Abstract
Objective:
To evaluate the effects of plyometric training on muscle-activation strategies and performance of the lower extremity during jumping exercises.
Subjects:
Twenty healthy National Collegiate Athletic Association Division I female athletes.
Design and Setting:
A pretest and posttest control group design was used. Experimental subjects performed plyometric exercises 2 times per week for 6 weeks.
Measurements:
We used surface electromyography to assess preparatory and reactive activity of the vastus medialis and vastus lateralis, medial and lateral hamstrings, and hip abductors and adductors. Vertical jump height and sprint speed were assessed with the VERTEC and infrared timing devices, respectively.
Results:
Multivariate analyses of variance revealed significant (P < .05) increases in firing of adductor muscles during the preparatory phase, with significant interactions for area, mean, and peak. A Tukey honestly significant difference post hoc analysis revealed significant increases in preparatory adductor area, mean, and peak for experimental group. A significant (P = .037) increase in preparatory adductor-to-abductor muscle coactivation in the experimental group was identified, as well as a trend (P = .053) toward reactive quadriceps-to- hamstring muscle coactivation in the experimental group. Pearson correlation coefficients revealed significant between-groups adaptations in muscle activity patterns pretest to posttest. Although not significant, experimental and control subjects had average increases of 5.8% and 2.0% in vertical jump height, respectively.
Conclusions:
The increased preparatory adductor activity and abductor-to-adductor coactivation represent preprogrammed motor strategies learned during the plyometric training. These data strongly support the role of hip-musculature activation strategies for dynamic restraint and control of lower extremity alignment at ground contact. Plyometric exercises should be incorporated into the training regimens of female athletes and may reduce the risk of injury by enhancing functional joint stability in the lower extremity.
Keywords: stretch-shortening exercises, electromyography
Female athletes involved in jumping and cutting activities are at greater risk for sustaining noncontact anterior cruciate ligament injuries when compared with their male counterparts.1–4 A variety of differences have been identified between the sexes5–10; however, recent findings from training studies of kinematic (motion) and kinetic (force) data suggest that lower extremity malalignment is related to inefficient neuromuscular control strategies.5 Plyometric training is an established technique for enhancing athletic performance but may also facilitate beneficial adaptations in the sensorimotor system that enhance dynamic restraint mechanisms11,12 and correct faulty jumping or cutting mechanics.
The dynamic restraint system relies on feed-forward and feedback motor control to anticipate and react to joint movements or loads.10 Feed-forward strategies employ muscle preactivation to “stress shield” articular structures and are organized based on previous experience with sport-specific activities.13 Functional training techniques with repetitive jumping and deceleration activities may create plastic neurologic adaptations to motor programs that improve coordination for both performance and dynamic restraint. The feedback motor- control process encompasses a number of reflexive pathways that continuously modify muscle activity to accommodate unanticipated events.10 Because the lower extremity is subjected to high joint loads and velocities during plyometric activities, these exercises are ideal for encouraging the reflexive pathways of feedback motor control.
Plyometric exercises are defined as eccentric loading immediately followed by a concentric contraction.14–19 These exercises have been credited with inducing neuromuscular adaptations to the stretch reflex, elasticity of muscle, and Golgi tendon organs.18,20 The stretch reflex is initiated during the eccentric loading phase and can facilitate greater motor-unit recruitment during the ensuing concentric contraction. The series and parallel connective-tissue components of muscle also store elastic energy, which can generate additional force if a muscle recoils quickly in the form of a concentric contraction. Lastly, Golgi tendon organs usually have a protective function against excessive tensile loads in the muscle; however, after plyometric training, Golgi tendon organ desensitization is thought to occur,21 allowing the elastic components of muscles to undergo greater stretch. When the stretch reflex and stored elastic energy are combined, a more powerful concentric force is created.18 Wilk et al18 suggested that muscular performance gains after plyometric training are attributed to these neural adaptations, rather than to morphologic changes. For this reason, plyometric training may enhance neuromuscular function and prevent knee injuries by increasing dynamic stability.22
Plyometric exercises may increase performance and decrease injury risk in competitive female athletes.5,23 During most functional activities, the knee joint is subjected to high abduction and adduction moments, and, therefore, a theorized relationship exists between these moments and knee injuries.24–27 Motion and forceplate data after plyometric training revealed that trained female athletes had lower abduction and adduction moments at the knee and lower landing forces when compared with untrained males.5 These results are believed to be evidence for increased dynamic restraint and functional knee stability. Furthermore, Hewett et al23 indicated that females who participated in a plyometric training program had a significant decrease in the number of serious knee injuries. Neuromuscular adaptations are believed to enhance dynamic knee stability and performance22; however, the specific adaptations responsible for the success of plyometric training are still theoretic. Our purpose was to examine the effects of plyometric training on muscle-activation strategies and performance in female athletes during jumping activities.
