Abstract
Background
Although many studies have shown that diabetes increases the risk for urinary incontinence, it is unclear whether poor glycemic control in women with diabetes is associated with incontinence. This study aims to determine the relationship between the hemoglobin A1c (HbA1c) level and urinary incontinence in a large, diverse cohort of older women.
Methods
We examined 6026 older women who responded to a survey (62% response rate) and were enrolled in the Diabetes and Aging Study, an ethnically stratified random sample of patients with diabetes enrolled in Kaiser Permanente Northern California. Our primary independent variable was the mean of all HbA1c measurements in the year preceding the survey. Outcomes included the presence/absence of incontinence and limitations in daily activities due to incontinence. We used modified Poisson regression and ordinal logistic regression models to account for age, race, body mass index, parity, diabetes treatment, duration of diabetes, and comorbidity.
Results
Sixty-five percent of women reported incontinence (mean age 59±10 years). After adjustment, HbA1c levels were not associated with the presence or absence of incontinence. However, among women reporting incontinence, HbA1c ≥9% was associated with more limitations due to incontinence than HbA1c <6% (adjusted odds ratio 1.67, 95% confidence interval: 1.09–2.57).
Conclusion
In this cross-sectional analysis, HbA1c level is not associated with the presence or absence of incontinence. However, for women with incontinence, poor glycemic control (HbA1c ≥9%) is associated with more limitations in daily activities due to incontinence. Longitudinal studies are needed to determine whether improving glycemic control to HbA1c <9% leads to fewer limitations in daily activities due to incontinence.
Introduction
Urinary incontinence is exceedingly common in older women and is associated with many poor outcomes.1,2 Depending on the definition of incontinence and the population studied, prevalence estimates as high as 69% have been reported.3,4 Further, incontinence is associated with social isolation,5,6 depressive symptoms,5,7 and poor self-rated health,7,8 all of which lower quality of life.1,6,7
Previous studies have identified diabetes mellitus as a potent risk factor for incontinence in women.9,10 However, among women with diabetes, it is unclear whether poor glycemic control and more severe hyperglycemia is associated with incontinence. Previous authors have hypothesized that poor glycemic control and hyperglycemia could lead to glycosuria and neuropathy, which may precipitate or worsen urinary incontinence symptoms.11–13 Studies to date have shown no evidence of an association between the level of glycemic control and incontinence.14,15 However, previous studies have included few women with poor glycemic control, limiting their power to explore the hyperglycemia–incontinence relationship in women at highest risk.14,15
Thus, we examined the relationship between hemoglobin A1c (HbA1c) and urinary incontinence in a large cohort of ethnically diverse women with diabetes mellitus (DM) enrolled in Kaiser Permanente Northern California (KPNC). Our objectives were to (1) determine whether HbA1c levels among women with DM were associated with the presence of urinary incontinence and (2) among women reporting incontinence, determine whether higher HbA1c levels were associated with more limitations in daily activities due to urinary incontinence. Since glycosuria occurs at serum blood sugar levels >180 mg/dL,16 which corresponds to a HbA1c level >8%,17 we hypothesized that patients with HbA1c >8% would be at higher risk for incontinence as well as more limitations in daily activities due to incontinence.
Methods
The Diabetes and Aging Study, a sub-study of the Diabetes Study of Northern California (DISTANCE),18 focuses on processes and outcomes of healthcare among older patients with diabetes. We conducted a lagged cross-sectional study, focusing on the relationship between self-reported incontinence and HbA1c levels in the year before self-report, using results from the DISTANCE survey and the KPNC electronic medical record (EMR).
