Abstract
Despite their crucial role in initiating steroid-hormone synthesis, the hypothalamic and pituitary hormones (LH, LHRH) and their receptors have received scant attention in genetic studies of hormone-related diseases. This study included 1,170 men diagnosed with prostate cancer (PC) in Los Angeles County between 1999 and 2003. LHRH and LH receptor genotypes were examined for association with PC survival. Additionally, associations with PC incidence were examined by comparing PC cases to control men of similar age and race/ethnicity. The LHR 312 G allele was found to be associated with increased PC mortality (p=0.01). Ten years after diagnosis, 16% of men carrying two copies of the G allele (genotype GG) had died of PC, compared to 11% of those with genotype AG and 9% of those with AA. In a case-control comparison, this same allele was significantly associated with decreased PC risk: OR=0.68 (95% CI: 0.49, 0.93) for genotype GG vs. AA. These results suggest that androgens may play opposing roles in PC initiation and progression, and highlight the need to include these important but overlooked genes in future studies of PC etiology, prognosis, and treatment.
Keywords: Prostate cancer, LHRH, LHR, genetic polymorphism, survival, association study
Introduction
Prostate cancer (PC) (OMIM: 176807) affects almost 240,000 men in the United States each year and is the second leading cause of cancer related death in this population [1]. Androgens are essential survival factors for prostate epithelial cells, and have long been recognized as the primary drivers of cell proliferation in both normal and malignant prostate. Genetic variations in the androgen receptor and androgen metabolic pathway have been studied with respect to PC incidence (reviewed in [2]) and survival [3]. Despite their importance in initiating androgen synthesis, the hypothalamic and pituitary hormones, luteinizing hormone-releasing hormone (LHRH) and luteinizing hormone (LH), have received scant attention in genetic studies.
LHRH is secreted, under physiologic conditions, in a pulsatile manner from the hypothalamus. LHRH receptors in the pituitary, upon brief exposures to high levels of LHRH, trigger release of LH. LH plays a critical role in initiating testicular androgen synthesis. The binding of LH to its receptors on testicular Leydig cells induces cAMP production and subsequent StAR-mediated cholesterol transport, which is the first and rate-limiting step in testicular androgen biosynthesis. This pathway is central to the development of PC and remains the primary therapeutic target in advanced PC.
In this study, we selected all validated non-synonymous SNPs having a minor allele frequency of at least 5% in the following genes: LHB (encoding the LH beta subunit), LHCGR (encoding LH receptor), GNRH1 (encoding LHRH), and GNRHR (encoding the LHRH receptor). Only two SNPs fulfilled these criteria, rs2293275 in LHCGR and rs6185 in GNRH1, which we will subsequently refer to as LHR312 and LHRH16. These SNPs were genotyped in a cohort of 1,170 men diagnosed with PC in Los Angeles County from 1999-2003. Genotypes were examined for association with PC survival. Additionally, associations with PC incidence were examined by comparing PC cases to control men of similar age and race/ethnicity. Cases and controls were participants in the Los Angeles site of the California Collaborative Prostate Cancer Study.
Methods
Study population
Study subjects were Los Angeles County participants from the California Collaborative Prostate Cancer Study, a population-based case control study that has been previously described in detail [4-6]. Briefly, men diagnosed with PC between 1999 and 2003 were identified by the Los Angeles County Cancer Surveillance Program and from Los Angeles County Cancer Registry records. Cases were ascertained to enrich the study population for advanced stage disease [7]. Cases were linked to the California Cancer Registry records in March 2013 to obtain vital status and cause of death. Controls living in the same neighborhood as the cases were identified using a standard neighborhood walk algorithm and were frequency matched to cases on age (±5 years) and race/ethnicity.
Written informed consent was obtained from all study participants. The study was approved by the Institutional Review Board of the University of Southern California.
This study included 1,170 prostate cancer cases and 538 controls. For the observed distributions of genotypes and survival probabilities shown in Table 1, minimal detectable hazard ratio for the log-rank test with a=0.05 and 80% power was 1.97 for LHR312 genotype GG vs. AA and 2.88 for LHRH16 genotype CC vs. GG. For case-control comparisons, minimal detectable effects correspond to odds ratios of 0.71 for LHR312 AG/GG vs. AA and 0.70 for LHRH16 GC/CC vs. GG.
Table 1.
