Abstract
Purpose
To investigate the hypothesis that non-steroidal anti-inflammatory drugs (NSAIDs) lower lung cancer risk.
Methods
We analysed pooled individual-level data from seven case–control and one cohort study in the International Lung Cancer Consortium (ILCCO). Relative risks for lung cancer associated with self-reported history of aspirin and other NSAID use were estimated within individual studies using logistic regression or proportional hazards models, adjusted for packyears of smoking, age, calendar period, ethnicity and education and were combined using random effects meta-analysis.
Results
A total of 4,309 lung cancer cases (mean age at diagnosis 65 years, 45% adenocarcinoma and 22% squamous-cell carcinoma) and 58,301 non-cases/controls were included. Amongst controls, 34% had used NSAIDs in the past (81% of them used aspirin). After adjustment for negative confounding by smoking, ever-NSAID use (affirmative answer to the study-specific question on NSAID use) was associated with a 26% reduction (95% confidence interval 8 to 41%) in lung cancer risk in men, but not in women (3% increase (−11% to 30%)). In men, the association was stronger in current and former smokers, and for squamous-cell carcinoma than for adenocarcinomas, but there was no trend with duration of use. No differences were found in the effects on lung cancer risk of aspirin and non-aspirin NSAIDs.
Conclusions
Evidence from ILCCO suggests that NSAID use in men confers a modest protection for lung cancer, especially amongst ever-smokers. Additional investigation is needed regarding the possible effects of age, duration, dose and type of NSAID and whether effect modification by smoking status or sex exists.
Keywords: NSAIDs, Aspirin, Lung cancer
Introduction
Worldwide, lung cancer is the leading cause of cancer mortality in men (∼951,000 deaths in 2008) and the second leading cause in women (427,000 deaths) [1,2]. Whilst tobacco control dominates strategies to reduce this burden, chemoprevention may also contribute, especially amongst former smokers. Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) may have anti-cancer properties, especially for cancers whose aetiology implicates the role of chronic inflammation, such as colorectal and lung. The anti-inflammatory effect of NSAIDs operates through inhibition of prostaglandins via suppression of cyclooxygenase-1 (COX-1) and COX-2, targeting the arachidonic acid metabolic pathway. Non-small-cell lung cancers in particular overexpress COX-2 [3, 4].
Aspirin reduces risk of adenocarcinoma of the colon, as demonstrated in randomised controlled trials (RCTs) [5, 6], an effect that may be restricted to COX-2 tumours [7]. In 2011, Rothwell et al. pooled data from 8 double-blind RCTs of daily aspirin in which the median scheduled treatment time was at least 4 years and analysed the effect of aspirin on cancer mortality as secondary endpoints (primary endpoints were cardiovascular diseases) [8]. Overall cancer mortality rates were 22% lower (95% CI: 13, 30) in those randomised to the aspirin group compared to the control group, and lung cancer-specific mortality rates were reduced by 29% (95% CI: 11, 42) in the aspirin group in the 20-year period after the trial commenced. No significant effect on lung cancer was observed in the initial 5-year period after randomisation (reduction of 8% (95% CI: −30, 35)). No trend with dose (above 75 mg/day) was observed, but the effect on all cancers was stronger in adenocarcinomas and increased with longer durations of treatment and was present in both smokers and non-smokers. The authors suggest that their intention-to-treat analyses were likely to have been conservative, as about 40% of patients in the aspirin group had stopped treatment by the end of the trial period.
Other studies of NSAIDs and lung cancer incidence or mortality have been observational in nature (over 15 published studies). A 2005 meta-analysis by Khuder et al. summarised evidence at the time and found that after adjusting for smoking, NSAIDs were associated with a RR of 0.68 (95% CI: 0.55, 0.85), stronger for small-cell than non-small-cell lung cancer although only two studies were able to stratify by histology [9]. As found in the meta-analysis, in settings where smokers are more likely to be prescribed aspirin for cardio-prevention—today common practice—confounding by smoking would lead to an underestimation of any real protective effect of NSAIDs; thus, accurate detailed smoking data are crucial in observational studies. Several further observational studies have been published, with variable findings including one null association [10], a protective effect of aspirin for non-small-cell lung cancer in women (men were not included) [11], the VITamin and Lifestyle cohort found a protective effect of NSAIDs in men, but not women, and for adeno-carcinomas and not squamous-cell carcinomas [12], a suggestion of a protective effect of low-dose aspirin in the Women's Health Study [13], and a protective effect of non-aspirin NSAIDs and not aspirin using UK GP prescription records [14], but no clear evidence of an effect in the Nurses' Health Study [15].
Thus, several uncertainties remain concerning whether the effects of aspirin and non-aspirin NSAIDs are equal in reducing lung cancer incidence, what lung cancer histologies are affected and whether there is effect modification by gender or smoking status. We investigated these associations using pooled individual-level data from the International Lung Cancer Consortium (ILCCO), for which there were over 4,000 cases with data on NSAID use.
Materials and methods
ILCCO was established in 2004 with the aims to share comparable data and maximise resource saving for lung cancer epidemiology research. Full details have been provided previously [16] and are available at http://ilcco.iarc.fr. For the current investigation, we included 8 studies from ILCCO that had data on aspirin or NSAID use prior to diagnosis, including American Health Foundation Tobacco Study (AHFTS) [17], New England Lung Cancer study (NELCS) [18], Danish Diet Cancer and Health Study (DDCHS) [19], Memorial Sloan-Kettering Cancer Center (MSKCC) study of never smokers and studies in Hawaii [20], Moffitt Cancer Center [21], Harvard [22] and the National Israel Cancer Control Center (NICCC) lung cancer study (Table 1). All 8 studies were approved by local ethical review boards. Six of the 8 studies were conducted in the US; all but one (DDCHS) had case–control designs with NSAID use recalled at the time of diagnosis/interview in cases/controls. DDCHS was a cohort study, in which self-reported NSAID use was collected in a baseline questionnaire, a median of 7 years prior to lung cancer diagnosis in cases. In the Harvard study, a question on NSAIDs was included from 2005 onwards; thus, only study participants recruited thereafter were included. In AHFTS, subjects interviewed between 1st January 1992 and 30th April 1997 (when NSAIDs were ascertained) were included, and as this was the only study where controls were hospital-based patients including some cancer patients, we excluded controls whose hospital admission was due to cancer or a condition for which aspirin is either prescribed (e.g. rheumatoid and osteoarthritis, cardiovascular conditions, migraine, general pain relief and possibly colorectal cancer) or contraindicated (e.g. peptic ulcers).
