature osteoblasts (NHOst cells). Human bone marrow-derived mesenchymal stem cells (MSCs) were purchased from Lonza Biosciences (Walkersville, MD) and cultured in Mesenchymal Stem Cell Growth Media (MGSCGM, Lonza Biosciences). Human MG63 cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modification of Eagle’s medium (cellgro®, MediaTech, Manassas, VA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA) and 1% penicillin-streptomycin (Gibco). Normal human osteoblasts (NHOst cells, Lonza) were cultured in Osteoblast Growth Media (Lonza). For all experiments, cells were plated at a density of 10,000 cells/cm2 and cultured at 37°C, 5% CO2, and 100% humidity. Media were changed 24 hours after plating and every 48 hours thereafter until cells reached confluence.
Recombinant human BMP2 (R&D Systems, Minneapolis, MN) was reconstituted in sterile 4 mM HCl containing 0.1% bovine serum albumin to a stock concentration of 100 μg/ml. The stock was diluted to final concentrations in culture medium. At confluence, cells were treated with 50, 100, or 200 ng/ml BMP2 unless noted otherwise.
Proteome Profiler Array
The effect of BMP2 on osteoblast apoptosis was investigated using a Human Apoptosis Proteome Profiler Array (R&D Systems), which examined changes in 35 apoptosis-related proteins. Confluent cultures of NHOst cells were treated with 200 ng/ml BMP2 for 6 hours. After incubation, cells were lysed and analyzed by modified Western blot. Membranes were imaged by chemiluminescence (VersaDoc, Bio-Rad, Hercules, CA) and changes in proteins determined by densitometry (QuantyOne, Bio-Rad).
Caspase-3
Cells were treated with BMP2 as described for 6 hours and assayed for caspase-3 activity using a commercial kit (R&D Systems) following manufacturer’s instructions. Briefly, cell monolayers were lysed in cold lysis buffer for 10 minutes at 4°C, and the cell lysates centrifuged at 10,000g for 1 minute. The resulting supernatant was combined with 2x reaction buffer and DEVD-pNA substrate and incubated at 37°C for 2 hours. Absorbance at 405 nm was determined using a microplate reader (VersaMax, Molecular Devices, Sunnyvale, CA).
BAX/BCL2 mRNA Levels
Cells were treated with BMP2 for 12 hours. RNA was isolated using TriZOL® (Invitrogen, Carlsbad, CA) and was quantified (Nanodrop Spectrophotometer, Thermo Scientific, Waltham, MA). 250 ng of RNA was used to synthesize cDNA libraries (High Capacity cDNA Reverse Transcription kit, Applied Biosystems, Carlsbad, CA). Levels of mRNA were quantified with real-time quantitative PCR (StepOnePlus, Applied Biosystems) using gene-specific primers (Table 1) and Power SybrGreen® Master Mix (Applied Biosystems). Starting mRNA quantities were determined by standard curve method. Levels of mRNA are presented as a ratio of BAX to BCL2.
Table 1.
Human primers used in Real-time PCR analysis
| Gene | Primer Sequence | |
|---|---|---|
| BAX | F | GACGAACTGGACAGTAACATGG |
| R | AAAGTAGAAAAGGGCGACAACC | |
| BCL-2 | F | CTGTTTGATTTCTCCTGGCTGTCTC |
| R | TCTACTGCTTTAGTGAACCTTTTGC | |
| NOG | QuantiTect Primer Assay, QT00210833 | |
| GAPDH | F | GCTCTCCAGAACATCATCC |
| R | TGCTTCACCACCTTCTTG | |
Total p53 Levels
Total p53 levels were determined in cells 24 hours after treatment using a sandwich ELISA (Human Total p53 DuoSet® IC, R&D Systems). After treatment, monolayers were rinsed with PBS and lysed [PBS containing 1 mM EDTA, 0.5% Triton™ X-100, 10 mM NaF, 150 mM NaCl, 20 mM β-glycerophosphate, 1 mM dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 3 μg/ml aprotinin]. Lysates were centrifuged at 2000g for 5 minutes and the supernatant assayed for total p53 following manufacturer’s instructions. Results were normalized to total protein content (Pierce 600nm Protein Assay, Thermo Scientific).
DNA Fragmentation
TUNEL Assay
DNA fragmentation was assessed by colorimetric terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL, TiterTACS™ in situ Microplate TUNEL Assay, R&D Systems). Confluent cultures were treated for 24 hours. Media were removed, and cells were fixed with 3.7% sucrose-buffered formaldehyde and processed following manufacturer’s instructions. Absorbance at 450 nm was determined using a microplate reader.
Radiometric Assay
At 60% confluence, cells were incubated with 1 μCi/ml 3H-thymidine (Perkin Elmer, Waltham, MA). At confluence, cultures were treated for 24 hours with BMP2. Cells were lysed [10mM Tris-HCl, 1mM EDTA, 0.2% Triton X-100] and subjected to three freeze-thaw cycles. Intact DNA was separated from fragmented DNA by ultracentrifugation at 13,000g for 15 minutes. Intact DNA (pellet) and fragmented DNA (supernatant) were counted by liquid scintillation counting (Beckman Coulter). Results are presented as percent fragmented DNA/total DNA.
