Abstract
Background
Accumulating evidences suggest that Hsp 70, the inducible component of Hsp70 family, might release from a living cell. Here we show that a pharmacological inhibitor of phospholipase C activity U73122 caused a 2–4 fold reduction of an intracellular level of Hsp70 in A431 human carcinoma cells.
Results
A depletion of Hsp70 under U73122 was a result of the protein release since it was detected in cell culture medium, as was established by immunoprecipitation and precipitation with ATP-agarose. The reduction of Hsp70 level was specifically attributed to the inhibition of PLC, since the non-active inhibitor, U73343, had no effect on Hsp70 level. The PLC-dependent decrease of Hsp70 intracellular level was accompanied by the enhanced sensitivity of A431 cells to the apoptogenic effect of hydrogen peroxide. Here for the first time we demonstrated one of the possibilities for a cell to export Hsp70 in PLC-dependent manner.
Conclusion
From our data we suggest that phospholipase C inhibition is one of the possible mechanisms of Hsp70 release from cells.
Background
The proteins belonging to Hsp70 family possess two major properties. First, they are known to elicit chaperone activity, i.e. to recognize misfolded and newly synthesized polypeptides and to participate in their intracellular transport and degradation. Another function is mainly attributed to the inducible member of Hsp70 family and constitutes protective capacity. This was convincingly demonstrated in vitro and in vivo, and the list of cytotoxic factors from which Hsp70 protects cells includes stimuli of apoptosis. The molecular mechanisms underlying the anti-apoptotic activity of Hsp70 are thoroughly investigated now. Among the proteins that may be affected by the over-expressing Hsp70 are APAF-1/caspase-9 [1], NFkappaB [2] and stress kinases [3], all known to participate in apoptotic signaling. Generally, Hsp70 is thought to interfere with almost all known signaling pathways, and vice versa, some of the latter can influence the rate of the chaperone expression through the stimulation of protein kinase C activity by the phorbol ester [4,5] or through the action of factors up-regulating cellular Ca2+ [6]. The chaperonic activity of Hsp70 can also be affected by proteins participating in cell signaling; it was shown that Bag-1 in Hsp70-overexpressing hamster fibroblasts was able to switch from Raf-1/ERK signaling cascade to that based on Hsp70 chaperonic mechanism [7]. Many cell signaling processes are related to the activity of phospholipase C (PLC). One of the latter, phospholipase Cγ1, belongs to the family of PLCs activated in cells exposed to a variety of extracellular ligands. PLC hydrolyzes the minor membrane phospholipid, phosphatidylinositol 4,5-bisphosphate. This reaction results in the generation of the two intracellular second messengers diacylglycerol and inositol 1,4,5-trisphosphate. The latter promotes the activation of protein kinase C and the release of Ca2+ from intracellular stores [8]. Several groups have demonstrated that PLC may also be activated in response to various stressful insults: exposure to heat, oxidants, UVC and mechanical stress [9-12]. Stress induced PLC activation was shown to be sufficient for preventing apoptosis induced by heat-shock and oxidants and significantly enhanced cell survival [11,12]. However, the mechanism of PLC activation in a cell physiology under stress conditions remains unclear. Since the expression and activity of Hsp70 can be controlled by the increase of intracellular Ca2+ and by protein kinase C, whereas PLC itself is able to regulate these factors, it seemed that both proteins are associated in cell signaling kaleidoscope. Moreover, both proteins are involved in a cell response to similar stressful factors. Based on these data we have suggested that Hsp70 and PLC might be functionally linked in their cellular activities, particularly in the process of a cell reaction to stress. To test this hypothesis we used the keratinocyte-derived A431 epidermoid carcinoma cells. In human skin and epidermal cell lines, such as A431 cells Hsp70 is normally expressed at high level [13], and these cells may be a convenient model for investigation of consequences of variations in the content of intracellular Hsp70. The goal of this study was to compare the level of Hsp70 in A431 cells treated with various factors known to activate or suppress PLC activity. Inhibition of PLC activity resulted in a substantial decrease of the content of the intracellular Hsp70. Simultaneously, Hsp70 was detected in cell culture medium as was established with the use of immuno-precipitation or precipitation with ATP-agarose. Two forms of Hsp70 were found in culture medium: one corresponding to Hsp70 and an additional one with the molecular mass of 98 kDa, the latter was proven to be the ubiquitinated form of Hsp70. It was also shown that the release of Hsp70 from A431 cells led to the increased sensitivity to the oxidative stress.
