Abstract
Background
The aim of this study was to evaluate the potential effect of intraperitoneal administration of leptin on the hepatic regeneration and the mitotic index.
Material/Methods
56 Sprague-Dawley rats were divided into 7 groups each containing 8 rats. Group 1 was evaluated as the sham group and no surgical procedure was performed on animals. The rats in groups 2, 3, and 4 (named C24, C48, C72, respectively) were given intraperitoneal injection of 2 ml/kg normal saline 60 minutes before the surgical procedure consisting of laparotomy and 70% hepatectomy. These groups were used as controls at 24, 48, and 72 hours. The rats in groups 5, 6, and 7 (named L24, L48, and L72, respectively) were given intraperitoneal injection of 20 μg/kg doses of recombinant mouse leptin 60 minutes before the same surgical procedure. These groups were evaluated as the experiment groups at 24, 48, and 72 hours. Blood samples were collected for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and the remaining tissue samples were obtained for liver histopathology, regeneration rate, and mitotic index (MI). The weights of the remaining livers were also noted.
Results
The values of AST and ALT were higher in the groups that were administered leptin and they had significantly higher mitotic index than the other groups. Leptin also significantly increased the regeneration ratio as compared to the control group. The weights of the remaining livers were also higher in the leptin groups.
Conclusions
Intraperitoneal administration of leptin was observed to increase liver regeneration and mitotic rate in 70% hepatectomized rats.
Keywords: liver regeneration, leptin, partial hepatectomy
Background
The liver is an organ that performs considerable metabolic functions regarding the activity of other organ systems [1]. The hepatocytes are quickly regenerated and proliferated following resection [2]. This regeneration, in turn, will last until the liver regains its original mass [3].
Leptin is a member of the super family of cytokines and influences body weight and homeostasis through effects on food intake and energy expenditure. It also plays an important role in the regulation of fatty acid metabolism in the liver, pancreas, skeletal muscle, and heart [4,5]. It is secreted from adipocytes and was originally used in the treatment of obesity by binding to its specific receptors, such as type-I cytokine receptor [6,7].
Hepatocyte dysfunction may be evaluated by measuring the increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) transaminases values that are usually present in serum [8].
Mitotic index is the ratio between the number of cells in mitosis and the total number of cells [9,10]. A wide range of markers are used to describe the hepatic regeneration criteria, including DNA synthesis and mitosis count, mitotic index, final hepatic volume, cell proliferation, and mitochondrial activity [11]. Therefore, the present study was planned with the aim of evaluating the potential effect of the intraperitoneal (i.p.) administration of leptin on the hepatic regeneration and the mitotic index in the rats that underwent 70% partial hepatectomy.
Material and Methods
Animals
Sprague-Dawley rats (n=56) weighing 230±30 g were used after 2 weeks of adaptation. The rats were housed in polycarbonate cages at a temperature − (21±1°C) and humiditiy − (45–55%) controlled room maintaned on a 12/12 reversed light cycle and were fed with a standard rat chow and allowed to drink water ad libitum. This study was approved by the Eskisehir Osmangazi University Institutional Animal Care and Use Committee (08.02.2007/12).
Experimental procedure
The animals were randomly selected and 7 groups were formed, each containing 8 animals. All of the rats were given i.m. administration of 4 mg/100gr ketamine hydrochloride (Parke-Davis) and 2 mg/100 gr xylazine (Bayer) anesthesia.
Group 1 (Sham): No surgical procedure was performed on the animals and they were used as sham. The blood samples were taken for detection of transaminases values only.
Group 2,3, and 4 (control groups; C24,C48, C72): I.p. injection of 2 ml/kg normal saline was administered 60 minutes before the surgical procedure consisting of laparotomy and 70% partial hepatectomy [12]. The rats of these groups were sacrificed at 24, 48, and 72 hour following the experimental procedure, respectively.
Group 5,6, and 7 (leptin groups; L24, L48, L72): I.p. injection of 20μg/kg doses of recombinant mouse leptin (Sigma Chemical Co., St. Louis, MO, USA) dissolved in phosphate-buffered saline (PBS) was administered 60 minutes before the same surgical procedure [12]. The rats of these groups were also sacrificed at 24, 48, and 72 hours following the experimental procedure, respectively.
