Abstract
Background
Von Willebrand factor (VWF) is a multimeric protein that binds platelets and collagen, facilitating hemostasis at sites of vessel injury. Measurement of VWF multimer distribution is critical for diagnosis of variant von Willebrand disease (VWD), particularly types 2A and 2B, but the typical measurement by gel electrophoresis is technically difficult and time consuming. A comparison of VWF collagen binding (VWF:CB) and VWF multimer distribution was performed to evaluate the utility of VWF:CB as a diagnostic test.
Methods
Participants were enrolled in the Zimmerman Program for the Molecular and Clinical Biology of VWD. VWF:CB was analyzed with type III collagen and multimer distribution by agarose gel electrophoresis. The study population included 146 healthy controls, 351 individuals with type 1 VWD, and 77 with type 2 VWD. Differences between normal and abnormal multimer groups were assessed with Mann-Whitney tests.
Results
The mean VWF:CB/VWF antigen ratio was 1.10 for individuals with normal multimer distribution and 0.51 for those with abnormal multimer distribution (P<0.001). Sensitivity of VWF:CB for multimer abnormalities was 100% for healthy controls, 99% for type 1, and 100% for type 2A and type 2B VWD using a VWF:CB/VWF antigen cutoff ratio of 0.6, and decreased to 99% for all with a ratio of 0.7. With the exception of individuals with novel or unclassified mutations, the VWF:CB was able to correctly categorize participants with variant VWD.
Conclusions
These findings suggest VWF:CB may substitute for multimer distribution in initial VWD testing, although further studies are needed to validate its clinical utility.
Keywords: von Willebrand disease, von Willebrand factor, collagen
Introduction
The function of von Willebrand factor (VWF) is dependent on the presence of high molecular weight multimers. The monomeric protein is co-translationally synthesized with C-terminal dimerization in the endoplasmic reticulum, then sent to the Golgi apparatus where N-terminal multimerization occurs (1). The 225 kD dimeric unit thus circulates in a multimeric structure of > 20,000 kD capable of structural modification under shear stress, for the purpose of recruiting platelets to sites of vascular injury (2, 3). Binding sites for platelet GPIb in the VWF A1 domain and for collagen in the VWF A1 and A3 domains facilitate this function.
Types 2A and 2B VWD, both lacking high molecular weight multimers, are characterized by excessive mucosal bleeding. Measurement of VWF multimer distribution is critical for accurate diagnosis of these subtypes of VWD to guide effective treatment (4). Type 1 VWD, characterized by low levels of VWF but a normal multimer distribution, typically responds to desmopressin, while replacement of VWF may be required for treatment of types 2A and 2B VWD (5).
Multimer distribution is classically analyzed by gel electrophoresis (6). Studies have demonstrated that VWF collagen binding (VWF:CB) can serve as a surrogate measure for the presence of high molecular weight multimers (7–9). VWF:CB to VWF antigen (VWF:CB/VWF:Ag) ratios of less than 0.6 or 0.7 have been considered to indicate abnormal results, with ratios above that presumed to represent a normal multimer distribution (8, 10). In clinical practice, however, collagen binding assays are not typically performed as part of the routine workup for VWD.
To clarify the diagnostic role of VWF:CB, we compared the VWF multimer distribution with VWF:CB for a population of healthy controls and individuals with VWD enrolled in the Zimmerman Program for the Molecular and Clinical Biology of VWD (Zimmerman Program). The results show that the type III collagen binding assay can substitute for electrophoretic analysis of VWF multimer distribution as a part of the initial workup of VWD.
Methods
Study Population
Informed consent was obtained for all participants following approval of the human research protocol by the Institutional Review Boards of the participating institutions. Healthy controls with no pre-existing diagnosis of a bleeding disorder were enrolled as a part of the Zimmerman Program from the local population of each of seven primary centers (Atlanta, Detroit, Iowa City, Indianapolis, Milwaukee, New Orleans, and Pittsburgh). VWD participants from eight primary centers and from numerous secondary centers (listed in the supplemental appendix) were enrolled if they had a pre-existing diagnosis of VWD, of any type, as determined by the treating physician at each center. The study population included 146 healthy controls, 351 individuals with type 1 VWD, and 77 with type 2 VWD (Table 1). Several participants had study laboratory findings that were not consistent with the original diagnosis, requiring reclassification as detailed below.
Table 1.
