Abstract
Objective:
To compare the effect of creatine supplementation on thermoregulation in males and females during exercise in a thermoneutral environment.
Design and Setting:
Male and female subjects participated in 30 minutes of cycle ergometry in nonsupplemented (NS) and creatine-supplemented (Cr) conditions at 70% to 75% of predetermined peak oxygen consumption.
Subjects:
Ten male and ten female subjects were evaluated with and without creatine supplementation.
Measurements:
Analyses were performed during exercise for core temperature and mean skin temperature using two 2 × 2 × 7 mixed-factorial analyses of variance (ANOVAs). We compared mean differences between NS and Cr conditions and sex for heart rate, systolic blood pressure, and diastolic blood pressure using 3 2 × 2 × 4 mixed-factorial ANOVAs. Three 2 × 2 mixed-factorial ANOVAs were computed to examine differences between sex and conditions for the following variables: nude body weight and blood urea nitrogen before and after exercise and urine specific gravity.
Results:
Significant time effects were found for core temperature, skin temperature, heart rate, and diastolic blood pressure. Time effect and difference between the sexes for systolic blood pressure were both significant. Differences in nude body weight and blood urea nitrogen before and after exercise were greater for males, but there was no difference between conditions. No significant difference between sex and condition for urine specific gravity was noted.
Conclusions:
Short-term creatine supplementation did not affect thermoregulation between the sexes when exercising in a thermoneutral environment. Differences in changes in nude body weight before and after exercise may be due to a higher sweating rate in males versus females. Differences in blood urea nitrogen before and after exercise between the sexes may be due to a reduced glomerular filtration rate coupled with greater muscle creatine breakdown in males.
Keywords: males, females, hypohydration, exercise
Creatine supplementation has been reported to increase intramuscular creatine stores by more than 20% in a relatively brief period (20 g·d−1 for 5 days).1 The relationship between elevated intramuscular creatine stores and the subsequent increased potential for the rephosphorylization of adenosine triphosphate have led investigators to examine the potential ergogenic effects of creatine supplementation. These ergogenic effects have been associated with anaerobic activities, such as resistance training, in which evidence supports alterations in skeletal muscle-fiber composition.2,3
Although the anaerobic ergogenic effects of creatine supplementation are well documented,2–8 controversy continues as to whether this supplement poses a health risk. Adverse effects of creatine supplementation have been reported,9 but many of these risks lack supporting or refutable scientific evidence. This lack of evidence is due, in part, to the investigative focus on creatine supplementation as an ergogenic aid and not on side effects.
With many athletes using creatine supplementation to enhance performance, we need to investigate possible adverse effects. Impaired thermoregulation as a consequence of creatine supplementation has been speculated to result from possible dehydration. This speculation has recently been refuted when thermoregulation was not affected by creatine-supplementation protocols of 7 and 28 days in males exercising in a hot environment.10,11
Although creatine supplementation remains a widely investigated topic, much information is available for males, but little is available for females.12–16 The potential exists for both sexes to supplement with creatine for performance enhancement, thereby making themselves susceptible to possible adverse effects. As a result, our purpose was to compare the thermoregulatory effects of 7 days of creatine supplementation in males and females exercising in a thermoneutral environment. We hypothesized that short-term creatine supplementation would not affect thermoregulation between the sexes in a thermoneutral environment. Subsequently, we speculated that anecdotal reports of dehydration associated with creatine supplementation are not supported and that normal hydration practices with exercise may be followed when supplementing with creatine.