METHODS
Research Design
This experiment was a pretest and posttest control-group design. The independent variables were time (pretraining, posttraining) and training group (control, plyometrics). The dependent variables were electromyography (EMG) signals (area, mean, peak, coactivation, pattern) and our performance measures for the thigh musculature (vertical jump height and sprint speed).
Subjects
Twenty National Collegiate Athletic Association Division I collegiate female soccer and field hockey players, 18 to 22 years of age, volunteered to participate in the study. Exclusionary criteria included any lower extremity reconstructive surgery in the past 2 years or unresolved musculoskeletal disorders that prohibited subjects from sport participation. All subjects participated in off-season training that involved practice 3 times per week and weight training 2 times per week. Random assignment was performed, and subjects were placed in either the control group (9 subjects: 7 soccer players, 2 field hockey players) or the experimental group (9 subjects: 7 soccer players, 2 field hockey players). The mean height for the control group was 165.66 ± 4.88 cm and for the experimental group, 164.54 ± 4.88 cm. The mean weight for the control group was 59.75 ± 3.62 kg and for the experimental group, 59.24 ± 3.62 kg. Any subject who missed more than 1 training session was removed from the study. All control subjects were asked to refrain from any plyometric-type training. After pretesting, 1 control subject was removed from the study because of an unrelated surgical procedure. One experimental subject was removed from the study after posttesting because of error in EMG calibration.
Instrumentation
Electromyographic Assessment
Testing methods and instrumentation were based on previous research conducted by Swanik et al.28 The EMG data were collected from 6 muscles: vastus medialis, vastus lateralis, medial hamstrings, lateral hamstrings, hip abductors, and hip adductors. Electrode placement was identified by palpating bony landmarks and the midlength of the contractile component during an isometric contraction. The skin was shaved, lightly abraded, and cleaned with 70% ethanol solution before 10-mm (diameter includes active portion of electrode surrounded by adhesive material), self-adhesive Ag/AgCl bipolar surface electrodes (Multi Bio Sensors Inc, El Paso, TX) were placed over the muscles 10 mm apart (center-to-center distance).29 Resistance between paired electrodes was measured with a standard multimeter3 and was less than 2 KΩ. One reference electrode was placed over the proximal tibia. The EMG activity was processed through the Noraxon Telemyo System (Noraxon USA Inc, Scottsdale, AZ).
Signals from the 6 muscles were passed from the leads to a battery-operated, 8-channel FM transmitter that was worn by the subject. A single-ended amplifier (impedance > 10 mΩ, gain = 1000) was used with a fourth-order Butterworth filter (10–500 Hz) and a common mode rejection ratio of 130 db at DC (minimum = 85 db across entire frequency of 10–500 Hz). A receiver with a sixth-order filter (gain = 2, total gain = 2000) was used to further amplify the signal. The signal was converted from analog to digital data with an A/D card (Keithley Metrabyte DAS-1000; Keithley Instruments, Inc, Tauton, MA). Once converted, the signal was passed to a computer, in which raw EMG data were sampled at a frequency of 1000 Hz and further analyzed with Myoresearch software (Noraxon USA). Before each test, the myoelectric signal was calibrated with the subject in a relaxed position to establish the baseline EMG activity. The EMG data were recorded during a drop jump and subsequent vertical jump.