Zimmerman program multimer distribution and VWF:CB/VWF:Ag ratios by VWD diagnosis.
| VWF:CB/VWF:Ag ratio | VWF:RCo/VWF:Ag ratio* | ||||
|---|---|---|---|---|---|
|
| |||||
| # participants | Mean ± 1 SD | Median (range) | Mean ± 1 SD | Median (range) | |
| All controls | 146 | 1.06 ± 0.14 | 1.05 (0.69–1.32) | 0.99 ± 0.19 | 1.01 (0.42–1.49) |
| Normal multimer | 144 | 1.06 ± 0.14 | 1.05 (0.69–1.32) | 1.00 ± 0.19 | 1.02 (0.48–1.49) |
| Abnormal multimer | 2 | 0.86 ± 0.09 | 0.86 (0.80–0.92) | 0.50 ± 0.10 | 0.50 (0.42–0.57) |
| Type 1 | 351 | 1.11 ± 0.17 | 1.12 (0.5–1.82) | 1.00 ± 0.24 | 1.00 (0.16–1.9) |
| Normal multimer | 342 | 1.12 ± 0.17 | 1.13 (0.5–1.82) | 1.01 ± 0.24 | 1.00 (0.23–1.9) |
| Abnormal multimer | 9 | 0.93 ± 0.13 | 0.98 (0.69–1.08) | 0.70 ± 0.28 | 0.74 (0.16–1.13) |
| Type 2A | 36 | 0.44 ± 0.30 | 0.31 (0.1–0.95) | 0.39 ± 0.23 | 0.34 (0.11–1.13) |
| Normal multimer | 2 | 0.87 ± 0.03 | 0.87 (0.85–0.89) | 0.47 ± 0.12 | 0.47 (0.38–0.56) |
| Abnormal multimer | 34 | 0.41 ± 0.28 | 0.29 (0.1–0.95) | 0.38 ± 0.23 | 0.31 (0.11–1.13) |
| Type 2B | 17 | 0.40 ± 0.14 | 0.43 (0.19–0.61) | 0.55 ± 0.27 | 0.47 (0.16–1.11) |
| Normal multimer | 0 | NA | NA | NA | NA |
| Abnormal multimer | 17 | 0.40 ± 0.14 | 0.43 (0.19–0.61) | 0.55 ± 0.27 | 0.47 (0.16–1.11) |
| Type 2M | 17 | 1.05 ± 0.12 | 1.00 (0.86–1.32) | 0.57 ± 0.19 | 0.54 (0.34–1.00) |
| Normal multimer | 16 | 1.06 ± 0.12 | 1.04 (0.86–1.32) | 0.58 ± 0.20 | 0.54 (0.34–1.00) |
| Abnormal multimer | 1 | 0.95 | 0.95 | 0.53 | 0.53 |
| Type 2N | 7 | 1.30 ± 0.56 | 1.10 (1.03–2.56) | 1.06 ± 0.22 | 1.02 (0.78–1.37) |
| Normal multimer | 7 | 1.30 ± 0.56 | 1.10 (1.03–2.56) | 1.06 ± 0.22 | 1.02 (0.78–1.37) |
| Abnormal multimer | 0 | NA | NA | NA | NA |
VWF:RCo values <10 IU/dL, the lower limit of detection in our laboratory, were assigned an arbitrary value of 5 for calculation purposes.
VWF testing
Blood was collected in 3.2% sodium citrate and frozen plasma shipped to a central reference laboratory (Hemostasis Reference Laboratory at the BloodCenter of Wisconsin) for VWF testing. VWF antigen (VWF:Ag), ristocetin cofactor activity (VWF:RCo), collagen binding with type III collagen (VWF:CB), and multimer distribution were performed on all samples as previously described (11). VWF:CB was performed on all index cases and healthy controls by an ELISA assay using type III human placental collagen (Southern Biotech) coated at 1 μg/mL in carbonate coating buffer (15 mmol/L sodium carbonate, 35 mmol/L sodium bicarbonate, 3 mmol/L sodium azide, pH 9.5) on Immulon Ib plates (Thermo Scientific). A polyclonal anti-VWF antibody (DAKO) was used for detection (12).
Multimer analysis was performed on all index cases and healthy controls by electrophoresis through a 0.65% high gelling temperature (HGT(P)) agarose gel (Lonza) containing 0.1% Lithium Dodecyl Sulfate (LiDS) at 120 V for 4 hours in a 0.1 mol/L Tris, 0.15 mol/L glycine, 0.1% LiDS (w/v) running buffer (13). Samples were transferred to an Immobilon-P membrane (Millipore), and VWF subsequently detected by Western blot using a monoclonal anti-VWF antibody (DAKO).
Quantitative multimer analysis was performed by performing gel electrophoresis as described above and transferring proteins to nitrocellulose (BioRad) by electroblotting at 5V for 2 hours in 0.05 mol/L sodium phosphate buffer, pH 7.8. Membranes were blocked with 0.05 mol/L Tris buffered saline containing 1% bovine serum albumin (w/v), pH 8.0, incubated with anti-VWF monoclonal antibodies AvW-1, AvW-15, and 105.4 (Hybridoma Core Laboratory, BloodCenter of Wisconsin) in 0.05 mol/L Tris buffered saline containing 0.05% Tween-20, pH 8.0, followed by peroxidase conjugated goat anti-mouse IgG (H+L) (Thermo Fisher Scientific). Protein was detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and visualized with the Fujifilm Luminescent Image Analyzer LAS-3000 (Fujifilm). Densitometry was performed using Multi Gauge Ver. 3.0 analysis software (Fujifilm).
An additional sample was collected in EDTA for DNA extraction. Full length VWF gene sequencing was performed for all index cases and healthy controls, including all intron-exon boundaries (14). A bleeding questionnaire was administered to each participant, including the healthy controls, utilizing questions sufficient to determine a bleeding score as published by the European Union group (15).
