Abstract
Objective
Sleep deficiency is an emerging concept denoting a deficit in the quantity or quality of sleep. This may be particularly salient for pregnant women since they report considerable sleep complaints. Sleep deficiency is linked with morbidity, including degradations in psychosocial functioning, (e.g., depression and stress), which are recognized risk factors for adverse pregnancy outcomes. We sought to describe the frequency of sleep deficiency across early gestation (10–20 weeks) and whether sleep deficiency is associated with reports of more depressive symptoms and stress.
Methods
Pregnant women (N=160) with no self-reported sleep or psychological disorder provided sleep data collected via diary and actigraphy during early pregnancy: 10–12, 14–16, and 18–20 weeks' gestation. Sleep deficiency was defined as short sleep duration, insufficient sleep, or insomnia. Symptoms of depression and stress were collected at the same three time points. Linear mixed effects models were used to analyze the data.
Results
Approximately 28%–38% met criteria for sleep deficiency for at least one time point in early gestation. Women who were sleep deficient across all time points reported more perceived stress than those who were not sleep deficient (p<0.01). Depressive symptoms were higher among women with diary-defined sleep deficiency across all time points (p=0.02).
Discussion
Sleep deficiency is a useful concept to describe sleep recognized to be disturbed in pregnancy. Women with persistent sleep deficiency appear to be at greater risk for impairments in psychosocial functioning during early gestation. These associations are important since psychosocial functioning is a recognized correlate of adverse pregnancy outcomes. Sleep deficiency may be another important risk factor for adverse pregnancy outcomes.
Introduction
Pregnant women experience myriad changes ranging from physical modifications to emotional and psychosocial alterations that can vary greatly as pregnancy progresses. One behavior noted to change dramatically in up to 75% of women is sleep.1 Emerging data implicate disturbed sleep as an important correlate to maternal and fetal health. Several recent publications have found significant, although modest associations between disturbed sleep during pregnancy and adverse pregnancy outcomes.2–8 Disturbed sleep is also a major risk factor for depressed mood9,10 and stress during pregnancy,11 as well as a predictor of depression or depressive mood postpartum.12–14 Studies indicate that depressed or stressed mothers are more likely to deliver preterm, deliver low birth weight babies (<2500 g), and increase the risk that their offspring will develop an adult-onset chronic disease.15–19
Few investigations, however, have assessed sleep in early gestation as a contributor to established risk factors or adverse pregnancy outcomes.2,20,21 One of our previous studies suggested that poor sleep quality in early gestation (14–16 weeks) was an independent risk factor for preterm birth and associated with prenatal stress.3 Facco and colleagues2 reported that 26% of their cohort had short sleep duration in early pregnancy, and that short sleep duration was associated with impaired glucose metabolism and risk for gestational diabetes mellitus. Similarly, Williams and colleagues observed that 13.4% of their cohort reported sleep duration ≤6 hours in early pregnancy, and that these women had higher blood pressure readings in the third trimester.20 We hypothesized that sleep during early gestation may be a critically important contributor to pregnancy outcomes as it may impact psychosocial and physiological pathways linked to adverse pregnancy outcomes.6 Sleep information from early gestation remains disproportionately underreported in the literature.
An emerging construct that may be applicable to the sleep disturbance experienced by pregnant women is “sleep deficiency.” In 2011, the National Center for Sleep Disorders Research (NCSDR) coined the term “sleep deficiency” to distinguish sleep disturbance between those with sleep disorders from those with problems resultant of “modern urban lifestyles.” They also set forth a challenge to develop an understanding of the mechanisms of the threat posed by sleep deficiency to health.22 Sleep deficiency, as defined by Czeisler23 is “a deficit in the quantity or quality of sleep obtained versus the amount needed for optimal health, performance, and well being.” Buxton and colleagues proposed specific sleep variables they believed best reflected this concept. They suggested that sleep deficiency is “the presence of short sleep duration or sleep insufficiency (never feeling rested upon awakening) or insomnia symptoms three of more times per week.”24 We contend that this concept is highly applicable to pregnant women, since many women complain of these specific sleep disturbances once becoming pregnant. Indeed, they are the most commonly reported sleep disturbances during pregnancy.20,25–27 They are reported substantially more often as compared to similar nonpregnant women.21 Hence, the goals of this study were to (1) characterize the prevalence of sleep deficiency in early gestation, and (2) examine whether the presence or absence of sleep deficiency during early gestation (characterized as persistent, intermittent, or no sleep deficiency) is associated with greater depressive symptoms and perceived stress.
Methods
Participants
Study participants were 184 pregnant women (<14 weeks) recruited from the greater Pittsburgh area during October 2008 through December 2012 (as part of a longitudinal study assessing pregnancy-related sleep disturbances in relation to perinatal outcomes. Recruitment was by self-referral, physician referral, local advertising, or via participation in University research registries. All women intended on keeping the pregnancy when enrolled. Women were excluded if they self-reported a diagnosis of any psychopathology, sleep disorder or were actively taking an anti-depressant medication or receiving psychotherapy. We were not able to objectively screen for obstructive sleep apnea/sleep disordered breathing or restless legs. Women with chronic diseases such as diabetes, human immunodeficiency virus (HIV) or uterine abnormalities were also excluded. Initial inclusion criteria required women <10 weeks pregnant. However, to facilitate enrollment, women were accepted into the study if they were less than 14 weeks pregnant. Approval was obtained from the University of Pittsburgh Institutional Review Board. All women provided written informed consent.
One-hundred sixty women with sufficient sleep data to ascertain the presence or absence of sleep deficiency for at least two of the three time points were included in analyses. Compared with those included in analyses, those excluded from analyses (n=24) were younger (26.59±4.75 vs. 29.64±4.76 years; p<0.01) and more likely to be divorced/never married (34.8% vs. 16.2%; p=0.03), have had at least one prior birth (65.2% vs. 41.3%; p=0.03), be of African American or “other” race/ethnicity (58.3% vs. 26.3%; p<0.01), have less than a college degree (69.6% vs. 32.5%; p<0.01), report a household income of <$20,000/don't know/refused to answer (60.9% versus 23.8%; p<0.01), and report being a current smoker (30.4% versus 11.9%; p=0.02). Participants excluded from analyses did not differ from those included in analyses on body mass index, sleeping habits, or exercise frequency.