We analyzed the integrated EMG data, expressed in microvolts·milliseconds. The raw signal was rectified and averaged over a 15-millisecond moving window. Because the EMG data integration requires 16 milliseconds of processing time, the EMG channels were synchronized to adjust for this delay.28
To indicate ground contact, the subjects landed on a vinyl switch mat (model 63515; Lafayette Instruments, Lafayette, IN) covered by a piece of 12-mm-thick Plyorobic Runway (model 4857P; M-F Athletic Co, Cranston, RI). On pilot testing, we found the switch mat to be ± 5 milliseconds of the Noraxon foot switch. This was considered acceptable because examining muscle-activation timing within 10 milliseconds is generally considered inconsequential due to variability in nerve length and conduction velocity.29–31 The switch mat was connected to the EMG computer to indicate ground contact at 2.27 kg of pressure. To normalize for time, a linear envelope was established based on the initial ground contact. Markers were placed 150 milliseconds before ground contact to capture the descent phase of the drop jump, and the absence of ground contact was used to indicate take-off on the subsequent vertical jump. Three drop-jump trials were performed, and the trials were combined using Myoresearch software to construct an ensemble average profile of the muscle activity during the specified linear envelope.28
The amplitude of muscle activity for each subject was normalized to the ensemble peak amplitude of the drop jumps based on the results of Yang and Winter.32 Using amplitude normalization, we converted the EMG data (microvolts·milliseconds) into a value that represents a percentage of the ensemble peak (percentage·milliseconds) and was calculated using Myoresearch software. Preparatory and reactive EMG phases of the jump were identified by time to compare subjects and analyze data. The preparatory phase encompassed 150 milliseconds before ground contact; the reactive phase consisted of initial ground contact to 350 milliseconds after ground contact.28
Vertical-Jump Height Assessment
Vertical-jump height was assessed using the VERTEC (Questtek Corp, Northridge, CA). The VERTEC device has 49 color-coded, movable acrylic vanes, which were spaced at 0.5-in (1.27-cm) intervals. The height of the VERTEC was adjusted in accordance with the manufacturer's guidelines. Subjects were instructed to jump as high and as fast as they could upon landing from the drop jump and attempt to make contact with their fingers at the highest vane. Subjects were given 3 attempts to reach their maximum height. The highest of the 3 trials was used as the comparative measure from pretest to posttest. Reliability for the VERTEC during running jumps, with 3 run-up steps and with a single-leg take-off, produced an intraclass correlation coefficient of 0.92. Standing jumps, which use both legs during take-off, have an intraclass correlation coefficient of 0.94.33
Sprint Speed Assessment
Sprint speed was assessed using an infrared timing device, consisting of a long-range transmitter and receiver. Sprint speed was displayed on the Polaris multievent timer (FarmTek, Inc, Dallas, TX). Subjects were asked to perform 3 repetitions of a shuttle run totaling 40 yards (36.57 m); the fastest sprint speed was used for the comparative measure from pretest to posttest.
Experimental Procedures
Subjects reported to the university Biokinetics Research Laboratory: Athletic Training Division for the pretest session. The university institutional review board approved all study procedures. Each subject signed the informed consent and completed the health history questionnaire; we then reviewed the questionnaire for inclusion and exclusion criteria. All data collection was performed by 1 tester to maximize reliability. Subjects were first asked to perform a warm-up, then a shuttle run totaling 40 yards. Each participant was allowed 1 practice trial before testing. Subjects performed 3 test repetitions, and the fastest sprint speed was recorded for data analysis. Subjects were allowed a 1-minute rest interval between test trials.
The EMG electrodes were then applied to the right leg, and subjects were instructed on the drop-jump procedures from a height of 18 in (45.72 cm). Each subject was allowed 3 practice trials before data collection. Subjects then performed 3 test repetitions with a 30-second rest interval between repetitions, and the highest vertical-jump height was recorded.
All subjects were then randomly assigned to either the control or experimental group. All subjects participated in regularly scheduled off-season strength training, practices, and games and tournaments, but the experimental group also participated in a plyometric program 2 times per week for 6 weeks (Table 1). Plyometric training sessions took approximately 20 to 30 minutes to perform. The training regimen was based on exercises determined to be sport specific as well as pilot testing performed with a similar caliber of athletes.
Table 1.
Sample of Plyometric Training Progression20

The experimental group was given instructions and illustrations of each plyometric exercise before the first training session. Wall touches were performed with the subjects facing a wall; the objective was to jump up as high and as fast as they could for 30 seconds. Three sets of wall touches were performed in the first week. Split squat jumps were performed with the subjects' feet facing forward in a lunge position. The objective was to jump straight up, land in the same position, and immediately repeat the jump. Two sets of 40 repetitions were performed in the first week. Lateral cone jumps were performed with the subjects standing next to a cone with their feet spread shoulderwidth apart; the objective was to jump back and forth over the cone as quickly as possible. Two sets of 30 repetitions were performed in the first week. Cone hops with 180° turns were performed with 4 cones spaced apart at 18-in (45.72-cm) intervals. Subjects began this exercise next to the first cone; they were instructed to jump over the cone, turning forward 180° while in the air, so they were facing the opposite direction when they landed. Subjects were to continue along the line of cones repeating this procedure. Jumping over all 4 cones was considered one repetition. Subjects performed 10 repetitions during the first week. Drop jumps (18-in [45.72 cm]) were added at week 4 of training. Subjects started on a box and were instructed to drop off the box; upon landing, subjects were to jump as high and as fast as they could. Initially subjects performed 20 repetitions of drop jumps.20 All subjects were posttested immediately after the 6-week training program. Sprint speed, EMG, and vertical-jump height analysis followed the same format as the pretest procedures.