Characteristics of prostate cancer cases
| All cases | PC death* | All cause death** | |
|---|---|---|---|
| All cases | 1170 | 134 (11%) | 426 (36%) |
| Age at diagnosis | |||
| <60 | 284 | 31 (11%) | 58 (20%) |
| 60-69 | 480 | 47 (10%) | 147 (31%) |
| 70+ | 406 | 56 (14%) | 221 (47%) |
| Stage at diagnosis | |||
| localized | 528 | 32 (6%) | 189 (36%) |
| local extension only | 428 | 24 (6%) | 99 (23%) |
| lymph node positive | 53 | 13 (25%) | 21 (40%) |
| distant | 89 | 44 (49%) | 66 (74%) |
| unknown | 7 | 21 (29%) | 52 (73%) |
| Grade | |||
| low | 725 | 38 (5%) | 215 (30%) |
| high | 415 | 85 (20%) | 189 (46%) |
| unknown | 30 | 11 (37%) | 21 (70%) |
| Race/ethnicity | |||
| African American | 349 | 40 (11%) | 132 (38%) |
| Hispanic | 327 | 38 (12%) | 114 (35%) |
| White | 494 | 56 (11%) | 180 (36%) |
| LHR321 genotype | |||
| AA | 283 | 24 (8%) | 99 (35%) |
| AG | 466 | 47 (10%) | 172 (37%) |
| GG | 340 | 50 (15%) | 121 (36%) |
| Total | 1089 | ||
| LHRH16 genotype | |||
| GG | 757 | 86 (11%) | 273 (36%) |
| GC | 295 | 37 (13%) | 115 (39%) |
| CC | 46 | 7 (15%) | 17 (37%) |
| Total | 1098 |
Number (%) of all cases who died from PC.
Number (%) of all cases who died from any cause.
Genotyping
SNPs were genotyped using DNA extracted from peripheral blood buffy coats. Genotypes were assayed using TaqMan allelic discrimination assays (Applied Biosystems) using the TaqMan Core Reagent Kit according to manufacturer’s recommendations. Fluorescent signals were measured using the ABI7900HT detection system. Each run included water blanks and 10% blind replicates. There were no discrepancies among replicates. Call rates were >98% for both assays.
Statistical analysis
Hazard ratios were estimated by fitting Cox proportional hazards models adjusted for race (black vs. non-black), age at diagnosis (≥70 vs. <70), tumor grade (high vs. low), and stage at diagnosis (regional/distant vs. local). Odds ratios were estimated from conditional logistic regression matching on race/ethnicity and socioeconomic status as previously described [5]. Logistic models were further adjusted for age and first-degree family history of PC.
Results
Survival
Table 1 describes the cohort of 1,170 cases included in this study. Age at diagnosis ranged from 42 to 94 years (median 66 years); 30% (349) were African American, 28% (327) were Hispanic, and 42% (494) were non-Hispanic white. Eight percent (89) were diagnosed with distant metastases, 41% (481) with regional disease, and 45% (528) with localized disease. Median follow-up was 10.0 years. At the time of linkage to the cancer registry, 36% had died, 11% from PC. PC-specific mortality appeared higher among men diagnosed at age 70 years or older, among those diagnosed with advanced or high-grade disease, and among those with LHR312 genotype GG.
Kaplan-Meier survival curves for PC-specific survival by LHR312 genotype are shown in Figure 1A. Genotype was signi-ficantly associated with PC-specific survival (Log rank test p=0.03). However, for approximately the first five years after diagnosis, survival was similar across genotypes. Poorer PC-specific survival among those with the GG genotype emerged only with long-term follow-up. At ten years follow-up, PC-specific survival was 84% among those with genotype GG, compared to 89% and 91% for those with genotypes AG and AA, respectively.
Figure 1.
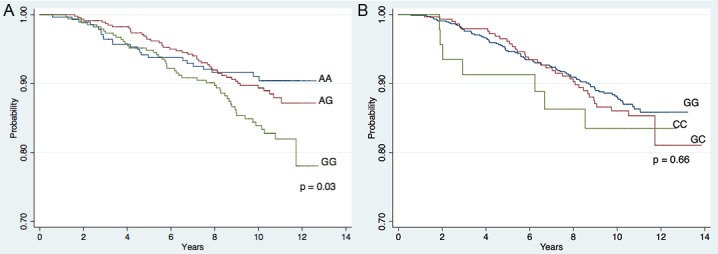
Kaplan-Meier Curves for PC-specific survival by genotype. A: Survival by LHR312 genotype; B: Survival by LHRH16 genotype. P-values are from the Log-Rank Test.
Hazard ratios for PC-specific survival and genotype are shown in Table 2. The significant association between PC-specific survival and LHR312 genotype remained after adjusting for stage, grade, age at diagnosis, and race. There was no evidence that the association between genotype and survival varied by grade or stage of disease, age, or race/ethnicity, however power to detect heterogeneity was low. LHR312 genotype was not associated with all-cause mortality (p trend = 0.82; data not shown).