Setting and participants
The DISTANCE survey enrolled an ethnically stratified, random sample of patients in the KPNC Diabetes Registry in 2005 and 2006.18 KPNC is a fully integrated health care delivery system that provides comprehensive medical care to ∼3.3 million members (∼30% of the northern California population). Except for the uninsured, the demographic characteristics of the KPNC's patient populations are similar to those of the overall population of northern California.19
The overarching aim of DISTANCE was to investigate ethnic and educational disparities in diabetes-related behaviors, care processes, and outcomes.18 DISTANCE was conducted among 40,735 patients (19,377 women) aged 30–75 years with both type 1 and 2 DM. The survey was conducted in one of 5 languages (English, Spanish, Cantonese, Mandarin, or Tagalog) and administered in one of 4 modes: computer-assisted telephone interview (CATI), web-based survey, self-administered written questionnaire, and short version of the written survey (overall response rate of 62%).18 Because the short version of the survey did not include questions about incontinence, we excluded the 2,393 short version respondents (12% of all respondents). From the remaining 6,652 women responding through CATI, web-based survey and long form written questionnaire, we excluded respondents who did not have a Hemoglobin A1c value in the year preceding the DISTANCE survey (n=602) and respondents who did not answer the impact of incontinence on daily activities question (n=24), leading to the final analytic cohort of 6,026 women with diabetes.
Measures: Independent variable and outcomes
Our primary independent variable was glycemic control as measured by glycosylated hemoglobin (HbA1c) from the KPNC EMR, categorized into a five-level variable (<6%, 6%–6.9%, 7%–7.9%, 8%–8.9%, and ≥9%). We obtained all HbA1c measures obtained for clinical purposes from the KPNC EMR in the 12-month period preceding the survey postmark date. On average, there were 2.1 HbA1c measurements and 90% of women had 1, 2, or 3 HbA1c measurements. HbA1c measures were averaged and this mean HbA1c value was categorized into our primary five-level independent variable. We conducted a sensitivity analysis substituting the most recent HbA1c measurement for the 12-month average HbA1c value and found no changes in our results.
Our two outcomes were (1) the presence or absence of occasional urinary incontinence and (2) limitations in daily activities due to incontinence. These outcomes were determined with questions derived from the 2001–2002 National Health and Nutrition Examination Survey (NHANES). The presence of occasional incontinence was determined with the question, “Do you experience occasional accidental leakage of urine?” For women who reported occasional urinary incontinence (n=3916), the limitations in daily activities due to incontinence was determined with the question, “During the past 12 months, how much did the leakage of urine affect your day-to-day activities? (not at all, slightly, moderately, quite a bit, or extremely).”20 Because of the small number of women who reported being extremely affected by urinary incontinence (n=130), we combined the quite a bit and extremely affected groups for our analysis. Previous studies suggest that patient-reported measures of incontinence are more closely associated with quality-of-life outcomes than objective measures of incontinence frequency or volume,21 suggesting subjective patient reports of incontinence may more accurately reflect the burden of incontinence.
Measures: Potential confounding factors
We accounted for a wide range of factors that may confound the relationship between glycemic control and incontinence including demographic factors (age and race/ethnicity), socioeconomic factors (educational attainment, income), health behaviors (smoking), comorbidities (diagnostic cost group [DxCG] comorbidity score using ICD9 diagnosis codes), stroke, diabetes-related factors (duration of diabetes, body mass index [BMI], and retinopathy), and incontinence related factors (parity and prior hysterectomy). Diabetes treatment was coded as a four-level variable: diet-controlled, pills only, insulin only, or pills and insulin. Several of these factors were obtained through the KPNC EMR (e.g., age, comorbidities, and BMI) while others were obtained through the DISTANCE survey (duration of diabetes and parity).
Statistical analysis: Presence/absence of incontinence
We determined the association between HbA1c and the presence of occasional incontinence using modified Poisson regression. We chose modified Poisson regression to estimate relative risk (RR) rather than logistic regression since our outcome was common, making the odds ratio (OR) less interpretable.22 Our primary independent variable was the HbA1c level categorized into a five-level variable and our outcome was the presence or absence of occasional incontinence. Initially, we accounted for all potential confounders but found that accounting for retinopathy, smoking, stroke, and prior hysterectomy did not affect our results, leading us to exclude these factors from our analysis. Thus, our final multivariate model accounted for age, race/ethnicity, BMI, parity, education, income, diabetes treatment, duration of diabetes, and the DxCG comorbidity score.