Results
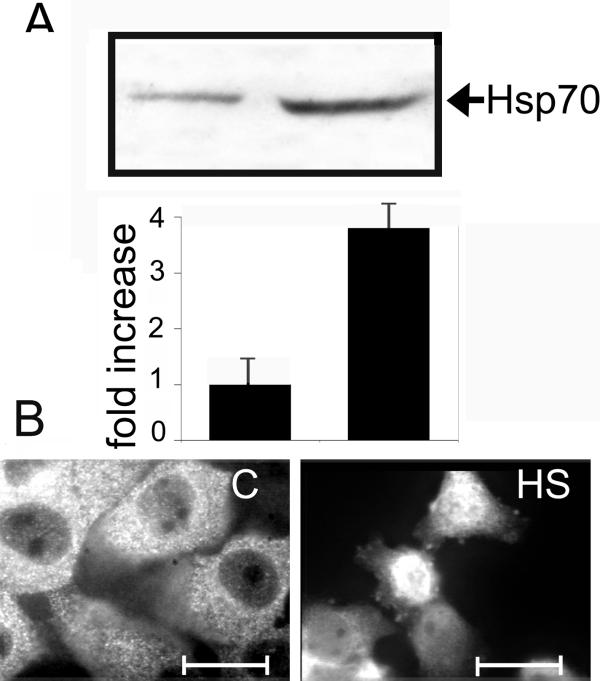
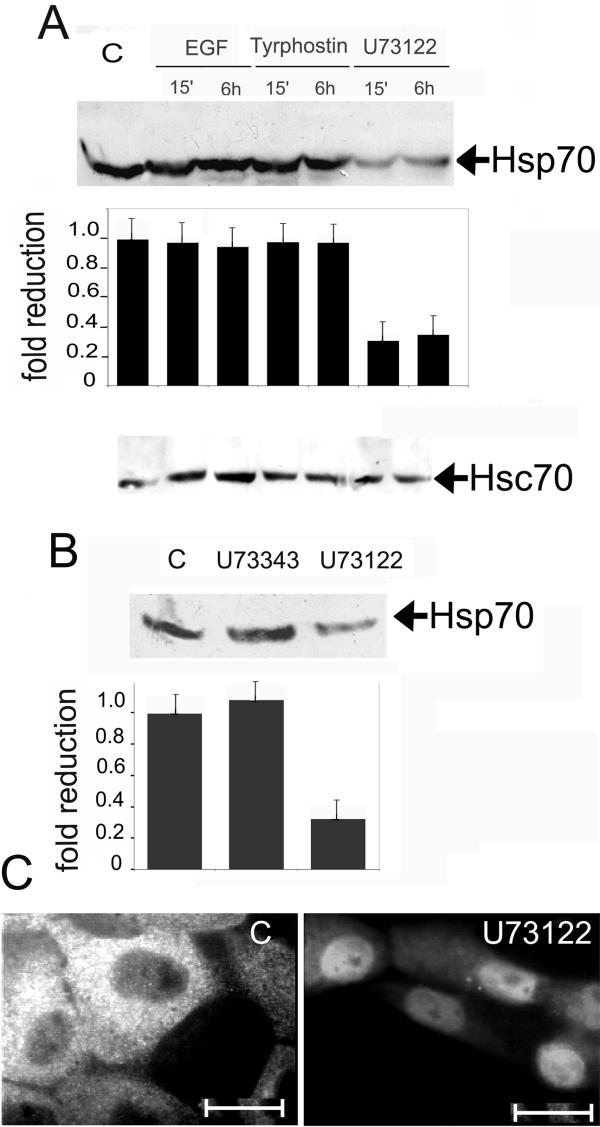
A431 keratinocyte-derived cells constitutively expressing inducible Hsp70 and its cognate form, Hsc70. To check whether these cells are capable of eliciting a typical stress response, we subjected them to heat shock at 42°C for 40 min. After recovery for 6 h at 37°C, we analyzed Hsp70 with the aid of immunofluorescence and immunoblotting, using 2H9 antibody, which had earlier been shown to recognize only the inducible member of the Hsp70 family. Although control cells contained rather high amounts of Hsp70, this value increased 3-5-fold after heat stress (Fig. 1A). Immunofluorescence analysis showed that Hsp70 was located in the cytoplasm in control cells, whereas after heat shock became concentrated in nuclei (Fig. 1B) showing the typical post-heat shock response [14]. In order to check whether changes in PLC activity affected the Hsp70 level, we studied its amount in A431 cells treated with EGF, tyrphostin AG1478 (a specific inhibitor of EGF receptor tyrosine kinase) and U73122 (an inhibitor of PLC activity). The level of Hsp70 was measured using Western blot analysis. Neither EGF, nor tyrphostin AG1478 altered significantly the intracellular level of Hsp70. In contrast, treatment of the cells with 1 uM U73122 resulted in a nearly 4-fold reduction in Hsp70 content (Fig. 2A). This decrease was observed 10 min after U73122 administration, and there was no recovery until >6 h. The non-active analog of U73122, U73343, had no effect on the Hsp70 level in A431 cells.