Table 1. ILCCO studies contributing to NSAID analysis: study design, number of subjects and study-specific NSAID question.
| Study | Setting | Lung cancer cases source | Controls/non-cases | Cases | Controls/non-cases | Questions pertaining to NSAID use |
|---|---|---|---|---|---|---|
| AHFTS | American Health Foundation Tobacco study | Multi-hospital cases, diagnosed 1992–1997 (when NSAIDs were ascertained) | Hospital patients with conditions unrelated to tobacco exposure. Controls with cancer, rheumatoid arthritis or osteoarthritis excluded | 977 | 683 | Have you ever taken any over-the-counter or prescription pain relievers (e.g. tylenol, aspirin, medipren) for at least once a month or more? |
| Boston | Boston, US | Massachusetts General Hospital, 2005–2008 diagnoses | Friends of cases | 768 | 123 | ‘How often do you take Aspirin or aspirin-containing products (bufferin, Bayer's aspirin, ASA acetylsalicylic acid)? How often do you take non-steroidal anti-inflammatory drugs (NSAIDs) (list of brandnames provided)?’ |
| DDCHS | Danish Diet Cancer and Health Study. 1993–1997 baseline questionnaire | Diagnoses during follow-up to end 2007. | Nationally representative cohort | 812 | 55396 | Self-reported analgesic use of at least one tablet per month in the past year++, with specific question on (i) aspirin (ii) paracetamol [not an NSAID] (ii) ibuprofen, low-dose aspirin, aspirin, non-aspirin NSAID. |
| Florida | Florida, US | Lee Moffitt Cancer Centre, 1999–2003 | Community residents attending cancer screening | 467 | 889 | ‘Has a doctor ever prescribed any of the following over-the-counter or prescription pain relievers on a weekly or daily basis: Aspirin or Ibuprofen?’ (brand names listed) |
| Hawaii | Hawaii, US | 1992–1997 | Health survey and Oahu health care financing participants, individually age-ethnicity matched | 627 | 588 | ‘Have you ever taken at least twice a week for 3 months or more: aspirin, acetaminophen, other pain relief, theophylline’. Specific drug and brand names listed. |
| MSKCC | Memorial Sloan-Kettering Cancer Centre, US | Lung cancer patients diagnosed 2005-08, never smokers | Hospital visitors who were never smokers | 102 | 101 | ‘Indicate which medications you have ever used now or in the past and the actual name of the medications.’ (List provided) |
| NELCS | New England Lung Cancer Study, New Hampshire and Vermont, US | Cancer registry and cancer hospital extracted lung cancer diagnoses, 2005-08 | Frequency age-sex matched, random selection from commercial database of general population | 276 | 251 | ‘Before reference date, did you take one of these 4 times/week for at least 6 months?’ (for all NSAIDs and some non-NSAIDs on a card). |
| NICCC | National Israeli Cancer Control Center | Lung cancers diagnosed 2007–2010 from multiple medical centres | Individually matched on clinic, age and sex (analysed as unmatched) | 280 | 270 | ‘Have you used aspirin during the past year (not including the month prior to diagnosis)? During the past year, did you use NSAIDs?’ A cue card listing drug names was shown. |
The questions asked pertaining to previous NSAID or aspirin use varied between studies (Table 1). All questions related to having taken any NSAIDs (i.e. prescription and non-prescription use), except the Moffitt study where the question referred to ever having been prescribed aspirin or ibuprofen. The specified minimum duration and intensity (times/week) of use varied between studies. Lifetime NSAID use was ascertained, except in the prospective DDCHS study in which participants were questioned about use in the year prior to the baseline questionnaire.
Individual-level data from each contributing study were pooled. For NSAID and aspirin use, study-specific variables were obtained, from which we coded each listed drug as aspirin (any drug containing acetylsalicylic acid), or non-aspirin NSAIDs, with the help of the US Food and Drug Administration Drugs@FDA online database that can be searched both by drug name and active ingredient. Non-aspirin NSAIDs included ibuprofen, naproxen, sulindac, indomethacin and diclofenac amongst others. We then generated, for both total NSAID and aspirin use, common variables pertaining to ‘ever use’, and where possible, total duration of use, age at first and last use, and average number of pills per week. We also extracted the predominant reason for use (taken as the reason pertaining to the longest period for subjects with multiple drug-use periods with different reasons). Finally, we explored whether any observed effect of NSAIDs/aspirin was likely to be drug specific or due to residual confounding by factors associated with use of pain relievers in general. To do this as a control measure, we also analysed the effect of acetaminophen, another common non-NSAID pain relief medication (where available within studies).
Variables for well-established lung cancer risk factors and participant characteristics were harmonised across studies. The variable ‘ever-use of NSAIDs’ indicates an affirmative answer to the question(s) asked based on the study-specific definition (as each study defined ‘ever use’ based on different minimum duration and frequency of NSAID use). For smoking, we created variables pertaining to smoking status as self-reported at the time of diagnosis/interview in cases/non-cases: never smokers (<100 cigarettes over lifetime), ex-smoker (stopped smoking at least 2 years previously) and current smokers (smoked within the past 2 years). For current and ex-smokers, smoking pack years were calculated as the intensity of smoking (packs per day) multiplied by years of smoking at that intensity, summed over all periods of smoking. Note that in DDCHS cohort, smoking data refer to exposures up to the baseline questionnaire, and updated smoking information was not obtained during follow-up.
Statistical methods
A two-stage approach to the analysis was taken: stage 1 being a within-study analysis and stage 2 combining study-specific effects across all studies. In the first stage, the relative risk (RR) for lung cancer associated with NSAID (or aspirin) use (or its characteristics) was estimated in each study as odds ratios (OR) from logistic regression models for case–control studies and conditional logistic regression for the Hawaiian matched case–control study. The prospective DDCHS study was analysed using a proportional hazards model in which RRs for time to first of lung cancer diagnosis/death/censoring, using age as the time-scale were estimated by hazard ratios.