Data Analysis
We calculated a 2-way multivariate analysis of variance (MANOVA) (group X time) with repeated measures on time to investigate significant differences when grouping the 3 EMG-dependent variables (area, mean, and peak). We performed a separate MANOVA for both the preparatory and reactive phases for each of the 6 muscles tested. The MANOVAs were also run for quadriceps:hamstrings and adductor: abductor coactivation in both the preparatory and reactive phases. When a finding was statistically significant as determined by the Wilks lambda criteria, we used 2-way univariate analysis of variance (group X time) with repeated measures on time to establish significant differences between individual dependent variables. When an interaction was significant, we conducted a test of simple main effects (Tukey honestly significant difference post hoc analysis). The MANOVAs were also performed to assess the statistical significance in vertical- jump height and sprint speed. Pearson correlation coefficients were used to analyze the patterns of EMG data (waveforms) based on shape and the variance ratio, which includes the variability of the EMG amplitude differences. The EMG pattern data were further divided into 10 periods within the drop-jump cycle and thus analyzed using the nonparametric Wilcoxon rank sum test. All data were analyzed using the Statistical Package for Social Sciences (version 10.0; SPSS Inc, Chicago, IL) for Microsoft Windows. The α level was set at P ≤ .05 for statistical significance.
RESULTS
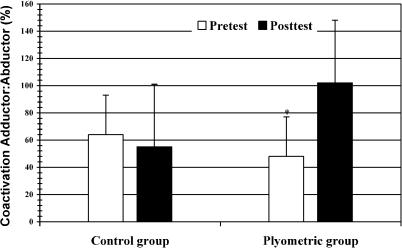
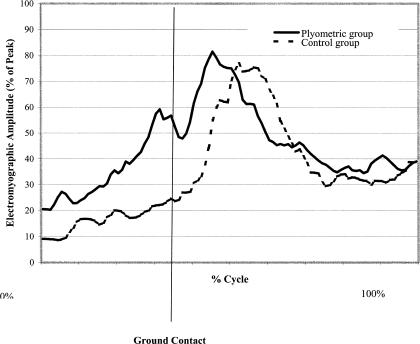
The group-by-session interaction in the hip adductor muscles during the preparatory phase was significant (F3,14 = 4.425, P = .022). A significant group-by-session interaction was also noted for 3 of the EMG-dependent variables: area (F1,16 = 6.580, P = .021), mean (F1,16 = 6.603, P = .021), and peak (F1,16 = 7.895, P = .013; Table 2). The experimental group had significant (P ≤ .05) increases in adductor muscle activity area, mean, and peak during the preparatory phase when compared with the control group. In the plyometric training group, the group-by-session interaction was significant, with an increase in preparatory adductor-to-abductor muscle coactivation (F1,15 = 5.267, P = .037; Figure 1). We noted a trend toward a group-by-session interaction in reactive quadriceps-to-hamstrings muscle coactivation (F1,16 = 4.346, P = .053). Significant changes in the muscle-activation patterns were seen from pretest to posttest between the experimental and control groups. The most prominent difference was observed after training, when the plyometric group demonstrated adductor muscle preactivation significantly earlier and with greater amplitude than the control group (Figure 2). No other significant differences in EMG data were identified.
Table 2.
Electromyography and Performance Variables (Mean ± SD)
Figure 1.
Coactivation ratio of adductor:abductor muscles for the control and plyometric training groups. A value of 100% on the vertical axis indicates equal levels of contraction in the adductor and abductor muscles. *P < .05 between groups.
Figure 2.
The pattern of electromyographic activity for the adductor muscles during a drop-jump task. At posttesting, the plyometric group demonstrated earlier and greater preactivation relative to ground contact. This suggests a change in the preprogrammed muscle-activation strategy that would benefit dynamic joint stability.
