Abstract
Understanding sources of individual differences in steroid hormone production has important implications for the evolution of reproductive and social behaviors. In females in particular, little is known about the mechanistic sources of these individual differences, despite established linkages between sex steroids and a variety of fitness-related traits. Using captive female dark-eyed juncos (Junco hyemalis) from two subspecies, we asked how variation in different components of the hypothalamo-pituitary-gonadal (HPG) axis related to variation in testosterone production among females, and we compared females to males in multiple components of the HPG axis. We demonstrated consistent individual differences in testosterone elevation in response to challenges with luteinizing hormone (LH) and gonadotropin-releasing hormone (GnRH). These hormone challenges led to more LH production but less testosterone production in females than males, and the sexes differed in some but not all measures of sensitivity to hormones along the HPG axis. Similar to findings in males, variation in testosterone production among females was not related to variation in LH production, gonadal LH-receptor mRNA abundance, or hypothalamic abundance of androgen receptor mRNA or aromatase mRNA. Rather, the primary source of individual variation in circulating steroids appears to the gonad, a conclusion further supported by positive correlations between testosterone and estradiol production. Unlike males, females did not differ by subspecies in any of the endocrine parameters that we assessed, suggesting some degree of independent evolution between the two sexes. Our results highlight the sources of physiological variation that may underlie the evolution of hormone-mediated phenotypes in females.
Keywords: testosterone, individual differences, hypothalamo-pituitary-gonadal axis, estrogen
1. INTRODUCTION
Sex steroid hormones regulate many reproductive and other fitness-related traits, and thus are thought to be proximate mediators of phenotypic evolution (Hau, 2007; Hau and Wingfield, 2011; Ketterson and Nolan, 1999; Ketterson et al., 1992; Zera et al., 2007). In the past several years, there has been growing interest in individual variation in testosterone, in part because identifying the sources of this variation may highlight the proximate mechanisms on which selection may act (Ball and Balthazart, 2008; Kempenaers et al., 2008; Williams, 2008). Individual male birds, for example, vary consistently in the amount of testosterone (T) produced in response to an experimental injection with gonadotropin-releasing hormone (GnRH (Jawor et al., 2006)), and these repeatable individual differences in T production correlate with immune function, aggression, parental care, ornamentation, and fitness measured in the wild (Greives et al., 2006; McGlothlin et al., 2008; McGlothlin et al., 2007; McGlothlin et al., 2010). Thus, there is ample evidence to suggest that individual variation in T in males may be a target of selection.
In females, however, there are many open questions: First, are females individually consistent in their response to stimulation of the hypothalamo-pituitary-gonadal (HPG) axis, providing the potential for evolution to shape female phenotype via changes at one or more tiers of the HPG axis? Evidence to date is mixed. On the one hand, females do produce testosterone (Ketterson et al., 2005; Staub and DeBeer, 1997) and individual differences in T production in females have been associated with fitness-related traits, including maternal yolk steroid deposition, body size, aggression towards predators and conspecifics, and the likelihood of successfully rearing young (Cain and Ketterson, 2012; Cain et al., 2011; Jawor et al., 2007; Muller et al., 2011). On the other hand, a number of studies on female birds have failed to find significant T elevation after stimulation of the HPG axis with GnRH (Goymann and Wingfield, 2004; Spinney et al., 2006; Wingfield et al., 1991) or significant repeatability of T elevation if elevation occurs (Jawor et al., 2007; but see Peluc et al., 2012), calling into question whether among-female variability in circulating testosterone should be seen as functional (see Kempenaers et al., 2008; Zera et al., 2007 for discussion).
The role of co-evolution is a second question that is central to understanding how testosterone may relate to the evolution of female phenotypes. Research on birds and fish has shown that female T levels are correlated with male T levels across species (Ketterson et al., 2005; Mank, 2007; Møller et al., 2005), suggesting that hormonal variation in females also may be shaped by intersexual genetic correlations and co-evolution between the sexes, instead of, or in addition to, any selection directly acting on females themselves. It is not clear, though, whether females and males share common sources of variation in T production, or whether the sexes diverge in patterns of variation, allowing one sex to evolve independently from the other to some degree. In addition, T is separated from estradiol (E2) by only one enzymatically-catalyzed reaction step (via aromatase, AROM), and E2 plays a central role in female reproduction as well (Williams, 2012b). As a consequence, T and E2 could potentially be co-regulated and thus co-evolve, due to shared dependence on upstream components of the endocrine cascade. However studies of female E2 function are quite rare, particularly in evolutionary endocrinology (Caro, 2012; Williams, 2012a), and so, much remains to be learned.
Our study aims to characterize variation in the regulation of sex steroid levels in females by measuring responses to a GnRH challenge in a controlled environment that can minimize extrinsic influences. This approach constitutes a key step in understanding how T and T-mediated traits evolve because it allows assessment of individual consistency of female sex steroid production and the relationships between T and E2, as well as examination of the sources of inter-female variation in HPG axis reactivity. Furthermore, direct comparisons of females and males will inform our understanding of the role of intersexual co-evolution.
The HPG axis consists of several interacting components that presumably contribute to variation in an individual's ability to produce sex steroids. At the top tier of the axis (hypothalamus), neurochemicals such as kisspeptin and gonadotropin-inhibitory hormone regulate the production and release of GnRH from the hypothalamus (Tsutsui et al., 2010). GnRH stimulates the release of gonadotropins (LH and FSH) from the pituitary, which regulate gametogenesis as well as production and release of sex steroids from the gonads (Adkins-Regan, 2008; Wingfield, 2012). At each tier of the axis, there is the potential for functional variation in the amount of hormone secreted, but there also may be functional variation in sensitivity to hormones secreted by other tiers of the axis, e.g. amount or binding affinity of GnRH-receptors, LH-receptors, etc. Adding further complexity is a web of negative feedback loops, such that hormones produced by each tier of the axis can down-regulate hormone production and sensitivity at other tiers of the axis. For example, high levels of testosterone binding to androgen receptors (ARs) in the hypothalamus leads to down-regulation of GnRH release (e.g. Krey and McGinnis, 1990). Negative feedback is also estrogen-dependent and mediated by hypothalamic AROM (Roselli and Resko, 2001; Tsai et al., 1994).
Each of these components of the HPG axis has been linked with seasonal, sexual, or experimental variation in the production of sex steroids, but rarely have multiple components of the HPG axis been analyzed as a complex. As a consequence, despite some theoretical discussion (Adkins-Regan, 2008), it is not entirely clear whether all of these components contribute equally to variation in sex steroid production, or whether one or more components is particularly influential in differentiating individuals at the high and low ends of the distribution of testosterone production. A handful of studies provide some insight regarding these sources of variation (Huffman et al., 2012; Maruska and Fernald, 2011; Maruska et al., 2011; Spinney et al., 2006); however, they do not directly address whether individual variation in testosterone production is primarily a consequence of variation at the hypothalamus, pituitary, gonad, or all three tiers of the axis. Examining individual variation in multiple components of the HPG axis simultaneously is a necessary step towards determining these functional sources of variation in sex steroids. Further, by revealing the degree to which multiple components are tightly correlated (integrated) or vary independently of one another, this approach can shed light upon the relative ease with which sex steroid signaling can evolve (Hau, 2007; Ketterson et al., 2009).
Here, we investigate several likely sources of natural variation in sex steroid production in females, and we assess the degree to which this variation is similar to or different from males. Using captive female dark-eyed juncos (Junco hyemalis), we performed a series of hormone injections to assess variation in and individual consistency of T production. We tested the hypothesis that sex steroid signaling is integrated, such that multiple components of the endocrine cascade are correlated with one another. We asked whether T production was correlated with E2 production, as predicted if androgen- and estrogen- mediated traits are co-regulated and might co-evolve. Furthermore, we probed the source(s) of inter-individual differences in the amount of T produced in response to a GnRH challenge using four measures: pituitary responsiveness to GnRH (assessed via circulating LH levels), gonadal sensitivity to LH (assessed as LH-R mRNA abundance measured via quantitative PCR), gonadal responsiveness to LH (assessed as T released in response to LH injection), and hypothalamic sensitivity to sex steroid-mediated feedback (assessed as abundance of mRNA for AR and for AROM in the rostral hypothalamus).