Statistical analysis: Limitations in daily activities due to incontinence
To determine the association between HbA1c and the patient-reported limitations in daily activities due to incontinence, we used ordinal logistic regression models with four levels of limitations (not at all, slightly, moderately, or quite a bit/extremely) as our outcome. Our independent variable was again the HbA1c level, categorized into a five-level variable. We accounted for the same potential confounding factors that we identified in the presence/absence of occasional incontinence analysis. The Brant test provided no evidence that the proportional odds assumption was violated (p=0.17). We estimated predicted probabilities of varying levels of limitations by HbA1c level, accounting for the effect of confounding factors used in the previous analysis. As a supplemental analysis, we calculated the mean HbA1c level for each of the four levels of limitations due to incontinence to determine whether the HbA1c levels were higher in women reporting greater limitations due to incontinence. We performed a test of trend by assessing the slope of the linear regression line between the categories of limitations due to incontinence and mean HbA1c.
The Diabetes and Aging Study, and DISTANCE were approved by the institutional review board at Kaiser Foundation Research Institute. The Committee of Human Research at the University of California, San Francisco reviewed this study and determined that it did not qualify as human research because all data used for analysis had been de-identified.
Results
Characteristics of the participants
Of the 6,026 women in our study, 64% (n=3,832) had HbA1c between 6% and 7.9% (Table 1). Women with higher HbA1c measurements were younger and more likely to be black or Latino (both p-values <0.001). Income and number of births were not different across women in different HbA1c categories. Women with higher HbA1c measurements had greater comorbidity burden, higher BMI, longer duration of diabetes, and were more likely to be treated with insulin (p<0.001 for all). Sixty-five percent of women (3,916 of 6,026) reported occasional urinary incontinence.
Table 1.
Characteristics of All Participants (n= 6,026)
| Characteristic | HbA1c <6% (n=547) | HbA1c 6–6.9% (n=2147) | HbA1c 7%–7.9% (n=1685) | HbA1c 8%–9% (n=836) | HbA1c 9%+ (n=811) | p* |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Mean age, years (±SD) | 59.7 (10.9) | 60.5 (9.9) | 59.5 (9.9) | 57.2 (9.9) | 54.5 (10.0) | <0.001 (trend) |
| Ethnicity, % | ||||||
| White | 37 | 26 | 22 | 21 | 16 | <0.001 |
| Black | 19 | 20 | 20 | 18 | 26 | |
| Latino | 17 | 16 | 18 | 22 | 26 | |
| Asian | 10 | 13 | 13 | 10 | 4 | |
| Filipino | 6 | 12 | 13 | 15 | 11 | |
| Other | 11 | 13 | 14 | 14 | 17 | |
| Socioeconomic status measures | ||||||
| Education, % | ||||||
| No degree/GED | 19 | 18 | 15 | 16 | 19 | 0.002 |
| HS/technical/AA | 56 | 56 | 56 | 58 | 59 | |
| Bachelors | 16 | 18 | 19 | 20 | 16 | |
| Post graduate | 9 | 8 | 10 | 6 | 6 | |
| Income, % | ||||||
| <$25,000 | 28 | 27 | 22 | 22 | 22 | 0.08 |
| $25,000–$49,999 | 30 | 31 | 33 | 32 | 35 | |
| $50,000–$79,999 | 22 | 23 | 24 | 26 | 25 | |
| ≥$80,000 | 20 | 19 | 21 | 20 | 18 | |
| DxCG comorbidity score (±SD) | 4.9 (6.3) | 4.4 (4.9) | 4.5 (4.6) | 5.2 (5.4) | 5.2 (5.0) | <0.001 (trend) |
| Number of births, % | ||||||
| 0 | 15 | 14 | 14 | 14 | 14 | 0.27 |
| 1–2 | 43 | 38 | 37 | 40 | 39 | |
| ≥3 | 42 | 48 | 49 | 46 | 47 | |
| Body mass index, % | ||||||
| <25 | 23 | 21 | 19 | 15 | 13 | <0.001 |
| 25–30 | 30 | 28 | 27 | 29 | 26 | |
| 305 | 21 | 22 | 26 | 24 | 28 | |
| >35 | 26 | 29 | 28 | 32 | 33 | |
| Diabetes duration in years (±SD) | 8.1 (8.2) | 9.7 (9.1) | 11.2 (9.0) | 12.2 (9.5) | 11.5 (8.2) | <0.001 (trend) |
| Diabetes treatment, % | ||||||
| Diet only | 32 | 17 | 4 | 2 | 1 | <0.001 |
| Pills only | 55 | 66 | 69 | 60 | 55 | |
| Insulin only | 9 | 9 | 11 | 15 | 17 | |
| Insulin and pills | 4 | 8 | 16 | 23 | 27 | |
p-values are analysis of variance (ANOVA) for categorical variables and test for trend for continuous variables.
