Abstract
Background
Plasmodium vivax is the second most common species among malaria patients diagnosed in Europe, but epidemiological and clinical data on imported P. vivax malaria are limited. The TropNetEurop surveillance network has monitored the importation of vivax malaria into Europe since 1999.
Objectives
To present epidemiological and clinical data on imported P. vivax malaria collected at European level.
Material and methods
Data of primary cases of P. vivax malaria reported between January 1999 and September 2003 were analysed, focusing on disease frequency, patient characteristics, place of infection, course of disease, treatment and differences between network-member countries.
Results
Within the surveillance period 4,801 cases of imported malaria were reported. 618 (12.9%) were attributed to P. vivax. European travellers and immigrants were the largest patient groups, but their proportion varied among the reporting countries. The main regions of infection in descending order were the Indian subcontinent, Indonesia, South America and Western and Eastern Africa, as a group accounting for more than 60% of the cases. Regular use of malaria chemoprophylaxis was reported by 118 patients. With 86 (inter-quartile range 41–158) versus 31 days (inter-quartile range 4–133) the median symptom onset was significantly delayed in patients with chemoprophylaxis (p < 0.0001). Common complaints were fever, headache, fatigue, and musculo-skeletal symptoms. All patients survived and severe clinical complications were rare. Hospitalization was provided for 60% and primaquine treatment administered to 83.8% of the patients, but frequencies varied strongly among reporting countries.
Conclusions
TropNetEurop data can contribute to the harmonization of European treatment policies.
Introduction
Plasmodium vivax, has a major adverse impact on global health [1], accounting for 70–80 million clinical cases annually. It is responsible for over 50% of malaria outside Africa, notably in Southeast Asia and Central and South America, and particularly on the Indian subcontinent. It also accounts for around 10% of cases in Eastern and Southern Africa but has only limited prevalence in West Africa, presumably due to the presence of Duffy-negative blood group variants that limit erythrocyte invasion by the parasite [2-5]. Similar to Plasmodium falciparum, P. vivax may cause severe anaemia, but major complications like cerebral malaria, hypoglycaemia, metabolic acidosis and respiratory distress observed in P. falciparum malaria, do not occur [3,6].
When imported to non-endemic areas, vivax malaria, despite its uncomplicated course, requires special attention for two reasons. First, diagnosis often is complicated by the late onset of symptoms which unlike those observed in falciparum malaria, may occur several months after arrival from endemic areas [7-10]. Second, case management is complicated by the fact that parasites can remain dormant in the liver as hypnozoites. Thus, even if blood stages of the parasite are cleared, reactivation of these liver forms may cause relapses within a few months [5,11].
Malaria is a notifiable disease in all European countries [12]. As shown by national surveillance reports [13-19], P. vivax is presently the second most frequent cause of imported malaria in most of Europe, except France [9,20], accounting for up to 40% of the annual cases in single countries. However, since P. vivax is less virulent than P. falciparum [3,6], surveillance reports primarily focus on P. falciparum infections. Epidemiological and clinical details of imported P. vivax malaria are, therefore, hardly described.
Since 1999, the importation of vivax malaria into Europe has also been systematically monitored by the TropNetEurop surveillance network. The present article summarises epidemiological and clinical data of the disease collected during the first 56 months of surveillance at European level, focusing on disease frequency, patient characteristics, place of infection, course of disease and treatment. Differences between network-member countries are highlighted.
Patients, materials and methods
The European network TropNetEurop was founded in 1999 to effectively detect emerging infections of potential regional, national or global impact at their point of entry into the domestic population, to link clinical and epidemiological knowledge and to serve as a platform for multi-centre research. Sentinel surveillance reporting is carried out by participating sites by use of a standardised and computerised reporting system. Electronic transmission of anonymous case information, comprising standardised demographic, epidemiological and clinical data, to the central database assures rapid detection of sentinel events. Presently 46 clinical sites from 16 European countries are organised in the network. Additional information about TropNetEurop and the reporting instruments can be received from the internet at http://www.tropnet.net.