By comparing females and males in these sources of variation along the HPG axis, we also tested the hypothesis that the sexes co-evolve. A recent study on captive male juncos showed that among-male variation in T production was primarily a consequence of gonadal response to the presence of LH, rather than individual differences at other tiers of the HPG axis (Bergeon Burns et al., under revision). If male and female mechanisms of sex steroid production have co-evolved, then we would predict similar patterns of variation in the two sexes. In contrast, if regulation of the production of gonadal sex steroids has evolved somewhat independently in females and males, then we might predict different sources of inter-individual differences in each sex. Bergeon Burns and colleagues (under revision) also compared variation along the HPG axis in two populations of junco that differ in a number of testosterone-mediated traits (Bergeon Burns et al., 2013; Nolan et al., 2002), and they found that males of the larger, more ornamented/aggressive white-winged junco subspecies (J. h. aikeni) showed reduced transcript abundance for gonadal LH-R and for hypothalamic AR, but no difference in T production, compared to the smaller, less ornamented/aggressive Carolina sub-species (J. h. carolinensis). Female white-winged juncos in the field are also larger and more ornamented than female Carolina juncos (Miller, 1941), though it is not yet known whether these differences persist in captivity and whether the subspecies difference in aggression extends to females. Furthermore, within population studies on male juncos suggest that ornamentation and body size evolve via correlational selection (McGlothlin et al., 2005) and that both traits are correlated with HPG axis reactivity (McGlothlin et al., 2008). If intersexual co-evolution constrains the evolution of the HPG axis, then we would expect to see population differences among females that are similar to those reported in males by Bergeon Burns et al. (under revision), with the prediction that females from the white-winged subspecies would have reduced abundance of gonadal LH-R mRNA and hypothalamic AR mRNA. If however, female HPG axes can evolve independently of male HPG axes, then we may not necessarily see signatures of these population differences in females.
2. MATERIAL AND METHODS
2.1 Animal capture and housing
We used mist nets and potter traps to capture 15 females and 19 males from the area surrounding Custer, South Dakota (“SD,” 43°46 N 103°36’W) and 14 females and 24 males from the area surrounding the University of Virginia's Mountain Lake Biological Station (“VA,” 37°22’N, 80°32’W). All animals were juveniles captured at the end of the summer in which they hatched, as evidenced by distinct juvenile plumage. Males were also used in a related study that investigated sources of variation in T production among populations and among individual males (Bergeon Burns et al., under revision). For logistical reasons, the two populations were tested in separate and sequential experiments (SD in Aug 2009- April 2010; VA in Aug 2010- April 2011), but otherwise, subjects were treated identically (i.e. same timeline, rooms, procedures, husbandry protocols, etc.). All subjects were transported to a large indoor aviary at Indiana University, where they overwintered as a mixed-sex flock on a photoperiod that mimicked their native environment. Before days reached a photostimulatory length, we moved birds into individual cages in one of four adjacent rooms, and we advanced photoperiod by 1 hour every other day to long days (16L:8D) to trigger gonadal recrudescence (beginning during the first week of March). All rooms included male and female birds, such that each individual in this study had visual, olfactory, and auditory cues from same- and opposite-sex conspecifics of their own population.
2.2 Experimental design
After subjects had been on long days for 3 weeks, we began hormone challenges. Briefly, each individual was removed from its cage and a baseline hormone sample was taken (latency from removal of cage to completion of bleed = 8.2 ± 0.4 min). Subjects then received each of three different injections separated by 5 days, randomized and counterbalanced for order.
The first challenge (measuring sex steroids as a function of GnRH) has been used extensively in this and other songbird species, to measure responsiveness of the HPG axis (DeVries et al., 2012; Goymann et al., 2007; Jawor et al., 2006, 2007; Muller et al., 2011). Each individual was injected intramuscularly with chicken GnRH (1.25 μg dissolved in 50 μL PBS; American Peptide, Sunnyvale, CA, USA), held in an individual capture bag, and bled a second time 30 min after the GnRH injection. Because this hormone challenge stimulated birds via GnRH, it measures the collective response of the pituitary and gonad. It is also thought to stimulate an approximately maximal output of testosterone, as even twice the dose of GnRH elicits a similar T response (Jawor et al., 2006).
The second challenge (measuring LH as a function of GnRH) was designed to focus solely on the pituitary's ability to produce gonadotropins (here, LH) in response to a standardized dose of GnRH. Each individual was injected with chicken GnRH, as above, but the post-challenge blood sample was collected after only 5 min. This time point was selected based upon pilot data from free-living males and females demonstrating that LH levels were significantly elevated above baseline only 5 min after GnRH challenge (Bergeon Burns and Ketterson, unpublished data).
The third challenge (measuring sex steroids as a function of LH) was designed to focus solely on the gonad's ability to produce sex steroids in response to standardized dose of LH. Each individual was injected intramuscularly with ovine LH (5 μg oLH-26 dissolved in 50 μl PBS, Lot # AFP5551B; obtained from NIDDK's National Hormone & Peptide Program and A. F. Parlow), held in an individual capture bag, and bled a second time 30 min after LH injection. This intramuscular dose and timing were selected based on pilot work showing elevated T in male juncos at this time point compared to baseline (Bergeon Burns et al., under revision).
We collected no more than 140 μL blood for each initial and post-challenge sample. Samples were stored on ice for no more than 5 h after collection, centrifuged, and plasma was drawn off the top and stored at −20°C until later hormone assays.
Five days after the final hormone challenge, all animals were euthanized with an overdose of isoflurane, followed by rapid decapitation. After weighing bodies to the nearest 0.1 g, brains and gonads were immediately dissected using RNAse-free sterile techniques, frozen on powdered dry ice, and transferred to −80°C for storage until later processing.
We scored the degree of plumage ornamentation by visually estimating the proportion of the outer tail feathers covered by white, as opposed to gray, following established methods. ‘Tail white’ score is the proportion of white on each of the right rectrices, summed over all six of these feathers (McGlothlin et al., 2005; Wolf et al., 2004). Prior work has shown that tail white functions in both aggressive and courtship interactions (e.g. Hill et al., 1999; Holberton et al., 1989).
2.3 Tissue processing and molecular methods
To assess reproductive status, we measured mass of frozen ovaries to the nearest 1 mg. In a random subset of females (n = 4 SD, 9 VA) we used calipers to measure the diameter of the largest follicle to the nearest 0.1 mm. Whole brains were dissected into functional regions, using anatomical landmarks as described in detail in previous studies (Soma et al., 1999; Soma et al., 2003). Briefly, we removed the hindbrain, cerebellum, optic tecta and optic chiasm, followed by removal of the entire diencephalon using forceps (from the tuberal area to the depth of the anterior commissure). Lastly, we bisected the diencephalon into rostral and caudal regions, focusing on the rostral portion of tissue that is largely limited to rostral portions of the hypothalamus, the preoptic area and the medial preoptic nucleus (hereafter, ‘rostral hypothalamus’).
