Abstract
Objective
Selective Serotonin Reuptake Inhibitors (SSRIs) are the most commonly prescribed medications for geriatric depression. The association of late-life depression and cognitive impairment has been well documented. However, there have been few placebo-controlled trials examining the impact of SSRIs on cognitive functioning.
Design
Pre-post neuropsychological data collected as part of an 8-week, double-blind, placebo-controlled trial of citalopram in depressed patients aged 75 years and older were used to examine change in cognitive functioning.
Setting
University affiliated outpatient psychiatry clinics.
Participants
One hundred seventy-four community dwelling men and women 75 years or older with non-psychotic unipolar depression.
Measurements
Neuropsychological assessments included mental status (Mini-Mental Status Exam), psychomotor speed (WAIS-III Digit Symbol Subtest), reaction time (Choice Reaction Time), visual-spatial skill (Judgment of Line Orientation), executive functioning (Stroop Color/Word Test), and memory (Buschke Selective Reminding Test).
Results
Differences in the pattern of change by treatment group depended on responder status. Citalopram non-responders were the only group to decline on verbal learning and psychomotor speed. Citalopram responders showed significant improvement in visuospatial functioning compared to non-responders in either condition, but their improvement was not greater than responders on placebo. Citalopram responders showed greater improvement on psychomotor speed than citalopram non-responders, but their improvement was not greater than placebo responders or non-responders.
Conclusions
Medication may have a deleterious effect on some aspects of cognition among patients age 75 and over who have not responded. This suggests that patients should not be maintained on a medication if they have not had an adequate response.
Keywords: cognitive functioning, cognitive impairment, geriatric depression, late-life depression, citalopram
Selective Serotonin Reuptake Inhibitors (SSRIs) are the first-line of treatment in the geriatric depressed due to the efficacy, safety and tolerability of its class. Cognitive impairment is common in late-life depression (LLD), particularly in memory (1–3), visuospatial functioning (2, 4), information processing speed (5, 6), and executive functioning (5, 7). It is important to consider the impact that antidepressant treatment can have on cognition when treating depressed older adults.
Research that has examined the impact of SSRIs on the cognitive functioning of depressed older adults has been inconclusive because most studies have been limited by methodological constraints including small sample size or lack of an age-matched control group for comparison (8–11). For instance, treatment of LLD with sertraline led to an improvement in short- and long-term memory storage and retrieval and speed of processing (11). Although these results suggest that some aspects of cognition (i.e. memory and processing speed) improve with antidepressant treatment, it is difficult to determine if the improvement was a function of repeat testing or medication because the design lacked a control group for comparison.
Studies using age-matched controls have shown that cognitive functioning of depressed older adults does not improve beyond the expected practice effect1 (12–14). For example, working and episodic memory, attention shifting, and processing speed did not improve following treatment with paroxetine to a greater degree than normal controls did with practice, regardless of responder status (14). Similarly, cognitive functioning showed no improvement beyond a practice effect among responders to either nortriptyline or paroxetine (13). These studies suggest that depressed older adults show little improvement as a function of treatment and cognitive impairment persists following an adequate trial of antidepressant medication.
Although such designs allow us to determine whether cognition changes as a function of antidepressant treatment, it does not allow us to conclude that the change (if any) is a result of treatment due to a lack of a placebo condition. However, there have been few placebo-controlled trials examining this issue. In one study, nortriptyline and phenelzine produced no change in cognition in depressed older adults when compared to placebo, and this effect did not depend on responder status (8). However, the small sample size and limited number of responders made it difficult to determine the impact of responder status on change in cognition. Furthermore, this study was restricted to the use of a TCA and MAO-I. In another study, patients taking duloxetine showed significant improvement in verbal learning and memory compared to the placebo group (15). Therefore, it is unclear what impact medication, SSRIs in particular, has on cognitive functioning.