Figure 1.
A431 cells originally normally respond to heat stress. A – A431 cells were heated at 42°C for 40 min and lysates of control and heat-shocked cells were analyzed by Western blot using 2H9 anti-Hsp70 antibody. The amount of Hsp70 production shown on the Western blot was quantified by densitometry and normalized to the total protein loaded into each lane of the gel. B – control and heat-stressed cells were stained with the 2H9 anti-Hsp70 monoclonal antibody. Scale bar indicates 5 μm.
Figure 2.
Effect of factors influencing PLC activity on the Hsp70 level in A431 cells. A – A431 cells were treated with EGF, Tyrphostin AG1478 and U73122, able to affect PLC activity, and lysates of cells were taken in 15 min and in 6 h after administration of the agents. The lysates were subjected to PAGE and examined by Western blot for Hsp70 and Hsc70 as indicated using 2H9 anti-Hsp70 and N69 anti-Hsc70 antibody respectively. The amount of Hsp70 shown on the Western blot was quantified by densitometry and normalized to the total protein loaded into each lane of the gel. B – Hsp70 level is reduced in the A431 cells treated with U73122; immunofluorescence with the use of 2H9 antibody. C – U73343 is not able to decrease the Hsp70 amount in A431 cells; lysates of the cells treated with U73122 and its non-active analog U73343 were analyzed by Western blot, using 2H9 antibody.
To prove the Western blotting data, immunofluorescence analysis was performed using the same antibody (Fig. 2B). In U7312-treated cells, Hsp70 almost completely disappeared from cytoplasm and partially passed into nuclei (Fig. 2B). The Hsc70 amount measured with the aid of Western blotting using N69 antibody remained constant after the treatment with U73122 (Fig. 2D).
According to the literature, some cells are able to excrete Hsp70 [15,16]. More recently, we have demonstrated such excretion from human T98 glioblastoma cells [14], and suggested that a reduction of intracellular Hsp70 content following PLC inhibition might be due to a release of the protein by A431 cells. To test this hypothesis, we analyzed cell culture medium using two kinds of affinity chromatography. The technique that was effective and quantitative for detecting Hsp70 in extremely diluted solutions proved to be chromatography on ATP-agarose [14]. We employed this method in the studies on A431 cells, and applied conditioned medium taken from control cells and from ones incubated with U73122 for 15 min on to column with ATP-agarose gel. The proteins bound to the gels were analysed with the aid of Western blotting using 2H9 antibody. Two bands corresponding to polypeptides 70 and 98 kDa were found on the blot and their intensity was much greater in the case of cells treated with U73122 (Fig. 3, left panel).
Figure 3.
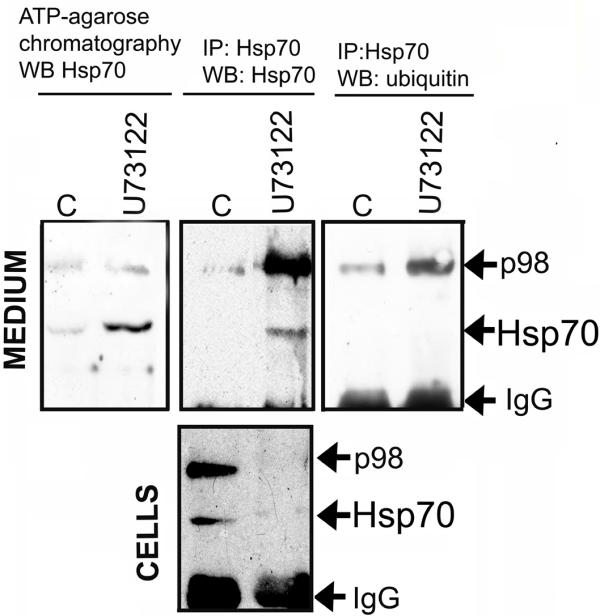
Inhibition of phospholipase C activity causes release of Hsp70 from A431 cells. The left panel: conditioned medium from A431 cells, control and treated with U73122 PLC inhibitor, was passed over the ATP-agarose column and the eluted protein was subjected to immunoblotting, using 2H9 antibody. The central and right panels: samples of conditioned medium were incubated with 2H9 antibody attached to Protein G-Sepharose, and the immunoprecipitated protein was studied with the help of immunoblotting, using 2H9 antibody (central panel) or anti-ubiquitin antibody (right panel). The lower panel: immunoprecipitation/immunoblotting with lysates of control and U73122-treated cells.