Table 2.
Hazard Ratios for PC-specific survival, by LHR and GnRHR genotype
| Hazard Ratio (95% CI)* | ||
|---|---|---|
|
|
||
| Unadjusted | Adjusted** | |
| LHR 312 genotype | ||
| AA | 1.00 (ref) | 1.00 (ref) |
| AG | 1.28 (0.72, 2.27) | 1.46 (0.80, 2.64) |
| GG | 1.90 (1.07, 3.36) | 2.25 (1.19, 4.26) |
| p trend = 0.01 | p trend = 0.01 | |
| LHRH 16 genotype | ||
| GG | 1.00 (ref) | 1.00 (ref) |
| GC | 1.02 (0.65, 1.59) | 0.91 (0.58, 1.44) |
| CC | 1.38 (0.60, 3.17) | 1.00 (0.43, 2.43) |
| p trend = 0.60 | p trend = 0.80 | |
Includes those cases with non-missing grade and stage (1000 for LHR 312 and 1010 for LHRH 16.
Cox Proportional Hazards models adjusted for race (Black vs. non-Black), age (≥70 vs. <70), grade (high vs. low), and stage (regional/distant vs. local).
Genotype at the LHRH16 locus was not significantly associated with PC survival (Figure 1B, Log rank test p=0.66). There appeared to be a modest increase in hazard for those with genotype CC vs. GG; however, power to detect such a difference was limited by the small number of men with genotype CC, and the apparent association disappeared after adjusting for stage, grade, age at diagnosis and race (Table 1). LHRH 16 genotype was not associated with all-cause mortality (Table 1, P trend = 0.57).
Incidence
Table 3 compares genotype frequencies among PC cases to those among controls. Odds of PC were approximately 30% lower for men who carried at least one LHR312 G allele, compared to men with the AA genotype. This association between PC and LHR genotype was observed in both racial groups, though the association was not statistically significant among African Americans. The frequency of the G allele was substantially lower among African Americans (33%) than among whites (65%). Genotype frequencies did not depart from Hardy-Weinberg equilibrium among controls in either racial group (p>0.05). There was no evidence of heterogeneity by stage or grade of disease. Odds ratios (and 95% confidence intervals) for genotype GG vs. AA were 0.65 (0.45, 0.95) and 0.72 (0.49, 1.07) for advanced and localized stage disease and 0.62 (0.41, 0.95) and 0.67 (0.47, 0.95) for high- and low-grade disease, respectively (data not shown).
Table 3.
LHR and LHRH genotypes among PC cases and controls
| All Races | Whites | African Americans | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Controls | Cases | OR (95% CI) | Controls | Cases | OR (95% CI) | Controls | Cases | OR (95% CI) | |
| Genotype | |||||||||
| LHR321 | |||||||||
| AA | 115 (20%) | 283 (26%) | 1.00 (ref) | 45 (11%) | 114 (15%) | 1.00 (ref) | 70 (45%) | 169 (52%) | 1.00 (ref) |
| AG | 272 (47%) | 466 (43%) | 0.70 (0.54, 0.93) | 204 (48%) | 339 (44%) | 0.65 (0.32, 0.97) | 68 (44%) | 127 (39%) | 0.78 (0.51, 1.17) |
| GG | 187 (33%) | 340 (31%) | 0.68 (0.49, 0.93) | 170 (41%) | 309 (41%) | 0.63 (0.32, 0.96) | 17 (11%) | 31 (9%) | 0.75 (0.38, 1.46) |
| 574 (100%) | 1089 (100%) | 419 (100%) | 762 (100%) | 155 (100%) | 327 (100%) | ||||
| LHRH16 | |||||||||
| GG | 360 (66%) | 757 (69%) | 1.00 (ref) | 241 (61%) | 471 (62%) | 1.00 (ref) | 119 (80%) | 286 (85%) | 1.00 (ref) |
| GC | 159 (30%) | 295 (27%) | 0.93 (0.73, 1.19) | 130 (35%) | 248 (32%) | 0.97 (0.73, 1.28) | 29 (19%) | 47 (14%) | 0.78 (0.46, 1.32) |
| CC | 19 (4%) | 46 (4%) | 1.21 (0.68, 2.16) | 18 (5%) | 44 (6%) | 1.26 (0.69, 2.29) | 1 (1%) | 2 (1%) | 0.47 (0.04, 5.32) |
| 538 (100%) | 1098 (100%) | 389 (100%) | 763 (100%) | 149 (100%) | 335 (100%) | ||||
Odds ratios calculated from conditional logistic regression, adjusting for age, 1st degree family history of PC, race/ethnicity, and SES.
