Abstract
It has long been known that bladder cancer (BC) incidence is approximately 4-fold higher in men than in women in the US, and a similar disparity also exists in other countries. The reason for this phenomenon is not known, which impedes progress in BC prevention. However, BC incidence is also significantly higher in male animals than in their female counterparts after treatment with aromatic amines, which are principal human bladder carcinogens. These animal studies and related studies in the context of available human data provide significant insight into what may drive the excessive BC risk in men, which is the focus of this article. The carcinogenicity and biotransformation of bladder carcinogens as well as the impact of sex hormones on these processes are discussed, highlighting the novel concept that the gender disparity in BC risk may result primarily from the interplay of androgen, estrogen and liver, with the liver functioning via its metabolic enzymes as the main decider of bladder exposure to carcinogens in the urine and the male and female hormones exerting opposing effects on carcinogenesis in the bladder and likely also on liver enzymes handling bladder carcinogens. The findings may facilitate further investigation into the mechanism of gender disparity in BC risk and may also have important implications for BC prevention.
Keywords: Androgen, aromatic amines, bladder carcinogenesis, carcinogen metabolism, estrogen
1. INTRODUCTION
Bladder cancer (BC) is the 4th most common cancer in US men, with 54,610 new cases to be expected in 2013 alone [1]. When calculated as health-care cost per patient (from diagnosis to death), BC ranked as the most expensive of all cancers in the US [2]. It has also long been known that BC incidence is approximately 4 times higher in men than in women in the US [3–5]. Globally, despite a 15-fold variation in men and 32-fold variation in women in age-standardized BC incidence among different countries, the gender disparity persists, with men being 2.6–6.7 times more likely and in Micro/Polynesia 20.5 times more likely to develop BC than do women, except in Eastern Africa where BC incidence is only 1.2 fold higher in men than in women [6]. While it is well recognized that tobacco smoking is the most important cause of BC, gender disparity in BC risk exists in both smokers and non-smokers. For example, in a US study of 283,394 men and 186,134 women (the NIH-AARP Diet and Health Study) followed over 11 years (1995 – 2006), the overall age-standardized BC risk in men was 3.7 times higher than in women, and the age-standardized BC risk in never-smokers was 4.3 times higher in men than in women [4]. Thus, the gender disparity in BC risk is apparently not due to a difference in tobacco smoking. Aromatic amines are widely recognized to be mainly responsible for BC induction by tobacco smoking [7–9], and non-smokers may also be exposed to these carcinogens through environmental and occupational exposure [7, 8, 10]. In line with the gender disparity in BC risk, the mortality of this disease in the US is also more than 3 fold higher in men than in women [11].
The molecular basis for the gender disparity in BC risk in human is not known, and the lack of such knowledge impedes progress in BC prevention. Nevertheless, animal studies with aromatic amines recapitulated the human phenomenon. Findings from these and related studies are discussed in the present article together with available human data, which provide important insight into the biological and molecular underpinning for the excessive BC risk in men. The studies cited in the present article were published over the past several decades and were identified by PubMed search and searching the reference lists of published papers. Arsenic in drinking water is also linked to increased BC risk [12], but it appears that BC incidence induced by arsenic may be somewhat higher in the female than in the male [13, 14]. These studies are not discussed in the present article.
2. THE INVERSE RELATIONSHIP OF CARCINOGENESIS IN THE BLADDER AND LIVER INDUCED BY AROMATIC AMINES
4-Aminobiphenyl (ABP, see Figure 1 for chemical structure) is a major human bladder carcinogen from tobacco smoke and other sources [7, 15, 16]. Indeed, levels of ABP-DNA adducts were up to 8-fold higher in bladder specimens or exfoliated urothelial cells in smokers than in non-smokers [17, 18]. In BALB/c mice fed with ABP (50–75 ppm in drinking water for 96 weeks), started at 4 weeks of age, BC developed in 20% of the male mice but in none of the female mice, whereas liver cancer developed in 33% of the female mice but in none of the male mice [19]. Approximately 80% of ABP-DNA adducts formed in human bladder cells and tissues are N-(deoxyguanosin-8-yl)-4-aminobiphenyl (dG-C8-ABP) [17, 20]. The dG-C8-ABP levels formed in the bladders and livers of mice exposed to ABP followed the same pattern of cancer formation in these organs. For example, in adult BALB/c mice fed with ABP at 55–75 ppm in drinking water for 4 weeks, dG-C8-ABP levels were 2–3 fold higher in the male bladders than in the female bladders, whereas levels of this adduct were 2–3 fold higher in the female livers than in the male livers [21]. Similar patterns of inverse association of ABP-DNA adduct levels in the bladder and liver were also seen in adult male and female C57BL/6 mice treated with ABP, irrespective of whether the carcinogen was applied topically or given in drinking water [22–24]. In adult C57 x IF F1 hybrid mice treated with another aromatic amine, 2-aminodiphenylene oxide (see Figure 1 for chemical structure), liver cancer incidence was also significantly higher in the female mice than in the male mice, whereas BC incidence was significantly higher in the male mice than in the female mice [25]. Interestingly, when ABP was administered to neonatal mice (either C57BL/6 or mixed background of C57BL/6 and 129/Sv strains) on day 8 and day 15 after birth by peritoneal injection and the mice were then followed without ABP for 12 months in one study [26] and 16 months in another study [27], the incidence of liver cancer in the male and female mice were 60–70% and 0–5% respectively, while BC development in these mice is unknown. The dramatically higher liver cancer incidence in the male mice than in the female mice stands in stark contrast to the markedly higher liver cancer incidence in female mice than in male mice when ABP treatment was initiated at 4 weeks of age as described above. The molecular basis for the dramatic shift of gender disparity in liver cancer development in relation to ABP dosing time is not known but may be related to a change in the expression of certain ABP-metabolizing enzymes. Studies with 2-acetamidofluorene (AAF, see Figure 1 for chemical structure), which is a carcinogenic metabolite of 2-aminofluorene, also an aromatic amine, seem to lend support to that belief. Chronic feeding of adult male F344 rats with AAF in the diet (200 ppm) caused liver cancer in 100% rats but no BC, whereas in rats fed concurrently with AAF and butylated hydroxytoluene (BHT, 300–6000 ppm), liver cancer incidence was lowered dose-dependently by BHT down to 56%, but BC incidence was elevated by BHT dose-dependently up to 44%, while BHT alone caused neither liver cancer nor BC [28]. Similar impact of BHT on liver and bladder carcinogenesis was shown in male F344 rats when dietary AAF was lowered to 50 ppm [29]. BHT treatment was also shown to reduce AAF-DNA adduct formation in the liver and to increase urinary excretion of glucuronic acid conjugates of AAF in rats exposed to AAF [30], suggesting that BHT may promote liver metabolism of AAF via UDP-glucuronosyltransferase (UGT). Indeed, BHT is known to modulate drug-metabolizing enzymes including UGT in the liver [31]. Glucuronic acid conjugates of aromatic amines and their metabolites are generally less toxic than their parent compounds, but are often unstable in urine and readily generate carcinogenic species, as discussed below. Thus, the inverse relationship of susceptibility to aromatic amines between bladder and liver may be linked to how liver metabolically handles these compounds.