AA, associate of arts; DxCG, diagnostic cost group; GED, general educational development, commonly known as a high school (HS) equivalency exam; HbA1c, hemoglobin A1c; SD, standard deviation.
The characteristics of the 3916 women reporting occasional urinary incontinence were similar to full cohort of 6026 women (Table 2). Again, women reporting occasional incontinence with higher HbA1c measurements were younger, more likely to be black and Latino, have higher comorbidity burden, have higher BMI, report longer duration of diabetes, and were more likely to be treated with insulin.
Table 2.
Characteristics of Women Reporting Occasional Incontinence (n= 3916)
| Characteristic | HbA1c <6% (n=343) | HbA1c 6-7% (n=1388) | HbA1c 7-8% (n=1109) | HbA1c 8-9% (n=552) | HbA1c 9%+ (n=524) | p* |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Mean age, years (±SD) | 59.8 (10.4) | 60.7 (9.6) | 59.1 (9.5) | 57.0 (9.8) | 54.4 (9.9) | <0.001 (trend) |
| Ethnicity, % | ||||||
| White | 41 | 30 | 25 | 23 | 18 | <0.001 |
| Black | 13 | 16 | 16 | 15 | 24 | |
| Latino | 20 | 16 | 20 | 22 | 28 | |
| Asian | 11 | 13 | 12 | 10 | 3 | |
| Filipino | 5 | 12 | 14 | 14 | 10 | |
| Other | 10 | 13 | 13 | 16 | 17 | |
| Socioeconomic measures | ||||||
| Education, % | ||||||
| No degree/GED | 19 | 18 | 16 | 15 | 18 | 0.04 |
| HS/technical/AA | 59 | 56 | 56 | 58 | 60 | |
| Bachelors | 13 | 18 | 19 | 21 | 16 | |
| Post graduate | 9 | 8 | 9 | 6 | 6 | |
| Income, % | ||||||
| <$25,000 | 26 | 27 | 22 | 23 | 21 | 0.19 |
| $25,000–$49,999 | 32 | 31 | 32 | 31 | 35 | |
| $50,000–$79,999 | 21 | 22 | 25 | 26 | 24 | |
| ≥$80,000 | 21 | 20 | 21 | 20 | 20 | |
| DxCG comorbidity score (±SD) | 4.7 (6.1) | 4.5 (4.9) | 4.5 (4.3) | 5.2 (4.9) | 5.2 (5.1) | 0.004 (trend) |
| Number of births, % | ||||||
| 0 | 13 | 11 | 12 | 13 | 13 | 0.07 |
| 1–2 | 45 | 37 | 36 | 41 | 39 | |
| ≥3 | 42 | 52 | 52 | 46 | 48 | |
| Body mass index, % | ||||||
| <25 | 18 | 19 | 16 | 14 | 9 | <0.001 |
| 25–30 | 33 | 27 | 26 | 25 | 26 | |
| 30–35 | 22 | 23 | 27 | 25 | 28 | |
| >35 | 27 | 31 | 31 | 36 | 37 | |
| Diabetes duration in years (±SD) | 7.7 (8.0) | 9.6 (8.8) | 10.9 (8.6) | 12.2 (9.5) | 11.6 (8.0) | <0.001 (trend) |
| Diabetes treatment, % | ||||||
| Diet only | 36 | 18 | 4 | 2 | 1 | <0.001 |
| Pills only | 55 | 66 | 69 | 58 | 53 | |
| Insulin only | 6 | 8 | 10 | 14 | 15 | |
| Insulin and pills | 3 | 8 | 17 | 26 | 31 | |
| Limitations due to incontinence | ||||||
| No effect | 50 | 44 | 43 | 40 | 41 | 0.27 |
| Slight effect | 33 | 33 | 34 | 36 | 36 | |
| Moderate effect | 9 | 12 | 12 | 11 | 11 | |
| Quite a bit/extreme effect | 8 | 11 | 11 | 13 | 12 | |
p-values are ANOVA for categorical variables and test for trend for continuous variables.
