Abstract
Individuals struggling with obesity often have difficulty maintaining dietary regimens. One source of dietary relapse is the reinstatement of previous feeding behaviors following the presentation of cues indicating the availability of palatable but highly caloric food reward. The drugs fenfluramine and sibutramine have previously been prescribed because they enhance satiety mechanisms and decrease meal size. However, it is unclear whether these anorectic agents are also effective in blocking the cue-induced reinstatement of food-seeking behaviors. In these three experiments, we compared the effects of systemic treatment of d-fenfluramine (3 mg/kg; N=11) and sibutramine (3 mg/kg; N=11) with that of the D1 antagonist SCH 23390 (6 μg/kg; N=11) at a dose that has previously been shown to attenuate cue-induced reinstatement. D-fenfluramine treatment blocked the cue’s ability to reinstate lever pressing as compared to the saline injection day. In contrast, sibutramine had no effect on cue-induced reinstatement; all animals reinstated their lever pressing during the first reinstatement test, and this was unaffected by sibutramine treatment. SCH 23390 treatment did not significantly reduce cue-induced reinstatement in this set of experiments. The results suggest that the motivational effects of d-fenfluramine is not limited to the promotion of satiety once a meal has been initiated, and demonstrate that some anorectic treatments may inhibit the effectiveness of conditioned cues to elicit relapse of food-seeking behavior.
Keywords: Fenfluramine, sibutramine, reinstatement, motivation, food-seeking
Introduction
Recent increases in medical costs and illness due to the current obesity epidemic have caused a large-scale effort aimed at understanding the environmental, biological, and behavioral elements that contribute to obesity. Insight has developed from investigations into the mechanisms underlying the role of hypothalamic and peripheral systems in maintaining a defended body weight (homeostasis; e.g., [1, 2]), as well as from research examining the role of food and food-associated cues as learned incentive objects that themselves promote approach and consumption (e.g., [3–6]). A main challenge for those intending to lose weight by dieting is to resist the temptation of preferred, highly caloric foods despite continued exposure to the food objects and with cues associated with them. Breaking with one’s diet can be viewed as a form of relapse, in the same way that someone with a history of drug abuse may relapse to drug-seeking behavior after a period of abstinence.
Traditional approaches to the preclinical testing of anorectic medications in animal models have tended to emphasize the impact of drug agents on satiety mechanisms. For example, several drugs that successfully reduce food intake and body weight in rats have been shown to reduce the size of meals by advancing the development of behaviors that indicate satiety (the “behavioral satiety sequence”; [7]). However, many medications that effectively enhance satiety mechanisms in animals and humans have had side-effects that have resulted in their withdrawal from the market (e.g., phen-fen combinations in the 1990s, sibutramine treatment from 1997–2010). Although clinical studies have occasionally reported declines in food craving when some of these medications have been given in humans [8, 9], there has been little examination of whether these agents might also impact food-seeking in animal reinstatement procedures that model dietary relapse in humans.
There are good reasons for this to be of interest. In a dieter’s battle to eat less, it would not only be advantageous to end meals early, but it would also be significant if the sight and smells of palatable food were less likely to initiate a meal (or a mid-day snack) in the first place. In animals, the influence of food-associated cues on food seeking can be examined through the use of reinstatement procedures [10, 11]. In these procedures, rats are trained to press a lever for a reinforcer (food or a drug of abuse) in the presence of cues. Subsequently, the behavior is extinguished until the rat responds at a fraction of the rate that was acquired during training. Then, reinstatement tests occur that examine the effect of presenting the cues that once predicted the reinforcer (cue-induced reinstatement) on the lever-pressing behavior. Reinstatement is measured as a function of an increase of lever-pressing behavior over the values obtained during extinction trials, even though the rat does not receive food pellets or drug delivery that is contingent upon their behavior during the reinstatement test.
Several recent pharmacotherapies for obesity have targeted serotonergic mechanisms in regulating food intake and body weight. Though no longer prescribed, fenfluramine and sibutramine are both pharmacological agents that increase serotonin within the synapse, and each has been previously prescribed to promote weight loss [12]. Both drugs have been reported to advance the satiety sequence in rats and reduce meal size [7, 13–15]. However, despite their known efficacy in promoting weight loss, there has been very little investigation into whether these drugs impact food-seeking behavior in animal reinstatement models. In one notable exception, it has been shown that d-fenfluramine treatment in rats blocks the reinstatement of food-seeking behavior that normally occurs in response to priming (non-contingent food delivery) or pharmacologically-induced stress [16]. These findings are intriguing because they suggest that serotonin-based pharmacotherapy for food intake may not only reduce meal size, but may also have the potential to reduce the seeking of food that is elicited by food-associated cues.