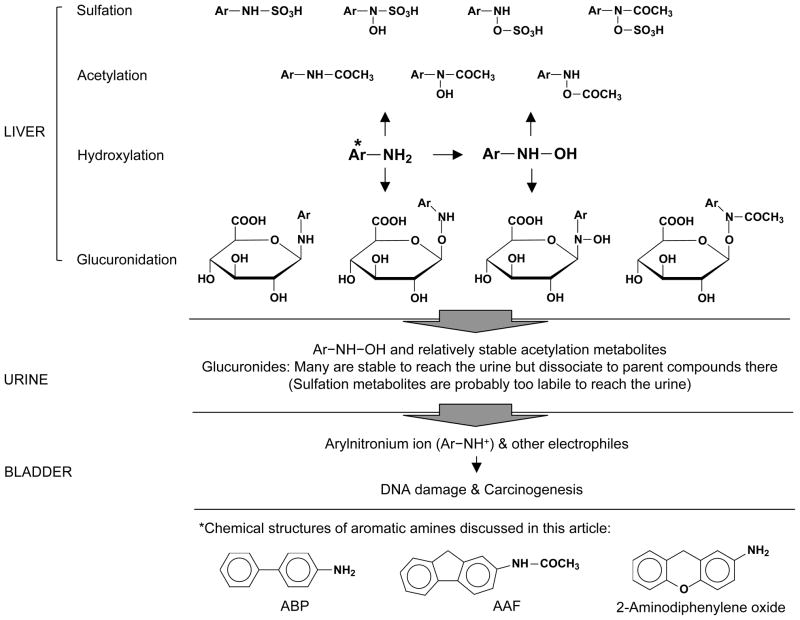
Figure 1.
Biotransformation of aromatic amines in the liver and delivery of carcinogenic metabolites to the bladder via urinary excretion. Metabolites formed in the liver may vary with different aromatic amines, and the metabolites shown herein may be incomplete. Some of the metabolites generated in the liver are known to be too unstable to reach the bladder. Arylnitronium ion is highly reactive and may be generated from N-hydroxy aromatic amines or other metabolites.
3. BIOTRANSFORMATION OF AROMATIC AMINES IN VIVO
Aromatic amines are not carcinogenic themselves but undergo extensive and complex metabolic activation and inactivation in vivo. While the details of their metabolisms are not fully known, liver is apparently the main site for the metabolism of these compounds.
3.1. Hydroxylation
Both N-hydroxylation (Figure 1) and ring-hydroxylation can occur to an aromatic amine as initial reactions, but only the former is considered a key activation step [32–34]. Cytochrome P450 (CYP) 1A2 was widely believed to be mainly responsible for N-hydroxylation of aromatic amines [35], but knockout of this enzyme in C57BL/6 mice did not show a significant impact on ABP-induced DNA adduct formation in both liver and bladder, nor did it alter the gender disparity in ABP-DNA adduct levels in these organs [23]. Thus, at least in mice, CYP1A2 is dispensable for metabolic activation of ABP, and there likely is a redundancy in enzymes for N-hydroxylation. For example, human CYP2A13 has also been shown to catalyze N-hydroxylation of ABP [36]. N-hydroxylation derivatives of aromatic amines are detected in the urine of animals exposed to aromatic amines [32], but it is not known whether these compounds are directly excreted or regenerated in the urine from unstable metabolites, nor is it known whether there is a difference between male and female animals in overall liver activity for N-hydroxylation of aromatic amines or in urinary levels of their N-hydroxylation products. Interestingly, CYP4B1which also activates aromatic amines was shown to be expressed at higher levels in the male bladder than in the female bladder in rats and that its expression in the rat bladder was stimulated by androgen [37]. This shows that bladder may be involved in the activation of aromatic amine and also suggests that similar enzymes in the liver may be regulated by sex hormones. Competing with N-hydroxylation in the liver are N- or N-O-acetylation by arylamine acetyltransferases (NATs) [38, 39] and N- or N-O-glucuronidation by UGTs [40, 41], and it is also known that the products of one of these reactions may serve as substrates for the other reactions (Figure 1). Moreover, sulfotransferases (SULTs) may catalyze N-, O- or N-O-sulfation of aromatic amines [42], hydroxylamines and the products of NATs-catalyzed reactions (hydroxamic acids) (Fig. 1) [43, 44]. These enzymes are discussed below in further detail.
3.2. Acetylation
Two NATs, NAT1 and NAT2, are expressed in human and animal cells, which show overlapping substrate specificity and participate in the metabolism of aromatic amines [45]. N-acetylation of aromatic amines by these enzymes generally is a detoxification reaction, but the enzymes also function as N, O-acyltransferase (transferring the acyl group from the nitrogen to the oxygen of N-hydroxy aromatic amines and their derivatives) (Figure 1), giving rise to potentially highly reactive electrophiles [46]. Human studies have shown that NAT2 slow acetylation status is associated with increased BC risk [47, 48]. However, in C57BL/6 mice or other mice, N-acetylation levels of ABP and AAF in the liver, bladder or other organs were not different between males and females, and in Nat1/2 gene knockout mice, whose tissue NATactivity was totally abolished, the gender disparity in dG-C8-ABP levels in the liver remained unchanged after ABP treatment, even though liver dG-C8-ABP levels were somewhat lower in the wild type mice than in the Nat1/2 knockout mice [26, 49]. Information on the effect of Nat1/2 gene knockout on bladder susceptibility to ABP or other aromatic amines is not available. However, in congenic mouse strains (C57BL/6 and B6.A) of rapid and slow acetylators (NAT2), after ABP treatment (28 days in drinking water), dG-C8-ABP levels were higher in the female livers than in the male livers, and higher in the male bladders than in the female bladders, as expected, and the acetylation status did not show a clear impact on dG-C8-ABP levels in both organs [24]. Collectively, the above studies indicate that NAT1/2 do not mediate the gender disparity in bladder/liver susceptibility to aromatic amines in mice.