Relationship between HbA1c and presence of occasional incontinence
We observed no association between HbA1c levels and the presence of occasional incontinence (Table 3). Across a wide spectrum of HbA1c levels from <6% to ≥9%, the proportion of women reporting occasional incontinence was stable between 67% and 71%. Our models confirmed the lack of association with no statistically significant risk ratios (RRs) in the unadjusted and adjusted analyses. For example, women with HbA1c ≥9% had a similar risk of incontinence to women with HbA1c <6% (unadjusted RR=1.05, 95% confidence interval [CI]: 0.95–1.16; adjusted RR 1.09, 95% CI: 0.98–1.21).
Table 3.
Hemoglobin A1c Levels and Occasional Incontinence
| HbA1c level | n (%) | Incontinence (%) | Unadjusted risk ratio (95% CI) | Adjusted* risk ratio (95% CI) |
|---|---|---|---|---|
| <6% | 547 (9) | 67 | Ref | Ref |
| 6–6.9% | 2147 (36) | 69 | 1.04 (0.95– 1.14) | 1.04 (0.95–1.14) |
| 7–7.9% | 1685 (28) | 71 | 1.07 (0.97–1.17) | 1.08 (0.99–1.19) |
| 8–8.9% | 836 (14) | 69 | 1.04 (0.94–1.16) | 1.06 (0.96–1.18) |
| ≥9% | 811 (13) | 70 | 1.05 (0.95–1.16) | 1.09 (0.98–1.21) |
Adjusted for age, race, body mass index, parity, education, income, diabetes treatment, years of diabetes, and DxCG comorbidity score.
CI, confidence interval.
Relationship between HbA1c and limitations in daily activities due to incontinence
Although HbA1c levels were not associated with the presence of incontinence, we found evidence that higher HbA1c levels were associated with greater limitations due to incontinence in both unadjusted and adjusted analyses. In the unadjusted analysis, women reporting no limitations due to incontinence had a mean (±standard deviation) HbA1c of 7.4% (±1.5); women reporting “slight” and “moderate” limitations due to incontinence had a mean HbA1c of 7.5% (±1.5 and 1.4, respectively) and women reporting “quite a bit” or “extreme” limitations had a mean HbA1c of 7.6% (±1.5), (p for trend=0.04). Thus, women reporting more limitations in activities due to incontinence had higher HbA1c levels.
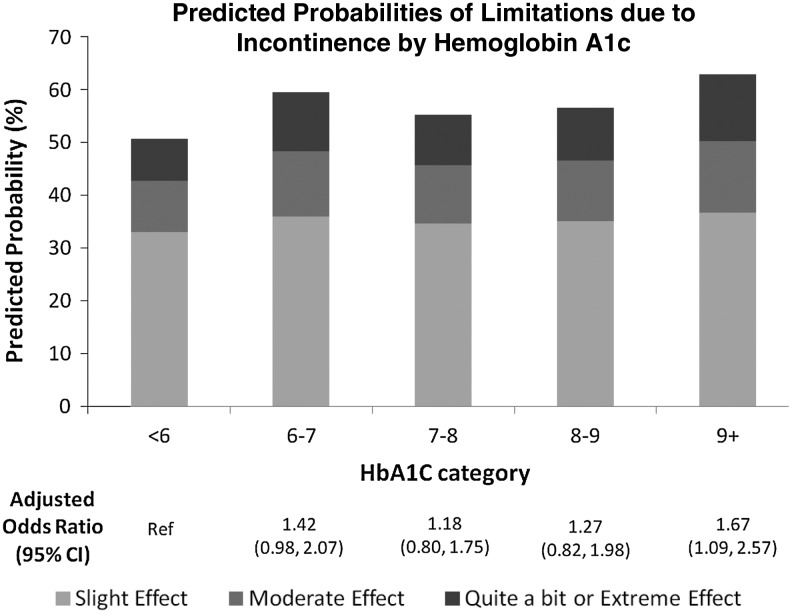
To account for possible confounding factors, we predicted the probability of our outcome (limitations in daily activities due to incontinence) by HbA1c levels using our multivariate ordinal logistic regression model (Fig. 1). After multivariate adjustment, women with a HbA1c ≥9% had an increased odds of more limitations in daily activities due to incontinence compared with women with HbA1c <6%, with a proportional OR of 1.67 (95% CI: 1.09–2.57). For women with intermediate HbA1c levels between 6%–6.9% through 8%–8.9%, there were no differences in limitations in daily activities due to incontinence.
