Abstract
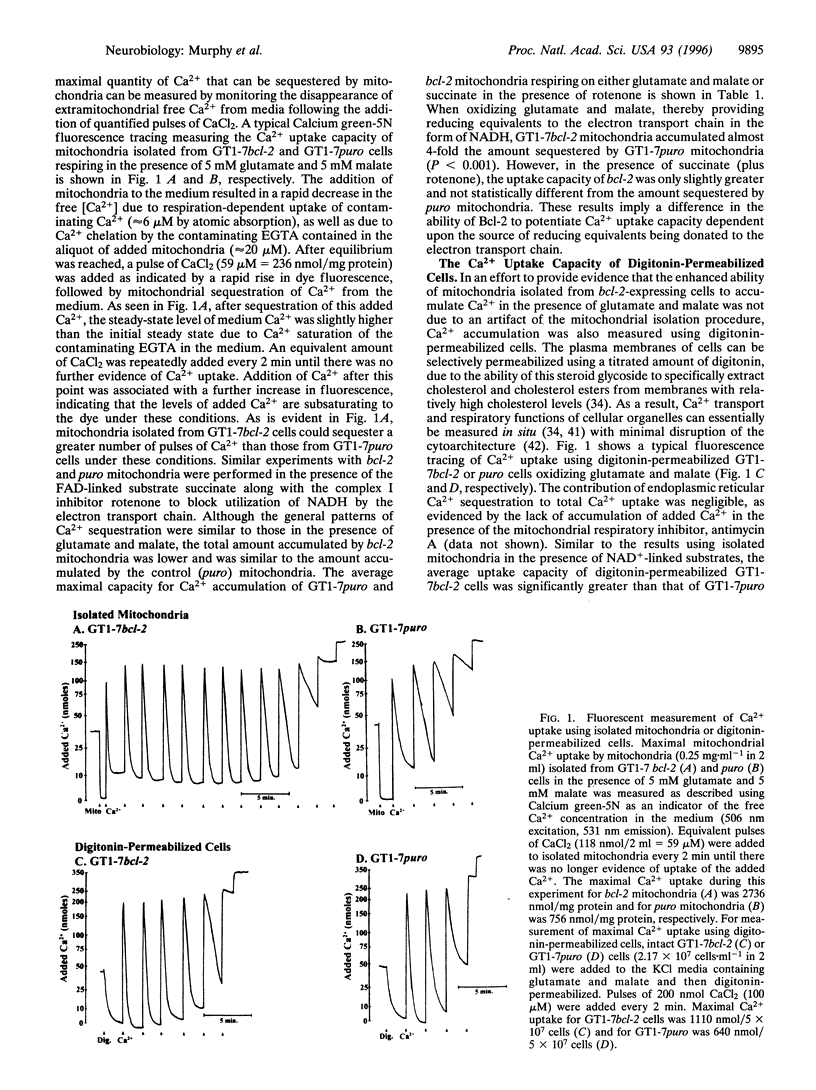
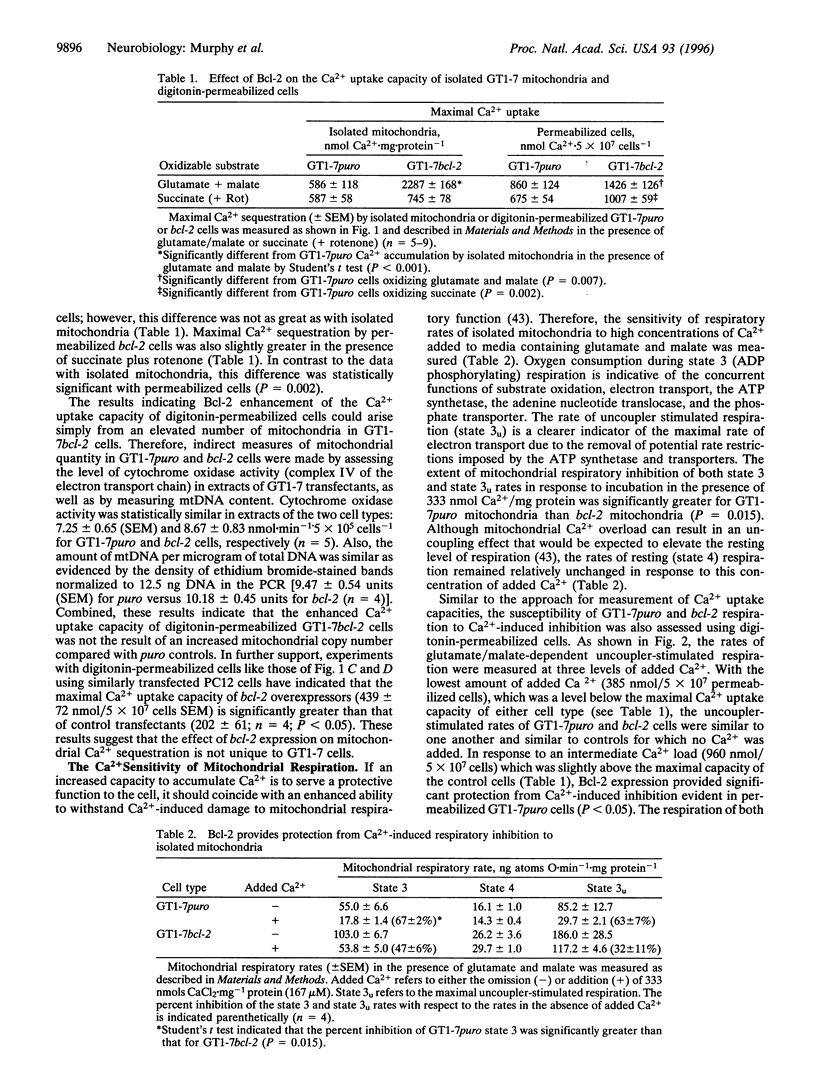
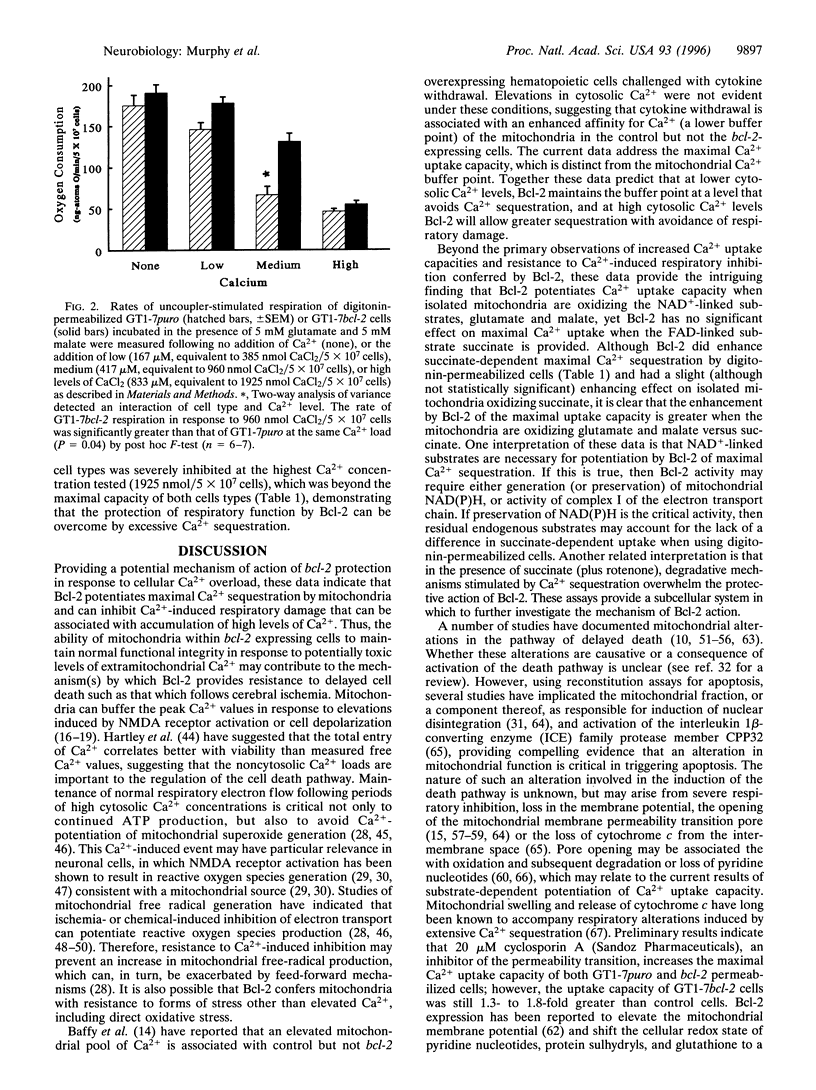
Expression of the human protooncogene bcl-2 protects neural cells from death induced by many forms of stress, including conditions that greatly elevate intracellular Ca2+. Considering that Bcl-2 is partially localized to mitochondrial membranes and that excessive mitochondrial Ca2+ uptake can impair electron transport and oxidative phosphorylation, the present study tested the hypothesis that mitochondria from Bcl-2-expressing cells have a higher capacity for energy-dependent Ca2+ uptake and a greater resistance to Ca(2+)-induced respiratory injury than mitochondria from cells that do not express this protein. The overexpression of bcl-2 enhanced the mitochondrial Ca2+ uptake capacity using either digitonin-permeabilized GT1-7 neural cells or isolated GT1-7 mitochondria by 1.7 and 3.9 fold, respectively, when glutamate and malate were used as respiratory substrates. This difference was less apparent when respiration was driven by the oxidation of succinate in the presence of the respiratory complex I inhibitor rotenone. Mitochondria from Bcl-2 expressors were also much more resistant to inhibition of NADH-dependent respiration caused by sequestration of large Ca2+ loads. The enhanced ability of mitochondria within Bcl-2-expressing cells to sequester large quantities of Ca2+ without undergoing profound respiratory impairment provides a plausible mechanism by which Bcl-2 inhibits certain forms of delayed cell death, including neuronal death associated with ischemia and excitotoxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. P., Darley-Usmar V. M., McCormack J. G., Stone D. Changes in mitochondrial matrix free calcium in perfused rat hearts subjected to hypoxia-reoxygenation. J Mol Cell Cardiol. 1993 Aug;25(8):949–958. doi: 10.1006/jmcc.1993.1107. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M., Dypbukt J. M., Bonfoco E., Zhivotovsky B., Orrenius S., Lipton S. A., Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995 Oct;15(4):961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Bernardi P., Broekemeier K. M., Pfeiffer D. R. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994 Oct;26(5):509–517. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd S. L., Nicholls D. G. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996 Jan;66(1):403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- CROFTS A. R., CHAPPELL J. B. CALCIUM ION ACCUMULATION AND VOLUME CHANGES OF ISOLATED LIVER MITOCHONDRIA. REVERSAL OF CALCIUM ION-INDUCED SWELLING. Biochem J. 1965 May;95:387–392. doi: 10.1042/bj0950387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron-Leslie L. A., Evans R. B., Cidlowski J. A. Bcl-2 inhibits glucocorticoid-induced apoptosis but only partially blocks calcium ionophore or cycloheximide-regulated apoptosis in S49 cells. FASEB J. 1994 Jun;8(9):639–645. doi: 10.1096/fasebj.8.9.8005391. [DOI] [PubMed] [Google Scholar]