3.3. Glucuronidation
UGTs catalyze the addition of glucuronic acid to the nitrogen or oxygen of aromatic amines and their metabolites, giving rise to N- and O-glucuronides (Figure 1) [34, 39, 40, 50]. The glucuronides may be relatively non-reactive and are excreted in the bile and urine. Urinary excretion of these compounds, however, is problematic for the bladder, as the conjugates, with the exception of the O-glucuronides of the ring hydroxylation products of aromatic amines [41], are highly labile in urine, especially in acidic urine [40, 41, 51, 52], delivering carcinogenic compounds to the bladder epithelium. The glucuronides may also be hydrolyzed by urinary β-glucuronidase [53–55], but studies have indicated that urinary β-glucuronidase may not contribute significantly to the instability of these glucuronides [54] or to bladder carcinogenesis [56]. UGTs are a large family of enzymes responsible for glucuronidation of a wide variety of substrates [57, 58]. Several human UGTs, including UGT1A1, 1A3, 1A4, 1A6, 1A9, 2B1, 2B2 and 2B7, have been shown to catalyze glucuronidation of one or more aromatic amines and their metabolites [59–61]. Liver mRNA level of UGT2B1 was found to be higher in adult male C57BL/6 mice than in their female counterparts, but liver mRNA level of UGT1A1 was higher in the female mice than in the male mice [62]. It was also reported that overall ABP N-glucuronidation activity was significantly higher in liver microsomes prepared from adult male C57BL/6 mice than from their female counterparts [22]. These results raise the possibility that certain UGTs in the liver may mediate in part the gender disparity in bladder/liver susceptibility to aromatic amines. Conceivably, higher UGT activity in the liver would protect the liver against aromatic amines but would render the bladder more susceptible to these compounds by generating more urinary glucuronide metabolites that are unstable. Interestingly, all UGT1As in human bladder epithelial cells and mouse bladder tissues were shown to be down regulated by androgen-mediated androgen receptor (AR) signaling [63], suggesting that androgen may sensitize bladder to carcinogens in part by suppressing UGT-mediated detoxification in the bladder. It is not known, however, whether any aromatic amine-metabolizing UGTs in the liver are modulated in a similar manner.
3.4. Sulfation
SULTs catalyze the sulfation of aromatic amines, and of hydroxylamines and hydroxamic acids formed from aromatic amines (Figure 1). Several human SULTs, including SULT1A1, 1A2, 1A3, 1B2 and 1C#2, have been shown to be active toward N-OH-ABP [64]. The sulfuric acid esters formed via the amine nitrogen or the oxygen attached to the amine nitrogen are highly unstable and rapidly react with DNA and other macromolecules. Indeed, it was previously shown that liver SULT activity correlates with liver toxicity and liver cancer development in rats treated by N-OH-AAF [65–67]. While significant SULT activity does not appear to exist in the bladder [68, 69], and the sulfuric acid esters of aromatic amines generated in the liver are thought to be too labile to survive the trip to the bladder [68], liver SULTs may alter the bioavailability of aromatic amines and their metabolites to the bladder by competing with other liver enzymes for substrates. Studies have shown that liver SULT activity toward N-OH-AAF and other aromatic amines is significantly higher in male rats than in female rats [44, 65], and in rats treated with N-OH-AAF, urinary levels of N-OH-AAF and glucuronides are significantly higher in the female rats, and urinary levels of sulfuric acid esters (formed from the ring hydroxylation metabolites, which are relatively stable) are significantly higher in the male rats [34]. A greater susceptibility of male rats than female rats to AAF-induced development of liver cancer and cirrhosis has also been reported [70], although sex-related bladder susceptibility to AAF-induced carcinogenesis in rats is not known. Interestingly, contrary to the rat results described above, in mice, as mentioned before, female liver is more susceptible than male liver and male bladder is more susceptible than female bladder to the genotoxicity and carcinogenicity of ABP and other aromatic amines. Whether this means that liver SULT activity toward aromatic amines in female mice is higher than in male mice is not clear. However, among the large number of SULTs that are expressed in mice, liver mRNA levels of SULT1A1, 1D1, 2A1/2 and 3A1, as well as PAPSs2 which synthesizes the enzyme cofactor 3′-phosphoadenosine 5′-phosphosulfate, are significantly higher in female C57BL/6 mice than in their male counterparts [71]. Collectively, the findings discussed above suggest that the expression of SULTs in the liver may be species- and gender-related, and also support the notion that certain SULTs in the liver may protect the bladder against aromatic amines at the cost of liver damage by generating metabolites that are highly electrophilic and rapidly consumed by nucleophiles (e.g. DNA, RNA and protein) in the liver, thus diverting the carcinogens away from entering the urine and from damaging the bladder. If certain liver SULTs play a part in the gender disparity in BC risk in human, aromatic amines may be more toxic to female liver than to male liver. While this seems to contradict the finding that liver cancer incidence in human is significantly higher in men than in women [3, 6, 72], the main risk factors of human liver cancer, including chronic infection with hepatitis viruses, cirrhosis and exposure to aflatoxins, may mask the potential effects of aromatic amines.
4. BLADDER CARCINOGENICITY AND METABOLISM OF N-BUTYL-N-(4-HYDROXYBUTYL)NITROSAMINE
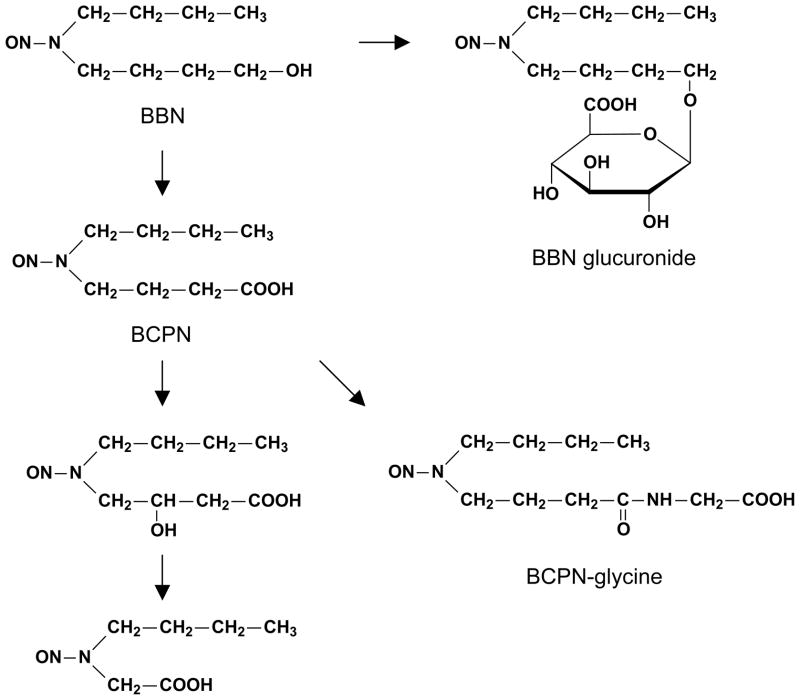
N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN, see Figure 2 for chemical structure) is a widely used experimental bladder carcinogen, albeit not a human carcinogen. BBN also causes higher incidence of BC in male animals than in their female counterparts. For example, when Wistar rats were treated with BBN in drinking water (0.05%) for 6 weeks and then observed for 18 weeks without BBN, BC incidence in the male rats was 3.2 times higher than in the female rats [73]. In C57BL/6 mice that were treated continuously with BBN (0.022% in in drinking water), male mice developed BC on average 63 days earlier than did female mice (average disease onsettime of 190 and 253 days respectively) [74]. Unlike aromatic amines, however, BBN has not been shown to cause liver cancer in animal studies. Also unlike the complex biotransformation undertaken by aromatic amines and the unstable nature of various metabolites as described above, BBN metabolism in vivo, while extensive, is relatively simple and the metabolites are relatively stable. BBN is converted primarily to N-butyl-N-(3-carboxypropyl)nitrosamine (BCPN), as a result of the oxidation of its alcoholic group to a carboxylic group by the alcohol/aldehyde dehydrogenase system, but is also O-glucuronided by UGT as a minor metabolite (Figure 2) [75, 76]. The specific enzymes for these reactions are not known. As with aromatic amines, however, BBN biotransformation also occurs primarily in the liver, and the metabolites are excreted in urine [77, 78], although bladder