The second stage involved combining study-specific estimates using meta-analytic random effects models, where between-study heterogeneity was examined using q-statistics and I2 values. These two stages were carried out for different levels of adjustment, beginning with minimal adjustment for age and sex, thereafter additionally adjusting for smoking status (current, ex (stopped smoking at least 2 years previously), never (less than 100 cigarettes ever-smoked)), smoking pack years (continuous), educational level and ethnicity. Sub-group analyses were also carried out by sex, histology (overexpression of COX-2 has been reported particularly in adenocarcinomas and not for small or squamous-cell carcinoma), age, smoking status and in subjects without a self-reported history of asthma (asthma is a counter-indication for aspirin use [23], and it is not known whether asthma may be associated with increased lung cancer risk). For the analysis of non-asthmatics in DDCHS, previous diagnosis of asthma was included as a time-varying exposure; thus, subjects were included in the non-asthma group up until the age at asthma diagnosis, if any. All analyses were conducted in Stata version 11.
Results
The pooled data from the 8 studies totalled 4,309 cases and 58,301 non-cases/controls. Amongst cases, mean age at lung cancer diagnosis was 65 years (inter-quartile range, 59–71 years), 49% were current smokers at the time of diagnosis and 36% were ex-smokers (Table 2). The most common histological types were adenocarcinomas (35%), squamous-cell carcinomas (25%) and small-cell carcinomas (16%) in current smokers; in ex- and never-smokers, there was a higher percentage of adenocarcinomas (52 and 72%, respectively) and less squamous-cell lung carcinomas (21 and 5%, data not shown in tables).
Table 2. Demographic and histological characteristics of included cases and non-cases.
| Cases | Non-cases/controlsa | ||
|---|---|---|---|
|
| |||
| Studies except DDCHS | DDCHS | ||
| N = 4,309 | N = 2,905 | N = 55,396 | |
| N (%) | N (%) | N (%) | |
| Age (years) | |||
| Mean (SD) | 64.5 (9.5) | 61.9 (10.8) | 56.1 (4.4)c |
| Sex | |||
| Male | 2,227 (51.7) | 1,584 (54.5) | 26,392 (47.6) |
| Female | 2,082 (48.3) | 1,321 (45.5) | 29,004 (52.4) |
| Educational level | |||
| Low | 862 (24.8) | 550 (20.1) | 12,255 (22.2) |
| Medium | 1,683 (48.4) | 1,191 (43.6) | 33,038 (59.8) |
| High | 935 (26.9) | 993 (36.3) | 9,917 (18.0) |
| Ethnicity | |||
| Caucasian | 3,689 (85.6) | 2,289 (78.8) | 55,396 (100%) |
| Hispanic/Latino | 65 (1.5) | 78 (2.7) | |
| Black | 122 (2.8) | 154 (5.3) | – |
| Asian | 248 (5.8) | 223 (7.7) | – |
| Native America | 15 (0.4) | 4 (0.1) | – |
| Hawaiian | 160 (3.7) | 147 (5.1) | – |
| Other | 10 (0.2) | 10 (0.3) | – |
| Smoking distribution | |||
| Never | 652 (15.1) | 1,100 (37.9) | 19,649 (35.5) |
| Ex | 1,566 (36.1) | 1,218 (41.9) | 14,560 (26.3) |
| Current | 2,101 (48.8) | 587 (20.2) | 21,187 (38.3) |
| Histologyb | |||
| Adenocarcinoma | 1,674 (45.4) | NA | NA |
| Squamous-cell carcinoma | 799 (21.7) | ||
| Large cell carcinoma | 125 (3.4) | ||
| Other non-small cell | 678 (18.4) | ||
| Small cell | 410 (11.1) | ||
Non-cases split by DDCHS inclusion/exclusion as 95% of non-cases are from this study
Distribution amongst non-missing histology. Missing for 2.5% of cases (N = 96)
Age at baseline questionnaire
Self-reported history of NSAID use by study and case/control status is shown in Table 3. For both cases and controls, the percentage of NSAID use varied between studies, generally increasing over calendar time from ∼30% in AHFTS to over 50% in the more recent studies. At MSKCC, there was a particularly high proportion at 86%, but the NSAID question did not specify a minimum duration or intensity of use. The prevalence of ever-use of NSAIDs in cases versus controls was lower in 4 studies, similar in 2 and higher in 2 studies (Table 3). Where data were available (4 studies), mean age at first NSAID use was 47.4, 56.2, 54.3 and 50.9 years in AHFTS, Florida, Hawaii and NELCS controls, respectively, and median duration of use ranged from 5 to 10 years between studies. The most common reasons for NSAID use, available in 3 studies, were cardioprotection, pain/headache and rheumatic diseases (Table 3), with use for cardioprotection generally more common in the later studies (amongst male controls, 68% in NELCS, 27% in Florida and 41% in AHFTS) and lower in women than in men (corresponding percentages in women: 46, 32 and 13%, respectively). Across all studies, the predominant type of NSAID used was aspirin: between 76 and 92% of male NSAID users were also aspirin users, whilst this percentage was lower in women (between 62 and 88%). Cardiovascular disease prevention was a more common reason for aspirin use than it was for any NSAID use (data not shown).
Table 3. Characteristics of NSAID use in cases and non-cases, by study.