All subjects exhibited a significant increase in both sprint speed (F1,16 = 32.495, P = .000) and vertical jump (F1,16 = 8.828, P = .009) over time. The average increases in vertical- jump height for the experimental (5.8%, mean = 2.54 ± 2.97 cm) and control groups (2%, mean = 0.84 ± 1.68 cm) were not significantly different. Table 2 presents means and standard deviations for all variables tested.
DISCUSSION
Our purpose was to evaluate the effects of plyometric training on muscle-activation strategies and performance in the hips and thighs of female athletes. Our findings suggest that plyometric training encourages early adductor preactivation that is of greater magnitude than in control subjects. Significant increases in adductor and abductor coactivation were also demonstrated, which together may position the decelerating knee joint in a more biomechanically neutral frontal-plane position. The significant changes identified in the muscle-activation patterns after plyometric training suggest that motor- control strategies can modify and may benefit dynamic joint stability. These neuromuscular adaptations corroborate previous kinematic and kinetic data.5 Our observations also support the use of plyometric training to enhance knee joint stability even if functional performance is not significantly improved.
Muscle-Activation Strategies
Dynamic knee stability is achieved with preparatory and reactive neuromuscular control.34,35 Preparatory muscle activity involves feed-forward processing, in which the planning of movements is based on sensory input from previous experiences.35 Reactive muscle activity involves the feedback process of motor control and the use of reflexive pathways to modify motor-unit recruitment.12 Increased muscle activity will augment muscle-stiffness properties,36 so that joint loads are absorbed within the tenomuscular unit rather than transmitted through articular structures. The most efficient strategies for regulation of muscle stiffness are not yet fully appreciated; however, increased muscle activation is a dynamic restraint mechanism capable of “stress shielding”13 vulnerable capsuloligamentous structures.37
The significant change in EMG activity after 6 weeks of plyometric training included increased adductor muscle-group amplitude during the preparatory phase of landing. The pattern of adductor muscle activation was also significantly different, with earlier preactivation and greater amplitude before landing. Hewett et al5 suggested that decreases in abduction and adduction knee moments after plyometric training resulted from altered muscular control of the lower extremity in the frontal plane. Olmstead et al38 found that the tensor fascia latae (hip abductor) worked in concert with the quadriceps during knee extension, whereas the gracilis (hip adductor) acted in synchrony with the medial hamstrings in knee flexion, indicating that the hip abductor and adductor musculature have direct roles in assisting with knee joint stability. Although increased abduction or adduction lower extremity alignment at landing creates a less stable position for the knee joint, a decrease in the abduction and adduction torques at the knee and hip may aid in stabilizing of the knee joint and preventing serious knee injuries.22 Therefore, the early adductor preactivation and increased amplitude of adductor EMG could provide greater functional knee stability at ground contact and ultimately decrease the incidence of knee injury.
Muscle coactivation, agonist and antagonist muscle synchrony, is also necessary to balance joint forces. Coactivation values of 100% indicate agonist and antagonist muscle synchrony, which also increases muscle and joint stiffness.37,39 Zhang and Wang40 reported that active contraction of hip abductor and adductor muscles could increase knee joint stiffness by about 58%, which is important in maintaining knee joint stability during functional tasks. Our results indicated a significant increase in preparatory adductor-to-abductor coactivation after training for the plyometric group (102%), whereas the control group remained low (55%). These results also support data from Hewett et al,5 who showed significant decreases in abduction and adduction moments at the knee joint in female athletes after plyometric training. Increased preparatory hip adductor-to-abductor muscular coactivation may increase hip and knee joint stiffness, thus decreasing adduction and abduction moments and enhancing dynamic restraint during functional activities.