cells also seem to have some capacity of converting BBN to BCPN [77]. In Wistar rats given a single oral dose of BBN, more than 40% of the dose was recovered as BCPN in 48-h urine, with a minor presence of BBN glucuronide and no presence of unchanged BBN in the urine [79]. Similar urinary BCPN recovery results were obtained in other strains of rats dosed with BBN [78]. BCPN may undergo further metabolism in the liver and the target tissues (Figure 2) [75, 80, 81], and in mice, a significant amount of BCPN is converted to a glycine derivative (BCPN-glycine, formed via the carboxyl group of BCPN) (Figure 2). Thus, in ICR mice given a single oral dose of BBN, 6% and 26% of the dose were recovered as BCPN and BCPN-glycine respectively in 48-h urine [81]. While the carcinogenicity of BCPN-glycine has not been investigated, and urinary BBN glucuronide may be too stable to be significantly toxic to the bladder, BCPN is directly genotoxic [82], and urinary BCPN is widely believed to be mainly responsible for BBN-induced bladder carcinogenesis [83]. There is also a good correlation between urinary levels of BCPN (in the case of mice, BCPN plus BCPN-glycine) and species-related variation in susceptibility to BBN-induced bladder carcinogenesis among rats, mice, hamsters and guinea pigs [78, 81, 84]. However, it is not known to what extent the gender disparity in BBN-induced bladder carcinogenesis is related to urinary levels of BCPN or other metabolites.
Figure 2.
BBN metabolism in the liver and urinary excretion of its metabolites. The specific enzymes catalyzing these reactions remain undefined.
5. THE EFFECTS OF SEX HORMONES ON BLADDER CARCINOGENESIS
Information on the effects of male and female hormones on BC development is limited and came almost exclusively from studies using BBN as a carcinogen. As mentioned above, in C57BL/6 mice, under an identical dosing condition, male mice developed BC on average 63 days earlier than did female mice, but the sex difference in BC development could be abolished by castrating the male mice, which was performed before carcinogen treatment, or treating the female mice with testosterone, which was administered during the carcinogen treatment period [74]. Also in C57BL/6 mice, AR knockout completely blocked BBN-induced BC development in both male and female mice, while BC incidence in the BBN-treated wild-type mice was 92% (male) and 42% (female) respectively, and in the male mice, AR knockout was more effective than castration; in the latter case, 50% mice developed BC [85]. These findings show that the androgen-AR signaling pathway is critical for BBN-induced BC development. Interestingly, in male Wistar rats which were treated with BBN in drinking water for 6 weeks and then observed for 18 weeks without BBN, castration performed immediately after the 6-week BBN treatment was more inhibitory (36.4% BC incidence) than performed before BBN treatment (50% BC incidence), compared to 60% BC incidence in the control group [73]. Thus, the promoting effect of the androgen-AR signaling pathway on bladder carcinogenesis occurs during the post-BBN progression phase. The above result also hints that androgen may actually inhibit the initiation of BBN-induced bladder carcinogenesis. Indeed, in female Wistar rats, under the same experiment conditions as described above for the male rats, the promoting effect of testosterone (50 mg/rat as a slow-releasing subcutaneous implant) on bladder carcinogenesis was less when given before BBN treatment (36.4% BC incidence) than when given after completion of BBN treatment (54.4% BC incidence), with 18.2% cancer incidence in the control group [73]. In contrast, treatment of male Wistar rats with diethylstilbestrol (50 mg/rats as a slow-releasing implant) either before or after BBN treatment resulted in strong and similar inhibition of BC development (7.1% vs. 5.9%, compared to 60% in the control group) [73], suggesting that the estrogen inhibits bladder carcinogenesis in the post-BBN progression phase. Likewise, spaying female Wistar rats before or after BBN treatment led to similar increase in BC incidence (30% vs. 25%, compared to 18.2% in the control group) [73]. It should also be noted that in the above-described study, in the absence of BBN, no BC developed in rats treated by testosterone, diethylstilbestrol, castration, spay or any combinations. Significant inhibitory effect on bladder carcinogenesis by another estrogen, ethinyl estradiol, given after BBN treatment, was also shown in male F344 rats [86]. Studies have shown that both AR and estrogen receptor are expressed in the bladder epithelium [87–89].
Several population-based studies have been published regarding the inhibitory effect of estrogen on BC development in human. In the US Nurses’ Health Study with 26 years of follow-up, McGrath et al. found that in postmenopausal women, early age at menopause was significantly associated with increased BC risk, but an inverse association of combined use of estrogen and progestin with BC risk was not statistically significant [90]. However, in the Los Angeles-Shanghai BC Study and the California Teachers Study, Davis-Dao et al. found that, in addition to 30% lower BC risk in parous women than in nulliparous women, combined use of estrogen and progestin as menopausal hormone therapy was protective against BC [91]. Yet, in the Breast Cancer Detection Demonstration Projects Follow-Up Study, Cantwell et al. did not find an association between BC risk and parity, age at menarche, age at first birth or oral contraceptive use, but did find an increased BC risk in women with early menopause, although this was not statistically significant [92]. Collectively, the above studies suggest an inhibitory effect of estrogen on BC development in human, and it is possible that some of the statistically insignificant associations noted above were due to limited sample size.
6. Conclusions
Liver plays an important role in BC development, as bladder carcinogens are primarily metabolized in the liver and carcinogenic metabolites are delivered to bladder through urinary excretion. Urinary delivery of carcinogens to bladder is consistent with bladder cancer originating almost exclusively from the inner surface (urothelium) of the bladder. Animal studies with bladder carcinogens recapitulate the gender disparity in BC risk seen in humans, and these studies have also shown a gender-related difference in liver metabolism of bladder carcinogens. Many lines of evidence strongly suggest that the gender-related difference in liver metabolism of bladder carcinogens translates into a difference in bladder exposure to the carcinogens, which in turn may function as one of the key drivers of the gender disparity in BC risk. There is evidence that certain isoforms of UGTs and/or SULTs in the liver may be the key mediators in this process. Furthers studies are needed to identify these enzymes and to understand their modulation by sex hormones. Moreover, it has also been demonstrated that androgen promotes and estrogen inhibits bladder carcinogenesis in the progression phase, while the underlining mechanisms remain to be elucidated. Taken together, the gender disparity in BC risk may resultprimarily from the interplay of androgen, estrogen and liver as illustrated in Figure 3.