Statistical analysis
Statistical analysis was performed using Stata 11.1 (StataCorp LP). Mann-Whitney tests were used to compare normal and abnormal multimer groups. ROC curves were generated separately for the healthy control group, the type 1 VWD group, the type 2A VWD group, and the type 2B VWD group.
Results
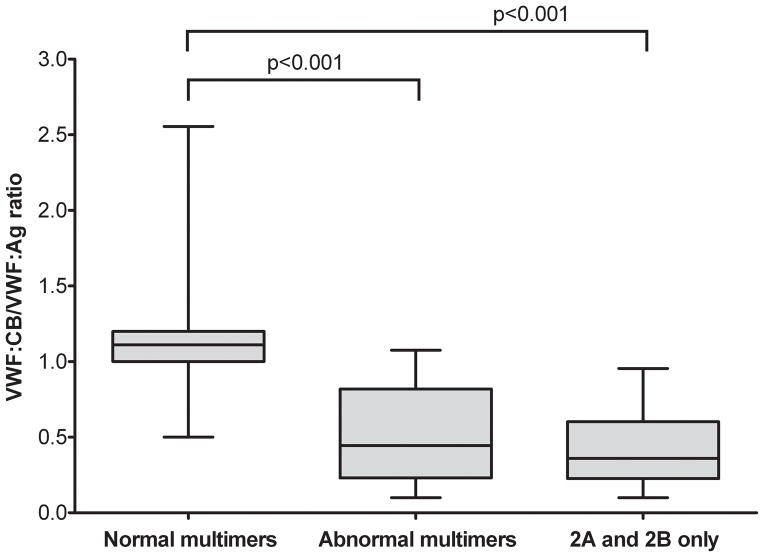
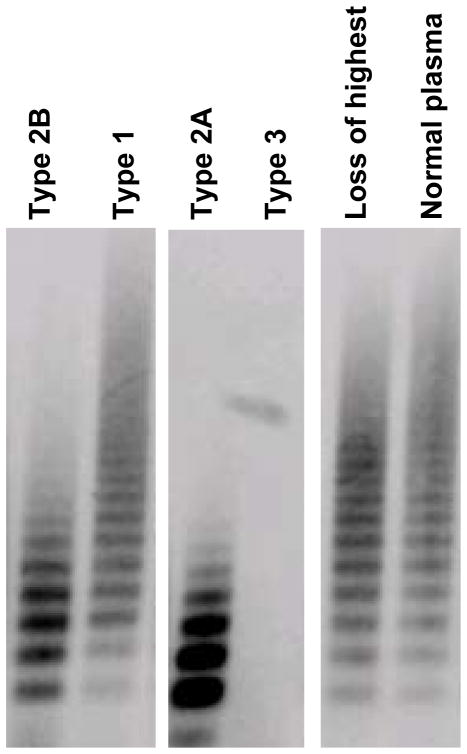
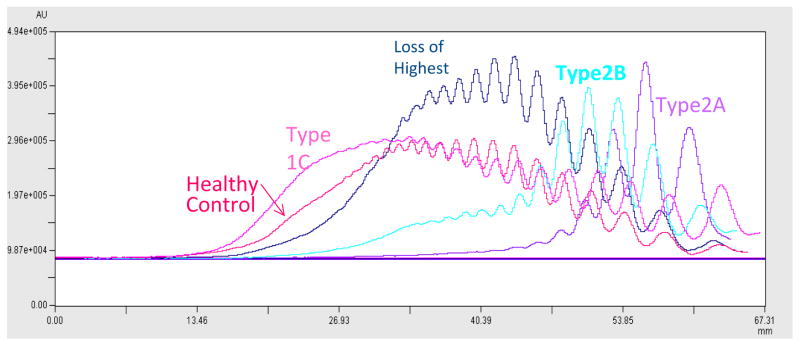
The study population included both healthy controls and individuals with VWD as detailed in Table 1. Individuals with VWD were enrolled based on a pre-existing diagnosis of VWD as made by their treating physician prior to study enrollment. Of these, 511 had a normal multimer distribution and 63 had an abnormal multimer distribution. There was a statistically significant difference in VWF:CB between those with a normal multimer distribution and those with an abnormal multimer distribution, with p < 0.001 (figure 1). Of those with abnormal multimers, 51 had a diagnosis of type 2A or 2B VWD while the remaining 12 would have been expected to have a normal multimer distribution given the lack of a documented type 2A or 2B VWD mutation. Some of these represent subtle abnormalities in multimer distribution with loss of only the highest molecular weight multimers, while others are either unclassified or novel mutations, as discussed below. Loss of only the highest multimers has been reported with sample processing or transport-related artifacts and may not represent true VWD (7). Examples of the different multimer distributions observed in this study are shown in figure 2. Quantitative analysis of the different distributions is shown in figure 3.
Figure 1. Collagen binding correlates with multimer distribution.