The present work summarises data of primary cases of P. vivax malaria reported within the network between January 1999 and September 2003. Relapse notifications were excluded from the database to avoid multiple-counting of importation events. Mixed-infections with P. vivax and other Plasmodium species were included. However, while analyses focusing on disease frequency, patient characteristics and place of infection were based on all observations, analyses with clinical end point were restricted to mono-infections elicited microscopically (confirmed cases) or by antibody detection (probable cases) in order to reduce bias. Patients with mixed plasmodial or other concomitant infections, cases diagnosed by clinical reasoning (suspected cases) and cases reported without specification of the underlying diagnostic proof (unclassified cases) were excluded from those analyses.
All analyses were done with the SAS software (release 8.01 by SAS Institute Inc., Cary, NC, USA). Missing values were assumed to be non-systematic. Incomplete cases were therefore disregarded in single calculations. However, sample size or missing data information is given with each result.
Results
Between January 1999 and September 2003, a total of 4,801 patients with travel-related malaria were reported within the TropNetEurop network. P. vivax was involved in 618 (12.9%) cases, either as sole pathogen (n = 585) or in mixed-infections with other species (n = 33), thus accounting for the second highest number of cases after P. falciparum. The reported proportion of P. vivax was rather steady with 10.9% in 1999, 12.6% in 2000, 15.1% in 2001, 12.3% in 2002 and 12.9% in 2003. All 16 TropNetEurop countries reported P. vivax malaria. However, as shown in table 1, the number of cases varied strongly between countries. Germany (24.3%), Spain (15.5%) and the UK (12.0%) reported most cases, whereas reports from Switzerland (1.8%), Poland (1.6%), Finland (1.0%), Ireland (1.0%) and Portugal (0.3%) were scarce. According to diagnostic information 557 (90.1%) of the 618 infections were confirmed, two (0.3%) were probable and eight (1.3%) were suspected cases. The remaining 51 (8.3%) could not be classified, due to missing information on the underlying diagnostic procedure. Those and the suspected cases were excluded from analyses with clinical endpoints in the latter.
Table 1.
Frequency of P. vivax malaria by year and reporting country
| 1999 | 2000 | 2001 | 2002 | 2003* | Total | |
| Country | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Austria | 2 (2.1) | 8 (4.9) | 5 (2.7) | 5 (3.9) | 3 (6.3) | 23 (3.7) |
| Belgium | 17 (10.4) | 8 (4.3) | 7 (5.5) | 2 (4.2) | 34 (5.5) | |
| Czech Rep. | 9 (9.6) | 13 (7.9) | 20 (10.8) | 5 (3.9) | 3 (6.3) | 50 (8.1) |
| Denmark | 14 (14.9) | 16 (9.8) | 12 (6.5) | 5 (3.9) | 1 (2.1) | 48 (7.8) |
| Finland | 1 (0.6) | 6 (4.7) | 6 (1.0) | |||
| France | 1 (1.1) | 1 (0.6) | 4 (2.2) | 7 (5.5) | 2 (4.2) | 15 (2.4) |
| Germany | 31 (33.0) | 35 (21.3) | 51 (27.6) | 22 (17.3) | 11 (22.9) | 150 (24.3) |
| Ireland | 2 (1.2) | 1 (0.5) | 1 (0.8) | 2 (4.2) | 6 (1.0) | |
| Italy | 8 (8.5) | 10 (6.1) | 16 (8.6) | 3 (2.4) | 37 (6.0) | |
| Norway | 4 (2.2) | 8 (6.3) | 1 (2.1) | 13 (2.1) | ||
| Poland | 3 (3.2) | 4 (2.2) | 3 (2.4) | 10 (1.6) | ||
| Portugal | 1 (0.8) | 1 (2.1) | 2 (0.3) | |||
| Spain | 15 (16.0) | 31 (18.9) | 23 (12.4) | 23 (18.1) | 4 (8.3) | 96 (15.5) |
| Sweden | 4 (4.3) | 8 (4.9) | 15 (8.1) | 8 (6.3) | 8 (16.7) | 43 (7.0) |
| Switzerland | 4 (4.3) | 1 (0.6) | 5 (3.9) | 1 (2.1) | 11 (1.8) | |
| UK | 3 (3.2) | 22 (13.4) | 22 (11.9) | 18 (14.2) | 9 (18.8) | 74 (12.0) |
| Total | 94 (100) | 164 (100) | 185 (100) | 127 (100) | 48 (100) | 616 (100) |
* Data reported between January and September 2003
Table 2 describes characteristics of the 618 patients. About 2/3 were male. The median age was 32 years (inter-quartile range 26–43). About 50% reported reception of pre-travel advice, 42.2% stated use of anti-malarial chemoprophylaxis. Among those with prophylaxis, 62.1% stated compliance with the recommended regimen.