METHODS
Experimental Design
Male and female subjects performed identical exercise sessions in a thermoneutral environment (temperature = 24.05 ± 1.63°C, relative humidity = 33.28 ± 17.21%) before and immediately after 7 days of creatine supplementation. Before the exercise sessions, we obtained anthropometric measurements for height, body mass, and body fat percentage (the latter via skinfold assessment using the chest, abdomen, and thigh for males and the triceps, suprailium, and thigh for females17). Additionally, subjects were evaluated for peak oxygen consumption (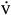 O2peak) via cycle ergometry. Within 5 days of the
O2peak) via cycle ergometry. Within 5 days of the 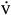 O2peak assessment, subjects engaged in a 30-minute cycle- ergometry exercise bout at an intensity of 70% to 75%
O2peak assessment, subjects engaged in a 30-minute cycle- ergometry exercise bout at an intensity of 70% to 75% 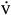 O2peak. This exercise session was considered the nonsupplemented (NS) condition.
O2peak. This exercise session was considered the nonsupplemented (NS) condition.
Following the NS condition, subjects engaged in 7 days of creatine supplementation at 20 g·d−1. Subjects were required to be free of creatine supplementation for at least 2 months before participating in the study. Two months were required because the washout period for muscle creatine levels to return to baseline after supplementation is approximately 1 month.18 None of our subjects indicated a history of previous creatine supplementation.
On the day immediately after the supplementation period (day 8), the subjects returned to the laboratory and were re- evaluated for a 30-minute cycle-ergometry exercise session at an intensity of 70% to 75% 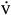 O2peak. This exercise session was considered the creatine-supplemented (Cr) condition. We chose not to blind the subjects to the creatine supplement to determine how subjects would react to knowingly taking a supplement. This design attempted to simulate what the subjects would have experienced if self-supplementing with creatine while engaging in outside (nonlaboratory) activities.
O2peak. This exercise session was considered the creatine-supplemented (Cr) condition. We chose not to blind the subjects to the creatine supplement to determine how subjects would react to knowingly taking a supplement. This design attempted to simulate what the subjects would have experienced if self-supplementing with creatine while engaging in outside (nonlaboratory) activities.
Subjects
The subjects were 10 males (age = 23.80 ± 3.58 years, height = 172.90 ± 8.63 cm, body mass = 70.90 ± 10.63 kg, body fat percentage = 9.30 ± 5.15%) and 10 females (age = 21.1 ± 2.9 years, height = 161.3 ± 3.2 cm, body mass = 62.40 ± 9.47 kg, body fat percentage = 22.35 ± 5.38%). The female subjects were evaluated during the follicular phase of their menstrual cycles. Menstrual phase must be considered when evaluating thermoregulation in women because women in the luteal phase exhibit increased core temperatures, delayed onset of sweating, and delayed peripheral vasodilation compared with the follicular phase.19 In this study, females self- reported the onset of menstrual bleeding, and menstrual phase was determined as follows. The follicular phase begins with the onset of menstrual bleeding and averages 15 days, with a range of 9 to 23 days. The ovulatory phase is 1 to 3 days, and the luteal phase lasts approximately 13 days, ending with the onset of menstrual bleeding.20 All subjects were informed of the purpose and possible risks involved in the investigation and were required to read and sign an informed consent prior to participation. All procedures were approved by the university's institutional review board.
Peak Oxygen Consumption Protocol
Before the testing sessions, the subjects were evaluated for 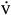 O2peak with a Monark cycle ergometer (model 818E; Varberg, Sweden), using the protocol described by Astrand.21 For the male subjects, a pedal rate of 50 revolutions per minute was maintained, with an initial workload of 100 W. This workload was increased by 50 W every 3 minutes until the criteria for
O2peak with a Monark cycle ergometer (model 818E; Varberg, Sweden), using the protocol described by Astrand.21 For the male subjects, a pedal rate of 50 revolutions per minute was maintained, with an initial workload of 100 W. This workload was increased by 50 W every 3 minutes until the criteria for 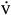 O2peak were met. For the female subjects, a pedal cadence of 50 revolutions per minute was maintained, with an initial workload of 50 W. This workload was increased by 25 W every 3 minutes until the criteria for
O2peak were met. For the female subjects, a pedal cadence of 50 revolutions per minute was maintained, with an initial workload of 50 W. This workload was increased by 25 W every 3 minutes until the criteria for 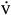 O2peak were met. The criteria for
O2peak were met. The criteria for 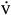 O2peak in both sexes were a plateau in oxygen consumption (
O2peak in both sexes were a plateau in oxygen consumption (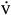 O2), an inability to maintain a minimum of 45 revolutions per minute for 1 minute, or volitional exhaustion.