Procedures
In this prospective observational study, sleep information was collected during T1 (10–12 weeks), T2 (14–16 weeks), and T3 (18–20 weeks of gestation) (defined as early gestation). Participants completed the Pittsburgh sleep diary daily28 and wore a wrist actigraph (Mini Mitter, Philips Respironics) for 2-weeks during each assessment period. Wrist actigraphy data (Minimitter Actiwatch 64; Minimitter) were analyzed in 1-minute epochs with Actiware version 5.04 software using sleep diary data to corroborate bedtime and wake time. Sleep diary data for bedtime and rise time and event markers were used to calculate sleep–wake variables. In cases where visual inspection showed an obvious discrepancy between sleep diary times and observed activity patterns, actigraphy bed, and/or rise times were edited to reflect what was reported in the sleep diary. The number of days from which data were available from sleep diaries was T1: 13.7±1.3 (range 4–14); T2: 13.7±0.6 (range 11–14); T3: 13.7±0.9 (range 8–14). The number of days from which data were available from actigraphy was: T1: 13.6±1.4 (range 3–14); T2: 13.8±1.1 (range 9–14); T3: 13.6±1.9 (range 9–14).
Sleep duration was defined as total sleep time from lights out to out of bed. Each 2-week collection period allowed for collection of all sleep patterns which often included aberrations due to illness, travel or “on call” nights. Thus, all available recording days were included in the descriptive analyses. Questionnaires on depressive and stress symptoms were completed at the end of T1, T2, and T3.
Definition of sleep deficiency
We used a modified definition of sleep deficiency set forth by Buxton et al.,24 which used the variables sleep duration, sleep insufficiency, and insomnia symptoms. A woman was sleep deficient if she met criteria on at least one of the sleep variables. Sleep duration was ascertained by averaging data for each 2-week period. Since the study design collected concurrent sleep diary and actigraphy data, we created two definitions of sleep deficiency: one utilized sleep duration from diary and the other utilized sleep duration from actigraphy. The rationale stems from the fact that the current literature is mainly comprised of studies that have examined subjective sleep duration; however, the potential advantage of using sleep duration derived from actigraphy is that it ‘provides objective sleep information from the participant's natural environment’, thus reducing recall bias29. Given this unique opportunity we chose to examine sleep deficiency with diary and actigraphy derived sleep duration. The median split was used to categorize the participants into short and average sleep duration30. Diary-assessed sleep duration was split at <7 / ≥7 hours, while actigraphy-assessed sleep duration was split at <6 / ≥6 hours. Sleep insufficiency was determined by question 5 on the Insomnia Symptom Questionnaire (ISQ)31 which asks “how often do you feel that your sleep is unrefreshing” with five response categories from “never” to “always.” Women were categorized into two groups based on their response: No (“never,” “rarely,” or “sometimes” unrefreshing) and Yes (“frequently” or “always” unrefreshing). Insomnia symptoms were assessed by calculating whether the woman had a positive case-definition of insomnia based on the ISQ scoring criteria31. The definition of insomnia is based on sleep symptoms (difficulty initiating/maintaining sleep) and daytime dysfunction as a result of the sleep symptoms occurring at least 3 times per week and for at least one month. Using the diary-assessed sleep duration, a woman was sleep deficient if she had sleep duration of <7 hours, slept insufficiency at least three times per week (frequently/always), or met case-definition criteria for insomnia. Using the actigraphy-assessed sleep duration, a woman was sleep deficient if she had sleep duration of <6 hours, slept insufficiency at least three times per week (frequently/always), or met case-definition criteria for insomnia.
Categorization of groups
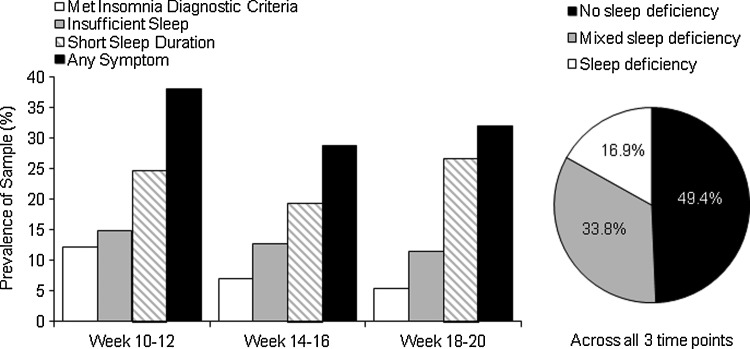
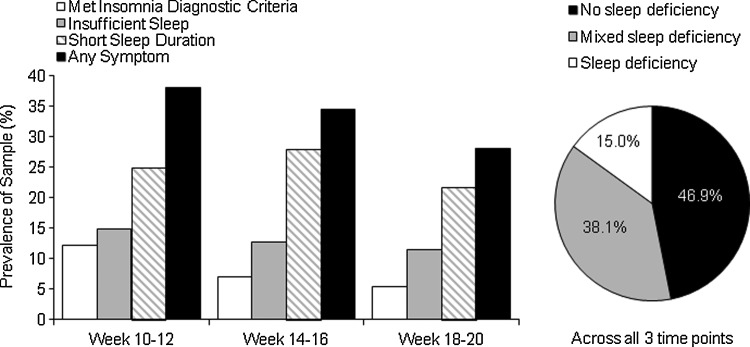
The presence or absence of sleep deficiency was evaluated across each of the three time points during early gestation. A total of 160 women from whom we had sleep data from at least two of the three time points were included in these analyses. First, we assessed the frequency of sleep deficiency at each time point (Fig. 1 and 2). Second, we were interested in the persistence of sleep deficiency. Three groups were identified: (1) sleep deficient, met criteria for sleep deficiency at all time points; (2) intermittent sleep deficient, met criteria for least one, but not all, time points; and (3) not sleep deficient, did not meet criteria for sleep deficiency at any time point.
FIG. 1.
Prevalence of diary-defined sleep deficiency criteria at each individual time point and its pattern across all three time points.
FIG. 2.
Prevalence of actigraphy-defined sleep deficiency criteria at each individual time point and its pattern across all three time points.
Outcome variables
The Inventory for Depressive Symptoms (IDS)-16 Item Inventory32 rates the nine criterion symptom domains (0–27) needed to diagnose a major depressive episode by Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). This commonly used questionnaire has a Cronbach's α ranging from .92–.94 in depressed and euthymic patients and a .79 in the current sample. For this report, the sleep items were removed in order to reduce collinearity with the predictor variable, sleep deficiency.
Perceived Stress Scale (PSS) 10-item33 was used to assess the degree to which situations in one's life are appraised as stressful during the last month. It is one of the most commonly administered subjective stress questionnaires with a Cronbach's α ranging from 0.78 to 0.91 in healthy, adult cohorts,34 and 0.89 in the current sample. Scores range from 0 (no stress) to 40 (very stressed).