Figure 3.
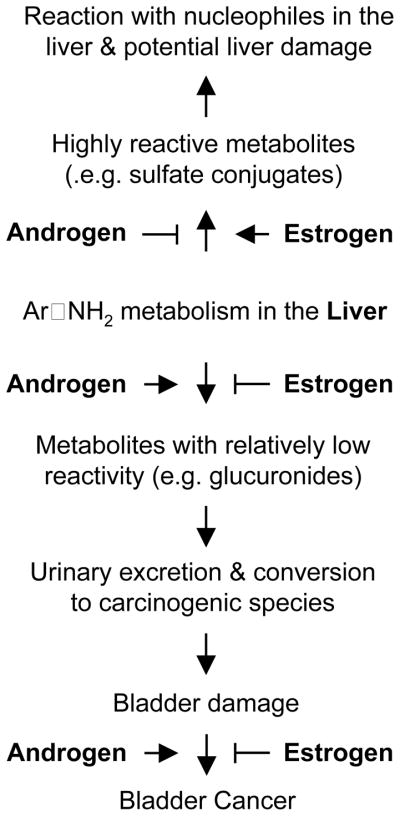
The potential interplay among androgen, estrogen and the liver, which may be mainly responsible for the gender disparity in BC risk. The illustration shows the potential opposing effects of androgen and estrogen on liver handling of aromatic amines and on carcinogenesis in the bladder.
Understanding the mechanism responsible for the gender disparity in BC risk has important implications for BC prevention. The findings described above suggest that approaches aimed at stimulating cytoprotection against carcinogens in the bladder epithelium may be highly effective for BC prevention. In this connection, we have recently shown that sulforaphane, a key chemopreventive ingredient in broccoli and other cruciferous vegetables [93], inhibits ABP-induced DNA damage in the bladder by activating Nrf2, a key transcriptional factor for stimulation of many cytoprotective and carcinogen-detoxifying genes, and that Nrf2 activation by sulforaphane occurs primarily in the epithelium, the principal site of BC development [94]. This result is also consistent with epidemiological studies showing a significant inverse association between the consumption of broccoli and other cruciferous vegetables and human BC risk [95, 96]. It is also conceivable that inhibitors of androgen-AR signaling or an estrogen may inhibit BC development in human. In fact, as mentioned before, epidemiological studies suggest the protective function of estrogen.
Acknowledgments
This work was supported in part by the National Institutes of Health (R01CA120533, R01CA124627, R01CA164574).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 3.Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartge P, Harvey EB, Linehan WM, Silverman DT, Sullivan JW, Hoover RN, Fraumeni JF., Jr Unexplained excess risk of bladder cancer in men. J Natl Cancer Inst. 1990;82:1636–1640. doi: 10.1093/jnci/82.20.1636. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Vineis P. Epidemiology of cancer from exposure to arylamines. Environ Health Perspect. 1994;102(Suppl 6):7–10. doi: 10.1289/ehp.94102s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riedel K, Scherer G, Engl J, Hagedorn HW, Tricker AR. Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers. J Anal Toxicol. 2006;30:187–195. doi: 10.1093/jat/30.3.187. [DOI] [PubMed] [Google Scholar]
- 9.Talaska G. Aromatic amines and human urinary bladder cancer: exposure sources and epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2003;21:29–43. doi: 10.1081/GNC-120021372. [DOI] [PubMed] [Google Scholar]
- 10.Skipper PL, Tannenbaum SR, Ross RK, Yu MC. Nonsmoking-related arylamine exposure and bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:503–507. [PubMed] [Google Scholar]
- 11.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo H-R, Tseng Y-C. Arsenic in drinking water and bladder cancer: comparison between studies based on cancer registry and death certificates. Environmental Geochemistry and Health. 2000;22:83–91. [Google Scholar]
- 13.Shen J, Wanibuchi H, Waalkes MP, Salim EI, Kinoshita A, Yoshida K, Endo G, Fukushima S. A comparative study of the sub-chronic toxic effects of three organic arsenical compounds on the urothelium in F344 rats; gender-based differences in response. Toxicol Appl Pharmacol. 2006;210:171–180. doi: 10.1016/j.taap.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CJ, Perfetti TA, Garg R, Hansch C. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol. 2003;41:807–817. doi: 10.1016/s0278-6915(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 16.Stabbert R, Schafer KH, Biefel C, Rustemeier K. Analysis of aromatic amines in cigarette smoke. Rapid Commun Mass Spectrom. 2003;17:2125–2132. doi: 10.1002/rcm.1161. [DOI] [PubMed] [Google Scholar]
- 17.Talaska G, al-Juburi AZ, Kadlubar FF. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc Natl Acad Sci U S A. 1991;88:5350–5354. doi: 10.1073/pnas.88.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talaska G, Schamer M, Skipper P, Tannenbaum S, Caporaso N, Unruh L, Kadlubar FF, Bartsch H, Malaveille C, Vineis P. Detection of carcinogen-DNA adducts in exfoliated urothelial cells of cigarette smokers: association with smoking, hemoglobin adducts, and urinary mutagenicity. Cancer Epidemiol Biomarkers Prev. 1991;1:61–66. [PubMed] [Google Scholar]
- 19.Schieferstein GJ, Littlefield NA, Gaylor DW, Sheldon WG, Burger GT. Carcinogenesis of 4-aminobiphenyl in BALB/cStCrlfC3Hf/Nctr mice. Eur J Cancer Clin Oncol. 1985;21:865–873. doi: 10.1016/0277-5379(85)90227-5. [DOI] [PubMed] [Google Scholar]
- 20.Beland FA, Beranek DT, Dooley KL, Heflich RH, Kadlubar FF. Arylamine-DNA adducts in vitro and in vivo: their role in bacterial mutagenesis and urinary bladder carcinogenesis. Environ Health Perspect. 1983;49:125–134. doi: 10.1289/ehp.8349125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirier MC, Beland FA. DNA adduct measurements and tumor incidence during chronic carcinogen exposure in rodents. Environ Health Perspect. 1994;102 (Suppl 6):161–165. doi: 10.1289/ehp.94102s6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Zoughool M, Succop P, Desai P, Vietas J, Talaska G. Effects of N-glucuronidation on urinary bladder genotoxicity of 4-aminobiphenyl in male and female mice. Environmental Toxicology and Pharmacology. 2006;22:153–159. doi: 10.1016/j.etap.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Tsuneoka Y, Dalton TP, Miller ML, Clay CD, Shertzer HG, Talaska G, Medvedovic M, Nebert DW. 4-aminobiphenyl-induced liver and urinary bladder DNA adduct formation in Cyp1a2(−/−) and Cyp1a2(+/+) mice. J Natl Cancer Inst. 2003;95:1227–1237. doi: 10.1093/jnci/djg025. [DOI] [PubMed] [Google Scholar]