Noggin Expression
Since soluble inhibitors regulate the actions of BMPs, we wanted to determine the effect of BMP2 treatment on mRNA levels of NOG, one of the most powerful inhibitors of BMPs. Cells were treated with BMP2 as described. Levels of mRNA for NOG were determined after 12 hours as described above and are presented as normalized to mRNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Noggin Silencing
shNOG-MG63 cells were examined to determine the role of Noggin in osteoblast apoptosis. MG63 cells silenced for Noggin (shNOG-MG63) were generated using lentiviral shRNA transduction particles (NM_005450, TRCN0000058563, Mission®, Sigma Aldrich, St. Louis, MO). MG63 cells were plated at 20,000 cells/cm2 and cultured overnight in full medium. Particles were added to the cells at a multiplicity of infection of 7.5 in full medium supplemented with 8 μg/ml hexadimethrine bromide (Sigma Aldrich) and incubated for 18 hours. After incubation, transduced cells were selected with full medium containing 0.25 μg/ml puromycin. The resulting cell line had a 70% reduction in NOG mRNA levels as determined by real-time qPCR and 72% reduction in secreted Noggin as determined by ELISA (data not shown).
BMP2 Signaling Pathway
Confluent cultures of NHOst cells were pre-treated for one hour with inhibitors of BMP2-dependent signaling pathways. 5Z-7-oxozeaenol (100 nM) was used to inhibit TAB/TAK1 signaling [Choo et al., 2006]; dorsomorphin (10 nM) was used to inhibit Smad signaling [Hao et al., 2008; Yu et al., 2008]; H-8 (1 μM) was used to inhibit PKA signaling [Rosado et al., 2002]. All inhibitors were purchased from EMD Chemicals (San Diego, CA). BMP2 was added to the media containing the inhibitor to a final concentration of 200 ng/ml. Cells were incubated and assayed as described above.
Statistical Analysis
Data are presented as mean ± SEM of six independent cultures per variable. Data were first examined by analysis of variance (ANOVA) and significant differences between groups determined using Bonferroni’s modification of Student’s t-test. P<0.05 was considered to be significant.
RESULTS
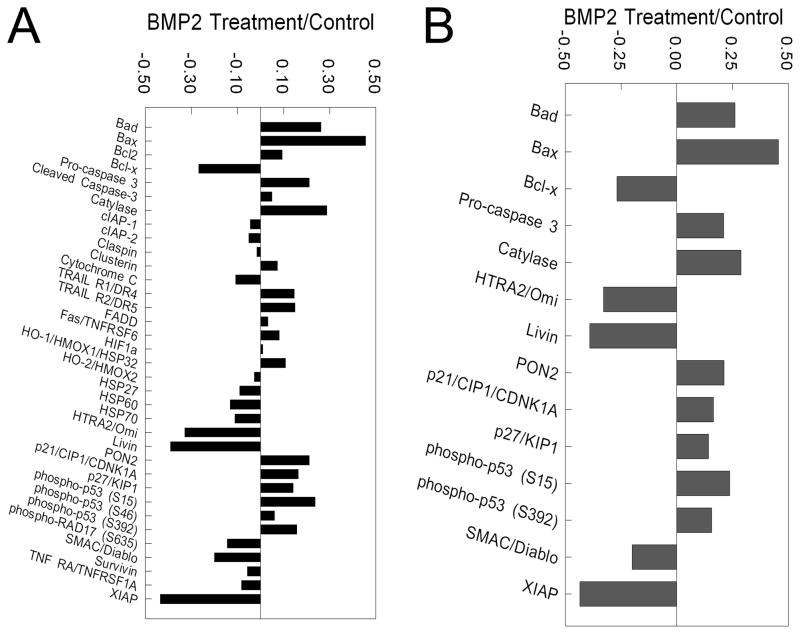
The results of the proteome profiler array showed that of the 35 apoptosis-related proteins assessed (Fig. 1A), 9 proteins were significantly higher and 5 proteins were lower in BMP2-treated NHOst cells than in untreated cells (Fig. 1B). Levels of pro-apoptotic proteins such as Bad, Bax, pro-caspase-3, and phosphorylated p53 (Ser 15) were increased more than 20% in NHOst cells treated with BMP2 than in control cells (Fig. 1B). Anti-apoptotic markers Bcl-x, Omi, Livin, and XIAP were 25% lower in BMP2-treated NHOst cells than in untreated cultures (Fig. 1B).
Figure 1. Effect of BMP2 treatment on apoptotic proteins in NHOst cells.
Confluent cultures of NHOst cells were treated for 6 hours with BMP2. Cell lysates were analyzed by modified Western blot for levels of 32 proteins known to be involved in apoptosis and densitometry analysis performed (A), and proteins with more than 15% changes after BMP2 treatment identified (B).