Revised Pregnancy Distress Questionnaire (NuPDQ)35 was used to assess pregnancy-related distress. Participants are asked to indicate if they are currently feeling bothered, upset, or worried about different aspects of pregnancy on a three-point scale ranging from ‘‘not at all’’ (0) to ‘‘very much’’ (2). An average pregnancy-specific distress score is calculated for each respondent by summing item responses and dividing by the total number of items. Pregnancy-specific distress scores range from 0 to 2.
Statistical approach
Descriptive analyses were used to describe the characteristics of the cohort. The distributions of all continuous measures were examined to check for normality. Means and standard deviations of all continuous measures, for the total sample are reported. The revised pregnancy distress questionnaire (NuPDQ) and body mass index (BMI) were log transformed as they were not normally distributed. Correlations are reported to examine the relationship between all sleep and nonsleep variables. The prevalence of “short sleepers,” “women with insomnia symptoms,” and “women with sleep insufficiency” are reported using both diary and actigraphy-assessed sleep duration at each time point (Fig. 1 and 2).
Linear mixed models (SAS PROC MIXED; SAS 9.2, SAS Institute) were used to examine whether depressive symptoms or stress levels differed between the three sleep deficiency groups across the three time points. An unstructured covariance pattern was used for all models. We also assessed whether depressive symptoms or stress levels differed between sleep deficiency groups at each individual time point, with pairwise comparisons of least square means performed if a significant between-group difference was found at that time point. Models were run unadjusted and following covariate adjustment for race/ethnicity (Caucasian, African American/other), marital status (married/living as married, divorced/never married), education (less than a college degree, college degree, some postgraduate work/postgraduate degree) and parity (no prior births, at least one prior birth). A two-sided p-value<0.05 was considered statistically significant.
Results
Table 1 presents the characteristics of the participants included in analyses (n=160) across the three diary-assessed sleep deficiency groups. Women were 29.6±4.8 years of age, with an average BMI of 26.5±6.0, and about 26% were African American or other racial group than Caucasian. Figures 1 and 2 depict the prevalence of each sleep deficiency symptom at each time point.
Table 1.
Participant Characteristics
| Variable | No sleep deficiency N=79 Mean (SD) | Intermittent sleep deficiency N=54 Mean (SD) | Persistent sleep deficiency N=27 Mean (SD) | Group difference |
|---|---|---|---|---|
| Age | 29.20(4.43) | 29.57(4.94) | 31.07(5.24) | 0.21 |
| BMI | 26.41(5.48) | 25.74(5.85) | 28.30(7.68) | 0.27 |
| % (n) | % (n) | % (n) | ||
| Race | Chi-square=2.93, p=0.23 | |||
| African American/Other | 20.3 (16) | 31.5 (17) | 33.3 (9) | |
| Caucasian | 79.7 (63) | 68.5 (37) | 66.7 (18) | |
| Smoker | Chi-square=1.36, p=0.51 | |||
| No | 91.1 (72) | 85.2 (46) | 85.2 (23) | |
| Yes (≥1 per day) | 8.9 (7) | 14.8 (8) | 14.8 (4) | |
| Exercise | Chi-square=4.83, p=0.09 | |||
| Never/<2 days/week | 34.2 (27) | 48.1 (26) | 55.6 (15) | |
| ≥2 days/week | 65.8 (52) | 51.9 (28) | 44.4 (12) | |
| Sleeping habits | **Chisquare=40.49, p<0.01 | |||
| Always get enough | 7.6 (6) | 14.8 (8) | 3.7 (1) | |
| Usually good, occasionally not enough | 64.6 (51) | 46.3 (25) | 18.5 (5) | |
| I often cannot sleep as much as I need | 24.1 (19) | 33.3 (18) | 48.1 (13) | |
| I never get enough sleep | 3.8 (3) | 0.0 (0) | 25.9 (7) | |
| I think I sleep too much | 0.0 (0) | 5.6 (3) | 3.7 (1) | |
| Marital status | ^Chi-square=7.40, p=0.03 | |||
| Married/living with partner | 91.1 (72) | 79.6 (43) | 70.4 (19) | |
| Divorced/never married | 8.9 (7) | 20.4 (11) | 29.6 (8) | |
| Education level | Chi-square=7.52, p=0.11 | |||
| <College degree | 25.3 (20) | 33.3 (18) | 51.9 (14) | |
| College degree | 31.6 (25) | 24.1 (13) | 25.9 (7) | |
| Some postgraduate work/postgraduate degree | 43.0 (34) | 42.6 (23) | 22.2 (6) | |
| Income | Chi-square=4.11, p=0.66 | |||
| <$20K/don't know/refused | 22.8 (18) | 22.2 (12) | 29.6 (8) | |
| $20K–49,999 | 16.5 (13) | 27.8 (15) | 25.9 (7) | |
| $50K–99,999 | 38.0 (30) | 33.3 (18) | 25.9 (7) | |
| ≥$100K | 22.8 (18) | 16.7 (9) | 18.5 (5) | |
| Parity | **Chi-square=11.59, p<0.01 | |||
| No previous births | 72.2 (57) | 46.3 (25) | 44.4 (12) | |
| At least one previous birth | 27.8 (22) | 53.7 (29) | 55.6 (15) |
Table reports % (n) or mean and standard deviation (SD) [Total sample=160].
No sleep deficiency is different from intermittent sleep deficiency and persistent sleep deficiency.
^No sleep deficiency is different from persistent sleep deficiency.