O2), an inability to maintain a minimum of 45 revolutions per minute for 1 minute, or volitional exhaustion.
Testing Sessions
The testing sessions consisted of 30 minutes of cycle ergometry exercise at an intensity of 70% to 75% of the previously determined 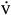 O2peak in a thermoneutral environment. Before exercise, we measured nude body weight, urine specific gravity, and serum blood urea nitrogen (BUN). The changes in nude body weight and BUN before and after exercise were used for comparison between the NS and Cr conditions. For urine specific gravity, preexercise values between the NS and Cr conditions were used for comparison.
O2peak in a thermoneutral environment. Before exercise, we measured nude body weight, urine specific gravity, and serum blood urea nitrogen (BUN). The changes in nude body weight and BUN before and after exercise were used for comparison between the NS and Cr conditions. For urine specific gravity, preexercise values between the NS and Cr conditions were used for comparison.
Nude body weight was assessed before and after exercise on a standard medical scale (model 439; Detecto, Webb City, MO) and used to determine sweating rate during exercise. Urine specific gravity was measured using a hand refractometer (model A 300 CL clinical; Atago Co, Tokyo, Japan). A blood sample drawn from the antecubital vein permitted analyses of BUN levels via reflectance spectrophotometry with the dry-chemistry technique (Ektachem DT-60; Kodak, Hartford, CT) following the manufacturer's guidelines. All preexercise measurements were repeated postexercise, and the differences were used for comparison between the NS and Cr conditions.
For the exercise bouts, baseline measurements were taken for core temperature (Tr), mean skin temperature (Tsk), systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR). Temperatures for Tr and Tsk were displayed on a bench top thermometer (DigiSense scanning thermometer; Cole-Parmer Instrument Co, Vernon Hills, IL). A thermistor was inserted approximately 10 cm beyond the anal sphincter to obtain Tr. For Tsk, skin-surface thermistors were placed on the right lateral aspect of the upper arm midway between the acromion and olecranon process, the right pectoralis major muscle approximately 1 inch (2.54 cm) below the center of the clavicle, the right anterior thigh midway between the anterior superior iliac spine and superior border of the patella, and the lateral aspect of the gastrocnemius at its greatest diameter. The Tsk was calculated using the formula proposed by Ramanathan (0.3[tchest + tarm] + 0.2[tthigh + tleg]).22
After baseline measurements were recorded, the subjects performed 30 minutes of cycle ergometry at 70% to 75% of their previously determined 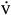 O2peak. During the exercise session, a pedal cadence of 60 revolutions per minute was maintained, and oxygen consumption was continuously monitored (TrueMax 2400 metabolic measurement system; ParvoMedics, East Sandy, UT) to ensure proper maintenance of the prescribed exercise intensity. The resistance required to maintain the pace was calculated.23 Measurements were obtained in 5- minute intervals for Tr and Tsk and every 10 minutes for HR and blood pressure.
O2peak. During the exercise session, a pedal cadence of 60 revolutions per minute was maintained, and oxygen consumption was continuously monitored (TrueMax 2400 metabolic measurement system; ParvoMedics, East Sandy, UT) to ensure proper maintenance of the prescribed exercise intensity. The resistance required to maintain the pace was calculated.23 Measurements were obtained in 5- minute intervals for Tr and Tsk and every 10 minutes for HR and blood pressure.