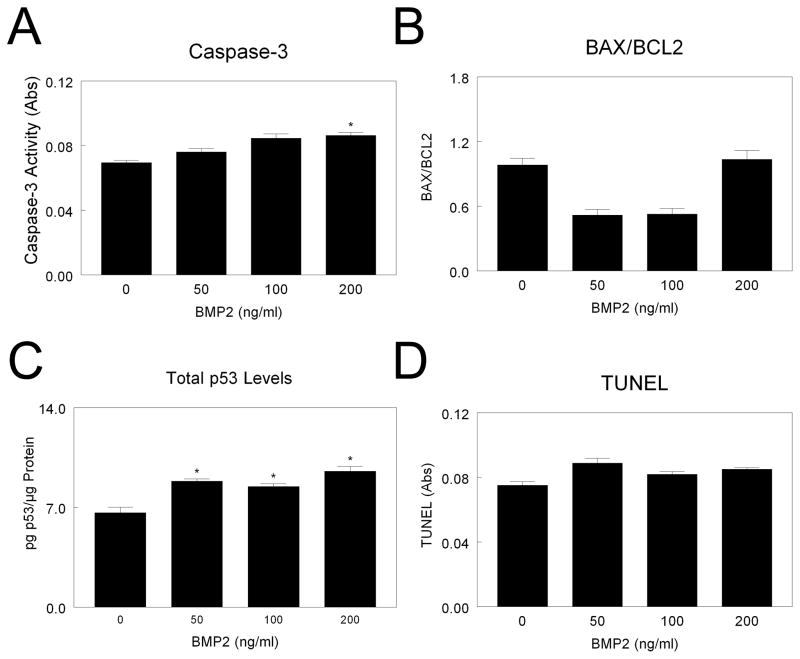
The effect of BMP2 on apoptosis varied with cell type. MSCs treated with 200 ng/ml BMP2 exhibited a small increase in caspase-3, with no effect at lower doses (Fig. 2A). Addition of BMP2 increased total p53 at all concentrations (Fig. 2C), but had no effect on BAX/BCL2 (Fig. 2B) or TUNEL (Fig. 2D). In MG63 cells, addition of 100 or 200 ng/ml BMP2 increased caspase-3 (Fig. 2A). However, BAX/BCL2 (Fig. 2B) and p53 (Fig. 2C) were only increased by 200 ng/ml BMP2. BMP2 had no effect on TUNEL in MG63 cells at the doses examined (Fig. 2D).
Figure 2. Effect of BMP2 on mesenchymal stem cell apoptosis.
MSCs were cultured until they reached confluence. Cells were treated with 50, 100, or 200 ng/ml BMP2 and caspase-3 (A), BAX/BCL2 (B), total p53 (C), and TUNEL (D) measured. *p< 0.05, versus control; #p< 0.05, versus 50 ng/ml BMP2; $p< 0.05, versus 100 ng/ml BMP2.
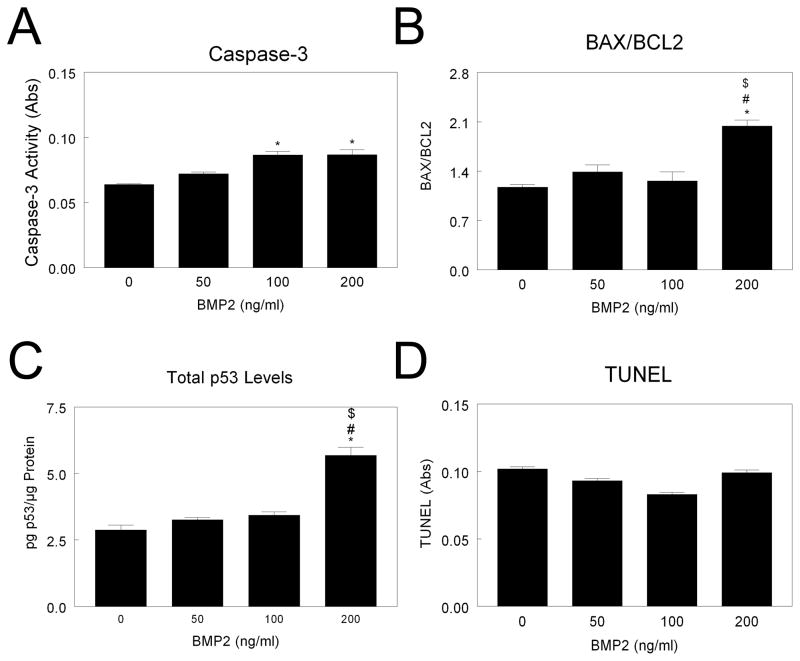
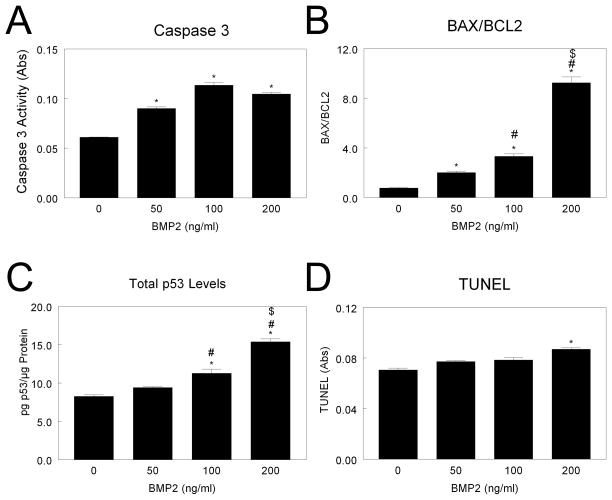
In contrast, BMP2 significantly increased caspase-3 in NHOst cells at all doses examined (Fig. 3A). BAX/BCL2 was increased after BMP2 treatment in a dose-dependent manner, with a 7-fold increase after 200 ng/ml BMP treatment (Fig. 3B). Levels of p53 were higher in 100 ng/ml and 200 ng/ml treated cultures than control cultures (Fig. 3C). TUNEL was increased in cultures treated with 200 ng/ml BMP2 (Fig. 3D), and this was confirmed by a 50% increase in percent fragmented DNA (data not shown).