The current experiments sought to examine the effects of the anorectic agents d-fenfluramine and sibutramine on cue-induced reinstatement of food-seeking in rats, and to compare their efficacy with that of blocking the dopamine D1 receptor (which has been previously shown to reduce food-seeking in a similar procedure [17]). Separate groups of food-restricted rats were trained to lever press for sugar pellet delivery in the presence of a light/tone cue. After learning was complete, the behavior was extinguished in the absence of pellet or cue delivery. Individual groups of rats were then tested under reinstatement conditions following systemic injections of d-fenfluramine, sibutramine, or the D1 antagonist SCH 23390, at doses previously shown to reduce food intake in feeding tests (sibutramine, fenfluramine) or alter incentive processes in rats (SCH 23390).
Methods
Subjects and housing
Male Sprague–Dawley rats (Harlan, Madison, WI) were dually housed in clear, polycarbonate cages with wire covers in a temperature and light controlled room (21 °C, 12-h light-dark cycle, lights on/off-7am/7pm). The subjects were handled daily upon arrival into the lab in an effort to reduce stress. All behavioral testing was conducted during the light phase. The following experiments conformed to NIH guidelines and were approved by the Wake Forest University Animal Care and Use Committee.
Apparatus
Six standard operant chambers (Med-Associates, St. Albans, VT, USA), fitted with a house light and two retractable levers, were used for the present experiments. Two identical 100-mA stimulus lights were located just above each lever; auditory stimuli were presented by a programmable speaker located on the wall opposite the levers and food hopper. Each chamber was enclosed in a sound-attenuating box equipped with a ventilation fan.
Behavioral procedure
Rats were food restricted by providing 8 gr of rat chow in the home cage daily until the animals reached 90% of their ad libitum body weight. Animals were then maintained at or slightly above their 90% weight by providing them with a daily ration of 12 g rat chow (on training days in which they earned large numbers of sugar pellets, see below) or the amount of chow needed to maintain them at 90% weight (usually 14–16 g), whichever was greater. Two days prior to the beginning of experimental training, rats were habituated to sugar pellets (45mg; BioServ) by supplementing their daily food ration with 2 g of the pellets. Rats were then acclimated to the operant chambers for two daily 30 min sessions, during which sugar pellets were delivered to the food hopper on a random-time 60 s schedule, with no levers present.
Operant training was initiated on the following day. Both levers were inserted into the chambers, and the rats were trained across 7 daily 20 min training sessions to lever press for sugar pellets. For the first day of training, active lever presses were reinforced on a fixed-ratio 1 schedule, in which each lever press resulted in the presentation of a 5 s light-tone cue (the light above the stimulus lever illuminated in conjunction with the presentation of an 80 dB, 3 kHz tone) and the delivery of a sugar pellet. Active lever presses during the cue presentation were recorded, but were not reinforced with a sugar pellet nor counted toward ratio requirements. Lever presses on the inactive lever, located on the opposite side of the food hopper, had no programmed consequences. Left/right positioning of the active lever was counterbalanced across the rats within each experimental group.
On the second day of operant training, rats were reinforced on a fixed-ratio 2 schedule, requiring two lever presses to achieve the same cue-reward presentation as on day 1. Training days 3–7 consisted of a variable-ratio (VR) 5 reinforcement schedule superimposed upon a fixed-interval (FI) 20 schedule of reinforcement. This VR-5, FI-20 schedule resulted in the first cue/pellet delivery on an average of 5 active lever presses. After receiving the initial reinforcement, a 20 s delay period passed before the reinforcement was available again. Following this rest interval, the VR-5 schedule resumed until another reinforcer was earned, and the schedule repeated. Similarly to previous reports [18, 19], this training procedure led to vigorous responding on the active lever by the seventh day of operant training.
Following the last day of VR-5, FI-20 training, rats underwent daily 20-min extinction sessions in which lever presses resulted in no programmed consequences. Extinction criterion was considered to be met when each individual rat lever pressed less than 10% compared to the last day of VR-5, FI-20 training on the previously active lever. One day after meeting criterion performance, each rat underwent the first of two 20-min reinstatement sessions. These reinstatement sessions consisted of the renewed presentation of the light-tone cues following the first lever press on the previously active lever. Further responding resulted in cue presentation on a VR-5 schedule. There was no sugar pellet delivery on reinstatement days, in order to specifically assess the cue-evoked incentive to lever press. The second reinstatement session for each rat was separated by a 48 hour period to assure complete drug metabolism between behavioral tests.