At the completion of the NS exercise session, subjects were supplemented for 7 days at 20 g·d−1 with creatine (n[aminoiminomethyl]-N-methyl glycine; Nutra Sense, Inc, Shawnee Mission, KS). We chose this dosage protocol because it significantly increases intramuscular creatine stores.1,18 After the creatine-supplementation protocol, subjects returned for testing under conditions identical to the NS condition testing. The Figure provides a timeline of the experimental protocol.
Timeline of the experimental protocol.
Statistical Analysis
Analyses were performed for NS and Cr condition, sex, and time. Using Tr and Tsk as the dependent variables, we computed a 2 × 2 × 7 mixed-factorial analysis of variance (ANOVA). The 3 independent variables included 2 within- subjects factors, condition (NS and Cr) and time (7 intervals in 5-minute increments), and a between-subjects factor, sex. For HR, SBP, and DBP, a 2 × 2 × 4 mixed-factorial ANOVA was calculated with the same independent variables; however, time was divided into 4 intervals of 10-minute increments. Finally, a total of three 2 × 2 mixed-factorial ANOVAs were computed with a between-subjects factor, sex, and a within- subjects factor, condition. Two of the 3 dependent variables examined differences before and after exercise (nude body weight and BUN), whereas urine specific gravity was examined by condition (NS versus Cr). The generalized least squares procedure (SPSS for Windows version 10.1; SPSS Inc, Chicago, IL) was used to calculate the statistics. For all analyses, the alpha level was set at P < .05. To test for basic assumptions, we computed the Mauchly test of sphericity for the within-subjects factors of NS and Cr condition and time. The test analyzed the similarity of the treatment differences across the time intervals for all subjects. We used the Greenhouse-Geiser statistic to adjust for degrees of freedom.
RESULTS
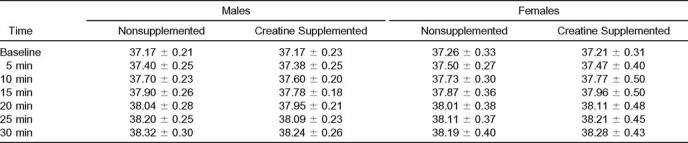
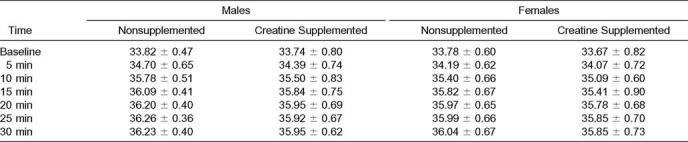
Using the Mauchly test of sphericity, all dependent variables were significantly different (P < .05). For Tr, no significant interaction was found among sex, condition (NS and Cr), and time (F2.57,46.30 = 2.57, P = .07). In addition, no significant interactions were found between sex and condition (F1,18 = 0.89, P = .36), condition and time (F2.57,46.30 = 0.19, P = .88), and time and sex (F1.55,27.99 = 1.27, P = .29). The analysis of the main effects revealed no significant mean difference in Tr between condition (F1,18 = 0.039, P = .85) or sex (F1,18 = 0.19, P = .67). However, significant differences were found among time intervals (F1.55,27.99 = 285.87, P = .00). Pairwise comparisons were computed to determine where significance occurred, showing significant increases in Tr at each time interval (Table 1).
Table 1.
Core Temperature Over Time During Exercise Sessions (°C)
For Tsk, we found similar results. No significant interactions existed among sex, condition, and time (Fcondition×time×sex(2.82,50.93) = 0.93, P = .48; Fcondition×sex(1,18) = 0.02, P = .88; Ftime×sex(2.61,46.89) = 1.82, P = .16; Fcondition×time(2.82,50.93) = 1.27, P = .29). Also, no significant main effects were noted for condition (F1,18 = 2.54, P = .13) or sex (F1,18 = 1.18, P = .29). The main effect for time for Tsk was significant (F2.61,46.89 = 305.76, P = .00). Skin temperature increased significantly with each time interval except from 20 to 30 minutes of exercise (Table 2).