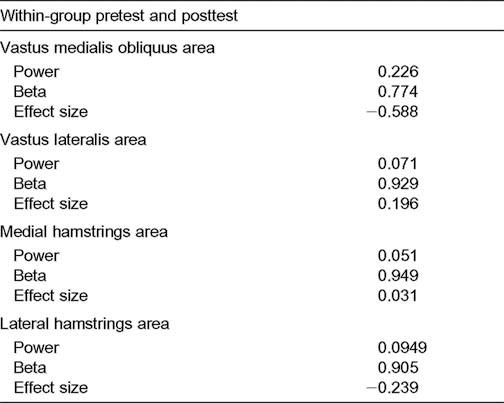
Dynamic restraint in the knee can also be achieved through reflexive or reactive neuromuscular control.34,35 Our findings indicated only a trend toward increased reactive muscle activation. This was observed after ground contact, when the plyometric group appeared to have more symmetric quadriceps- to-hamstrings muscle coactivation. Baratta et al41 suggested that individuals with hypertrophied quadriceps muscles, such as high-performance athletes, have less coactivation of the hamstrings muscles because of an inhibitory effect on reciprocal antagonistic muscles. Less quadriceps and hamstrings coactivation can increase strain on the anterior cruciate ligament and predispose athletes to noncontact injuries.42,43 However, plyometric training may produce neuromuscular adaptations that encourage more symmetric quadriceps and hamstrings coactivation and balance joint loads for dynamic restraint.44 The lack of significant differences in reactive muscle activation in the quadriceps and hamstrings muscle groups may have been due to our subject sample and their high level of physical condition. Although the sport-specific plyometric exercises were a novel activity for the subjects, their previous experience with physical conditioning may have limited the potential for enhanced reflexive-induced EMG changes,20 and feed-forward processing may have dominated the motor patterns at posttesting. Treatment effects are more likely when a larger population is represented, so a limitation to our study may have been the small sample size and variability of EMG data. Further analysis of the reactive EMG variables confirmed that observing significant differences in the reactive strategies of collegiate athletes may require large numbers of subjects. Calculations revealed that the within-group effect size from plyometric training ranged from 0.03 to 0.59 and the power ranged from .05 to .23 (Table 3).
Table 3.
Power and Effect Size for Plyometric Group
Functional Performance
Both groups demonstrated small but statistically insignificant improvements in vertical-jump height and sprint speed over time. Our findings differ from those of other authors,5,45–47 who observed significant between-group differences in ver-tical-jump performance. However, these studies differed in the frequency, intensity, time, and type of plyometric exercises and used sedentary control subjects or lower-caliber athletes for group comparisons.
Field45 noted improvements with a plyometric training program in athletes who were similar but of a slightly lower caliber. Blattner and Noble47 saw significant increases in vertical- jump height after isokinetic and plyometric training, but neither regimen was more effective than the other. Pestolesi46 reported a mean increase of 0.45 in (1.14 cm) in vertical jump after 6 weeks of jump training with high school athletes, whereas our collegiate group had a mean increase of 1 in (2.54 cm). We used a plyometric training program with a higher volume of repetitions (weekly foot contacts)45 and smaller rest interval than that recommended by Chu.20 However, initial pilot testing on a similar cohort of athletes was unable to induce any adaptations with a lower intensity protocol.45 The plyometric conditioning protocol was subsequently intensified, and we were able to observe neuromuscular but not performance adaptations.
We avoided selection bias by using high-caliber athletes in the control group, and the participation of subjects in off-season training may have limited the potential for observing group differences or a larger plyometric-training effect size. Testing a larger sample may negate this study limitation because the experimental group had a greater mean increase in vertical-jump height, compared with the control group, which may be clinically relevant to elite athletes.
CONCLUSIONS
Our purpose was to examine the effect of plyometric training on muscle activation in female athletes. Plyometric training induced beneficial neuromuscular adaptations in the hip adductor muscles that may assist with knee stability. Adductor muscle preactivation and adductor and abductor coactivation both increased after plyometric training. These neuromuscular adaptations, combined with previous kinematic and kinetic data,5 strongly support the use of plyometric training to enhance dynamic restraint and functional stability at the knee joint. These observations also suggest that more emphasis should be placed on hip-muscle performance and coordination in the training regimen of female athletes to minimize the risk of knee injuries.
ACKNOWLEDGMENTS
We thank the members of the 2001–2002 women's soccer and field hockey teams at St Joseph's University for their commitment to, and enthusiasm for, the plyometric training program.