- Chen J., Graham S. H., Chan P. H., Lan J., Zhou R. L., Simon R. P. bcl-2 is expressed in neurons that survive focal ischemia in the rat. Neuroreport. 1995 Jan 26;6(2):394–398. doi: 10.1097/00001756-199501000-00040. [DOI] [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini P., Chernyak B. V., Petronilli V., Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996 Mar 22;271(12):6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- Dessi F., Ben-Ari Y., Charriaut-Marlangue C. Ruthenium red protects against glutamate-induced neuronal death in cerebellar culture. Neurosci Lett. 1995 Dec 1;201(1):53–56. doi: 10.1016/0304-3940(95)12128-q. [DOI] [PubMed] [Google Scholar]
- Dugan L. L., Sensi S. L., Canzoniero L. M., Handran S. D., Rothman S. M., Lin T. S., Goldberg M. P., Choi D. W. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995 Oct;15(10):6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens J. A. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated CA2+ and Na+: implications for neurodegeneration. J Neurochem. 1994 Aug;63(2):584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- Ellerby L. M., Ellerby H. M., Park S. M., Holleran A. L., Murphy A. N., Fiskum G., Kane D. J., Testa M. P., Kayalar C., Bredesen D. E. Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. J Neurochem. 1996 Sep;67(3):1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- Faulk E. A., McCully J. D., Tsukube T., Hadlow N. C., Krukenkamp I. B., Levitsky S. Myocardial mitochondrial calcium accumulation modulates nuclear calcium accumulation and DNA fragmentation. Ann Thorac Surg. 1995 Aug;60(2):338–344. doi: 10.1016/0003-4975(95)00446-r. [DOI] [PubMed] [Google Scholar]
- Fiskum G., Craig S. W., Decker G. L., Lehninger A. L. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochman N., Givelber H. Automated, simultaneous microdetermination of calcium and magnesium by atomic absorption. Clin Chem. 1970 Mar;16(3):229–234. [PubMed] [Google Scholar]
- González-Flecha B., Cutrin J. C., Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest. 1993 Feb;91(2):456–464. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekar P. G., Kanthasamy A. G., Borowitz J. L., Isom G. E. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J Neurochem. 1995 Nov;65(5):2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Gunter K. K., Sheu S. S., Gavin C. E. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994 Aug;267(2 Pt 1):C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Hartley D. M., Kurth M. C., Bjerkness L., Weiss J. H., Choi D. W. Glutamate receptor-induced 45Ca2+ accumulation in cortical cell culture correlates with subsequent neuronal degeneration. J Neurosci. 