Total RNA was extracted from the rostral hypothalamus and ovary using the Trizol method (Invitrogen, Carlsbad, CA). RNA was treated with DNAse (Promega, Madison, WI, USA), and cDNA was synthesized using Superscript III (Invitrogen) from 1 μg of total RNA from each sample. We performed qPCR using a Strategene MX3000P equipped with MxPro software (v.4.10, Agilent, Santa Clara, CA, USA). Each sample was run in duplicate, and each reaction included a 1:10 dilution of cDNA (2.5 μL), PerfeCta SYBR green low ROX (12.5 μL), and forward and reverse primers (0.3 μM) in a total volume of 25 μL. All reactions proceeded through the following thermal profile: 10 min at 95°C; 40 cycles of 95°C for 30s, 60°C for 1 min, and 70°C for 30s; and a final dissociation step to assess product specificity (95°C for 1 min, 55°C for 30s, and 95°C for 1 min). We used the 2−ΔΔCt method of quantification (Livak and Schmittgen, 2001), in which the abundance of each gene of interest is expressed as the fold change in expression relative to a pooled standard, normalized by the expression of a reference gene (glyceraldehyde-3-phosphate dehydrogenase, GAPDH). We assessed amplification efficiencies for each gene using a 5-point standard curve and corrected for imperfect efficiencies in MxPro (efficiencies ranged from 93% to 116%). All primer sequences have been used before in songbirds (see ESM Table 1 for sequence information). We confirmed high sequence identity (≥ 95%) in these genes using sequences from the dark-eyed junco transcriptome (Peterson et al., 2012).
2.4 Hormone assays
We measured plasma LH using the heterologous radioimmunoassay (RIA) that has been used extensively in songbirds (Follett et al., 1972; Sharp et al., 1987; Wingfield et al., 1991), including female juncos (Jawor et al., 2007). This RIA employed a double-antibody, post-precipitation process with antisera raised against purified chicken LH. Each sample was run in duplicate (20 μL each) on a single assay. Intra-assay variability was 12.1 ± 1.0% and the minimum detectable concentration was 0.078 ng/mL.
We measured plasma T and E2 using separate commercially available enzyme immunoassays (Enzo #901–065 and Cayman Chemical # 582251, respectively). For details on kit accuracy, cross-reactivity, detection limits, and parallelism, see ESM Table 2. Each kit followed roughly the same procedures, beginning with either 30 μL of plasma (for T EIA) or 40 μL of plasma (for E2 EIA; see elaboration below). We spiked each sample with a trace amount of tritiated T or E2 (~2000 CPM). We extracted hormones using diethyl ether (2x extractions for T; 3x extractions for E2), to facilitate later correction for incomplete recoveries (for T: 84 ± 0.5%; for E2: 70 ± 0.5%). We dried each sample with nitrogen gas and reconstituted in ethanol and assay buffer (350 μL total volume for T; 200 μL total volume for E2). Each sample was plated in duplicate (100 μL per well for T; 50 μL per well for E2) following the manufacturers’ guidelines. T samples were assayed on 4 plates, and E2 samples on one plate. We report the following coefficients of variation for T: intraplate = 8.3% (range: 4.2 – 13.4%), interplate = 24.8%; and for E2: intraplate = 8.7%. For T, we used a plate correction factor to standardize between plates, following Jawor et al. (2006). One pre-injection T sample was undetectable by our assay, and we used the kit sensitivity to assign a T value, corrected for plasma volume.
Because we performed the estradiol assay as the last of three assays, we did not have sufficient plasma volume to assess E2 before and after each injection type for all females. Therefore, we did not attempt to examine patterns of individual variation in female E2. Instead we asked first whether GnRH or LH injections significantly elevated estradiol levels, and second whether post-challenge estradiol levels were correlated with post-challenge testosterone levels. To accomplish this with limited plasma, we combined samples across individuals, as follows. We separately rank-ordered all baseline samples and all post-challenge samples within each injection type according to their plate-corrected plasma T levels. We then pooled samples of the as yet un-assayed plasma according to the rank of their already determined concentration of T, combining only as many samples as needed to accumulate 40 μL. This process resulted in 31 plasma ‘pools’ (n = 8 pre-LH injection, n = 7 pre-GnRH injection, n = 8 post-LH injection, n = 8 post-GnRH injection). Each resultant pool consisted of plasma from 1-4 individual plasma samples (mean ± se = 2.1 ± 0.1). Based upon the exact volume of plasma from each initial sample, we calculated a ‘composite T’ value for each pool, and used it to relate plasma testosterone and plasma estradiol levels. While this approach did not allow us to assess whether E2 elevation was significant using a repeated measures design, it did allow us to ask whether plasma samples with high or low T have similarly high or low E2, and whether pre- and post- injection plasma samples differed in E2.
2.5 Statistical analyses
All statistical analyses used JMP v. 10.0.0 (SAS Institute, Cary, NC, USA), and we report results as mean ± se, unless otherwise noted. All tests are two-tailed. We tested for normality with Shapiro-Wilks tests, and when necessary, we used a natural log-transformation on hormone data and a log2 transformation on gene expression data. Sample sizes are n = 29 females, 43 males, except that one female died of unknown causes between hormone sampling and tissue harvesting, and so we were unable to collect tissues from this individual.
We tested for population differences among females in tail white and body mass using unpaired t-tests. Because we injected the same concentration of hormone into all females, but females varied in mass both within and between populations, we also tested for relationships between body mass and post-injection hormone levels, while controlling for population. One female was an outlier in body mass (3.5 standard deviations above the mean), and she was removed from these analyses. Finding no relationship between body mass and hormone levels (see §3.1), we did not include body mass in subsequent analyses.
To test for sex differences and population differences among females along the HPG axis, we used a single MANOVA with population nested within sex, including the following dependent variables: abundance of mRNA for AR and AROM in the rostral hypothalamus, abundance of mRNA for LH-R in the gonad, T levels pre- and post-LH injection, T levels pre- and post-GnRH injection, and LH levels pre- and post-GnRH injection. We could not find a transformation that normalized measures of gene expression when the sexes were considered together, so we selected the transformation that most closely approached normality because MANOVA results are robust even when assumptions of normality are violated (Gupta et al., 2008). We used post-hoc pairwise F-tests to identify particular variables that differed between males and females, or between populations among females. Finding no significant population differences among females (see §3.2), populations were pooled for all subsequent analyses. We did not perform post-hoc F-tests to examine population differences among males, as these data have been reported elsewhere (Bergeon Burns et al., under revision), and were summarized above (§1).
To test whether GnRH or LH injections lead to T elevation in females, we used a repeated measures linear mixed model, with bird as a random factor and timepoint (pre vs. post injection) and injection type (GnRH or LH) as fixed effects. Similarly, to test whether GnRH injection lead to LH elevation in females, we used a repeated measures linear mixed model, with bird as a random factor and timepoint (pre vs. post injection) as a fixed effect.
We used Pearson correlation to ask whether T levels induced by LH injection were correlated with T levels induced by GnRH injection. Likewise, we used Pearson correlations to determine whether LH levels induced by GnRH challenge were correlated with T levels induced by either GnRH or LH injections. We opted to use non-parametric statistics for estradiol-related analyses due the pooling of samples. Thus, we used a Mann Whitney U test to assess whether post-injection plasma pools had higher E2 levels than pre-injection (baseline) plasma pools, and we used a Spearman rank correlation to assess the relationship between plasma testosterone and estradiol in post-injection plasma pools.
We used a stepwise model selection process to ask which endocrine parameters best explained inter-female variation in the amount of T produced in response to a GnRH challenge. Due to known functional connectedness between different tiers of the HPG axis and the potential for issues of co-linearity, we initially tested for significant correlations among the variables of interest, specifically: measures of gonadal LH sensitivity (LH-R mRNA abundance), gonadal response to LH injection (plasma T after LH challenge), pituitary response to GnRH (plasma LH after GnRH challenge), and androgen sensitivity in the rostral hypothalamus (AR and AROM mRNA abundance). We found a significant positive relationship only between the two measures of hypothalamic androgen sensitivity (r = 0.77, n = 28 females, p < 0.0001), and we collapsed these two variables using principal components. The first principal component had an eigenvalue = 1.77, explained 88.6% of the variance in these data, and loaded positively with eigenvectors = 0.71 for both AR and AROM mRNA abundance. Thus, our initial model attempted to relate T levels measured after GnRH challenge by breaking the GnRH challenge into its constituent parts, including LH levels after GnRH challenge, T levels after LH challenge, gonadal LH-R abundance, and hypothalamic sensitivity to androgens (PC1). We then used backward stepwise model selection based upon the minimum Akaike Information Criterion, corrected for finite sample size (AICc), to select the best model. E2 data were not included in this stepwise analysis due to the pooling of samples described above.