Drug Treatment
30 min prior to the two reinstatement sessions, rats received i.p injections of the drug or the vehicle solution (sterile isotonic saline). The order of the reinstatement trials (i.e. vehicle versus drug) were counterbalanced across rats within each experiment. On drug reinstatement days, rats in Experiment 1 (n=10) were treated with (+)-fenfluramine hydrochloride (3 mg/kg; Sigma), while rats in Experiment 2 (N=11) received injections of sibutramine (3 mg/kg; Tocris Bioscience). Previous studies have determined that, at these doses, fenfluramine and sibutramine are effective in attenuating appetite when injected systemically [12, 14, 20]. Rats in Experiment 3 (n=11) received injections of SCH23390 (6 μg/kg; Tocris Bioscience). SCH23390 has been previously shown to inhibit cue-induced reinstatement [17], and served as a positive control for these experiments.
Data Analyses
Reinstatement was operationally defined as a significant enhancement of lever presses on the previously active lever compared to the last day of extinction training. Saline-reinstatement days were the trials in which rats received systemic injections of the saline vehicle prior to reinstatement testing. Drug reinstatement days were defined as those trials in which the d-fenfluramine, sibutramine, or SCH23390 were systemically injected prior to cue-evoked reinstatement tests. For each group of rats, lever presses were analyzed with two separate repeated measures ANOVAs. The first compared activity on the active and inactive levers across the final extinction day and the saline reinstatement day, to verify successful reinstatement in the absence of drug treatment. A separate analyses was conducted to assess lever pressing across days in which rats received drug or saline treatment prior to reinstatement testing. Post-hoc pair-wise comparisons utilized Tukey’s HSD, as appropriate.
Results
Experiment 1: The effects of d-fenfluramine on the cue-evoked reinstatement of food-seeking
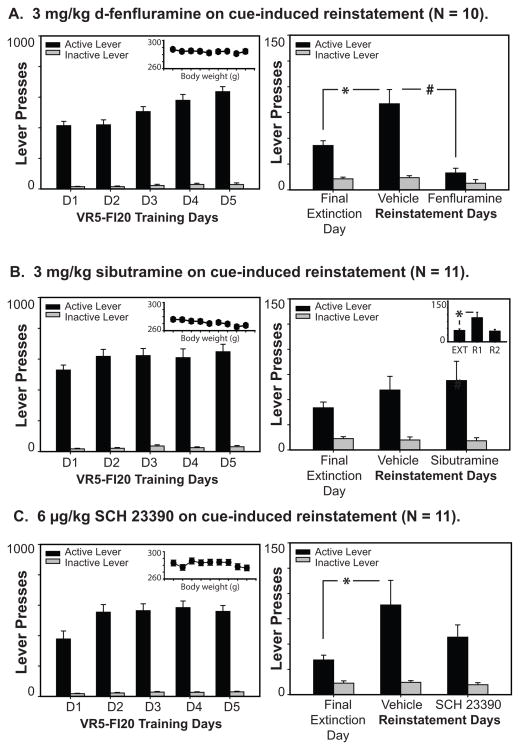
For all experiments, rats discriminated the reinforced lever from the lever that was never reinforced across all phases of the task (see Figure 1). Rats in Experiment 1 reinstated their responding on the active lever following response-contingent presentation of the light/tone cue on the saline reinstatement day; lever pressing was significantly increased on the previously active lever as compared to the final day of extinction (main effect of lever: F1,9 = 34.98, p < .001; main effect of testing day: F1,9 = 11.39, p = .008; lever X day interaction: F1,9 = 13.69, p = .005). Systemic injection of d-fenfluramine at 3 mg/kg blocked this cue-induced reinstatement of food-seeking behavior. Specifically, on days that rats received fenfluramine, they significantly reduced their activity on the previously reinforced lever in the presence of the food-associated cues (main effect of drug treatment: F1,9 = 17.21, p = .002; drug X lever interaction: F1,9 = 23.403, p = .001). Post-hoc analyses utilizing Tukey’s HSD demonstrated that these significant effects were driven by changes in activity on the previously reinforced lever (p < .05); lever presses on the inactive lever were not significantly different when compared across the final extinction day and the saline reinstatement day, nor across the saline and drug reinstatement tests (all p’s > .05).
Figure 1.