LHRH16 genotype was not associated with PC incidence, either overall or when stratified by race, stage, or grade of disease. The C allele was less frequent among African Americans than among whites (10% vs. 22%). There were no departures from Hardy-Weinberg equilibrium among either racial group (p>0.05).
Discussion
In this cohort of 1,170 prostate cancer cases, diagnosed more than a decade ago, we found that the LHR312 G allele is associated with increased PC mortality (p=0.01). Ten years after diagnosis, 16% of men carrying two copies of the G allele (genotype GG) had died of PC, compared to 11% of those with genotype AG and 9% of those with AA. Yet, in a case-control comparison, this same allele was significantly associated with decreased PC risk.
The LHR312 G and A alleles respectively encode serine and asparagine at position 312 in exon 10, an exon that is critical for LH receptor activation [8]. The SNP lies adjacent to a potential N-linked glycosylation site: 313N-314K-315T, however it has not been found to affect glycosylation at 313Asn [9].
Although in vitro evidence for functionality is lacking, association studies provide evidence that the SNP may have subtle functional effects. The few association studies that have examined this polymorphism consistently link the G allele to decreased LH receptor signaling. The A allele was over-represented in two independent samples of patients with breast cancer [9], a disease associated with increased steroid hormone exposure. Conversely, the G allele has been overrepresented in conditions associated with decreased testosterone production, such as testicular maldescent and male infertility without maldescent [10], testicular cancer [11], and male genital under-masculinization [12].
In light of these reports, our results suggest that modestly impaired LH signaling (marked by the LHR321 G allele) may be associated with decreased PC risk but worse PC prognosis. This first observation (association of the G allele with decreased risk) is in agreement with the androgen hypothesis of PC causation and progression, which has been universally accepted since the seminal studies of Huggins [13] more than seven decades ago. Within the last decade, however, a more nuanced appreciation of the relationship between androgens and PC has emerged. A number of studies from around the world have reported that testosterone levels around the time of PC diagnosis are not positively associated with prognosis. One recent study reported a U-shaped association, with both low and high serum testotsterone levels being associated with high risk PC [14]. Most studies have found that low testosterone levels are associated with higher Gleason scores, higher PSA levels, more poorly differentiated disease, and increased risk of biochemical recurrence [15-18]. Consistent with these studies, we found that high-grade disease was more common among men carrying a G (low activity) allele (39%) compared to those with genotype AA (31%) (p=0.02, data not shown).
Strengths of this study include the large sample size of more than 1,000 PC cases ascertained via cancer registry from a multi-ethnic population, with more than 10 years of follow-up. The main study limitations are related to the incomplete assessment of genetic variation in the LH gene pathway and to the unavailability of clinical data on PC treatment history for cancer registry-identified cases. Our results highlight the need to include these important overlooked genes in future studies of PC etiology, prognosis, and treatment.
Acknowledgements
Financial support was received from grant number 99-00524V-10258 (to S.A.I.) from the Cancer Research Fund, under Interagency Agreement #97-12013 (University of California contract #98-00924V) with the Department of Health Services Cancer Research Program, and by grant R01CA84979 (to S.A.I.) from the National Cancer Institute, National Institutes of Health. Cancer incidence data used in this publication have been collected by the Los Angeles Cancer Surveillance Program of the University of Southern California with Federal funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. N01-PC-35139, and the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, and grant number 1U58DP000807-3 from the Centers for Disease Control and Prevention. We are grateful to the men who participated in this study, without whom this research would not be possible.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Schleutker J. Polymorphisms in androgen signaling pathway predisposing to prostate cancer. Mol Cell Endocrinol. 2012;360:25–37. doi: 10.1016/j.mce.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Lindström S, Adami HO, Bälter KA, Xu J, Zheng SL, Stattin P, Grönberg H, Wiklund F. Inherited variation in hormone-regulating genes and prostate cancer survival. Clin Cancer Res. 2007;13:5156–61. doi: 10.1158/1078-0432.CCR-07-0669. [DOI] [PubMed] [Google Scholar]
