Abstract
Two types of fat, white adipose tissue (WAT) and brown adipose tissue (BAT), exist in mammals including adult humans. While WAT stores excess calories and an excessive accumulation of fat causes obesity, BAT dissipates energy to produce heat through non-shivering thermogenesis for protection against cold environments and provides the potential for the development of novel anti-obesity treatments. The hypothalamus plays a central role in the control of energy balance. Specifically, recent observations indicate the importance of the dorsomedial hypothalamus (DMH) in thermoregulation. We have found that the orexigenic neuropeptide Y (NPY) in the DMH has distinct actions in modulating adiposity and BAT thermogenesis. Knockdown of NPY in the DMH elevates the thermogenic activity of classic BAT and promotes the development of brown adipocytes in WAT, leading to increased thermogenesis. These findings identify a novel potential target for combating obesity.
Keywords: dorsomedial hypothalamus, neuropeptide Y, adipogenesis, brown adipose tissue, thermogenesis, energy expenditure
Introduction
Brown adipose tissue (BAT) is a unique tissue that combusts chemical energy (lipids and glucose) to produce heat through non-shivering thermogenesis for protection against cold environments. Traditionally, BAT is considered to be present primarily in small mammals and human infants. Recently, using a combination of 18fluoro-labeled 2-deoxyglucose (18FDG) positron emission tomography and computed tomography (PET-CT) technology, active BAT has been detected in adult humans.1 Adult humans have increased BAT activity during cold exposure, but decreased BAT activity when they are overweight or obese.1 Animal studies have further shown that BAT possesses a remarkable capability for utilizing both lipids and glucose in the process of cold-induced BAT thermogenesis.2 Thus, although the critical role for BAT in adult humans in the maintenance of energy balance remains to be determined, the findings of active BAT in adult humans have led to a great interest in its practical potential for treating obesity/diabetes (e.g., searching for ways to elevate BAT activity and/or to turn WAT into BAT (browning of WAT) that burns calories instead of storing them).
Brown versus white adipose tissue
White adipose tissue and BAT are two distinct fat tissues. WAT is the primary body fat and consists of unilocular adipocytes that contain a large lipid droplet. WAT stores excess calories when energy intake exceeds energy expenditure. An excessive accumulation of WAT over time results in overweight and obesity, conditions that have been linked to various life-threatening diseases such as cardiovascular disease and type 2 diabetes. White adipocytes also secrete a number of hormones and/or cytokines such as leptin, which acts on the brain as a feedback signal of energy stores to regulate energy balance. Leptin limits food intake and elevates energy expenditure through both increasing anorectic (e.g., the proopiomelanocortin-derived neuropeptide alpha-melanocyte stimulating hormone) and decreasing orexigenic (e.g., neuropeptide Y, NPY) actions. Circulating leptin levels generally correspond to WAT mass. Although obese subjects have elevated leptin levels, they are commonly leptin resistant. In contrast to WAT, BAT is comprised of multilocular, mitochondrial-rich adipocytes that contain multiple small lipid droplets. BAT conducts non-shivering thermogenesis through BAT-specific mitochondrial uncoupling-protein 1 (UCP1). In brown adipocytes, while a process of β-oxidation of fatty acids generates the proton gradient, UCP1 mediates the proton influx back into the mitochondrial matrix and dissipates oxidative energy as heat instead of the synthesis of ATP. Therefore, the capability of energy-burning BAT provides a potential approach to increase caloric utilization and thus may be a target for obesity treatment.