3. RESULTS
3.1 Body mass, ornamentation, and reproductive status
South Dakota females were significantly heavier than Virginia females (unpaired t-test: t = 2.24, p = 0.038, SD: 22.4 ± 0.2 g; VA: 20.8 ± 0.7 g), but body mass was unrelated to hormonal response to any of the three challenges (F < 1.06, p > 0.31). South Dakota females also had significantly more white on their tails than Virginia females (unpaired t-test, t = 9.08, p < 0.0001, SD: 3.0 ± 0.1, VA: 2.0 ± 0.1). Visual assessment of ovaries did not show signs of yolky follicles. However, ovarian mass (49 ± 3 mg, n = 28 females) and measurements of the largest follicle from a subset of females (2.0 ± 0.1 mm for n = 13 females) were both consistent with breeding females who are not actively preparing to lay a clutch (e.g. Wingfield et al., 1991).
3.2 Comparison of endocrine parameters with males and between populations
Nested MANOVA revealed an overall significant effect of sex, but no overall effect of population (Whole model: Wilks’ λ = 0.032, F = 14.4, p < 0.0001; Sex: F = 134.5, p < 0.0001; Population [sex]: λ = 0.69, F = 1.33, p = 0.18). Post-hoc tests revealed significant sex differences in several endocrine parameters; however, there were no significant population differences among females in any of these (Table 1), and we therefore excluded ‘population’ from all subsequent analyses on females. Females expressed significantly less AROM mRNA in the rostral hypothalamus and significantly less LH-R mRNA than did males, but the sexes did not differ in abundance of AR mRNA in the rostral hypothalamus. As expected, females also had significantly lower initial and post-challenge T levels than males. The sexes did not differ in pre-challenge LH levels, but females had significantly higher post-challenge LH levels.
Table 1.
Nested MANOVA and post-hoc F-tests on endocrine parameters.
| F | p | |
|---|---|---|
| AROM mRNA in rostral hypothalamus | ||
| SDF vs. VAF | 0.83 | 0.37 |
| M vs. F | 5.61 | 0.021* |
| AR mRNA in rostral hypothalamus | ||
| SDF vs. VAF | 1.59 | 0.21 |
| M vs. F | 0.76 | 0.39 |
| LH-R mRNA in gonad | ||
| SDF vs. VAF | 0.022 | 0.88 |
| M vs. F | 11.46 | 0.0012* |
| Testosterone, pre-GnRH challenge | ||
| SDF vs. VAF | 1.45 | 0.23 |
| M vs. F | 211.27 | <.0001* |
| Testosterone, post-GnRH challenge | ||
| SDF vs. VAF | 0.55 | 0.46 |
| M vs. F | 888.14 | <.0001* |
| Testosterone, pre-LH challenge | ||
| SDF vs. VAF | 0.54 | 0.46 |
| M vs. F | 141.43 | <.0001* |
| Testosterone, post-LH challenge | ||
| SDF vs. VAF | 0.10 | 0.75 |
| M vs. F | 841.80 | <.0001* |
| LH, pre-GnRH challenge | ||
| SDF vs. VAF | 0.026 | 0.87 |
| M vs. F | 0.14 | 0.71 |
| LH, post-GnRH challenge | ||
| SDF vs. VAF | 0.03 | 0.85 |
| M vs. F | 8.04 | 0.0061* |
F = Female, M = Male, SD = South Dakota, VA = Virginia. Significant results (p < 0.05) denoted with asterisk.
3.3 Hormonal response to GnRH and LH injections
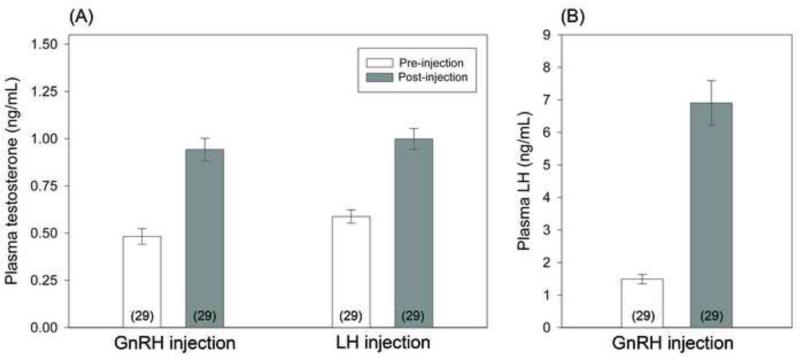
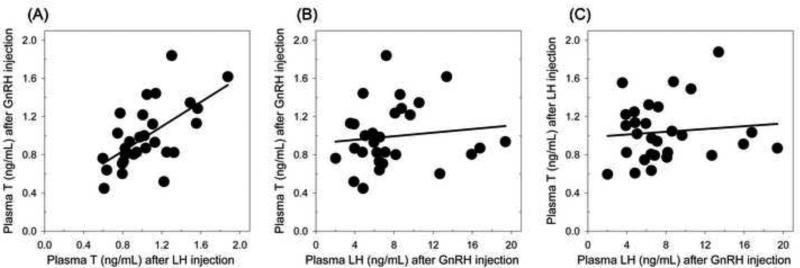
With respect to T levels in females, repeated measures linear mixed models revealed a significant effect of timepoint (pre versus post: F = 99.0, p < 0.001) and injection type (GnRH versus LH: F = 4.52, p = 0.036), but no significant interaction (F = 1.35, p = 0.25; whole model: R2adj = 0.56, p < 0.001; Figure 1A). The significant effect of injection type appears to relate to marginally higher pre- and post-injection T levels in relation to the LH challenge; however, there was no significant difference between baseline T levels before each injection (Tukey test, p = 0.10) or between elevated levels after each injection (Tukey tests, p = 0.90). Both injection types lead to significant elevation in T (Tukey test, pre versus post, p < 0.0001). Post-injection T levels were strongly correlated among individuals between the two injection types (Pearson r = 0.60, n = 29 females, p = 0.0005; Figure 2A), consistent with this apparently similar T elevation after each injection type.
Figure 1. Female hormonal response to GnRH and LH challenges.
(A) Testosterone levels in plasma collected 30 min after GnRH injection and LH injection were significantly higher than baseline testosterone levels. (B) Plasma LH levels collected 5 min after GnRH injection were significantly higher than baseline. Data show back-transformed means and error bars represent one standard error. Sample sizes shown in parentheses.
Figure 2. Correlations among hormonal responses.
(A) Plasma T levels following GnRH and LH injections were positively correlated among females, but (B) there was no relationship between plasma T and plasma LH after GnRH injection (B) or after LH injection (C) among females.
Repeated measures linear mixed models also revealed that females significantly elevated LH levels in response to GnRH injection (R2adj = 0.75, F1,28 = 147.9, p < 0.0001; Figure 1B), but post-challenge LH levels were unrelated to T levels induced by either GnRH injection (Pearson r = 0.20, n = 29 females, p = 0.31; Figure 2B) or LH injection (Pearson r = 0.16, n = 29 females, p = 0.40; Figure 2C).