Figure 3. Effect of BMP2 on MG63 cell apoptosis.
MG63 cells were cultured until they reached confluence. Cells were treated with 50, 100, or 200 ng/ml BMP2 and caspase-3 (A), BAX/BCL2 (B), total p53 (C), and TUNEL (D) measured. *p< 0.05, versus control; #p< 0.05, versus 50 ng/ml BMP2; $p< 0.05, versus 100 ng/ml BMP2.
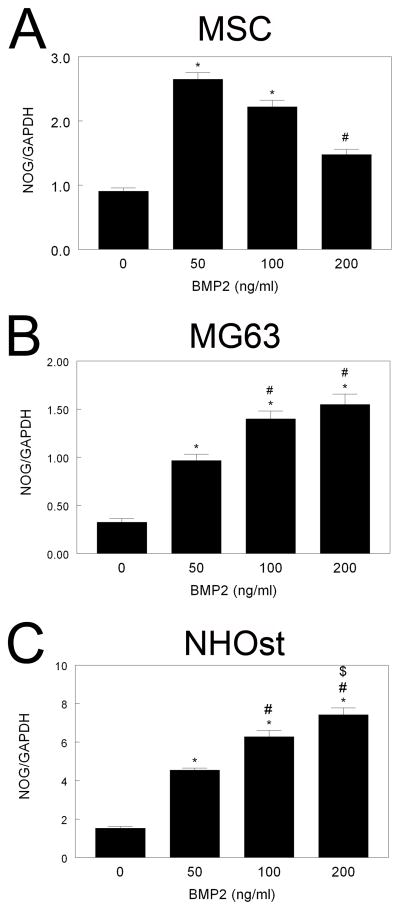
Noggin expression was regulated by BMP2 in all cell types examined. In MSCs, NOG was 1.5-fold higher than control after BMP2 treatment, but decreased with increasing BMP2 dose (Fig. 5A). In MG63 cells, treatment with BMP2 induced a greater than 2-fold increase in NOG mRNA levels, an effect that increased with increased BMP2 dose (Fig. 5B). A similar effect was seen in NHOst, with a 3-fold increase in NOG mRNA levels after 200 ng/ml BMP2 (Fig. 5C).
Figure 5. Effect of BMP2 treatment on NOG mRNA levels.
Confluent cultures of MSCs (A), MG63 cells (B), and NHOst cells (C) were treated for 12 hours with 50, 100, or 200 ng/ml BMP2 and levels of mRNA for NOG measured by real-time qPCR. *p< 0.05, versus control; #p< 0.05, versus 50 ng/ml BMP2; $p< 0.05, versus 100 ng/ml BMP2.
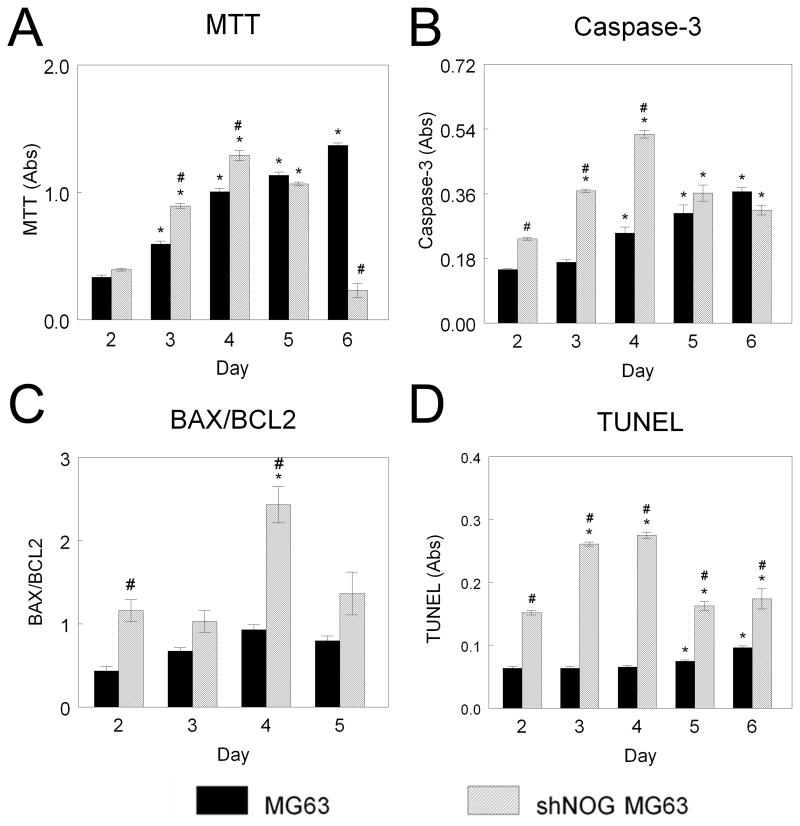
Reduced Noggin expression resulted in increased expression of apoptotic markers and increased apoptosis. shNOG-MG63 cells had higher levels of apoptotic markers than wild type MG63 cells throughout the culture period. Wild type MG63 cells exhibited time-dependent increases in MTT activity (Fig. 6A). In contrast, shNOG-MG63 cells had increased MTT levels until day 5, when cells underwent apoptosis and MTT was reduced to 20% of levels seen in control (Fig. 6A). Caspase-3 was higher in shNOG-MG63 cells at days 2, 3, and 4 than in wild type MG63 cells (Fig. 6B). BAX/BCL2 was also 100% higher in shNOG-MG63 cells at day 2 and 4 than in MG63 cells (Fig. 6C). shNOG-MG63 cells had higher TUNEL than MG63 cells at all times examined (Fig. 6D). TUNEL was 100% higher in shNOG-MG63 cells at day 2 and 200% higher at day 3 and 4 in comparison to MG63 cells (Fig. 6D).