Table 2.
Mean Skin Temperatures Over Time During Exercise Sessions (°C)
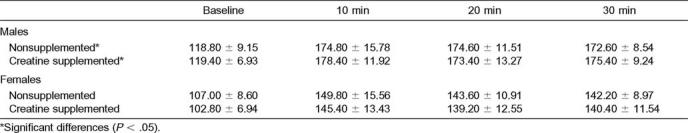
For HR, all interactions were nonsignificant, with time having a significant main effect (F1.35,24.34 = 1468.90, P = .00). Heart rate increased significantly over each time interval. For SBP, condition, sex, and time did not interact, but the interactions between condition and sex (F1,18 = 4.81, P = .04) and time and sex (F2.17,38.98 = 7.80, P = .00) were significant. A least significant difference post hoc analysis was performed to determine where the significance occurred. For condition and sex, males had higher SBP for the NS and Cr conditions. For time and sex, males had significantly higher SBP across all time intervals. For DBP, a significant main effect was found for time. Baseline DBP was significantly lower than at 10, 20, and 30 minutes of exercise. Additionally, DBP at 10 minutes was significantly lower than at 20 and 30 minutes of exercise (Tables 3–5).
Table 3.
Mean Heart Rate Over Time During Exercise Sessions (beats per minute)

Table 5.
Mean Diastolic Blood Pressure Over Time During Exercise Sessions (mm Hg)

Table 4.
Mean Systolic Blood Pressure Over Time During Exercise Sessions (mm Hg)
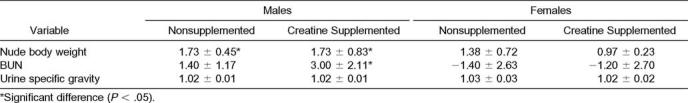
For changes in nude body weight and BUN, the difference between the sexes was significant (Fnude body weight(1,18) = 13.45, P = .00; FBUN(1,18) = 28.86, P = .00), with males having higher mean scores than females. No significant differences were found for urine specific gravity between sex and condition (Table 6).
Table 6.
Changes in Nude Body Weight and Blood Urea Nitrogen (BUN) from Preexercise to Postexercise for the Nonsupplemented and Creatine-Supplemented Conditions and Urine Specific Gravity for the Nonsupplemented and Creatine-Supplemented Conditions
DISCUSSION
We investigated the differences in the thermoregulatory responses of males and females after 1 week of creatine supplementation. Recent investigators10,11 have refuted anecdotal reports of dehydration as a consequence of creatine supplementation; however, these studies are few, and the authors did not examine sex differences. Kern et al10 suggested that body- water changes after 28 days of creatine supplementation may aid in thermoregulation during exercise, whereas Volek et al11 reported that short-term (7 days') creatine supplementation did not affect thermoregulatory measures. Each of these groups used males as subjects and examined thermoregulation in the heat (temperature 37°C, humidity 25%,10 and temperature 37°C, humidity 80%, respectively11).