- 4.Rowland GW, Schwartz GG, John EM, Ingles SA. Protective effects of low calcium intake and low calcium absorption vitamin D receptor genotype in the California Collaborative Prostate Cancer Study. Cancer Epidemiol Biomarkers Prev. 2013;22:16–24. doi: 10.1158/1055-9965.EPI-12-0922-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, John EM, Ingles SA, Stern MC. Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer: results from a multiethnic case-control study. Carcinogenesis. 2012;33:2108–18. doi: 10.1093/carcin/bgs242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catsburg C, Joshi AD, Corral R, Lewinger JP, Koo J, John EM, Ingles SA, Stern MC. Polymorphisms in carcinogen metabolism enzymes, fish intake, and risk of prostate cancer. Carcinogenesis. 2012;33:1352–1359. doi: 10.1093/carcin/bgs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi AD, John EM, Koo J, Ingles SA, Stern MC. Fish intake, cooking practices, and risk of prostate cancer: results from a multi-ethnic case-control study. Cancer Causes Control. 2012;23:405–420. doi: 10.1007/s10552-011-9889-2. [DOI] [PubMed] [Google Scholar]
- 8.Müller T, Gromoll J, Simoni M. Absence of exon 10 of the human luteinizing hormone (LH) receptor impairs LH, but not human chorionic gonadotropin action. J Clin Endocrinol Metab. 2003;88:2242–2249. doi: 10.1210/jc.2002-021946. [DOI] [PubMed] [Google Scholar]
- 9.Piersma D, Verhoef-Post M, Look MP, Utterlinden AG, Pols HA, Berns EM, Themmen AP. Polymorphic variations in exon 10 of the luteinizing hormone receptor: functional consequences and associations with breast cancer. Mol Cell Endocrinol. 2007;276:63–70. doi: 10.1016/j.mce.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Simoni M, Tuttelmann F, Michel C, Böckenfeld Y, Nieschlag E, Gromoll J. Polymorphisms of the luteinizing hormone/chorionic gonadotropin receptor gene: association with maldescended testes and male infertility. Pharmacogenet Genomics. 2008;18:193–200. doi: 10.1097/FPC.0b013e3282f4e98c. [DOI] [PubMed] [Google Scholar]
- 11.Kristiansen W, Aschim EL, Andersen JM, Witczak SD, Haugen TB. Variations in testosterone pathway genes and susceptibility to testicular cancer in Norwegian men. Int J Androl. 2012;35:819–827. doi: 10.1111/j.1365-2605.2012.01297.x. [DOI] [PubMed] [Google Scholar]
- 12.Mongan NP, Hughes IA, Lim HN. Evidence that luteinizing hormone receptor polymorphisms may contribute to male undermasculinisation. Eur J Endocrinol. 2002;147:103–107. doi: 10.1530/eje.0.1470103. [DOI] [PubMed] [Google Scholar]
- 13.Huggins C, Stevens RE, Hodges CV. The effect of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 14.Salonia A, Abdollah F, Capitanio U, Suardi N, Briganti A, Gallina A, Bolombo R, Ferrai M, Catagna G, Rigatti P, Montorsi F. Serum sex steroids depict a nonlinear u-shaped association with high-risk prostate cancer at radical prostatectomy. Clin Cancer Res. 2012;18:3648–3657. doi: 10.1158/1078-0432.CCR-11-2799. [DOI] [PubMed] [Google Scholar]
- 15.Taira AV, Merrick GS, Galbreath RW, Butler WM, Wallner KE, Allen ZA, Lief JH, Adamovich E. Pretreatment serum testosterone and androgen deprivation: effect on disease recurrence and overall survival in prostate cancer patients treated with brachytherapy. Int J Radiat Oncol Biol Phys. 2009;74:1143–1149. doi: 10.1016/j.ijrobp.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Xylinas E, Ploussard G, Durand X, Fabre A, Salomon L, Allory Y, Vordos D, Hoznek A, Abbour CC, de la Taille A. Low pretreatment total testosterone (<3 ng/ml) predicts extraprostatic disease in prostatatectomy specimens from patients with preoperative localized prostate cancer. BJU Int. 2011;107:1400–3. doi: 10.1111/j.1464-410X.2010.09816.x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Cruz E, Piqueras M, Huguet J, Peri L, Izquierdo L, Musquera M, Franco A, Alvarez-Vijande R, Ribal MJ, Alcaraz A. Low testosterone levels are related to poor prognosis factors in men with prostate cancer prior to treatment. BJU Int. 2012;110:E541–6. doi: 10.1111/j.1464-410X.2012.11232.x. [DOI] [PubMed] [Google Scholar]
- 18.Roder MA, Christensen IJ, Berg KD, Gruschy L, brasso K, Iversen P. Serum testosterone level as a predictor of biochemical failure after radical prostatectomy for localized prostate cancer. BJU Int. 2012;109:520–524. doi: 10.1111/j.1464-410X.2011.10335.x. [DOI] [PubMed] [Google Scholar]