| N | Ever-NSAID Use N (%) | Years of use (Median, IQR) | Age at first use | Age at last use | Reason for use amongst users (row %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Men | Women | ||||||||||||||
|
|
|
||||||||||||||
| CVD | Pain | Headache | Rheumatic | Fever/other | CVD | Pain | Headache | Rheumatic | Fever/other | ||||||
| AHFTS | |||||||||||||||
| Cases | 977 | 279 (28.6) | 6 (2, 17) | 47.9 (17.1) | 60.4(11.4) | 25 | 26 | 28 | 10 | 11 | 14 | 18 | 34 | 21 | 13 |
| Controls | 683 | 223 (32.7) | 9 (3, 20) | 47.4 (16.5) | 60.2 (12.1) | 41 | 16 | 25 | 5 | 12 | 13 | 24 | 38 | 17 | 9 |
| Boston | |||||||||||||||
| Cases | 768 | 535 (69.7) | 9.5 (3,15) | - | - | - | - | - | - | - | - | - | - | - | - |
| Controls | 123 | 88 (70.4) | 10 (5,15) | - | - | - | - | - | - | - | - | - | - | - | - |
| DDCHS | |||||||||||||||
| Cases | 812 | 277 (34.1) | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Controls | 55396 | 18,958 (34.2) | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Floridaa | |||||||||||||||
| Cases | 467 | 143 (30.6) | 5 (2,10) | 61 3 (12.3) n = 35 | - | 52 | 18 | 0 | 1 | 29 | 29 | 23 | 0 | 2 | 46 |
| Controls | 889 | 236 (26.5) | 3(1,7) | 56.2 (8.3) n = 55 | - | 27 | 21 | 2 | 3 | 48 | 32 | 26 | 1 | 3 | 38 |
| Hawaii | |||||||||||||||
| Cases | 627 | 183 (29.2) | 7 (3,17) | 52.2 (15.6) | 63.7 (10.1) | - | - | - | - | - | - | - | - | - | - |
| Controls | 588 | 197 (33.5) | 5 (2,11) | 54.3 (15.0) | 63.3 (11.2) | - | - | - | - | - | - | - | - | - | - |
| MSKCC | |||||||||||||||
| Cases | 102 | 88 (86.3) | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Controls | 101 | 85 (84.2) | - | - | - | - | - | - | - | - | - | - | - | - | - |
| NELCS | |||||||||||||||
| Cases | 276 | 128 (46.4) | 8 (2,15) | 51.9 (13.0) | 62.1 (9.7) | 69 | 11 | 11 | 6 | 4 | 44 | 25 | 12 | 14 | 5 |
| Controls | 251 | 116 (46.2) | 10 (4,17) | 50.9 (13.4) | 62.3 (10.2) | 68 | 19 | 5 | 6 | 2 | 46 | 22 | 7 | 19 | 6 |
| NICCC | |||||||||||||||
| Cases | 280 | 171 (61.1) | 5 (1,10) | - | - | - | - | - | - | - | - | - | - | - | - |
| Controls | 270 | 179 (66.3) | 4 (3,5) | - | - | - | - | - | - | - | - | - | - | - | - |
| Total | |||||||||||||||
| Cases | 4309 | ||||||||||||||
| Controls | 58,301 | ||||||||||||||
NSAID use only refers to aspirin and ibuprofen use. CVD=NSAID use was for cardiovascular disease prevention
Given the known associations of smoking, age, education and ethnicity with lung cancer, and plausible association of each of these factors with NSAID use, they were considered as potential confounders. Their associations with the odds of having taken NSAIDs were examined amongst controls in each study, and patterns of use were broadly similar across studies (except for MSKCC, see online supplementary table), with NSAID use being almost twofold more likely in men than in women (the percentage of controls who had used NSAIDs was 7.3% higher (95% CI: 3.8, 10.9) in men than in women in all studies excluding DDCHS where it was 9.6% higher in women (8.7, 10.3)), at older ages and in current and ex-smokers compared to never smokers (associations mutually adjusted for age, sex, smoking status, education and ethnicity). The only exceptions to this were amongst women in DDCHS and in the earlier AHFTS study in which use declined with age as it did in all AHFTS controls.
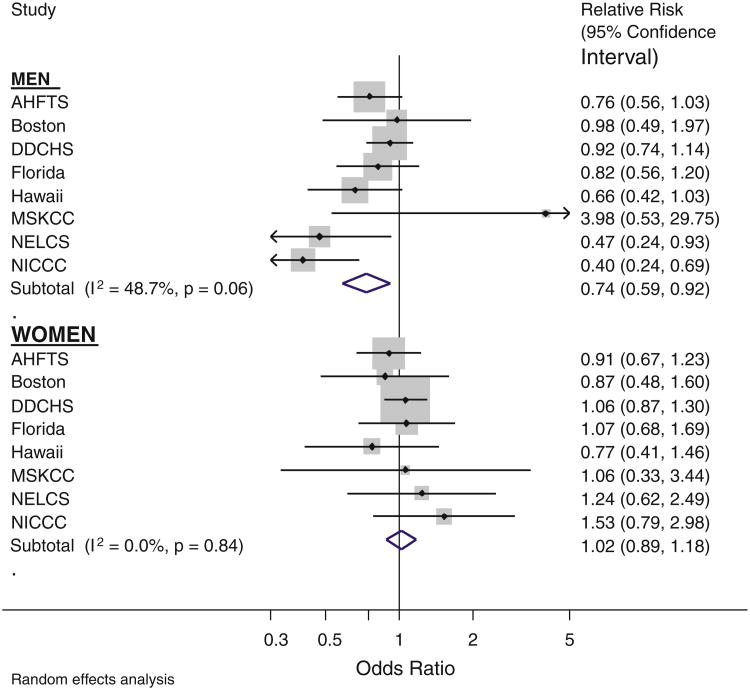
For the association of ever-use of NSAIDs and lung cancer, there was strong evidence of sex differences (p = 0.003 test for heterogeneity); thus, sex-specific results are presented from here on. In men, the age-adjusted combined association was suggestive of a protective effect with a 20% lower lung cancer risk (95% CI: 7, 32) associated with ever-use of NSAIDs (data not shown). Negative confounding by smoking was present within studies and thus additionally adjusting for smoking (current, never, ex), pack years of smoking, ethnicity and education strengthened this association to a combined estimate of 26% lower risk (95% CI: 8, 41) (Fig. 1). Hereafter, all estimates are adjusted for these factors. Although between-study heterogeneity was present for men (p = 0.06, Fig. 1), all but one study-specific point estimate were below the null value of 1. NICCC was the study contributing to the greatest heterogeneity (having the lowest odds ratio of 0.40), and in sensitivity analyses, removing this study gave an overall relative risk that was only slightly smaller in magnitude, with a risk reduction of 19% (95% CI: 4, 32) and there was no remaining heterogeneity (p = 0.29). In contrast, for women the combined relative risk, both before (data not shown) and after adjustment for smoking, was consistent with no association between NSAID use and lung cancer risk, with a RR of 1.07 (95% CI: 0.94, 1.25), and study-specific estimates were not significantly heterogeneous (Fig. 1). For analyses in both men and women, the DDCHS study had the largest statistical weight in the meta-analysis: weights of 23.3% in men and 49% in women (larger in women because of the higher prevalence of NSAID use amongst women in DDCHS). However, sensitivity analyses removing DDCHS did not change overall estimates.
Fig. 1. Effect of ever-NSAID use adjusted for age, smoking status, packyears, calendar year, education and ethnicity, by sex.