This graph shows VWF:CB/VWF:Ag ratios on the y axis for individuals enrolled in the Zimmerman Program with either normal multimer distribution (n=511), abnormal multimer distribution (n=63), or a diagnosis of either type 2A or 2B VWD (n=53). VWF:CB/VWF:Ag was significantly different (P<0.001) for those with normal multimer distribution as compared to either those with abnormal multimer distribution or those with type 2A or 2B VWD. The box extends from the 25% to the 75% percentile and the line represents the median for each group. Whiskers represent the highest and lowest value.
Figure 2. Normal and abnormal multimer distributions seen in Zimmerman program samples.
This figure shows characteristic multimer patterns observed in our study. These include type 2B VWD with loss of high molecular weight multimers, type 1 VWD with full spectrum of multimers, type 2A VWD, with loss of high and intermediate molecular weight multimers, type 3 VWD with no visible multimers, a sample with loss of the highest molecular weight multimers, and a normal plasma sample with all multimers present.
Figure 3. Densitometry analysis of multimer distribution.
Densitometry was measured for a healthy control and four individuals, one each having type 1C, type 2A, type 2B, and loss of only the highest multimers. Tracings for the individuals with type 2 VWD clearly demonstrate lack of the high molecular weight multimers, whereas the participant only missing the highest molecular weight multimers appears closer to the healthy control.
Collagen binding with type III collagen was highly sensitive to defects in multimer distribution (table 2). We examined cut-off ratios of 0.6, 0.7, and 0.8. The sensitivity was 99% using the lowest cutoff value (0.6) but only dropped to 97% using the highest cutoff value (0.8). These results included those individuals with abnormal multimer distribution but normal collagen binding who lacked VWF gene mutations or have novel mutations. ROC curves were generated for each group and yielded high correlation, with the area under the ROC curve 0.90 for the healthy controls, 0.82 for the type 1 VWD individuals, 0.98 for the type 2A VWD individuals, and 0.99 for the type 2B VWD individuals.
Table 2.
Sensitivity of VWF:CB/VWF:Ag ratio for detection of multimer abnormalities.
| VWF:CB/VWF:Ag cutoff | Healthy Controls | Type 1 | Type 2A | Type 2B |
|---|---|---|---|---|
| 0.6 | 100% | 99% | 100% | 100% |
| 0.7 | 99% | 99% | 99% | 99% |
| 0.8 | 97% | 97% | 97% | 97% |
For healthy controls and for type 1 VWD, a normal VWF:CB/VWF:Ag ratio and a normal multimer distribution are expected. For types 2A and 2B VWD, a decreased VWF:CB/VWF:Ag ratio and an abnormal multimer distribution are expected.
With regard to correct classification as type 2A or 2B VWD, the sensitivity of the VWF:CB/VWF:Ag ratio was 100% when all cases with an unclear phenotype were excluded, as discussed in detail below. All healthy controls had normal VWF:CB/VWF:Ag ratios, and all individuals with type 2A and 2B VWD had low VWF:CB/VWF:Ag ratios. Specificity was only 99% for the individuals with type 1 VWD, owing to 4 individuals with a low VWF:CB/VWF:Ag ratio (all ≤5 IU/dL) but normal multimer distribution and no documented type 2 VWD mutation on DNA sequencing. Omission of individuals with low VWF:Ag would have raised the specificity to 100%. No difference was seen in VWF:CB/VWF:Ag ratio for individuals with type 2A VWD as compared to those with type 2B VWD (p=NS).
VWF:CB and multimer distribution in healthy controls
VWF:CB was then compared to multimer distribution for each group of participants based on their clinical diagnosis. 146 healthy controls were available for analysis. Only 2 (1.4%) had an abnormal multimer distribution, both with loss only of the highest molecular weight multimers, a pattern associated in our laboratory with sample processing artifacts (7). All controls had normal VWF:CB/VWF:Ag ratios, as did both individuals who had abnormal multimer distribution. The lowest VWF:CB/VWF:Ag ratio seen in this group was 0.69, consistent with the use of a cutoff ratio of 0.6–0.7 for diagnosis of variant VWD. Both individuals with abnormal multimer distribution had low VWF:RCo/VWF:Ag ratios (0.42 and 0.57) but normal bleeding scores (of 0 and −1, respectively) as evaluated by the European Union bleeding score questionnaire (15). Neither had a mutation in the VWF coding region.
VWF:CB and multimer distribution in type 1 VWD
Next, VWF:CB was compared to multimer distribution for individuals with type 1 VWD. Of the 342 participants with type 1 VWD with normal multimers, four had a VWF:CB/VWF:Ag ratio of <0.7, but 3 of these had very low VWF:Ag (≤5 IU/dL), where the sensitivity of the ratio would be expected to be less optimal. Nine individuals with type 1 VWD had an abnormal multimer distribution, with loss of the highest molecular weight multimers (6 participants), loss of all high molecular weight multimers (1 participant) or a shift from high to low molecular weight multimers with relatively increased staining of the low molecular weight bands (2 participants). Of the seven individuals with loss of high molecular weight multimers, 5 had known type 1 VWD mutations and normal VWF:CB/VWF:Ag ratios, possibly representing sample processing or transport related artifact (7), as no multimer issues have been previously reported for these mutations (table 3). One individual had a low VWF:RCo/VWF:Ag ratio and an unclassified mutation, p.R1374H, which has been alternately classified as type 1, type 2A, and type 2M VWD (16–18). One participant had no coding sequence mutation found. Two individuals had a full spectrum of multimers with a shift from high to low molecular weight multimers; both with novel A1 domain mutations (p.L1365P and p.V1934G) that are currently being investigated. The significance of this multimer pattern is unclear. None of the individuals with type 1 VWD with abnormal multimer distribution had mutations exclusively associated with type 2 VWD.