Table 2.
Characteristics of patients with P. vivax malaria
| N | % | |
| Sex (n = 615) | ||
| Male | 422 | 68.6 |
| Female | 193 | 31.4 |
| Pre-travel advice (n = 387) | ||
| Yes | 208 | 53.7 |
| No | 179 | 46.3 |
| Prophylaxis (n = 554) | ||
| None | 320 | 57.8 |
| Chloroquine | 39 | 7.0 |
| Proguanil | 6 | 1.1 |
| Proguanil + Chloroquine | 45 | 8.1 |
| Mefloquine | 123 | 22.2 |
| Doxycycline | 12 | 2.2 |
| Proguanil + Atovaquone | 3 | 0.5 |
| Other | 6 | 1.1 |
| Compliance (n = 190) | ||
| Yes | 118 | 62.1 |
| No | 72 | 37.9 |
| Patient classification (n = 615) | ||
| Immigrants/Refugees | 121 | 19.7 |
| Foreign visitors | 36 | 5.8 |
| Europeans living in EC | 406 | 66.0 |
| European expatriates | 52 | 8.5 |
| Median | IQ-Range | |
| Age (n = 606) | 32 | 26–43 |
IQ-Range = Inter-quartile range
The majority of patients who imported vivax malaria into Europe were Europeans living and working in Europe (66.0%). Immigrants and refugees, summarising both those of overseas origin who may have lived in the reporting country for many years and very recent immigrants, made up the second largest group (19.7%), followed by European expatriates (8.5%) and foreign visitors (5.8%). Analysing patient classifications by reporting country revealed that immigrants and refugees accounted for distinctly more than the overall 20% proportion in Norway (61.5%), Italy (45.9%), France (40.0%), Spain (31.3%) and Denmark (25.0%).
Reasons for travel differed for Europeans and immigrants. While Europeans living in Europe mainly travelled for tourism (71.4%), followed by business (7.8%), missionary work (7.0%), research (6.3%), visits to relatives or friends (6.0%), military missions (0.8%) or other reasons (0.8%). The main reasons for travel in the immigrant group were immigration to Europe (47.0%) or visits to relatives or friends in the former home country (44.4%).
Figure 1 marks 16 geographical regions from which P. vivax malaria was imported from during the 56 month surveillance period. The main regions of infection were the Indian subcontinent (17.0%), Indonesia (12.1%), South America (11.4%) and Western Africa (11.4%), as a group accounting for 52% of the cases. However, while the Indian subcontinent was the main region of infection each year, the others switched places in the annual ranking order. Further regions of importance were Eastern Africa (10.0%), Southeast Asia (8.6%), and Oceania (8.5%), contributing another 27% of the cases. Main countries of infection were Indonesia (12.1%), India (8.7%), Papua New Guinea (8.0%), Pakistan (7.8%) and Ecuador (5.7%).
Figure 1.