FIG. 1.
Limitations due to incontinence.
Discussion
Among a large and racially/ethnically diverse cohort of women with diabetes, we found that urinary incontinence is exceedingly common, with 65% of women reporting occasional urinary incontinence. Although HbA1c was not associated with the presence of occasional incontinence, very poor glycemic control (HbA1c ≥9%) was associated with more limitations in daily activities due to incontinence even after accounting for a wide range of potential confounding factors. Our cross-sectional study cannot establish causal relationships; however, one potential explanation for our findings is that for women who are continent, HbA1c >9% may lead to glycosuria and frequent urination but not incontinence. However, for women with baseline incontinence, poor glycemic control and glycosuria may worsen preexisting incontinence and may lead to greater limitations in daily activities.
There are two important clinical implications of our results. First, our study adds to literature by confirming that racially and ethnically diverse older women with diabetes are at high risk of urinary incontinence.23 Combined with previous studies from the Diabetes and Aging Study, which showed a strong relationship between urinary incontinence and health-related quality of life,24 our results reinforce the importance of asking about, diagnosing, and treating urinary incontinence in diverse older women with diabetes.25
Second, for older women with diabetes and incontinence, improving glycemic control has often been advocated as a means of improving urinary incontinence symptoms.11,13 Our cross-sectional results cannot establish how changing HbA1c levels may affect urinary incontinence. However, our results, showing that HbA1c >9% is associated with greater limitations in daily activities due to incontinence, suggest that a longitudinal study is needed to determine whether improving glycemic control to HbA1c <9% can improve urinary incontinence symptoms.
Previous studies examining the relationship between glycemic control and incontinence have focused on the presence of incontinence and found no evidence of an association between HbA1c levels and urinary incontinence.14,15 Jackson and colleagues studied 218 women age 55–75 years enrolled in Group Health and found no associations between HbA1c (categorized into a three-level variable: ≤7.5%, 7.6%–8.5% and >8.5%) and either any incontinence or severe incontinence.14 Phelan and colleagues reported that among 2,994 overweight/obese women with diabetes who volunteered for a 4-year intensive weight loss trial, baseline cross-sectional analysis did not show any evidence of an association between HbA1c and incontinence.15 This study confirms and extends these previous studies, showing that while HbA1c does not predict the presence/absence of occasional incontinence, HbA1c >9% does predict more limitations due to incontinence.
Our study should be interpreted in light of its strengths and limitations. The first major strength of our study is that it examines a large, diverse, real-world clinical population with uniform access to care. A second strength is the nearly comprehensive assessment of glycemic control and data on many potentially important confounding factors from the Kaiser EMR and DISTANCE survey. A third strength is that our lagged cross-sectional study design (HbA1c levels obtained in the year before survey administration) makes reverse causation (greater limitations due to incontinence leading to poorer glycemic control) less likely.