Associations of ever-use of aspirin (prevalence 28%, i.e. with or without use of other non-aspirin NSAIDs) and of non-aspirin NSAID use exclusively (6.6% across studies) with lung cancer were very similar (Table 4). In men, relative risk point estimates were 0.73 and 0.76 for aspirin and non-aspirin NSAIDs, respectively, and in women, there were null associations for both. Thus, in further analyses, we focus on use of all types of NSAIDs together. Furthermore, although there was heterogeneity in the definition of ever-NSAID use (see Methods section), individual exclusion of each study did not change the point estimate. (In Table 4, although MSKCC appears to be an outlier, the statistical weight contribution of this small study was <3%, thus, it did not greatly influence the combined estimate and was not excluded.)
Table 4. Study-specific and random effects combined relative risks for ever use of aspirin and of non-aspirin NSAIDs use by sex.
| Study | Men | Women | ||
|---|---|---|---|---|
|
|
|
|||
| Any aspirin versus no NSAIDs | Non-aspirin NSAID versus no NSAIDs | Aspirin versus no NSAIDs | Non-aspirin NSAID versus no NSAIDs | |
| Relative riska(95% confidence interval) | ||||
| AHFTS | 0.64 (0.46, 0.90) | 1.32 (0.74, 2.36) | 0.98 (0.68, 1.41) | 0.81 (0.53, 1.25) |
| Boston | 0.88 (0.42, 1.82) | 1.69 (0.48, 5.96) | 0.63 (0.32, 1.23) | 1.52 (0.68, 3.39) |
| DDCHS | 0.93 (0.74, 1.17) | 0.82 (0.49, 1.38) | 1.04 (0.83, 1.29) | 1.14 (0.82, 1.59) |
| Florida | 0.89 (0.60, 1.34) | 0.41 (0.15, 1.16) | 1.05 (0.64, 1.71) | 1.19 (0.47, 3.02) |
| Hawaii | 0.65 (0.41, 1.02) | 0.78 (0.24, 2.53) | 0.72 (0.36, 1.43) | 1.16 (0.24, 5.66) |
| MSKCC | 4.29 (0.54, 34.0) | 2.58 (0.14, 46.7) | 1.18 (0.36, 3.88) | 0.74 (0.19, 2.94) |
| NELCS | 0.56 (0.27, 1.14) | 0.17 (0.04, 0.70) | 1.34 (0.62, 2.88) | 0.98 (0.30, 3.17) |
| NICCC | 0.41 (0.24, 0.70) | 0.19 (0.02, 2.23) | 1.60 (0.82, 3.12) | –b |
| Combined estimate | 0.73 (0.57, 0.92) | 0.76 (0.46, 1.25) | 1.02 (0.87, 1.19) | 1.05 (0.83, 1.32) |
| I2 (%) | 49.5% | 43.2% | 0% | 0% |
| Between-study heterogeneity (p) | p = 0.054 | p = 0.09 | p = 0.62 | p = 0.84 |
Relative risks estimated by odds ratios for all studies except DDCHS where they are hazard ratios, adjusted for age, smoking category (never, ex, current), smoking pack years (continuous), ethnicity, year of birth, 3 category educational level
Excluded as only one exposed subject
Duration of NSAID use was available in 5 studies. Amongst NSAID users, in individual studies between 27 and 52% of men had taken NSAIDs for at least 10 years, and durations were similar amongst female NSAID users (corresponding range, 25–49%). In men, NSAID users of less than 5 years use had a 21% risk reduction (95% CI: −6, 41), i.e. nearly as large as the reduction observed in users with over 10 years of use (26% reduction) (Table 5). Large heterogeneity in the long duration category was caused by a single study (Florida), and after removing it, the combined estimate of 10? years NSAID use was stronger, with lung cancer risk 43% lower (95% CI: 23, 58). Male NSAID users who took at least 7 pills per week (i.e. usually daily) had a similar reduction in risk to men who took fewer than 7 pills per week. Amongst women, associations of NSAID use by duration or pills per week were close to the null value of no effect (RRs in Table 5).
Table 5. Random effects combined relative risks for ever-NSAID use, by sex and further subsets.
| Contributing studies | Subset/category | Men | Women | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| RR (95% CI) | Heterogeneity p | RR (95% CI) | Heterogeneity p | ||||
| All | All 8 | 0.74 (0.59, 0.92) | 0.06 | 1.03 (0.89, 1.18) | 0.84 | ||
| By characteristics of NSAIDs use | |||||||
| Total duration | Boston, Hawaii, NELCS, Florida, AHFTS | Never | 1 | 1 | |||
| <5 years | 0.79 (0.59, 1.06) | 0.37 | 1.00 (0.72, 1.37) | 0.36 | |||
| 5–9 years | 0.84 (0.59, 1.21) | 0.69 | 0.94 (0.59, 1.48) | 0.56 | |||
| ≥10 years | 0.74 (0.43, 1.28) | 0.01 | 0.91 (0.68, 1.21) | 0.94 | |||
| Tablets per week | All except Hawaii | Never | 1 | 1 | – | ||
| <7 | 0.70 (0.46, 1.07) | 0.03 | 0.98 (0.82, 1.17) | 0.87 | |||
| ≥7 | 0.79 (0.56, 1.12) | 0.01 | 1.13 (0.93, 1.37) | 0.68 | |||
| Ever versus never-NSAID use, stratified by other lung cancer risk factors | |||||||
| Age | All | <65 years | 0.63 (0.44, 0.91) | 0.008 | 1.01 (0.96, 1.19) | 0.69 | |
| ≥65 | 0.91 (0.69, 1.19) | 0.54 | 1.13 (0.92 1.39) | 0.95 | |||
| Amongst subjects with no diagnosis | previous asthma | NELCS, DDCHS, Hawaii, MSKCC | 0.85 (0.68,1.07) | 0.28 | 1.06 (0.89, 1.27) | 0.69 | |
| Ever versus never-NSAID use and relative risks of lung cancer histologies, according to smoking status | |||||||
| Risk of histology-specific lung cancer, by smoking status | All 8 | All participants | |||||
| RR of small cell-lung carcinoma | 0.78a (0.55,1.09) | 0.39 | 1.18a (0.84, 1.66) | 0.51 | |||
| RR of non-small-cell lungb | 0.77 (0.59, 1.00) | 0.03 | 1.00 (0.86, 1.17) | 0.84 | |||
| RR of adenocarcinoma | 0.92 (0.72, 1.18) | 0.28 | 0.99 (0.84, 1.16) | 0.93 | |||
| RR of squamous-cell carcinoma | 0.71 (0.50, 1.02) | 0.17 | 1.06 (0.87, 1.29) | 0.55 | |||
| All 8 | Never smokersc RR of lung cancer (652 cases) | 1.07 (0.67, 1.69) | 0.40 | 0.95 (0.70, 1.29) | 0.96 | ||
| RR of lung adenocarcinoma (297 cases) | 1.95 (1.01, 3.75) | 0.87 | 1.02 (0.68, 1.52) | 0.96 | |||
| All except MSKCC | Current/Ex-smokers | ||||||
| RR of lung cancer | 0.67 (0.52, 0.88) | 0.02 | 1.05 (0.89, 1.23) | 0.71 | |||