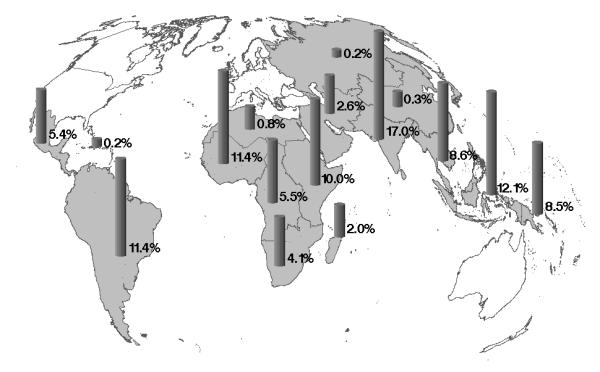
Geographical origin of P. vivax malaria imported to Europe between January 1999 and September 2003 (n = 618)
Exclusion of patients with concomitant plasmodial or other infections and cases with suspected or unknown diagnostic status left 526 patients for the analysis of clinical endpoints. Symptom information was given for 487 of those. The most frequent complaints were fever, headache, fatigue and musculo-skeletal symptoms, affecting 95.5%, 51.3%, 32.6% and 29.6% of the patients, respectively. However, a variety of other symptoms was noted, too. Further information on the course of the disease is summarised in table 3. The median time from end of journey to symptom onset was 60 days (inter-quartile range 8–149). However, with 86 versus 31 days the median onset of symptoms was significantly delayed in patients with chemoprophylaxis (p<0.0001 Wilcoxon rank test). More than half of the patients (60.1%) were hospitalised, although in-patient treatment was distinctly less common in Ireland (0%), Switzerland (9.1%), Belgium (15.6%) and Spain (26.1%). The median length of hospitalization was four days (inter-quartile range 2–5). Information whether complications occurred during the course of the disease, was given for 270 of the 526 patients. Complications were reported in 30 of them, 22 mentioning recrudescence or relapse, one G6PD-deficiency and seven indicating severe disease. Specific complications in the latter group were serious spleno- or hepatomegaly (3 patients), spleen-rupture (1 patient), pancytopenia (1 patient), macrohaematuria (1 patient) and psychosis (1 patient). All 618 patients survived.
Table 3.
Course of disease in 526 patients with confirmed or probable P. vivax mono-infection
| Median | IQ-Range | |
| Days from end of journey to onset (nmiss = 80) | 60 | 8–149 |
| with chemoprophylaxis | 86 | 41–158 |
| without chemoprophylaxis | 31 | 4–133 |
| Days in hospital (nmiss = 400) | 4 | 2–5 |
| N | % of non missing | |
| In-patients (nmiss = 7) | 312 | 60.1 |
| Complications (nmiss = 256) | 30 | 11.1 |
| indicating treatment failure | 23 | 8.5 |
| indicating severe disease | 7 | 2.6 |
| Fatalities (nmiss = 0) | 0 | 0.0 |
Nmiss= Number of cases disregarded due to missing data; IQ-Range = Inter-quartile range
Treatment information was given for 518 confirmed and probable mono-infections. Table 4 presents frequencies of drugs used in the treatment of P. vivax malaria. Although primaquine and chloroquine was the most frequently used drug combination, 84 (16.2%) patients, including 61 males older than four, were not treated with primaquine. Least frequent use of primaquine was reported from France (0.0%), Ireland (20%), Poland (40.0%) and Finland (50.0%).
Table 4.
Drugs used in the treatment of 518 patients with P. vivax malaria
| Drugs | No. of patients | % |
| Primaquine | 434 | 83.8 |
| Chloroquine | 426 | 82.2 |
| Quinine | 48 | 9.3 |
| Mefloquine | 36 | 6.9 |
| Atovaquone/Proguanil | 12 | 2.3 |
| Artemether/Lumefantrine | 4 | 0.8 |
| Proguanil | 3 | 0.6 |
| Artemisinin-derivates | 2 | 0.4 |
| Pyriniethamine/Sulfadoxine | 1 | 0.2 |
Note. Multiple entries per patient were possible
Discussion
Since network membership is self-selected, TropNetEurop surveillance data may not be representative for the whole of Europe. However, since most major referral centres of the continent are represented in the network and the number of patients treated within the network is large (~62,000 patients/year), approximate representativeness can be assumed. One objective of the present work was to look at differences between TropNetEurop-member countries. However, since some countries contributed only small numbers of cases to our database (see table 1) differences found in between country comparisons should rather be understood as hints than taken for proof.