Table 3.
Sequence variations found in Zimmerman Program type 1 and type 2A VWD subjects with inconsistent multimer results and normal VWF:CB.
| Sequence Variation | # of individuals | VWD type(s) * | Multimer distribution |
|---|---|---|---|
| p.R924Q† | 2 | Type 1 | loss of HMWM |
| p.R1374H | 1 | Type 1 (2A, 2M) | loss of HMWM |
| p.Y1584C† | 2 | Type 1 | loss of HMWM |
| p.C2693Y‡ | 1 | Type 1 | loss of HMWM |
| p.L1365P‡ | 1 | Type 1 | shift high to low |
| p.V1934G†‡ | 1 | Type 1 | shift high to low |
| p.M1304R‡ | 1 | Type 2A (2M) | Normal, shift high to low |
| p.R1374C† | 1 | Type 2A (1, 2M) | Normal, shift high to low, loss of HMWM |
| p.C524Y†‡ | 1 | Type 2A | shift high to low |
| p.Y1349C‡ | 1 | Type 2A | shift high to low |
| p.R2287W† | 1 | Type 2A (1) | loss of HMWM |
Sequence variations are listed as the VWD type determined at time of enrollment in the Zimmerman Program for affected individuals. VWD types in parentheses represent other reported classifications for that sequence variation;
also seen in Zimmerman Program subject(s) with type 1 VWD and normal multimers;
novel mutation not previously described; HMWM=loss of high molecular weight multimers; shift high to low=shift from high molecular weight multimers to low molecular weight multimers.
VWF:CB and multimer distribution in type 2 VWD
The comparison of multimer distribution to VWF:CB for individuals with type 2A VWD showed that the vast majority had loss of high and/or intermediate molecular weight multimers or a shift from high to low molecular weight bands, as would be expected for this diagnosis. Six participants with loss of high molecular weight multimers had VWF:CB/VWF:Ag ratios >0.7, but none of those individuals had VWF mutations exclusively associated with type 2A VWD (table 3). Two had the p.R1374C mutation, which was seen in individuals with type 1, type 2A, and type 2M VWD enrolled in our study. One had a p.M1304R mutation which has also been seen in patients categorized as both type 2A and type 2M VWD (Jorge Di Paola, University of Colorado, personal communication). One had a novel mutation, p.C524Y, which has not yet been characterized by our group. One had the p.R2287W mutation, a known type 1 VWD mutation normally associated with a normal multimer pattern. The last individual with normal collagen binding had no mutation found and also may not represent true type 2A VWD, particularly given that this individual also had a normal VWF:RCo/VWF:Ag ratio. Two individuals with type 2A VWD had normal multimer distribution, both with unclassified mutations (one with p.M1304R and one with p.R1374C). Characterization of these novel mutations is in progress as they may not represent typical type 2A VWD and therefore might be better classified in a different category (or termed “unclassifiable”). All participants with known type 2A VWD mutations had abnormal multimer distribution and VWF:CB/VWF:Ag ratios less than 0.7.
All of the 17 participants with type 2B VWD had low VWF:CB/VWF:Ag ratios and corresponding abnormal multimer distribution, but 4 of the individuals with type 2B had VWF:RCo/VWF:Ag ratios greater than 0.7. This suggests that either von Willebrand factor collagen binding or multimer analysis is required to differentiate this group, as the VWF:RCo/VWF:Ag ratio alone would have been insufficient for diagnosis. All participants in this category had known type 2B VWD mutations on DNA sequencing.
All of the 17 individuals with type 2M VWD enrolled in the Zimmerman program had normal VWF:CB/VWF:Ag ratios. Seven individuals with type 2N VWD were enrolled, none of whom had abnormal multimer distribution or decreased VWF:CB/VWF:Ag ratio.
Discussion
This study shows that, in a population of previously diagnosed individuals with type 2A and 2B VWD, the VWF:CB assay provides a sensitive screen for detection of abnormal multimer distribution. This is important because of both the technical challenges in testing multimer distribution and the subjective nature of this non-quantitative assay. No participants in this study would have been erroneously diagnosed as normal or as type 1 VWD on the basis of the VWF:CB assay if they had a type 2A or type 2B VWD mutation. Several individuals with type 1 VWD had low VWF:CB/VWF:Ag ratios, primarily due to very low VWF:Ag values (≤5 IU/dL); these individuals would then require further evaluation. However, as screening tests should be more inclusive, this likely would mean repeat testing for a few patients rather than misdiagnosis of VWD in an unaffected person. An alternate approach would be to omit reporting VWF:CB/VWF:Ag ratios for patients whose VWF:Ag is significantly reduced.