Both WAT and BAT are innervated by the sympathetic nervous system (SNS), activation of which induces lipolysis in WAT and produces thermogenesis in BAT. Most intriguingly, sympathetic activation via treatment with β3-adrenergic receptor (β3-AR) agonists or cold stress causes the development of brown adipocytes in various white fat depots such as retroperitoneal, mesenteric, epididymal, and inguinal depots.3 These findings have been driving an exciting topic—browning of white adipocytes. So far, two main hypotheses or mechanisms of white to brown adipocyte transformation have been proposed. The theory of reversible physiological trans-differentiation between WAT and BAT implies the plasticity of white and/or brown adipocytes in all or most fat depots in rodents.4 This theory is supported particularly by the evidence of a lack of proliferating cells in β3-AR agonist-induced white to brown adipocytes and transdifferentiating intermediate paucilocular adipocytes in cold-induced brown adipocytes in WAT.4 Thus, β3-AR agonist– or cold-induced multilocular fat cells (brown adipocytes) in WAT are likely derived directly from unilocular white adipocytes. In contrast, Petrovic et al. have argued against this view and proposed two different progenitor cells in BAT and WAT.5 Specifically, classical brown adipocytes such as interscapular BAT are derived from adipomyocytes (a myf-5 lineage) that share their origin with myocytes, whereas atypical brown adipocytes termed brite adipocytes (or beige cells) develop from an origin (a non-myf-5 lineage) that shares a common precursor with white adipocytes.5 Consistent with this view, Wu et al. have recently identified these beige cells as a distinct type of thermogenic fat cell in both mouse and human.6 Nevertheless, despite the clear evidence showing the formation of brown or brown-like adipocytes in WAT, the underlying mechanisms (transdifferentiation versus a distinct brown-like fat cell) remain a topic of debate.
Hypothalamic signaling in BAT thermogenesis
The hypothalamus plays a central role in the regulation of body weight and is comprised of several distinct nuclei that integrate peripheral and central signals of body energy status to modulate food intake and energy expenditure to maintain body weight or energy homeostasis. For instance, lesions of the ventromedial hypothalamus (VMH) result in hyperphagia and obesity, whereas lesions of the dorsomedial hypothalamus (DMH) cause hypophagia, weight loss, and reduced linear growth. A variety of evidence has also shown that the hypothalamus serves as an important relay involved in thermoregulation and regulates BAT non-shivering thermogenesis to influence body temperature. In the early 1980s, Perkins et al. initially reported that electrical stimulation of the VMH increased interscapular BAT temperature while β-adrenergic blockade almost abolished this increase,7 suggesting the importance of the VMH in BAT sympathetic activation and thermogenesis. On the basis of this and other similar findings, the VMH has been proposed as an important hypothalamic region involved in the regulation of BAT thermogenesis,8 although negative results have also been reported.9
By a detailed characterization of the role of various hypothalamic nuclei in body temperature control, Zaretskaia et al. actually demonstrated that the DMH, not the VMH, is an important hypothalamic region of thermoregulation.9 They found that disinhibition of neurons in the DMH, but not the VMH, by unilateral microinjection of the γ-aminobutyric acid A (GABAA) receptor antagonist bicuculline (10 pmol/50 nl), increased interscapular BAT and core body temperature.9 Thus, the authors interpreted the findings of the thermogenic effects of the VMH to be likely derived from the effects of neighboring DMH neurons due to the possibility of diffusion of the large volume of VMH injection, or electrical stimulation or disruptions of fibers of passage through the VMH.10 In support of the role for the DMH, the neural circuitry studies showed that DMH neurons were heavily detected by the transneuronal tracer (pseudorabies virus) that was injected into the interscapular BAT whereas VMH neurons were not or rarely detected.11 Overall, data from the laboratories of Dimicco, Morrison, and others have provided strong evidence indicating that the DMH acts as an intermediate relay receiving inputs from the hypothalamic preoptic area (POA), a neuronal site integrating central and peripheral thermal signals, and sending the outputs to the rostral raphe pallidus (rRP) in the medulla, the area containing premotor neurons that innervate SNS to interscapular BAT, to modulate BAT thermogenic function.10,11
Recent reports have also shown the roles for other hypothalamic nuclei including the paraventricular nucleus (PVN), lateral hypothalamus (LH), and arcuate nucleus (ARC) in the regulation of BAT activity and thermogenesis. Melanocortin-4 (MC4) receptor–expressing neurons in the PVN, at least in part, influence sympathetic outflow to interscapular BAT, and acute parenchymal microinjection of the MC3/4 receptor agonist melanotan II into the PVN increases interscapular BAT temperature in hamsters,12 whereas Madden and Morrison have shown that neurons in the PVN inhibit sympathetic outflow to BAT likely via activation of a GABAergic input to BAT sympathetic premotor neurons in the raphe pallidus.13 Moreover, an orexinergic projection from the perifornical hypothalamus to rRP increases BAT thermogenesis in rats.14 Arcuate nucleus NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN of mice.15 Together, the role of hypothalamic signaling in the regulation of BAT thermogenesis is likely more complicated (including multiple hypothalamic nuclei) than once thought. The complete neural mechanisms and pathways of hypothalamic control of thermoregulation remain to be elucidated.