Thermoregulation depends on several factors, including the hydration state of the individual.10 Therefore, dehydration as a consequence of creatine supplementation may adversely affect thermoregulatory measures in exercising individuals. Dehydration during performance results in an increased Tr, coupled with increased cardiovascular strain. The increased cardiovascular strain is manifested via a rise in HR throughout exercise, resulting in higher HR levels at exhaustion versus the euhydrated state. Additionally, there are reductions in stroke volume and possible hypovolemia, which may have additional consequences relative to skin blood flow and subsequent thermoregulation during exercise.24,25
In our investigation, males and females exhibited similar responses for Tr, Tsk, and HR, suggesting that creatine supplementation did not adversely affect thermoregulation between the sexes. Females may rely more heavily on the cardiovascular aspect of thermoregulation versus males, resulting in larger increases in HR and less evaporative weight loss in females.26 Although cardiovascular processes for thermoregulation may differ, in our study Tr was similar between the sexes. Additionally, both sexes were exercising at the same relative intensity (70% to 75%  O2peak), which may have reduced the sex differences in thermoregulation. Exercising at similar absolute intensities might have revealed a greater tolerance to heat for the males versus the females because males would be working at a lower relative intensity.27
O2peak), which may have reduced the sex differences in thermoregulation. Exercising at similar absolute intensities might have revealed a greater tolerance to heat for the males versus the females because males would be working at a lower relative intensity.27
The stabilization of Tsk at 20 minutes of exercise may indicate a plateau in heat exchange via cutaneous vasodilation. Although females may rely more on cutaneous vasodilation for thermoregulation, increased cutaneous blood flow provides a mechanism for heat transfer from the core to the environment for both males and females.26,27 However, the continued rise in Tr and HR for each sex suggests that cutaneous vasodilation was ineffective, in isolation, in providing adequate thermoregulation.
The continued rise in HR during the NS and Cr exercise sessions suggests similar cardiovascular strain for each condition. Had the creatine supplementation induced a dehydrated state, HR near the end of the exercise bout for the Cr condition would have been greater than for the NS condition. Additionally, the lack of difference in HR between the sexes can be attributed to males and females both exercising at the same relative intensity. Although the males may have been working at a higher absolute workload to achieve the 75%  O2peak, this difference in workload has been shown to be of consequence during exposure to hot and humid environments but not in thermoneutral environments.19
O2peak, this difference in workload has been shown to be of consequence during exposure to hot and humid environments but not in thermoneutral environments.19
Differences in blood pressure were found between the NS and Cr conditions and the sexes. For both sexes, DBP decreased during each exercise bout. Males had higher SBP than females for both the NS and Cr conditions. Elevations in blood pressure have been speculated to be associated with creatine supplementation because of fluid retention28; however previous investigations have not supported these speculations.11,15 In our study, both sexes exhibited normal blood-pressure responses to exercise and did not differ between the NS and Cr conditions. The difference in blood pressure between the sexes appears to have been due to males having a higher baseline blood pressure, which resulted in a higher exercise blood pressure.
Nude body weight changed more in males before and after exercise than in females as a result of higher sweating rates, yet no preexercise and postexercise differences were noted between supplementation conditions. The higher sweating rate for males indicates a greater reliance on the sweating mechanism for thermoregulation versus reliance on cardiovascular components of thermoregulation for females.26
Our data do not support dehydration with short-term creatine supplementation for either sex. Dehydration would result in decreased sweat rates and a higher HR at the end of the exercise bout. Additionally, urine specific gravity values for both sexes for the NS and Cr conditions were normal. We did not measure changes in total body water, so we cannot support the speculation that the fluid retention associated with creatine supplementation may aid in thermoregulation.10 However, neither males nor females were adversely affected by the creatine supplementation, and the lack of differences in nude body weight before and after exercise between conditions suggests adequate fluid-volume availability for the sweating mechanism.
Males had a greater change in BUN during exercise, yet changes in BUN for the sexes were not different between the NS and Cr conditions. Differences in BUN between the sexes may be attributable to a greater decrease in glomerular filtration rate in males, thereby elevating plasma urea concentration. The glomerular filtration rate has been reported to decrease as much as 50% during heavy exercise, with greater decreases occurring during hypohydration.29 Both sexes were euhydrated at each testing condition, and, therefore, normal reductions in renal blood flow would be expected.
The greater amounts of muscle mass in males may have also contributed to higher levels of BUN due to the continual breakdown of muscle creatine. Muscle creatine breakdown occurs at a relatively constant rate of 1.6% per day.30 Because creatine is a nitrogen-containing compound, its continual breakdown coupled with a decreased glomerular filtration rate would result in elevated levels of BUN.