| RR of non-small-cell | 0.66 (0.49, 0.89) | 0.02 | 0.99 (0.82, 1.35) | 0.46 | |||
| RR of adenocarcinoma | 0.83 (0.63, 1.10) | 0.23 | 0.97 (0.76, 1.22) | 0.93 | |||
| RR of squamous-cell carcinoma | 0.65 (0.44, 0.95) | 0.10 | 0.92 (0.62, 1.35) | 0.46 | |||
Estimated from a single logistic regression model with adjustment for study, due to small numbers and lack of convergence within study-specific analyses
Non-small-cell lung cancers: adenocarcinoma (58.4%), squamous-cell carcinoma (28%), large cell (4.3%), other (9.5%)
652 cases overall, of which 71% of known histology were adenocarcinomas, too few cases to analyse other histologies separately
Other than effect modification by sex upon which all previous results have been based, further effect modifiers of the NSAID-lung cancer association were examined separately within each sex. Amongst men, the inverse association was stronger at younger than older ages (RR = 0.63 under 65 years, RR = 0.91 over 65 years, Table 5); a slightly weaker association was observed when restricting to male non-asthmatics amongst the 4 studies with asthma history information (RR = 0.85); the association was stronger in current smokers (RR = 0.58) than in ex-smokers (RR = 0.72, data not shown) and was not present in never smokers (RR = 1.07, Table 5), the latter category having the smallest number of cases. Amongst male current- and ex-smokers combined, the effect was stronger for squamous-cell carcinomas (RR = 0.65 (95% CI: 0.44, 0.95)) than adenocarcinomas (RR 0.83 (95% CI: 0.63, 1.10)) of the lung. In women, the overall null association held in subsets defined by smoking status and for lung cancer risk by histology.
In 4 studies (Hawaii, NELCS, AHFTS and DDCHS), there were also data on acetaminophen use. In these 4 studies, past NSAID was inversely associated with lung cancer risk in men (RR 0.79, 95% CI: 0.67, 0.93) whilst past acetaminophen use not (RR = 1.11, 95% CI: 0.92, 1.33). In women, neither ever-NSAID use (RR 1.00, 95% CI: 0.85, 1.17) nor ever acetaminophen use (RR = 1.02, 95% CI: 0.87, 1.20) were associated with lung cancer risk.
Discussion
Pooled data from the ILCCO studies suggest that NSAID use is protective against lung cancer in men, with an average risk reduction of 26% (95% CI: 8 to 41). This inverse association was slightly stronger in current smokers and former smokers than in never smokers, and it was also slightly stronger for squamous-cell and small-cell lung cancers than for adenocarcinomas. Amongst men, we found that aspirin and non-aspirin NSAID use were associated with a similar reduction in risk, but use of the latter was less common, and thus, confidence intervals were wider. That a risk reduction was restricted to NSAID use and not acetaminophens, another pain reliever, suggests that lung cancer risk factors associated with use of pain relief in general and that were not controlled for did not influence the results (although there remains the possibility of confounding by other factors) and suggests that recall bias did not account for the association because any misclassification in the recall of pain relief use is not expected to have differed by type of pain relief (NSAID or other). Reverse causality is also unlikely to explain an inverse association as, if anything, recent use of pain relief would be expected to be higher in cases in the period before diagnosis, and not lower as observed. If asthma, a contraindication for the use of NSAIDs, was a risk factor for lung cancer, as is under-debate, there would be the possibility that the effect in men is partly confounded; however, an inverse association was observed in non-asthmatic men.
The overall findings for men are consistent and of a similar magnitude to previously reported protective effects—our estimate of a 24% reduction for NSAID use is similar to that found in RCTs of aspirin, i.e. a 29% reduction in lung cancer mortality associated with long-term aspirin use in Rothwell et al's pooled analysis and a 36% reduction in British doctors [24, 25]. Khuder's meta-analysis also reported a similar reduction for aspirin of 32% (95% CI: 15, 45) for both sexes combined, but with no differences according to sex. We observed suggestions, although not strong, of a greater reduction the longer the duration of NSAID use, but no dose–response effect for number of tablets per day. These analyses were likely to be influenced by random variation, having only 1,200 cases with data on NSAID use and the number of tablets per day not the best indicator of dose. The protective effect in men was similar for both aspirin and non-aspirin NSAIDs. Previous studies have found conflicting results—notably a UK study with reliable prescription data found a protective effect only for non-aspirin NSAIDs and not for aspirin, except within a subgroup of patients with a history of angina or myocardial infarction [26]. Their lack of association for aspirin may be partly explained by residual confounding by smoking, which was unlikely to be a problem in the present study.
The stronger association for squamous-cell carcinoma than for adenocarcinoma is in contrast to what we expected and to findings in a recent prospective study [27], as adenocarcinomas have the greatest expression of COX-2 compared to other histologies [28, 29]. Alternatively, stronger effects for squamous-cell carcinomas and for current/former smokers may be explained by the raised proliferative activity in their tumours, as measured by Ki-67 labelling index [30], that COX inhibitors have been shown to reduce in current and former smokers [31].