The purpose of this study was to examine the impact of antidepressant treatment on change in cognitive functioning. To accomplish this aim, we used neuropsychological (NP) data collected as part of the Old-Old Depression Study (16), a large (n=174), randomized, double-blind, placebo-controlled trial of citalopram in depressed older adults (age > 75). These data provided us with the methodological strength to address two questions: 1) Do patients treated with citalopram show differential change in cognitive functioning over the 8 weeks when compared to patients treated with placebo? 2) Does change in cognitive performance depend on responder status? To our knowledge, this is the first attempt to approach these issues using a placebo-controlled trial of an SSRI in an old-old (>75 years old) depressed population.
METHOD
The procedures used in the multi-site, randomized, placebo-controlled trial (RCT) have been previously described (17–19). Briefly, 174 community dwelling men and women 75 years or older meeting DSM-IV criteria (based on SCID interview) for non-psychotic unipolar depression (single or recurrent) with a baseline 24-item Hamilton Rating Scale for Depression (HRSD) score ≥ 20 participated in this 8-week RCT. All patients began the trial with a one-week single-blind placebo lead-in with the baseline visit conducted at the end of the lead-in. Patients were randomized to citalopram 20 mg/d or matched placebo only if they continued to meet inclusion and exclusion criteria at the end of the placebo lead-in. At the end of week four, patients with a HRSD score > 10 had the dose increased to two pills per day, i.e., 40 mg of citalopram or 2 placebo pills.
Neuropsychological Test Battery
The test battery was designed to assess a number of cognitive functions pertinent to aging and major depression including mental status, psychomotor speed, reaction time, visual-spatial skill, attention, and memory. Three of the tests (Choice Reaction Time, Judgment of Line Orientation, Stroop) were presented on a Macintosh laptop computer and were written in the PsyScope programming language (20) whereas the other three tests (Mini-Mental State Exam, Buschke Selective Reminding Test, Digit Symbol) were administered by hand. The tests included the 30-item Folstein Mini-Mental State Exam (MMSE) (21) to estimate global cognitive functioning, the WAIS-III Digit Symbol Subtest (22) as a measure of psychomotor speed, the Choice Reaction Time (CRT) test adapted from Thorne, et al. (23), the Stroop Color/Word Test (24) to assess the response inhibition component of executive functioning, the Judgment of Line Orientation (JOLO) (25) as a measure of spatial judgment, and the Buschke Selective Reminding Test (SRT) (26) as a measure of verbal learning.
Missing Data
Missing data at baseline ranged from 0.6% on the MMSE and Buschke SRT to 9.8% on the JOLO and from 11.5% on the MMSE to 19.0% on the Stroop at follow-up. To accommodate missing data, we used multiple imputation using the PROC MI and MIANALYZE procedures in SAS. Multiple imputation is a simulation technique that replaces each missing datum with a set of m > 1 plausible values (27). This report is based on five imputed data sets (m =5), which is sufficient to obtain excellent results unless rates of missing data are exceptionally high (28). The imputed data sets are analyzed using standard statistical analyses and results from the analyses from the m complete data sets are combined using Rubin’s rules (27, 29) to generate valid statistical inferences that reflect uncertainty due to missing values and improve the accuracy of the results.
Statistical Analyses
Prior to testing for differences in change in NP test performance, we used the PROC REG and PROC LOGISTIC procedures in SAS to test for differences at baseline between the two treatment conditions as well as the four treatment condition by responder status groups (see below). There were no differences on age, education, gender, baseline depression severity, responder status, or on any of the NP tests with the exception of baseline scores on the MMSE and Digit Symbol subtest of the WAIS-III. Therefore, we adjusted for baseline Digit Symbol and MMSE scores in the two treatment group analyses. When comparing the four patient groups (responder status by treatment condition), we found differences on education and baseline MMSE, CRT, and Buschke SRT scores. We therefore included these variables as covariates in the four group analyses. We also adjusted for site of study in all analyses, which we know from previous reports to be associated with outcome (19).