Our study also has limitations. First, the observed association between HbA1c ≥9% and more activity limitations due to incontinence may represent a correlation between two markers of diabetes severity. However, we believe this is unlikely to completely explain our findings since this association remained after adjusting many diabetes severity factors (duration of diabetes, types of treatment, retinopathy, and comorbidity burden). Second, like all surveys, DISTANCE survey respondents likely differ from nonrespondents. Thus, our results may not be generalizable to women with diabetes who do not respond to surveys. However, unlike many surveys where little is known about nonrespondents, all invited participants in DISTANCE were Kaiser Diabetes Registry patients. Analysis showed that respondents and nonrespondents had similar demographic profiles.18
Further, we did not assess the type of urinary incontinence (i.e., stress, urge, overflow, mixed or functional incontinence) and due to the small numbers of patients with type 1 diabetes, we could not determine whether the relationship between glycemic control and urinary incontinence differed between patients with type 1 and type 2 diabetes. Although previous studies suggest a strong relationship between urinary incontinence and health-related quality of life (HRQOL), this study is unable to determine whether glycemic control is associated with HRQOL. Next, although our questions are derived from NHANES and have face validity, they have not been independently validated. In addition, we had limited number of events for women with HbA1c <6%, suggesting that we may not have had the power to detect clinically important associations. Because we focused on occasional incontinence, we cannot preclude an association between HbA1c and more frequent incontinence.
Finally, patients may not be representative of the entire U.S. population of patients with diabetes, since every patient was fully insured and received care in a single integrated delivery system. The KPNC system provides coordinated and uniform access to care, including comprehensive care management. As such, our results may underestimate rates of urinary incontinence and inadequate glycemic control in the under- and uninsured, or among populations cared for in nonintegrated health systems. However, while the burden of incontinence and hyperglycemia may be somewhat lower in the study population, it is unlikely that this would bias the association between those two factors.
Conclusion
In summary, 65% of women with diabetes report occasional incontinence, suggesting that providers should ask women with diabetes about incontinence. Similar to previous studies, we found no association between HbA1c levels and the presence/absence of incontinence. However, for women reporting incontinence, HbA1c ≥9% was associated with more patient-reported limitations in daily activities due to urinary incontinence. Longitudinal studies are needed to further explore the relationship between levels of glycemic control and urinary incontinence.
Acknowledgments
Dr. Sei J. Lee was supported by the Hellman Family Award for Early Career Faculty at University of California–San Francisco. Dr. Lee was also supported by K23AG040779 and KL2RR024130 from the National Center for Research Resources, a component of the U.S. National Institutes of Health. Drs. Andrew Karter and Elbert Huang were supported through the Diabetes and Aging Study (R01DK081796). Dr. Huang was also supported by P30DK092949 and Dr. Karter was also supported by P30DK092924 through the National Institute of Diabetes and Digestive and Kidney Disease.
We thank Mr. Howard H. Moffet of the Division of Research at Kaiser Permanente Northern California who assisted with the conception of the DISTANCE study and acquisition of the data. We also thank Ms. Irena Stijacic-Cenzer of the Division of Geriatrics at the University of California–San Francisco and San Francisco Veterans Affairs Medical Center and Ms. Jennifer Liu of the Division of Research at Kaiser Permanente Northern California for performing the statistical analysis. They received no compensation for their contributions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Holroyd-Leduc JM. Straus SE. Management of urinary incontinence in women: Scientific review. JAMA. 2004;291:986–995. doi: 10.1001/jama.291.8.986. [DOI] [PubMed] [Google Scholar]
- 2.Mardon RE. Halim S. Pawlson LG. Haffer SC. Management of urinary incontinence in Medicare managed care beneficiaries: results from the 2004 Medicare Health Outcomes Survey. Arch Intern Med. 2006;166:1128–1133. doi: 10.1001/archinte.166.10.1128. [DOI] [PubMed] [Google Scholar]