Blood samples were collected from the inferior vena cava for the AST and ALT values and the remaining liver tissue was totally resected for the histological examination. The total amount of the remaining liver tissues were also weighed and noted.
The hepatic regeneration ratio was calculated using Child’s Formula [13].
Histological examinations
All of the specimens were carefully excised and fixed in neutral buffered formalin for histologic analyses. After the fixation, the tissues were embedded in paraffin and serial sections (4 μm) were prepared for each of the paraffin blocks; on average, 50 sections were collected per rat. Sections were stained for the assessment of liver histology and mitotic index (MI) with hematoxylin and eosin (H&E). Digital images were obtained by an Olympus BX-61 (Olympus America Inc. NY, USA) microscope with a DP70 digital camera. The mitosis rate was determined by random evaluation of at least 1000 hepatocytes and is expressed as a percentage (number of cells with mitosis/total hepatocytes ×100) [14].
Statistical analysis
The data were analyzed by SPSS for Windows 17.0 and SigmaStat 3.5 programs. Shapiro-Wilk test was used to determine the conformance of data with normal distribution. The comparison of the average values of the groups was performed by using ANOVA for parametric tests and Tukey HSD (post hoc tests) test for multiple comparisons. Analysis of the nonconforming data with the normal distribution was performed by using Kruskal-Wallis and Mann-Whitney U tests, which are nonparametric, and the results were summarized as mean ±SD. P<0.05 was considered as statistically significant.
Results
Biochemical analyses
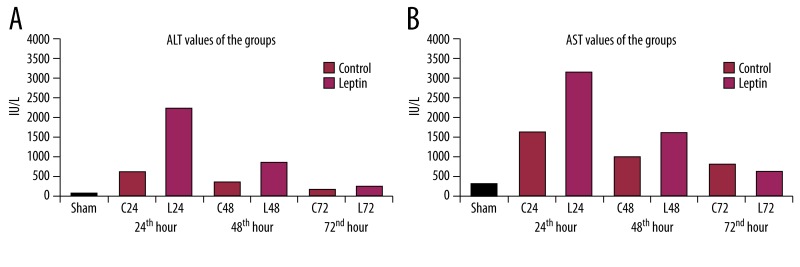
The biochemical analyses shows that there was a statistically significant difference (p<0.01) between the sham and the other groups, revealing that hepatectomy of 70% produced significant parenchymal injury in terms of increased AST and ALT levels (IU/L). However, the values of the control and leptin groups revealed that leptin produced more pronounced injury until 48 hours, but these levels returned to similar values as control at 72 hours. There was no statistically significant difference (p>0.05) established between the 72-hour leptin group (L72) and the control group (C72) in respect to AST and ALT values (Figure 1).
Figure 1.
Transaminase values of the groups. Leptin produced a transient elevation in AST (A) and ALT (B) levels however they returned to similar levels as control ones at 3rd day.
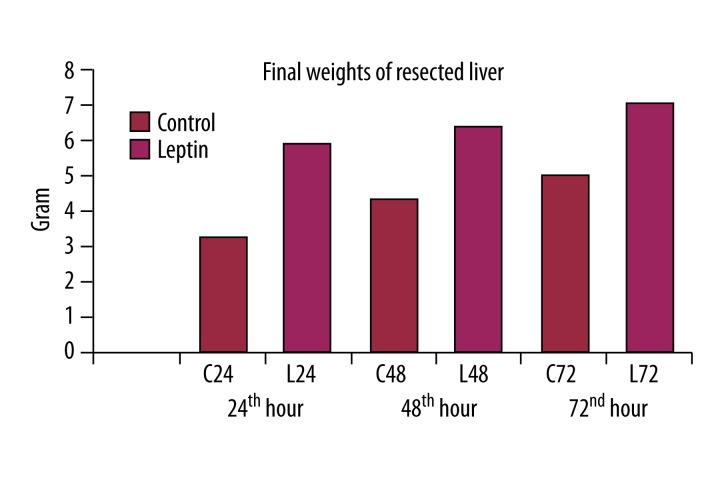
Final weights of the resected liver
Leptin administration significantly increased the final weights of the resected liver as compared to control groups (p<0.01) (Figure 2).