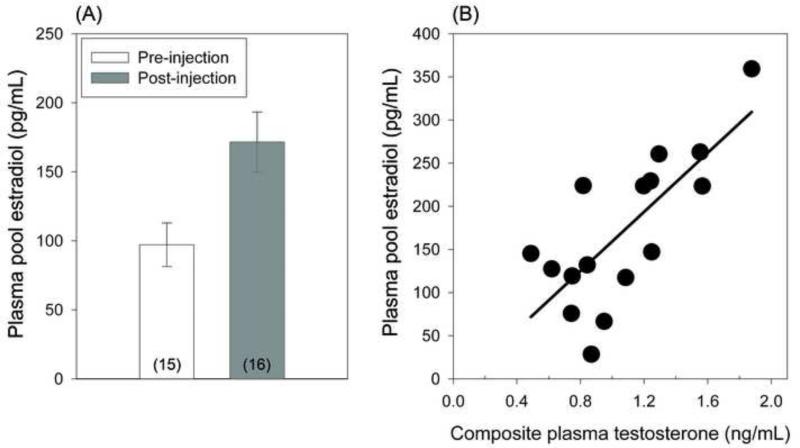
Plasma pools collected after females were challenged with GnRH or LH had significantly higher levels of estradiol than baseline plasma samples (Mann Whitney: Z = −2.39, p = 0.017, n= 16 post, n = 15 pre; Figure 3A). Among post-challenge plasma pools, concentrations of estradiol and testosterone were significantly positively correlated (Spearman's r = 0.63, p = 0.0094; Figure 3B).
Figure 3. Female testosterone and estradiol.
(A) Plasma levels of estradiol were significantly higher than baseline after GnRH or LH injection (mean ± one standard error). Sample sizes of plasma pools shown in parentheses. (B) Levels of estradiol and testosterone among post-injection plasma pools were positively correlated. Estradiol was directly assayed using pooled plasma, and the composite T value was mathematically calculated for each plasma pool based upon already assayed T levels from the individual samples used to make each plasma pool.
3.4 Sources of inter-female variation in testosterone production
The best fit model revealed that testosterone levels following LH challenge were predictive of testosterone levels following GnRH challenge (R2adj = 0.34, F1,27 = 15.5, p = 0.0005, AICc = 8.29). None of the other endocrine parameters we assessed predicted significant variation in female T levels (i.e., no relationships between T and post-GnRH challenge levels of LH, abundance of gonadal LH-R mRNA, or androgen sensitivity in the rostral hypothalamus).
4. DISCUSSION
Our results reveal consistent individual differences in testosterone production in response to hormonal challenges in captive female songbirds. Similar to recent findings in males (Bergeon Burns et al., under revision), these individual differences do not appear to be the consequence of correlated variation across multiple tiers of the HPG axis because the amount of T a female produced in response to a GnRH challenge was not related to pituitary LH production, gonadal LH-R mRNA abundance, or hypothalamic AR and AROM mRNA abundance. Instead, individual variation in testosterone production appears to be primarily a function of the ovary, as evidenced by our finding that stimulation of an individual's pituitary with a peripheral GnRH injection led to roughly the same amount of T production as a more direct stimulation of the gonad with a peripheral LH injection (Figure 1A, 2A). We also found that plasma estradiol levels were significantly higher after these hormonal challenges, and post-injection T and E2 levels were positively correlated. Compared to males, hormonal activation of the HPG axis led to less T production and more LH production in females, and the sexes differed in some measures of sensitivity to hormones along the HPG axis as well. Unlike males, females from the two subspecies of juncos did not differ in any endocrine parameter that we assessed. Applying these findings in an evolutionary context, we note that these analyses of the sources of inter-female variation in sex steroid production can serve as a guide to the sources of variation that might underlie the evolution of hormone-mediated phenotypes. Furthermore, by comparing these patterns of variation to those observed in males, we gain insights into sexual conflict and co-evolution along the HPG axis.
4.1 Sources of consistent individual differences in female HPG axis reactivity
We found positive correlations between post-injection T levels following GnRH and LH challenges (Figure 2A, 1A). In addition, the amount of LH produced in response to GnRH challenge was not related to the amount of T produced in response to LH or GnRH injection (Figure 2B, 2C). Combined, these findings led us to conclude that much of the variation in T production among females lies downstream of the pituitary and is likely inherent to the ovary. Thus, pituitary output of LH does not appear to be a signal that conveys continuous quantitative information regarding the amount of T to be released by the gonad. This finding regarding LH is perhaps unsurprising, as hormones are often described as operating via step-function, such that quantitative variation is meaningful primarily with respect to thresholds (Adkins-Regan, 2005).
T secretion was not related to gonadal gene expression for LH-R, and it was also not related to abundance of hypothalamic AR mRNA or AROM mRNA, suggesting that individual variation in T production also may not lie in individual differences in gene expression related to sensitivity to LH in the gonads or sensitivity to steroid-mediated feedback at the top of the HPG axis, respectively. In other words, among-individual variation in HPG axis reactivity was not predicted by variation across multiple levels of the axis (i.e. a “high T” female was not also a “high LH” female or a “high LH-R female,” etc.). Although it is possible that analyses of protein levels might reveal relationships that our investigation of transcript abundance did not, these results suggest that while the tiers of the HPG axis are functionally connected (e.g. an LH surge is required to initiate gonadal sex steroid production), individual differences in T are largely a function of some aspect of the gonad (see also further discussion below). Consequently, our findings indicate that selection could theoretically modify T titers without necessarily effecting correlated changes across multiple components of the HPG axis.
Exactly which component of the gonad explains this variation is still unresolved, though there are several non-mutually exclusive possibilities. For example, glucocorticoid receptors are known to be expressed in gonadal tissue (Lattin et al., 2012), and acute stress may inhibit T secretion by acting directly at the level of the gonad (Deviche et al., 2012). Because each individual was held in a capture bag for the 30 min between injection and post-challenge sampling, HPA axes were surely activated, providing an opportunity for elevated CORT levels to act on the gonad to dampen the stimulatory effect of LH or GnRH on sex steroid secretion. Gonadotropin-inhibitory hormone (GnIH) and GnIH receptors are also present in the gonad, and can inhibit T secretion as well (McGuire and Bentley, 2010). In addition, enzymes involved in steroid biosynthesis (steroidogenic acute regulatory protein, 3-beta-hydroxysteroid dehydrogenase, etc.) have been associated with differences in T in certain medical conditions (Payne and Youngblood, 1995) and in association with different social statuses (Huffman et al., 2012). We are not aware of any study, however, that has linked any of these receptors or enzymes with individual variation in T, and our ongoing research is exploring individual variation in these factors that might promote or suppress T secretion by acting at the level of the gonad.
We also found that plasma samples collected after LH or GnRH challenges had significantly higher estradiol than baseline plasma samples, and that plasma T levels were positively correlated with plasma E2 levels (Figure 3). Thus, while we were not able to assess whether E2 production showed similar individual consistency or similar sources of variation compared to testosterone, the correlation between the two sex steroids certainly suggests that a female capable of producing high levels of T also may be capable of producing high levels of E2.
Our study suggests that the gonad is one major source of variation in individual differences in sex steroid levels in females, but there are a number of relevant factors that we did not investigate, including hormone transport and clearance mechanisms (Ball and Balthazart, 2008; Hau and Wingfield, 2011; Wingfield, 2012) or the contribution of the adrenals (Staub and DeBeer, 1997). For example, the adrenal glands are also known to produce and secrete androgens, primarily prohormones (e.g. DHEA), which are converted to other steroids in target tissues. It is feasible that adrenal secretions may have contributed to the individual differences in sex steroids measured in response to LH or GnRH challenges, though appreciable LH-dependent adrenal secretion of T and E2 is thought to be rare in healthy females in reproductive condition (Bernichtein et al., 2008). Further, we cannot yet say whether individual differences in sex steroid production are also related to one's tendency to release GnRH, the abundance of GnRH released in response to a particular environmental stimulus, the abundance of pituitary GnRH-receptors, or the mechanisms by which the HPG axis interacts with other endocrine axes. In the future, we hope to see additional studies that employ a similar inter-individual approach across these multiple levels, including assessments of both mRNA and protein abundance.
