Abstract
The hypothalamic supraoptic and paraventricular nucleus contain magnocellular neurosecretory cells (MNCs) that project to the posterior pituitary gland where they secrete either oxytocin or vasopressin (the anti-diuretic hormone) into the circulation. Oxytocin is important for delivery at birth and is essential for milk ejection during suckling. Vasopressin primarily promotes water reabsorption in the kidney to maintain body fluid balance, but also increases vasoconstriction. The profile of oxytocin and vasopressin secretion is principally determined by the pattern of action potentials initiated at the cell bodies. While it has long been known that the activity of MNCs depends upon afferent inputs that relay information on reproductive, osmotic and cardiovascular status, it has recently become clear that activity depends critically on local regulation by glial cells, as well as intrinsic regulation by the MNCs themselves. Here, we provide an overview of recent advances in our understanding of how intrinsic and local extrinsic mechanisms integrate with afferent inputs to generate appropriate physiological regulation of oxytocin and vasopressin MNC activity.
Keywords: Paraventricular nucleus, supraoptic nucleus, oxytocin, vasopressin, osmoregulation, reproduction
Introduction
Oxytocin and vasopressin (the anti-diuretic hormone) are secreted into the systemic circulation from the posterior pituitary gland. While oxytocin and vasopressin have many peripheral and central functions (1), circulating oxytocin is primarily known for its essential role in milk let-down during suckling and for its involvement in the normal progression of parturition, whereas circulating vasopressin principally acts in the kidney to promote water reabsorption, and on the vasculature to increase blood pressure via vasoconstriction. At least in the rat, oxytocin complements vasopressin actions in the kidney via promotion of natruiresis (2) to help maintain plasma osmolality.
There has been only one oxytocin receptor identified to date (3, 4). The many different cellular actions of oxytocin are mediated by activation of various G-protein (Gq/11, Gs and Gi) coupled intracellular signalling pathways (5, 6), the best characterised of which is activation of phospholipase C via Gq/11 (7). By contrast, the effects of vasopressin are mediated by V1a, V1b (V3) and V2 receptors, which are differentially distributed throughout the body. In the periphery, Gq/11-coupled V1a receptors are highly expressed in vascular smooth muscle and induce vasoconstriction in response to strong hypotensive or hypovolaemic stimuli (8); Gq/11-coupled V1b receptors are expressed in the anterior pituitary gland and mediate vasopressin-induced secretion of adrenocorticotrophic hormone (9); and Gs-coupled V2 receptors are highly expressed in the collecting duct principal cells of the kidney and mediate water and sodium reabsorption by increasing insertion of aquaporin-2 (10) and epithelial sodium channels (11) into the luminal membrane.
While regulation of receptor numbers and signal transduction can modulate the efficacy of hormone signalling, the temporal organisation of the effects oxytocin and vasopressin are largely determined by the profile of hormone secretion. The secretion of both hormones is principally regulated by the action potential discharge of the oxytocin- and vasopressin-synthesising neurones (magnocellular neurosecretory cells; MNCs) within the hypothalamus.
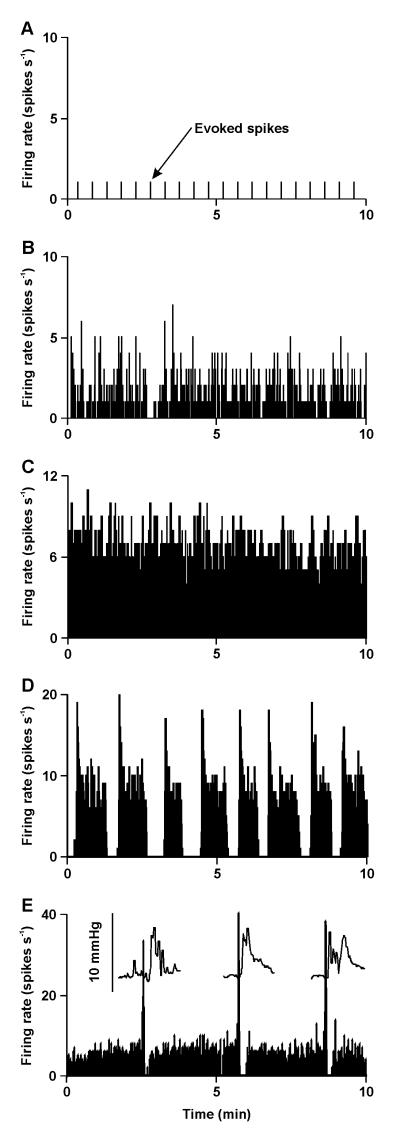
Under basal conditions, vasopressin MNCs maintain plasma vasopressin concentrations at ~1 - 3 pg ml−1 (12) to cause the reabsorption of ~30 litres of water from the urine each day in humans. Despite being exposed to the same prevailing physiological conditions, vasopressin MNCs display a wide range of activity patterns (13); some fire no spontaneous spikes (silent), some fire spikes irregularly, some fire continuously and some fire in a rhythmic ‘phasic’ pattern of bursts (Figure 1), during which there is clear spike frequency adaptation (a reduction in firing rate with time), separated by silent periods that each last tens of seconds (14, 15). It is the integrated output of the population of vasopressin MNCs produces the circulating concentration of vasopressin (16).
Figure 1. Activity patterning in magnocellular neurosecretory cells in vivo.
The ratemeter records show individual examples of the spontaneous activity (averaged in 1 s bins) of MNCs displaying each of the activity patterns typical of magnocellular neurosecretory cells (MNCs) in urethane anaesthetised rats. Under basal conditions, MNCs exhibit a range of activity patterns from silence (A), through irregular activity (B) to continuous activity (C) and, in vasopressin MNCs only, phasic activity (D). In A, action potentials were evoked in a silent MNC by electrical stimulation of the posterior pituitary gland every 30 s (evoked spikes). The recording in E is from an oxytocin MNC in a urethane-anesthetised rat being suckled during lactation and shows the typical high frequency bursts evident in oxytocin MNCs during parturition and suckling. The insets in E show the intramammary pressure increase for milk ejection that follows each burst of activity in the oxytocin MNC. Data for E was kindly provided by Prof J.A. Russell, University of Edinburgh.
Similarly to vasopressin MNCs, oxytocin MNCs display a range of activity patterns under basal conditions: silent, irregular or continuous firing that maintains circulating concentrations of oxytocin relatively constant at ~1 – 3 pg ml−1 (12). However, during parturition and lactation, oxytocin MNCs adopt a firing pattern comprised of high frequency co-ordinated bursts (17) that cause large pulses in oxytocin concentrations to induce rhythmic contraction of the uterus for birth and contraction of the milk ducts for milk ejection (Figure 1E).
The adoption and maintenance of the appropriate hormone secretion profile for the prevailing physiological conditions relies upon afferent inputs that relay information on reproductive and osmotic status. However, the regulation of activity is more complex than passive following of afferent input activity. It has recently become clear that the activity of MNCs is strongly influenced by glial cells and also that the patterning of activity is dependent upon intrinsic regulation by the MNCs themselves. A further layer of complexity is evident at a population level because hormone output is determined by the integrated activity of the population as a whole; for vasopressin MNCs this requires that some MNCs adopt different activity patterns in response to the same physiological conditions, whereas oxytocin MNCs must adopt the same activity pattern across the whole population under specific circumstances. Nevertheless, the biological message encoded by the neuronal population as a whole can be deduced from the physiological response to the secreted hormone, which allows interpretation of the function of activity patterning across the population of MNCs. In this review, we provide an overview of the means by which local and intrinsic mechanisms translate afferent information into appropriate activity patterns that generate the required physiological response.
The magnocellular neurosecretory system
Anatomy of the hypothalamo-neurohypohysial system
There are over 100,000 MNCs in the human (18) and ~10,000 in the rat (19). While MNCs are principally located in the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN) (Figure 2A – C), there are substantial numbers in some smaller accessory cell groups, of which the largest is the nucleus circularis (accessory magnocellular nucleus), located approximately mid-way between the PVN and SON (20).
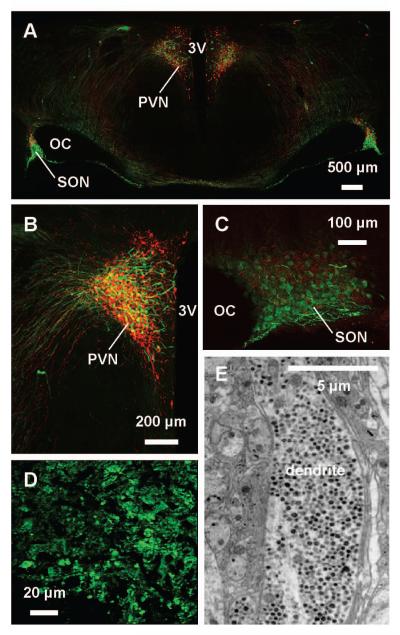
Figure 2. The magnocellular neurosecretory system.
A – C, Photomicrographs of coronal sections of rat hypothalamus (A), in which vasopressin magnocellular neurosecretory cells (MNCs) are immunostained with fluorescent green and oxytocin MNCs with fluorescent red. MNC cell bodies are principally found in the hypothalamic supraoptic nucleus (SON) (B), lateral to the optic chiasm (OC), and paraventricular nucleus (PVN) (C), lateral to the third cerebral ventricle (3V). The SON contains only MNCs that project to the posterior pituitary gland, whereas the PVN also contains parvocellular oxytocin and vasopressin MNCs (as well as other parvocellular neurones) that project elsewhere in the brain. D, Photomicrograph of vasopressin axon terminals in the posterior pituitary gland. E, Electron micrograph showing MNC dendrites densely packed with dense-core vesicles (small black dots). We thank Dr Vicky Tobin, University of Edinburgh, for generation of the immunohistochemistry images.
In terms of neuronal phenotypes, the SON contains essentially only MNCs, which synthesise either oxytocin or vasopressin. By contrast, the PVN also contains parvocellular neurones that project to the median eminence as well as to other brain regions (21). MNCs each project a single axon caudally and medially to collect in the hypothalamo-neurohypophysial tract, which courses through the internal zone of the median eminence to the posterior (neural lobe of the) pituitary gland (the neurohypophysis) where oxytocin and vasopressin are secreted into the general circulation (22). Within the posterior pituitary gland, each axon arborises extensively to give rise to several thousand neurosecretory axon swellings and terminals (Figure 2D). MNC axon terminals are tightly packed with dense-core (neurosecretory) vesicles that contain vasopressin or oxytocin, as well as a distinct population of microvesicles that contain glutamate (23). While has been widely accepted that MNCs project only to the posterior pituitary gland, it has recently been shown that some oxytocin MNCs (particularly those with cell bodies in the nucleus circularis) project axon collaterals to (at least) forebrain areas, including the central amygdala and nucleus accumbens (24).
Most MNCs have between one and three dendrites (25). In the SON, most dendrites course ventrally to the ventral glial lamina (a layer of glial cells on the ventral surface of the SON), where they form a plexus of dendritic bundles (26). In the PVN, the dendrites mainly track dorsally and medially towards the subependymal region of the third ventricle (27). MNC cell bodies and dendrites are also densely packed with dense-core vesicles (Figure 2E).
Secretion from magnocellular neurosecretory cells
Secretion of oxytocin and vasopressin from axon swellings and terminals in the posterior pituitary gland occurs by exocytosis of dense-core vesicles in response to action potential invasion of the neurosecretory membrane. Once released into the extracellular space, the hormones enter the general circulation by diffusion through fenestrated capillaries in the posterior pituitary gland (28). MNC terminals do not sustain intrinsic repetitive action potential discharge (29) and so hormone secretion is principally determined by the frequency and pattern of action potential discharge initiated at the cell bodies. Nevertheless, various factors can modulate stimulus-secretion coupling at MNC terminals in the posterior pituitary gland, including ionic conditions (30), purines (31) and neuropeptides (32).
The secretory response to increasing action potential firing rate is highly non-linear (Figure 3); in response to trains of electrical stimuli, oxytocin and vasopressin release from isolated posterior pituitary glands is facilitated as the frequency of stimulation increases (33). This ‘frequency facilitation’ of hormone release differs between oxytocin and vasopressin terminals. Frequency facilitation of oxytocin release is most marked between ~5 and 25 Hz, but release continues to increase (albeit with a slower rate of increase) beyond ~50 Hz. By contrast, frequency facilitation of vasopressin release is maximal at ~13 Hz and sustained higher frequency stimulation actually results in less vasopressin release (33, 34). Frequency facilitation of hormone release has been ascribed to increased calcium entry through voltage-gated channels to enhance exocytosis, as a consequence of action potential broadening during high frequency firing, depolarisation due to accumulation of potassium in the extracellular space, and a progressively more extensive invasion of the neurosecretory terminal field in the posterior pituitary gland (35). Frequency facilitation of hormone release is not sustained upon continuous stimulation, with a more pronounced ‘fatigue’ in facilitation of vasopressin release than oxytocin release (36). This fatigue is rapidly reversed when stimulation is stopped for a few tens of seconds. These properties of MNC terminals are important for generating the appropriate secretion of oxytocin and vasopressin to meet the prevailing physiological demands. A corollary of frequency facilitation of release is that MNCs firing at less than ~4 spike s−1 likely releases little or no hormone from the posterior pituitary gland in vivo (34).
Figure 3. Frequency facilitation of oxytocin and vasopressin release from magnocellular neurosecretory cells terminals.
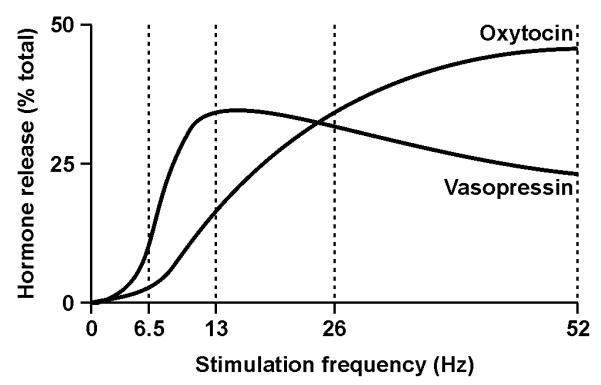
Isolated posterior pituitary glands were electrically stimulated with 156 pulses delivered at each of the four frequencies indicated in a balanced order of presentation. Evoked hormone release is expressed as a percentage of the total release evoked by the four stimulations. Note that hormone release is facilitated at higher frequencies, that little hormone is released at frequencies of < 4 Hz and that frequency facilitation of vasopressin release peaks at a lower frequency than for oxytocin release. Modified from (33), with permission.
Hence, while the profile of posterior pituitary secretion is orchestrated by the pattern of action potential discharge at the cell bodies, the terminals actively modulate the spread of the signal within the neurosecretory terminal field, as well as the efficacy of stimulus-secretion coupling, to fine-tune the gain of the secretory output generated.