According to TropNetEurop data, P. vivax is the second most frequent cause of malaria importation, accounting for 12.9% of the case imports into Europe since 1999. Analysis of data from 612 patients treated for P. vivax malaria in Europe between January 1999 and September 2003 yielded that 118 patients had contracted the disease despite compliant chemoprophylaxis. However, since standard chemoprophylactics do not act against hypnozoites [11,21-23], this does not indicate drug failure. Primaquine, which acts against hypnozoites [11,21-24] and was shown to be effective in the prevention of falciparum and vivax malaria [25-27] has been proposed in North America as an optional agent for regular or terminal prophylaxis in certain travellers [23,28-30]. The fact that primaquine prophylaxis was not reported in any of the TropNetEurop cases reflects the fact that it is either very effective, or uncommonly used in Europe. Most likely the latter is true, since primaquine is presently not licensed for prophylactic use. About half of the patients treated for vivax malaria in Europe were Europeans, who contracted the disease on holiday. Immigrants and refugees made up the second largest patient group, with an overall proportion of about 20%. In Norway, Italy, France, Spain and Denmark they accounted for even more of the cases. The fact that more than 40% of the immigrants had contracted the disease on a visit to their former home country reveals that even those who may have lived in Europe for many still contribute considerably to malaria importation.
It seems peculiar that Western Africa was found to be a major region of infection, accounting for more cases than Eastern Africa. P. vivax prevalence was reported to be very limited in Western Africa, due to the presence of Duffy-negative blood-group variants [2-5]. Because our surveillance data lack a true denominator it cannot be excluded, that the larger number of patients returning from Western Africa with vivax malaria results from higher travel activity to that region. However, since international tourist arrivals counted by the world tourist organisation in 1999 and 2000 http://www.world-tourism.org were twice as high in Eastern Africa, this appears to be an unlikely explanation. Another hypothetical explanation would be that a major part of the vivax cases reported from Western Africa might be misclassified infections with Plasmodium ovale, which is more common in that region and difficult to distinguish from P. vivax microscopically.
Analysis of the course of the disease revealed that half of the patients fell ill later than 60 days after arrival from an endemic area. The most common complaints were fever, headache, fatigue, and musculo-skeletal symptoms. The finding that symptom onset was significantly delayed in patients with chemoprophylaxis is consistent with recently published findings [10]. This can be explained by the activity of standard prophylactics against blood stages but not against hypnozoites. No fatalities and only few severe clinical complications were reported, which emphasises P. vivax's limited virulence as compared to P. falciparum [3,6]. More than half of the patients received in-patient treatment, indicating differences in national treatment policies rather than severe disease, since hospitalization rates varied greatly among TropNetEurop member countries.
None of the blood schizonticides used in the treatment of malaria affects hypnozoites of P. vivax, thus radical cure without relapses can only be achieved by additional administration of primaquine [11,21,23,31]. However, since P. vivax strains differ in their innate sensitivity to primaquine, anti-relapse treatment may fail, especially when underdosed [21,32-34]. On the other hand, omission of antirelapse treatment does not necessarily lead to relapses [35]. Both uncertainties might be used as an argument to restrict primaquine use to the treatment of recurrent episodes of the disease, even in patients without G6PD-deficiency [36]. Still, relapses may seriously threaten patients' health, whereas primaquine, which is highly effective in relapse prevention [31,32], is well tolerated [25-28]. This indicates that unrestricted use of primaquine in patients without G6PD-deficiency might offer additional health benefits. However, this has not been evaluated in systematic risk benefit analyses. Our data on complications support the finding that primaquine treatment is well tolerated but not perfectly preventive. Within the network, 16.2% of the patients did not receive primary anti-relapse treatment with primaquine. Contraindications like G6PD-deficiency, young age or pregnancy could be ruled out as an explanation in most of these cases [21]. Primaquine relapse prevention was found to be common in most TropNetEurop member countries, with France, Ireland, Poland, and Finland showing clearly lower utilization of the drug. This indicates heterogeneity of national or site-specific treatment policies. An inquiry among members of TropNetEurop confirmed that some sites actually do limit primaquine to the treatment of recurrent episodes.