4.2 Implications for evolution and regulation of testosterone- and estrogen-mediated phenotypes
In the past few years, several researchers have emphasized the need to understand the causes and consequences of individual variation in hormones and hormone-mediated traits (Ball and Balthazart, 2008; Kempenaers et al., 2008; Williams, 2008). Despite a growing focus on individual variation (e.g. Bergeon Burns et al., 2013; McGlothlin et al., 2010; Rosvall et al., 2012; While et al., 2010), we still have a limited understanding of the mechanistic sources of individual differences in hormone levels, particularly in females, where studies on evolutionary physiology and functional variation in hormones have been particularly rare (Caro, 2012; Williams, 2012a).
Our results, however, revealed nearly 4-fold and 13-fold variation in post-challenge testosterone and estradiol levels, respectively, suggesting that there is ample variability in both T and E2 that could be important in the regulation and evolution of female mating behavior, fertility, or other fitness-related traits. For example, T and E2 are elevated in relation to oogenesis (Amiri et al., 1996), suggesting that individual differences in HPG axis reactivity may reflect variability in readiness to breed, e.g. if females that produce more sex steroids are better equipped to initiate oogenesis under appropriate environmental conditions (Williams, 2012a). Hormonal variation may instead exceed that which can account for individual differences in reproductive output, such that this variation is functionally relevant due to hormonal pleiotropy on other traits (Williams, 2005). For example, elevated E2 may have an excitatory effect on mating and sexual behavior by directly facilitating female neural response to male song (Hunt and Wingfield, 2004; Maney et al., 2006; Maney et al., 2008, but see Wingfield and Monk, 1994). Likewise, natural variation in and experimental manipulations of sex steroids have been linked with variation in female aggression, maternal care, immune function, and yolk steroid allocation (Cain and Ketterson, 2012; Jawor et al., 2007; Muller et al., 2011; Rosvall, 2013; Williams, 2005; Zysling et al., 2006).
A key issue in making sense of individual variation is determining whether it is a result of intrinsic or extrinsic sources. Most research has focused on extrinsic factors, such as the environment or other factors that change over time, including age or experience (Kempenaers et al., 2008). Our work can eliminate several of these factors in our system because all females in this study hatched the previous spring and had no prior breeding experience, thereby minimizing age and experiential contributions to HPG axis reactivity. All females were held in captivity on identical light regimes and in individual cages, thereby limiting variability related to season and social conditions, respectively. Because we minimized or eliminated many common extrinsic sources of variability (age, season, experience, social conditions) and we showed empirically that body mass did not predict hormonal response, we think it is most parsimonious to conclude that the individually consistent T elevation we observed relates to some intrinsic aspect of each individual, though the amount of individual variation explained may well change if these extrinsic sources were varied.
Further, this individual consistency suggests the potential for a heritable component because repeatability is the mathematical upper boundary for heritability (Boake, 1989; Falconer, 1989). While we did not measure true repeatability (i.e. multiple measures of the exact same trait), our finding that gonadal output is individually consistent is analogous to repeatability, again suggesting that the individual variation reported here may reflect true individual differences. Others have reported repeatable variation in response to a GnRH challenge in male animals (Bergeon Burns et al., under revision; Jawor et al., 2006), and a few studies suggest high heritability of T production (Mills et al., 2009; Prove, 1978; Robison et al., 1994). In addition, artificial selection has been shown to affect other endocrine systems (e.g. HPA, Evans et al., 2006), which can change rapidly during population divergence (Atwell et al., 2012). Thus, it seems likely that individual variation in HPG axis reactivity (and in this case, factors relating to reactivity of the gonad in particular) may indeed be a target of evolutionary change related to a number of steroid-mediated traits. Follow-up studies investigating the genetic or developmental factors that underlie this hormonal variation, including maternal effects and organizational effects of hormones, may well reveal the proximate mechanisms by which phenotypic change occurs.
Despite previous attempts to characterize the female T response to a GnRH challenge (Goymann and Wingfield, 2004; Jawor et al., 2007; Spinney et al., 2006; Wingfield et al., 1991), our study is among the few to show a significant elevation in T in females (Figure 1A), and it is likewise one of the first to show that this elevation is repeatable in females (Figure 2A). Two recent studies on captive female birds have shown that GnRH challenges elevated T while females were actively yolking eggs (Muller et al., 2011; Peluc et al., 2012), and one study showed weak, but significant, repeatability of T elevation (Peluc et al., 2012). Notably, our results suggest that female juncos are capable of pituitary and gonadal hormonal responses, even in the absence of yolky follicles. This finding differs from a previous study on free-living juncos that reported significant T elevation in response to a GnRH challenge only in the week preceding egg-laying (Jawor et al., 2007), though our findings concur with a study on free-living Northern cardinals (Cardinalis cardinalis) indicating that the ovary is capable of T production well outside of the egg laying period (DeVries et al., 2011). The size of the ovaries in our study was similar to other female songbirds in breeding condition (e.g. Wingfield et al., 1991), suggesting that these females possessed photostimulated ovaries that were physiologically capable of breeding, but they had not yet undergone exponential ovarian growth and development that is dictated by supplementary cues (Farner et al., 1966; Farner and Wingfield, 1980). In sum, the results of this study combined with findings from the field suggest that there are indeed extrinsic components to HPG axis reactivity, layered atop the intrinsic variability we characterized in this controlled study.
4.3 Comparison with males and implications for sexual conflict and co-evolution
High levels of circulating T are thought to be disadvantageous for females, and T levels appear to co-evolve between the two sexes (Ketterson et al., 2005; Mank, 2007; Møller et al., 2005). Consistent with this view and with previous research (Cornil et al., 2011; Dawson et al., 1985), we found that females had lower baseline T, post-challenge T, and hypothalamic AROM than males, but we did not find sex differences in AR mRNA abundance in the rostral hypothalamus. Females had higher post-challenge LH than did males, possibly related to the integral role of LH surges in ovulation (Farner and Wingfield, 1980), though females had less gonadal LH-R mRNA than males. Like males (Bergeon Burns et al., under revision), individual variation in female T production appeared to be a function of the gonad, but was not correlated with plasma LH levels or with gene expression related to hypothalamic sensitivity to androgen-mediated feedback or gonadal sensitivity to LH. Finding that variation in T lies within the sexually differentiated gonad may suggest a greater potential for independent evolution between the sexes than if we had found that multiple tiers of the HPG axis collectively contributed to individual differences in T production. However, within the gonad, both sexes use the same biochemical pathways to synthesize sex steroids, and both sexes locally express glucocorticoid and GnIH receptors, meaning that there still may be co-evolution between the sexes even at the level of the gonad.
Females did not show any of the population differences in endocrine physiology that we observed in males. Specifically, males from the larger and more ornamented South Dakota population showed reduced gene expression for hypothalamic AR and gonadal LH-R, compared to males in the Virginia population (Bergeon Burns et al., under revision), but there were no population differences in females in any parameter we assessed, even though females from the South Dakota were likewise larger and more ornamented than Virginia females. Thus, unlike the morphological features we assessed (tail white and body mass), components of the female HPG axis appear to be buffered from those ecological or evolutionary processes that generated population differentiation among males, be it the result of weak intersexual co-evolution, sex specific developmental effects, or sexual dimorphism in the evolutionary drivers acting on the HPG axis.
