SUMMARY
Sharpin-deficient mice display a multiorgan chronic inflammatory phenotype suggestive of altered leukocyte migration. We therefore studied the role of SHARPIN in lymphocyte adhesion, polarization and migration. We found that SHARPIN localizes to the trailing edges (uropods) of both mouse and human chemokine-activated lymphocytes migrating on ICAM-1, which is one of the major endothelial ligands for migrating leukocytes. SHARPIN-deficient cells adhere better to ICAM-1 and show highly elongated tails when migrating. The increased tail lifetime in SHARPIN-deficient lymphocytes decreases the migration velocity. The adhesion, migration and uropod defects in SHARPIN deficient lymphocytes were rescued by reintroducing SHARPIN into the cells. Mechanistically we show that SHARPIN interacts directly with LFA-1, a leukocyte counter-receptor for ICAM-1, and inhibits the expression of intermediate and high-affinity forms of LFA-1. Thus SHARPIN controls lymphocyte migration by endogenously maintaining LFA-1 inactive to allow adjustable detachment of the uropods in polarized cells.
INTRODUCTION
SHARPIN is a widely expressed cytosolic protein that, together with HOIP and HOIL-1L, forms the linear ubiquitin chain assembly complex (LUBAC) (Gerlach et al., 2011; Ikeda et al., 2011; Tokunaga et al., 2011). The ternary LUBAC complex catalyzes the formation of linear polyubiquitin chains, which regulate cell signaling, most notably the canonical NF-κB activation in response to stimuli like TNF.
A spontaneous SHARPIN-null mutation in Sharpincpdm mice manifests with a progressive multi-organ inflammatory phenotype (HogenEsch et al., 1993; Seymour et al., 2007). The most prominent feature in these mice is chronic proliferative dermatitis resembling psoriasis, but the mice also display leukocytosis, inflammation in many other organs (gastrointestinal track, liver etc), splenomegaly, abnormal development of Peyer's patches, hypoimmunoglobulinemia, and defective Th1 cytokine production. The complex Sharpincpdm phenotype is partially linked to signaling and cytokine defects. Thus, the skin inflammation can be reversed by crossing Sharpincpdm mice with TNF-deficient mice (Gerlach et al., 2011). This is thought to be due to elimination of the increased LUBAC-dependent cell death sensitivity of Sharpincpdm keratinocytes upon TNF triggering, and subsequent alleviation of secondary inflammatory response. Sharpincpdm mice also have decreased secretion of proinflammatory cytokines (e.g. IFN-γ, IL-6 and IL-12) and increased secretion of Th2-type cytokines (IL-5) (Renninger et al., 2010; Wang et al., 2012; Zak et al., 2011). However, the non-cutaneous lymphoid aberrations are not affected by TNF elimination (Gerlach et al., 2011), mice deficient for HOIL-1 (another LUBAC component) do not appear to display any overt inflammatory phenotype (Tokunaga et al., 2009), and pharmacological inhibition of NF-κB signaling only partially alleviates the skin inflammation (Liang et al., 2011). Moreover, although blockade of IL-5 virtually eliminates eosinophils from Sharpincpdm mice, it does not affect the inflammation phenotype (Renninger et al., 2010). It is therefore tempting to speculate that other, non-LUBAC dependent functions of SHARPIN contribute to the dysregulated leukocyte accumulation seen in Sharpincpdm mice.
Chemokine-triggered leukocyte migration on endothelial cells is one crucial step during the leukocyte traffic into sites of inflammation (Ley et al., 2007; Vestweber, 2012). Given that SHARPIN has previously been reported to inhibit β1-integrins in cancer cells (Rantala et al., 2011), we asked whether SHARPIN could regulate leukocyte locomotion in inflammation. Here we observed that SHARPIN strongly localizes to the trailing edges (called uropods in leukocytes) of migrating lymphocytes, and controls uropod detachment and cell locomotion. In migrating lymphocytes the leading edge and the mid-cell zone are thought to display active LFA-1 (lymphocyte function associated antigen-1, a heterodimeric αLβ2-integrin expressed on practically all leukocytes) capable of binding to intercellular adhesion molecule-1 (ICAM-1) and other ligands. Uropods, in contrast, express inactive LFA-1, which facilitates cellular detachment (Morin et al., 2008; Smith et al., 2005; Stanley et al., 2008). However, the mechanisms of uropodial LFA-1 deactivation have remained unknown. We now show that SHARPIN regulates lymphocyte polarity by directly interacting and deactivating LFA-1. Thus, our data reveal SHARPIN as a new regulator of uropod detachment, which is the final check-point in leukocyte extravasation, and suggest a new mechanism which dynamically controls deactivation of leukocyte integrins.
RESULTS
Impaired Uropod Detachment in SHARPIN-Deficient Lymphocytes
SHARPIN-deficient Sharpincpdm mice manifest with aberrant leukocyte infiltrations in many organs, and therefore we studied whether SHARPIN would play a role in leukocyte locomotion. We found that endogenous SHARPIN is preferentially localized to the detached uropods, together with an established uropod marker CD44, in chemokine CXCL12 (C-X-C-motif ligand 12) -activated wild-type splenocytes migrating on recombinant mouse ICAM-1 (Figure 1A). Sharpincpdm splenocytes, which, as expected, lacked anti-mouse SHARPIN antibody reactivity, were flatter, and displayed a loss of distinctive uropod structures, showing instead elongated trailing edges adherent to the substrate.