To test for differences in change in NP test performance, we used data from the multiply imputed data sets and adopted a partial or regressed change approach to analyzing two time-point data (30) using the PROC REG procedure in SAS. According to this approach, the endpoint NP test score is treated as the outcome variable and the baseline test score is treated as a covariate. This effectively removes all correlation of the endpoint score from the baseline score and represents an improvement over simple change scores (subtracting baseline from endpoint), which tend to overcorrect the endpoint score by the baseline score due to unreliability of measurement (30). We first tested for differences in endpoint scores between treatment conditions using a dummy coded (citalopram = 1, placebo = 0) variable. To test whether change in NP test performance depends on responder status (50% reduction from baseline HRSD score), we again used a dummy coded variable to designate the four patient groups (citalopram responders, citalopram non-responders, placebo responders, and placebo non-responders). Each covariate was centered at its respective mean so the intercept corresponded to the mean of the reference group at endpoint and the unstandardized regression weights reflected the difference between the groups included in the model and the reference group (excluded from the model). All significance tests were evaluated at the 5% level.
RESULTS
Descriptive Statistics
Table 1 presents baseline demographic and clinical characteristics of the total sample, placebo and citalopram groups, and the four groups of patients classified by treatment condition and responder status. The average study participant was 79.57 years and completed about 2 years of college. Approximately 58% of the sample were women, average baseline depression severity was 24.32 on the 24-item HRSD, and 40% of the sample was classified as responders. The average MMSE score of the sample at baseline was 27.99 and 6.9% had a score of 24 or below.
Table 1.
Baseline Clinical and Demographic Characteristics of the total sample, the citalopram and placebo conditions, and the four patients groups classified by treatment condition and responder status.
| Variable | Total Sample (n=174) | Citalopram (n=84) | Placebo (n=90) | Citalopram Responders | Citalopram Non-responders | Placebo Responders | Placebo Non-responders |
|---|---|---|---|---|---|---|---|
| Age | 79.57 (4.36) | 79.82 (3.97) | 79.33 (4.69) | 79.30 (3.74) | 80.19 (4.08) | 80.05 (5.42) | 78.89 (4.13) |
| Women (%) | 58 | 54 | 62 | 60 | 49 | 61 | 63 |
| Education, Yr | 13.77 (3.27) | 13.82 (2.76) | 13.72 (3.67) | 12.76 (2.61) | 14.56 (2.67) | 13.30 (4.17) | 13.99 (3.30) |
| HRSD | 24.32 (4.12) | 24.40 (4.31) | 24.25 (3.93) | 24.04 (4.50) | 23.96 (4.13) | 23.33 (3.46) | 24.82 (4.10) |
| Responder (%) | 40 | 41 | 38 | 100 | 0 | 100 | 0 |
| MMSE | 27.99 | 28.37 (1.61) | 27.62 (2.46) | 28.46 (1.40) | 28.31 (1.74) | 27.35 (2.38) | 27.79 (2.50) |
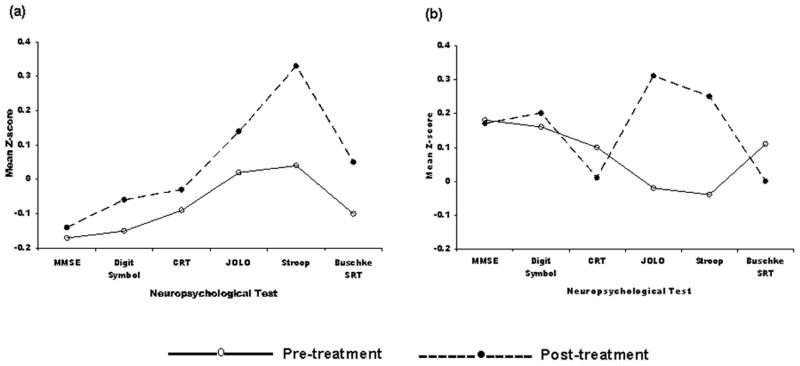
To facilitate interpretation of the pattern of change in NP test performance as a function of treatment group, all NP scores were converted to z-scores based on mean values at baseline in the total sample. As can be seen in Figure 1, NP test performance improved on each test in the placebo group, which is consistent with a practice effect. For the purposes of this report, practice effects refer to improvement in performance due to repeat testing and are defined on the basis of the performance of the placebo group. Differences from the placebo group (both positive and negative) reflect deviations from a practice effect and represent either improvement beyond a practice effect or the absence of a practice effect, possibly even decline. As can be seen in Figure 1, the citalopram group improved on some tests and declined on others.