Supplementary Material
Acknowledgments
We are grateful to the University of Virginia's Mountain Lake Biological Station, Black Hills National Forest, and the Gorsuchs for access to field sites; to M Boser, R Hanauer, S Jayaratna, KE Cain, R Kiley, MP Peterson, K Roth, EM Schultz, R Stewart, E Swanger, C Taylor, S Wanamaker, C Wood, and E Zeller for assistance in the field and lab; to DL Maney and D Abebe for LH primer sequences; and to AF Parlow and NIDDK's National Hormone and Peptide Program for supplying ovine LH. This study was approved by the Bloomington Institutional Animal Care and Use Committee under protocols 06-242 and 09-037. The authors were supported by: NIH NRSA fellowship (F32HD068222) to KAR; NSF predoctoral fellowship and NSF DDIG (IOS-0909834) to CMBB; NSF IOS-0820055 to EDK, including an REU supplement; and NIH training grant (T32HD049336; “Common Themes in Reproductive Diversity”) to KAR, CMBB, and EDK; NSF IOS-0744705 to TPH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adkins-Regan E. Hormones and animal social behavior. Princeton University Press; Princeton, NJ: 2005. [Google Scholar]
- Adkins-Regan E. Do hormonal control systems produce evolutionary inertia? Phil Trans Royal Soc B. 2008;363:1599–1609. doi: 10.1098/rstb.2007.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri BM, Maebayashi M, Hara A, Adachi S, Yamauchi K. Ovarian development and serum sex steroid and vitellogenin profiles in the female cultured sturgeon hybrid, the bester. J Fish Biol. 1996;48:1164–1178. [Google Scholar]
- Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED. Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol. 2012;23:960–969. doi: 10.1093/beheco/ars059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Phil Trans Royal Soc B. 2008;363:1699–1710. doi: 10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeon Burns CM, Rosvall KA, Hahn TP, Demas GE, Ketterson ED. Examining sources of variation in HPG axis function among individuals and populations of the dark-eyed junco. Horm Behav. doi: 10.1016/j.yhbeh.2013.10.006. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeon Burns CM, Rosvall KA, Ketterson ED. Neural steroid sensitivity and aggression: comparing individuals of two songbird subspecies. J Evol Biol. 2013;26:820–831. doi: 10.1111/jeb.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernichtein S, Alevizaki M, Huhtaniemi I. Is the adrenal cortex a target for gonadotropins? Trends Endocrinol Metab. 2008;19:231–238. doi: 10.1016/j.tem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Boake CRB. Repeatability - its role in evolutionary studies of mating behavior. Evol Ecol. 1989;3:173–182. [Google Scholar]
- Cain KE, Ketterson ED. Competitive females are successful females; phenotype, mechanism, and selection in a common songbird. Behav Ecol Sociobiol. 2012;66:241–252. doi: 10.1007/s00265-011-1272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain KE, Rich MS, Ainsworth K, Ketterson ED. Two sides of the same coin? Consistency in aggression to conspecifics and predators in a female songbird. Ethology. 2011;117:786–795. doi: 10.1111/j.1439-0310.2011.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro SP. Avian ecologists and physiologists have different sexual preferences. Gen Comp Endocrinol. 2012;176:1–8. doi: 10.1016/j.ygcen.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J, Charlier TD. Organizing Effects of Sex Steroids on Brain Aromatase Activity in Quail. Plos One. 2011;6 doi: 10.1371/journal.pone.0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Follett BK, Goldsmith AR, Nicholls TJ. Hypothalamic gonadotropin-releasing hormone and pituitary and plasma FSH and prolactin during photostimulation and photorefractoriness in intact and thyroidectomized starlings (Sturnus vulgaris). J Endocrinol. 1985;105:71–77. doi: 10.1677/joe.0.1050071. [DOI] [PubMed] [Google Scholar]
- Deviche P, Gao S, Davies S, Sharp PJ, Dawson A. Rapid stress-induced inhibition of plasma testosterone in free-ranging male rufous-winged sparrows, Peucaea carpalis: Characterization, time course, and recovery. Gen Comp Endocrinol. 2012;177:1–8. doi: 10.1016/j.ygcen.2012.02.022. [DOI] [PubMed] [Google Scholar]
- DeVries MS, Holbrook AL, Winters CP, Jawor JM. Non-breeding gonadal testosterone production of male and female Northern Cardinals (Cardinalis cardinalis) following GnRH challenge. Gen Comp Endocrinol. 2011;174:370–378. doi: 10.1016/j.ygcen.2011.09.016. [DOI] [PubMed] [Google Scholar]
- DeVries MS, Winters CP, Jawor JM. Testosterone elevation and response to gonadotropin-releasing hormone challenge by male Northern Cardinals (Cardinalis cardinalis) following aggressive behavior. Horm Behav. 2012;62:99–105. doi: 10.1016/j.yhbeh.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J Evol Biol. 2006;19:343–352. doi: 10.1111/j.1420-9101.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Introduction to quantitative genetics. 3rd ed Longman Scientific & Technical; Essex, UK: 1989. [Google Scholar]
- Farner DS, Follett BK, King JR, Morton ML. A quantitative examination of ovarian growth in white-crowned sparrow. 130. Biological Bulletin. 1966:67. doi: 10.2307/1539953. [DOI] [PubMed] [Google Scholar]
- Farner DS, Wingfield JC. Reproductive endocrinology of birds. Annu Rev Physiol. 1980;42:457–472. doi: 10.1146/annurev.ph.42.030180.002325. [DOI] [PubMed] [Google Scholar]
- Follett BK, Scanes CG, Cunningh Fj. Radioimmunoassay for avian luteinizing-hormone. J Endocrinol. 1972;52:359–&. [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male-male androgen responsiveness - Revisiting the Challenge Hypothesis. Horm Behav. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Competing females and caring males. Sex steroids in African black coucals, Centropus grillii. Anim Behav. 2004;68:733–740. [Google Scholar]
- Greives TJ, McGlothlin JW, Jawor JM, Demas GE, Ketterson ED. Testosterone and innate immune function inversely covary in a wild population of breeding Dark-Eyed Juncos (Junco hyemalis). Funct Ecol. 2006;20:812–818. [Google Scholar]
- Gupta AK, Harrar SW, Fujikoshi Y. MANOVA for large hypothesis degrees of freedom under non-normality. Test. 2008;17:120–137. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays. 2007;29:133–144. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hau M, Wingfield JC. Hormonally-Regulated Trade-Offs: Evolutionary Variability and Phenotypic Plasticity in Testosterone Signaling Pathways. In: Flatt T, Heyland A, editors. Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-Offs. Oxford University Press; Oxford, UK: 2011. pp. 349–361. [Google Scholar]
- Hill JA, Enstrom DA, Ketterson ED, Nolan V, Ziegenfus C. Mate choice based on static versus dynamic secondary sexual traits in the dark-eyed junco. Behav Ecol. 1999;10:91–96. [Google Scholar]
- Holberton RL, Able KP, Wingfield JC. Status signaling in dark-eyed juncos, Junco hyemalis - plumage manipulations and hormonal correlates of dominance. Anim Behav. 1989;37:681–689. [Google Scholar]
- Huffman LS, Mitchell MM, O'Connell LA, Hofmann HA. Rising StARs: Behavioral, hormonal, and molecular responses to social challenge and opportunity. Horm Behav. 2012;61:631–641. doi: 10.1016/j.yhbeh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Hunt KE, Wingfield JC. Effect of estradiol implants on reproductive behavior of female Lapland longspurs (Calcarius lapponicus). Gen Comp Endocrinol. 2004;137:248–262. doi: 10.1016/j.ygcen.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, et al. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis). Gen Comp Endocrinol. 2006;149:182–189. doi: 10.1016/j.ygcen.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, et al. Testosterone response to GnRH in a female songbird varies with stage of reproduction: implications for adult behaviour and maternal effects. Funct Ecol. 2007;21:767–775. [Google Scholar]
- Kempenaers B, Peters A, Foerster K. Sources of individual variation in plasma testosterone levels. Phil Trans Royal Soc B. 2008;363:1711–1723. doi: 10.1098/rstb.2007.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integr Comp Biol. 2009;49:365–379. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V. Adaptation, exaptation, and constraint: A hormonal perspective. Am Nat. 1999;154:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Sandell M. Testosterone in females: Mediator of adaptive traits, constraint on sexual dimorphism, or both? Am Nat. 2005;166:S85–S98. doi: 10.1086/444602. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Wolf L, Ziegenfus C. Testosterone and avian life histories - effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am Nat. 1992;140:980–999. [Google Scholar]