Remarkably, MNC cell bodies and dendrites also release oxytocin and vasopressin by exocytosis (37) in response to mobilisation of intracellular calcium stores (38). A number of other neuropeptides are also synthesised by MNCs. For vasopressin MNCs, these include the κ-opioid peptide, dynorphin (39), as well as apelin (40), galanin (41), neuroendocrine regulatory peptides (NERPs) (42, 43), pituitary adenylate cyclase-activating polypeptide (PACAP) (44) and secretin (45), each of which are co-localised with vasopressin in dense-core vesicles (46). Similarly, oxytocin MNCs also synthesise other neuropeptides, including proenkephalin A-derived μ-opioid peptides (47) and dynorphin (48). In addition to neuropeptides, MNCs express high levels of nitric oxide synthase (49) and their dense-core vesicles contain large amounts of ATP (50) that is presumably secreted along with the neuropeptides during periods of activity. The physiological function of these co-released substances will be described in more detail later this review.
The glutamate contained within MNC microvesicles is also released in response to action potential invasion of the posterior pituitary gland. However, high frequency action potential discharge is thought to trigger release of neuropeptides in preference to classical neurotransmitters (51) and so, if glutamate is released from the axon terminals and this release is regulated as it is in other neurons, the profile of glutamate secretion is likely to be distinct from that of hormone secretion, with glutamate more readily released at low firing rates.
Activity patterning in magnocellular neurosecretory cells
Continuous activity in oxytocin and vasopressin magnocellular neurosecretory cells
While oxytocin and vasopressin MNCs can display markedly different action potential firing patterns, particularly when facing specific physiological challenges such as dehydration or pregnancy and lactation, most MNCs fire continuously under basal conditions, regardless of phenotype (52). While water deprivation for as little as six hours was initially reported to induce phasic activity in the majority of vasopressin neurons (51), we have found that the majority of MNCs are still continuously active after 48 h of water deprivation (13).
Although both oxytocin and vasopressin MNCs display continuous activity, there are marked differences in the rate and organisation of firing in continuous oxytocin and vasopressin MNCs in vivo. Oxytocin MNCs generally fire action potentials at a slower rate (~2 – 4 spikes s−1) than vasopressin MNCs (~4 – 6 spikes s−1) under basal conditions. The difference in basal spontaneous firing rate might be caused by differences in the organisation of continuous firing between the two cell types; these differences can be revealed by calculating the probability of the next action potential firing with time after the preceding action potential to generate a hazard function.
The hazard function reflects the post-spike excitability of MNCs (54), and shows that vasopressin MNCs are generally more excitable soon after each action potential than oxytocin MNCs (55) (Figure 4). For 10 – 20 ms after each action potential, both oxytocin and vasopressin MNCs are unexcitable and will not fire a further spontaneous action potential (the relative refractory period). Thereafter, excitability increases progressively in both oxytocin and vasopressin MNCs. However, while oxytocin MNC post-spike excitability increases from zero at ~20 – 30 ms after each spike to reach a steady-state hazard from ~70 ms after each spike, vasopressin MNC post-spike excitability overshoots (in both continuous and phasic vasopressin MNCs) before returning to a similar steady-state excitability as evident in oxytocin MNCs (55). These differences in post-spike excitability might contribute to the differences in spontaneous firing rate between oxytocin and vasopressin MNCs because chronic blockade of the overshoot of post-spike excitability in vasopressin MNCs (54) reduces vasopressin MNCs firing rate to ~1 spike s−1 and the firing rate of these MNCs is increased to ~4 spikes s−1 by acute reversal of the blockade (52).
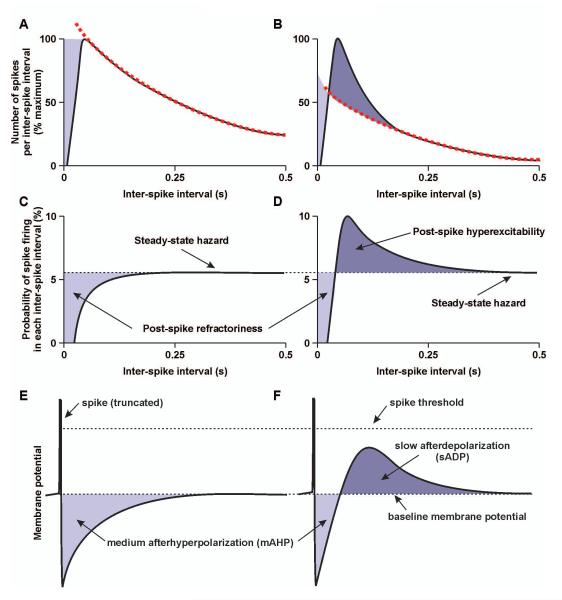
Figure 4. Post-spike excitability and post-spike potentials in magnocellular neurosecretory cells.
A and B, Schematic representations of inter-spike interval distribution of oxytocin (A) magnocellular neurosecretory cells (MNCs) and vasopressin MNCs (B). The tail of the distribution is fit by a single exponential decay (dashed line); for vasopressin MNCs, but not oxytocin MNCs, the exponential does not fit the peak of the distribution. C and D, Schematic representations of hazard functions for oxytocin MNCs (C) and vasopressin MNCs (D). Hazard functions are the probability of the next spike firing with time after the preceding spike and represent the post-spike excitability of neurones. Hazards are calculated from the inter-spike distributions using the formula: h[i-1, i] = n[i-1, 1] / (N – n[0, i-1]), where h[i-1, i] is the hazard at inter-spike interval i, n[i-1, 1] is the number of spikes in inter-spike interval, i, n[0, i-1] is the total number of spikes preceding the current inter-spike interval and N is the total number of spikes in all inter-spike intervals. E and F, Schematic representations of individual spikes from an oxytocin MNC (E) and a vasopressin MNC (F), with the associated changes in membrane potential caused by the medium afterhyperpolarization (mAHP) and slow afterdepolarization (sADP) (not to scale). MNCs exhibit a prominent post-spike mAHP that initially hyperpolarises the cell after each spike, making it less likely to reach spike threshold (post-spike refractoriness). Vasopressin MNCs also exhibit a prominent post-spike sADP that is lower amplitude and longer-lasting than the mAHP; the sADP increases causes post-spike hyperexcitability by bringing the membrane potential closer to spike threshold, making it more likely that on-going (stochastic) synaptic input will reach spike threshold to trigger a further spike. If a spike does not fire, the probability of spike firing returns a steady-state hazard that is inferred to reflect the baseline membrane potential and the on-going synaptic input activity.
Phasic activity in vasopressin magnocellular neurosecretory cells
While many vasopressin MNCs are silent, irregular or continuous under basal and stimulated conditions, it is generally accepted that phasic activity is the most efficient for secretion of vasopressin from the posterior pituitary gland (35).
Phasic activity is characterised by periods of activity that last at least 15 s (with the probability of burst termination being essentially stochastic thereafter) separated by silent periods that last for at least 10 s (with the probability of the next burst starting being essentially stochastic thereafter) (56). At the onset of bursts, vasopressin MNCs typically achieve firing rates of ~15 – 25 spikes s−1 for the first 5 – 10 s, before accommodating to a steady-state firing rate of ~ 6 spikes s−1 for the remainder of the burst (14, 15).
Given that vasopressin release is maximal at ~13 Hz (33, 34), the largest secretion of vasopressin is likely achieved during the first 5 – 10 s of each burst. As alluded to earlier, vasopressin release fatigues during continuous stimulation but this fatigue is reversed when stimulation is stopped for a few tens of seconds (36). Hence, the silent periods between bursts probably reset vasopressin MNCs to release further vasopressin at the onset of the next burst, when the typical firing rate is again in the range that is most efficient for vasopressin release.
The importance of the silent periods and burst duration for efficient vasopressin release is illustrated by the protection of these parameters from change during chronic osmotic stimulation (13, 53, 57, 58). By contrast, the firing rate within bursts has consistently been reported to be increased during chronic osmotic stimulation (13, 53, 57, 58). Hence, the increased intra-burst firing rate will keep phasic vasopressin MNC activity in the optimal range for efficient vasopressin release for longer during each burst but there will still be adequate time for recovery from fatigue of release between bursts.
The secretory profile of vasopressin depends on the integrated activity of the entire population of vasopressin MNCs, rather than the activity of any particular individual MNC. Phasic bursts are not synchronous across the population of vasopressin MNCs (14); this, coupled with the dampening effect of the (less efficient) continuous release from continuously-active vasopressin MNCs likely accounts for the smooth profile of vasopressin concentrations in the bloodstream in the face of highly variable vasopressin secretion from each phasic MNC. Hence, to maintain the appropriate level of vasopressin in the bloodstream (under both basal and stimulated conditions), individual vasopressin MNCs must not only sense the prevailing physiological conditions but co-ordinate their response with their neighbours to prevent over- or under-recruitment of MNCs to the actively-secreting phasic MNC pool. The mechanisms by which this co-ordination is achieved are unknown but might involve somato-dendritic release from MNCs.
While recruitment of all vasopressin MNCs to phasic activity has been reported during prolonged water deprivation (53), we and others have found that chronic osmotic stimulation does not increase the proportion of MNCs displaying phasic activity compared to the proportion evident under basal conditions it is unlikely (13, 57, 58). Plasma vasopressin concentrations are ~1 pg ml−1 in conscious rats and this increases to ~10 pg ml−1 during chronic osmotic stimulation (59). However, plasma concentrations can approach 100 pg ml−1 in response to extreme acute osmotic stimuli (60). Presumably, these very high concentrations are achieved when all vasopressin MNCs adopt phasic activity. Hence, it is likely that only the most extreme stimulation would induce phasic activity in all vasopressin MNCs because this would be expected to increase plasma vasopressin to concentrations well above the reported range. Thus, even under chronic stimulation, individual vasopressin MNCs must not only sense the changing physiological conditions but co-ordinate their individual response with those of their neighbours to mount the appropriate physiological response across the population of MNCs (16).
Generation of phasic activity in vasopressin magnocellular neurosecretory cells
While phasic activity was first observed in anaesthetised rats in the early 1970s (61), the mechanisms that generate phasic activity patterning are still the subject of debate. Phasic bursts were originally demonstrated to result from activation of an intrinsic after-potential in MNCs recorded from brain slices, specifically an afterdepolarisation (ADP) that sustains regenerative activity during each burst (62). However, the most recent data from organotypic cultures prepared from juvenile rats suggest that phasic activity is driven by glutamatergic synaptic inputs because EPSP frequency and amplitude appear to be increased during phasic bursts (63). It is likely that both processes contribute to the generation of phasic activity in vivo, as comprehensively reviewed elsewhere (14, 15).
Excitatory synaptic input activity is clearly essential for action potential discharge in vasopressin MNCs in vivo because administration of glutamate receptor antagonists will silence phasic MNCs (64-66), even under stimulated conditions (66). Hence, it is probable that the final event that causes membrane potential to cross threshold and trigger each action potential in vivo is an EPSP. Nevertheless, the probability of action potential firing in vivo is influenced by the occurrence of action potentials, as revealed by the hazard function analyses of post-spike excitability described above. These changes in post-spike excitability modulate the probability that any EPSP will reach threshold to trigger the next action potential and are thought to result from changes in post-spike potentials that follow each action potential in MNCs (55) (Figure 4): a large-amplitude fast after-hyperpolarisation (fAHP) that lasts <10 ms (67), a small-amplitude medium AHP (mAHP) that lasts several hundred ms (68, 69) and a very small-amplitude slow AHP (sAHP) that lasts several seconds (68, 70) as well as a large-amplitude fast ADP (fADP) that lasts a few hundred ms (71) and low-amplitude slow ADP (sADP) that lasts several seconds (72, 73). The influence of these post-spike potentials on activity patterning in phasic MNCs waxes as wanes because each has a different amplitude and time-course that allow them to dominate membrane potential at different times following each action potential, and to summate over different timescales to impact activity at different times over the course of bursts.
Briefly, for ~10 s after termination of the previous burst, MNCs remain silent while the neurone recovers from a prolonged post-burst hyperpolarisation (56) that is probably generated by sAHP summation. Thereafter, bursts are stochastically initiated (56), presumably when EPSPs cross threshold to generate an action potential. The large fADP that follows each action potential bootstraps further action potential firing to establish the burst (71) and probably, along with increased EPSP frequency and amplitude (63), supports the very fast firing evident in the first few seconds of each burst. The longer-lasting sADP summates to establish a plateau potential that maintains membrane potential relatively close to threshold to sustain activity during bursts (56). The influence of the sADP is moderated by the mAHP, which summates to induce spike frequency adaptation over the first ~5 – 20 s of bursts (67, 69), as well as by activity-dependent inhibition of the sADP (74). In addition to the sADP and mAHP, the fAHP and sAHP probably also modulate the amplitude of the plateau potential: the fAHP paradoxically increases firing rate during bursts via activation of a hyperpolarisation-activated inward current (IH) (75) and sAHP summation decreases firing rate (70). As stated above, bursts typically last at least 15 – 20 s, with the probability of burst termination being essentially stochastic thereafter (56). Hence, it appears that the dynamic interaction of the various post-spike potentials establishes each burst but that the opposing influences of various AHPs and ADPs eventually reach a steady state to maintain a relatively stable plateau potential, during which time burst termination occurs stochastically as stochastic spontaneous EPSPs eventually fail to reach threshold (or when a burst of IPSCs fires (74, 75)), at which point the plateau potential decays decreasing the probability of on-going EPSPs reaching threshold and the burst terminates.
Counter-intuitively, GABA might also be involved in initiating, as well as terminating, phasic bursts; bursts of action potential-independent miniature IPSCs (mISPCs) that last a few seconds have been recorded from SON MNCs in hypothalamic slices, and current injections simulating IPSC bursts initiated bursts of action potential discharge in silent MNCs via a rebound depolarization, as well as terminating on-going action potential discharge by driving the plateau potential away from threshold (77). However, these observations are difficult to reconcile with the observation that local antagonism of SON GABAA receptors does not excite vasopressin MNCs in vivo (78) and the recent in vitro finding that GABA is always excitatory on vasopressin MNCs (but not oxytocin MNCs) from adult rats (79).
Burst firing of oxytocin magnocellular neurosecretory cells during birth and lactation
While oxytocin is essential for milk ejection when the young suckle, it does not appear to be essential for delivery of the young at parturition because transgenic mice lacking oxytocin deliver live young that die of starvation unless fostered by mice with oxytocin (80). Nevertheless, oxytocin is required for the normal progression of birth because administration of an oxytocin receptor antagonist to mice during parturition delays (but does not prevent) the delivery of subsequent pups (81, 82). Oxytocin is the most powerful uterotonic agent known, but also stimulates release of prostaglandin F2α (PGF2α) from the endometrium (83). At the end of pregnancy, PGF2α release triggers luteolysis in rats and thus causes withdrawal of progesterone to induce labour. There is a striking increase in the expression of uterine oxytocin receptors at the end of pregnancy (84), which is also triggered by PGF2α release; deletion of the PGF2α receptor prevents the rise in uterine oxytocin receptor expression, rendering the uterus insensitive to oxytocin and thus causing delivery to fail (85). Hence, the interplay of oxytocin and PGF2α appears to be required for normal delivery.