Another method for isolation of Hsp70 from the conditioned medium was immunoprecipitation with 2H9 antibody. The antibody was added to the medium samples and the immune complexes were precipitated with Protein G-Sepharose. Proteins attached to the immunosorbent were analyzed with the aid of Western blotting using 2H9 antibody. Comparison of immunoprecipitates from conditioned medium of control and U73122-treated cells showed that Hsp70 was present in detectable amounts only in the medium from U73122-treated cells (Fig. 3, central upper panel). An additional band with the molecular mass of nearly 98 kDa recognized by the monoclonal antibody to Hsp70 was also detected in immunoprecipitates from conditioned medium. To prove that the culture medium was not contaminated with Hsp70, the immunoprecipitation was performed with free DMEM. There were no bands on blots with these samples (data not shown). To reveal relationship between the content of intracellular Hsp70 and its amount in cell medium, the lysates obtained from the cells incubated 15 min with U73122 and from control cells were assayed by immunoprecipitation, using 2H9 antibody (Fig. 3, lower panel). The immunoblotting data showed that the accumulation of Hsp70 in culture medium was paralleled to reduction of the endogenous protein content. The result of immunoprecipitation showed that the band of protein with the mass of 98 kDa was much more abundant than that in the material attached to ATP-agarose. We suggest that the monoclonal antibody 2H9 reacted more readily with this protein and to a lesser extent with the principal antigen, Hsp70. In the attempt to identify the 98 kDa component, we followed Jiang and coauthors [17], who demonstrated that a member of Hsp70 family, the Hsc70 cognate heat shock protein, could be ubiquitinated. Since the 98 kDa protein found in culture medium together with Hsp70 was recognized by monoclonal 2H9 antibody in both immunoprecipitation and Western blotting assays, this protein might be an ubiquitinated form of Hsp70. To test this, immunoprecipitates obtained after the reaction of 2H9 antibody with culture medium of non-treated and U73123-treated cells were probed by Western blotting with anti-ubiquitin antibody (Fig 3, right panel). Immunoblot analysis demonstrated that p98 did indeed represent an ubiquitinated form of Hsp70. Thus, inhibition of PLC forced A431 to liberate Hsp70 both in non-modified and ubiquitinated forms.
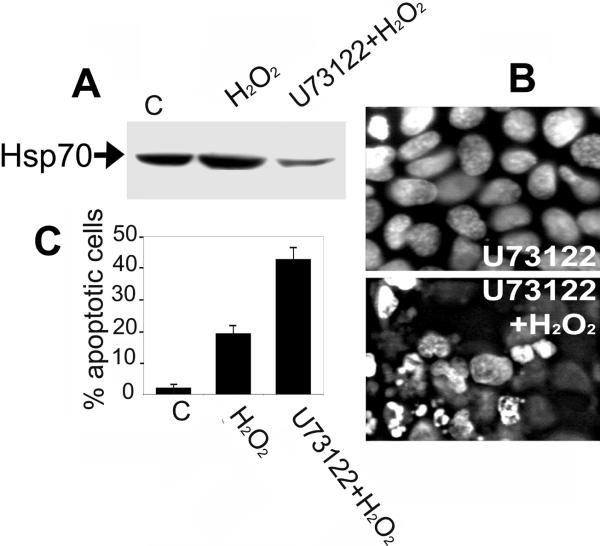
Since the reduction of Hsp70 amount might lower cell resistance to apoptotic stimuli, we compared the effect of hydrogen peroxide on control and U73122-treated A431 cells. H2O2 was chosen due to its apoptogenic effect that was convincingly demonstrated in numerous cell models. Treatment of A431 cells with 200 uM H2O2 did not significantly alter the original level of Hsp70 or its level in cells exposed to H2O2, but in the cells pre-treated with U73122, the Hsp70 level was lower than in the two other cell groups (Fig. 4A). Treatment with U73122 alone had no influence on the cell viability and growth dynamics (Fig. 4B). Examination of the kinetics of the response to 200 uM of H2O2 treatment revealed that, within 5 h, the U-73122-treated cells began to round up and detach from the plastic. Cells stained with DAPI displayed features typical of apoptosis including condensation and fragmentation of nuclei (Fig. 4B). The percentage of apoptotic cells was nearly twice higher than that in the population of control cells (Fig. 4C). Thus, the decrease of the Hsp70 amount correlated well with an elevation of sensitivity of A431 cells to hydrogen peroxide.