Figure 1.
Change in cognitive performance from pre to post-treatment in the (a) placebo condition and (b) citalopram condition across six neuropsychological tests.
Hypothesis Testing
Table 2 shows the unadjusted means and standard deviations for all NP tests both pre and post-treatment for the citalopram and placebo groups as well as the four patient groups (treatment group by responder status). Adjusting for site and baseline MMSE and Digit Symbol, there was a statistically significant difference between the placebo and citalopram conditions at endpoint on the Buschke SRT. Specifically, patients treated with citalopram scored lower at endpoint than patients treated with placebo [B=−2.74 SE=1.41, t(1087)=−1.94, 95% CI −5.52, 0.03, p=0.05].
Table 2.
Unadjusted neuropsychological test performance scores at baseline and endpoint for the citalopram and placebo subsamples and the four patient groups classified according to treatment condition and responder status
| NP Test | Citalopram (n=84) | Placebo (n=90) | Citalopram Responders | Citalopram Non-responders | Placebo Responders | Placebo Non-responders | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| MMSE | 28.37 (1.61) | 28.35 (1.67) | 27.62 (2.46) | 27.67 (2.15) | 28.46 (1.40) | 28.59 (1.26) | 28.31 (1.74) | 28.19 (1.88) | 27.35 (2.38) | 27.58 (2.28) | 27.79 (2.50) | 27.78 (2.06) |
| Digit Symbol | 45.17 (16.83) | 45.86 (16.91) | 40.14 (16.22) | 41.49 (17.98) | 45.38 (17.21) | 48.92 (16.20) | 45.02 (16.59) | 43.75 (17.11) | 39.87 (17.23) | 42.20 (19.02) | 40.31 (15.57) | 41.04 (17.32) |
| Stroop | 0.87 (0.58) | 0.69 (0.54) | 0.82 (0.64) | 0.64 (0.61) | 0.84 (0.61) | 0.61 (0.61) | 0.89 (0.55) | 0.75 (0.47) | 0.92 (0.72) | 0.63 (0.66) | 0.77 (0.59) | 0.65 (0.58) |
| CRT | 6.76 (0.31) | 6.79 (0.36) | 6.82 (0.37) | 6.80 (0.39) | 6.77 (0.34) | 6.77 (0.36) | 6.74 (0.29) | 6.80 (0.36) | 6.90 (0.43) | 6.83 (0.50) | 6.77 (0.32) | 6.78 (0.31) |
| JOLO | 19.49 (5.61) | 21.55 (5.41) | 19.76 (6.63) | 20.50 (5.75) | 19.21 (5.46) | 22.59 (5.09) | 19.69 (5.72) | 20.82 (5.53) | 18.71 (7.18) | 19.93 (5.90) | 20.42 (6.18) | 20.85 (5.63) |
| Buschke SRT | 36.37 (8.78) | 35.23 (10.73) | 34.23 (10.83) | 35.71 (11.86) | 37.04 (7.66) | 37.46 (10.61) | 35.90 (9.47) | 33.68 (10.57) | 31.68 (9.46) | 33.78 (11.47) | 35.83 (11.33) | 36.92 (11.96) |
Note. Table values are means and standard deviations. MMSE=Mini Mental Status Exam, total number correct; Stroop=Color/Word Test, interference effect; CRT=Choice Reaction Time, median reaction time of correct responses, log transformed; JOLO=Judgment of Line Orientation, total number correct; Buschke SRT=Buschke Selective Reminding Test, immediate recall, total number correct
We next compared the four groups of patients classified according to treatment condition and responder status on endpoint NP test performance. As can be seen in Table 2, citalopram responders scored significantly higher than both citalopram non-responders [B=−2.54, SE=0.97, t(80.46)=−2.54, 95% CI −4.38, −0.53, p=0.01] and placebo non-responders [B=−2.47, SE=0.89, t(217.31)=−2.77, 95% CI −4.23, −0.71, p=0.01] on the JOLO at endpoint. However, citalopram responders were not statistically significantly different than placebo responders at endpoint [B=−1.81, SE=1.02, t(111.56)=−1.78, 95% CI −3.83, 0.20, p=0.08]. Looking at endpoint performance on the Buschke SRT, citalopram non-responders were the only group to decline from pre-test to post-test. Specifically, citalopram non-responders scored lower (3.64 points) than placebo non-responders at study end [B=−3.64 SE=1.83, t(472.15)=−1.99, 95% CI −7.23, −0.05, p=0.05]. Similarly, citalopram non-responders were the only group to decline from pre-test to post-test on the Digit Symbol. In particular, citalopram non-responders scored lower than citalopram responders at endpoint [B=−5.62 SE=2.65, t(233.31)=−2.12, 95% CI −10.84, −0.40, p=0.04].