Figure 1. SHARPIN Regulates Uropod Detachment in Migrating Lymphocytes.
(A) CXCL12-stimulated, wild-type and Sharpin-deficient (Sharpincpdm) mouse splenocytes migrating on recombinant mouse ICAM-1 (5 μg/ml) immunostained for SHARPIN and CD44. Representative micrographs and quantitation of mean fluorescence intensities (MFI) along the cell axis (mean±sem (sem in grey color); n >10 cells/staining; statistical comparisons between the 20 first frontal and 20 last posterior measurement points) are shown. Bars, 10 μm.
(B, C) Adhesion (B) and spreading (C) of wild-type and Sharpincpdm splenocytes and thymocytes on ICAM-1 (5 μg/ml).
(D, E) Migration of CXCL12-stimulated, wild-type and Sharpincpdm (D) splenocytes and (E) purified CD4+ T cells on different concentrations of ICAM-1. Representative cell tracks and cumulative data are shown. See also Video S1 and Video S2.
(F) Phenotype of the uropods in cells migrating on high ICAM-1 concentration (50 μg/ml) was determined using image analyses. Extended uropod defines an uropod length > 33% of cell body diameter, polarity index is the ratio of the longest leading edge-to-uropod distance divided by the longest width of the cell and uropod lifetime is the time difference between uropod appearance and disappearance. Bars, 10 μm.
(G) Expression of SHARPIN, CD44, F-actin and talin in purified, CXCL12-stimulated CD4+ wild-type and Sharpincpdm cells migrating on high ICAM-1 concentration (50 μg/ml). Representative micrographs and quantitations as in (A) (n = 5-8 cells/staining) are shown. Bars, 10 μm.
Data are representative from or are the mean±sem of 3 (A-D, F) and 2 (E, G) independent experiments. In A, F and G the white arrows point to the uropods/tails. *p<0.05, **p<0.01, ***p<0.001 (Student's two-tailed t-test).
SHARPIN-deficient splenocytes and thymocytes adhered to and spread on ICAM-1 significantly better than wild-type cells (Figure 1B-C). Furthermore, lymphocyte migration on ICAM-1 was regulated by SHARPIN in a substrate concentration dependent manner (Figure 1D). When low levels of ICAM-1 substrate (0.5-5 μg/ml) were used, CXCL12-stimulated Sharpincpdm lymphocytes migrated significantly faster than wild-type cells. However, at a high ICAM-1 concentration (50 μg/ml), SHARPIN-deficient lymphocytes migrated much slower than the wild-type cells. Purified CD4+ T cells from Sharpincpdm mice also migrated faster on low ICAM-1 concentration and slower on high ICAM-1 concentration than purified wild-type CD4+ cells (Figure 1E and Video S1). Sharpincpdm cells migrating on high ICAM-1 concentration had more extended tails than wild-type cells and showed an abnormally strongly polarized morphology (Figure 1F). Moreover, the uropod/tail lifetime was significantly longer for lymphocytes lacking SHARPIN, and consequently the SHARPIN-deficient cells showed impaired detachment from the matrix (Figure 1F and Video S2). Wild-type cells migrating on high ICAM-1 concentration (50 μg/ml) showed preferential localization of SHARPIN and CD44 to uropods and phalloidin staining revealed typical cortical F-actin enriched in the uropods, whereas talin (an integrin activator normally polarized to the cell front (Gomez-Mouton et al., 2001)) accumulated at the leading edges (Figure 1G). In Sharpincpdm cells, in contrast, CD44 and talin both distributed more evenly throughout the cells, and the strongly elongated tails were positive for filamentous actin throughout the tails.
Lymphocytes bind to ICAM-1 mainly via LFA-1 (Hogg et al., 2010). Integrin-mediated cell migration is dependent on receptor expression levels, receptor activity and ligand density (following a bell-shaped curve), and detachment of trailing edges of cells requires disassembly of integrin-containing adhesions (Constantin et al., 2000; Franco et al., 2004; Palecek et al., 1997). Therefore, the increased migration speed on low ICAM-1 concentration and the elongated tails on high ICAM-1 concentration in Sharpincpdm lymphocytes could be indicative of higher LFA-1 expression levels or activity. We found that αL/β2 (LFA-1) integrin was expressed on practically all thymocytes and splenocytes (92-95%, n = 7-8 mice/group) of both wild-type and Sharpincpdm mice, and the levels were comparable (thymocytes and CD4+ splenocytes) or slightly lower (total splenocytes) on Sharpincpdm cells (Figure 2A and B). The expression of other integrins (αM/CD11b, αX/CD11c, α4/CD49d) and the numbers of CD4+ and CD8+ T cells, B220+ B cells, CD4+CD25+FoxP3+ T regulatory cells, and cells expressing chemokine receptors (CXCR4, CCR7, CCR9) and activation markers (CD25, L-selectin) were also similar in spleen and thymus of wild-type and Sharpincpdm mice, with the exception of minor changes in the numbers of CD4+ cells, αM/CD11b and CD49d positive cells among total splenocytes (Figure S1A-F and data not shown). Notably, among CD4+ splenocytes the expression of αM/CD11b and CD49d was similar in both genotypes. Together these data show that the lymphocyte phenotypes of wild-type and Sharpincpdm lymphocytes are relatively similar, and that SHARPIN preferentially localizes to uropods in migrating lymphocytes and regulates uropod stability, adhesion to the substrate and migration velocity both in total splenocytes and purified CD4+ T cells.