Effects of d-fenfluramine (A), sibutramine (B), and SCH 23390 (C) on cue-induced reinstatement of lever-pressing behavior. The left panels depict the average number of active and inactive lever presses for the five days of VR-5, FI-20 training for both experiments, and demonstrate that rats reliably discriminate between the reinforced and non-reinforced inactive lever during training as well as subsequent testing. Insets depict the body weights for each group across the five days of VR-5, FI-20 training, the first two days of extinction, and the reinstatement tests (from left to right). The panels on the right show total average active and inactive lever responding on the final extinction day and two reinstatement test days. Fenfluramine treatment significantly inhibited cue-induced reinstatement of food-seeking (A), whereas neither sibutramine nor SCH 23390 treatment significantly altered reinstatement as tested here (B & C). For sibutramine treatment, the inset shows that reinstatement was reliably seen on the first day of reinstatement testing, regardless of drug treatment. No differences in lever pressing were observed on the inactive lever across test conditions for any of these experiments. Stars (*) indicate a significant increase in lever pressing on the saline reinstatement day when compared to the final extinction day (as determined by Tukey HSD); the number sign (#) indicates a significant decrease in lever pressing activity on the drug restatement test as compared to that observed on the saline reinstatement day.
Experiment 2: The effects of sibutramine on the cue-evoked reinstatement of food-seeking
Rats treated with 3 mg/kg of sibutramine discriminated responding on the active lever across testing days (main effect of lever: F1,10 = 23.67, p = .001), but failed to demonstrate successful reinstatement of activity on the active lever as assessed between the final extinction session and the saline reinstatement test (main effect of testing day: F1,10 = 0.032, p = .86; lever X day interaction: F1,10 = 0.207, p = .66). Neither was there evidence that sibutramine affected lever pressing as compared to the saline reinstatement test (main effect of drug treatment: F1,10= 0.032, p = .86; drug X day interaction: F1,10 = 0.207, p = .66). To allay concerns that the reinstatement procedure did not work in this group of animals, we reanalyzed the data in temporal order, comparing the final extinction day with lever presses on the first reinstatement test, regardless of the drug treatment that each rat received on that day. As can be seen in the inset to Figure 1B, reinstatement in the presence of the light/tone cue occurred on the first day regardless of drug treatment (main effect of testing day: F1,10 = 9.146, p = .013; day X lever interaction, F1,10 = 7.496, p = .021), but was extinguished by the second test. This suggests that the reinstatement procedure itself was effective, but that sibutramine did not impact the cues’ effectiveness of enhancing food-seeking.
Experiment 3: The effects of D1 receptor antagonism on the cue-induced reinstatement of food-seeking behavior
Rats significantly reinstated their lever pressing behavior on the active lever during the saline reinstatement test, as compared to the final extinction day (main effect of lever: F1,10= 13.490, p = .004; main effect of testing day: F1,10 = 5.799, p = .037; lever X day interaction: F1,10 = 5.567, p = .028). Tukey’s post-hoc analysis verified that this effect was driven by a significant increase in lever pressing on the active, but not the inactive lever, on the saline reinstatement test day. However, the decrease in lever pressing observed when rats were systemically treated with 6 μg/kg SCH 23390 approached but did not achieve significance (main effect of lever: F1,10= 13.636, p = .004; main effect of drug: F1,10= 3.463, p = .092; drug X lever interaction: F1,10= 2.619, p = .137).
Discussion
These experiments are the first to test the effects of either d-fenfluramine or sibutramine treatment on cue-induced relapse to food-seeking behavior in a rodent model. Despite similar effects of these drugs on reducing meal size and advancing satiety processes [7, 13–15], the two drugs had differential effects on cue-induced reinstatement as tested here. Systemic treatment of d-fenfluramine blocked the reinstatement of lever pressing caused by the contingent presentation of food-associated cues in a similar manner to that previously shown following treatment with SCH 23390, a D1 receptor antagonist that is known to regulate incentive processes and that has been previously shown to block cue-induced reinstatement when injected either systemically or centrally [17, 19]. These results are consistent with a recent demonstration of d-fenfluramine’s efficacy in attenuating priming- and stress-induced relapse to food-seeking in a similar reinstatement model [16]. It is unlikely that the inhibition of reinstatement caused by fenfluramine was due to locomotor inhibition, as drug treatment did not significantly reduce pressing on the inactive lever. Furthermore, Pickens and colleagues [16] have reported that although fenfluramine treatment reduces lever-pressing in conditions of both food-reinforced responding and reinstatement tests (at the same dose used here), rats are not impaired in their ability to lever press at high rates or approach/enter the food cup.