Figure 4.
The release of Hsp70 from A431 cells makes the latter sensitive to apoptosis-inducing action of hydrogen peroxide. A – data of immunoblotting with 2H9 antibody of control cells and cells treated with hydrogen peroxide and with U73122. B – DAPI staining of A431 cells treated with U73122 alone or in combination with hydrogen peroxide. C – the number of apoptotic cells in population of control cells and cells treated with U73122 alone and with combination of U73122 and hydrogen peroxide.
Discussion
The goal of this study was to establish a possible relationship between two anti-stress proteins, Hsp70 and phospholipase C. The protective effect resulting from PLC activation was confirmed using different approaches. First, mouse embryonic fibroblasts genetically deficient in PLCγ1 cell viability following heat, or H2O2 treatment was reduced, while the reconstitution of the enzyme protects cells from stress agents [11,12]. Second, overexpression of PLCγ1 was shown to inhibit apoptosis induced by UV-irradiation and by superoxides [10-12]. Third, inhibition of PLC or activation of protein kinase C, or both, during ischemia impaired significantly the postischemic myocardial recovery [18].
On the other hand, it was shown that different kinds of cell damaging factors such as, heat, oxidants, osmotic shock, mechanical stress resulted in PLC activation [9-12]. Hsp70 is known to be one of the most powerful anti-stress and anti-apoptotic proteins; its protective activity was convincingly demonstrated in a great number of experiments in vitro and in vivo [19]. There are many cross-points, where Hsp70 and PLC can be functionally linked to each other, and to check this A431 epidermoid cell line was chosen. These cells were found to exhibit a normal heat shock response, accumulation of Hsp70 and its transient transport to the nucleus, Fig. 1. It was suggested that activation and/or suppression of PLC activity might cause changes in the amount or intracellular distribution of Hsp70. EGF that normally triggers PLC activation failed to affect the content of Hsp70; however, a selective inhibition of PLC activity with U73122 caused a pronounced decrease of the chaperone amount. This reduction occurred in 10 min after administration of the inhibitor and caused considerable loss of the protein in cells and its partial reallocation from the cytoplasm to nuclei (fig. 2B). Since the decrease of intracellular Hsp70 level might be due to a release of the protein, we analyzed conditioned medium with the aid of two methods, immunoprecipitation and affinity chromatography on ATP-agarose, the latter known to specifically bind Hsp70 and similar proteins [20]. As shown by immunoblotting with anti-Hsp70 antibody, immunoprecipitates and eluates from ATP-agarose after passing extracts of U73122-treated cells contained two bands; one of them was identified as Hsp70. For the last few years the idea that Hsp70, earlier considered to be exclusively intracellular molecule, can be released and/or imported by mammalian cells has become rather popular. It dates back to the data of Tytell and coworkers who demonstrated that Hsp70 was able to migrate from glia to giant axon of the sea squid [15]. Recently, we have shown that human glioma cells could export Hsp70 into the culture medium regardless of whether cells were in normal conditions or subjected to heat shock [14]. Finally, Dybdahl with coauthors demonstrated that Hsp70 could be fluxed to blood of patients undergoing arterial surgery; moreover, this efflux of Hsp70 seemed to be relevant for the physiology of the whole organism [21]. Hsp70 plays a major role in protection of cells against a variety of harmful agents, therefore it seemed worth checking whether a reduction of the chaperone amount might affect cell resistance to one of the former factors, superoxide. The data of experiments with the combined treatment of A431 cells with hydrogen peroxide and U73122 showed that the loss of Hsp70 rendered the cells sensitive to H2O2 (fig. 4). Our discovery of PLC-dependent extracellular transport of Hsp70 accompanied by loss of cell resistance to risky factors and observations of Dybdahl and others have allowed us to suggest that there are certain loci in the whole organism in which cells affected by a factor similar to U73122 might start to release Hsp70. This protein accumulating in such loci and known to be a strong chaperone or chaperokine [21,22] may affect markedly function of adjacent cells in an unpredictable way. For instance, Hsp70 expressed by glial cells could penetrate neurons and protect them from various stressors including heat stress [14,23].