Acknowledgments
Acknowledgments
All the authors of this paper are members of TropNetEurop, the European Network on Imported Infectious Disease Surveillance http://www.tropnet.net/
Contributor Information
N Mühlberger, Email: muehlberger@bbges.de.
T Jelinek, Email: Jelinek@bbges.de.
J Gascon, Email: JGASCON@clinic.ub.es.
M Probst, Email: meike.probst@charite.de.
T Zoller, Email: thomas.zoller@charite.de.
M Schunk, Email: schunk@lrz.uni-muenchen.de.
J Beran, Email: jiri.beran@vakcinace.cz.
I Gjørup, Email: ida.gjorup@dadlnet.dk.
RH Behrens, Email: Ron.Behrens@lshtm.ac.uk.
J Clerinx, Email: jclerinx@poliklin.itg.be.
A Björkman, Email: anders.bjorkman@ks.se.
P McWhinney, Email: pmcw@doctors.org.uk.
A Matteelli, Email: amatteelli@bsnet.it.
R Lopez-Velez, Email: rlopezvelez@hrc.insalud.es.
Z Bisoffi, Email: zeno.bisoffi@sacrocuore.it.
U Hellgren, Email: urban.hellgren@medhs.ki.se.
S Puente, Email: spuente@hciii.insalud.es.
ML Schmid, Email: Matthias.Schmid@nuth.northy.nhs.uk.
B Myrvang, Email: Bjorn.Myrvang@ulleval.no.
ML Holthoff-Stich, Email: ml.ho-stich@missioklinik.de.
H Laferl, Email: herman.laferl@KFJ.magwien.gv.at.
C Hatz, Email: hatz@keep.touch.ch.
H Kollaritsch, Email: herwig.kollaritsch@univie.ac.at.
A Kapaun, Email: annette_kapaun@med.uni-heidelberg.de.
J Knobloch, Email: juergen.knobloch@med.uni-tuebingen.de.
J Iversen, Email: johan.iversen@inet.uni2.dk.
A Kotlowski, Email: akotl@immt.gdynia.pl.
DJM Malvy, Email: denis.malvy@chu-bordeaux.fr.
P Kern, Email: peter.kern@medizin.uni-ulm.de.
G Fry, Email: tropical@iol.ie.
H Siikamaki, Email: heli.siikamaki@fimnet.fi.
MH Schulze, Email: mhschulze@yahoo.com.
G Soula, Email: Jean.Delmont@mail.ap-hm.fr.
M Paul, Email: mpaul@eucalyptus.am.poznan.pl.
J Gómez i Prat, Email: jordigp.pbcn@ics.scs.es.
V Lehmann, Email: vidar.lehmann@helse-bergen.no.
O Bouchaud, Email: olivier.bouchaud@bch.ap-hop-paris.fr.
S da Cunha, Email: saraiva@huc.min-saude.pt.
J Atouguia, Email: jma@ihmt.unl.pt.
G Boecken, Email: tropmed.marine@t-online.de.