- 24.Flammang TJ, Couch LH, Levy GN, Weber WW, Wise CK. DNA adduct levels in congenic rapid and slow acetylator mouse strains following chronic administration of 4-aminobiphenyl. Carcinogenesis. 1992;13:1887–1891. doi: 10.1093/carcin/13.10.1887. [DOI] [PubMed] [Google Scholar]
- 25.Clayson DB, Lawson TA, Pringle JA. The carcinogenic action of 2-aminodiphenylene oxide and 4-aminodiphenyl on the bladder and liver of the C57 X IF mouse. Br J Cancer. 1967;21:755–762. doi: 10.1038/bjc.1967.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugamori KS, Brenneman D, Sanchez O, Doll MA, Hein DW, Pierce WM, Jr, Grant DM. Reduced 4-aminobiphenyl-induced liver tumorigenicity but not DNA damage in arylamine N-acetyltransferase null mice. Cancer Lett. 2012;318:206–213. doi: 10.1016/j.canlet.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura S, Kawabe M, Ward JM, Morishima H, Kadlubar FF, Hammons GJ, Fernandez-Salguero P, Gonzalez FJ. CYP1A2 is not the primary enzyme responsible for 4-aminobiphenyl-induced hepatocarcinogenesis in mice. Carcinogenesis. 1999;20:1825–1830. doi: 10.1093/carcin/20.9.1825. [DOI] [PubMed] [Google Scholar]
- 28.Maeura Y, Weisburger JH, Williams GM. Dose-dependent reduction of N-2-fluorenylacetamide-induced liver cancer and enhancement of bladder cancer in rats by butylated hydroxytoluene. Cancer Res. 1984;44:1604–1610. [PubMed] [Google Scholar]
- 29.Williams GM, Tanaka T, Maruyama H, Maeura Y, Weisburger JH, Zang E. Modulation by butylated hydroxytoluene of liver and bladder carcinogenesis induced by chronic low-level exposure to 2-acetylaminofluorene. Cancer Res. 1991;51:6224–6230. [PubMed] [Google Scholar]
- 30.Grantham PH, Weisburger JH, Weisburger EK. Effect of the antioxidant butylated hydroxytoluene (BHT) on the metabolism of the carcinogens N-2-fluorenylacetamide and N-hydroxy-N-2-fluorenylacetamide. Food Cosmet Toxicol. 1973;11:209–217. doi: 10.1016/s0015-6264(73)80487-0. [DOI] [PubMed] [Google Scholar]
- 31.Cha YN, Heine HS. Comparative effects of dietary administration of 2(3)-tert-butyl-4-hydroxyanisole and 3,5-di-tert-butyl-4-hydroxytoluene on several hepatic enzyme activities in mice and rats. Cancer Res. 1982;42:2609–2615. [PubMed] [Google Scholar]
- 32.Radomski JL, Brill E. The role of N-oxidation products of aromatic amines in the induction of baldder cancer in the dog. Arch Toxikol. 1971;28:159–175. doi: 10.1007/BF00330245. [DOI] [PubMed] [Google Scholar]
- 33.McMahon RE, Turner JC, Whitaker GW. The N-hydroxylation and ring-hydroxylation of 4-aminobiphenyl in vitro by hepatic mono-oxygenases from rat, mouse, hamster, rabbit and guinea-pig. Xenobiotica. 1980;10:469–481. doi: 10.3109/00498258009033782. [DOI] [PubMed] [Google Scholar]
- 34.Weisburger EK, Grantham PH, Weisburger JH. Differences in the Metabolism of N-Hydroxy-N-2-Fluorenylacetamide in Male and Female Rats. Biochemistry. 1964;3:808–812. doi: 10.1021/bi00894a014. [DOI] [PubMed] [Google Scholar]
- 35.Hammons GJ, Milton D, Stepps K, Guengerich FP, Tukey RH, Kadlubar FF. Metabolism of carcinogenic heterocyclic and aromatic amines by recombinant human cytochrome P450 enzymes. Carcinogenesis. 1997;18:851–854. doi: 10.1093/carcin/18.4.851. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima M, Itoh M, Sakai H, Fukami T, Katoh M, Yamazaki H, Kadlubar FF, Imaoka S, Funae Y, Yokoi T. CYP2A13 expressed in human bladder metabolically activates 4-aminobiphenyl. Int J Cancer. 2006;119:2520–2526. doi: 10.1002/ijc.22136. [DOI] [PubMed] [Google Scholar]
- 37.Imaoka S, Yoneda Y, Sugimoto T, Ikemoto S, Hiroi T, Yamamoto K, Nakatani T, Funae Y. Androgen regulation of CYP4B1 responsible for mutagenic activation of bladder carcinogens in the rat bladder: detection of CYP4B1 mRNA by competitive reverse transcription-polymerase chain reaction. Cancer Lett. 2001;166:119–123. doi: 10.1016/s0304-3835(00)00572-3. [DOI] [PubMed] [Google Scholar]
- 38.Kirlin WG, Trinidad A, Yerokun T, Ogolla F, Ferguson RJ, Andrews AF, Brady PK, Hein DW. Polymorphic expression of acetyl coenzyme A-dependent arylamine N-acetyltransferase and acetyl coenzyme A-dependent O-acetyltransferase-mediated activation of N-hydroxyarylamines by human bladder cytosol. Cancer Res. 1989;49:2448–2454. [PubMed] [Google Scholar]
- 39.Miller EC. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978;38:1479–1496. [PubMed] [Google Scholar]
- 40.Babu SR, Lakshmi VM, Huang GP, Zenser TV, Davis BB. Glucuronide conjugates of 4-aminobiphenyl and its N-hydroxy metabolites. pH stability and synthesis by human and dog liver. Biochem Pharmacol. 1996;51:1679–1685. doi: 10.1016/0006-2952(96)00165-7. [DOI] [PubMed] [Google Scholar]
- 41.Ciotti M, Lakshmi VM, Basu N, Davis BB, Owens IS, Zenser TV. Glucuronidation of benzidine and its metabolites by cDNA-expressed human UDP-glucuronosyltransferases and pH stability of glucuronides. Carcinogenesis. 1999;20:1963–1969. doi: 10.1093/carcin/20.10.1963. [DOI] [PubMed] [Google Scholar]
- 42.Ramaswamy SG, Jakoby WB. Amine N-sulfotransferase. J Biol Chem. 1987;262:10039–10043. [PubMed] [Google Scholar]
- 43.Gilissen RA, Hume R, Meerman JH, Coughtrie MW. Sulphation of N-hydroxy-4-aminobiphenyl and N-hydroxy-4-acetylaminobiphenyl by human foetal and neonatal sulphotransferase. Biochem Pharmacol. 1994;48:837–840. doi: 10.1016/0006-2952(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 44.Gilissen RA, Ringer DP, Stavenuiter HJ, Mulder GJ, Meerman JH. Sulfation of hydroxylamines and hydroxamic acids in liver cytosol from male and female rats and purified aryl sulfotransferase IV. Carcinogenesis. 1992;13:1699–1703. doi: 10.1093/carcin/13.10.1699. [DOI] [PubMed] [Google Scholar]