Figure 2. SHARPIN and Inactive LFA-1 Co-Localize in Uropods.
(A, B) Expression of LFA-1 on isolated wild-type and Sharpincpdm thymocytes and total and CD4-positive splenocytes. Shown are (A) representative FACS plots, and (B) the specific mean fluorescence intensities (MFI) for CD11a.
(C) Immunostainings of cytospin preparations of resting human PBMC with anti-SHARPIN and control Abs. DAPI was used to visualize nuclear morphology. An arrowhead points to a representative lymphocyte. Bars, 10 μm.
(D) Immunostainings of freshly isolated, CXCL12-stimulated human PBMC migrating on recombinant human ICAM-1 (5 μg/ml) for SHARPIN (a non-polarized and polarized cell are shown).
(E-F) Immunostainings of GFP-SHARPIN transfected, CXCL12-stimulated human PBMC migrating on ICAM for (E) CD44, and (F) talin.
(G-I) Immunostainings of freshly isolated, CXCL12-stimulated human PBMC migrating on ICAM-1 for SHARPIN and, (G) CD11a (total), (H) extended LFA-1 (KIM127 epitope) and (I) activated LFA-1 (24 epitope).
(J) Quantitations of mean fluorescence intensities (MFI) of the indicated stainings along the cell axis (mean±sem (sem in grey color); n > 4-8 cells/staining; statistical comparisons between the 20 first frontal and 20 last posterior measurement points) are shown.
In (D-I) the white arrows point to the uropods, and the bars are 5 μm. Data in (A-B) are from 4-9 mice/genotype and in (C-I) representative of at least 3 independent experiments with different blood cell donors. *p<0.05, **p<0.01, ***p<0.001 (Student's two-tailed t-test). See also Figure S1.
SHARPIN Preferentially Co-Localizes with Inactive LFA-1 in Uropods
To analyze the possible role of SHARPIN in regulating LFA-1 activity we used human cells, for which reporter antibodies for LFA-1 activity are available. In freshly isolated human peripheral blood mononuclear cells (PBMC) and in CXCL12-stimulated PBMC binding to recombinant ICAM-1, stainings with specific anti-SHARPIN antibodies (Figure S1G-H) showed SHARPIN to be evenly distributed in the cytoplasm of non-polarized cells (Figure 2C-D). In contrast, in polarized cells (>100 cells analyzed) endogenous SHARPIN was always concentrated in, but not restricted to, the uropods (Figure 2D and Figure S1I). GFP-SHARPIN transfected into PBMC also preferentially localized in CD44-positive uropods, and was less abundant in the talin-positive leading edges (Figure 2E-F). Leukocytes migrating in a lymphoid tissue in vivo also showed enrichment of endogenous SHARPIN in the uropods (Figure S3J).
In human cells migrating on ICAM-1, LFA-1 was found both at the leading edge and in the uropod, and endogenous SHARPIN (as well as GFP-SHARPIN) and LFA-1 partially colocalized in the uropods (Figure 2G and Figure S1K-L). KIM127 and 24 are unique reporter mAbs, which specifically detect the extended, intermediate affinity, and high-affinity forms of LFA-1, respectively (Dransfield and Hogg, 1989; Hogg et al., 2010; Robinson et al., 1992). On freshly isolated, migrating PBMC the KIM127 and 24 signals concentrated to the leading edge and mid-cell zones, whereas SHARPIN mainly localized to the uropod (Figure 2H-I). Thus, our quantitative data (Figure 2J) show that SHARPIN is enriched in uropods together with inactive LFA-1 in contrast to the integrin activator talin and active LFA-1, which mainly localize to the leading edge and mid-cell zones.
SHARPIN Interacts with LFA-1
SHARPIN has been shown to physically interact with CD29 cell adhesion receptors in cancer cells (Rantala et al., 2011). To study whether SHARPIN can physically interact with leukocyte integrins, we co-transfected plasmids encoding αL and β2 chains of LFA-1 with GFP-SHARPIN (or GFP only as a control) into HEK293 cells. An anti-αL antibody specifically co-immunoprecipitated GFP-SHARPIN, but not GFP (Figure 3A). Endogenous LFA-1 and endogenous SHARPIN from Jurkat cells and freshly isolated human PBMC also co-immunoprecipitated. LFA-1 was also detected in reciprocal immunoprecipitations with anti-SHARPIN antibody (Figures 3B-C). Activation of PBMC for 2-30 min with CXCL12 did not alter the protein levels of SHARPIN or LFA-1 (Figure S2).
Figure 3. SHARPIN Directly Binds to the Conserved Membrane Proximal Sequence of αL.
(A-C) Co-immunoprecipitation of SHARPIN with LFA-1 in (A) HEK 293 cells overexpressing αL and β2 integrins and GFP or GFP-SHARPIN, (B) Jurkat cells (endogenous proteins) and (C) freshly isolated PBMC (endogenous proteins).
(D) The integrin constructs used in the binding studies (ECD, extracellular domain; TMD, transmembrane domain and CYPD; cytoplasmic domain) and the sequences of the transmembrane and intracellular domains of αL, αM, αX, αD and β2 integrins. The conserved membrane proximal area in α-tails is highlighted in red.