DISCUSSION
This was the first study to examine the impact of antidepressant treatment on change in cognitive functioning in depressed adults 75 years and older using data from an eight-week, randomized, placebo-controlled trial. While the placebo group showed a distinct practice effect from baseline to endpoint on all NP tests, the citalopram group improved on some tests but declined on others. However, the pattern of change depended on responder status. Specifically, citalopram non-responders were the only group to decline in performance on verbal learning (Buschke SRT) and psychomotor speed (Digit Symbol). Citalopram responders showed significant improvement in visuospatial functioning (JOLO) when compared to non-responders in either condition, but their improvement was not greater than responders on placebo. Similarly, citalopram responders showed greater improvement on psychomotor speed (Digit Symbol) than citalopram non-responders, but their improvement was not greater than placebo responders or non-responders. The findings indicate that the practice effect is impaired in some domains among non-responders on medication. Therefore, these findings suggest that patients should not be maintained on a medication if they have not had an adequate response.
One possible explanation for the observed decline in verbal learning and psychomotor speed is that the overall level of cognitive functioning in the sample was low and the citalopram group had a disproportionately high number of cognitively impaired patients. This might explain why there was inconsistent improvement in the citalopram condition and why this study differs from previous placebo-controlled trials (8, 15). However, the average MMSE score at baseline for the sample was 28, which is well within normal limits. Moreover, although there was a significant difference in MMSE scores between the treatment groups at baseline, it was the citalopram group that scored higher at baseline than the placebo group.
Another possibility is that brain lesions, which are associated with age (31) and other risk factors such as hypertension and diabetes (32), were disproportionately represented in the citalopram condition. White matter hyperintensities (WMH) that are characteristic of LLD may interrupt frontal-striatal pathways that mediate cognitive functions that are commonly impaired in LLD. Furthermore, cognitive impairment is associated with the presence of WMH in LLD and deficits worsen as the lesions become more severe (2, 5). However, there were no statistically significant differences in the percentage of patients in the citalopram group and the placebo group classified as having high lesion load, which was defined as a deep white matter hyperintensity rating of 2 or a subcortical gray matter rating of 3 on the Fazekas modified Coffey Rating Scale for Signal Hyperintensities (33).
The decline in verbal learning may be particularly attributed to the anticholinergic effects of SSRIs. SSRIs have unique non-serotonergic pharmacological profiles that are associated with distinct effects on cognitive functioning (34). Paroxetine, for example, may cause impairment in delayed verbal recall in healthy middle-aged adults and elderly subjects whereas sertraline is associated with improvement in immediate and delayed verbal recall and verbal fluency (35, 36). Although administration of citalopram is associated with improvement in working memory in depressed adults (37) and increased memory consolidation in healthy adults (38), it is still unclear what effect citalopram can have on cognitive functioning of the geriatric depressed, a population that is especially vulnerable to the adverse effects of antidepressant medication (39).
The observed decline in verbal learning and psychomotor speed in the citalopram group is consistent with a recent report from an epidemiological study of elderly depressed patients examining the relationship between depressive symptoms, cognitive impairment, and antidepressant use (40). Findings revealed that baseline depression scores predicted future mild cognitive impairment but only among those using antidepressant medications at baseline. Taken together, these findings support the contention that non-responders should not be maintained on medication that may have a negative impact on some aspects of cognitive functioning, which may facilitate the development of mild cognitive impairment (MCI) (41).