As described earlier, the mean firing rate of oxytocin MNCs is generally ~2 – 4 spikes s−1. However, during parturition and suckling, intermittent high-frequency bursts occur every 5 – 10 min that are responsible for rhythmic contraction of the uterus and of the milk ducts. These bursts are superimposed upon this slow/irregular firing (86-89); bursts each last only 1 – 2 s, but the action potential activity is intense, often with firing rates of 50 – 100 spikes s−1. After each burst, oxytocin MNCs typically fall silent for several seconds, presumably to allow the neurones to recover from fatigue of hormone secretion. Because these bursts occur every few minutes, last for only a couple of seconds and are followed by a period of inactivity, the overall increase in firing rate is only about 1 – 2 spikes min−1 for each oxytocin MNC.
Oxytocin MNCs exhibit bursts only at parturition and during lactation; all other physiological stimuli increase the activity of oxytocin MNCs in a graded manner. This sustained release does not trigger uterine contraction or milk ejection; rather, sustained activation, such as elicited by hyperosmotic stimulation, actually inhibits bursting (90). Furthermore, pulses of oxytocin do not increase natriuresis in the kidney (91). Hence, these superimposed firing patterns allow oxytocin MNCs to simultaneously perform two distinct physiological functions.
Each burst triggers the transient release of a bolus of oxytocin from the posterior pituitary gland that causes the episodic contraction of the uterus, critical for delivery of the young, or of the milk ducts, essential for milk ejection (89). Administration of the μ-opioid receptor agonist, morphine, or the L-type calcium channel blocker, verapamil, silences oxytocin MNCs (92, 93) and prevents stimulation of oxytocin secretion (92, 94). Morphine administration interrupts parturition for up to an hour (95). Similarly, verapamil injected during lactation interrupts milk ejections for up to an hour (92). The morphine-induced interruption of parturition can be overcome by episodic administration of oxytocin to mimic the endogenous secretion profile, but not by continuous administration of the same amount of oxytocin (95). Similarly, episodic contraction of the milk ducts can be precipitated by repeated systemic injection of oxytocin, but not by strong stimulation of sustained oxytocin secretion (96). Hence the pattern of oxytocin release from the posterior pituitary gland generates the pattern of uterine and milk duct contraction.
Generation of burst firing in oxytocin magnocellular neurosecretory cells
The electrical activity of SON MNCs is correlated with intrauterine pressure during parturition (Figure 1E) and the phase relationship between MNC firing and uterine activity suggests that uterine contractile activity increases the acitivty of oxytocin MNCs, rather than simple uterine distension (97). These observations are consistent with the bursting activity of oxytocin MNCs being driven by afferent input activity in response to peripheral signals. However, the mechanisms that cause burst firing in oxytocin MNCs have been most extensively studied in lactation, when milk-ejection bursts occur every 5 – 10 min during suckling of the young, and have been extensively reviewed elsewhere (98). It is more difficult to explain how afferent inputs translate the continuous suckling of the young into episodic activation of oxytocin MNCs. Nevertheless, it has long been believed that the initial trigger for bursts is gated by afferent input because milk-ejection bursts only occur during active suckling in vivo. Furthermore, the number of action potentials in each burst increases as the number of pups suckled increases (99), consistent with afferent gating of bursts.
However, burst-like activity (although with lower intra-burst firing rates) has been induced in vivo in virgin female rats by mobilisation of calcium release from oxytocin MNC intracellular stores (38) and in vitro by α1-adrenoceptor agonist administration in a low-calcium medium to MNCs from lactating rats (76) and male rats (100), suggesting that bursting might be an emergent property of the oxytocin MNCs themselves within their local environment. As with phasic activity in vasopressin MNCs, burst firing of oxytocin MNCs likely arises from a combination of intrinsic and extrinsic processes.
During basal firing, the intervals between consecutive action potentials in oxytocin MNCs are typically at least 20 ms (55, 101). By contrast, during suckling-induced milk-ejection bursts in anaesthetised rats, almost half of the intervals between consecutive action potentials are less than 20 ms (101). Moreover, analysis of action potential discharge patterns in vivo shows that short inter-spike intervals are usually followed by long inter-spike intervals but that during bursts, short intervals are more likely to be followed by further short intervals (38). Taken together, these observations suggest that post-spike potentials in oxytocin MNCs change to favour the emergence of bursting activity during parturition and lactation. Indeed, over the course of pregnancy, the proportion of oxytocin MNCs that express a sADP increases to be more than double that evident in virgin rats (102, 103); this might cause the depolarisation that occurs during pharmacologically-induced bursts (76) and hence the ability of oxytocin MNCs to fire action potentials with very short inter-spike intervals during endogenous bursts. Whether a similar plasticity occurs in the fADP of oxytocin MNCs has yet to be determined. Counter-intuitively, the mAHP and sAHP are also increased in oxytocin MNCs of late pregnant and lactating rats (102-104), a change that appears to be dependent on central oxytocin receptors (105) as well as on prolonged calcium transients in oxytocin MNCs (104). The increased mAHP and sAHP might limit the duration of oxytocin MNC bursts; in this scenario, burst initiation results in summation of sADPs (and perhaps fADPs) to cause a marked depolarisation that triggers rapid action potential discharge while progressive activation of the mAHP would quickly reverse this depolarisation to reduce firing rate and terminate the burst (analogous to, but more pronounced than, spike frequency adaptation in phasic vasopressin MNCs). The concurrent activation of the more prolonged sAHP might then cause the silent period evident for a few seconds after each burst in vivo.
While plasticity in post-spike potentials favours the emergence of bursting activity during parturition and lactation, such changes are unlikely to trigger each individual burst. Synaptic glutamate release on oxytocin MNCs is enhanced during lactation (106), suggesting that increased excitatory synaptic efficacy might contribute to burst firing. The ability of mISPC bursts to trigger action potential discharge described above is evident in oxytocin MNCs as well as vasopressin MNCs (77), suggesting that a rebound depolarization after a burst of IPSPs might cause bursting in oxytocin MNCs. Indeed bursts of IPSPs might be more effective in late-pregnant and lactating rats because a switch in GABAA receptor subunit expression increases the duration of IPSCs at these times (107). In addition, the sustained outward potassium current that facilitates a rebound depolarization following a burst of IPSCs is enhanced in oxytocin MNCs from lactating rats (103, 108), favouring enhanced summation of IPSPs during birth and lactation when bursts of action potentials are required for delivery of the offspring and for delivery of milk to the new-born.
Co-ordination of burst firing across oxytocin magnocellular neurosecretory cells
While the adoption of burst firing by oxytocin MNCs is remarkable, an even more striking feature of these bursts is that they are co-ordinated across the population of oxytocin MNCs located in two distinct bilateral nuclei (17, 109); while bursts of action potentials from each individual oxytocin MNC will release oxytocin from that MNC, co-ordination among MNCs is required for the increased release to be translated into pulsatile release of oxytocin from the population of MNCs; this pattern of secretion causes the rhythmic contraction of the uterus during normal parturition as well as the episodic contraction of the milk ducts for milk ejection (97).
Although bursts are certainly co-ordinated, they are not entirely synchronous; there are no leaders and followers, and there is no synchrony between individual action potentials across the population (17, 109). Hence, the recruitment of the entire population of oxytocin MNCs to co-ordinated bursts of activity during parturition and lactation does not result from pacemaker activity within the population.
As explained earlier, bursts of mIPSCs have been recorded from SON MNCs that can initiate bursts of action potentials (77). Remarkably, some of these bursts were co-ordinated between recorded pairs of MNCs (77). Hence, co-ordinated bursts of mIPSPs might contribute to the co-ordination of bursts across the population of MNCs as well as the generation of bursts in individual MNCs.
Somato-dendritic secretion from magnocellular neurosecretory cells
MNC cell bodies and dendrites are densely packed with dense-core vesicles from which they release oxytocin and vasopressin by exocytosis (37). Somato-dendritic release is can be activity dependent (110, 111). However, it has been estimated that, on average, each MNC releases less than one vesicle every second from the somato-dendritic compartment (112). Hence, somato-dendritic exocytosis of individual dense-core vesicles from MNCs is unlikely to be triggered by individual action potentials. Indeed, sustained depolarisation, such as can be elicited in vitro by high extracellular potassium concentrations, markedly increases somato-dendritic release (38). Furthermore, somato-dendritic secretion can also be triggered by increasing intracellular calcium levels (38, 113, 114), which allows some stimuli, such as a α-melanocyte stimulating hormone, to increase somato-dendritic secretion while inhibiting electrical activity (115). Hence, the profile of somato-dendritic release does not necessarily follow the profile of secretion into the bloodstream.
Somato-dendritic vasopressin release
The membranes of vasopressin-containing neurosecretory vesicles contain V1a and V1b receptors (116) that are presumably inserted into the cell membrane and exposed to high local concentrations of vasopressin during exocytosis. Antagonism of V1a-receptors within the SON increases the activity of phasic MNCs in vivo (117), an effect that is evident from the onset of bursts (110), implying that inhibition by endogenous vasopressin emerges very rapidly at the onset of bursts, or that phasic MNCs are tonically inhibited by endogenous vasopressin. Given that vasopressin can be recovered from the extracellular fluid of the SON under basal conditions (118) and that phasic activity can be triggered in some silent MNCs by local administration of a V1a-receptor antagonist (117), it seems likely that at least some MNCs are tonically inhibited by endogenous vasopressin.
Exogenous vasopressin inhibits EPSC amplitude (119, 120), possibly via retrograde inhibition of excitatory synaptic transmission (121) as well as by autocrine inhibition. In addition, vasopressin increases IPSC frequency (122). Hence, somato-dendritic vasopressin release, which itself increases somato-dendritic release (123), provides a mechanism for autocrine/retrograde inhibition of vasopressin MNC activity (121, 124) to restrain the activity of the population of vasopressin MNCs.
While local administration of vasopressin inhibits vasopressin MNCs displaying robust phasic activity or continuous activity (117, 125), it has also been reported to excite vasopressin MNCs displaying irregular activity (125), suggesting that somato-dendritically released vasopressin should drive vasopressin MNCs towards phasic activity. Intracellular calcium levels are increased during phasic bursts (126, 127) and, consistent with the effects of vasopressin on phasic activity, application of vasopressin blocks spontaneous intracellular calcium oscillations in MNCs but triggers oscillations in non-oscillatory MNCs (128), providing a mechanism for the differential effects of vasopressin on MNC activity. The inhibitory actions of vasopressin are probably mediated by V1a-receptors because V1a-receptor antagonism increases the activity of phasic MNCs in vivo (110, 117), whereas the excitatory actions might be mediated by V1b-receptors. While there is no evidence to support the involvement of V1b-receptors in the excitation, vasopressin MNCs express V1b-receptors (116) and vasopressin activates phospholipase C (PLC) in vasopressin MNCs. Given that V1b-receptor activation stimulates PLC to increase adrenocorticotropic hormone secretion from anterior pituitary corticotrophs (129), it is possible that V1b-receptor mediated activation of PLC might trigger vasopressin-induced excitation of MNCs (130).
However, while exogenous vasopressin can drive MNCs towards phasic activity (125), most vasopressin MNCs do not display phasic activity under basal conditions (52, 53). Hence, while the activity of some MNCs are tonically regulated by endogenous vasopressin (117), it appears unlikely that there is sufficient vasopressin within the supraoptic nucleus to drive all vasopressin MNCs to adopt phasic activity. It has been estimated that each MNC only secretes one vesicle per second under basal conditions (112), and that this is sufficient to maintain extracellular concentrations of vasopressin in the PVN and SON that can be measured by microdialysis (131). Hence, vasopressin MNCs might be continuously exposed to vasopressin at levels that reflect the average activity of the vasopressin MNC population as whole. This has led to the proposal that somato-dendritic vasopressin might also act as a ‘population feedback signal’ that optimises activity across vasopressin MNCs via paracrine actions (112, 132). However, to date, there is no evidence that vasopressin released by one MNC can impact the activity of neighbouring MNCs, likely due to the mopping-up of vasopressin by extracellular peptidases (74). Indeed, when two vasopressin MNCs are recorded by a single extracellular electrode (and are thus presumably only tens of μm apart), there is no synchronisation of the onset or termination of phasic bursts between the two MNCs (14). Hence, it appears that somato-dendritic release of vasopressin from one MNC does not affect the activity of its neighbours, at least under basal conditions.
Neuropeptides co-released with vasopressin
Many other neuropeptides are also synthesised by MNCs (133) but the physiological significance of relatively few have been characterised to any great extent (124). For vasopressin MNCs, the co-localised neuropeptides with the best characterised effects on MNC activity are apelin (40), dynorphin (39), galanin (41), NERPs (42, 43), PACAP (44) and secretin (45). Similarly to vasopressin itself, vasopressin MNCs express receptors for most (and perhaps all) of these neuropeptides (44, 45, 134-138), providing a mechanism for autocrine feedback regulation of vasopressin MNCs by these co-released neuropeptides. Apelin was originally isolated from bovine stomach but is also expressed throughout the nervous system (139). Vasopressin MNCs express apelin (139, 140) as well as apelin receptors (APJ receptors) (138). While apelin is synthesised by vasopressin MNCs, there is a marked segregation of apelin and vasopressin labelling within MNCs (143), suggesting that the two peptides might be stored in distinct neurosecretory pools, and could therefore be differentially released in response to specific stimuli.
APJ receptor knockout mice have compromised responses to osmotic challenges (141). Furthermore, centrally-administered apelin inhibits vasopressin MNCs (40) to reduce basal vasopressin release (40, 142), suggesting that apelin might be an autocrine inhibitor of vasopressin MNCs. Consistent with this role, dehydration increases apelin and APJ receptor expression within the SON (40, 137), which might permit apelin to prevent over-excitation of vasopressin MNCs. However, these observations are confounded by the finding that intra-SON administration of apelin directly excites vasopressin MNCs (including phasic MNCs) by activation of non-specific cation channels (138). In addition, apelin reduces somato-dendritic vasopressin release (138), which would be expected to relieve the inhibitory vasopressin tone from at least some vasopressin MNCs.
It is possible that, similar to exogenous vasopressin (125), the effects of apelin depend on the activity of the vasopressin MNCs; the observations of apelin inhibition were made in lactating rats (40) in which vasopressin MNCs are hyperactive to preserve body water in the face of the increased demands of milk production (144), whereas the excitatory actions of apelin were evident under basal conditions (138).