Figure 6. Effect of NOG silencing on MG63 cell apoptosis.
MG63 and shNOG-MG63 cells were harvested at various time points after plating. MTT (A), caspase-3 (B), BAX/BCL2 (C), and TUNEL (D) were assessed. *p< 0.05, versus day 2; #p< 0.05, versus MG63 cells.
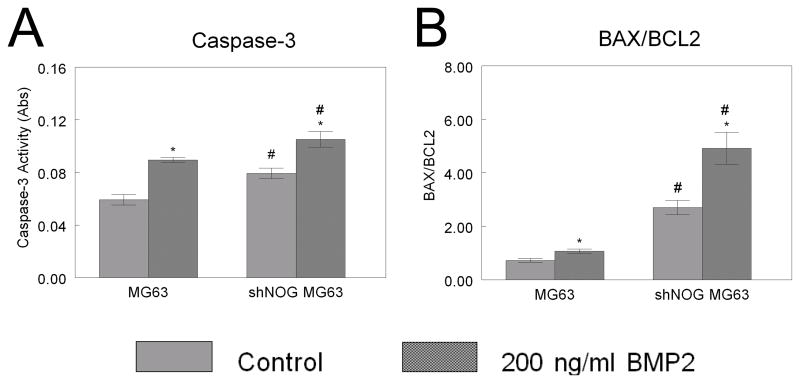
The increased apoptosis in shNOG-MG63 cells was enhanced by BMP2. MG63 cells treated with 200 ng/ml BMP2 had a 25% increase in caspase-3; shNOG-MG63 cells had a higher baseline level of caspase-3, which was increased 25% by addition of BMP2 (Fig. 7A). Likewise, BAX/BCL2 increased in MG63 cells treated with BMP2. Baseline BAX/BCL was higher in shNOG-MG63 cells and was synergistically increased by exogenous BMP2 (Fig. 7B).
Figure 7. Effect of NOG silencing on BMP2-induced MG63 cell apoptosis.
MG63 and shNOG-MG63 cells were treated with 200 ng/ml BMP2. Caspase-3 (A) and BAX/BCL2 (B) were assessed. *p< 0.05, versus control; #p< 0.05, versus MG63 cells.
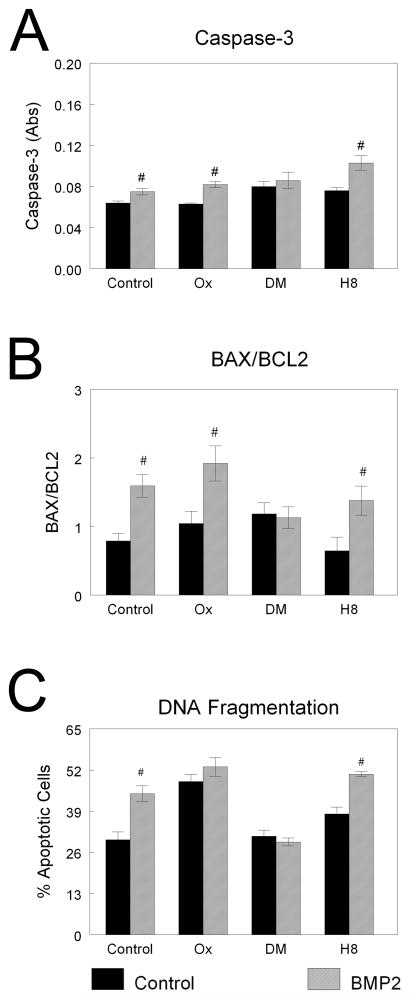
The effect of BMP2 on apoptosis was via a Smad-dependent signaling pathway. In NHOst cells, the stimulatory effect of 200 ng/ml BMP2 on caspase-3 activity was blocked by dorsomorphin, but not by 5Z-7-oxozeaenol or H-8 (Fig. 8A). Likewise, only dorsomorphin blocked the BMP2-induced increase in BAX/BCL2 (Fig. 8B). However, increased DNA fragmentation after BMP2 treatment was inhibited by both dorsomorphin and 5Z-7-oxozeaenol (Fig. 8C), indicating that TAB/TAK1 signaling also played a role.
Figure 8. Effect of BMP pathway inhibition on BMP2-induced NHOst cell apoptosis.
Confluent cultures of NHOst cells were pre-treated with inhibitors to TAB1/TAK (5Z-7-oxozeaenol, Ox), Smad (dorsomorphin, DM), or PKA (H8) before treatment with 200 ng/ml BMP2. Caspase-3 (A), BAX/BCL2 (B), and DNA fragmentation (C) were measured. #p< 0.05, versus untreated cells.