In contrast, we observed no association between NSAID use as reported by women and lung cancer risk, neither overall nor in any subgroup. These sex differences were not explained by differences in type of NSAID used (aspirin or not). Data on duration, dose and reasons for use were limited to a few studies, and thus, there was a lack of power to thoroughly investigate these reasons for the sex difference. One possibility that may contribute, but needs further exploration, is that, as the reasons for taking NSAIDs differ between men and women, with women less likely to be prescribed for cardioprotection and more likely for inflammatory conditions (arthralgia, arthritis, joint pain), types, durations, doses and frequency of use (e.g. intermittent for menstrual pain) may differ. Alternatively true sex differences may exist, modified by estradiol's effect on COX activity in women [32]. Previous studies have conflicting results. A similar result of a protective effect in men but none in women was found in the prospective VITAL study [33], and no clear protective effect was seen in the Nurses' Health Study [34]. In the Women's Health Study RCT of 100 mg aspirin every other day, followed for 10 years, lung cancer was the only cancer site with a suggestion of reduced incidence (RR 0.78 (95% CI: 0.59, 1.03)), which was statistically significant for lung cancer mortality (RR 0.70 (95% CI: 0.50, 0.99)) and was stronger for small-cell than non-small-cell lung cancers (NSCLC) [35]. A protective effect on NSCLC in Caucasian and African American women was found in Detroit [36], and with Rothwell's pooled analysis that found an effect on overall cancer mortality in women [37].
The pooling of data from 8 studies offered several strengths. Firstly, with over 2,000 cases in each sex, we had increased power to detect associations than in any single study (we had 92% power to detect an odds ratio of 0.8, with 35% NSAID use). Observing a consistent association across studies and countries provides additional robustness to the findings. Our results benefit from being based predominantly in community settings, in which NSAID use is at levels taken by the general population, rather than in trial settings where participants may be more motivated to participate and adhere to allocated intervention arms. Where hospital controls were included, this group only provided an appropriate comparison group if NSAID use in this sample was representative of that in the general population, an assumption that may not hold and would falsely bias results towards an apparent protective effect of NSAIDs if there were an overrepresentation of controls with conditions for which NSAIDs are prescribed. We excluded hospital controls admitted due to conditions for which NSAIDs are prescribed in an attempt to obtain a control group whose prior NSAID use was more representative of the general population. As users of NSAIDs often differ from non-users in other risk factors for lung cancer, the ability to control for them is crucial in observational studies. With detailed smoking data, we were able to do this for smoking, a negative confounder. In adjusting for smoking as a confounder, because initiation of smoking was prior to NSAID use (90% of smokers had started smoking by age 25 whereas mean age at first NSAID use was 51 years (where data were available), it was considered as a confounder (smokers more likely to later take NSAIDs)). However, for subjects who changed their smoking status from current to former, it is possible that this occurred as a result of NSAID prescription (both part of healthy behaviour advice) and thus that quitting smoking (at an average age of 53 years) would be a downstream variable and should not be controlled for. Potential weaknesses of our analyses include possible differential recall between cases and controls, but whether cases have a greater or lesser tendency to report NSAID use is unclear, and if this tendency was greater, it would have resulted in an underestimation of the effect. Although exposure status was based on recalled self-reports, we think it is unlikely to have been measured with more error in women, so this would not account for sex differences. Further, prevalence of ever-NSAID use was consistent with NHANES estimates during the periods when the studies were conducted with the exception of higher rates in the MSKCC and Boston. Details of NSAID use varied across studies. In particular, we have not been able to conduct a thorough investigation of the effects of age at use, dosage, or time since last use.
If NSAIDs are protective against lung cancer, the preventive potential is attractive, especially for aspirin given its protective effect on several other common chronic diseases including colon cancer and cardiovascular disease [38, 39]. Smokers who take aspirin would have a large absolute benefit for these combined endpoints, but amongst this group, the lung cancer risk reduction associated with aspirin is small in comparison to that that could be attained by quitting smoking [40]. Further, the adverse side effects of possible gastrointestinal bleeding or aspirin-induced development of asthma cannot be overlooked.
Our results are consistent with a protective effect of NSAIDs, most of which were aspirin, on lung cancer in men. Confirmation of the association of aspirin and NSA-IDs on lung cancer is still needed, and RCTs specifically designed with cancer as the endpoint and conducted within the general population are needed. These would clarify outstanding questions as recently outlined in a review by Cuzick et al. including effects by lung cancer histology, potential effect modification by sex and by smoking status, issues of safe doses, age at use, duration of use and other potential modifying factors [41].
Supplementary Material
Acknowledgments
The ILCCO data management is supported by Canadian Cancer Society (CCSRI no. 020214) and Cancer Care Ontario Research Chair Award. Individual ILCCO studies were funded or supported by various institutions and organisations. NEL-CS: Grant Number P20RR018787 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); MSKCC: Steps for Breath and the Labrecque Foundation, the Society of Memorial Sloan-Kettering Cancer Center. The authors would like to thank the following for their contribution with data management: Urvi Mujumdar and Radhai Rastogi (MSKCC) and Katja Boll (DDCHS).
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s10552-011-9847-z) contains supplementary material, which is available to authorized users.
Contributor Information
Valerie A. McCormack, Email: mccormackv@iarc.fr, Section of Environment and Radiation, International Agency for Research on Cancer, 150 cours Albert Thomas, 69008 Lyon, France.
Rayjean J. Hung, Samuel Lunenfeld Research Institute of Mount Sinai Hospital, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Darren R. Brenner, Samuel Lunenfeld Research Institute of Mount Sinai Hospital, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Heike Bickeboller, Department of Genetic Epidemiology Medical School, Georg-August-University of Gottingen, Gottingen, Germany.
Albert Rosenberger, Department of Genetic Epidemiology Medical School, Georg-August-University of Gottingen, Gottingen, Germany.
Joshua E. Muscat, Pennsylvania State Cancer Institute, Pennsylvania State College of Medicine, Hersey, PA, USA
Philip Lazarus, Pennsylvania State Cancer Institute, Pennsylvania State College of Medicine, Hersey, PA, USA.
Anne Tjønneland, Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark.
Søren Friis, Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark.
David C Christiani, Harvard School of Public Health, Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA.
Eun-mi Chun, Harvard School of Public Health, Massachusetts General Hospital/Harvard Medical School, Boston, MA, USA.
Loic Le Marchand, Cancer Research Center of Hawaii, University of Hawaii, Honolulu, HI, USA.
Gad Rennert, Department of Community Medicine and Epidemiology, Carmel Medical Center and Bruce Rappaport Faculty of Medicine, Israel Institute of Technology and Clalit Health Services National Cancer Control Center, Haifa, Israel.