While oxytocin MNCs also express APJ receptors, their activity is little affected by apelin administration under basal conditions (138), suggesting that apelin might only be effective against oxytocin MNCs under stimulated conditions.
Probably the best-characterised autocrine modulator of vasopressin MNC activity is the κ-opioid peptide, dynorphin (112). While κ-opioid binding sites have been demonstrated in the cortex of the kidney (145), as has mRNA for the κ-opioid receptor, the major diuretic effect of peripherally-applied κ-opioids appear to be mediated by inhibition of vasopressin MNCs (52, 54) to reduce vasopressin release because κ-opioids do not elicit diuresis in Brattleboro rats, which do not secrete vasopressin (146). Vasopressin MNCs express dynorphin (39) and κ-opioid receptors (135), but unlike apelin, these are located in the same neurosecretory vesicles as vasopressin (46, 147) but at much lower levels than vasopressin itself (148).
The κ-opioid inhibition of MNC activity is mediated by a reduction of evoked EPSP amplitude (149), mini EPSC frequency (150) and sADP amplitude (151). The inhibitory effects of exogenous κ-opioids reflect the actions of endogenous κ-opioid peptides because administration of a κ-opioid receptor antagonist prolongs phasic bursts in vivo (52, 56) and in vitro (56, 74) by increasing sADP amplitude (74) to increase plateau potential amplitude during phasic bursts (56), indicating that endogenous κ-opioid peptides reduce sADP amplitude to decrease plateau potential amplitude and thus decrease the probability of spike firing during bursts. Furthermore, κ-opioid receptor antagonism also prevents retrograde inhibition of EPSCs (150), indicating that endogenous κ-opioid peptides depress excitatory synaptic transmission, which would further reduce the probability of spike firing during bursts.
In contrast to the tonic vasopressin inhibition of phasic MNCs, endogenous κ-opioid inhibition of phasic MNCs is absent at the onset of each burst but emerges as bursts progress (110), suggesting that the progressive activity-dependent activation of κ-opioid receptors over the course of each burst helps shape phasic activity by contributing to the termination of phasic bursts (14, 15). Vasopressin MNCs displaying irregular activity appear to be more sensitive to endogenous κ-opioid inhibition than phasic MNCs (13, 152). Hence MNCs exhibiting increased irregular activity might be more sensitive than phasic MNCs to κ-opioids, which would cause more rapid termination of activity than evident in phasic MNCs, to generate the short periods of relatively high frequency firing often evident in irregular vasopressin MNCs. By contrast to endogenous κ-opioid inhibition of phasic and irregular vasopressin MNCs, vasopressin MNCs displaying continuous activity are not inhibited by endogenous κ-opioids (52), even under stimulated conditions (13). While continuously-active MNCs can be inhibited by exogenous κ-opioid peptides, they are not affected by a κ-opioid receptor antagonist delivered at the same doses that excite phasic MNCs (13, 52), suggesting that continuously-active vasopressin MNCs express functional κ-opioid receptors but are not exposed to endogenous dynorphin. Hence, changes in somato-dendritic dynorphin release might be involved in the transition between firing patterns in individual vasopressin MNCs (152).
Like dynorphin, galanin is co-expressed in vasopressin-containing neurosecretory vesicles. However, some neurosecretory vesicles contain galanin, but not vasopressin, and these vesicles are differentially targeted to the dendrites of vasopressin MNCs (41). Thus, galanin might be specifically involved in autocrine/paracrine regulation of MNCs.
While central galanin administration increases peripheral vasopressin secretion under basal conditions, it inhibits secretion during acute osmotic stimulation (153, 154), perhaps by inhibition of SFO neurone activity (155), modulation of monoaminergic inputs (156), presynaptic inhibition of mEPSC frequency (157), inhibition of the sADP (158) and/or direct MNC hyperpolarisation (158). The inhibition of mEPSC frequency is more profound in MNCs from dehydrated rats (157), perhaps as a result of GAL1 receptor up-regulation in MNCs during dehydration (136). Hence, galanin might provide a restraining influence on MNC activity, particularly under stimulated conditions, to prevent over-excitation of vasopressin MNCs.
NERPs 1-3 are co-localised with vasopressin in MNCs (42, 43) and the MNC expression of all three NERPs is increased during dehydration (43, 159), suggesting a role for regulation of vasopressin MNC activity by somato-dendritic release of NERPs.
MNCs express PACAP and its cognate receptor, both of which are up-regulated in the SON during dehydration (44). PACAP depolarises MNCs to increase their firing rate (126) but also increases somato-dendritic vasopressin release, which will presumably limit the duration of the PACAP-induced excitation (114).
MNCs express secretin as well as secretin receptors and central secretin administration increases plasma vasopressin concentrations (45), suggesting that somato-dendritic secretin might stimulate systemic vasopressin release. Chronic osmotic stimulation also increases plasma secretin concentrations (45) and, similar to vasopressin, secretin increases insertion of aquaporin-2 into the luminal membrane of the kidney to increase water reabsorption (160). Hence, secretin might act at multiple levels to assist in maintaining body fluid balance (161). However, systemic secretin stimulates vasopressin (and oxytocin) MNC activity via activation of noradrenergic inputs (162) and so the increased secretin levels evident during dehydration (160) would be expected to have a positive feedback effect; presumably this positive feedback loop is overridden by other negative feedback effects to prevent runaway activation of vasopressin secretion.
Somato-dendritic oxytocin release
Similar to vasopressin receptor expression by vasopressin MNCs, oxytocin MNCs express oxytocin receptors (163) to provide a potential mechanism for autocrine feedback regulation of activity. By contrast to the inhibitory effects of vasopressin on vasopressin MNC activity, the feedback effects of oxytocin appear to be stimulatory and are important for the positive feedback regulation of oxytocin secretion evident during parturition and lactation (164).
Oxytocin release into the SON increases immediately prior to each milk-ejection burst in MNCs (165). Central oxytocin administration facilities milk-ejection bursts in suckling rats (166) as does oxytocin injection into the SON or PVN (167).
While the overall effect of somato-dendritic oxytocin is excitation, the effects are complex and might be involved in burst generation by combined excitatory and inhibitory processes. Oxytocin suppresses IPSC amplitude, but not frequency (168), which would be expected to excite oxytocin MNCs by shifting MNC membrane potential closer to the threshold for spike initiation. Counter-intuitively, oxytocin also induces retrograde inhibition of EPSCs (169) by triggering release of endocannabinoids from the postsynaptic MNC (170), which (in combination with mAHP and sAHP activation (102-104)) might contribute to termination of milk ejection bursts and to the post-burst silence in oxytocin MNCs. Hence, somato-dendritically released oxytocin likely has a postsynaptic positive feedback effect to contribute to activity during bursts while activating endocannabinoid release for slower retrograde inhibition of excitatory synaptic drive to terminate bursts.
Remarkably, unilateral injection of oxytocin into one nucleus facilitates bursting of MNCs in contralateral nuclei (167), suggesting that somato-dendritic oxytocin release contributes to co-ordination of bursts across the population of oxytocin MNCs as well as to the generation of bursts in each MNC. Bursting has been modelled as an emergent behaviour of oxytocin MNCs sparsely connected by dendro-dendritic interactions (171). MNC dendrites tend to be separated from one another by glial processes under basal conditions but in late pregnancy and lactation, MNC dendrites are found in close apposition with other dendrites, forming dendritic bundles (172, 173). Each MNC has between one and three dendrites (25) and, in the model, each MNC has two dendrites that are each in different dendritic bundles, forming a sparse network of connections that allow somato-dendritic interactions between MNCs via oxytocin-induced release of endocannabinoids (171). This model replicates many of the features of co-ordinated milk ejection bursts in oxytocin MNCs, with bursting being initiated at any of the dendritic bundles (each of which contains only a few dendrites) and spreads rapidly through the remaining bundles (171).
Priming of somato-dendritic oxytocin release
The consequences of somato-dendritic oxytocin release are more complex than simple autoregulation of the electrical activity of the cells of origin. Somato-dendritic secretion does not always follow the action potential discharge that drives systemic secretion; e.g. systemic osmotic stimulation leads to prompt and long-lasting activation of oxytocin (and vasopressin) secretion into the systemic circulation but the associated increase somato-dendritic oxytocin (and vasopressin) release, is delayed by at least an hour compared to secretion into the circulation (174). Remarkably, after exposure of the SON to thapsigargin to mobilise calcium release from intracellular stores, somato-dendritic peptide release in response to osmotic stimulation (and other forms of stimulation) is dramatically potentiated in vivo and in vitro (38). This effect is long-lasting; priming oxytocin MNCs enhances somato-dendritic release in response to subsequent activity-dependent release for at least 90 min after exposure to thapsigargin. Priming in oxytocin (and vasopressin) MNCs is not a consequence of long-lasting elevation of intracellular calcium induced by thapsigargin. The thapsigargin-induced elevation of intracellular calcium lasts for ~5 min (175) but the potentiation of somato-dendritic release is only evident after 30min.
Thapsigargin induces an increase in intracellular calcium by inhibiting the endoplasmic reticulum calcium-ATPase to block the refilling of inositol triphosphate (IP3)-sensitive intracellular calcium stores. As a consequence, thapsigargin might also open store-operated cation channels to increase the effectiveness of subsequent stimuli such as depolarisation. Store-operated cation channels in MNCs are calcium permeable and are activated at membrane potentials more negative than –40mV (176). The irreversible depletion of intracellular calcium stores will permanently activate these currents. The resulting small but persistent calcium current might contribute to the activation of the calcium-sensitive exocytotic machinery, or increase voltage-dependent calcium influx by increasing the membrane potential range at which calcium influx occurs through voltage-gated channels.
Priming does not occur as a result of changes in translation/protein processing, but involves a translocation of dense-cored vesicles closer to the dendritic membrane. Thapsigargin treatment increases the number of dense-core vesicles located in the readily-releasable pool near the plasma membrane (177), indicating that the thapsigargin-induced priming involves a relocation of dense-core vesicles closer to sites of secretion, probably via actions on actin filaments to allow dense-core vesicles to leave the reserve pool (178).
Perhaps the most striking feature of priming is that oxytocin itself induces priming in oxytocin MNCs (38) (Figure 5) and oxytocin facilitates somato-dendritic oxytocin release (179). In contrast, vasopressin does not induce priming in vasopressin MNCs (113) and does not facilitate somato-dendritic vasopressin release (179). Somato-dendritic oxytocin release precedes milk-ejection bursts in oxytocin MNCs (165) and central oxytocin administration promotes bursting (180). Remarkably, induction of priming allows electrical stimulation to induce milk-ejection burst-like activity in oxytocin MNCs recorded from virgin female rats (38). Hence, oxytocin-induced priming might be required for bursting behaviour in oxytocin MNCs.
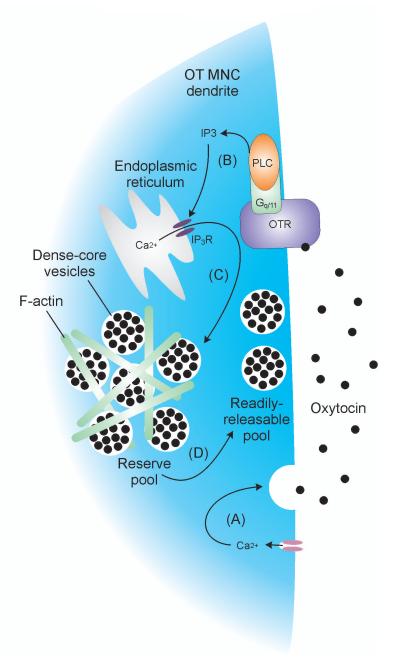
Figure 5. Priming somato-dendritic oxytocin release.
Calcium influx triggers somato-dendritic oxytocin release (A). Oxytocin activates oxytocin receptors on the plasma membrane to increase intracellular inositol triphosphate (IP3) concentrations (B). IP3 increases calcium release from the endoplasmic reticulum (C) to mobilise dense-core vesicles from the reserve pool to the readily-releasable pool (D), ‘priming’ oxytocin MNCs to release increased amounts of oxytocin in response to subsequent stimuli.
Neuropeptides co-released with oxytocin
By contrast to neuropeptides synthesised by vasopressin MNCs, the effects of somato-dendritic release of other neuropeptides synthesised by oxytocin MNCs are poorly characterised. Oxytocin MNCs also synthesise proenkephalin A-derived μ-opioid peptides (47) and dynorphin (48), as well as μ- (181) and κ-opioid receptors (182). While oxytocin MNCs are inhibited by administration of μ- and κ-opioid receptor agonists (96) and release from chronic opioid inhibition increases somato-dendritic oxytocin release (183, 184), it is unclear whether the endogenous opioid peptides are released from the soma and dendrites in sufficient quantities to alter oxytocin MNC activity under basal conditions; neither μ- (185) nor κ-opioid (52) receptor antagonism significantly affect the activity of oxytocin MNCs in vivo. It appears that the principal function of these endogenous opioid peptides might be as auto-inhibitors of secretion from the neurosecretory terminals in the posterior pituitary gland (32, 186).
Non-exocytotic somato-dendritic neurotransmitter/neuromodulator release
In addition to neuropeptides, MNC neurosecretory vesicles contain large amounts of ATP (50) and MNCs express various P2X receptors (187-189) as well as P2Y receptors (190), providing further potential mechanisms for somato-dendritic regulation of MNC activity (124). ATP injection into the SON induces anti-diuresis in anaesthetised rats (191) presumably via increased vasopressin secretion because ATP increases vasopressin (and oxytocin) secretion from hypothalamic explants (192). The ATP-induced increase in hormone secretion likely arises from a direct ATP-induced depolarization of MNCs (193) in combination with an ATP-induced increase in glutamate and GABA release from presynaptic neurones (194). While ATP is released by MNCs themselves, ATP can also be released from neighbouring glia (195) as well from noradrenergic afferents (196) to excite MNCs.
ATP is rapidly catabolised to curtail its actions (197), which generates extracellular adenosine. In anesthetised rats, adenosine A1 receptor antagonism increases the firing rate of phasic vasopressin MNCs (198). While the firing rate of continuously-active oxytocin and vasopressin MNCs are not affected by A1 receptor antagonism, the variability of firing is increased in both (198), suggesting that both are sensitive to endogenous adenosine feedback, although the physiological impact of this subtle action has yet to be determined. Endogenous adenosine causes activity-dependent enhancement of the mAHP in all MNCs (199) and in phasic MNCs this deepens spike frequency adaptation to shorten bursts (198) and might contribute to termination of milk-ejection bursts in oxytocin MNCs. Inhibition of the mAHP likely does not account for all of the effects of endogenous adenosine on MNCs because A1 receptor antagonism also inhibits activity-dependent depression of IPSCs and EPSCs via presynaptic receptor activation (200). MNCs also express A2A receptors and activation of these receptors causes a depolarisation-induced increase in firing rate (201). Nevertheless, the major functional role of endogenous adenosine appears to be inhibition because inhibition of adenosine uptake strongly inhibits spike discharge (202).