(E) Pull-down experiments with recombinant GST-SHARPIN and recombinant full-length LFA-1 and LFA-1 lacking the cytoplasmic tails. GST only is a loading control. (F) Interaction of different GST-SHARPIN with the full-length and tail-less LFA-1 heterodimers was determined using far western overlay assays.
(G) Pull-down assays with synthetic biotinylated peptides corresponding to the cytoplasmic sequences of αL, αM, αD and β2 with lysates of GFP-SHARPIN transfected cells. αL cons, the conserved membrane proximal part of αL.
Data are representative of at least 3 independent experiments. See also Figure S2.
Purified recombinant full-length LFA-1, but not LFA-1 protein lacking the cytoplasmic domains, bound to recombinant GST-SHARPIN, but not GST alone, in pull-down assays (Figure 3D and E). Far Western analyses confirmed this interaction (Figure 3F). The full-length cytoplasmic sequence of αL, but not of β2, and the conserved membrane proximal part of the αL-tail, synthesized as a biotinylated peptide, bound to GFP-SHARPIN from cell lysates (Figure 3G). The cytoplasmic sequences of other leukocyte integrins αM and αD (αX was insoluble) also interacted with GFP-SHARPIN (Figure 3G). All these experiments clearly show that SHARPIN directly binds to the cytoplasmic domain of αL.
SHARPIN Maintains LFA-1 in an Inactive Conformation and Regulates Lymphocyte Extravasation
Direct interaction between SHARPIN and LFA-1, and the co-localization of inactive LFA-1 and SHARPIN in the uropods led us to study whether SHARPIN could be involved in LFA-1 inactivation. Knock-down of SHARPIN in human PBMC (on average 50-70%) increased the adhesion of these cells to human recombinant ICAM-1, and improved their migration on low ICAM-1 concentration and inhibited their migration on high ICAM-1 concentration (Figures 4A and B). Conversely, over-expression of SHARPIN significantly reduced the expression of the LFA-1 activation epitopes KIM127 and 24 on cultured, CXCL12-stimulated PBMC in FACS analyses (Figure 4C and Figure S3A). Moreover, transiently expressed human LFA-1 was more active in Sharpincpdm mouse embryonic fibroblasts (MEFs) than in wild-type MEFs (Figure 4D and Figure S3B). These functional data show that SHARPIN inhibits LFA-1.
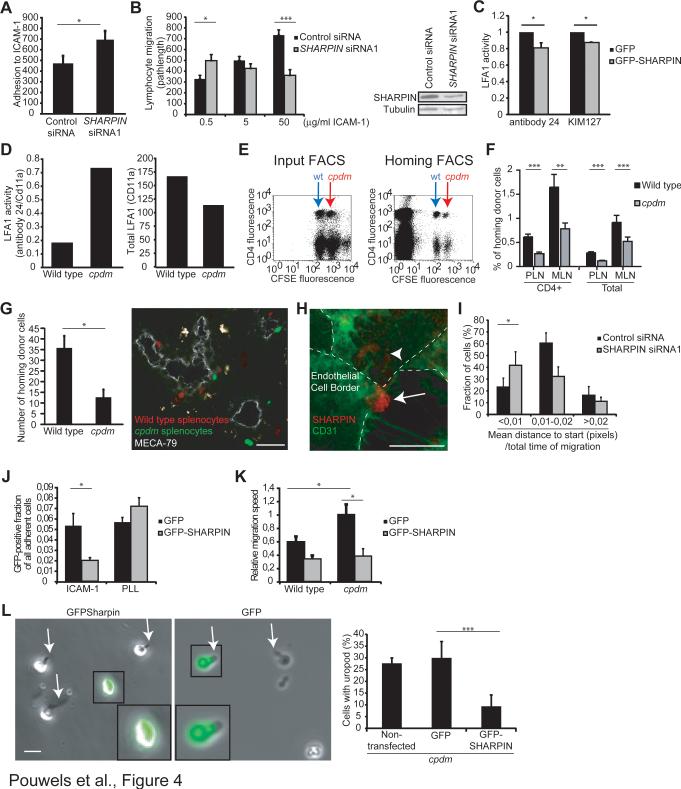
Figure 4. Sharpin De-Activates LFA-1, Improves Lymphocyte Extravasation and Re-Expression of SHARPIN Rescues the Uropod and Migration Defects of SHARPIN-Deficient Cells.
(A-B) Adhesion (A) and migration (B) of human PBMC transfected with SHARPIN siRNA or control siRNA on recombinant human ICAM-1, and the efficacy of knockdown on SHARPIN protein levels.
(C) FACS analyses of the reporter mAb 24 and KIM127 cell surface binding to GFP-SHARPIN and control (GFP only) expressing, CXCL12-stimulated human PBMC. The results are normalized against total CD11a expression.
(D) Cell surface expression of 24 reporter epitope (normalized to total αL expression; left panel) and total αL expression (MFI, right panel) on wild-type and Sharpincpdm MEFs expressing human LFA-1 was determined using FACS (a representative experiment).
(E) Representative FACS plots of the input population and transferred cells isolated from mesenteric lymph nodes of wild-type recipient mice in the homing experiments after 2 h. Wild-type (indicated by blue arrow) and Sharpincpdm (indicated by red arrow) donor splenocytes are CFSE dim and bright, respectively, and the cells were co-stained with Alexa 647-labeled anti-CD4.