DISCUSSION
This study demonstrates that BMP2 modulates apoptosis in a cell-dependent manner. In MSCs, treatment with this morphogen had only small effects on apoptotic signaling. The immature osteoblast MG63 cells were sensitive to treatment with BMP2, but only at the highest dose used in our study. However, in NHOst cells, BMP2 induced apoptosis in a dose-dependent manner, an important fact given the exceedingly high doses used clinically.
BMP2 had little effect on apoptosis in MSCs in our study. BMPs are known to induce stem cell differentiation into chondrocytes, osteoblasts, and adipocytes in a dose-dependent manner [Ryoo et al., 2006]. Moreover, several studies have indicated that BMP signaling is also required for maintenance of the stem cell niche and MSC survival [Edgar et al., 2007; Solmesky et al., 2009]. This evidence suggests that BMPs affect maintenance and differentiation, but not apoptosis, in MSCs, in agreement with the results of our study.
Differential regulation of BMP2 effects were seen in cells committed to the osteoblast lineage. BMP2 increased BAX/BCL2, caspase-3, and TUNEL in immature osteoblast-like MG63 cells, confirming results using other immature osteoblast cell lines [Hay et al., 2004; Hay et al., 2001]. However, this effect was only at the highest dose tested. In committed osteoblasts, the effect was more robust, and occurred at lower doses. These observations suggest that the effect of BMP2 on osteoblast apoptosis is dependent on maturation state. In addition to up-regulating of apoptotic markers, BMP2 down-regulated anti-apoptotic markers. In NHOst, anti-apoptotic Bcl-x was lower in cells treated with BMP2 than in untreated cells, confirming results seen in myeloma cells [Kawamura et al., 2002] and gastric cancer cells [Zhang et al., 2012]. Taken together, this emphasizes that the effect of BMP2 on cell apoptosis is conserved among cells of diverse origins, particularly those that are terminally differentiated.
BMPs are tightly regulated intracellularly by Smads [Kawabata et al., 1998] and extracellularly by diverse families of soluble inhibitors, including Cerberus, Twisted gastrulation, Chordin, Crossveinless, and Noggin [Walsh et al., 2010]. The present study demonstrates that Noggin, a powerful inhibitor of BMP signaling, was regulated by treatment with BMP2, an effect dependent on cell type, and that silencing Noggin resulted in cell apoptosis. Our results are in agreement with a number of studies in developmental biology. Loss of function Noggin transgenic mice exhibit increased apoptosis during palatogenesis [He et al., 2010]. Overexpression of Noggin in transgenic mice reduces apoptosis in eyelid epithelium [Sharov et al., 2003] and during limb development [Guha et al., 2002]. Noggin has also been found to regulate apoptosis during neural crest development [Anderson et al., 2006]. Interestingly, it has also been shown that the BMP inhibitor Sclerostin can also induce apoptosis in MSCs [Sutherland et al., 2004] and that administration of Gremlin during chick limb development inhibits apoptosis [Merino et al., 1999]. In a clinical study, it was suggested that mRNA levels of BMP inhibitors during fracture healing might predict whether the facture will result in a non-union [Chang et al., 2003; Cho et al., 2002; Fajardo et al., 2009]. These results demonstrate the complex regulation of BMPs and their inhibitors are critical in determining the final signaling outcome in tissue formation, regeneration, and homeostasis.
While it is clear that BMP signaling regulates cell survival, the pathways by which this occurs are unclear. In our study, inhibitors to Smad signaling blocked BMP-induced apoptosis in NHOst cells. Smad signaling also modulated apoptosis in colon cancer cells [Beck et al., 2007], but there is conflicting evidence on the pathway involved in osteoblast apoptosis. It has been suggested that this effect occurs through PKC-dependent, Smad-independent signaling [Hay et al., 2001]; however this study used dominant negative Smad1 expression to establish this and did not examine the contributions of Smad 5 or 8. Here, we used dorsomorphin, which inhibits the ability of the BMP receptor to phosphorylate Smad1/5/8. Dorsomorphin is commonly used in the literature to inhibit canonical Smad-BMP signaling, particularly during zebrafish embryogenesis [Cannon et al., 2010; Hong and Yu, 2009] but also during mammalian processes [Rath et al., 2011; Yu et al., 2008]. Several studies have reported that dorsomorphin (and its analog LDN193189) may inhibit pathways other than BMP signaling [Cannon et al., 2010; Vogt et al., 2011]. However, the dose of dorsomorphin shown to inhibit these pathways in C2C12 cells was higher (500 nM) than in our study [Boergermann et al., 2010]. There were no off-target effects demonstrated with 5 or 50 nM dorsomorphin, suggesting that the 10 nM dose used in this study may not induce off-target effects.