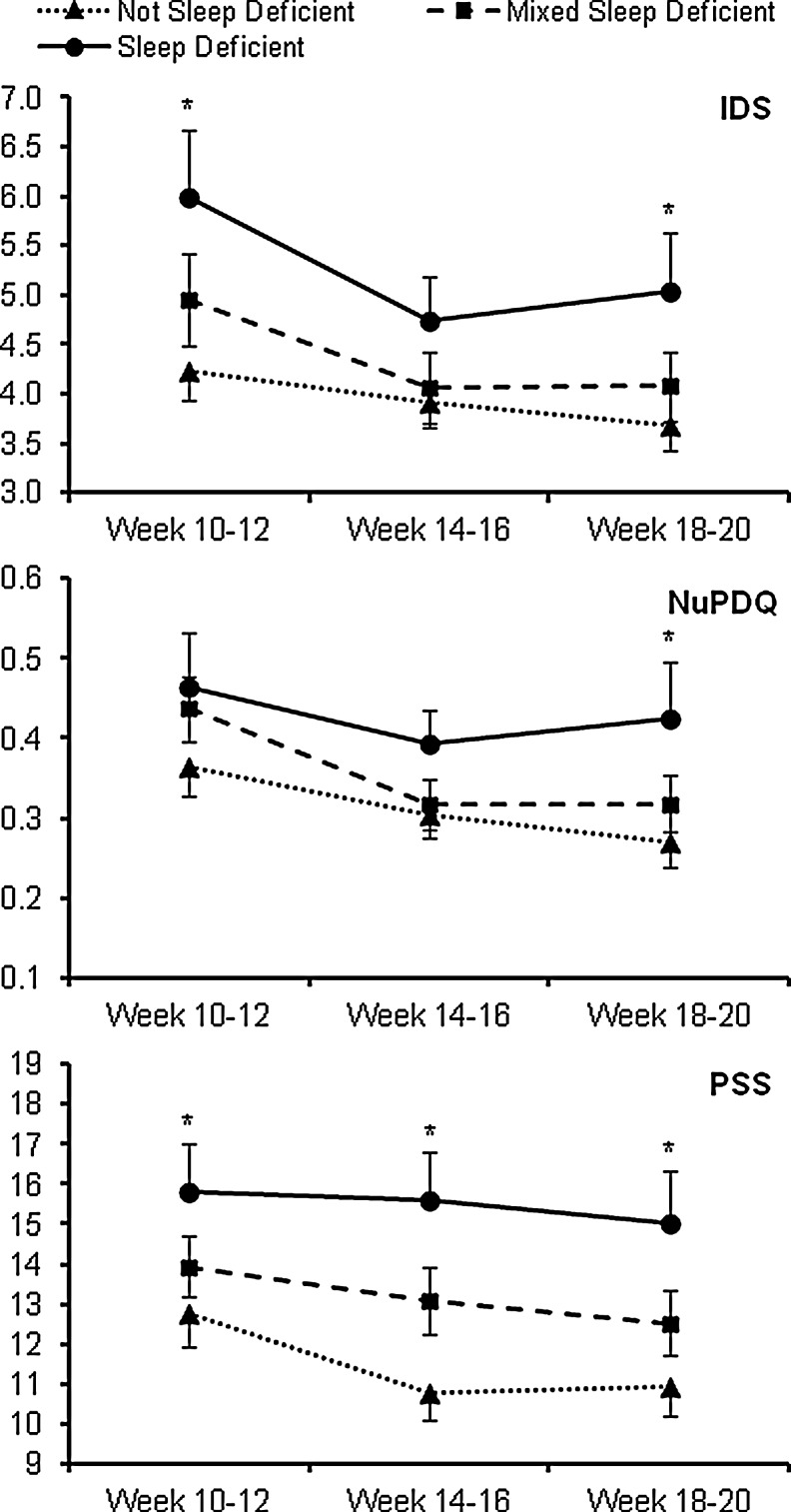
Depressive symptoms and stress levels across the three time points among the diary-derived sleep deficiency groups are presented in Fig. 3. For each outcome, a significant time effect was observed (F2,157=8.44–10.15, each p<0.01), but no interaction effect was noted (F4,157=0.43–1.42, p=0.23–0.79). Across all time points, NuPDQ did not differ by sleep deficiency group (F2,157=2.51, p=0.09), whereas IDS and PSS (Perceived Stress Scale) significantly differed by sleep deficiency group (F2,157=3.85, p=.02 and F2,157=5.53, p<0.01, respectively). NuPDQ scores did not differ between sleep deficiency groups at any individual time point. IDS scores differed by sleep deficiency group at T1 and T3 (T1: F2,157=4.37, p=0.01; T3: F2,157=2.95, p=0.05), with those with sleep deficiency having significantly higher IDS scores than those with no sleep deficiency (p=<0.01 to 0.02) at T1 and T3. PSS scores differed by sleep deficiency group at each individual time point (T1: F2,157=3.51, p=0.03; T2: F2,157=6.13, p<0.01; T3: F2,157=4.15, p=0.02); at each of the time points, those with sleep deficiency had significantly higher PSS scores than those with no sleep deficiency (p<0.01 for each). Following adjustment for race/ethnicity, marital status, education, and parity, IDS scores across all time points no longer differed by sleep deficiency group (F2,152=1.91, p=0.15). The differences in PSS scores across all time points by sleep deficiency group persisted following covariate adjustment (F2,152=3.10, p=0.048), though differences in PSS scores among sleep deficiency groups was only found at T2 (F2,152=3.43, p=0.04).
FIG. 3.
Depressive symptoms and stress levels across time among different categories of diary-defined sleep deficiency. Data shown are untransformed raw values prior to analysis. IDS, Inventory of Depressive Symptomatology; NuPDQ, Revised Pregnancy Distress Questionnaire; PSS: Perceived Stress Scale. *significant difference between “no sleep deficiency” and “sleep deficiency” groups (p<0.01).