Another possible target for exogenous Hsp70 is dendritic cells, monocytes and macrophages. In this case Hsp70 induced CD40, TLR2 and TLR4 receptors which are necessary steps toawrds activation of the innate immune system [see [24] for review]. The mechanism of Hsp70 export by cells remains unclear. Proteins could integrate into the artificial lipid bilayer and a transmembrane ion flow was detected, suggesting involvement of an ion pathway [25]. It was demonstrated that in rat red blood cells, an hsp70-like protein is located in the cytosol and exported via exosomes during the in vivo reticulocyte maturation [26]. The presence of Hsp70 has been detected in vesicles (named exosomes) leaking from mammalian and avian immature red cells (i.e. reticulocytes), as well as from differentiating avian erythroleukemia cells. It is proposed that Hsp70 takes part in an exosome formation and/or release in immature red cells [27]. In this regard numerous data are to be mentioned that prove the PLC also is implicated in regulation of exocytosis.
First, it was shown that exocytosis of vesicles may be blocked by drugs depleting Ca2+ stores and by inhibitors of PLC [28]. Second, phosphatidylinositol transfer proteins were found to be essential component for PLC-mediated hydrolysis of PIP2 and for the regulation of exocytosis [29]. Finally, according to our preliminary data pre-treatment of A431 cells with brefeldin, an inhibitor of vesicular transport, stopped the reduction of the Hsp70 in U73122-treated cells (Evdonin et al., unpublished observations). Based on these data we suggest that release of Hsp70 provoked by PLC inhibition occurs via vesicular transport.
Another interesting finding in this study is that a considerable part of Hsp70 released from A431 cells is in the ubiquitinated form. This fact agrees well with data indicating that the ubiquitination of Hsc70 did not trigger proteasome-dependent degradation [17], hence, the only way for cell to remove the protein is to excrete it. A number of groups [30,31] demonstrated that ubiquitination serves not only as a signal for proteasome-dependent degradation, but also as a trigger for different transport events.
Conclusions
In conclusion, the data presented here show that Hsp70 can be released from viable A431 cells in the PLC-dependent manner, and that the exodus of Hsp70 results in elevated sensitivity of the cells to oxidative stress. These observations also prove that orchestration between cell mitogen signaling and stress signaling is necessary for the cell survival in response to stress.
Methods
Materials
Culture medium and fetal calf serum were purchased in Gibco Life Science (USA), reagents for electrophoresis, ATP-agarose, protein G-Sepharose, PLC inhibitor U73122, its non-active analog U73343 anti ubiquitin antibody and secondary antibodies conjugated with horseradish peroxidase or labeled with Cy3 were from Sigma (St Louis, USA). Tyrphostin AG1478 was purchased from Calbiochem (Lucerne, Switzerland).
Cells
A431 cells were grown in the Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum on 10 cm plastic dishes. The cells were treated with 100 ng/ml EGF, 1 uM U73122, 1 uM U73343 or 10 nM Tyrphostine AG1478 for indicated periods of time. To detect Hsp70 in culture medium cells were washed with DMEM, and fresh medium was added followed by the administration of a particular factor.
SDS-PAGE, immunoprecipitation, and immunoblot analysis
For immunoprecipitation studies, cells were washed with phosphate-buffered saline (PBS, pH 7.4), lysed on ice in RIPA buffer (250 μl), and then spun at 13 K for 10 min. To the supernatant 10 μl of anti-HSP antibody were added, and the mixture was gently rotated at 4°C for 4 h. Protein G-agarose (10 μl) was then added, and the incubation was continued for an additional 90 min. The protein G-agarose was collected by centrifugation and the beads were washed three times with 50 mM Tris-HCl, pH 8.0, containing 150 mM NaCl, 1% (v/v) Nonidet P-40. The protein G-agarose slurry was suspended in 2 × Laemmli SDS sample buffer and heated at 90°C for 5 min. Immunoprecipitated proteins were analyzed on 12 % polyacrylamide gel followed by the transfer onto the polyvinylidene difluoride membrane (0.4 μm) in 25 mM Tris, 192 mM glycine, and 20% (v/v) methanol [32,33]. Membranes were incubated successively 30 min in 5% (w/v) nonfat dry milk, 1 h in 10 mM Tris-HCl, pH 8.0, 125 mM NaCl, 0.2% (v/v), Tween 20 and in the same solution containing antiHsp70 2H9 [1:1000, [34]], N69 anti-Hsc70 antibodies (1:1000), or anti-ubiquitin antibody (1:100). After washing in 0.05% Tween-PBS blots were incubated 30 min in secondary antibodies with peroxidase diluted 5000-fold in above solution. Protein bands on blots were visualized using enhanced chemiluminescence as described by the manufacturer (Amersham, UK). To equalize amounts of protein loaded on acrylamide gel protein concentration was measured with the use of dye-binding method [35].