The ability of BMP2 to induce bone formation makes it attractive as a regenerative strategy [Chang et al., 2003; Kraiwattanapong et al., 2005]. However, the mechanisms regulating the adverse effects often seen after BMP2 treatment must be elucidated. In cells of mesenchymal origin, BMP2 can initiate signaling that induces proliferation, differentiation, or apoptosis. BMPs can dimerize as homodimers or heterodimers to transduce downstream signals [Marazzi et al., 1997]. BMP heterodimers have a higher affinity for type II BMP receptors than homodimers; homodimeric BMP2 or BMP4 have high affinity for type I receptors [Kirsch et al., 2000; Sebald et al., 2004]. BMPR1b is a type I receptor shown to be required for the apoptotic effect of BMP2 on osteoblasts [Hay et al., 2004]. Therefore, we hypothesize that BMPs likely function in vivo mainly as heterodimers, activating specific signaling that results in bone maintenance. It is likely that delivery of exogenous BMP2 results in high levels of homodimers, which bind to BMPR1b with high affinity, initiating signaling that causes apoptosis and bone resorption. Because the adult skeleton has low levels of Noggin [Wu et al., 2003], the inhibitor is not able to regulate this signaling. By this mechanism, the body is able to prevent excessive, uncontrolled bone formation. Clinically, the large doses of BMP2 delivered may stimulate endogenous production of BMP2. This effect, coupled with low levels of Noggin in bone to control the process, may explain the adverse effects seen with BMP2 use. By modulating both BMP ligands and inhibitors, it may be possible to induce bone formation while minimizing potential complications.
Taken together, the results suggest that the effect of BMP2 treatment on osteoblast and progenitor apoptosis depends on the maturation state of the cell. While in progenitor cells BMP2 has minimal effects on cell apoptosis, in mature osteoblasts these potent factors induce apoptosis, especially at higher doses. The data suggest that in progenitor cells BMP2 acts more as a differentiation factor than an apoptogen and that in terminally differentiated cells, BMP2 induces apoptosis. It is possible that in clinical cases where complications arise with BMP2 administration, the osteogenic induction effect in MSCs is less robust than the apoptosis induced in mature osteoblasts, resulting in osteolysis.
Figure 4. Effect of BMP2 on normal human osteoblast apoptosis.
NHOst cells were cultured until they reached confluence. Cells were treated with 50, 100, or 200 ng/ml BMP2 and caspase-3 (A), BAX/BCL2 (B), total p53 (C), and TUNEL (D) measured. *p< 0.05, versus control; #p< 0.05, versus 50 ng/ml BMP2; $p< 0.05, versus 100 ng/ml BMP2.
Acknowledgments
Contract grant sponsor: NIAMS; Contract grant number: AR052102
The authors would like to thank Christian Tan, Rahul Basu, David Haithcock, and Christine Wasilewski for their technical contributions to this work. This study was supported by a grant from the Department of Defense.
References
- Anderson RM, Stottmann RW, Choi M, Klingensmith J. Endogenous bone morphogenetic protein antagonists regulate mammalian neural crest generation and survival. Dev Dyn. 2006;235:2507–20. doi: 10.1002/dvdy.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19:1465–72. doi: 10.1016/j.cellsig.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boergermann JH, Kopf J, Yu PB, Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol. 2010;42:1802–7. doi: 10.1016/j.biocel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JE, Upton PD, Smith JC, Morrell NW. Intersegmental vessel formation in zebrafish: requirement for VEGF but not BMP signalling revealed by selective and non-selective BMP antagonists. Br J Pharmacol. 2010;161:140–9. doi: 10.1111/j.1476-5381.2010.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SC, Chuang HL, Chen YR, Chen JK, Chung HY, Lu YL, Lin HY, Tai CL, Lou J. Ex vivo gene therapy in autologous bone marrow stromal stem cells for tissue-engineered maxillofacial bone regeneration. Gene Ther. 2003;10:2013–9. doi: 10.1038/sj.gt.3302106. [DOI] [PubMed] [Google Scholar]
- Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–20. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- Choo MK, Kawasaki N, Singhirunnusorn P, Koizumi K, Sato S, Akira S, Saiki I, Sakurai H. Blockade of transforming growth factor-beta-activated kinase 1 activity enhances TRAIL-induced apoptosis through activation of a caspase cascade. Mol Cancer Ther. 2006;5:2970–6. doi: 10.1158/1535-7163.MCT-06-0379. [DOI] [PubMed] [Google Scholar]
- Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40:1389–98. doi: 10.1016/j.bone.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo M, Liu CJ, Egol K. Levels of expression for BMP-7 and several BMP antagonists may play an integral role in a fracture nonunion: a pilot study. Clin Orthop Relat Res. 2009;467:3071–8. doi: 10.1007/s11999-009-0981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha U, Gomes WA, Kobayashi T, Pestell RG, Kessler JA. In vivo evidence that BMP signaling is necessary for apoptosis in the mouse limb. Dev Biol. 2002;249:108–20. doi: 10.1006/dbio.2002.0752. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012;23:61–7. doi: 10.1016/j.cytogfr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E, Lemonnier J, Fromigue O, Guenou H, Marie PJ. Bone morphogenetic protein receptor IB signaling mediates apoptosis independently of differentiation in osteoblastic cells. J Biol Chem. 2004;279:1650–8. doi: 10.1074/jbc.M300969200. [DOI] [PubMed] [Google Scholar]