We are the first to report on the sex differences in thermoregulation while subjects were supplemented with creatine. Previous investigators have focused on thermoregulation and fluid volume changes in males, reporting no adverse effects.10,11,31
Although our findings are limited by the lack of subject blinding and uncontrolled (but monitored) temperature and relative humidity, we believe this study design had minimal effects on the results because of similar findings of other researchers.10,11 We investigated the effects of short-term creatine supplementation using a dosage regimen that has been reported to rapidly increase muscle creatine stores.1,18 Our data do not support adverse effects on thermoregulation between the sexes. The blood-pressure differences between the sexes did not appear to be a result of the creatine supplementation; rather, the males had higher initial blood pressure readings compared with the females. These results support previous findings that creatine supplementation does not adversely affect thermoregulatory capabilities, and we provide new data on sex differences during exercise in a thermoneutral environment.
ACKNOWLEDGMENTS
The creatine monohydrate was donated by Nutra Sense, Inc, Shawnee Mission, KS.
REFERENCES
- 1.Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- 2.Volek JS, Duncan ND, Mazzetti SA, et al. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. 1999;31:1147–1156. doi: 10.1097/00005768-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Willoughby DS, Rosene JM. Effects of oral creatine and resistance training on myosin heavy chain expression. Med Sci Sports Exerc. 2001;33:1674–1681. doi: 10.1097/00005768-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance in muscle metabolism during maximal exercise in humans. Am J Physiol. 1996;271:E31–E37. doi: 10.1152/ajpendo.1996.271.1.E31. [DOI] [PubMed] [Google Scholar]
- 5.Odland LM, MacDougall JD, Tarnopolsky MA, Elorriaga A, Borgmann A. Effect of oral creatine supplementation on muscle [Pcr] and short-term maximum power output. Med Sci Sports Exerc. 1997;29:216–219. doi: 10.1097/00005768-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 6.McNaughton LR, Dalton B, Tarr J. The effects of creatine supplementation on high-intensity exercise performance in elite performers. Eur J Appl Physiol Occup Physiol. 1998;78:236–240. doi: 10.1007/s004210050413. [DOI] [PubMed] [Google Scholar]
- 7.Kreider RB, Ferreira M, Wilson M, et al. Effects of creatine supplementation on body composition, strength, and sprint performance. Med Sci Sports Exerc. 1998;30:73–82. doi: 10.1097/00005768-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Becque MD, Lochmann JD, Melrose DR. Effects of oral creatine supplementation on muscular strength and body composition. Med Sci Sports Exerc. 2000;32:654–658. doi: 10.1097/00005768-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Terjung RL, Clarkson P, Eichner ER, et al. American College of Sports Medicine roundtable: the physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Kern M, Podewils LJ, Vukovich M, Buono MJ. Physiological response to exercise in the heat following creatine supplementation. JEPonline (serial online) 2001;4 Available at: http://www.css.edu/users/tboone2/asep/jan3.htm. Accessed September 5, 2001. [Google Scholar]