DAPI staining
DAPI staining was performed as described previously [12]. Briefly, prior to staining, the cells were fixed with 4% paraformaldehyde for 30 min at room temperature and washed with PBS. DAPI was added to the fixed cells for 30 min, after what they were examined by fluorescence microscopy. Apoptotic cells were identified by a typical pattern with condensed and fragmented nuclei. The percentage of apoptotic cells was calculated as related to a number of total cells multiplied by 100; minimum 1000 cells were counted for each treatment.
Immunofluorescence
Cells grown on uncoated glass coverslips were washed 3 times in PBS. The cells were then fixed with 3.7% formaldehyde for 15 min at room temperature, followed by a brief wash in PBS. The cells were incubated in a blocking solution containing 3% bovine serum albumin in PBS. For permeabilization 0.1% Triton X-100 was added into blocking and the solutions of antibodies. 2H9 anti-Hsp70 antibody was diluted 1:500 in PBS and cells were incubated in this solution 1 h at room temperature. After washing in PBS cells were incubated with goat anti-rabbit Cy3 conjugate, diluted 1:1000, for 30 min at room temperature. The cells were then washed in PBS, and staining was and visualized with the aid of KodakE400 microscope equipped with epifluorescence optics and digital camera.
Precipitation with ATP-agarose
The conditioned medium from A431 was collected and TritonX100, MgCl2 and Tris HCl pH 7,6 were added to give concentrations of 0,1%, 1 mM, 20 mM respectively (buffer A). The protein solution was passed through 100 μl ATP-agarose column, followed by washing with a buffer A without Triton X-100. A column was eluted with the buffer A, containing 3 mM ATP. Eluted proteins were resolved by SDS-Page electrophoresis followed by blotting with 2H9 antibody.
Abbreviations
The abbreviations used are: PLC – phospholipase C; EGF – epidermal growth factor.
Acknowledgments
Acknowledgements
We would like to thank Dr. Natalia Tsupkina for the help in manipulations with cells. The work was supported in part by grants 01-04-49079, 01-04-48845 from Russian Fund for Basic Research and CRIG 067604 from Wellcome Trust.
Contributor Information
Anton L Evdonin, Email: evdonin@mail.ru.
Irina V Guzhova, Email: guzhova@mail.cytspb.rssi.ru.
Boris A Margulis, Email: margulis@mail.cytspb.rssi.ru.
Natalia D Medvedeva, Email: medved@mail.cytspb.rssi.ru.
References
- Beere HM., Wolf BB, Cain K, Mosser DD, Mahboubi A, Kruwana T, Tailor P., Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Darieva ZA, Rocha Melo A, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress and Chaperones. 1997;2:132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of Hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;19:7146–7159. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Aten RF, Behrman HR. Physiological and pharmacological inhibitors of luteinising hormone-dependent steroidogenesis induced heat shock protein 70 in rat luteal cells. Endocrinology. 1995;136:1775–1781. doi: 10.1210/en.136.4.1775. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Smallridge RC, Galloway RJ, Kiang JG. Increases in HSF1 translocation and synthesis in human epidermoid A-431 cells: role of protein kinase C and [Ca2+]i. J Investig Med. 1996;44:144–153. [PubMed] [Google Scholar]
- Yamamoto N, Smith MW, Maki A, Berezesky IK, Trump BF. Role of cytosolic Ca2+ and protein kinases in the induction of the hsp70 gene. Kidney Int. 1994;45:1093–1104. doi: 10.1038/ki.1994.146. [DOI] [PubMed] [Google Scholar]
- Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nature Cell Biol. 2001;3:276–282. doi: 10.1038/35060068. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwhof C, Van Wamel JT, Noordzij LA, Aydin S, Harper JC, Van Der Laarse A. Mechanical stress stimulates phospholipase C activity and intracellular calcium ion levels in neonatal rat cardiomyocytes. Cell Calcium. 2001;29:73–83. doi: 10.1054/ceca.2000.0158. [DOI] [PubMed] [Google Scholar]
- Lee YH, Kim S, Kim J, Young Kim K, Kim MJ, Ryu SH, Suh P. Overexpression of phospholipase C-gamma1 suppresses UVC-induced apoptosis through inhibition of c-fos accumulation and c-Jun N-terminal kinase activation in PC12 cells. Biochim Biophys Acta. 1999;1440:235–243. doi: 10.1016/S1388-1981(99)00128-6. [DOI] [PubMed] [Google Scholar]