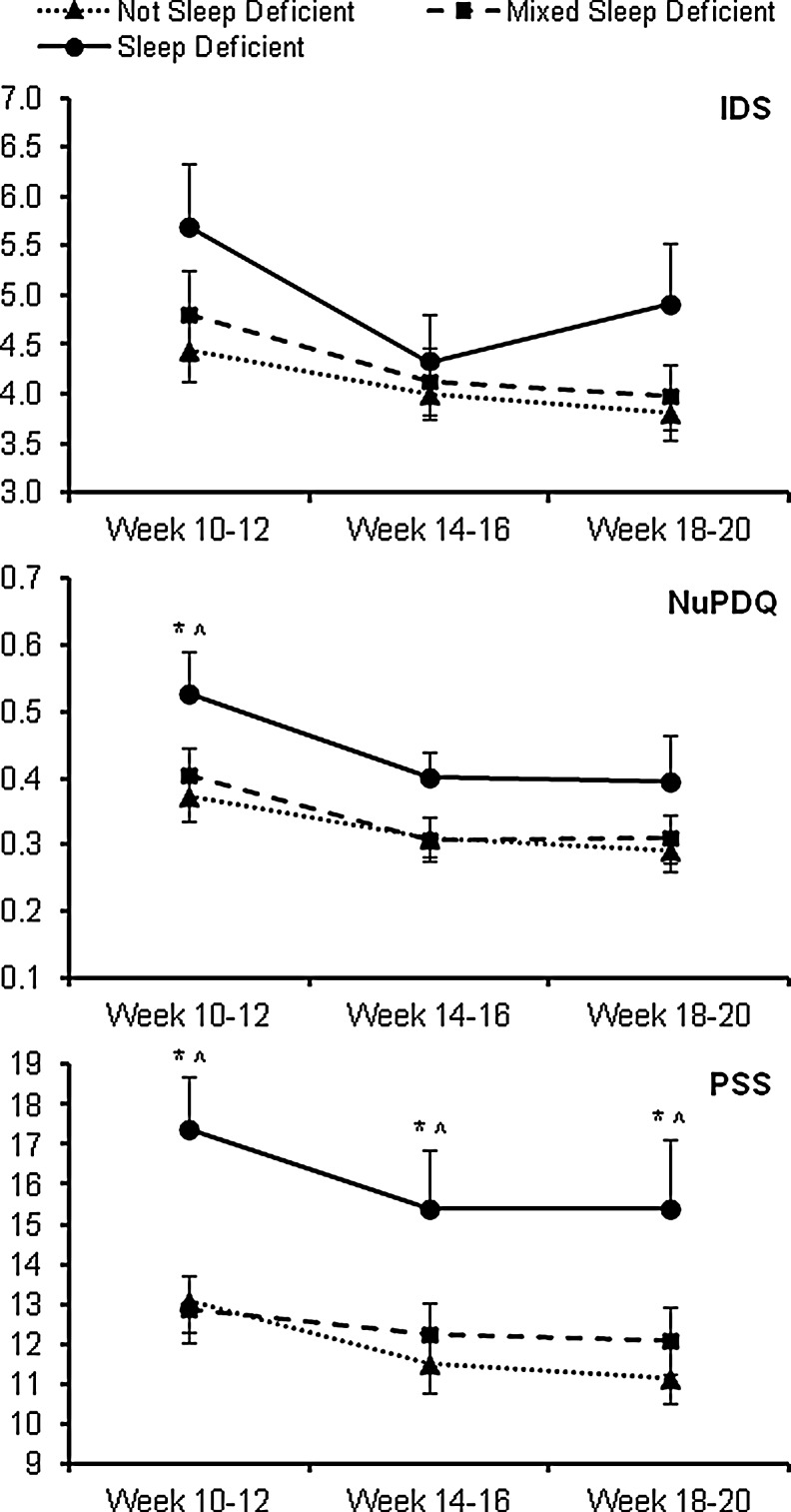
Similarly, depressive symptoms and stress levels across the three time points among the actigraphy-derived sleep deficiency groups are presented in Fig. 4. For each outcome, a significant time effect was observed (F2,157=9.75–14.22, each p<0.01), but no interaction effect was noted (F4,157=0.80–1.68, p=0.16–0.53). Across all time points, IDS and NuPDQ did not differ by sleep deficiency group (F2,157=1.71, p=0.18 and F2,157=2.86, p=0.06, respectively), whereas PSS significantly differed by sleep deficiency group (F2,157=5.31, p<0.01). IDS scores did not differ between sleep deficiency groups at any individual time point. At T1, NuPDQ scores differed by sleep deficiency group (F2,157=3.96, p=0.02), with those with persistent sleep deficiency having significantly higher NuPDQ scores than those with no sleep deficiency (p≤0.01) or intermittent sleep deficiency (p=0.02). PSS scores differed by sleep deficiency group at each individual time point (T1: F2,157=6.36, p<0.01; T2: F2,157=3.19, p=0.04; T3: F2,157=4.03, p=0.02); at each of the time points, those with persistent sleep deficiency had significantly higher PSS scores than those with no sleep deficiency (p≤0.01) or intermittent sleep deficiency (p<0.01–0.04). Following adjustment for race/ethnicity, marital status, education, and parity, differences in PSS scores across all time points by sleep deficiency groups was reduced to marginal significance (F2,152=2.83, p=0.06), with differences in PSS scores among sleep deficiency groups found only at T1 (F2,152=4.57, p=0.01).
FIG. 4.
Depressive symptoms and stress levels across time among different categories of actigraphy-defined sleep deficiency. Data shown are untransformed raw values prior to analysis. IDS: Inventory of Depressive Symptomatology; NuPDQ: Revised Pregnancy Distress Questionnaire; PSS: Perceived Stress Scale. *significant difference between “no sleep deficiency” and “sleep deficiency” groups; ^significant difference between “mixed sleep deficiency” and “sleep deficiency” groups (p<0.01).
Discussion
The goal of this paper was to describe the degree to which women in early gestation met criteria for sleep deficiency, and whether women with sleep deficiency reported greater depressive symptomatology and stress. Although a newly emerging construct, we believe that sleep deficiency is applicable to pregnant women given the hypothesis that (1) “sleep deficiency is thought to reflect a deficit in quantity or quality of sleep versus the amount needed for optimal health,”23 (2) the three variables suggested to define sleep deficiency (i.e., short sleep duration, insufficient sleep or insomnia symptoms)24 are commonly reported by pregnant women in early gestation, and (3) these sleep variables are associated with morbidity.20,21,25 Our results indicate that sleep insufficiency and short sleep duration are prevalent among women in early gestation, more so than in comparable nonpregnant women.21 Insomnia, characterized by the ISQ,31 which is defined by the DSM-IV36 and research diagnostic criteria (RDC),37 was less frequent. These data shed light on the frequency and chronicity of sleep deficiency in early gestation. It is clear that a substantial number of women have at least one symptom cross-sectionally (25%–40%), and this is consistent with previous literature.2,10,21,26,38 What is new here is that less than 20% of women have persistent sleep deficiency. These findings add to the existing literature by providing evidence that a portion, but not all pregnant women, sleep poorly in early gestation,39–41 and these women may benefit from targeted clinical intervention. The diversity in sleep disturbances may provide additional information as to why there is such variability regarding psychological or physical health outcomes during pregnancy. Depression19 and stress,42,43 for example, are both independently associated with poor pregnancy outcomes, yet the evidence as to how these risk factors may lead to morbidity is unclear. We speculate that sleep deficiency may either mediate or independently add to these associations.3,44,45 Future studies need to include both depressed and nondepressed or stressed and nonstressed cohorts in order to effectively answer whether sleep deficiency is a predictor or mediator.
Sleep deficiency in early pregnancy may be clinically, as well as physiologically, important. Clinically, sleep treatment and/or intervention decisions are contingent on the particular aspects of sleep disrupted. For instance, the behavioral techniques used to treat difficulty initiating sleep are different than those used to treat difficulty maintaining sleep. It is also unclear as to whether the sleep disturbances reported in early pregnancy are analogous to those reported in later pregnancy given the change in hormone concentrations and body habitus, for example, in late pregnancy. Presently, the majority of published reports on sleep disturbances and/or symptoms of insomnia are from women in late pregnancy in which the rates are reported to reach upwards of >60%.26,46,47 Moreover, these reports do not clinically identify insomnia, only the presence of symptoms. For example, Fernandez-Alonso and colleagues used the Insomnia Severity Index to ascertain the frequency of insomnia in late pregnancy.46 While this is a commonly used instrument, the focus is on severity rather than a case definition of insomnia. Also, since a majority of women indicate experiencing symptoms of insomnia at least once in the last month,40,48 it may not be very sensitive or specific in identifying primary insomnia in pregnant women.