(F) Percentages of wild-type and Sharpincpdm CD4-positive and total lymphocytes in the mesenteric (MLN) and peripheral (PLN) lymph nodes of wild-type recipient mice after a 2 h recirculation period (n = 7 mice/genotype).
(G) Microscopic analyses of homing of wild-type (red) and Sharpincpdm (green) cells to PLN after a 30 min recirculation. MECA-79 positive high endothelial venules are shown in grey. A representative micrograph and pooled data (n = 7 recipient mice; 187 homed wild-type cells) are shown. Bar, 50 μm.
(H, I) Transmigration of human PBMC transfected with SHARPIN siRNA or control siRNA through an endothelial monolayer in in vitro flow assays. (H) Expression of SHARPIN in a PBMC transmigrating through CD31+ endothelial monolayer (endothelial borders are shown by a dashed line, and the arrow indicates the leukocyte uropod, and the arrowhead the PBMC cell body below the endothelium). (I) Net displacement of PBMC after transmigration (n = 40 control siRNA and n = 45 SHARPIN siRNA transfected cells from 3 independent experiments with different blood and endothelial cell donors). See also Video S3.
(J) Adhesion assays determining the binding of wild-type and Sharpincpdm splenocytes expressing GFP-SHARPIN or GFP alone to ICAM-1 and poly-L-lysine (PLL, a nonintegrin binding ligand).
(K) Migration velocity of wild-type and Sharpincpdm splenocytes expressing GFPSHARPIN or GFP alone on ICAM-1.
(L) Uropod formation on Sharpincpdm cells expressing SHARPIN-GFP or GFP alone after CXCL12-stimulation and migration on ICAM-1 (50 μg/ml).
Data are the mean±sem of 3 (A, I), 2 (B, G, J-L), 2-5 (C) independent experiments. *p<0.05, **p<0.01, ***p<0.001 (Student's two-tailed t-test). See also Figure S3.
To study the role of SHARPIN in lymphocyte migration in vivo we performed competitive homing assays, in which the ability of lymphocytes to move from the blood through the vessel wall into the tissue is measured. Splenocytes from wild-type and Sharpincpdm mice were isolated, labeled with two different intensities of a fluorescent dye (CFSE) and injected intravenously into wild-type recipients. After a 2 h recirculation period, Sharpincpdm lymphocytes (both total splenocytes and CD4+ lymphocytes) homed to peripheral (PLN) and mesenteric (MLN) lymph nodes with about half the efficiency of their wild-type counterparts (7 mice/group, Figure 4E-F). In an independent experiment the reduced lymph node homing of Sharpincpdm splenocytes and CD4+ T cells was confirmed, and we observed that, instead, Sharpincpdm cells accumulated more in the spleen than wild-type cells (Figure S3C). Also in microscopic analyses fewer Sharpincpdm lymphocytes accumulated in PLN parenchyma than wild-type cells 30 min after intravenous transfer (Figure 4G). Thus, absence of SHARPIN impairs lymphocyte homing to lymph nodes.
The function of SHARPIN in lymphocyte transmigration was studied using time-lapse microscopy in in vitro flow chamber assays, in which human PBMC are perfused with a defined shear stress over cultured confluent endothelial monolayers. After adhesion, control siRNA silenced human PBMC transmigrated (transition from white/grey to black cells in phase contrast images) through the endothelium preferentially at the endothelial cell junctions. SHARPIN localized to the uropods during the transmigration (Figure 4H). After completion of the transmigration, the majority of cells swiftly moved onwards from the transmigration site (Figures 4I and Video S3). SHARPIN-silenced cells transmigrated with similar kinetics, but thereafter they migrated less persistently away from the transmigration sites, and often had to make repetitive efforts to leave the transmigration spot below the endothelium (Figure 4I and Video S3).
SHARPIN Rescues Migration and Uropod Detachment Defects of SHARPIN-Deficient Cells
Sharpincpdm splenocytes expressing GFP-SHARPIN bound 60% less to ICAM-1 (5 μg/ml) than Sharpincpdm splenocytes expressing GFP only (Figure 4J), showing that the increased adhesion of Sharpincpdm cells on ICAM-1 was SHARPIN-dependent. Binding of Sharpincpdm splenocytes to a non-integrin-dependent ligand (poly-L-lysine) was not affected by SHARPIN.
The migration velocity of GFP-expressing Sharpincpdm lymphocytes on ICAM-1 (5 μg/ml) was significantly higher than that of GFP-expresing wild-type cells (Figure 4K). Importantly overexpression of GFP-SHARPIN in Sharpincpdm cells reduced the migration velocity significantly by 60% compared to GFP only. In fact, wild-type and Sharpincpdm cells overexpressing SHARPIN had comparable migration velocities. Moreover, expression of GFP-SHARPIN, but not GFP alone, in Sharpincpdm cells increased the number of cells with detached uropods (Figure 4L). Thus, SHARPIN is causally involved in regulating uropod formation, polarization and lymphocyte motility on ICAM-1.
DISCUSSION
This study identifies a novel function for SHARPIN in regulating cell polarization and uropod detachment in migrating lymphocytes. We also describe SHARPIN as the first endogenous molecule capable of inhibiting LFA-1 by binding directly to the integrin α-tail. These data show that the inactive LFA-1 conformation is not merely a passive default state in the leukocytes, but is actively maintained through adjustable intracellular protein interactions.