Figure 2.
Leptin significantly increased the final weights of the resected livers.
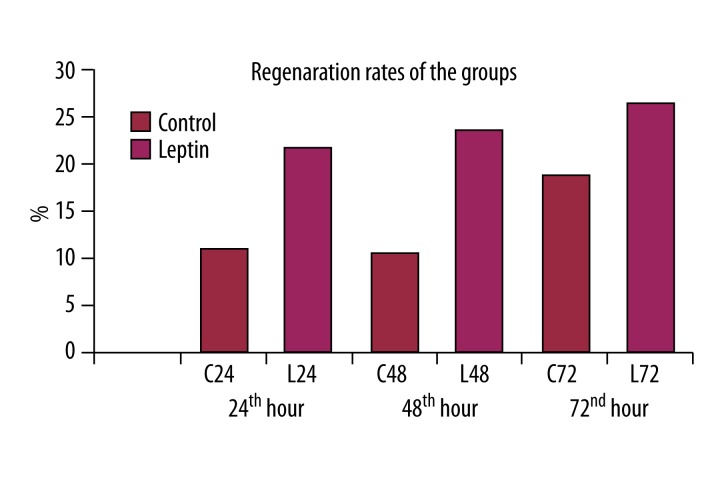
Regeneration ratio
The comparison of the regeneration ratios of the groups revealed that leptin administration significantly increased the regeneration ratio (p<0.05) (Figure 3).
Figure 3.
Leptin produced a significant increase in regeneration ratio following 70% hepatectomy.
Histological results
The liver sections of the animals in the C24 group demonstrated that 70% hepatectomy caused diffuse replication of hepatocytes at 24 hours (Figure 4A). There was also diffuse vacuolization and mitotic figures at 48 hours in the control group (Figure 4B). The progression of mitosis and polymorphonuclear cell infiltration were also observed at 72 hours in this group (Figure 4C).
Figure 4.
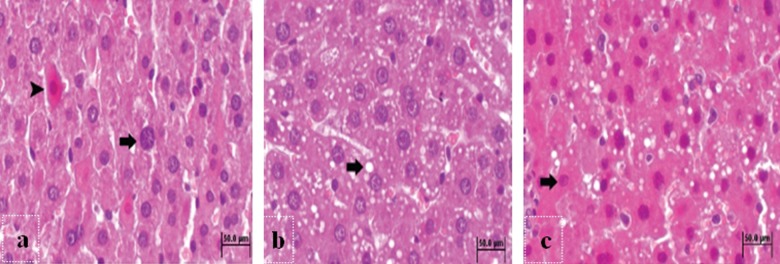
Histopathologic sections of the control groups at 24th (A), 48th (B) and 72nd (C) hours. (A) The hepatocytes in the neolobules during replication phase (arrow) and hepatocytes with eosinophilic cytoplasm (arrow head) in the liver sections of C24 group. H&E. Bar 50 μm. (B) Diffuse vacuolization (arrow) of hepatocytes in the liver sections of C48 group. H&E. Bar 50 μm. (C) The hepatocytes in the neolobules with eosinophilic cytoplasm (arrow) in the liver sections of C72 group. H&E. Bar 50 μm.
A highly diffused replication was observed in the liver sections of the 24-hour leptin group (Figure 5A), whereas the liver sections of the 48-hour leptin group showed increased number of hepatocyte counts in different phases of mitosis (Figure 5B). The liver sections of the 72-hour leptin group demonstrated the progression of mitosis (Figure 5C). In all of the leptin groups, the vacuolization of the hepatocytes was partially reduced and polymorphonuclear cell infiltration was rarely observed.
Figure 5.