Assessing sleep deficiency in early pregnancy is also physiologically important. First, the variables that comprise sleep deficiency are linked to immune aberrations during pregnancy.21,49 Second, sleep disturbance is associated with depression and stress, particularly during pregnancy.18,26,50–52 Likewise, both depression and stress, which are often chronic, can result in cumulative sleep disturbance and immunological alterations.42,53 It is unclear at the present time whether a subset of women entered our study with subclinical levels of depression or substantial stress, which resulted in sleep deficiency. Sleep deficiency was most strongly and consistently associated with stress symptoms. This could be a reflection of initial hormone fluctuations that may negatively impact mood and sleep.54 While we a priori excluded women with self-reported psychopathology and/or current treatment, it is possible that some women may have developed significant depressive symptoms during pregnancy or failed to get diagnosed.55 The two stress measures we used identify distinct forms of stress. The NuPDQ is specifically designed to assess pregnancy-related distress,35 while the PSS assesses general perceived stress. We contend that the dramatic fluctuation in hormones may impact some women's ability to adjust to this life event. The significant association observed only at T1 may be a reflection of this phenomenon. A more in-depth understanding of these bidirectional relationships is warranted given the consistent evidence that they are all associated with adverse pregnancy outcomes.12,56,57
These data contribute to what is currently known about insomnia in early gestation. We found that 5%–12% of women in early gestation met the case-definition for insomnia based on the ISQ.31 This is consistent with our previous report of insomnia symptoms in a separate cohort of nondepressed, pregnant women at 20 weeks' gestation.26 However, it must be noted that the current study identified a case-definition of insomnia based on DSM-IV36 and research diagnostic criteria (RDC),37 whereas in our prior study26 we only considered women positive for insomnia if they indicated difficulty initiating/maintaining sleep in the previous 7 days. One can conclude from these two studies that a subset of women in early gestation are at risk for insomnia. Given the evidence relating insomnia to various morbidities in nonpregnant individuals,58–60 this subset of women may be particularly vulnerable for subsequent maternal and fetal morbidity.
We also report that a substantial number of women report insufficient sleep in early pregnancy. Distinct from short sleep duration, insufficient sleep has recently been linked to poor self-rated health61. Women who reported insufficient sleep for 14 or more out of the past 30 days had the highest risk of poor health. This has implications for pregnant women given that insufficient sleep (sleep deficiency) is more prevalent in women who have depression or stress, are from a lower socioeconomic rung or are not of Caucasian race.62 These, as well as other correlates such as diet, are associated with cardiovascular disease, diabetes and overweight/obesity (i.e., cardiometabolic disease).62 Cardiometabolic diseases are a primary risk factor for adverse pregnancy outcomes including preeclampsia and preterm birth.63–66
Lastly, and clinically relevant, is the sizeable incidence of short sleep duration observed among this cohort. This finding is in agreement with some,2,67 but in contrast to popular belief and the current literature.20,47 In a recent study of Turkish women in their first trimester, for example, the average sleep duration was 8.3±2.0 hours per night.47 It is important to note that this information was ascertained from the Women's Health Initiative Insomnia Rating Scale, which asks women to recall their typical sleep duration. Our study, on the other hand, ascertained 6 weeks of prospective sleep data. These data will allow further examination of weekly and daily patterns of sleep duration in pregnant women. We hypothesize that this will be a relevant correlate of morbidity given emerging evidence that variability in sleep is associated with poor health outcomes.68–70
The current findings contribute to the existing knowledge of sleep in early gestation, a time period that has been often overlooked but may be important to pathophysiology during pregnancy6. Failed remodelling of maternal vessels perfusing the placenta that is proposed as causally relevant to the adverse pregnancy outcomes, preeclampsia, intrauterine growth restriction and preterm birth occurs between 10 and 20 weeks of gestation.71 The pathophysiological consequences of disturbed sleep could impact placental development6. However, we acknowledge that this study has limitations. First, the study does not provide information on sleep architecture or electroencephalogram (EEG) spectral frequencies in early gestation. While previous studies have reported few changes in sleep architecture across pregnancy,72,73 emerging evidence from studies utilizing power spectral analysis of the EEG suggest that there are different sleep phenotypes not identifiable via traditional sleep stage scoring.74 This has yet to be evaluated in pregnant women. Also, as indicated by the few reports and the small sample sizes, measuring sleep in pregnant women via polysomnography is cost-prohibitive and involves high participant burden. We contend that the use of self-report diaries concurrent with actigraphy provides a more comprehensive assessment of sleep during pregnancy. Sleep diaries give investigators the participant's perspective of the previous evening's events, while actigraphy allows for the collection of concurrent yet objective information on these sleep habits in the participants' natural sleep environment.29 Investigators are afforded the ability to collect data over a long period of time, which provides the opportunity to assess patterns and variability. The incongruent findings between sleep duration measured by self-report and actigraphy and depressive/stress symptoms is not surprising given the equivocal findings reported in other investigations.29 Martin and Hakim note that actigraphy is good at characterizing sleep duration in depressed, insomnia, and sleep-disordered breathing patients,29 but it is not consistently correlated with depressive symptoms in otherwise healthy cohorts.75,76 Sleep duration from diaries, on the other hand, is more consistently associated with depressive symptoms.76 The use of the median split for sleep duration for the two methods insured that there would be comparable groups with short and average sleep duration. This was also viewed as a suitable decision since self-reported sleep duration is often overestimated in comparison to actigraphy.29 This hypothesis is supported by the similar percentages of women with short sleep duration at each time point. With regards to stress, Lee and Hsu recently reported that actigraphy derived sleep duration was not associated with perceived stress.67 Unfortunately, they did not report on self-reported sleep duration. They did however, observe that poor sleep quality was significantly associated with stress. Another limitation is the reliance on self-report for presence or absence of psychopathology, sleep disorders and medication/treatment use. While the prevalence of sleep disordered breathing/obstructive sleep apnea, for example, is lowest in early pregnancy, we cannot rule out that some women may have had an undiagnosed sleep disorder. Lastly, we do not have sleep information past 20 weeks' gestation. Thus, we cannot compare sleep in early pregnancy to later pregnancy. There have been, however, a few studies that indicate that sleep progressively gets worse as the pregnancy advances.21,26 Future studies should include sleep data from women across the entire gestational period.