Increased LFA-1 activation epitopes associated with lack of SHARPIN expression and diminished LFA-1 activation with increased SHARPIN expression. Since SHARPIN did not alter LFA-1 expression levels, these data directly implicate SHARPIN in regulation of LFA-1 activity. Because there is always a dynamic balance between active and inactive LFA-1 on the cell membrane, SHARPIN could help to maintain LFA-1 in an inactive conformation by preventing activation and/or by deactivating the active conformation. Thus, in non-adherent and freshly adherent cells, which do not have uropods and which display even localization of SHARPIN throughout the cytoplasm, SHARPIN may function in regulating general LFA-1 adhesiveness and activation.
In migrating lymphocytes, adhesive cell-substrate contacts need to be disengaged at the trailing edge to allow net forward movement of the cell (Smith et al., 2005). We showed that in migrating cells SHARPIN preferentially redistributes to uropods, to which inactive LFA-1 is recruited (Morin et al., 2008; Smith et al., 2005). The effects of SHARPIN on lymphocyte migration were dependent on the ligand concentration. Increased LFA-1 activation in the absence of SHARPIN enhanced cell migration on low ICAM-1 concentration and inhibited migration on high ICAM-1 concentration and in transmigration assays. Similarly, constitutively active LFA-1 mutant T-cells (lacking the cytoplasmic domain) adhere better to ICAM-1 but show impaired transendothelial migration in vitro and migrate worse to tissues in vivo than wild type cells (Lu and Springer, 1997; Semmrich et al., 2005). A small molecule agonist of LFA-1 also enhances ligand binding and inhibits transendothelial migration of lymphocytes accompanied by uropod elongation and impaired de-adhesion (Yang et al., 2006). Also T cells carrying a constitutively active mutant (an engineered point mutation in the extracellular domain) adhere better to ICAM-1 and yet home worse than wild-type cells to PLN and MLN in vivo (Park et al., 2010). These data strongly implicate the role of LFA-1 activity regulation also in in vivo homing, although the contribution of slight changes in the LFA-1 expression levels was not formally excluded. In fact, LFA-1-dependent uropod elongation has very recently been proposed to be a final common step during transendothelial migration (Hyun et al., 2012). Therefore, a release mechanism is needed before the cell can migrate further into the tissue, and we postulate that SHARPIN, which is enriched in the uropod of extravasating leukocytes, facilitates homing, and enhances cell movement after transendothelial migration, could play an important role in this process.
The results that SHARPIN negative cells showed impaired short-term (30 min- 2h) homing to lymph nodes and yet SHARPIN-deficient mice suffer from chronic inflammation may have several explanations. The chronic inflammatory phenotype develops during a much longer time period (the first 4-6 weeks of postnatal life) (HogenEsch et al., 1993), during which many other LFA-1 mediated inflammatory functions apart from the initial entry may become relevant. For instance, LFA-1-ICAM-1 interaction contributes to T-cell co-stimulation (Wulfing and Davis, 1998) and lymphocyte exit from the lymph nodes into lymphatic vasculature (Reichardt et al., 2013), and therefore dysregulated LFA-1 activity in Sharpin cpdm cells may modulate these processes. Finally, in vivo SHARPIN-deficient cells localized better than wild-type cells to spleen (which is one of the affected organs in Sharpincpdm mice), implicating that the contribution of LFA-1 activity regulation may vary in different organs.
Activation of LFA is essential for its function (Abram and Lowell, 2009; Gahmberg et al., 2009; Hogg et al., 2010; Kinashi, 2007). LFA-1 is normally found in an inactive (i.e. incompetent for ligand binding) form on leukocytes, and only after receiving activation signals, does it become functionally competent. Critical for the activation is the interaction of the cytoplasmic domain of the β chain with talin and kindlins, which leads to conformational changes of the extracellular domains in the integrin heterodimer (Moser et al., 2009; Shattil et al., 2010). LFA-1 activation can be indirectly inhibited through certain signaling molecules, such as Cdc42, PIP5K1γ90, cytokine growth differentiation factor-15 and E3 ubiquitin ligase Cbl-b, which interfere with the upstream activation signals (Bolomini-Vittori et al., 2009; Hogg et al., 2010; Kinashi, 2007; Wernimont et al., 2010; Zhang et al., 2003). In contrast, SHARPIN directly binds to the cytosolic membrane proximal sequence of the α-tail of LFA-1. Certain artificial deletions of the αL cytoplasmic tail increase LFA-1 activity and cell adhesion to ICAM-1 (Lu and Springer, 1997; Semmrich et al., 2005). These mutants are predicted to be unable to bind SHARPIN, which could explain why these mutants are constitutively active.