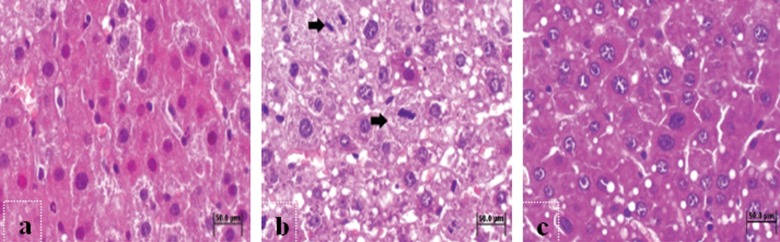
Histopathologic sections of the leptin groups at 24th (A), 48th (B) and 72nd (C) hours. (A) Diffuse replication and mitosis in the liver sections of L24 group. H&E. Bar 50 μm. (B) Hepatocytes at different phases of mitosis (arrow) in the liver sections of L48 group. H&E. Bar 50 μm. (C) Mitosis and reduced vacuolization of the hepatocytes in liver sections of L72 group. H&E. Bar 50 μm.
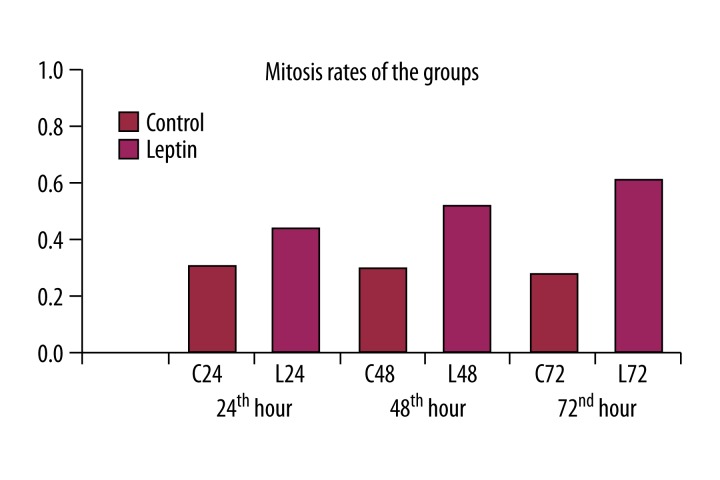
The mitotic index values of the groups administered leptin were statistically higher as compared to control groups at the same hour (p<0.001). In other words, leptin significantly increased mitotic ratio (Figure 6).
Figure 6.
Leptin produced significantly increased mitosis rates at 24th, 48th and 72nd hr as compared to control groups.
Discussion
Hepatic resections have become more reliable due to the recent improvements in pre-operative diagnosis, surgical techniques, and post-operative care. However, hepatocellular dysfunction may develop in parenchymal hepatic diseases, and post-hepatectomy liver failure may be seen and result in high mortality (60–90%) in patients with hepatic dysfunction and related complications [2].
The term “leptin” originates from leptos in Greek, which means “thin”. Leptin is a protein hormone of 167 amino acids and 16 kDa, and was originally discovered as a product of obese (ob) gene in 1994 by cloning from mice by Friedman et al. [4,5]. Its signals are expressed via OBR receptors in ventromedial hypothalamus. After being released in circulation, it passes through the blood-brain barrier and binds to the receptors in the arcuate nucleus of ventromedial hypothalamus, thereby inhibiting the release of neuropeptide-Y and agouti-related peptide (AgRP), stimulating food intake [4]. Although it is regarded as an anorexigenic hormone, it has high obesity values as a result of resistance to its effects [15].
The actual mechanism of leptin to increase hepatic fibrosis is not known. The role of leptin in the molecular pathogenesis of fibrosis in mammals was recently demonstrated in glomerulosclerosis and hepatic fibrosis. In peripheral tissues, leptin enables the homeostatic control of body weight via glucose metabolism and the inhibitory effects in insulin secretion [16].
Hepatic regeneration has generally been investigated by studies in which 2/3 of the hepatic mass was resected by surgical procedure in rodents; this technique is known as 2/3 partial hepatectomy. Since rodents have a hepatic structure with multiple lobes, 3 out of 5 (2/3 of the mass) may be easily resected by surgical procedure. The remaining structure is reorganized at close-range to the original mass with 5 lobes [17]. In our study, the 70% hepatectomy model was applied as described by Higgins and Anderson [18].