DMH NPY in BAT thermogenesis
Lesions of the DMH result in hypophagia and weight loss, suggesting that the output of the DMH is primarily orexigenic. Consistent with this view, recent studies indicate that DMH NPY serves as one of the important mediators of the actions of DMH. NPY-expressing neurons have been found in the DMH, particularly in the compact subregion, in both normal growing rats and nonhuman primates.16 Using the rat model, we have determined the actions of NPY in the DMH in the control of food intake and energy expenditure. Although detailed neural circuits of DMH NPY in the control of energy balance remain to be determined, evidence has shown that NPY neurons in this DMH region project to the nucleus of the solitary tract in the hindbrain of rats, and through this pathway, at least in part, DMH NPY modulates food intake.17 Consistent with such a role, while NPY overexpression in the DMH of Otsuka Long Evans Tokushima fatty (OLETF) rats contributes to increases in food intake and body weight, leading to obesity and type 2 diabetes, knockdown of NPY in the DMH using adeno-associated virus (AAV)–mediated NPY-specific RNAi (AAVshNPY) significantly ameliorates these alterations in OLETF rats.17 DMH NPY knockdown also prevents high-fat diet–induced increases in food intake and body weight.18
In addition, while DMH NPY overexpression causes a reduction of Ucp1 expression in interscapular BAT, DMH NPY knockdown results in increased expression of Ucp1 in interscapular BAT,18 suggesting that DMH NPY appears also to modulate interscapular BAT activity or thermogenesis. To test this hypothesis, we conducted an experiment to directly examine the effect of DMH NPY knockdown on interscapular BAT temperature using a radio transmitter device (E-Mitter, Mini Mitter, Bend, Oregon). We buried an E-Mitter device under interscapular BAT in rats with DMH NPY knockdown and monitored fat temperature across multiple days. We found that, at room temperature (23 ± 1 °C), interscapular BAT temperature was significantly increased by an average of 0.43 °C during the dark, and there was a trend for increases during the light in NPY knockdown rats receiving DMH injection of AAVshNPY compared to rats receiving DMH injection of the control vector AAVshCTL. Thus, these results demonstrated that in addition to the feeding effect, DMH NPY has actions in modulating interscapular BAT thermogenesis to affect energy expenditure. Although the neural circuitry of POA–DMH–rRP–BAT in BAT thermogenesis has been documented,10,11 whether DMH NPY is involved in this neural pathway remains to be determined.
DMH NPY knockdown promotes browning of white adipocytes
To examine the adiposity effect of DMH NPY, we evaluated WAT depots in rats with DMH NPY knockdown and noted that the color of subcutaneous inguinal fat in NPY knockdown rats became significantly dark or brown compared to that of control rats,18 implying the possibility that DMH NPY knockdown promotes the development of brown adipocytes in inguinal WAT. Follow-up studies evaluated this possibility using a number of approaches. First, hematoxylin and eosin staining showed that inguinal adipocytes of NPY knockdown rats formed new large clusters containing multilocular adipocytes (brown fat–like adipocytes).18 Second, immunohistochemistry with a specific antibody against the BAT marker UCP1 detected UCP1 immunostaining in these newly formed fat cells and in a number of unilocular adipocytes around these clusters.18 Third, real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analyses identified UCP1 at both mRNA and protein levels in this inguinal fat, respectively.18 Finally, a study of sympathetic denervation on inguinal fat determined that DMH NPY knockdown alters sympathetic outflow to inguinal fat. Norepinephrine (NE) levels were significantly increased in the inguinal fat of NPY knockdown rats, implying that DMH NPY knockdown causes increased sympathetic outflow to inguinal fat.18 Injection of the neurotoxin 6-hydroxydopamine (6-OHDA) into inguinal fat area significantly lowered NE levels (i.e., causing a sympathetic denervation).18 This sympathetic denervation prevents DMH NPY knockdown–induced browning of inguinal WAT as shown by the finding that the number of brown fat–like adipocytes (multilocular adipocytes) and UCPl expression was largely abrogated in the inguinal fat with 6-OHDA treatment.18 Overall, these results provide strong evidence that DMH NPY knockdown promotes the development of brown adipocytes in inguinal WAT (browning of inguinal WAT) through affecting the local SNS. The origin of these fat cells (derived from beige/brite cells or transdifferentiated from white adipocytes) remains to be determined.