1993 May;13(5):1993–2000. doi: 10.1523/JNEUROSCI.13-05-01993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennet T., Bertoni G., Richter C., Peterhans E. Expression of BCL-2 protein enhances the survival of mouse fibrosarcoid cells in tumor necrosis factor-mediated cytotoxicity. Cancer Res. 1993 Mar 15;53(6):1456–1460. [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., Raff M. C. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994 Apr 15;13(8):1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Raff M. C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995 Apr 27;374(6525):814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Petit P., Zamzami N., Vayssière J. L., Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995 Oct;9(13):1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Lam M., Dubyak G., Chen L., Nuñez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnik M. D., Zahos P., Geschwind M. D., Federoff H. J. Expression of bcl-2 from a defective herpes simplex virus-1 vector limits neuronal death in focal cerebral ischemia. Stroke. 1995 Sep;26(9):1670–1675. doi: 10.1161/01.str.26.9.1670. [DOI] [PubMed] [Google Scholar]
- Littauer A., de Groot H. Release of reactive oxygen by hepatocytes on reoxygenation: three phases and role of mitochondria. Am J Physiol. 1992 Jun;262(6 Pt 1):G1015–G1020. doi: 10.1152/ajpgi.1992.262.6.G1015. [DOI] [PubMed] [Google Scholar]
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996 Jul 12;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M., Jr, Stys P. K. Elemental composition and water content of rat optic nerve myelinated axons and glial cells: effects of in vitro anoxia and reoxygenation. J Neurosci. 1995 Oct;15(10):6735–6746. doi: 10.1523/JNEUROSCI.15-10-06735.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah S. P., Zhong L. T., Liu Y., Roghani A., Edwards R. H., Bredesen D. E. The protooncogene bcl-2 inhibits apoptosis in PC12 cells. J Neurochem. 1993 Mar;60(3):1183–1186. doi: 10.1111/j.1471-4159.1993.tb03275.x. [DOI] [PubMed] [Google Scholar]
- Martinou J. C., Dubois-Dauphin M., Staple J. K., Rodriguez I., Frankowski H., Missotten M., Albertini P., Talabot D., Catsicas S., Pietra C. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994 Oct;13(4):1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Mellon P. L., Windle J. J., Goldsmith P. C., Padula C. A., Roberts J. L., Weiner R. I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990 Jul;5(1):1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Fiskum G. Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem. 1984 Mar;137(2):360–367. doi: 10.1016/0003-2697(84)90098-8. [DOI] [PubMed] [Google Scholar]