- 3.Holroyd-Leduc JM. Straus SE. Management of urinary incontinence in women: Clinical applications. JAMA. 2004;291:996–999. doi: 10.1001/jama.291.8.996. [DOI] [PubMed] [Google Scholar]
- 4.Swithinbank LV. Donovan JL. du Heaume JC, et al. Urinary symptoms and incontinence in women: Relationships between occurrence, age, and perceived impact. Br J Gen Pract. 1999;49:897–900. [PMC free article] [PubMed] [Google Scholar]
- 5.Fultz NH. Herzog AR. Self-reported social and emotional impact of urinary incontinence. J Am Geriatr Soc. 2001;49:892–899. doi: 10.1046/j.1532-5415.2001.49179.x. [DOI] [PubMed] [Google Scholar]
- 6.Sims J. Browning C. Lundgren-Lindquist B. Kendig H. Urinary incontinence in a community sample of older adults: prevalence and impact on quality of life. Disabil Rehabil. 2011;33:1389–1398. doi: 10.3109/09638288.2010.532284. [DOI] [PubMed] [Google Scholar]
- 7.Ko Y. Lin SJ. Salmon JW. Bron MS. The impact of urinary incontinence on quality of life of the elderly. Am J Manag Care. 2005;11(4 Suppl):S103–111. [PubMed] [Google Scholar]
- 8.Johnson TM., 2nd Kincade JE. Bernard SL. Busby-Whitehead J. Hertz-Picciotto I. DeFriese GH. The association of urinary incontinence with poor self-rated health. J Am Geriatr Soc. 1998;46:693–699. doi: 10.1111/j.1532-5415.1998.tb03802.x. [DOI] [PubMed] [Google Scholar]
- 9.Brown JS. Grady D. Ouslander JG. Herzog AR. Varner RE. Posner SE. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 10.Sampselle CM. Harlow SD. Skurnick J. Brubaker L. Bondarenko I. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol. 2002;100:1230–1238. doi: 10.1016/s0029-7844(02)02241-x. [DOI] [PubMed] [Google Scholar]
- 11.Vischer UM. Bauduceau B. Bourdel-Marchasson I, et al. A call to incorporate the prevention and treatment of geriatric disorders in the management of diabetes in the elderly. Diabetes Metab. 2009;35:168–77. doi: 10.1016/j.diabet.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Araki A. Ito H. Diabetes mellitus and geriatric syndromes. Geriatr Gerontol Int. 2009;9:105–114. doi: 10.1111/j.1447-0594.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 13.Ouslander JG. Management of overactive bladder. N Engl J Med. 2004;350:786–799. doi: 10.1056/NEJMra032662. [DOI] [PubMed] [Google Scholar]
- 14.Jackson S. Scholes D. Boyko E. Abraham L. Fihn S. Urinary incontinence and diabetes in postmenopausal women. Diabetes Care. 2005;28:1730–1738. doi: 10.2337/diacare.28.7.1730. [DOI] [PubMed] [Google Scholar]
- 15.Phelan S. Kanaya AM. Subak LL, et al. Prevalence and risk factors for urinary incontinence in overweight and obese diabetic women: Action for health in diabetes (look ahead) study. Diabetes Care. 2009;32:1391–1397. doi: 10.2337/dc09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post TW. Rose BD. Urinalysis in the diagnosis of renal disease; Waltham, MA: 2009. UpToDate, [Google Scholar]
- 17.Nathan DM. Kuenen J. Borg R. Zheng H. Schoenfeld D. Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffet HH. Adler N. Schillinger D, et al. Cohort profile: The Diabetes Study of Northern California (DISTANCE)—objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol. 2009;38:38–47. doi: 10.1093/ije/dyn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JS. Vittinghoff E. Lin F. Nyberg LM. Kusek JW. Kanaya AM. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose: findings from the National Health and Nutrition Examination Survey (NHANES) 2001–2002. Diabetes Care. 2006;29:1307–1312. doi: 10.2337/dc05-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang AJ. Brown JS. Thom DH. Fink HA. Yaffe K Study of Osteoporotic Fractures Research. Urinary incontinence in older community-dwelling women: The role of cognitive and physical function decline. Obstet Gynecol. 2007;109:909–916. doi: 10.1097/01.AOG.0000258277.01497.4b. [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Shaw C. Tansey R. Jackson C. Hyde C. Allan R. Barriers to help seeking in people with urinary symptoms. Fam Pract. 2001;18:48–52. doi: 10.1093/fampra/18.1.48. [DOI] [PubMed] [Google Scholar]
- 24.Laiteerapong N. Karter AJ. Liu JY, et al. Correlates of quality of life in older adults with diabetes: The diabetes and aging study. Diabetes Care. 2011;34:1749–1753. doi: 10.2337/dc10-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths AN. Makam A. Edwards GJ. Should we actively screen for urinary and anal incontinence in the general gynaecology outpatients setting? A prospective observational study. J Obstet Gynaecol. 2006;26:442–444. doi: 10.1080/01443610600747272. [DOI] [PubMed] [Google Scholar]