In contrast to the effects of d-fenfluramine treatment, injections of the anorectic agent sibutramine did not affect cue-induced reinstatement. The dose chosen for examination was based upon the known efficacy of sibutramine on food consumption, as shown in our laboratory [14] and others [15, 20]. The 3 mg/kg dose tested here advances satiety processes and decreases food intake by 50% or more for as long as a 2-hr test period, but it does not cause locomotor impairments that have been reported at higher doses [20]. The current results suggest that in the absence of sensory and gustatory feedback of the food reinforcer, sibutramine does not reduce the learned incentive value of food-associated cues.
We did not replicate the previously-reported finding that D1 receptor antagonism with SCH 23390 significantly suppressed responding in a cue-induced reinstatement task. Though the pattern of behavior observed was similar to that shown following systemic D1 antagonism at an identical dose [17] and intracranial injections of SCH 23390 into the nucleus accumbens [19], the suppression in active lever responding did not achieve statistical significance here. It is possible that the lack of effect was due to procedural differences between this study and Ball and colleague’s experiments (which reported significant inhibition of cue-induced reinstatement following systemic SCH 23390 injections at the same dose), or may have resulted from a lack of power in discriminating the difference between conditions. Power did not appear to be a factor for the main experimental groups of this study, however, as fenfluramine’s drug effect was statisticially validated, and sibutramine showed no trend toward inhibiting cue-induced reinstatement (animals pressed, on average, slightly more times following sibutramine treatment than during the saline reinstatement test).
That d-fenfluramine and sibutramine did not both impact cue-induced reinstatement of food-seeking suggests two things. First, these data show that not all drugs that are effective at reducing meal size and advancing satiety processes may also be effective at reducing food-seeking behavior in the presence of cue incentives that can motivate the start of a meal or snack. Second, the mechanisms that fenfluramine and sibutramine utilize to increase neurotransmitter tone may have distinguishable effects on appetitive behavior. Fenfluramine treatment acts to increase serotonergic tone by directly promoting neurotransmitter release, whereas sibutramine acts by blocking reuptake of both serotonin and norepinephrine at their reuptake protein [21–23]. The latter mechanism is therefore dependent upon constitutive activity of serotonin processes, whereas the former is not. Given that difference, it will be interesting to determine if selective receptor activation of serotonin receptors that have been implicated in food intake and proposed as potential pharmacological targets for obesity (such as the 5-HT2C and 5-HT1B receptors) may also inhibit food-seeking behavior in this reinstatement procedure.
It should be noted that simply because a drug alters the incentive value of food or food cues does not, on its own, mean that an agent may be effective in the treatment of obesity. For instance, drugs that block dopamine D1 receptors often affect incentive processes in both rats and humans, including attenuating reinstatement caused by food-associated cues [17]. However, when D1 antagonists are administered either systemically [24] or centrally (e.g., into the nucleus accumbens, [25]), there is little effect on overall food consumption when food is made freely available. To further complicate this picture, dopamine agonists often enhance incentive processes (such as responding to a conditioned cue), but reduce food intake (at least when administered acutely). Recently, it has been shown that the blockade of dopamine D3 receptors alters motivation to approach food incentives in humans, and that it reduces lever-pressing for food availability in rodent models [26, 27]. Whether targeting the D3 receptor will have efficacy in promoting weight loss remains to be determined.
As the effort continues to develop efficacious weight loss medicines, the current data suggests that it may be important to consider the effects of new drug therapies on both the advancement of satiety processes, as well as their potential to inhibit the impact of food-associated cues to cause relapse to food-seeking behavior. The former mechanism allows for caloric reduction over the course of a meal, whereas the latter may provide some resistance to environmental triggers that cause relapse. Fenfluramine may be one such drug, but it was withdrawn from the weight loss market in 1997. As drugs continue to be developed, and combination therapies are tested, it may be of some use to consider the combination of low dose anorectic medication with agents that are effective in blocking the relapse to food-seeking in the presence of food-predictive cues. It remains to be determined if any of the agents that are currently under development or on the market (such as lorcaserin or the phentermine/topiramate combination) serve dual roles in inhibiting food-seeking and promoting satiety mechanisms.
Highlights.