- 45.Sim E, Hickman D, Coroneos E, Kelly SL. Arylamine N-acetyltransferase. Biochem Soc Trans. 1992;20:304–309. doi: 10.1042/bst0200304. [DOI] [PubMed] [Google Scholar]
- 46.Bartsch H, Dworkin M, Miller JA, Miller EC. Electrophilic N-acetoxyaminoarenes derived from carcinogenic N-hydroxy-N-acetylaminoarenes by enzymatic deacetylation and transacetylation in liver. Biochim Biophys Acta. 1972;286:272–298. doi: 10.1016/0304-4165(72)90265-6. [DOI] [PubMed] [Google Scholar]
- 47.Marcus PM, Vineis P, Rothman N. NAT2 slow acetylation and bladder cancer risk: a meta-analysis of 22 case-control studies conducted in the general population. Pharmacogenetics. 2000;10:115–122. doi: 10.1097/00008571-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Marcus PM, Hayes RB, Vineis P, Garcia-Closas M, Caporaso NE, Autrup H, Branch RA, Brockmoller J, Ishizaki T, Karakaya AE, Ladero JM, Mommsen S, Okkels H, Romkes M, Roots I, Rothman N. Cigarette smoking, N-acetyltransferase 2 acetylation status, and bladder cancer risk: a case-series meta-analysis of a gene-environment interaction. Cancer Epidemiol Biomarkers Prev. 2000;9:461–467. [PubMed] [Google Scholar]
- 49.Sugamori KS, Brenneman D, Grant DM. In vivo and in vitro metabolism of arylamine procarcinogens in acetyltransferase-deficient mice. Drug Metab Dispos. 2006;34:1697–1702. doi: 10.1124/dmd.106.010819. [DOI] [PubMed] [Google Scholar]
- 50.Radomski JL, Hearn WL, Radomski T, Moreno H, Scott WE. Isolation of the glucuronic acid conjugate of n-hydroxy-4-aminobiphenyl from dog urine and its mutagenic activity. Cancer Res. 1977;37:1757–1762. [PubMed] [Google Scholar]
- 51.Babu SR, Lakshmi VM, Hsu FF, Zenser TV, Davis BB. Glucuronidation of N-hydroxy metabolites of N-acetylbenzidine. Carcinogenesis. 1995;16:3069–3074. doi: 10.1093/carcin/16.12.3069. [DOI] [PubMed] [Google Scholar]
- 52.Rothman N, Talaska G, Hayes RB, Bhatnagar VK, Bell DA, Lakshmi VM, Kashyap SK, Dosemeci M, Kashyap R, Hsu FF, Jaeger M, Hirvonen A, Parikh DJ, Davis BB, Zenser TV. Acidic urine pH is associated with elevated levels of free urinary benzidine and N-acetylbenzidine and urothelial cell DNA adducts in exposed workers. Cancer Epidemiol Biomarkers Prev. 1997;6:1039–1042. [PubMed] [Google Scholar]
- 53.Fripp PJ. The Origin of Urinary Beta-Glucuronidase. Br J Cancer. 1965;19:330–335. doi: 10.1038/bjc.1965.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zenser TV, Lakshmi VM, Davis BB. Human and Escherichia coli beta-glucuronidase hydrolysis of glucuronide conjugates of benzidine and 4-aminobiphenyl, and their hydroxy metabolites. Drug Metab Dispos. 1999;27:1064–1067. [PubMed] [Google Scholar]
- 55.Kadlubar FF, Miller JA, Miller EC. Hepatic microsomal N-glucuronidation and nucleic acid binding of N-hydroxy arylamines in relation to urinary bladder carcinogenesis. Cancer Res. 1977;37:805–814. [PubMed] [Google Scholar]
- 56.Paigen K, Peterson J, Paigen B. Role of urinary beta-glucuronidase in human bladder cancer. Cancer Res. 1984;44:3620–3623. [PubMed] [Google Scholar]
- 57.King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr Drug Metab. 2000;1:143–161. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- 58.Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677–685. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- 59.Zenser TV, Lakshmi VM, Hsu FF, Davis BB. Metabolism of N-acetylbenzidine and initiation of bladder cancer. Mutat Res. 2002;506–507:29–40. doi: 10.1016/s0027-5107(02)00149-5. [DOI] [PubMed] [Google Scholar]
- 60.Green MD, King CD, Mojarrabi B, Mackenzie PI, Tephly TR. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab Dispos. 1998;26:507–512. [PubMed] [Google Scholar]
- 61.Mackenzie PI, Rodbourn L, Iyanagi T. Glucuronidation of carcinogen metabolites by complementary DNA-expressed uridine 5′-diphosphate glucuronosyltransferases. Cancer Res. 1993;53:1529–1533. [PubMed] [Google Scholar]
- 62.Buckley DB, Klaassen CD. Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos. 2007;35:121–127. doi: 10.1124/dmd.106.012070. [DOI] [PubMed] [Google Scholar]
- 63.Izumi K, Zheng Y, Hsu JW, Chang C, Miyamoto H. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: a potential mechanism of androgen-induced bladder carcinogenesis. Mol Carcinog. 2013;52:94–102. doi: 10.1002/mc.21833. [DOI] [PubMed] [Google Scholar]
- 64.Yasuda S, Idell S, Fu J, Carter G, Snow R, Liu MC. Cigarette smoke toxicants as substrates and inhibitors for human cytosolic SULTs. Toxicol Appl Pharmacol. 2007;221:13–20. doi: 10.1016/j.taap.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 65.DeBaun JR, Miller EC, Miller JA. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970;30:577–595. [PubMed] [Google Scholar]
- 66.Weisburger JH, Yamamoto RS, Williams GM, Grantham PH, Matsushima T, Weisburger EK. On the sulfate ester of N-hydroxy-N-2-fluorenylacetamide as a key ultimate hepatocarcinogen in the rat. Cancer Res. 1972;32:491–500. [PubMed] [Google Scholar]
- 67.DeBaun JR, Smith JY, Miller EC, Miller JA. Reactivity in vivo of the carcinogen N-hydroxy-2-acetylaminofluorene: increase by sulfate ion. Science. 1970;167:184–186. doi: 10.1126/science.167.3915.184. [DOI] [PubMed] [Google Scholar]
- 68.Chou HC, Lang NP, Kadlubar FF. Metabolic activation of the N-hydroxy derivative of the carcinogen 4-aminobiphenyl by human tissue sulfotransferases. Carcinogenesis. 1995;16:413–417. doi: 10.1093/carcin/16.2.413. [DOI] [PubMed] [Google Scholar]
- 69.Pacifici GM, Franchi M, Bencini C, Repetti F, Di Lascio N, Muraro GB. Tissue distribution of drug-metabolizing enzymes in humans. Xenobiotica. 1988;18:849–856. doi: 10.3109/00498258809041723. [DOI] [PubMed] [Google Scholar]