REFERENCES
- 1.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 2.Ireland ML, Gaudette M, Crook S. ACL injuries in the female athlete. J Sport Rehabil. 1997;6:97–110. [Google Scholar]
- 3.Malone TR, Hardaker WT, Garrett WE, Feagin JA, Bassett FH. Relationship of gender to anterior cruciate ligament injuries in intercollegiate basketball players. J South Orthop Assoc. 1993;2:36–39. [Google Scholar]
- 4.Zelisko JA, Noble HB, Porter M. A comparison of men's and women's professional basketball injuries. Am J Sports Med. 1982;10:297–299. doi: 10.1177/036354658201000507. [DOI] [PubMed] [Google Scholar]
- 5.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes: decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24:765–773. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 6.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 7.Moller-Nielsen J, Hammar M. Women's soccer injuries in relation to the menstrual cycle and oral contraceptive use. Med Sci Sports Exerc. 1989;21:126–129. [PubMed] [Google Scholar]
- 8.Souryal TO, Freeman TR. Intercondylar notch size and anterior cruciate ligament injuries in athletes: a prospective study. Am J Sports Med. 1993;21:535–539. doi: 10.1177/036354659302100410. [DOI] [PubMed] [Google Scholar]
- 9.Souryal TO, Moore HA, Evans JP. Bilaterality in anterior cruciate ligament injuries: associated intercondylar notch stenosis. Am J Sports Med. 1988;16:449–454. doi: 10.1177/036354658801600504. [DOI] [PubMed] [Google Scholar]
- 10.Swanik CB, Lephart SM, Giannantonio FP, Fu FH. Reestablishing proprioception and neuromuscular control in the ACL-injured athlete. J Sport Rehabil. 1997;6:182–206. [Google Scholar]
- 11.Swanik KA, Swanik CB, Lephart SM, Huxel K. The effects of functional training on the incidence of shoulder injury in intercollegiate swimmers. J Sport Rehabil. 2002;11:142–154. [Google Scholar]
- 12.Swanik KA, Lephart SM, Swanik CB, Lephart SP, Stone DA, Fu FH. The effects of shoulder plyometric training on proprioception and selected muscle performance characteristics. J Shoulder Elbow Surg. 2002;11:579–586. doi: 10.1067/mse.2002.127303. [DOI] [PubMed] [Google Scholar]
- 13.Lephart SM, Ferris C, Riemann B, Myers J. Gender differences in neuromuscular patterns and landing strategies. Paper presented at: 2001 Kentucky Sports Medicine Research Retreat, The Gender Bias with ACL Injuries; April 2001; Lexington, KY. [Google Scholar]
- 14.Anderson FC, Pandy MG. Storage and utilization of elastic strain energy during jumping. J Biomech. 1993;26:1413–1427. doi: 10.1016/0021-9290(93)90092-s. [DOI] [PubMed] [Google Scholar]
- 15.Bosco C, Komi PV. Potentiation of the mechanical behavior of the human skeletal muscle through prestretching. Acta Physiol Scand. 1979;106:467–472. doi: 10.1111/j.1748-1716.1979.tb06427.x. [DOI] [PubMed] [Google Scholar]
- 16.Cavagna GA, Saibene FP, Margaria R. Effect of negative work on the amount of positive work performed by an isolated muscle. J Appl Physiol. 1965;20:157–158. doi: 10.1152/jappl.1965.20.1.157. [DOI] [PubMed] [Google Scholar]
- 17.Komi PV, Bosco C. Utilization of stored elastic energy in leg extensor muscles by men and women. Med Sci Sports. 1978;10:261–265. [PubMed] [Google Scholar]
- 18.Wilk KE, Voight ML, Keirns MA, Gambetta V, Andrews JR, Dillman CJ. Stretch-shortening drills for the upper extremities: theory and clinical application. J Orthop Sports Phys Ther. 1993;17:225–239. doi: 10.2519/jospt.1993.17.5.225. [DOI] [PubMed] [Google Scholar]
- 19.Wilt F. Plyometrics: what it is and how it works. Athl J. 1975;55:76–90. [Google Scholar]
- 20.Chu DA. Jumping Into Plyometrics. 2nd ed. Chicago, IL: Human Kinetics; 1998. [Google Scholar]
- 21.Hutton RS, Atwater SW. Acute and chronic adaptations of muscle proprioceptors in response to increased use. Sports Med. 1992;14:406–421. doi: 10.2165/00007256-199214060-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hewett TE. Neuromuscular and hormonal factors associated with knee injuries in female athletes: strategies for intervention. Sports Med. 2000;29:313–327. doi: 10.2165/00007256-200029050-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. Am J Sports Med. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan TS, Lloyd DG. Muscle activation at the human knee during isometric flexion-extension and varus-valgus loads. J Orthop Res. 1997;15:11–17. doi: 10.1002/jor.1100150103. [DOI] [PubMed] [Google Scholar]