Delineation of multiple functions and the physiological role of SHARPIN remains an important area of future investigation. It will be critical to determinate whether SHARPIN can simultaneously function as a component of the LUBAC complex and an inhibitor of integrins, possibly implicating a role for linear ubiquitination/NF-κB signaling in the regulation of integrin signaling, or whether these two binding modes are mutually exclusive. Moreover, we have shown that binding of SHARPIN to the conserved segment of the α-cytoplasmic tails of collagen and fibronectin-binding α2β1 and α5β1-integrins in cancer cells prevents recruitment of the integrin activators talin and kindlin with the β1-tail via an yet unknown mechanism (Rantala et al., 2011). Whether similar mechanisms operate in inhibition of LFA-1 by SHARPIN remains to be determined but also additional mechanisms could be involved. SHARPIN thus inhibits both β1- and β2-integrins, although the activity regulation of β1-integrins in adherent epithelial and mesenchymal cells is fundamentally different from that of β2-integrins in professionally motile leukocytes. Activity regulation of β1-integrins is modulatory, since these extracellular matrix receptors are always at least partially active and strongly coupled to actin. In sharp contrast, activation of β2-integrins needs to be fully switchable from inactive to active within seconds to allow extravasation of blood-borne leukocytes. In addition, many of the integrin activators are specific for β2-integrins over β1-integrins; (e.g. the GTPase Rap1 regulating LFA-1 vesicle traffic, RAPL regulating LFA-1 transport, and adaptor 14-3-3 binding to phosphorylated β2-integrin) and the Rap1-RAPL dependent LFA-1 activation involves binding to the alpha-subunit cytoplasmic region of LFA-1 immediately adjacent to the SHARPIN binding site (Hogg et al., 2010; Katagiri et al., 2003). This suggests that SHARPIN mediated LFA-1 inactivation may involve mechanisms that do not play a role in the regulation of β1-integrins. However, this remains to be investigated in detail. The specific physiological contribution of SHARPIN mediated NF-κβ activation (via LUBAC) and integrin inactivation in different lymphoid and non-lymphoid tissues and at different time points also needs to be determined in future using in vivo imaging, specific inhibitors and cell-type specific conditional knockouts.
In conclusion, we have shown that SHARPIN preferentially localizes to uropods in migrating lymphocytes, directly binds to the cytoplasmic tail of αL-integrin, and keeps LFA-1 inactive in these cells. Thereby SHARPIN regulates uropod detachment and locomotion and transendothelial migration of inflammatory cells. Our findings challenge the prevailing dogma that the inactive LFA-1 is the passive default form of the molecule by showing that active interaction with endogenous SHARPIN is required to maintain LFA-1 in the non-activated state.
EXPERIMENTAL PROCEDURES
Mice, Cells and Transfections
Spontaneously SHARPIN-deficient C57BL/KaLawRij-SHARPINcpdm/RijSunJ (abbreviated Sharpincpdm) mice (HogenEsch et al., 1993; Seymour et al., 2007) were maintained using heterozygote breedings. Sex-matched littermate wild-type and Sharpincpdm animals (6-8 wk) were used in all experiments. The animal studies were approved by the Animal Experiment Board in Finland.
Mouse splenocytes and thymocytes were isolated using mechanical teasing, and CD4+ T cells purified by negative selections. Human PBMC and mouse lymphocytes were transfected with plasmids and siRNAs using human and mouse T cell Nucleofection kits (Amaxa).
Adhesion and Migration Assays and Uropod Analyses
Cells were allowed to adhere to precoated wells (5 μg/ml ICAM-1 or poly-L-lysine), washed, fixed, stained with propidium iodide and quantified with Acumen Explorer laser scanner (TTP LabTech Ltd). For migration assays cells were imaged at 1-2 min intervals with or without CXCL12-stimulation on ICAM-1 precoated (0.5-50 μg/ml) wells. Cell area, migration speed and path length analyses were done with NIH ImageJ software. The extended uropods (uropod length > 33% of cell body diameter) and the duration of uropod attachment was scored form the time lapse videos. The polarization index of cells was calculated as the ratio of x to y, where x is the longest distance across cells (from head to tail) and y is the greatest width perpendicular to x.
Human and mouse leukocytes adhering or migrating on ICAM-1 were stained for immunofluorescence using earlier described protocols (Rantala et al., 2011). Migrating cells were stained with KIM127 and 24 using a 80 s exposure of live cells before fixation (Smith et al., 2005). For FACS analysis cells were labelled using indirect or direct staining techniques, and analysed using FACSCalibur with CellQuest software (BD Biosciences).
Immunoblottings, Immunoprecipitations, Pull-Downs and Far Western Assays
Immunoblotting and immunoprecipitations were done as described (Mattila et al., 2005). For the pull-down analysis, the cells were lysed or the recombinant protein diluted in a 2% β-octylglucoside containing lysis buffer, incubated with 2.5 μM biotinylated integrinpeptides bound to streptavidin-sepharose beads, washed, and immunoblotted. For Far Western assays purified proteins were spotted on nitrocellulose membranes, incubated with different GST-SHARPIN proteins in blocking buffer, washed and detected with anti-GST antibody.
Statistical Analysis
The numerical data are presented as mean±sem. Each individual experiment used cells from different persons or different mice. All statistical analyses were performed with two-tailed Student's t-test. p < 0.05 was considered significant.
Supplementary Material
Highlights.
SHARPIN preferentially localizes to uropods and controls their detachment in migrating lymphocytes
SHARPIN regulates lymphocyte polarity and locomotion
A direct interaction with SHARPIN is needed to maintain LFA-1 in the inactive state
ACKNOWLEDGMENTS
We thank Ms. Petra Laasola, Laura Lahtinen, Jenni Siivonen, Riikka Sjöroos, Etta-Liisa Väänänen, Kathleen Silva and Sari Mäki for expert technical help. Dr. Hans-Jürgen Kreienkamp is acknowledged for providing mice.