- 11.Volek JS, Mazzetti SA, Farquhar WB, Barnes BR, Gomez AL, Kraemer WJ. Physiological responses to short-term exercise in the heat after creatine loading. Med Sci Sports Exerc. 2001;33:1101–1108. doi: 10.1097/00005768-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Cox G, Mujika I, Tumilty D, Burke L. Acute creatine supplementation and performance during a field test simulating match play in elite female soccer players. Int J Sport Nutr Exerc Metab. 2002;12:33–46. doi: 10.1123/ijsnem.12.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Ziegenfuss TN, Rogers M, Lowery L, et al. Effect of creatine loading on anaerobic performance and skeletal muscle volume in NCAA Division I athletes. Nutrition. 2002;18:397–402. doi: 10.1016/s0899-9007(01)00802-4. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton KL, Meyers MC, Skelly WA, Marley RJ. Oral creatine supplementation and upper extremity anaerobic response in females. Int J Sport Nutr Exerc Metab. 2000;10:277–289. doi: 10.1123/ijsnem.10.3.277. [DOI] [PubMed] [Google Scholar]
- 15.Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med Sci Sports Exerc. 2000;32:291–296. doi: 10.1097/00005768-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Tarnopolsky MA, MacLennan DP. Creatine monohydrate supplementation enhances high-intensity exercise performance in males and females. Int J Sport Nutr Exerc Metab. 2000;10:452–463. doi: 10.1123/ijsnem.10.4.452. [DOI] [PubMed] [Google Scholar]
- 17.Adams G. Exercise Physiology Laboratory Manual. 3rd ed. Boston, MA: WCB McGraw-Hill; 1998. [Google Scholar]
- 18.Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol. 1996;81:232–237. doi: 10.1152/jappl.1996.81.1.232. [DOI] [PubMed] [Google Scholar]
- 19.Fisher M, Paolone V, Rosene J, Drury D, Van Dyke A, Moroney D. The effect of submaximal exercise on recovery hemodynamics and thermoregulation in men and women. Res Q Exerc Sport. 1999;70:361–368. doi: 10.1080/02701367.1999.10608056. [DOI] [PubMed] [Google Scholar]
- 20.Genuth SM. The reproductive glands. In: Berne RM, Levy MN, editors. Physiology. 4th ed. St. Louis, MO: Mosby; 1998. pp. 965–1013. [Google Scholar]
- 21.Astrand P. Work test with the bicycle ergometer. In: Heyward VH, editor. Advanced Fitness Assessment and Exercise Prescription. 3rd ed. Champaign, IL: Human Kinetics; 1998. p. 63. [Google Scholar]
- 22.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 23.Swain D, Leutholtz B. Metabolic Calculations Simplified. Baltimore, MD: Lippincott, Williams, & Wilkins; 1997. [Google Scholar]
- 24.Gonzalez-Alonson J, Mora-Rodriguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am J Physiol Heart Circ Physiol. 2000;278:H321–H330. doi: 10.1152/ajpheart.2000.278.2.H321. [DOI] [PubMed] [Google Scholar]
- 25.Sawka MN, Young AJ, Francesconi RP, Muza SR, Pandolf KB. Thermoregulatory and blood responses during exercise at graded hypohydration levels. J Appl Physiol. 1985;59:1394–1401. doi: 10.1152/jappl.1985.59.5.1394. [DOI] [PubMed] [Google Scholar]
- 26.Paolone AM, Wells CL, Kelly GT. Sexual variations in thermoregulation during heat stress. Aviat Space Environ Med. 1978;49:715–719. [PubMed] [Google Scholar]
- 27.Kenney WL, Johnson JM. Control of skin blood flow during exercise. Med Sci Sports Exerc. 1992;24:303–312. [PubMed] [Google Scholar]
- 28.Volek JS, Kraemer WJ. Creatine supplementation: its effect on human muscular performance and body composition. J Strength Cond Res. 1996;10:200–210. [Google Scholar]
- 29.Cianflocco AJ. Renal complications of exercise. Clin Sports Med. 1992;11:437–451. [PubMed] [Google Scholar]
- 30.Volek JS, Duncan ND, Mazzetti SA, Putukian M, Gomez AL, Kraemer WJ. No effect of heavy resistance training and creatine supplementation on blood lipids. Int J Sport Nutr Exerc Metab. 2000;10:144–156. doi: 10.1123/ijsnem.10.2.144. [DOI] [PubMed] [Google Scholar]
- 31.Ziegenfuss TM, Lowery LM, Lemon PWR. Acute fluid volume changes in men during three days of creatine supplementation. JEPonline (serial online) 1998;1 Available at: http://www.css.edu/users/tboone2/asep/jan3.htm. Accessed September 5, 2001. [Google Scholar]