- 25.Kowalk DL, Duncan JA, Vaughan CL. Abduction-adduction moments at the knee during stair ascent and descent. J Biomech. 1996;29:383–388. doi: 10.1016/0021-9290(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd DG, Buchanan TS. A model of load sharing between muscles and soft tissues at the human knee during static tasks. J Biomech Eng. 1996;118:367–376. doi: 10.1115/1.2796019. [DOI] [PubMed] [Google Scholar]
- 27.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 28.Swanik CB, Lephart SM, Giraldo JL, DeMont RG, Fu FH. Reactive muscle firing of anterior cruciate ligament-injured females during functional activities. J Athl Train. 1999;34:121–129. [PMC free article] [PubMed] [Google Scholar]
- 29.DeLuca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- 30.Cram JR, Kasman GS, Holtz J. Introduction to Surface Electromyography. Gaithersburg, MD: Aspen Publishers, Inc; 1998. [Google Scholar]
- 31.Straub SJ. Skill Level Differences in Lower Extremity Kinematics and Neuromuscular Characteristics of Female Gymnasts During Drop Landings. [dissertation] Philadelphia, PA: Temple University; 2002. [Google Scholar]
- 32.Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65:517–521. [PubMed] [Google Scholar]
- 33.Young W, MacDonald C, Heggen T, Fitzpatrick J. An evaluation of the specificity, validity, and reliability of jumping tests. J Sports Med Phys Fitness. 1997;37:240–245. [PubMed] [Google Scholar]
- 34.Dietz V, Noth J, Schmidtbleicher D. Interaction between pre-activity and stretch reflex in human triceps brachii during landing from forward falls. J Physiol. 1981;311:113–125. doi: 10.1113/jphysiol.1981.sp013576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn TG, Gillig SE, Ponser SE, Weil N, Utz SW. The learning process in biofeedback: is it feed-forward or feedback? Biofeedback Self Regul. 1986;11:143–156. doi: 10.1007/BF00999982. [DOI] [PubMed] [Google Scholar]
- 36.Sinkjaer T, Arendt-Nielsen L. Knee stability and muscle coordination in patients with anterior cruciate ligament injuries: an electromyographic approach. J Electromyogr Kinesiol. 1991;1:209–217. doi: 10.1016/1050-6411(91)90036-5. [DOI] [PubMed] [Google Scholar]
- 37.Bach TM, Chapman AE, Calvert TW. Mechanical resonance of the human body during voluntary oscillations about the ankle joint. J Biomech. 1983;16:85–90. doi: 10.1016/0021-9290(83)90049-0. [DOI] [PubMed] [Google Scholar]
- 38.Olmstead TG, Wevers HW, Bryant JT, Gouw GJ. Effect of muscular activity on valgus/varus laxity and stiffness of the knee. J Biomech. 1986;19:565–577. doi: 10.1016/0021-9290(86)90162-4. [DOI] [PubMed] [Google Scholar]
- 39.Dyhre-Poulsen P, Simonsen EB, Voigt M. Dynamic control of muscle stiffness and H reflex modulation during hopping and jumping in man. J Physiol. 1991;437:287–304. doi: 10.1113/jphysiol.1991.sp018596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang LQ, Wang G. Dynamic and static control of the human knee joint in abduction-adduction. J Biomech. 2001;34:1107–1115. doi: 10.1016/s0021-9290(01)00080-x. [DOI] [PubMed] [Google Scholar]
- 41.Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D'Ambrosia R. Muscular coactivation: the role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16:113–122. doi: 10.1177/036354658801600205. [DOI] [PubMed] [Google Scholar]
- 42.Cowling EJ, Steel JR. Is lower limb muscle synchrony during landing affected by gender? Implications for variations in ACL injury rates. J Electromyogr Kinesiol. 2001;11:263–268. doi: 10.1016/s1050-6411(00)00056-0. [DOI] [PubMed] [Google Scholar]
- 43.Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med. 1986;14:83–87. doi: 10.1177/036354658601400114. [DOI] [PubMed] [Google Scholar]
- 44.Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15:207–213. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- 45.Field RW. Off-season plyometric conditioning for the collegiate soccer player. Natl Strength Cond Assoc J. 1991;13:27–28. [Google Scholar]
- 46.Pestolesi TJ. Selected Training Programs to Improve Vertical Jump in High School Athletes [master's thesis] Long Beach, CA: California State University; 1989. [Google Scholar]
- 47.Blattner SE, Noble L. Relative effects of isokinetic and plyometric training on vertical jumping performance. Res Q. 1979;50:583–588. [Google Scholar]