In addition to substances released by exocytosis, MNCs also release nitric oxide (NO). MNCs express high levels of nitric oxide synthase (NOS) (49) and neuronal NOS is upregulated in the SON during dehydration (203). Inhibition of NOS increases osmotically-stimulated secretion of oxytocin and vasopressin (204, 205), suggesting that MNC NO production restrains peripheral hormone secretion in response to osmotic stimulation. This restraint likely arises from NO inhibition of the firing rate of SON MNCs (206, 207) by increasing IPSC frequency and amplitude (207, 208). NO also restrains MNC responses to other stimuli (205, 209), suggesting that NO generation is a general inhibitory feedback regulator of MNC activity, regardless of the specific stimulus and of the phenotype of MNC.
MNCs also synthesize and release carbon monoxide (CO) in an activity-dependent manner; both oxytocin and vasopressin MNCs express the CO-synthesizing enzyme heme-oxygenase I, and this expression is enhanced during dehydration (210). In contrast to NO, CO appears to act as an excitatory gaseous transmitter, promoting firing activity in these neurons (210). Thus, there appears to be a balance between these two functionally opposing gaseous tranmsittser in the regulation of MNC activity, particularly during conditions of high hormonal demand.
Glial regulation of magnocellular neurosecretory cell activity
Glia have long been regarded as non-excitable cells that support and nourish neurones. However, it is now clear that glia are important participants in neuronal communication (Figure 6). In particular astrocytes actively modulate signalling at the tripartite synapse (211, 212). Activation of astrocytic α1-adrenoceptors releases ATP as a gliotransmitter at the tripartite synapse; this ATP activates P2X7 receptors on postsynaptic MNCs to increase calcium influx as well as activation of phosphotidyl inositol 3-kinase, and thus increase AMPA receptor insertion into the postsynaptic membrane to increase MNC sensitivity to synaptic glutamate (195). Similarly, presynaptic glutamate release can also liberate glial ATP via activation of metabotropic glutamate receptors (213, 214). Hence, astrocytes are active components of excitatory synaptic signalling to MNCs.
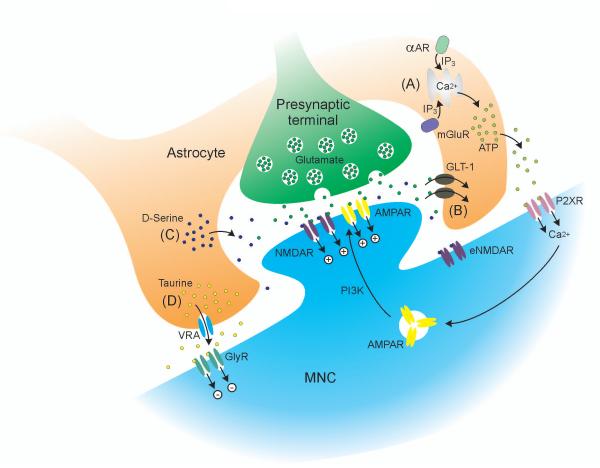
Figure 6. Glial regulation of magnocellular neurosecretory cell activity under basal conditions.
A, α1-adrenoreceptor (αAR) or group 1 and 5 metabotropic glutamate receptor (mGluR) activation increases intracellular calcium in astrocytes via inositol triphosphate (IP3) to trigger ATP release. ATP activates magnocellular neurosecretory cell (MNC) P2X receptors (P2XR) to increase calcium influx, which activates phosphotidyl inositol 3-kinase (PI3K) to increase AMPA receptor (AMPAR) insertion into the postsynaptic membrane, mediating long-term potentiation of glutamate synapses (synaptic scaling). B, Astrocyte glutamate transporters (GLT-1) remove glutamate from the extracellular space to limit activation of extrasynaptic NMDA receptors (eNMDAR). C, Astrocytes release D-serine to act as a co-agonist with glutamate at postsynaptic NMDARs on MNCs. D, Astrocytes release taurine through volume-regulated anion channels (VRA) to activate extrasynaptic glycine receptors (GlyR) to hyperpolarise the MNC via chloride influx.
Remarkably, astrocytes withdraw their processes from around MNCs during dehydration (215) and lactation (216) and this ‘glial retraction’ modulates the MNC responses to the prevailing physiological conditions (Figure 7). Indeed, glia retract only from oxytocin MNCs during lactation (217), suggesting that this might have a functional impact on bursting activity of oxytocin MNCs. However, preventing glial retraction by disruption of neural cell adhesion molecules does not alter milk-ejection bursts in oxytocin MNCs (218). Nevertheless, glial retraction has been shown to impact the excitability of MNCs in both dehydration and lactation, and might increase dendritic bundling to enhance intercellular communication between oxytocin MNCs and facilitate co-ordinated bursting during birth and lactation.
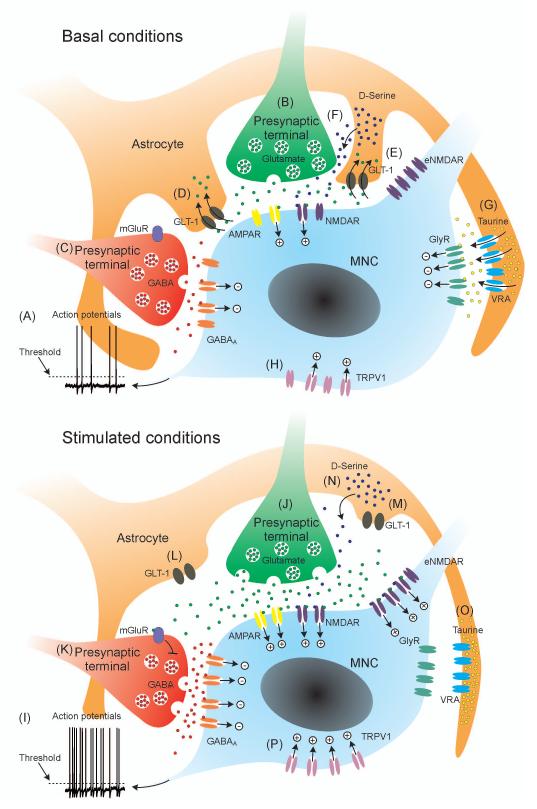
Figure 7. Integrated physiological modulation of magnocellular neurosecretory cell activity.
Under basal conditions (top panel) various excitatory and inhibitory intrinsic, local and afferent mechanisms integrate to generate low frequency action potential discharge (A) in magnocellular neurosecretory cells (MNC) to maintain relatively low and constant circulating oxytocin and vasopressin concentrations. Excitatory inputs, principally but not exclusively from glutamate neurones (B) depolarise the MNC for a short time after each synaptic event; if threshold is reached, post-spike potentials (not shown) will modulate membrane potential. In parallel with excitatory drive, inhibitory inputs, principally but not exclusively from GABA neurones (C) will transiently hyperpolarise the MNC. Astrocytes express glutamate transporter-1 (GLT-1), which prevents synaptically-released glutamate reaching metabotropic glutamate receptors (mGluR) on other afferent inputs (D) and extrasynaptic NMDA receptors (NMDAR) on MNCs (E). Astrocytes release D-Serine that acts as a co-agonist at NMDARs to permit glutamate activation (F) and taurine that activates extrasynaptic glycine receptors to tonically hyperpolarise MNCs (G). Some stretch-inactivated TRPV1 channels are active to tonically depolarise MNCs. Under stimulated conditions, each of these mechanisms is altered to increase the probability of action potential discharge (I). Glutamate release is increased to increase EPSC frequency (J). GABA release is also increased to increase IPSC frequency (K) to dampen the gain of the excitatory drive. Withdrawal of astrocytic processes (glial retraction) reduces the physical barrier to neurotransmitter diffusion and reduces the efficacy of GLT-1 to allow spillover of glutamate to activate mGluR on neighbouring GABA synaptic terminals (L), presumably to prevent over-inhibition. Glial retraction also allows glutamate to activate extrasynaptic NMDARs (eNMDAR) to induce a tonic depolarisation (M). A further consequence of glial retraction is to reduce the efficacy of D-serine released from astrocytes (N), presumably to limit excitation. When astrocytes shrink (e.g in hyperosmotic conditions), volume-regulated anion (VRA) channels close to reduce glycine release and reverse the tonic hyperpolarization mediated by GlyR (O). MNC shrinkage opens TRPV1 channels to increase the tonic depolarisation. The net effect of these changes is to drive baseline membrane potential towards threshold and increase noise in the baseline membrane potential, increasing the probability that action potential threshold will be reached. See text for details of the specific physiological conditions that modulate each of the mechanisms.
Glia act as a physical barrier to neurotransmitter/neuromodulator diffusion and also mop-up the major neurotransmitters via high affinity glutamate and GABA transporters (219, 220). When glia retract during lactation, extracellular neurotransmitter concentrations rise, increasing tonic activation of presynaptic metabotropic glutamate receptors (mGluRs), which inhibits further transmitter release (220, 221) to reduce synaptic efficacy at glutamatergic inputs on MNCs. Glutamatergic synaptic efficacy is also reduced by glial retraction during lactation due to a reduction in D-serine, which is released from glia to act as a co-agonist at the NMDA receptor (NMDAR) (222). Glial retraction during lactation decreases the physical barrier to diffusion away from the synaptic cleft, which also allows synaptically-released glutamate to more easily diffuse away from its site of release and activate presynaptic metabotropic glutamate receptors on GABAergic terminals to inhibit GABA release (223) to disinhibit MNCs. A further consequence of glial retraction is a switch in presynaptic kainate receptor (KAR)-mediated facilitation of GABA transmission in virgin rats (mediated by presynaptic GluK1-containing KARs), to KAR-inhibition of GABA transmission in lactating rats when extracellular glutamate levels are increased to activate metabotropic KARs (224).
In addition to activation of conventional postsynaptic receptors within the synaptic cleft, glutamate can also activate extrasynaptic receptors to induce a persistent ‘tonic’ current that influences global neuronal excitability, shown by a sustained outward shift in holding current during bath application of NMDAR blockers (225). Glia surrounding MNCs express the glutamate transporter, GLT-1 (220, 226), and GLT-1 blockers enhance the tonic NMDA current in MNCs. The tonic NMDA current is also enhanced during dehydration, as well as in rats with heart failure (227), a pathological condition characterized by abnormally elevated levels of circulating vasopressin. In both cases, the enhanced tonic NMDA current is likely the result of a diminished efficacy of GLT-1 (225, 227). Thus, enhanced activation of extrasynaptic NMDA receptors, due to blunted glial GLT-1 clearance, contributes to increased MNC activity and hormone release under physiological (dehydration) and pathological (heart failure) conditions.
Recently, extrasynaptic NMDARs (eNMDARs) were shown to participate in functional crosstalk with GABAA receptors. Activation of eNMDARs resulted in a transient and reversible potentiation of postsynaptic GABAA receptors, via a calcium- and phosphorylation-dependent mechanism (228). This inter-receptor crosstalk likely acts as a compensatory, counterbalancing mechanism to dampen glutamate-mediated over-excitation (227). Hence, glia play a key role determining the status of the glutamate/GABA synaptic balance in MNCs, acting at pre-, post-, and extrasynaptic loci.
In addition to transporters for glutamate (221), glia express GABA transporters (219) that also modulate ambient GABA levels. Similarly to glutamate, GABA generates a persistent hyperpolarisation via the activation of extrasynaptic receptors (219, 229). Counter-intuitively, dehydration increases intra-SON GABA release (as well as glutamate release), evident as an increase in the frequency of spontaneous IPSCs (and EPSCs) in MNCs from dehydrated rats (230). This increased GABA might dampen the glutamate-driven hyperosmotic excitation of MNCs to prevent over-excitation, as it does during acute osmotic stimulation (231).
Glia surrounding MNCs also release taurine to inhibit MNCs via extrasynaptic glycine receptors (232). Glial taurine release is increased in hypo-osmotic conditions to increase MNC inhibition and is reduced in hyperosmotic conditions to disinhibit MNCs. However, mice that lack the taurine transporter concentrate urine normally during dehydration, but continue to generate hyperosmotic urine upon rehydration, when the urine of wild-type littermates rapidly returns to normal osmolality (233). Hence, decreased glial taurine release appears to have little impact on MNC activity during chronic dehydration whereas hypo-osmolality-induced increases in taurine release inhibit MNCs.
Afferent inputs to magnocellular neurosecretory cells
Afferent inputs are essential for action potential firing in MNCs in vivo; selective pharmacological antagonism of individual excitatory inputs (64, 65) silences these neurones, even during intense stimulation (66, 185). These synaptic inputs arise from various afferent projections from many other brain areas (Figure 8) that have been extensively described elsewhere (35, 234).
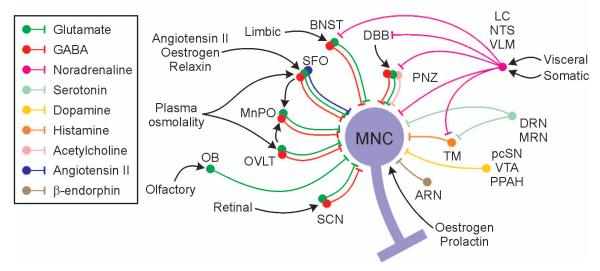
Figure 8. Schematic representation of some of the major peripheral and afferent inputs to magnocellular neurosecretory cells.
See text for details of the physiological functions of each of the inputs. Abbreviations: ARN: arcuate nucleus; BNST: bed nucleus of the stria terminalis; DBB: diagonal band of Broca; DRN: dorsal raphe nucleus; LC: locus coeruleus; MnPO: median preoptic nucleus; MRN: median raphe nucleus; NTS: nucleus tractus solitarius; OB: olfactory bulb; OVLT: organum vasculosum of the lamina terminalis; pcSN: pars compacta of the substantia nigra; PNZ: perinuclear zone; PPAH: preoptic periventricular /anterior hypothalamic region; SCN: suprachiasmatic nucleus; SFO: subfornical organ; TM: tuberomammillary nucleus; VLM: ventrolateral medulla; VTA: ventral tegmental area.
Perinuclear inputs
The region immediately dorsal to the SON, the perinuclear zone, is such a rich source of glutamatergic and GABAergic innervation of the SON that stimulation of this zone is frequently used in in vitro electrophysiology experiments to elicit evoked excitatory and inhibitory postsynaptic potentials/currents (EPSP/Cs and IPSP/Cs) in SON MNCs. While a large proportion of perinuclear zone axons enter the SON, many of these neurones have extensive axon collaterals that project elsewhere (235), including the parvocellular and magnocellular PVN (236), providing a potential mechanisms for co-ordination of activity between the different magnocellular nuclei. Indeed, stimulation of the perinuclear zone elicits glutamatergic EPSPs in MNCs of the ipsilateral and contralateral PVN (237). In addition to perinuclear zone inputs, stimulation of different sites shows that the PVN is essentially ringed in the horizontal plane by glutamatergic and GABAergic projections from an area that includes parts of the anterior hypothalamic area, the posterior bed nucleus of the stria terminalis (BNST) and the dorsomedial hypothalamus (238, 239).