The liver reaches its former size by the extension of the lobules and addition of new lobules. A wide range of markers have been used to describe hepatic regeneration criteria, including DNA synthesis and mitosis count, mitotic index, final hepatic volume, cell proliferation, and mitochondrial activity [11]. In the present study, we used the final resected weight, regeneration ratio, and mitotic index criteria for the determination of regeneration.
Xu et al. [19] reported an increase of 108.0% in the remaining hepatic tissue 3 days after 75% hepatic resection. In our study, we observed increased liver weights of 29%, 33%, and 46% at 24, 48, and 72 hours in the control groups, but liver weights increased 87%, 162%, and 160% in leptin groups, respectively, in accordance with results from other investigations.
In recent years, leptin has been demonstrated to be a potent mitogen for many cell lines in the peripheral tissues. It also increases re-epithelization in vitro and in vivo and induces angiogenesis [6,7].
Sinusoidal endothelial cells and Kupffer cells are described as the main target of the fibrogenic effect of leptin. On the other hand, the fibrogenic response was significantly reduced in the mice with leptin-resistance or lack of leptin. This fibrogenic effect demonstrates that leptin is a potent mitogen and inhibitor of apoptosis [15]. These findings indicate that leptin may play a central role in the profibrogenic response in the liver [5,16,20]. Therefore, in accordance with our findings, leptin may have a direct role in the regenerative process following 70% hepatectomy [6,7].
Leptin production is increased in infection and inflammation as part of the host acute phase response. Recent studies have shown that leptin regulates the functions of non-specific immune response cells such as secretion of cytokines from macrophages and the phagocytosis of neutrophiles. In our study, polymorphonuclear leukocytes (PMNL) infiltration seen in the control groups was observed to be decreased in the leptin-administered group following hepatectomy.
In the present study, diffuse vacuolization as well as mitotic figures was observed in the control group at 48 hours. However, vacuolization was partially reduced in leptin groups, so hepatocytes were considered to be protected from histological injury by leptin administration.
Serum transaminases are highly sensitive in indicating the hepatocyte damage. The levels of serum transaminases are increased in all kinds of liver injury, independent of cause. In our study, 70% hepatectomy produced high levels of AST and ALT in all groups. There was a statistically significant difference between the sham and the other groups (p<0.01). Moreover, a statistically significant difference was established between the control and the leptin groups. Although leptin produced significantly increased values of transaminases at 24 and 48 hours as compared to control groups (p<0.05), these elevated values declined to similar levels as control at 72 hours (p>0.05). Thus, it produced a transient parenchymal injury in terms of transaminase elevation. This result may suggest that leptin may not prevent cellular injury at an early period (i.e., the first 2 days) following hepatectomy – and it may even exacerbate cellular injury. However, this effect is temporary and disappears towards the third day.
Mitotic cells are not observed under normal conditions, but they are frequently observed within 24 to 48 hours following hepatectomy [9,21]. In the present study, we did not observe any cell mitosis in the sham group. The liver sections of the leptin groups showed diffuse replication at 24 hours, increased number of hepatocyte counts in different phases of mitosis at 48 hours, and progression of mitosis at 72 hours. The mitotic index is the ratio between the number of cells in mitosis and the total number of cells. Selzner et al. [9] reported the mitotic index values at 24 and 48 hours were 2% and 3%, respectively, whereas Akcan et al. [10] found it was 2.6% and 6.4%, respectively. In our study, the mitotic index was established as 2.6%, 2.6%, and 2.2% at 24, 48, and 72 hours in control groups, respectively; and 4.4%, 5.0%, and 5.6% in leptin groups, respectively. The difference between the control and the leptin groups was also statistically significant (p<0.001).
Conclusions
I.p. administration of leptin was observed to increase the mitosis rate, regeneration ratio, and final liver weight following 70% hepatectomy. Although it seems to produce a transient increase in transaminase values, it augments the liver regeneration and ameliorates histopathological injury following hepatectomy. Further studies are necessary to adopt these results and leptin usage in clinical area.