We are not the first to report on the utility of the VWF:CB assay in VWD diagnosis. In a recent review, the VWF:CB assay was championed by Favaloro for its ability to screen for multimer defects and reduce misdiagnosis of type 2 VWD (19). Federici and colleagues utilized the combination of VWF:RCo/VWF:Ag and VWF:CB/VWF:Ag ratios to categorize patients as type 1, type 2A, type 2B, or type 2M VWD (8). Adcock and colleagues also reported efficacy using the VWF:CB/VWF:Ag ratio to distinguish abnormal multimer distribution in types 2A and 2B VWD (9). It should be noted, however, that variability in collagen preparations and collagen coating techniques may hamper interpretation of the VWF:CB (10, 20, 21). We chose a preparation of type III collagen that has been well validated in our lab. Other groups have advocated for a combination of types I and III collagen (20).
One of the limitations of our study is that it used a single collagen source, and a single multimer technique, which may limit generalizability of these results. Another limitation is the retrospective nature of the analysis, in that participants had a pre-existing diagnosis of VWD prior to study entry. A number of abnormal multimer distributions were seen in participants with normal VWF:Ag, VWF:CB, and no corresponding type 2A or type 2B VWD mutation. Quantitative multimer results for similar samples with only loss of the highest molecular weight multimers were normal, suggesting transport related artifact or sample processing artifact as a possible explanation (7). The current study design prevented confirmation of this hypothesis, but future work will incorporate repeat sampling of participants and additional collagen coating conditions.
Multimer distribution is assessed by running plasma samples on an agarose gel, transferring to a nitrocellulose membrane, detecting the VWF present and subjective assessment of results (13). Results take several days to obtain and are non-quantitative. This is a difficult, labor-intensive, and costly technique. In contrast, the VWF collagen binding assay is ELISA based, using purified collagen coated on an ELISA plate (22). Results are available within hours and are quantitative. The technique is easy, and minimal labor is required, with the potential for automation of the assay. Even with the recent introduction of quantitative multimer techniques (23), VWF:CB remains more efficient and less costly. In our laboratory, the VWF:CB can be performed for less than half the cost of multimer distribution. Use of international, cross-referenced plasma samples such as those offered by the WHO allows collagen binding results to be standardized across laboratories (24).
This suggests that the real-world application of the VWF:CB may actually improve the detection of variant VWD, avoiding misclassification of patients as variant VWD when multimer distribution is normal. Pre-analytical factors such as sample processing may result in loss of the highest molecular weight multimers, leading to a report of an abnormal finding, potential misdiagnosis, or costly additional testing. Acquired multimer abnormalities, as seen in patients with ventricular septal defect, aortic stenosis, or other cardiac abnormalities, may also lead to loss of the highest molecular weight multimers in the absence of a genetic defect in VWF (25–27). In addition, the VWF:CB will detect mutations in VWF-collagen interactions, a functional defect that may not be detected by either analysis of multimer distribution or VWF:RCo. Several such mutations that exclusively or disproportionately affect VWF:CB have been reported (12, 28–30).
The normal VWF:RCo/VWF:Ag ratio in several of the patients with type 2B VWD suggests that VWF:CB may actually outperform the VWF:RCo in this group. In addition, the VWF:RCo assay is known to have a high coefficient of variation (CV), unlike the VWF:CB (31–33). CVs in our laboratory for the VWF:CB are ≤5%. One recent study demonstrated greater sensitivity to loss of high molecular weight multimers with collagen binding assays as compared to monoclonal antibody-based VWF activity assays, suggesting that such a VWF activity assay cannot substitute for either VWF:RCo or VWF:CB in assessment of multimer distribution (34).