These data provide novel information regarding an emerging concept proposed by the Center for Sleep Disorders Research: sleep deficiency. Its definition is distinct from traditional definitions of sleep or circadian rhythm disorders. This concept is applicable to pregnant women, as it is appreciative of the fact that pregnant women do not experience sleep disturbance in isolation and they often report clusters of sleep problems. Up to 40% of women in early gestation reported short sleep duration, insufficient sleep, or met case definition for insomnia. These sleep difficulties may contribute to maternal and fetal morbidity.
Acknowledgments
The authors would like to thank Ms. Annette Wood for her excellent data management; Ms. Alyssa Haney and Ms. Kerith Kiewra for their assistance with subject recruitment; and Ms. Joann Broadus for her administrative assistance. Lastly, the authors would like to extend a special thank you to all the women who have participated in the study.
Support for this was provided by National Institute for Nursing Research, grant R00 NR010813.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lee KA. Sleep during pregnancy and postpartum. In: Lee-Chiong T, editor. Encyclopedia of Sleep Medicine. Hoboken, NJ: John Wiley nd Sons, Inc; 2006. pp. 629–635. [Google Scholar]
- 2.Facco FL. Grobman WA. Kramer J. Ho KH. Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203:142–145. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okun ML. Schetter CD. Glynn LM. Poor sleep quality is associated with preterm birth. Sleep. 2011;34:1493–1498. doi: 10.5665/sleep.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C. Enquobahrie D. Frederick IO. Abetew D. Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: A pilot study. BMC Womens Health. 2010;10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reutrakul S. Zaidi N. Wroblewski K, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34(11):2454–2457. doi: 10.2337/dc11-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okun ML. Roberts JM. Marsland AL. Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet Gynecol Surv. 2009;64:273–280. doi: 10.1097/OGX.0b013e318195160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Keeffe M. St-Onge MP. Sleep duration and disorders in pregnancy: implications for glucose metabolism and pregnancy outcomes. Int J Obes (Lond) 2012;37:765–770. doi: 10.1038/ijo.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.August EM. Salihu HM. Biroscak BJ. Rahman S. Bruder K. Whiteman VE. Systematic review on sleep disorders and obstetric outcomes: Scope of current knowledge. Am J Perinatol. 2012;30:323–334. doi: 10.1055/s-0032-1324703. [DOI] [PubMed] [Google Scholar]
- 9.Jomeen J. Martin C.R. Assessment and relationship of sleep quality to depression in early pregnancy. J Reproduct Infant Psychol. 2007;25:97–99. [Google Scholar]
- 10.Skouteris H. Germano C. Wertheim EH. Paxton SJ. Milgrom J. Sleep quality and depression during pregnancy: A prospective study. J Sleep Res. 2008;17:217–220. doi: 10.1111/j.1365-2869.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 11.Strange LB. Parker KP. Moore ML. Strickland OL. Bliwise DL. Disturbed sleep and preterm birth: A potential relationship? Clin Exp Obstet Gynecol. 2009;36:166–168. [PubMed] [Google Scholar]
- 12.Bei B. Milgrom J. Ericksen J. Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–538. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal D. Gay C. Lee K. Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Womens Ment Health. 2009;12:229–237. doi: 10.1007/s00737-009-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okun ML. Hanusa BH. Hall M. Wisner KL. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behav Sleep Med. 2009;7:106–117. doi: 10.1080/15402000902762394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alder J. Fink N. Bitzer J. Hosli I. Holzgreve W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 16.Austin MP. Leader L. Maternal stress and obstetric and infant outcomes: Epidemiological findings and neuroendocrine mechanisms. Aust N Z J Obstet Gynaecol. 2000;40:331–337. doi: 10.1111/j.1479-828x.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 17.Coussons-Read ME. Okun ML. Schmitt MP. Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 18.Dole N. Savitz DA. Hertz-Picciotto I. Siega-Riz AM. McMahon MJ. Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 19.Wisner KL. Sit DK. Hanusa BH, et al. Major depression and antidepressant treatment: Impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams MA. Miller RS. Qiu C. Cripe SM. Gelaye B. Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep. 2010;33:1363–1371. doi: 10.1093/sleep/33.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okun ML. Coussons-Read ME. Sleep disruption during pregnancy: How does it influence serum cytokines? J Reprod Immunol. 2007;73:158–165. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 22.National Center on Sleep Disorders Research. Bethesda, MD: National Institutes of Health; 2011. National institutes of health sleep disorders research plan. [Google Scholar]
- 23.Czeisler CA. Impact of sleepiness and sleep deficiency on public health—Utility of biomarkers. J Clin Sleep Med. 2011;7:S6–S8. doi: 10.5664/JCSM.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buxton OM. Hopcia K. Sembajwe G, et al. Relationship of sleep deficiency to perceived pain and functional limitations in hospital patient care workers. J Occup Environ Med. 2012;54:851–858. doi: 10.1097/JOM.0b013e31824e6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facco FL. Kramer J. Ho KH. Zee PC. Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115:77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 26.Okun ML. Kiewra K. Luther JF. Wisniewski SR. Wisner KL. Sleep disturbances in depressed and nondepressed pregnant women. Depress Anxiety. 2011;28:676–85. doi: 10.1002/da.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okun ML. Luther JF. Wisniewski SR. Sit D. Prairie BA. Wisner KL. Disturbed sleep, a novel risk factor for preterm birth? J Womens Health (Larchmt) 2012;21:54–60. doi: 10.1089/jwh.2010.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monk TH. Reynolds CF. Kupfer DJ, et al. The Pittsburgh sleep diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- 29.Martin JL. Hakim AD. Wrist actigraphy. Chest. 2011;139:1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacCallum RC. Zhang S. Preacher KJ. Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 31.Okun ML. Kravitz HM. Sowers MF. Moul DE. Buysse DJ. Hall M. Psychometric evaluation of the Insomnia Symptom Questionnaire: A self-report measure to identify chronic insomnia. J Clin Sleep Med. 2009;5:41–51. [PMC free article] [PubMed] [Google Scholar]
- 32.Rush AJ. Gullion CM. Basco MR. Jarrett RB. Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S. Kamarck T. Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 34.Cohen S. Janicki-Deverts D. Who's stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol. 2012;42:1320–1334. [Google Scholar]