Because the pattern of synaptic potentials recorded is essentially stochastic, it has been proposed that activity in these local inputs might generate background ‘noise’ in MNC membrane potential, against which the ability of brainstem and forebrain inputs to evoke specific responses is enhanced by a phenomenon referred to as ‘stochastic resonance’ (35). However, at least for the glutamatergic inputs, it appears that the perinuclear zone might play an active role in relaying information from distant inputs, such as the brainstem noradrenergic input (240, 241). At first glance, a local excitatory relay for a distant excitatory input might appear redundant but, in addition to monosynaptic excitatory postsynaptic potentials (EPSPs), single electrical stimuli applied to the perinuclear zone evoke multiple EPSPs in some SON MNCs (239), suggesting that there are local polysynaptic excitatory circuits within the perinuclear zone that modulate activity of SON MNCs. Hence, relaying the excitatory signals through local glutamatergic neurones might amplify the distant inputs to drive a stronger response in MNCs.
While perinuclear inputs are generally regarded as being principally glutamatergic or GABAergic, a large proportion of neurones in the perinuclear zone of the SON also synthesise acetylcholine (242). SON MNCs express nicotinic receptors (243) that mediate synaptic activation by perinuclear zone stimulation in vitro (244) and central administration of the muscarinic receptor agonist, carbachol, markedly increases the firing rate of both oxytocin and vasopressin MNCs in the SON (245). It is likely that PVN MNCs are similarly innervated because they are directly excited via nicotinic receptors in vitro (246). While the physiological significance of this prominent cholinergic innervation has not been well-characterised, it has been proposed to contribute to the osmotic stimulation of vasopressin secretion (247).
Forebrain inputs and osmoregulation
Among the major afferent inputs to MNCs are those that lie adjacent to the third ventricle: the median preoptic nucleus (MnPO), the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO). These regions convey information on the osmotic status of the animal to the MNCs (248, 249). Lesion of the region anterior and ventral to the third ventricle (AV3V, which encompasses the MnPO and OVLT) reduces oxytocin and vasopressin release in response to hyperosmotic stimulation (as does lesion of the SFO) by reducing the osmotically-stimulated increase in MNC action potential discharge (250). These neurones also promote thirst via projections to other brain regions, including the anterior cingulate cortex and insular cortex (251) and lesion of the AV3V has long been known to abolish osmotically-driven thirst (252). Thus, the AV3V appears to be a centre for co-ordinating the appropriate physiological and behavioural responses to changes in plasma osmolality and so are often referred to as the ‘osmoregulatory complex’.
While the principal action of vasopressin is antidiuresis in the kidney to conserve body water when plasma osmolality increases, increasing osmolality also activates oxytocin MNCs (231) and consequently the secretion of oxytocin (253), which (at least in the rat) is also involved in regulating the osmotic status of the animal via promotion of natruiresis in the kidney (2).
The OVLT and the SFO lack a blood-brain barrier and so neurones in these regions can be directly influenced by plasma osmolality, as well as factors circulating in the blood. OVLT (254) and SFO (255) neurones are directly osmosensitive; the former increase their action potential discharge via hyperosmotic depolarisation mediated by activation of transient receptor potential vanilloid 1 (TRPV1) channels (254).
In addition to intrinsic osmosensitivity, neurones of the osmoregulatory complex express receptors for many different circulating hormones (256), including AT1 receptors for angiotensin II (257). Angiotensin II production is increased via the renin-angiotensin system when blood pressure to the kidneys is reduced. Its principal actions are to increase arterial blood pressure by vasoconstriction of arterioles, and to increase renal retention of salt and water directly, as well as by stimulation of aldosterone secretion from the adrenal glands. Oxytocin and vasopressin release are also potently stimulated by circulating angiotensin II via the SFO (258) to provide a further level of co-ordination of the whole-body response to reduced kidney perfusion.
The OVLT and SFO project directly to the PVN and SON and indirectly via the MnPO, which also has reciprocal connections with both the OVLT and the SFO (232). While the overall effect of osmotic stimulation via the osmosensory complex is excitation of oxytocin and vasopressin MNCs and consequent secretion of hormones from the posterior pituitary gland, the projection neurones are both excitatory and inhibitory; while a direct osmosensitive glutamatergic input to the SON from the OVLT has been identified in vitro (259), both MnPO and OVLT stimulation can elicit GABA-mediated inhibition of SON MNCs in vivo (231, 260). In addition to the components mediated by glutamate and GABA, several peptides are also expressed in forebrain projections to the MNCs, principal among these is angiotensin II itself in the SFO projection to the MNCs, which mediates at least some of the SFO-induced excitation of MNCs (261).
It might appear counter-intuitive to have an excitatory stimulus relayed by a mixed population of excitatory and inhibitory afferent neurones. However, it is important that the osmotic response of MNCs is graded over a wide physiological range to allow the appropriate homeostatic response to variations in the osmotic status of the organism. To this end, it appears that the excitatory component of the osmosensory complex input provides the drive to increase MNC firing rate when plasma osmolality exceeds the osmotic threshold for vasopressin secretion of ~280 mOsmol kg−1, while the critical function of inhibitory component is to reduce the gain of the excitatory response to allow a graded response to increasing plasma osmolality over the ‘physiological’ range of 280 – 340 mOsmol kg−1 (231). Indeed, mathematical modelling indicates that the graded increase in MNC firing rate in response to increasing osmolality in vivo probably involves an equal activation of excitatory and inhibitory osmosensory inputs (231).
While afferent inputs are required for osmotic activation of MNCs, MNCs are themselves osmosensitive via stretch-inactivated TRPV1 channels (262). The full osmotic response of MNCs requires concurrent activation of afferent inputs and intrinsic TRPV1 channels (263, 264).
Noradrenergic inputs
Among the most prominent projections to MNCs in the PVN and SON are the ascending brainstem noradrenergic projections from the A1 cell group in the ventrolateral medulla (VLM) and the A2 cell group in the nucleus tractus solitarius (NTS). While these areas have been implicated in osmoregulation of MNC activity (264), they are widely accepted as primarily relaying visceral information from the periphery to the MNCs. Indeed, osmotic activation of these brain areas might be downstream of hypothalamic activation because water deprivation activates pre-autonomic PVN neurones that project to the brainstem (265, 266) and intra-carotid hypertonic saline infusion increases renal sympathetic nerve activity concomitant with an increase in Fos protein expression in OVLT neurones that project to the PVN (267, 268).
Noradrenergic inputs are well characterised as relaying cardiovascular information arising from baroreceptors and chemoreceptors whose afferents terminate in the NTS, as well as the area postrema and nucleus ambiguus (269). In addition to cardiovascular regulation, these inputs are also involved in mediating reproductive, gastrointestinal and somatosensory influences on MNC activity (35, 270). VLM neurones project preferentially to vasopressin MNCs (271), while NTS inputs project preferentially to oxytocin MNCs (272). Interruption of synaptic transmission through the VLM inhibits NTS excitation of vasopressin MNCs, but not oxytocin MNCs, suggesting that the NTS projection to oxytocin MNCs is predominantly direct while that to the vasopressin MNCs is mediated via the VLM (272).
Noradrenaline directly excites SON MNCs via a α1-adrenergic receptor mediated depolarisation (273, 274). Intra-SON antagonism of α1-adrenergic receptors profoundly inhibits oxytocin MNCs in vivo (185), indicating that oxytocin MNCs, at least, are under excitatory noradrenergic tone. For vasopressin MNCs, both excitation and inhibition by noradrenaline have been reported, with low doses of noradrenaline being excitatory via a α1-adrenergic receptors, while high doses are inhibitory via β-adrenergic receptors (275). Furthermore, excitation of vasopressin MNCs by stimulation of the VLM projection is reduced by α2-adrenergic receptor activation, indicating that excitatory transmitter release is likely inhibited by presynaptic α2-adrenergic receptors (276). The NTS input to oxytocin MNCs is likely also under presynaptic α2-adrenergic receptor modulation because stimulation of oxytocin release is reduced by α2-adrenergic receptor activation (185).
While most neurones that project to MNCs from the VLM and NTS are characterised as noradrenergic, various neuropeptides are co-expressed with noradrenaline in different subsets of neurones: the NTS noradrenergic neurones co-express enkpehalin (277) and galanin (278) and the VLM noradrenergic neurones co-express enkpehalin (277), neuropeptide Y (279) and substance P (280). Furthermore, an appreciable number of NTS neurones that project to MNCs do not express noradrenaline (281), but rather express the neuropeptides, inhibin β, somatostatin and the μ-opioid, encephalin, in varying proportions (282).
Indeed excitation of vasopressin MNCs by A1 stimulation is not completely blocked by noradrenergic antagonists (283), indicating that it might result from the actions of co-transmitters (or of non-noradrenergic neurones), including ATP, which excites MNCs via P2X receptor-mediated depolarisation (284) and potentiates noradrenergic stimulation of oxytocin and vasopressin release from hypothalamic explants (192), possibly via a synergistic increase in intracellular calcium concentrations (285). Other possible candidates for mediating A1 stimulation of MNCs, at least in part, are neuropeptide Y, which also potentiates noradrenergic stimulation of oxytocin and vasopressin release (286) and substance P, which synergises with ATP to cause the release of oxytocin and vasopressin (286).
Noradrenergic involvement in cardiovascular regulation of vasopressin magnocellular neurosecretory cells
Vasopressin secretion is tonically inhibited by afferent inputs from stretch receptors (baroreceptors) in the left atrium, aortic arch and carotid sinus. A reduction in baroreceptor activity, such as occurs during haemorrhage, increases vasopressin (and oxytocin) MNC activity as well as plasma vasopressin (and oxytocin) concentrations (287), while an increase in baroreceptor activity inhibits MNCs (288).
Haemorrhage increases noradrenaline levels in the SON (289) and lesion of the locus coeruleus, which contains the A6 cell group, reduces haemorrhage-induced oxytocin and vasopressin secretion (290) and inhibits baroreceptor activation of MNCs (291). However, there is little evidence of a direct projection from the locus coeruleus to MNCs and noradrenaline depletion within the SON or PVN does not prevent baroreceptor-induced inhibition of vasopressin MNCs (292). The locus coeruleus (and NTS) projects to the diagonal band of Broca (293) and it is likely that this relays baroreceptor-mediated effects on MNC activity via activation of inputs (294-298) that inhibit vasopressin MNCs via GABA interneurones in the perinuclear zone (299).
The defence of blood pressure by vasopressin does not operate in isolation from the homeostatic regulation of plasma osmolality; hypovolaemia or hypotension increases the gain of the secretory response to increasing osmolality and hypervolaemia or hypertension decreases the gain of the response (300). These changes in osmotic gain might be caused by plasticity in the vasopressin MNCs themselves because chronic osmotic stimulation prevents baroreflex inhibition of MNCs by changing the chloride gradient in MNCs to switch GABA from being inhibitory to being excitatory (301).
Noradrenergic involvement in reproductive regulation of oxytocin magnocellular neurosecretory cell activity
The noradrenergic innervation of oxytocin MNCs is increased in lactation (302), suggesting that remodelling of the noradrenergic input to MNCs over the course of pregnancy and lactation might be involved in signalling the reproductive status of the organism. Afferents from the uterus and vagina terminate in the NTS (303) and noradrenergic NTS neurones express Fos protein in response to activation of stretch receptors in the birth canal during fetal expulsion (304). Noradrenergic (and non-noradrenergic) neurones of the VLM and NTS retrogradely-labelled from the SON express Fos protein during parturition (305) and noradrenaline levels are increased within the SON at parturition (306). While NTS noradrenergic neurones are also activated during lactation, suckling (rather than the presence of pups) increases Fos protein expression in VLM noradrenergic neurones without further increasing expression in NTS neurones (307), suggesting that the specific noradrenergic pathways activated by uterine contraction and suckling of the young might be different.
While noradrenaline directly excites MNCs, its effects in pregnancy and lactation are likely more complex than simple excitation. Noradrenaline release within the SON is increased in late pregnancy (308). Noradrenaline facilitates oxytocin release within the SON and PVN during pregnancy and lactation (309, 310) and oxytocin increases noradrenaline release within the SON (311). Hence, the noradrenergic input to oxytocin MNCs might facilitate the emergence of oxytocin autoexcitation to promote bursting activity in oxytocin MNCs necessary for delivery of the young and of milk to the new-born. Indeed, stimulation of central noradrenergic (and histaminergic) receptors is necessary for intranuclear release of oxytocin in response to suckling (312).
Noradrenergic inputs might also be important for the co-ordination of bursts across the population of oxytocin MNCs. Bilateral retrograde tracer injection into the right and left SONs results in co-localisation within individual neurones only in the VLM and anterograde tracer injection into the VLM labels axons that branch at the optic chiasm to innervate both SONs (180). Critically, lesion of the optic chiasm dramatically reduces the co-ordination of bursts between oxytocin MNCs recorded from the left and right SON (180). Hence, VLM neurones project to MNCs on both sides of the brain and are likely involved in co-ordinating bursts in oxytocin MNCs.
While direct noradrenergic projections to oxytocin MNCs contribute to the co-ordination of bursts, there might also be indirect actions of noradrenaline neurones on bursting in oxytocin MNCs. There is a dense noradrenergic projection to the bed nucleus of the stria terminalis (BNST) (313) where noradrenaline is released (314). Stimulation of the BNST (with oxytocin) facilitates bursting in oxytocin MNCs in the SON (315) and the cyclical activity of BNST neurones is correlated with milk-ejection bursts in oxytocin MNCs (316), suggesting that the BNST might also be involved in gating milk ejection bursts.
Noradrenergic involvement in gastrointestinal regulation of magnocellular neurosecretory cell activity
Peripheral administration of the gastrointestinal peptide, cholecystokinin (CCK), causes a dose-dependent increase in oxytocin, but not vasopressin, secretion into the bloodstream (317). The increased oxytocin secretion is caused by an increase in the firing rate of oxytocin MNCs (55, 318).