We examined if two anorectic agents block cue-induced reinstatement of food-seeking
D-fenfluramine treatment blocked cue-driven reinstatement of lever-pressing in rats
In contrast, sibutramine treatment did not affect cue-induced reinstatement
Future pharmacotherapy for obesity should consider effects on appetitive motivation
Acknowledgments
This work was supported by DA030618 (WEP), a Undergraduate Research Grant from the national chapter of Psi Chi (RTF), and the Wake Forest University Department of Psychology. We would like to thank Kara A. Clissold for technical assistance during the execution of this project, and Elizabeth G. Guy for valuable comments on earlier drafts of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 2.Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int J Obes (Lond) 2011;35:1455–1465. doi: 10.1038/ijo.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M. Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1266–1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlayson G, King N, Blundell JE. Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev. 2007;31:987–1002. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halford JC, Wanninayake SC, Blundell JE. Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav. 1998;61:159–168. doi: 10.1016/s0091-3057(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 8.Hanotin C, Thomas F, Jones SP, Leutenegger E, Drouin P. Efficacy and tolerability of sibutramine in obese patients: a dose-ranging study. Int J Obes Relat Metab Disord. 1998;22:32–38. doi: 10.1038/sj.ijo.0800540. [DOI] [PubMed] [Google Scholar]
- 9.Wurtman RJ, Wurtman JJ. Carbohydrate craving, obesity and brain serotonin. Appetite. 1986;7(Suppl):99–103. doi: 10.1016/s0195-6663(86)80055-1. [DOI] [PubMed] [Google Scholar]
- 10.Calu DJ, Chen YW, Kawa AB, Nair SG, Shaham Y. The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halford JC, Harrold JA, Lawton CL, Blundell JE. Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets. 2005;6:201–213. doi: 10.2174/1389450053174550. [DOI] [PubMed] [Google Scholar]
- 13.Clifton PG, Lee MD, Dourish CT. Similarities in the action of Ro 60–0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 2000;152:256–267. doi: 10.1007/s002130000504. [DOI] [PubMed] [Google Scholar]
- 14.Pratt WE, Connolly ME. Contrasting effects of systemic and central sibutramine administration on the intake of a palatable diet in the rat. Neurosci Lett. 2010;484:30–34. doi: 10.1016/j.neulet.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Tallett AJ, Blundell JE, Rodgers RJ. Sibutramine-induced anorexia: potent, dose-dependent and behaviourally-selective profile in male rats. Behav Brain Res. 2009;198:359–365. doi: 10.1016/j.bbr.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Pickens CL, Cifani C, Navarre BM, Eichenbaum H, Theberge FR, Baumann MH, Calu DJ, Shaham Y. Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology (Berl) 2012;221:341–353. doi: 10.1007/s00213-011-2585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball KT, Combs TA, Beyer DN. Opposing roles for dopamine D1- and D2-like receptors in discrete cue-induced reinstatement of food seeking. Behav Brain Res. 2011;222:390–393. doi: 10.1016/j.bbr.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 18.Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Guy EG, Choi E, Pratt WE. Nucleus accumbens dopamine and mu-opioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res. 2011;219:265–272. doi: 10.1016/j.bbr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Higgs S, Cooper AJ, Barnes NM. Reversal of sibutramine-induced anorexia with a selective 5-HT(2C) receptor antagonist. Psychopharmacology (Berl) 2011;214:941–947. doi: 10.1007/s00213-010-2106-2. [DOI] [PubMed] [Google Scholar]
- 21.Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587:49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heal DJ, Aspley S, Prow MR, Jackson HC, Martin KF, Cheetham SC. Sibutramine: a novel anti-obesity drug. A review of the pharmacological evidence to differentiate it from d-amphetamine and d-fenfluramine. Int J Obes Relat Metab Disord. 1998;22(Suppl 1):S18–28. discussion S29. [PubMed] [Google Scholar]
- 24.Clifton PG, Rusk IN, Cooper SJ. Effects of dopamine D1 and dopamine D2 antagonists on the free feeding and drinking patterns of rats. Behav Neurosci. 1991;105:272–281. doi: 10.1037//0735-7044.105.2.272. [DOI] [PubMed] [Google Scholar]
- 25.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 26.Mogg K, Bradley BP, O’Neill B, Bani M, Merlo-Pich E, Koch A, Bullmore ET, Nathan PJ. Effect of dopamine D(3) receptor antagonism on approach responses to food cues in overweight and obese individuals. Behav Pharmacol. 2012;23:603–608. doi: 10.1097/FBP.0b013e3283566a4a. [DOI] [PubMed] [Google Scholar]
- 27.Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA, Ashby CR, Jr, Gardner EL, Volkow ND. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav. 2008;89:499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]