- Krey LC, McGinnis MY. Time-courses of the appearance-disappearance of nuclear androgen plus receptor complexes in the brain and adenohypophysis following testosterone administration-withdrawal to castrated male rats relationships with gonadotropin secretion. Journal of Steroid Biochemistry. 1990;35:403–408. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- Lattin CR, Waldron-Francis K, Richardson JW, de Bruijn R, Bauer CM, Breuner CW, et al. Pharmacological characterization of intracellular glucocorticoid receptors in nine tissues from house sparrow (Passer domesticus). Gen Comp Endocrinol. 2012;179:214–220. doi: 10.1016/j.ygcen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Mank JE. The evolution of sexually selected traits and antagonistic androgen expression in actinopterygiian fishes. Am Nat. 2007;169:142–149. doi: 10.1086/510103. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology. 2011;26:412–423. doi: 10.1152/physiol.00032.2011. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Levavi-Sivan B, Biran J, Fernald RD. Plasticity of the reproductive axis caused by social status change in an African cichlid fish: I. Pituitary gonadotropins. Endocrinology. 2011;152:281–290. doi: 10.1210/en.2010-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J Evol Biol. 2008;21:39–48. doi: 10.1111/j.1420-9101.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am Nat. 2007;170:864–875. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Parker PG, Nolan V, Ketterson ED. Correlational selection leads to genetic integration of body size and an attractive plumage trait in dark-eyed juncos. Evolution. 2005;59:658–671. [PubMed] [Google Scholar]
- McGlothlin JW, Whittaker DJ, Schrock SE, Gerlach NM, Jawor JM, Snajdr EA, et al. Natural selection on testosterone production in a wild songbird population. Am Nat. 2010;175:687–701. doi: 10.1086/652469. [DOI] [PubMed] [Google Scholar]
- McGuire NL, Bentley GE. A functional neuropeptide system in vertebrate gonads: Gonadotropin-inhibitory hormone and its receptor in testes of field-caught house sparrow (Passer domesticus). Gen Comp Endocrinol. 2010;166:565–572. doi: 10.1016/j.ygcen.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Miller AH. Speciation in the avian genus Junco. University of California Publications in Zoology. 1941;44:173–434. [Google Scholar]
- Mills SC, Grapputo A, Jokinen I, Koskela E, Mappes T, Oksanen TA, et al. Testosterone-mediated effects on fitness-related phenotypic traits and fitness. Am Nat. 2009;173:475–487. doi: 10.1086/597222. [DOI] [PubMed] [Google Scholar]
- Møller AP, Garamszegi LZ, Gil D, Hurtrez-Bousses S, Eens M. Correlated evolution of male and female testosterone profiles in birds and its consequences. Behav Ecol Sociobiol. 2005;58:534–544. [Google Scholar]
- Muller W, Groothuis TGG, Goerlich VC, Eens M. GnRH - a missing link between testosterone concentrations in yolk and plasma and its intergenerational effects. Plos One. 2011;6 doi: 10.1371/journal.pone.0022675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan V, Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Titus RC, et al. Dark-eyed Junco (Junco hyemalis). In: Poole A, editor. The Birds of North America Online. Cornell Lab of Ornithology; Ithaca, NY. USA: 2002. [Google Scholar]
- Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52:217–225. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- Peluc SI, Reed WL, McGraw KJ, Gibbs P. Carotenoid supplementation and GnRH challenges influence female endocrine physiology, immune function, and egg-yolk characteristics in Japanese quail (Coturnix japonica). Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology. 2012;182:687–702. doi: 10.1007/s00360-011-0638-3. [DOI] [PubMed] [Google Scholar]
- Peterson MP, Whittaker DJ, Ambreth S, Sureshchandra S, Buechlein A, Podicheti R, et al. De novo transcriptome sequencing in a songbird, the dark-eyed junco (Junco hyemalis): Genomic tools for an ecological model system. 2012 doi: 10.1186/1471-2164-13-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prove E. Courtship and testosterone in male zebra finches (Taeniopygia guttata castanotis). Journal of Comparative Ethology. 1978;48:47–67. [Google Scholar]
- Robison OW, Lubritz D, Johnson B. Realized heritability estimates in boars divergently selected for testosterone levels. J Anim Breed Genet. 1994;111:35–42. doi: 10.1111/j.1439-0388.1994.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J Steroid Biochem Mol Biol. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Rosvall KA. Life history trade-offs and behavioral sensitivity to testosterone: an experimental test when female aggression and maternal care co-occur. PLos One. 2013 doi: 10.1371/journal.pone.0054120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, et al. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proceedings of the Royal Society B: Biological Sciences. 2012;279:3547–3555. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PJ, Dunn IC, Talbot RT. Sex-differences in the LH responses to chicken LHRH-I and LHRH-II in the domestic-fowl. J Endocrinol. 1987;115:323–331. doi: 10.1677/joe.0.1150323. [DOI] [PubMed] [Google Scholar]
- Soma KK, Bindra RK, Gee J, Wingfield JC, Schlinger BA. Androgenmetabolizing enzymes show region-specific changes across the breeding season in the brain of a wild songbird. J Neurobiol. 1999;41:176–188. [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: Relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Spinney LH, Bentley GE, Hau M. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis). Horm Behav. 2006;50:762–771. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Staub NL, DeBeer M. The role of androgens in female vertebrates. Gen Comp Endocrinol. 1997;108:1–24. doi: 10.1006/gcen.1997.6962. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Hayes TB, Licht P. Role of aromatization in testosterone-induced inhibition of luteinizing-hormone secretion in female turtles, Trachemys scripta. Biol Reprod. 1994;50:144–151. doi: 10.1095/biolreprod50.1.144. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Kriegsfeld LJ, Osugi T, Seong JY, Vaudry H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol. 2010;22:716–727. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- While GM, Isaksson C, McEvoy J, Sinn DL, Komdeur J, Wapstra E, et al. Repeatable intra-individual variation in plasma testosterone concentration and its sex-specific link to aggression in a social lizard. Horm Behav. 2010;58:208–213. doi: 10.1016/j.yhbeh.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Williams TD. Mechanisms underlying the costs of egg production. Bioscience. 2005;55:39–48. [Google Scholar]
- Williams TD. Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Phil Trans Royal Soc B. 2008;363:1687–1698. doi: 10.1098/rstb.2007.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TD. Hormones, life-history, and phenotypic variation: Opportunities in evolutionary avian endocrinology. Gen Comp Endocrinol. 2012a;176:286–295. doi: 10.1016/j.ygcen.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Williams TD. Physiological Adaptations for Breeding in Birds. Princeton University Press; Princeton, NJ USA: 2012b. [Google Scholar]
- Wingfield JC. Regulatory mechanisms that underlie phenology, behavior, and coping with environmental perturbations: an alternative look at biodiversity. Auk. 2012;129:1–7. [Google Scholar]
- Wingfield JC, Hegner RE, Lewis DM. Circulating Levels of luteinizing-hormone and steroid-hormones in relation to social-status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Journal of Zoology. 1991;225:43–58. [Google Scholar]
- Wingfield JC, Monk D. Behavioral and hormonal responses of male song sparrows to estradiol-treated females during the non-breeding season. Horm Behav. 1994;28:146–154. doi: 10.1006/hbeh.1994.1012. [DOI] [PubMed] [Google Scholar]
- Wolf WL, Casto JM, Nolan V, Ketterson ED. Female ornamentation and male mate choice in dark-eyed juncos. Anim Behav. 2004;67:93–102. [Google Scholar]
- Zera AJ, Harshman LG, Williams TD. Evolutionary endocrinology: The developing synthesis between endocrinology and evolutionary genetics. Annu Rev Ecol Evol Syst. 2007;38:793–817. [Google Scholar]
- Zysling DA, Greives TJ, Breuner CW, Casto JM, Demas GE, Ketterson ED. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis). Horm Behav. 2006;50:200–207. doi: 10.1016/j.yhbeh.2006.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.