A possible improvement in the diagnostic evaluation for VWD would combine the VWF:Ag, VWF:RCo, and VWF:CB as an initial screen (likely with the addition of factor VIII activity). Discrepancy between either VWF:Ag and VWF:RCo or VWF:Ag and VWF:CB might then prompt analysis of multimer distribution, but such analysis could be reserved for those patients with an abnormality on the initial testing. Our results corroborate the hypothesis that no patients, at least in our study, would have been missed by this mechanism. However, prospective analysis of this approach and standardization of collagen binding assays are required to validate elimination of the VWF multimer distribution from the initial diagnostic panel.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Zimmerman Program investigators, staff, and subjects who participated in this study. This work was supported by the National Institutes of Health K08 grant HL102260 (VF) and program project grant HL081588 (RRM, SLH). This work was also supported in part by the Midwest Athletes Against Childhood Cancer, the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- VWF
von Willebrand factor
- VWD
von Willebrand disease
- VWF:CB
VWF collagen binding
- VWF:Ag
VWF antigen
- VWF:RCo
VWF ristocetin cofactor activity
- HGT
high gelling temperature
- LiDS
lithium dodecyl sulfate
- HMWM
high molecular weight multimers
References
- 1.Haberichter SL, Shi Q, Montgomery RR. Regulated release of VWF and FVIII and the biologic implications. Pediatr Blood Cancer. 2006;46:547–53. doi: 10.1002/pbc.20658. [DOI] [PubMed] [Google Scholar]
- 2.Meyer D, Obert B, Pietu G, Lavergne JM, Zimmerman TS. Multimeric structure of factor VIII/von willebrand factor in von willebrand’s disease. J Lab Clin Med. 1980;95:590–602. [PubMed] [Google Scholar]
- 3.Dent JA, Galbusera M, Ruggeri ZM. Heterogeneity of plasma von willebrand factor multimers resulting from proteolysis of the constituent subunit. J Clin Invest. 1991;88:774–82. doi: 10.1172/JCI115376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budde U, Pieconka A, Will K, Schneppenheim R. Laboratory testing for von willebrand disease: Contribution of multimer analysis to diagnosis and classification. Semin Thromb Hemost. 2006;32:514–21. doi: 10.1055/s-2006-947866. [DOI] [PubMed] [Google Scholar]
- 5.Federici AB, Mazurier C, Berntorp E, Lee CA, Scharrer I, Goudemand J, et al. Biologic response to desmopressin in patients with severe type 1 and type 2 von willebrand disease: Results of a multicenter european study. Blood. 2004;103:2032–8. doi: 10.1182/blood-2003-06-2072. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri ZM, Zimmerman TS. Variant von willebrand’s disease: Characterization of two subtypes by analysis of multimeric composition of factor VIII/von willebrand factor in plasma and platelets. J Clin Invest. 1980;65:1318–25. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favaloro EJ, Facey D, Grispo L. Laboratory assessment of von willebrand factor. use of different assays can influence the diagnosis of von willebrand’s disease, dependent on differing sensitivity to sample preparation and differential recognition of high molecular weight VWF forms. Am J Clin Pathol. 1995;104:264–71. doi: 10.1093/ajcp/104.3.264. [DOI] [PubMed] [Google Scholar]
- 8.Federici AB, Canciani MT, Forza I, Cozzi G. Ristocetin cofactor and collagen binding activities normalized to antigen levels for a rapid diagnosis of type 2 von willebrand disease--single center comparison of four different assays. Thromb Haemost. 2000;84:1127–8. [PubMed] [Google Scholar]
- 9.Adcock DM, Bethel M, Valcour A. Diagnosing von willebrand disease: A large reference laboratory’s perspective. Semin Thromb Hemost. 2006;32:472–9. doi: 10.1055/s-2006-947860. [DOI] [PubMed] [Google Scholar]
- 10.Favaloro EJ. An update on the von willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin Thromb Hemost. 2007;33:727–44. doi: 10.1055/s-2007-1000364. [DOI] [PubMed] [Google Scholar]
- 11.Flood VH, Gill JC, Morateck PA, Christopherson PA, Friedman KD, Haberichter SL, et al. Common VWF exon 28 polymorphisms in african americans affecting the VWF activity assay by ristocetin cofactor. Blood. 2010;116:280–6. doi: 10.1182/blood-2009-10-249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flood VH, Lederman CA, Wren JS, Christopherson PA, Friedman KD, Hoffmann RG, et al. Absent collagen binding in a VWF A3 domain mutant: Utility of the VWF:CB in diagnosis of VWD. J Thromb Haemost. 2010;8:1431–3. doi: 10.1111/j.1538-7836.2010.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery RR, Hathaway WE, Johnson J, Jacobson L, Muntean W. A variant of von willebrand’s disease with abnormal expression of factor VIII procoagulant activity. Blood. 1982;60:201–7. [PubMed] [Google Scholar]
- 14.Bellissimo DB, Christopherson PA, Flood VH, Gill JC, Friedman KD, Haberichter SL, et al. VWF mutations and new sequence variations identified in healthy controls are more frequent in the african-american population. Blood. 2012;119:2135–40. doi: 10.1182/blood-2011-10-384610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, et al. A quantitative analysis of bleeding symptoms in type 1 von willebrand disease: Results from a multicenter european study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 16.Castaman G, Eikenboom JC, Rodeghiero F, Briet E, Reitsma PH. A novel candidate mutation (Arg611-->His) in type I ‘platelet discordant’ von willebrand’s disease with desmopressin-induced thrombocytopenia. Br J Haematol. 1995;89:656–8. doi: 10.1111/j.1365-2141.1995.tb08383.x. [DOI] [PubMed] [Google Scholar]