Footnotes
Source of support: Departmental sources
References
- 1.Tralhão JG, Abrantes AM, Hoti E, et al. Hepatectomy and liver regeneration: from experimental research to clinical application. ANZ J Surg. 2013 doi: 10.1111/ans.12201. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Vetelainen R, Vliet AK, Gulik TM. Severe steatosis increases hepatocellular injury and impairs liver regeneration in a rat model of partial hepatectomy. Ann Surg. 2007;245:44–50. doi: 10.1097/01.sla.0000225253.84501.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uyanoğlu M, Canbek M, Aral E, et al. Effects of carvacrol upon the liver of rats undergoing partial hepatectomy. Phytomedicine. 2008;15:226–29. doi: 10.1016/j.phymed.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Mutschler J, Abbruzzese E, Wiedemann K, et al. Functional polymorphism in the Neuropeptide Y Gene Promoter (rs16147) is associated with serum leptin levels and waist-hip ratio in women. Ann Nutr Metab. 2013;62(4):271–76. doi: 10.1159/000346799. [DOI] [PubMed] [Google Scholar]
- 5.Chuang JH, Wang PW, Tai MH. An adipocentric view of liver fibrosis and cirrhosis. Chang Gung Med J. 2004;27:855–68. [PubMed] [Google Scholar]
- 6.Yamauchi H, Uetsuka K, Okada T, et al. Impaired liver regeneration after partial hepatectomy in db/db mice. Exp Toxicol Pathol. 2003;54(4):281–86. doi: 10.1078/0940-2993-00265. [DOI] [PubMed] [Google Scholar]
- 7.Picard C, Lambotte L, Starkel P, et al. Steatosis is not sufficient to cause an impaired regenerative response after partial hepatectomy in rats. J Hepatol. 2002;36:645–52. doi: 10.1016/s0168-8278(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 8.Aoki T, Murakami M, Niiya T, et al. Capacity of hepatic regeneration following a second partial hepatectomy in rats. Hepatol Res. 2001;21:228–41. doi: 10.1016/s1386-6346(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 9.Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: Disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35–42. doi: 10.1002/hep.510310108. [DOI] [PubMed] [Google Scholar]
- 10.Akçan A, Kucuk C, Ok E, et al. The effect of amrinone on liver regeneration in experimental hepatic resection model. J Surg Res. 2006;130:66–72. doi: 10.1016/j.jss.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Michalopoulos GK, De Frances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 12.Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin-the classical, resistin-the controversical, adiponectin-the promising, and more to come. Best Pract Res Clin Endoc Metab. 2005;19:525–46. doi: 10.1016/j.beem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukocyte Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 14.Hou Z, Yanaga K, Kamohara Y, et al. A new supressive agent against interleukin-1β and tumor necrosis factor-α enhances liver regeneration after partial hepatectomy in rats. Hepatol Res. 2003;26:40–46. doi: 10.1016/s1386-6346(02)00334-0. [DOI] [PubMed] [Google Scholar]
- 15.Tsochatzis EA, Papatheodoridis GV, Archimandritis AJ. Adipokines in nonalcoholic steatohepatitis: from pathogenesis to implications in diagnosis and therapy. Mediators Inflamm. 2009;2009:831670. doi: 10.1155/2009/831670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handy JA, Saxena NK, Fu P, et al. Adiponectin activation of AMKP disrupts leptin mediated hepatic fibrosis via supressors of cytokine signaling (SOC-3) J Cell Biochem. 2010;110:1195–207. doi: 10.1002/jcb.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins GM, Anderson RM. Experimental pathology of the liver. Restoration of the liver of the white rat following surgical removal. Arch Pathol. 1931;12:186. [Google Scholar]
- 19.Xu HS, Rosenlof LK, Jones RS. Bile secretion and liver regeneration in partially hepatectomized rats. Ann Surg. 1993;218(2):176–82. doi: 10.1097/00000658-199308000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canbakan B, Tahan V, Balci H, et al. Leptin in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7(3):249–54. [PubMed] [Google Scholar]
- 21.Laconi S, Curreli F, Diana S, et al. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine A kinetic study. J Hepatol. 1999;31:1069–74. doi: 10.1016/s0168-8278(99)80320-1. [DOI] [PubMed] [Google Scholar]