To assess whether this transformed inguinal BAT has similar BAT function, we conducted an additional experiment to examine the effect of DMH NPY knockdown on inguinal fat temperature using a radio transmitter device that was buried under the inguinal fat. We found that, at room temperature c(23 ± 1 °C), inguinal fat temperature was significantly increased by an average of 0.3 °C during the dark period in rats with DMH NPY knockdown compared to control animals, indicating that the transformed inguinal BAT has thermogenic actions.
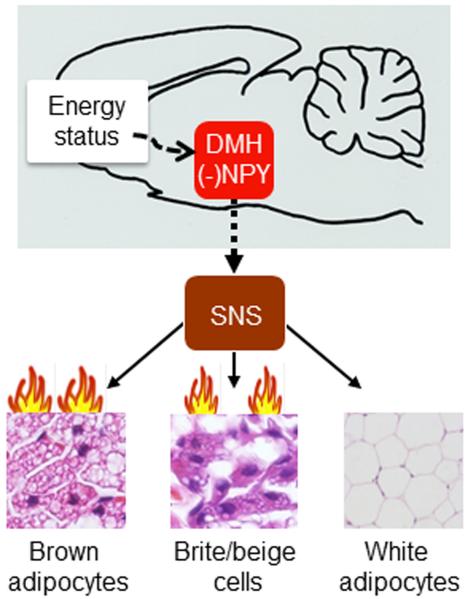
In summary, recent observations have underscored the significance of the DMH in brain thermoregulation and BAT thermogenesis. The neural circuitry of POA-DMH-rRP-BAT has been implicated in BAT thermogenesis and body temperature control. DMH NPY serves as an important neural signal to regulate energy expenditure and BAT thermogenesis (Fig. 1), but it is unclear whether DMH NPY is involved in the POA–DMH–rRP–BAT system. Moreover, knockdown of NPY in the DMH elevates BAT thermogenesis and promotes browning of WAT, providing potential molecular insight into the central mechanism controlling BAT/WAT development and functions. A detailed characterization of this neural pathway and molecular mechanism could have a significant impact on the prevention and treatment of obesity and diabetes.
Figure 1.
Dorsomedial hypothalamic (DMH) neuropeptide Y (NPY) modulation of brown/white adipocyte formation and thermogenesis through the sympathetic nervous system (SNS). Knockdown of NPY in the DMH elevates thermogenic activity of interscapular brown adipose tissue. DMH NPY knockdown promotes the development of brown adipocytes (likely brite/beige cells) in the inguinal white adipose tissue and increases fat thermogenesis. A complete DMH NPY signaling pathway remains to be determined (indicated as a dashed line with arrow head).
Acknowledgments
This work was supported by U.S. National Institute of Diabetes and Digestive and Kidney Diseases Grants DK074269 and DK087888.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Enerback S. Human brown adipose tissue. Cell metabolism. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. doi:10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nature medicine. 2011;17:200–205. doi: 10.1038/nm.2297. doi:10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 3.Himms-Hagen J, et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. The American journal of physiology. 1994;266:R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 4.Cinti S. Between brown and white: novel aspects of adipocyte differentiation. Annals of medicine. 2011;43:104–115. doi: 10.3109/07853890.2010.535557. doi:10.3109/07853890.2010.535557. [DOI] [PubMed] [Google Scholar]
- 5.Petrovic N, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of biological chemistry. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. doi:10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. doi:10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981;289:401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. doi:10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 9.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain research. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- 10.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. doi:10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 11.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Frontiers in bioscience: a journal and virtual library. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song CK, et al. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;295:R417–428. doi: 10.1152/ajpregu.00174.2008. doi:10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;296:R831–843. doi: 10.1152/ajpregu.91007.2008. doi:10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. doi:10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi YC, et al. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell metabolism. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. doi:10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Bi S. Dorsomedial hypothalamic NPY modulation of adiposity and thermogenesis. Physiology & behavior. 2013 doi: 10.1016/j.physbeh.2013.03.022. doi:10.1016/j.physbeh.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, et al. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. doi:10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell metabolism. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. doi:10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]