- Murphy A. N., Kelleher J. K., Fiskum G. Submicromolar Ca2+ regulates phosphorylating respiration by normal rat liver and AS-30D hepatoma mitochondria by different mechanisms. J Biol Chem. 1990 Jun 25;265(18):10527–10534. [PubMed] [Google Scholar]
- Myers K. M., Fiskum G., Liu Y., Simmens S. J., Bredesen D. E., Murphy A. N. Bcl-2 protects neural cells from cyanide/aglycemia-induced lipid oxidation, mitochondrial injury, and loss of viability. J Neurochem. 1995 Dec;65(6):2432–2440. doi: 10.1046/j.1471-4159.1995.65062432.x. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Farschon D. M., Reed J. C. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994 Oct 21;79(2):353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Nieminen A. L., Saylor A. K., Tesfai S. A., Herman B., Lemasters J. J. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J. 1995 Apr 1;307(Pt 1):99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraidathathu T., de Groot H., Kehrer J. P. Production of reactive oxygen by mitochondria from normoxic and hypoxic rat heart tissue. Free Radic Biol Med. 1992 Oct;13(4):289–297. doi: 10.1016/0891-5849(92)90176-h. [DOI] [PubMed] [Google Scholar]
- Pastorino J. G., Snyder J. W., Hoek J. B., Farber J. L. Ca2+ depletion prevents anoxic death of hepatocytes by inhibiting mitochondrial permeability transition. Am J Physiol. 1995 Mar;268(3 Pt 1):C676–C685. doi: 10.1152/ajpcell.1995.268.3.C676. [DOI] [PubMed] [Google Scholar]
- Petit P. X., Lecoeur H., Zorn E., Dauguet C., Mignotte B., Gougeon M. L. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol. 1995 Jul;130(1):157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S., Reynolds I. J. Calcium green-5N, a novel fluorescent probe for monitoring high intracellular free Ca2+ concentrations associated with glutamate excitotoxicity in cultured rat brain neurons. Neurosci Lett. 1993 Nov 12;162(1-2):149–152. doi: 10.1016/0304-3940(93)90582-6. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Hastings T. G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995 May;15(5 Pt 1):3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. Pro-oxidants and mitochondrial Ca2+: their relationship to apoptosis and oncogenesis. FEBS Lett. 1993 Jun 28;325(1-2):104–107. doi: 10.1016/0014-5793(93)81423-w. [DOI] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Sciamanna M. A., Zinkel J., Fabi A. Y., Lee C. P. Ischemic injury to rat forebrain mitochondria and cellular calcium homeostasis. Biochim Biophys Acta. 1992 Apr 7;1134(3):223–232. doi: 10.1016/0167-4889(92)90180-j. [DOI] [PubMed] [Google Scholar]