- Hay E, Lemonnier J, Fromigue O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem. 2001;276:29028–36. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Matsui M, Yu X, Chai Y, Klingensmith J, Chen Y. Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev Biol. 2010;347:109–21. doi: 10.1016/j.ydbio.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–94. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409–18. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Kawamura C, Kizaki M, Ikeda Y. Bone morphogenetic protein (BMP)-2 induces apoptosis in human myeloma cells. Leuk Lymphoma. 2002;43:635–9. doi: 10.1080/10428190290012182. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Nickel J, Sebald W. BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J. 2000;19:3314–24. doi: 10.1093/emboj/19.13.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiwattanapong C, Boden SD, Louis-Ugbo J, Attallah E, Barnes B, Hutton WC. Comparison of Healos/bone marrow to INFUSE(rhBMP-2/ACS) with a collagen-ceramic sponge bulking agent as graft substitutes for lumbar spine fusion. Spine (Phila Pa 1976) 2005;30:1001–7. doi: 10.1097/01.brs.0000160997.91502.3b. discussion 1007. [DOI] [PubMed] [Google Scholar]
- Lee YS, Chuong CM. Activation of protein kinase A is a pivotal step involved in both BMP-2- and cyclic AMP-induced chondrogenesis. J Cell Physiol. 1997;170:153–65. doi: 10.1002/(SICI)1097-4652(199702)170:2<153::AID-JCP7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J. 2007;7:609–14. doi: 10.1016/j.spinee.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Marazzi G, Wang Y, Sassoon D. Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev Biol. 1997;186:127–38. doi: 10.1006/dbio.1997.8576. [DOI] [PubMed] [Google Scholar]
- Merino R, Rodriguez-Leon J, Macias D, Ganan Y, Economides AN, Hurle JM. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development. 1999;126:5515–22. doi: 10.1242/dev.126.23.5515. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & Growth Factor Reviews. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Int J Dev Biol. 2006;50:511–21. doi: 10.1387/ijdb.052101xn. [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–8. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- Pajni-Underwood S, Wilson CP, Elder C, Mishina Y, Lewandoski M. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development. 2007;134:2359–68. doi: 10.1242/dev.001677. [DOI] [PubMed] [Google Scholar]
- Rath B, Nam J, Deschner J, Schaumburger J, Tingart M, Grassel S, Grifka J, Agarwal S. Biomechanical forces exert anabolic effects on osteoblasts by activation of SMAD 1/5/8 through type 1 BMP receptor. Biorheology. 2011;48:37–48. doi: 10.3233/BIR-2011-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi AH. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005;16:249–50. doi: 10.1016/j.cytogfr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Reddi AH, Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972;69:1601–5. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado E, Schwartz Z, Sylvia VL, Dean DD, Boyan BD. Transforming growth factor-beta1 regulation of growth zone chondrocytes is mediated by multiple interacting pathways. Biochim Biophys Acta. 2002;1590:1–15. doi: 10.1016/s0167-4889(02)00194-5. [DOI] [PubMed] [Google Scholar]
- Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–7. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Sebald W, Nickel J, Zhang JL, Mueller TD. Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol Chem. 2004;385:697–710. doi: 10.1515/BC.2004.086. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Weiner L, Sharova TY, Siebenhaar F, Atoyan R, Reginato AM, McNamara CA, Funa K, Gilchrest BA, Brissette JL, Botchkarev VA. Noggin overexpression inhibits eyelid opening by altering epidermal apoptosis and differentiation. EMBO J. 2003;22:2992–3003. doi: 10.1093/emboj/cdg291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–82. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Smoljanovic T, Bojanic I, Delimar D. Adverse effects of posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2009;18:920–3. doi: 10.1007/s00586-009-0959-z. author reply 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmesky LJ, Abekasis M, Bulvik S, Weil M. Bone morphogenetic protein signaling is involved in human mesenchymal stem cell survival in serum-free medium. Stem Cells Dev. 2009;18:1283–92. doi: 10.1089/scd.2009.0020. [DOI] [PubMed] [Google Scholar]
- Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, Latham JA. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–35. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFss and BMP pathways. Cell Signal. 2011;23:1831–42. doi: 10.1016/j.cellsig.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Vukicevic S, Sampath KT. Bone morphogenetic proteins: regeneration of bone and beyond. Basel: Birkha\0308user; 2004. p. xi.p. 310. [Google Scholar]
- Walsh DW, Godson C, Brazil DP, Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends Cell Biol. 2010;20:244–56. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Wu XB, Li Y, Schneider A, Yu W, Rajendren G, Iqbal J, Yamamoto M, Alam M, Brunet LJ, Blair HC, Zaidi M, Abe E. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest. 2003;112:924–34. doi: 10.1172/JCI15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi Y, Sakiyama J, Kameda T, Iba H, Suzuki A, Ueno N, Kuroiwa A. BMP-2/-4 mediate programmed cell death in chicken limb buds. Development. 1996;122:3725–34. doi: 10.1242/dev.122.12.3725. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ge Y, Sun L, Cao J, Wu Q, Guo L, Wang Z. Effect of bone morphogenetic protein-2 on proliferation and apoptosis of gastric cancer cells. Int J Med Sci. 2012;9:184–92. doi: 10.7150/ijms.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yang S, Zhou GQ, Yang J, Ji D, Sabatakos G, Zhu T. Downregulation of cAMP-dependent protein kinase inhibitor gamma is required for BMP-2-induced osteoblastic differentiation. Int J Biochem Cell Biol. 2006;38:2064–73. doi: 10.1016/j.biocel.2006.05.015. [DOI] [PubMed] [Google Scholar]