- 70.Reuber MD. Influence of age and sex on carcinoma and cirrhosis of the liver in AXC strain rats ingesting 0.025% N-2-fluorenyldiacetamide. Pathol Microbiol (Basel) 1975;43:31–37. doi: 10.1159/000162792. [DOI] [PubMed] [Google Scholar]
- 71.Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci. 2006;93:242–255. doi: 10.1093/toxsci/kfl050. [DOI] [PubMed] [Google Scholar]
- 72.Edgren G, Liang L, Adami HO, Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2012;27:187–196. doi: 10.1007/s10654-011-9647-5. [DOI] [PubMed] [Google Scholar]
- 73.Okajima E, Hiramatsu T, Iriya K, Ijuin M, Matsushima S. Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol Res. 1975;3:73–79. doi: 10.1007/BF00256185. [DOI] [PubMed] [Google Scholar]
- 74.Bertram JS, Craig AW. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur J Cancer. 1972;8:587–594. doi: 10.1016/0014-2964(72)90137-5. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki E, Aoki J, Okada M. Metabolic fate of N-butyl-N-(4-hydroxybutyl) nitrosamine and N, N-dibutylnitrosamine in the guinea pig, with reference to their carcinogenic effects on the urinary bladder. Gann. 1981;72:547–551. [PubMed] [Google Scholar]
- 76.Okada M, Ishidate M. Metabolic fate of N-n-butyl-N-(4-hydroxybutyl)-nitrosamine and its analogues. Selective induction of urinary bladder tumours in the rat. Xenobiotica. 1977;7:11–24. doi: 10.3109/00498257709036241. [DOI] [PubMed] [Google Scholar]
- 77.Airoldi L, Bonfanti M, Magagnotti C, Fanelli R. Development of an experimental model for studying bladder carcinogen metabolism using the isolated rat urinary bladder. Cancer Res. 1987;47:3697–3700. [PubMed] [Google Scholar]
- 78.Suzuki E, Mochizuki M, Okada M. Relationship of urinary N-butyl-N-(3-carboxypropyl)nitrosamine to susceptibility of animals to bladder carcinogenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine. Gann. 1983;74:360–364. [PubMed] [Google Scholar]
- 79.Suzuki E, Okada M. Metabolic fate of N-butyl-N-(4-hydroxybutyl)nitrosamine in the rat. Gann. 1980;71:856–862. [PubMed] [Google Scholar]
- 80.Airoldi L, Magagnotti C, Bonfanti M, Fanelli R. Alpha-oxidative metabolism of the bladder carcinogens N-nitrosobutyl(4-hydroxybutyl)amine and N-nitrosobutyl(3-carboxypropyl)amine within the rat isolated bladder. Carcinogenesis. 1990;11:1437–1440. doi: 10.1093/carcin/11.8.1437. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki E, Anjo T, Aoki J, Okada M. Species variations in the metabolism of N-butyl-N-(4-hydroxybutyl) nitrosamine and related compounds in relation to urinary bladder carcinogenesis. Gann. 1983;74:60–68. [PubMed] [Google Scholar]
- 82.Nagao M, Suzuki E, Yasuo K, Yahagi T, Seino Y. Mutagenicity of N-butyl-N-(4-hydroxybutyl)nitrosamine, a bladder carcinogen, and related compounds. Cancer Res. 1977;37:399–407. [PubMed] [Google Scholar]
- 83.Hashimoto Y, Suzuki E, Okada M. Induction of urinary bladder tumors in ACI-N rats by butyl(3-carboxypropyl)nitrosoamine, a major urinary metabolite of butyl-(4-hydroxybutyl)nitrosoamine. Gann. 1972;63:637–638. [PubMed] [Google Scholar]
- 84.Hirose M, Fukushima S, Hananouchi M, Shirai T, Ogiso T. Different susceptibilities of the urinary bladder epithelium of animal species to three nitroso compounds. Gann. 1976;67:175–189. [PubMed] [Google Scholar]
- 85.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, Yeh S, Messing EM, Chang C. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 86.Shirai T, Tsuda H, Ogiso T, Hirose M, Ito N. Organ specific modifying potential of ethinyl estradiol on carcinogenesis initiated with different carcinogens. Carcinogenesis. 1987;8:115–119. doi: 10.1093/carcin/8.1.115. [DOI] [PubMed] [Google Scholar]
- 87.Imada S, Akaza H, Ami Y, Koiso K, Ideyama Y, Takenaka T. Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. Eur Urol. 1997;31:360–364. doi: 10.1159/000474484. [DOI] [PubMed] [Google Scholar]
- 88.Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol. 2000;15:1261–1270. doi: 10.14670/HH-15.1261. [DOI] [PubMed] [Google Scholar]
- 89.Makela S, Strauss L, Kuiper G, Valve E, Salmi S, Santti R, Gustafsson JA. Differential expression of estrogen receptors alpha and beta in adult rat accessory sex glands and lower urinary tract. Mol Cell Endocrinol. 2000;170:219–229. doi: 10.1016/s0303-7207(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 90.McGrath M, Michaud DS, De Vivo I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol. 2006;163:236–244. doi: 10.1093/aje/kwj028. [DOI] [PubMed] [Google Scholar]
- 91.Davis-Dao CA, Henderson KD, Sullivan-Halley J, Ma H, West D, Xiang YB, Gago-Dominguez M, Stern MC, Castelao JE, Conti DV, Pike MC, Bernstein L, Cortessis VK. Lower risk in parous women suggests that hormonal factors are important in bladder cancer etiology. Cancer Epidemiol Biomarkers Prev. 2011;20:1156–1170. doi: 10.1158/1055-9965.EPI-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cantwell MM, Lacey JV, Jr, Schairer C, Schatzkin A, Michaud DS. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer. 2006;119:2398–2401. doi: 10.1002/ijc.22175. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 94.Ding Y, Paonessa JD, Randall KL, Argoti D, Chen L, Vouros P, Zhang Y. Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis. 2010;31:1999–2003. doi: 10.1093/carcin/bgq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91:605–613. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 96.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:938–944. doi: 10.1158/1055-9965.EPI-07-2502. [DOI] [PubMed] [Google Scholar]