References
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Weatherall DJ, Miller LH, Baruch DI, Marsh K, Doumbo OK, Casals-Pascual C, Roberts DJ. Malaria and the red cell. Hematology (Am Soc Hematol Educ Program) 2002:35–57. doi: 10.1182/asheducation-2002.1.35. [DOI] [PubMed] [Google Scholar]
- Ridley RG. Chemotherapeutic hope on the horizon for Plasmodium vivax malaria? Proc Natl Acad Sci USA. 2002;99:13362–133624. doi: 10.1073/pnas.232483699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunaweera ND, Wijesekera SK, Wanasekera D, Mendis KN, Carter R. The paroxysm of Plasmodium vivax malaria. Trends Parasitol. 2003;19:188–193. doi: 10.1016/S1471-4922(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Kain KC, Harrington MA, Tennyson S, Keystone JS. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis. 1998;27:142–149. doi: 10.1086/514616. [DOI] [PubMed] [Google Scholar]
- Filler S, Causer LM, Newman RD, Barber AM, Roberts JM, MacArthur J, Parise ME, Steketee RW, Dorsey G, Gandhi M, Oyugi JH, Rosenthal PJ, Romi R, Sabatinelli G, Majori G, Svenson JE, MacLean JD, Gyorkos TW, Keystone J, Juckett G, Boccolini D, Kain KC, Harrington MA, Tennyson S, Keystone JS. Malaria surveillance – United States, 2001. MMWR Surveill Summ. 2003;52:1–14. [PubMed] [Google Scholar]
- Danis M, Legros F, Thellier M, Caumes E. Données actuelles sur le paludisme en France métropolitaine. Méd Trap (Mars) 2002;62:214–218. [PubMed] [Google Scholar]
- Schwartz E, Parise M, Kozarsky P, Cetron M. Delayed onset of malaria – implications for chemoprophylaxis in travelers. N Engl J Med. 2003;349:1510–1516. doi: 10.1056/NEJMoa021592. [DOI] [PubMed] [Google Scholar]
- Baird JK, Rieckmann KH. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 2003;19:115–120. doi: 10.1016/S1471-4922(03)00005-9. [DOI] [PubMed] [Google Scholar]
- Legros F, Danis M. Surveillance of malaria in European Union countries. Euro Surveill. 1998;3:45–47. doi: 10.2807/esm.03.05.00103-en. [DOI] [PubMed] [Google Scholar]
- Romi R, Boccolini D, Majori G. Malaria incidence and mortality in Italy in 1999–2000. Euro Surveill. 2001;6:143–147. doi: 10.2807/esm.06.10.00378-en. [DOI] [PubMed] [Google Scholar]
- Strauss R, Pfeifer C. Malaria in Austria 1990–2000. Euro Surveill. 2003;8:91–96. doi: 10.2807/esm.08.04.00408-en. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Warhurst DC, Blaze M, Smith V, Williams J. Malaria imported into the United Kingdom in 1996. Euro Surveill. 1998;3:40–42. doi: 10.2807/esm.03.04.00107-en. [DOI] [PubMed] [Google Scholar]
- RKI Reiseassoziierte Infektionskrankheiten in Deutschland 2001. Epidemiols Bull. 2002. pp. 285–296.
- Overview of malaria notifications in the Netherlands, 1995–9. Eurosurveill Weekl. 2000;4:38. [Google Scholar]
- Malaria in Denmark, 1999. Eurosurveill Weekl. 2000;4:38. [Google Scholar]
- Imported malaria in Finland, 2001. Euro Surveill Weekl. 2002;6:17. [Google Scholar]
- Legros F, Gay F, Belkaid M, Danis M. Imported malaria in continental France in 1996. Euro Surveill. 1998;3:37–38. doi: 10.2807/esm.03.04.00105-en. [DOI] [PubMed] [Google Scholar]
- WHO The use of antimalarial drugs – Report of an informal consultation (13 to 17 November 2000) 2001. p. 144.