Hedy S. Rennert, Department of Community Medicine and Epidemiology, Carmel Medical Center and Bruce Rappaport Faculty of Medicine, Israel Institute of Technology and Clalit Health Services National Cancer Control Center, Haifa, Israel
Angeline S. Andrew, Norris Cotton Cancer Center, Department of Community & Family Medicine, Dartmouth Medical School, Lebanon, NH, USA
Irene Orlow, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Bernard Park, Hackensack University Medical Center, Hackensack, NJ, USA.
Paolo Boffetta, Tisch Cancer Institute and Institute for Translational Epidemiology, Mount Sinai School of Medicine, New York, NY, USA; International Prevention Research Institute, Lyon, France.
Eric J. Duell, Unit of Nutrition, Environment and Cancer, Cancer Epidemiology Research Program, Catalan Institute of Oncology (ICO), Bellvitge Biomedical Research Institute (IDIBELL), Barcelona, Spain
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Na-kamura S, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58(17):3761–3764. [PubMed] [Google Scholar]
- 4.Sandler AB, Dubinett SM. COX-2 inhibition and lung cancer. Semin Oncol. 2004;31(2 Suppl 7):45–52. doi: 10.1053/j.seminoncol.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 7.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 9.Khuder SA, Herial NA, Mutgi AB, Federman DJ. Non-steroidal antiinflammatory drug use and lung cancer: a meta-analysis. Chest. 2005;127(3):748–754. doi: 10.1378/chest.127.3.748. [DOI] [PubMed] [Google Scholar]
- 10.Kelly JP, Coogan P, Strom BL, Rosenberg L. Lung cancer and regular use of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs 25. Pharmacoepidemiol Drug Saf. 2008;17(4):322–327. doi: 10.1002/pds.1532. [DOI] [PubMed] [Google Scholar]
- 11.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2008;17(1):148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slatore CG, Au DH, Littman AJ, Satia JA, White E. Association of nonsteroidal anti-inflammatory drugs with lung cancer: results from a large cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1203–1207. doi: 10.1158/1055-9965.EPI-08-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the women's health study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Diaz S, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of lung cancer. Int J Cancer. 2007;120(7):1565–1572. doi: 10.1002/ijc.22514. [DOI] [PubMed] [Google Scholar]
- 15.Feskanich D, Bain C, Chan AT, Pandeya N, Speizer FE, Colditz GA. Aspirin and lung cancer risk in a cohort study of women: dosage duration and latency. Br J Cancer. 2007;97(9):1295–1299. doi: 10.1038/sj.bjc.6603996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung RJ, Christiani DC, Risch A, Popanda O, Haugen A, Zienolddiny S, et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3081–3089. doi: 10.1158/1055-9965.EPI-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muscat JE, Chen SQ, Richie JP, Jr, Altorki NK, Citron M, Olson S, et al. Risk of lung carcinoma among users of nonsteroidal antiinflammatory drugs. Cancer. 2003;97(7):1732–1736. doi: 10.1002/cncr.11242. [DOI] [PubMed] [Google Scholar]
- 18.Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect. 2009;117(11):1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjonneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, et al. Study design, exposure variables, and socioeconomic determinants of participation in Diet, cancer and health: a population-based prospective cohort study of 57, 053 men and women in Denmark. Scand J Public Health. 2007;35(4):432–441. doi: 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- 20.Le Marchand L, Sivaraman L, Pierce L, Seifried A, Lum A, Wilkens LR, et al. Associations of CYP1A1, GSTM1, and CYP2E1 polymorphisms with lung cancer suggest cell type specificities to tobacco carcinogens. Cancer Res. 1998;58(21):4858–4863. [PubMed] [Google Scholar]
- 21.Gallagher CJ, Muscat JE, Hicks AN, Zheng Y, Dyer AM, Chase GA, et al. The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(4):823–828. doi: 10.1158/1055-9965.EPI-06-0823. [DOI] [PubMed] [Google Scholar]
- 22.Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Chris-tiani DC. Dietary intake of Cruciferous vegetables, Glu-tathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control. 2004;15(10):977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- 23.Babu KS, Salvi SS. Aspirin and asthma. Chest. 2000;118(5):1470–1476. doi: 10.1378/chest.118.5.1470. [DOI] [PubMed] [Google Scholar]
- 24.Peto R, Gray R, Collins R, Wheatley K, Hennekens C, Jamrozik K, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988;296(6618):313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Diaz S, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of lung cancer. Int J Cancer. 2007;120(7):1565–1572. doi: 10.1002/ijc.22514. [DOI] [PubMed] [Google Scholar]
- 27.Slatore CG, Au DH, Littman AJ, Satia JA, White E. Association of nonsteroidal anti-inflammatory drugs with lung cancer: results from a large cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1203–1207. doi: 10.1158/1055-9965.EPI-08-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58(17):3761–3764. [PubMed] [Google Scholar]
- 29.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58(22):4997–5001. [PubMed] [Google Scholar]
- 30.Haga Y, Hiroshima K, Iyoda A, Shibuya K, Shimamura F, Iizasa T, et al. Ki-67 expression and prognosis for smokers with resected stage I non-small cell lung cancer. Ann Thorac Surg. 2003;75(6):1727–1732. doi: 10.1016/s0003-4975(03)00119-x. [DOI] [PubMed] [Google Scholar]
- 31.Kim ES, Hong WK, Lee JJ, Mao L, Morice RC, Liu DD, et al. Biological activity of celecoxib in the bronchial epithelium of current and former smokers. Cancer Prev Res (Phila) 2010;3(2):148–159. doi: 10.1158/1940-6207.CAPR-09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol. 2009;36(6):524–531. doi: 10.1053/j.seminoncol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slatore CG, Au DH, Littman AJ, Satia JA, White E. Association of nonsteroidal anti-inflammatory drugs with lung cancer: results from a large cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1203–1207. doi: 10.1158/1055-9965.EPI-08-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feskanich D, Bain C, Chan AT, Pandeya N, Speizer FE, Colditz GA. Aspirin and lung cancer risk in a cohort study of women: dosage, duration and latency 1. Br J Cancer. 2007;97(9):1295–1299. doi: 10.1038/sj.bjc.6603996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the women's health study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 36.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2008;17(1):148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 38.Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med. 2002;136(2):157–160. doi: 10.7326/0003-4819-136-2-200201150-00015. [DOI] [PubMed] [Google Scholar]
- 39.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 40.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.