This study should be interpreted in the context of several limitations. First, there were statistically significant differences between the two treatment conditions as well as the four patient groups at baseline on several NP tests. However, these differences were adjusted for in the statistical models by including those tests as covariates. Second, it may be possible that including a small number of MCI patients (MMSE ≤ 24) in this study (n=12) might have influenced our results. However, we ran the analyses with and without this group of patients and the results were not different. Third, there was missing data, as is typically the case in clinical trials, and we accommodated for missing data using multiple imputation, a far superior method compared to traditional approaches using mean substitution or complete case analysis. Fourth, a somewhat limited NP battery was used. Only one aspect of executive functioning (i.e. response inhibition) was evaluated and no formal test of attention was included in the study. These limitations, however, are balanced by using data from the only randomized, placebo-controlled clinical trial of antidepressant treatment among depressed patients age 75 or older. Moreover, unlike other studies, there were an approximately equal number of responders in both treatment conditions, allowing for an adequate test of whether change in cognitive function across two treatments depends on responder status.
Our findings indicate that citalopram may interfere with the normal practice effect in verbal learning and psychomotor speed among patients who do not respond on medication. While responders on medication may improve in some domains, their improvement does not exceed the expected practice effect observed in patients randomized to placebo. This raises the important clinical issue that, although two treatments may be equivalent with regard to response, they may have differential effects on cognitive functioning, especially in a cognitively vulnerable population. Our findings suggest that non-responders should not be maintained on medication that may have a negative effect on some aspects of cognitive functioning.
Acknowledgments
This research was supported by a grant from Forest Laboratories and National Institute of Mental Health grants T32 MH20004 (Steven P. Roose) and K23 MH075006 (Joel R. Sneed).
Footnotes
References
- 1.Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 2.Kramer-Ginsberg E, Greenwald BS, Krishnan KRR, et al. Neuropsychological Functioning and MRI Signal Hyperintensities in Geriatric Depression. Am J Psychiatry. 1999;156:438–444. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- 3.Gallassi R, Di Sarro R, Morreale A, et al. Memory impairment in patients with late-onset major depression: The effect of antidepressant therapy. Journal of Affective Disorders. 2006;91:243–250. doi: 10.1016/j.jad.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 5.Lesser I, Boone K, Mehringer C, et al. Cognition and white matter hyperintensities in older depressed patients. American Journal of Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 6.Nebes RD, Butters MA, Mulsant BH, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Medicine. 2000;30:679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- 7.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression.[see comment] American Journal of Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 8.Georgotas A, McCue RE, Reisberg B, et al. The Effects of Mood Changes and Antidepressants on the Cognitive Capacity of Elderly Depressed Patients. International Psychogeriatrics. 1989;1:135–143. doi: 10.1017/s1041610289000141. [DOI] [PubMed] [Google Scholar]
- 9.Nebes RD, Pollock BG, Mulsant BH, et al. Cognitive effects of paroxetine in older depressed patients. J Clin Psychiatry. 1999;60:26–29. [PubMed] [Google Scholar]
- 10.Bondareff W, Alpert M, Friedhoff AJ, et al. Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. American Journal of Psychiatry. 2000;157:729–736. doi: 10.1176/appi.ajp.157.5.729. [DOI] [PubMed] [Google Scholar]
- 11.Doraiswamy PM, Krishnan KR, Oxman T, et al. Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci. 2003;58:M1137–1144. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- 12.Portella MJ, Marcos T, Rami L, et al. Residual cognitive impairment in late-life depression after a 12-month period follow-up. International J Geriatric Psychiatry. 2003;18:571–576. doi: 10.1002/gps.895. [DOI] [PubMed] [Google Scholar]