The study was supported by the grants from the ERC, Finnish Academy, Sigrid Juselius Foundation, Cancer Reasearch UK, the Wellcome Trust, and by the NIH AR49288 grant. JP has been funded by the Finnish Cancer Institute and the Instrumentarium Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes 3 figures, 3 videos, one table and Extended Experimental Procedures.
REFERENCES
- Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, Martinello M, Constantin G, Laudanna C. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009;10:185–194. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin a subunits. EMBO J. 1989;8:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Gahmberg CG, Fagerholm SC, Nurmi SM, Chavakis T, Marchesan S, Gronholm M. Regulation of integrin activity and signalling. Biochim Biophys Acta. 2009;1790:431–444. doi: 10.1016/j.bbagen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- Gomez-Mouton C, Abad JL, Mira E, Lacalle RA, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Manes S, Martinez AC. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HogenEsch H, Gijbels MJ, Offerman E, van Hooft J, van Bekkum DW, Zurcher C. A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am J Pathol. 1993;143:972–982. [PMC free article] [PubMed] [Google Scholar]
- Hogg N, Patzak I, Willenbrock F. The insider's guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2010;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012;209:1349–1362. doi: 10.1084/jem.20111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- Kinashi T. Integrin regulation of lymphocyte trafficking: lessons from structural and signaling studies. Adv Immunol. 2007;93:185–227. doi: 10.1016/S0065-2776(06)93005-3. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Liang Y, Seymour RE, Sundberg JP. Inhibition of NF-kappaB signaling retards eosinophilic dermatitis in SHARPIN-deficient mice. J Invest Dermatol. 2011;131:141–149. doi: 10.1038/jid.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CF, Springer TA. The alpha subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- Mattila E, Pellinen T, Nevo J, Vuoriluoto K, Arjonen A, Ivaska J. Negative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat Cell Biol. 2005;7:78–85. doi: 10.1038/ncb1209. [DOI] [PubMed] [Google Scholar]
- Morin NA, Oakes PW, Hyun YM, Lee D, Chin YE, King MR, Springer TA, Shimaoka M, Tang JX, Reichner JS, et al. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205:195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell- substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Park EJ, Peixoto A, Imai Y, Goodarzi A, Cheng G, Carman CV, von Andrian UH, Shimaoka M. Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood. 2010;115:1572–1581. doi: 10.1182/blood-2009-08-237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala JK, Pouwels J, Pellinen T, Veltel S, Laasola P, Mattila E, Potter CS, Duffy T, Sundberg JP, Kallioniemi O, et al. SHARPIN is an endogenous inhibitor of beta1-integrin activation. Nat Cell Biol. 2011;13:1315–1324. doi: 10.1038/ncb2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renninger ML, Seymour RE, Whiteley LO, Sundberg JP, Hogenesch H. Anti-IL5 decreases the number of eosinophils but not the severity of dermatitis in Sharpin-deficient mice. Exp Dermatol. 2010;19:252–258. doi: 10.1111/j.1600-0625.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MK, Andrew D, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher EC. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J Immunol. 1992;148:1080–1085. [PubMed] [Google Scholar]
- Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, Bartsch B, Laschinger M, Hogg N, Pfeffer K, et al. Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med. 2005;201:1987–1998. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour RE, Hasham MG, Cox GA, Shultz LD, Hogenesch H, Roopenian DC, Sundberg JP. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8:416–421. doi: 10.1038/sj.gene.6364403. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Smith A, McDowall A, Nicol A, Zicha D, Hogg N. Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell. EMBO J. 2008;27:62–75. doi: 10.1038/sj.emboj.7601959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sokolovska A, Seymour R, Sundberg JP, Hogenesch H. SHARPIN is essential for cytokine production, NF-kappaB signaling, and induction of Th1 differentiation by dendritic cells. PLoS One. 2012;7:e31809. doi: 10.1371/journal.pone.0031809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernimont SA, Legate KR, Simonson WT, Fassler R, Huttenlocher A. PIPKI gamma 90 negatively regulates LFA-1-mediated adhesion and activation in antigen-induced CD4+ T cells. J Immunol. 2010;185:4714–4723. doi: 10.4049/jimmunol.1001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. Novel insights into leukocyte extravasation. Curr Opin Hematol. 2012;19:212–217. doi: 10.1097/MOH.0b013e3283523e78. [DOI] [PubMed] [Google Scholar]
- Yang W, Carman CV, Kim M, Salas A, Shimaoka M, Springer TA. A small molecule agonist of an integrin, alphaLbeta2. J Biol Chem. 2006;281:37904–37912. doi: 10.1074/jbc.M606888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak DE, Schmitz F, Gold ES, Diercks AH, Peschon JJ, Valvo JS, Niemisto A, Podolsky I, Fallen SG, Suen R, et al. Systems analysis identifies an essential role for SHANK-associated RH domain-interacting protein (SHARPIN) in macrophage Toll-like receptor 2 (TLR2) responses. Proc Natl Acad Sci USA. 2011;108:11536–11541. doi: 10.1073/pnas.1107577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shao Y, Fang D, Huang J, Jeon MS, Liu YC. Negative regulation of T cell antigen receptor-mediated Crk-L-C3G signaling and cell adhesion by Cbl-b. J Biol Chem. 2003;278:23978–23983. doi: 10.1074/jbc.M212671200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.