While CCK can directly activate MNCs via activation of stretch-inactivated cation channels (319), the excitation of oxytocin MNCs by peripheral CCK is blocked by gastric vagotomy (317), destruction of peripheral fibres (320, 321), or by peripheral administration of CCK-A (but not CCK-B) receptor antagonists (322). Thus peripheral CCK appears to excite oxytocin MNCs in vivo by activating CCK-A receptors located on gastric vagal afferent fibres. These vagal signals are likely mediated by noradrenergic neurones because peripheral CCK increases noradrenaline levels in the SON (323) and the increased firing rate seen in oxytocin MNCs after peripheral CCK is blocked by iontophoretic administration of an α1-adrenoreceptor antagonist (324). Similarly, neurotoxic lesion of noradrenergic neurones abolishes CCK-induced oxytocin secretion and Fos protein expression in MNCs (185, 325). While both NTS and VLM neurones (but not other areas that project to MNCs) are activated by CCK, it has been demonstrated that only noradrenergic neurones of the A2 (NTS) cell group express Fos protein after peripheral CCK (325). Hence, peripheral CCK acts via CCK-A receptors on gastric vagal afferents to activate the A2 cell group projection to oxytocin MNCs to increase oxytocin secretion.
While peripheral CCK increases oxytocin secretion into the blood, the physiological significance of this response is unknown. CCK-induced oxytocin secretion can be mimicked by gastric distension (318) and gastric vagotomy reduces both CCK-induced inhibition of food intake and CCK-induced oxytocin secretion (317), suggesting that peripheral oxytocin might mediate CCK effects on food intake. However, the CCK-induced inhibition of food intake and gastric emptying is longer lasting than its stimulation of oxytocin secretion (326), and peripheral oxytocin administration does not induce food intake (317), indicating that peripheral oxytocin release does not mediate the CCK-induced inhibition of food intake. Nevertheless, oxytocin might play a pivotal role in food intake, and thus body weight regulation, via release into the brain because central (but not peripheral) oxytocin receptor antagonist administration blunts CCK-induced inhibition of food intake (327).
While these initial studies focussed on oxytocin as a satiety factor because this is the major role played by CCK in the regulation of food intake, there has been a recent resurgence in interest in central oxytocin as a long-term regulator of bodyweight because disruption of the SIM1 gene causes profound monogenic obesity in humans via increased food intake (328) and SIM1 knockout mice lack PVN and SON oxytocin MNCs (329, 330).
Serotoninergic inputs
Retrograde tracing studies suggest that MNCs receive a sparse serotoninergic projection from the dorsal and median raphe nuclei (331). However, these projections were not confirmed by anterograde labelling using Phaseolus vulgaris leucoagglutinin (PHA-L) injection into the dorsal or median raphe nucleus (332, 333). Nevertheless, intracerebroventricular (ICV) serotonin administration increases plasma oxytocin and vasopressin levels (334). PHA-L injections show a moderate innervation of the regions surrounding the PVN and SON (332, 333), which might mediate the serotonin excitation of MNCs.
Similar to noradrenaline, serotonin release in the SON is increased by intravenous injection of CCK (335) and so serotonin might play a similar role to noradrenaline in mediating the transfer of visceral information to MNCs. However, serotonin is not a mere adjunct to the noradrenergic inputs because, unlike noradrenaline, serotonin concentrations in the SON do not change over the course of parturition (306) and serotonin antagonists do not interfere with the progress of parturition in rats (336), suggesting that serotonin inputs might not be specifically involved in activation of oxytocin MNCs during parturition. However, intravenous CCK increases intra-SON serotonin concentrations more in late pregnant rats than in virgin rats (308), suggesting that serotonin might have a facilitatory role supporting oxytocin MNC activity during parturition.
Dopaminergic inputs
The main dopaminergic projections to MNCs arise from the A9 cell group of the pars compacta of the substantia nigra, the A10 cell group of the ventral tegmental area, as well as the A14 and A15 cell groups in the preoptic periventricular /anterior hypothalamic region (337, 338). Central dopamine administration increases oxytocin and vasopressin secretion under basal conditions (339) via an enhancement of MNC activity (340). While dopaminergic synapses have been visualised on MNCs in the PVN and SON using electron microscopy (341), and dopamine depolarises MNCs via D2 receptors (342), more recently it has become clear that dopamine also modulates MNC activity via activation of presynaptic D4 receptors, which inhibits GABA release onto MNCs (343, 344). Hence, dopaminergic stimulation of neurohypophysial hormone release appears to result from a combination of direct depolarisation and a reduction of inhibitory GABA influences.
The physiological role of dopaminergic inputs to MNCs have not been well-characterised but the facilitation of MNC activity is also evident under stimulated conditions, enhancing oxytocin MNC activity during lactation, as well as vasopressin MNC activity during water diuresis evoked by alcohol administration (340). Furthermore, dopamine release within the SON is increased during the second half of parturition (306), suggesting that increased activation of dopaminergic inputs might specifically contribute to excitation of oxytocin MNCs during parturition.
However, dopaminergic regulation of MNC activity is likely more complex than simple excitation because D4 receptor activation also inhibits glutamate release onto MNCs (345); this restraint of excitatory inputs might prevent over-excitation by dopaminergic inputs, or refine the temporal profile of the excitation.
Histaminergic inputs
Histaminergic projections to MNCs arise from the tuberomammillary nucleus (346) and appear to contribute to delivery of the young at parturition (347). The tuberomammillary nucleus receives afferent inputs from many brain areas (348), including synaptic inputs from noradrenergic and serotoninergic neurones (349).
Histamine has differential effects on oxytocin and vasopressin MNCs. Histamine excites vasopressin MNCs via an H1 receptor-mediated reduction of the K+ leak current (350), inhibition of EPSCs (351) and by enhancing the sADP (352) via H1-receptor activation of a non-specific cation current (353). In contrast to its effects on vasopressin MNCs, histamine was initially reported to inhibit oxytocin MNCs via H2 receptor activation of chloride-mediated hyperpolarisation (354). However, central histamine administration increases oxytocin secretion into the circulation (355). Histamine neurones express elevated levels of mRNA for the histamine synthetic enzyme, histidine decarboxylase, in late pregnant and lactating rats (347) when excitation would be expected and stimulation of central histaminergic (and noradrenergic) receptors is necessary for intranuclear release of oxytocin in response to suckling (312). Hence, it appears that the direct histaminergic inhibition of oxytocin MNCs is overcome by induction of somato-dendritic oxytocin release, possibly to facilitate bursting during parturition and lactation.
Histamine might also be involved in co-ordination of milk-ejection bursts across the population of oxytocin MNCs; hypothalamic lesions incorporating the tuberomammillary nucleus disrupts co-ordination of milk-ejection bursts between pairs of oxytocin MNCs recorded from the left and right SON of anaesthetised rats (356).
Endogenous opioid peptide inputs
Proopiomelanocortin (POMC) neurones in the brain are predominantly located in the hypothalamic arcuate nucleus and POMC fibres originating in the arcuate nucleus have been visualised in the PVN and SON, particularly in the oxytocin-rich parts of these nuclei (357). Oxytocin MNCs are under active opioid restraint over the course of pregnancy, presumably to protect the offspring from oxytocin secretion triggered by other stimuli such as inflammation (164, 358, 359). Administration of morphine inhibits oxytocin MNCs by inhibition of noradrenergic inputs (335), as well as by direct hyperpolarization of oxytocin MNCs (360). The endomorphins, which are highly potent and specific endogenous μ-opioid peptides, are present in the hypothalamus (361). While ICV endomorphin 1 inhibits both oxytocin and vasopressin MNCs, it potently inhibits oxytocin MNCs by a direct action (similar to exogenous agonists) whereas its weaker inhibition of vasopressin MNCs is mediated by indirect actions (362). While MNCs develop dependence on chronic morphine (363, 364), at least in part through changes in their intrinsic properties (360, 365), they do not develop dependence on chronic endomorphin 1 (362), similar to other neuronal responses to chronic activation by peptides (366), suggesting that release from on-going endogenous opioid inhibition is unlikely to trigger the increase in oxytocin MNC activity required for parturition.
Suprachiasmatic inputs
Oxytocin and vasopressin are secreted in a circadian pattern (12) that is mediated by a mixed excitatory (glutamatergic) and inhibitory (GABAergic) projection to the SON from the suprachiasmatic nucleus (367) and might be entrained to the light-dark cycle by a retinal projection via the perinuclear zone (368). These inputs are likely of particular importance in preventing night-time enuresis but might also impact the time of day at which delivery of the offspring occurs.
Olfactory inputs
MNCs receive direct afferent inputs from the main and accessory olfactory bulb (369-371) that excite MNCs via glutamatergic receptor activation (372). Remarkably, activation of olfactory inputs increase the incidence of dye coupling between MNCs but only in animals expressing maternal behaviour (373, 374), suggesting that these inputs might be important in the generation of the appropriate physiological responses of MNCs for maternal care of the offspring.
Hormonal regulation of magnocellular neurosecretory cell activity
Steroid regulation of oxytocin magnocellular neurosecretory cell activity
During pregnancy, the maternal brain, including oxytocin MNCs, adapts to maintain the pregnancy and to prepare for birth, lactation and for nurturing of the baby (164). In the rat, parturition uses about one third of the oxytocin stored in the pituitary gland and in preparation for the demands of parturition (and lactation), pituitary oxytocin stores increase during pregnancy.
Circulating oestrogen and progesterone levels increase markedly over the course of pregnancy (164) and oestrogen promotes oxytocin gene expression (375), suggesting that the high sex steroid levels of pregnancy might stimulate oxytocin synthesis in preparation for parturition. However, oxytocin MNCs do not express oestrogen receptor α (ERα), and express little ERβ (376). Nevertheless, oestrogen does appear to directly activate the oxytocin gene promoter, but not via the classical oestrogen response element; while oestrogen action is dependent on the presence of ER, it does not involve ER binding to DNA, rather oestrogen actions appear to be mediated by a nuclear orphan receptor binding site in the oxytocin gene promoter (377).
MNCs strongly express the non-classical ER, GPR30 (378), which might mediate rapid actions of oestrogen in vivo to enhance the excitation evident upon removal of opioid inhibitory tone (379) and in vitro to promote somato-dendritic oxytocin (and vasopressin) secretion (380) and to potentiate the kainate-induced depolarisation in MNCs recorded from slices from rats in late lactation (381).
While MNCs do not express the ER, many of their inputs express ER, particularly those from the osmoreceptor complex (382). Oestrogen potentiates systemic oxytocin and vasopressin secretion induced by hyperosmotic stimulation (383). Hence, oestrogen might promote fluid retention in the face of the decreased plasma osmolality that develops over the course of pregnancy (384).
Prolonged progesterone treatment inhibits oxytocin MNCs (385, 386) and progesterone administration at the end of pregnancy delays parturition by inhibiting oxytocin secretion (387). However, oxytocin MNCs do not express progesterone receptors (PRs), even at the end of pregnancy (388), and so these inhibitory effects are likely mediated by afferent inputs.
The primary metabolite of progesterone, allopreganolone, is an allosteric modulator of GABAA receptors and the expression of the α1 subunit of the GABAA receptor by oxytocin MNCs increases through pregnancy (389), a process that is driven by the elevated progesterone levels of pregnancy (390). While increased α1 subunit expression would normally decrease IPSC duration, α1 subunit-containing GABAA receptors are more sensitive to allopregnanolone, which is abundant in late pregnancy and prolongs IPSC duration (107). As parturition approaches, despite the continued presence of progesterone, the subunit expression switches to favour α2 subunits, which removes the allopregnanolone sensitivity to decrease IPSC duration in time for parturition and this persists into lactation (107), removing the enhanced inhibitory influence on oxytocin MNCs in time for delivery and lactation.
By contrast to their inhibitory effects, allopregnanolone (and to a lesser extent, progesterone) increase somato-dendritic oxytocin release (as well as some vasopressin release) by a GABA-dependent mechanism (391), which would be expected to contribute to the bursting of oxytocin MNCs during partition and lactation.
Regulation of oxytocin secretion by non-steroidal pregnancy hormones
In addition to sex steroids, several hormones alter the activity of oxytocin MNCs; the best characterised of these is relaxin. Ovarian relaxin secretion increases over the course of pregnancy until term, becoming detectable in mid-pregnancy and continuing to increase until delivery (392). While relaxin ripens the cervix, softens the ligaments of the pubic symphysis and inhibits uterine contraction (384) in preparation for parturition, it also increases the firing rate of oxytocin MNCs to increase systemic secretion (393). However, this excitation is unlikely to be involved in stimulating oxytocin secretion during parturition because the excitation is lost in late pregnancy (and lactation) (394). Rather it appears that relaxin excitation of MNCs contributes to resetting the control of body fluid balance over the course of pregnancy, when there is a marked increase in blood volume and a decrease in plasma osmolality due to mild hyponatraemia in the face of sodium retention (384); late-pregnant relaxin knockout mice do not exhibit reduced plasma osmolality (395). Vasopressin MNCs are also excited by relaxin (393). While relaxin receptors are expressed in the PVN and SON (396), circulating relaxin is unlikely to enter the brain and so its effects on MNCs might be mediated via activation of receptors in the SFO (396).
Oxytocin MNCs express prolactin receptors (397) and are inhibited by central administration of prolactin in virgin female rats (398). Prolactin also inhibits oxytocin MNCs in vitro, with both excitatory and inhibitory effects reported (399), with the inhibition probably mediated by activation of potassium channels (400). The effects of prolactin on oxytocin MNCs appear to depend on the physiological status of the individual because prolactin increases oxytocin secretion in lactating rats, although this stimulatory effect might be mediated by actions on the neurosecretory terminals in the posterior pituitary gland, rather than by modulation of action potential firing rate (401).
Kisspeptins are a recently discovered group of peptides secreted by the placenta that are agonists at the GPR54 receptor (402). Peripheral kisspeptin administration excites oxytocin MNCs via activation of vagal afferents (321) to increase circulating oxytocin levels (402, 403). While placental secretion of kisspeptin increases dramatically over the course of pregnancy in humans (404), the impact of placental kisspeptin on oxytocin MNCs during pregnancy or lactation has yet to be determined.
Stress responses of oxytocin magnocellular neurosecretory cells in pregnancy
Oxytocin MNCs are activated by a wide range of stressors, including behavioural, immune and visceral stressors (359). Clearly, stress activation of oxytocin MNCs would increase the risk of pre-term delivery, particularly in late pregnancy when uterine oxytocin receptor expression is upregulated in preparation for delivery (84). To reduce the risk of inappropriate activation of uterine contraction, a wide range of stressors are less effective at causing oxytocin secretion in late pregnancy, including behavioural stressors (405), hyperosmotic stimulation (406) and immune challenge (358). The reduced stress responses of oxytocin MNCs in late pregnancy is due, at least in part, to active inhibition by endogenous opioid peptides (304), which might be induced by allopregnanolone (407).
Oxytocin, vasopressin and behaviour
The largest explosion of research on oxytocin and vasopressin in recent years has been the investigation of the impact of these neuropeptides on behaviour when released into the brain. The effects of oxytocin (and to a lesser extent, vasopressin) on social behaviour appear to be via actions on the brain reward pathway and have been extensively reviewed elsewhere (408, 409). These remarkable findings have led to a focus on dysfunction of the central oxytocin system as a key element of behavioural disorders, and for stimulation of central oxytocin receptors as a potential therapy for autism spectrum disorder (410), as well as for drug addiction (411). In addition to effects on social behaviour, central oxytocin has again come to prominence as a regulator of food intake (412).