- 35.Lobel M. Stony Brook, NY: State University of New York at Stony Brook; 1996. The Revised Prenatal Distress Questionnaire (NUPDQ) [Google Scholar]
- 36.American Psychiatric Association. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Text revision ed. [Google Scholar]
- 37.Edinger JD. Bonnet MH. Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 38.Baratte-Beebe KR. Lee K. Sources of midsleep awakenings in childbearing women. Clin Nurs Res. 1999;8:386–397. doi: 10.1177/10547739922158377. [DOI] [PubMed] [Google Scholar]
- 39.Dzaja A. Arber S. Hislop J, et al. Women's sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Krystal AD. Insomnia in women. Clin Cornerstone. 2003;5:41–50. doi: 10.1016/s1098-3597(03)90034-2. [DOI] [PubMed] [Google Scholar]
- 41.Santiago JR. Nolledo MS. Kinzler W. Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;134:396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 42.Coussons-Read ME. Lobel M. Carey JC, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wadhwa PD. Sandman CA. Porto M. Dunkel-Schetter C. Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. Am J Obstet Gynecol. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 44.Hall M. Buysse DJ. Reynolds CF. Kupfer DJ. Baum A. Sleep quality predicts symptoms of depression and psychosocial distress in healthy young adults. Sleep Res. 1996;25:162. [Google Scholar]
- 45.Hall M. Baum A. Buysse DJ. Prigerson HG. Kupfer DJ. Reynolds CF. Sleep as a mediator of the stress-immune relationship. Psychosom Med. 1998;60:48–51. doi: 10.1097/00006842-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Alonso AM. Trabalon-Pastor M. Chedraui P. Perez-Lopez FR. Factors related to insomnia and sleepiness in the late third trimester of pregnancy. Arch Gynecol Obstet. 2012;286:55–61. doi: 10.1007/s00404-012-2248-z. [DOI] [PubMed] [Google Scholar]
- 47.Kizilirmak A. Timur S. Kartal B. Insomnia in pregnancy and factors related to insomnia. ScientificWorldJournal. 2012;2012:197093. doi: 10.1100/2012/197093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ancoli-Israel S. Roth T. Characteristics of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22:S347–S353. [PubMed] [Google Scholar]
- 49.Okun ML. Hall M. Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14:560–567. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 50.Field T. Hernandez-Reif M. Diego M. Risk factors and stress variables that differentiate depressed from nondepressed pregnant women. Infant Behav Dev. 2006;29:169–174. doi: 10.1016/j.infbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Glazier RH. Elgar FJ. Goel V. Holzapfel S. Stress, social support, and emotional distress in a community sample of pregnant women. J Psychosom Obstet Gynaecol. 2004;25:247–255. doi: 10.1080/01674820400024406. [DOI] [PubMed] [Google Scholar]
- 52.Field T. Diego M. Hernandez-Reif M. Figueiredo B. Schanberg S. Kuhn C. Sleep disturbances in depressed pregnant women and their newborns. Infant Behav Dev. 2007;30:127–133. doi: 10.1016/j.infbeh.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Christian LM. Franco A. Glaser R. Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steiner M. Dunn E. Born L. Hormones and mood: From menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 55.Burt VK. Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry. 2002;63:9–15. [PubMed] [Google Scholar]
- 56.Field T. Diego M. Hernandez-Reif M, et al. Chronic prenatal depression and neonatal outcome. Int J Neurosci. 2008;118:95–103. doi: 10.1080/00207450601042144. [DOI] [PubMed] [Google Scholar]
- 57.Kamysheva E. Skouteris H. Wertheim EH. Paxton SJ. Milgrom J. A prospective investigation of the relationships among sleep quality, physical symptoms, and depressive symptoms during pregnancy. J Affect Disord. 2009;123:317–320. doi: 10.1016/j.jad.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Kripke DF. Garfinkel L. Wingard DL. Klauber MR. Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 59.Specchio LM. Prudenzano MP. de Tommaso M, et al. Insomnia, quality of life and psychopathological features. Brain Res Bull. 2004;63:385–391. doi: 10.1016/j.brainresbull.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Vgontzas AN. Liao D. Pejovic S, et al. Insomnia with short sleep duration and mortality: The Penn State cohort. Sleep. 2010;33:1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geiger SD. Sabanayagam C. Shankar A. The relationship between insufficient sleep and self-rated health in a nationally representative sample. J Environ Public Health. 2012;2012:518263. doi: 10.1155/2012/518263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: Implications for cardiometabolic disease risk. Soc Sci Med. 2013;79:7–15. doi: 10.1016/j.socscimed.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilbert WM. Young AL. Danielsen B. Pregnancy outcomes in women with chronic hypertension: A population-based study. J Reprod Med. 2007;52:1046–1051. [PubMed] [Google Scholar]
- 64.Manten GT. Sikkema MJ. Voorbij HA. Visser GH. Bruinse HW. Franx A. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy. 2007;26:39–50. doi: 10.1080/10641950601146574. [DOI] [PubMed] [Google Scholar]
- 65.Solomon CG. Seely EW. Hypertension in pregnancy. Endocrinol Metab Clin North Am. 2006;35:157–171. doi: 10.1016/j.ecl.2005.09.003. vii. [DOI] [PubMed] [Google Scholar]
- 66.Wadhwa PD. Culhane JF. Rauh V. Barve SS. Stress and preterm birth: Neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5:119–125. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- 67.Lee SY. Hsu HC. Stress and health-related well-being among mothers with a low birth weight infant: The role of sleep. Soc Sci Med. 2012;74:958–965. doi: 10.1016/j.socscimed.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mezick EJ. Matthews KA. Hall M, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34:1346–54. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buysse DJ. Cheng Y. Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okun ML. Reynolds CF., III Buysse DJ, et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burton GJ. Charnock-Jones DS. Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009;138:895–902. doi: 10.1530/REP-09-0092. [DOI] [PubMed] [Google Scholar]
- 72.Driver HS. Shapiro CM. A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep. 1992;15:449–453. doi: 10.1093/sleep/15.5.449. [DOI] [PubMed] [Google Scholar]
- 73.Lee KA. Zaffke ME. McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95:14–18. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 74.Mongrain V. Carrier J. Paquet J. Belanger-Nelson E. Dumont M. Morning and evening-type differences in slow waves during NREM sleep reveal both trait and state-dependent phenotypes. PLoS One. 2011;6:e22679. doi: 10.1371/journal.pone.0022679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ancoli-Israel S. Cole R. Alessi C. Chambers M. Moorcroft W. Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 76.Paudel ML. Taylor BC. Diem SJ, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56:1228–1235. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]