- 13.Butters MA, Becker JT, Nebes RD, et al. Changes in Cognitive Functioning Following Treatment of Late-Life Depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 14.Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of Psychiatric Research. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 15.Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164:900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- 16.Roose SP, Sackeim HA, Krishnan KRR, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: A randomized, placebo-controlled trial. American Journal of Psychiatry. 2004;161:2050–2059. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- 17.Sneed JR, Roose SP, Keilp JG, et al. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. American Journal of Geriatric Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 18.Sneed JR, Keilp JG, Brickman AM, et al. The specificity of neuropsychological impairment in predicting antidepressant non-response in the very old depressed. International Journal of Geriatric Psychiatry. 2008;23:319–323. doi: 10.1002/gps.1889. [DOI] [PubMed] [Google Scholar]
- 19.Roose SP, Sackeim HA, Krishnan KRR, et al. Antidepressant Pharmacotherapy in the Treatment of Depression in the Very Old: A Randomized, Placebo-Controlled Trial. Am J Psychiatry. 2004;161:2050–2059. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- 20.Cohen JD, MacWhinney B, Flatt M, et al. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavioral Research, Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale 3rd Revision (WAIS-III) San Antonio: Psychological Corporation; 1997. [Google Scholar]
- 23.Thorne DR, Genser SG, Sing HC, et al. The Walter Reed Performance Assessment Battery. Neurobehavioral Toxicology and Teratology. 1998;7:415–418. [PubMed] [Google Scholar]
- 24.MacLeod C. A half-century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 25.Benton AL, Sivan AB, Hamsher K, et al. Contributions to Neuropsychological Assessment. New York: Oxford; 1983. [Google Scholar]
- 26.Buschke H, Fuld P. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 27.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analyst’s perspective. Multivariate Behavioral Research. 1998;33:545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- 28.Schafer JL. Multiple imputation: A primer. Statistical Methods in Medical Research. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 29.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 30.Cohen J, Cohen P, West SG, et al. Applied multiple regression/correlation for the behavioral sciences. 3. Mahwah, NJ: Lawrence Erlbaum Publishers; 2003. [Google Scholar]
- 31.Taylor WD, MacFall JR, Steffens DC, et al. Localization of age-associated white matter hyperintensities in late-life depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:539–544. doi: 10.1016/S0278-5846(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 32.Murray AD, Staff RT, Shenkin SD, et al. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. American Journal of Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 34.Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. Journal of the American Geriatrics Society. 2008;56:1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt JAJ, Kruizinga MJ, Riedel WJ. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. Journal of Psychopharmacology. 2001;15:173–179. doi: 10.1177/026988110101500304. [DOI] [PubMed] [Google Scholar]
- 36.Furlan PM, Kallan MJ, Ten Have T, et al. Cognitive and psychomotor effects of paroxetine and sertraline on healthy elderly volunteers. Geriatr Psychiatry. 2001:9. [PubMed] [Google Scholar]
- 37.Zobel AW, Schulze-Rauschenbach S, von Widdern OC, et al. Improvement of working but not declarative memory is correlated with HPA normalization during antidepressant treatment. J Psychiatr Res. 2004;38:377–383. doi: 10.1016/j.jpsychires.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Harmer CJ, Bhagwagar Z, Cowen PJ, et al. Acute administration of citalopram facilitates memory consolidation in healthy volunteers. Psychopharmacology. 2002;163:106–110. doi: 10.1007/s00213-002-1151-x. [DOI] [PubMed] [Google Scholar]
- 39.Baldwin D, Johnson FN. Tolerability and safety of citalopram. Rev Contemp Pharmacother. 1995;6:315–325. [Google Scholar]
- 40.Ravaglia G, Forti P, Lucicesare A, et al. Prevalent depressive symptoms as a risk factor for conversion to mild cognitive impairment in an elderly Italian cohort. Am J Geriatr Psychiatry. 2008;16:834–843. doi: 10.1097/JGP.0b013e318181f9b1. [DOI] [PubMed] [Google Scholar]
- 41.Devanand DP, Pelton GH, Marston K, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. International Journal of Geriatric Psychiatry. 2003;18:123–130. doi: 10.1002/gps.802. [DOI] [PubMed] [Google Scholar]