- Shimazaki K., Ishida A., Kawai N. Increase in bcl-2 oncoprotein and the tolerance to ischemia-induced neuronal death in the gerbil hippocampus. Neurosci Res. 1994 Jul;20(1):95–99. doi: 10.1016/0168-0102(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kosaka H., Kamiike W., Matsuda H., Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995 Apr 27;374(6525):811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- Sun D., Gilboe D. D. Ischemia-induced changes in cerebral mitochondrial free fatty acids, phospholipids, and respiration in the rat. J Neurochem. 1994 May;62(5):1921–1928. doi: 10.1046/j.1471-4159.1994.62051921.x. [DOI] [PubMed] [Google Scholar]
- Tatsumi H., Katayama Y. Regulation of the intracellular free calcium concentration in acutely dissociated neurones from rat nucleus basalis. J Physiol. 1993 May;464:165–181. doi: 10.1113/jphysiol.1993.sp019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J. F., Beconi M., Barilla J., Chavez U. B., McCord J. M. Mitochondrial generation of oxygen radicals during reoxygenation of ischemic tissues. Free Radic Res Commun. 1991;12-13 Pt 2:681–689. doi: 10.3109/10715769109145847. [DOI] [PubMed] [Google Scholar]
- Vayssiere J. L., Petit P. X., Risler Y., Mignotte B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11752–11756. doi: 10.1073/pnas.91.24.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanović S., Zamoyska R. Anti-CD3-induced cell death in T cell hybridomas: mitochondrial failure and DNA fragmentation are distinct events. Eur J Immunol. 1991 Feb;21(2):419–424. doi: 10.1002/eji.1830210225. [DOI] [PubMed] [Google Scholar]
- Werth J. L., Thayer S. A. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994 Jan;14(1):348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Reynolds I. J. Mitochondria and Na+/Ca2+ exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J Neurosci. 1995 Feb;15(2):1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidan E., Sims N. R. The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J Neurochem. 1994 Nov;63(5):1812–1819. doi: 10.1046/j.1471-4159.1994.63051812.x. [DOI] [PubMed] [Google Scholar]
- Zamzami N., Marchetti P., Castedo M., Decaudin D., Macho A., Hirsch T., Susin S. A., Petit P. X., Mignotte B., Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995 Aug 1;182(2):367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N., Marchetti P., Castedo M., Zanin C., Vayssière J. L., Petit P. X., Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995 May 1;181(5):1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N., Susin S. A., Marchetti P., Hirsch T., Gómez-Monterrey I., Castedo M., Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996 Apr 1;183(4):1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. T., Sarafian T., Kane D. J., Charles A. C., Mah S. P., Edwards R. H., Bredesen D. E. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]