- 17.Hilbert L, Gaucher C, Mazurier C. Identification of two mutations (Arg611Cys and Arg611His) in the A1 loop of von willebrand factor (vWF) responsible for type 2 von willebrand disease with decreased platelet-dependent function of vWF. Blood. 1995;86:1010–8. [PubMed] [Google Scholar]
- 18.Goodeve A, Eikenboom J, Castaman G, Rodeghiero F, Federici AB, Batlle J, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von willebrand disease in the european study, molecular and clinical markers for the diagnosis and management of type 1 von willebrand disease (MCMDM-1VWD) Blood. 2007;109:112–21. doi: 10.1182/blood-2006-05-020784. [DOI] [PubMed] [Google Scholar]
- 19.Favaloro EJ. Toward a new paradigm for the identification and functional characterization of von willebrand disease. Semin Thromb Hemost. 2009;35:60–75. doi: 10.1055/s-0029-1214149. [DOI] [PubMed] [Google Scholar]
- 20.Favaloro EJ. Collagen binding assay for von willebrand factor (VWF:CBA): Detection of von willebrands disease (VWD), and discrimination of VWD subtypes, depends on collagen source. Thromb Haemost. 2000;83:127–35. [PubMed] [Google Scholar]
- 21.Mendelboum Raviv S, Szekeres-Csiki K, Jenei A, Nagy J, Shenkman B, Savion N, et al. Coating conditions matter to collagen matrix formation regarding von willebrand factor and platelet binding. Thromb Res. 2012;129:e29–35. doi: 10.1016/j.thromres.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 22.Brown JE, Bosak JO. An ELISA test for the binding of von willebrand antigen to collagen. Thromb Res. 1986;43:303–11. doi: 10.1016/0049-3848(86)90150-7. [DOI] [PubMed] [Google Scholar]
- 23.Haberichter SL, Jacobi PM, Flood VH, Christopherson PA, Gill JC, Bellissimo DB, et al. Quantitative analysis of VWF multimer structure: Discrimination between VWD subtypes [abstract] Blood. 2011;118:1215. [Google Scholar]
- 24.Hubbard AR, Hamill M, Beeharry M, Bevan SA, Heath AB SSC Sub-Committees on Factor VIII/Factor IX von Willebrand factor of ISTH. Value assignment of the WHO 6th international standard for blood coagulation factor VIII and von willebrand factor in plasma (07/316) J Thromb Haemost. 2011;9:2100–2. doi: 10.1111/j.1538-7836.2011.04471.x. [DOI] [PubMed] [Google Scholar]
- 25.Gill JC, Wilson AD, Endres-Brooks J, Montgomery RR. Loss of the largest von willebrand factor multimers from the plasma of patients with congenital cardiac defects. Blood. 1986;67:758–61. [PubMed] [Google Scholar]
- 26.Pareti FI, Lattuada A, Bressi C, Zanobini M, Sala A, Steffan A, et al. Proteolysis of von willebrand factor and shear stress-induced platelet aggregation in patients with aortic valve stenosis. Circulation. 2000;102:1290–5. doi: 10.1161/01.cir.102.11.1290. [DOI] [PubMed] [Google Scholar]
- 27.Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, et al. Acquired von willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–9. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 28.Ribba AS, Loisel I, Lavergne JM, Juhan-Vague I, Obert B, Cherel G, et al. Ser968Thr mutation within the A3 domain of von willebrand factor (VWF) in two related patients leads to a defective binding of VWF to collagen. Thromb Haemost. 2001;86:848–54. [PubMed] [Google Scholar]
- 29.Riddell AF, Gomez K, Millar CM, Mellars G, Gill S, Brown SA, et al. Characterization of W1745C and S1783A: 2 novel mutations causing defective collagen binding in the A3 domain of von willebrand factor. Blood. 2009;114:3489–96. doi: 10.1182/blood-2008-10-184317. [DOI] [PubMed] [Google Scholar]
- 30.Flood VH, Gill JC, Christopherson PA, Bellissimo DB, Friedman KD, Haberichter SL, et al. Critical von willebrand factor A1 domain residues influence type VI collagen binding. J Thromb Haemost. 2012;10:1417–24. doi: 10.1111/j.1538-7836.2012.04746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitchen S, Jennings I, Woods TA, Kitchen DP, Walker ID, Preston FE. Laboratory tests for measurement of von willebrand factor show poor agreement among different centers: Results from the united kingdom national external quality assessment scheme for blood coagulation. Semin Thromb Hemost. 2006;32:492–8. doi: 10.1055/s-2006-947863. [DOI] [PubMed] [Google Scholar]
- 32.Meijer P, Haverkate F. An external quality assessment program for von willebrand factor laboratory analysis: An overview from the european concerted action on thrombosis and disabilities foundation. Semin Thromb Hemost. 2006;32:485–91. doi: 10.1055/s-2006-947862. [DOI] [PubMed] [Google Scholar]
- 33.Favaloro EJ. Evaluation of commercial von willebrand factor collagen binding assays to assist the discrimination of types 1 and 2 von willebrand disease. Thromb Haemost. 2010;104:1009–21. doi: 10.1160/TH10-06-0360. [DOI] [PubMed] [Google Scholar]
- 34.Favaloro EJ, Bonar R, Chapman K, Meiring M, Funk Adcock D. Differential sensitivity of von willebrand factor (VWF) ‘activity’ assays to large and small VWF molecular weight forms: A cross-laboratory study comparing ristocetin cofactor, collagen-binding and mAb-based assays. J Thromb Haemost. 2012;10:1043–54. doi: 10.1111/j.1538-7836.2012.04729.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.