- Bai X-C, Liu A-L, Deng F, Zou Z-P, Bai J, Ji Q-S, Luo S-Q. Phospholipase C-γ1 is required for survival in heat stress: involvement of protein kinase C-dependent Bcl-2 phosphorylation. J Biochem. 2002;131:207–212. doi: 10.1093/oxfordjournals.jbchem.a003089. [DOI] [PubMed] [Google Scholar]
- Wang X-T, Mcgulloch KD, Wang X-J, Carpenter G, Holbrook NJ. Oxidative stress-induced phospholipase C-g1 activation enhances cell survival. J Biol Chem. 2001;276:28364–28371. doi: 10.1074/jbc.M102693200. [DOI] [PubMed] [Google Scholar]
- Trautinger F, Trautinger I, Kindas-Mugge I, Metze D, Luger TA. Human keratinocytes in vivo and in vitro constitutively express the 72-kD heat shock protein. J Invest Dermatol. 1993;101:334–338. doi: 10.1111/1523-1747.ep12365491. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Kislyakova K, Moskaliova O, Fridlandskaia II, Tyttel M, Cheetham M, Margulis BA. In vitro studies show that Hsp70 can be released by glia and that exogenous HSP 70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/S0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Tyttel M, Greenberg SG, Llasek RJ. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986;363:161–164. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Feramisco JR. Disruption of the three cytoskeletal networks in mammalian cells does not affect transcription, translation, or protein translocation changes induced by heat shock. Mol Cell Biol. 1985;5:1229–1237. doi: 10.1128/mcb.5.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Meerson FZ, Malyshev IY, Zamotrinsky AV, Kopylov YU. The role of hsp70 and IP3-DAG mechanism in the adaptive stabilization of structures and heart protection. J Mol Cell Cardiol. 1996;28:835–843. doi: 10.1006/jmcc.1996.0078. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, Flo Th, Waage A, Quereshi N, Sellevold OFM, Espevik T, Sundan A. Inflammatory Response After Open Heart Surgery Release of Heat-Shock Protein 70 and Signaling Through Toll-Like Receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Bolch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular hsp70. Role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Kelty JD, Noseworthy PA, Feder ME, Robertson RM, Ramirez JM. Thermal preconditioning and heat-shock protein 72 preserve synaptic transmission during thermal stress. J Neurosci. 2002;22:1–6. doi: 10.1523/JNEUROSCI.22-01-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin RPA, Lundvuist A, More SH, Von Bonin A, Kiessling R, Ljungren H-G. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–135. doi: 10.1016/S1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- Zaric J, Glisin V, Popovic Z. Evidence for HSP70-like protein in the RBC membrane of the hereditarily anemic Belgrade laboratory (b/b) rat. Mol Cell Biochem. 1998;178:119–125. doi: 10.1023/A:1006877026611. [DOI] [PubMed] [Google Scholar]
- Alder GM, Austen BM, Bashford CL, Mehlert A, Pasternak CA. Heat shock proteins induced pores in membranes. Biosci Rep. 1990;10:509–518. doi: 10.1007/BF01116611. [DOI] [PubMed] [Google Scholar]
- Mathew A, Bell A, Johnstone RM. Hsp-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem J. 1995;308:823–830. doi: 10.1042/bj3080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Meunier FA, Ashton AC, Martsushita H, Hirst WD, Lelianova VG, Wilkin GP, Dolly JO, Ushkaryov YA. Vesicle exocytosis stimulated by alpha-latrotoxin is mediated by latrophilin and requires both external and stored Ca2+ EMBO J. 1998;17:3909–3920. doi: 10.1093/emboj/17.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockroft S. Phosphatidylinositol transfer proteins: requirements in phospholipase C signaling and in regulated exocytosis. FEBS Lett. 1997;410:44–48. doi: 10.1016/S0014-5793(97)00414-6. [DOI] [PubMed] [Google Scholar]
- Strous GJ, Gent J. Dimerization, ubiquitylation and endocytosis go together in growth hormone receptor function. FEBS Lett. 2002;529:102–109. doi: 10.1016/S0014-5793(02)03187-3. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicularbody sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Laemli UK. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrilamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasunskaia EB, Fridlandskaia II, Guzhova IV, Bozhkov VM, Margulis BA. Accumulation of major stress protein 70 kDa protect myeloid and lymphoid cells from death by apoptosis. Apoptosis. 1997;2:156–163. doi: 10.1023/A:1026460330596. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]