- Kain KC, Shanks GD, Keystone JS. Malaria chemoprophylaxis in the age of drug resistance. I. Currently recommended drug regimens. Clin Infect Dis. 2001;33:226–234. doi: 10.1086/321817. [DOI] [PubMed] [Google Scholar]
- Canadian recommendations for the prevention and treatment of malaria among international travellers. Committee to Advise on Tropical Medicine and Travel (CATMAT), Laboratory for Disease Control. Can Commun Dis Rep. 2000;26 Suppl 2:1–42. [PubMed] [Google Scholar]
- Arnold J, Alving AS, Hockwald RS, Clayman CB, Dern RJ, Beutler E, Flanagan CL, Jeffery GM. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, P-F-6 strain) J Lab Clin Med. 1955;46:391–397. [PubMed] [Google Scholar]
- Fryauff DJ, Baird JK, Basri H, Sumawinata I, Purnomo , Richie TL, Ohrt CK, Mouzin E, Church CJ, Richards AL, et al. Randomised placebo-controlled trial of primaquine for prophylaxis of falciparum and vivax malaria. Lancet. 1995;346:1190–1193. doi: 10.1016/S0140-6736(95)92898-7. [DOI] [PubMed] [Google Scholar]
- Soto J, Toledo J, Rodriquez M, Sanchez J, Herrera R, Padilla J, Berman J. Primaquine prophylaxis against malaria in nonimmune Colombian soldiers: efficacy and toxicity. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129:241–244. doi: 10.7326/0003-4819-129-3-199808010-00013. [DOI] [PubMed] [Google Scholar]
- Baird JK, Lacy MD, Basri H, Barcus MJ, Maguire JD, Bangs MJ, Gramzinski R, Sismadi P, Krisin , Ling J, Wiady I, Kusumaningsih M, Jones TR, Fryauff DJ, Hoffman SL. Randomized, parallel placebo-controlled trial of primaquine for malaria prophylaxis in Papua, Indonesia. Clin Infect Dis. 2001;33:1990–1997. doi: 10.1086/324085. [DOI] [PubMed] [Google Scholar]
- Paul MA, McCarthy AE, Gibson N, Kenny G, Cook T, Gray G. The impact of Malarone and primaquine on psychomotor performance. Aviat Space Environ Med. 2003;74:738–745. [PubMed] [Google Scholar]
- Juckett G. Malaria prevention in travelers. Am Fam Physician. 2000;61:2535–2536. [PubMed] [Google Scholar]
- Shanks GD, Kain KC, Keystone JS. Malaria chemoprophylaxis in the age of drug resistance. II. Drugs that may be available in the future. Clin Infect Dis. 2001;33:381–385. doi: 10.1086/321866. [DOI] [PubMed] [Google Scholar]
- Pukrittayakamee S, Chantra A, Simpson JA, Vanijanonta S, Clemens R, Looareesuwan S, White NJ. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother. 2000;44:1680–1685. doi: 10.1128/AAC.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay NJ, Desai S, Kamtekar KD, Kadam VS, Dalvi SS, Kshirsagar NA. Efficacies of 5- and 14-day primaquine regimens in the prevention of relapses in Plasmodium vivax infections. Ann Trop Med Parasitol. 1999;93:809–812. doi: 10.1080/00034989957790. [DOI] [PubMed] [Google Scholar]
- Jelinek T, Nothdurft HD, Von Sonnenburg F, Loscher T. Long-term efficacy of primaquine in the treatment of vivax malaria in nonimmune travelers. Am J Trop Med Hyg. 1995;52:322–324. doi: 10.4269/ajtmh.1995.52.322. [DOI] [PubMed] [Google Scholar]
- da Silva Rdo S, Pinto AY, Calvosa VS, de Souza JM. Esquemas terapêuticos encurtados para o tratament de malária por Plasmodium. Rev Soc Bras Med Trop. 2003;36:235–239. [PubMed] [Google Scholar]
- Yadav RS, Ghosh SK. Radical curative efficacy of five-day regimen of primaquine for treatment of Plasmodium vivax malaria in India. J Parasitol. 2002;88:1042–1044. doi: 10.1645/0022-3395(2002)088[1042:RCEOFD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nicolas X, Granier H, Laborde JP, Talarmin F, Klotz F. Plasmodium vivax: actualités thérapeutiques. Presse Méd. 2001;30:767–771. [PubMed] [Google Scholar]