Oxytocin and vasopressin do not cross the blood-brain-barrier and so centrally-acting oxytocin must be released within the brain. While there are known oxytocin-containing efferents from some parvocellular PVN neurones (21), these projections are generally sparse and so most research in recent years has focussed on the potential for somato-dendritic release to function as a long-range signalling system within the brain (413). However, the recent identification of axon collaterals from oxytocin MNCs that project to areas of the brain involved in behaviour (24) has identified a new avenue for MNCs to communicate with the rest of the brain.
Concluding remarks
Here, we have highlighted the interaction of afferent inputs with local and intrinsic mechanisms in the regulation of magnocellular neurosecretory activity responses to physiological challenges. Many peripheral inputs converge on the same afferent inputs. However, it is not clear whether this convergence is upon the same neurones within the afferent input population, or upon different individuals within the population and this question remains one of the challenges for the field.
Hypothalamic MNCs are among the most studied and best characterised neurones in the brain, yet each passing year opens new perspectives on the control and function of these neurones. The wide-ranging peripheral effects of circulating oxytocin and vasopressin (1) are mirrored in the increasingly diverse central actions of these peptides that must result from central release. Many of the central (neuropeptide) and peripheral (hormonal) effects of these peptides might represent two arms of a co-ordinated whole-organism physiological and behavioural response to challenges to homeostasis. Whether achieved by co-ordinated somato-dendritic and axon terminal release, or by release from centrally-projecting axon collaterals, MNCs are ideally placed to respond physiological and environmental conditions and, as the endocrine system has long been known to do, orchestrate the whole body response to maintain homeostasis.
Acknowledgements
CHB was supported by the Health Research Council of New Zealand and the Marsden Fund of the Royal Society of New Zealand; JSB by Alberta Innovates and the Canadian Institutes of Health Research: ML by the Biotechnology and Biological Sciences Research Council; JES by R01 HL112225 and R21 HL113801 from the National Institutes of Health.
References
- 1.Cunningham ET, Jr., Sawchenko PE. Reflex control of magnocellular vasopressin and oxytocin secretion. Trends Neurosci. 1991;14:406–11. doi: 10.1016/0166-2236(91)90032-p. [DOI] [PubMed] [Google Scholar]
- 2.Verbalis JG, Mangione MP, Stricker EM. Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology. 1991;128:1317–22. doi: 10.1210/endo-128-3-1317. [DOI] [PubMed] [Google Scholar]
- 3.Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356:526–9. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- 4.Rozen F, Russo C, Banville D, Zingg HH. Structure, characterization, and expression of the rat oxytocin receptor gene. Proc Natl Acad Sci USA. 1995;92:200–4. doi: 10.1073/pnas.92.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 6.Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, Dayanithi G. Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16:e138–56. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku CY, Qian A, Wen Y, Anwer K, Sanborn BM. Oxytocin stimulates myometrial guanosine triphosphatase and phospholipase-C activities via coupling to G alpha q/11. Endocrinology. 1995;136:1509–15. doi: 10.1210/endo.136.4.7895660. [DOI] [PubMed] [Google Scholar]
- 8.Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120:989–1002. doi: 10.1378/chest.120.3.989. [DOI] [PubMed] [Google Scholar]
- 9.Aguilera G, Subburaju S, Young S, Chen J. The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog Brain Res. 2008;170:29–39. doi: 10.1016/S0079-6123(08)00403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marples D, Frokiaer J, Nielsen S. Long-term regulation of aquaporins in the kidney. Am J Physiol. 1999;276:F331–9. doi: 10.1152/ajprenal.1999.276.3.F331. [DOI] [PubMed] [Google Scholar]
- 11.Bankir L, Bichet DG, Bouby N. Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am J Physiol Renal Physiol. 2010;299:F917–28. doi: 10.1152/ajprenal.00413.2010. [DOI] [PubMed] [Google Scholar]
- 12.Forsling ML, Montgomery H, Halpin D, Windle RJ, Treacher DF. Daily patterns of secretion of neurohypophysial hormones in man: effect of age. Exp Physiol. 1998;83:409–18. doi: 10.1113/expphysiol.1998.sp004124. [DOI] [PubMed] [Google Scholar]
- 13.Scott V, Bishop VR, Leng G, Brown CH. Dehydration-induced modulation of kappa-opioid inhibition of vasopressin neurone activity. J Physiol. 2009;587:5679–89. doi: 10.1113/jphysiol.2009.180232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CH. Rhythmogenesis in vasopressin cells. J Neuroendocrinol. 2004;16:727–39. doi: 10.1111/j.1365-2826.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29:108–15. doi: 10.1016/j.tins.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Leng G, Brown C, Sabatier N, Scott V. Population dynamics in vasopressin cells. Neuroendocrinology. 2008;88:160–72. doi: 10.1159/000149827. [DOI] [PubMed] [Google Scholar]
- 17.Belin V, Moos F, Richard P. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nuclei in suckled rats: direct proof with paired extracellular recordings. Exp Brain Res. 1984;57:201–3. doi: 10.1007/BF00231147. [DOI] [PubMed] [Google Scholar]
- 18.Manaye KF, Lei DL, Tizabi Y, Davila-Garcia MI, Mouton PR, Kelly PH. Selective neuron loss in the paraventricular nucleus of hypothalamus in patients suffering from major depression and bipolar disorder. J Neuropathol Exp Neurol. 2005;64:224–9. doi: 10.1093/jnen/64.3.224. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- 20.Fisher AW, Price PG, Burford GD, Lederis K. A 3-dimensional reconstruction of the hypothalamo-neurohypophysial system of the rat. The neurons projecting to the neuro/intermediate lobe and those containing vasopressin and somatostatin. Cell Tissue Res. 1979;204:343–54. doi: 10.1007/BF00233647. [DOI] [PubMed] [Google Scholar]
- 21.Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–14. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- 22.Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–8. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- 23.Meeker RB, Swanson DJ, Greenwood RS, Hayward JN. Ultrastructural distribution of glutamate immunoreactivity within neurosecretory endings and pituicytes of the rat neurohypophysis. Brain Res. 1991;564:181–93. doi: 10.1016/0006-8993(91)91454-9. [DOI] [PubMed] [Google Scholar]
- 24.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 25.Stern JE, Armstrong WE. Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. J Neurosci. 1998;18:841–53. doi: 10.1523/JNEUROSCI.18-03-00841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong WE, Scholer J, McNeill TH. Immunocytochemical, Golgi and electron microscopic characterization of putative dendrites in the ventral glial lamina of the rat supraoptic nucleus. Neuroscience. 1982;7:679–94. doi: 10.1016/0306-4522(82)90074-4. [DOI] [PubMed] [Google Scholar]
- 27.Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 28.Hatton GI. Pituicytes, glia and control of terminal secretion. J Exp Biol. 1988;139:67–79. doi: 10.1242/jeb.139.1.67. [DOI] [PubMed] [Google Scholar]
- 29.Bourque CW. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol. 1990;421:247–62. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrero HG, Lemos JR. Ionic conditions modulate stimulus-induced capacitance changes in isolated neurohypophysial terminals of the rat. J Physiol. 2010;588:287–300. doi: 10.1113/jphysiol.2009.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemos JR, Wang G. Excitatory versus inhibitory modulation by ATP of neurohypophysial terminal activity in the rat. Exp Physiol. 2000;85(Spec No):67S–74S. doi: 10.1111/j.1469-445x.2000.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz-Miranda S, Dayanithi G, Custer E, Treistman SN, Lemos JR. μ-Opioid receptor preferentially inhibits oxytocin release from neurohypophysial terminals by blocking R-type Ca2+ channels. J Neuroendocrinol. 2005;17:583–90. doi: 10.1111/j.1365-2826.2005.01346.x. [DOI] [PubMed] [Google Scholar]
- 33.Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- 34.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290:433–40. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–55. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 36.Bicknell RJ, Brown D, Chapman C, Hancock PD, Leng G. Reversible fatigue of stimulus-secretion coupling in the rat neurohypophysis. J Physiol. 1984;348:601–13. doi: 10.1113/jphysiol.1984.sp015128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–9. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–9. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- 39.Watson SJ, Akil H, Fischli W, Goldstein A, Zimmerman E, Nilaver G, wimersma Griedanus TB. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982;216:85–7. doi: 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]
- 40.De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A. 2004;101:10464–9. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landry M, Vila-Porcile E, Hokfelt T, Calas A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci. 2003;17:579–89. [PubMed] [Google Scholar]
- 42.Yamaguchi H, Sasaki K, Satomi Y, Shimbara T, Kageyama H, Mondal MS, Toshinai K, Date Y, González LJ, Shioda S, Takao T, Nakazato M, Minamino N. Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J Biol Chem. 2007;282:26354–60. doi: 10.1074/jbc.M701665200. [DOI] [PubMed] [Google Scholar]
- 43.Fujihara H, Sasaki K, Mishiro-Sato E, Ohbuchi T, Dayanithi G, Yamasaki M, Ueta Y, Minamino N. Molecular characterization and biological function of neuroendocrine regulatory peptide-3 in the rat. Endocrinology. 2012;153:1377–86. doi: 10.1210/en.2011-1539. [DOI] [PubMed] [Google Scholar]
- 44.Gillard ER, Leon-Olea M, Mucio-Ramirez S, Coburn CG, Sanchez-Islas E, de Leon A, Mussenden H, Bauce LG, Pittman QJ, Curras-Collazo MC. A novel role for endogenous pituitary adenylate cyclase activating polypeptide in the magnocellular neuroendocrine system. Endocrinology. 2006;147:791–803. doi: 10.1210/en.2005-1103. [DOI] [PubMed] [Google Scholar]
- 45.Chu JYS, Lee LTO, Lai CH, Vaudry H, Chan YS, Yung WH, Chow BKC. Secretin as a neurohypophysial factor regulating body water homeostasis. Proc Natl Acad Sci USA. 2009;106:15961–6. doi: 10.1073/pnas.0903695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitnall MH, Gainer H, Cox BM, Molineaux CJ. Dynorphin-A-(1-8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983;222:1137–9. doi: 10.1126/science.6648526. [DOI] [PubMed] [Google Scholar]
- 47.Martin R, Moll U, Voigt KH. An attempt to characterize by immunocytochemical methods the enkephalin-like material in oxytocin endings of the rat neurohypophysis. Life Sci. 1983;33(Suppl 1):69. doi: 10.1016/0024-3205(83)90446-0. [DOI] [PubMed] [Google Scholar]
- 48.Eriksson M, Ceccatelli S, Uvnas-Moberg K, Iadarola M, Hokfelt T. Expression of Fos-related antigens, oxytocin, dynorphin and galanin in the paraventricular and supraoptic nuclei of lactating rats. Neuroendocrinology. 1996;63:356–67. doi: 10.1159/000126976. [DOI] [PubMed] [Google Scholar]
- 49.Kadowaki K, Kishimoto J, Leng G, Emson PC. Up-regulation of nitric oxide synthase (NOS) gene expression together with NOS activity in the rat hypothalamo-hypophysial system after chronic salt loading: evidence of a neuromodulatory role of nitric oxide in arginine vasopressin and oxytocin secretion. Endocrinology. 1994;134:1011–7. doi: 10.1210/endo.134.3.7509733. [DOI] [PubMed] [Google Scholar]
- 50.Poisner AM, Douglas WW. Adenosine triphosphate and adenosine triphosphatase in hormone-containing granules of posterior pituitary gland. Science. 1968;160:203–4. doi: 10.1126/science.160.3824.203. [DOI] [PubMed] [Google Scholar]
- 51.Hokfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–79. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 52.Brown CH, Ludwig M, Leng G. Kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–8. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–40. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- 54.Brown CH, Leng G. In vivo modulation of post-spike excitability in vasopressin cells by kappa-opioid receptor activation. J Neuroendocrinol. 2000;12:711–4. doi: 10.1046/j.1365-2826.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- 55.Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–80. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus kappa-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95:3235–44. doi: 10.1152/jn.00062.2006. [DOI] [PubMed] [Google Scholar]
- 57.Dyball RE, Pountney PS. Discharge patterns of supraoptic and paraventricular neurones in rats given a 2 per cent NaCl solution instead of drinking water. J Endocrinol. 1973;56:91–8. doi: 10.1677/joe.0.0560091. [DOI] [PubMed] [Google Scholar]
- 58.Walters JK, Hatton GI. Supraoptic neuronal activity in rats during five days of water deprivation. Physiol Behav. 1974;13:661–7. doi: 10.1016/0031-9384(74)90237-6. [DOI] [PubMed] [Google Scholar]
- 59.Ciosek J. Vasopressin and oxytocin release as influenced by thyrotropin-releasing hormone in euhydrated and dehydrated rats. J Physiol Pharmacol. 2002;53:423–37. [PubMed] [Google Scholar]
- 60.Verbalis JG, Baldwin EF, Robinson AG. Osmotic regulation of plasma vasopressin and oxytocin after sustained hyponatremia. Am J Physiol. 1986;250:R444–51. doi: 10.1152/ajpregu.1986.250.3.R444. [DOI] [PubMed] [Google Scholar]
- 61.Wakerly JB, Lincoln DW. Phasic discharge of antidromically identified units in the paraventricular nucleus of the hypothalamus. Brain Res. 1971;25:192–4. doi: 10.1016/0006-8993(71)90580-4. [DOI] [PubMed] [Google Scholar]
- 62.Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–2. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- 63.Israel J-M, Poulain DA, Oliet SHR. Glutamatergic inputs contribute to phasic activity in vasopressin neurons. J Neurosci. 2010;30:1221–32. doi: 10.1523/JNEUROSCI.2948-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nissen R, Hu B, Renaud LP. N-methyl-D-aspartate receptor antagonist ketamine selectively attenuates spontaneous phasic activity of supraoptic vasopressin neurons in vivo. Neuroscience. 1994;59:115–20. doi: 10.1016/0306-4522(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 65.Nissen R, Hu B, Renaud LP. Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J Physiol. 1995;484:415–24. doi: 10.1113/jphysiol.1995.sp020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown CH, Bull PM, Bourque CW. Phasic bursts in rat magnocellular neurosecretory cells are not intrinsically regenerative in vivo. Eur J Neurosci. 2004;19:2977–83. doi: 10.1111/j.0953-816X.2004.03408.x. [DOI] [PubMed] [Google Scholar]
- 67.Greffrath W, Magerl W, Disque-Kaiser U, Martin E, Reuss S, Boehmer G. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurones. J Neuroendocrinol. 2004;16:577–88. doi: 10.1111/j.1365-2826.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- 68.Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol. 1998;513:493–506. doi: 10.1111/j.1469-7793.1998.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol. 1996;494:389–98. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci. 2004;24:7718–26. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teruyama R, Armstrong WE. Calcium-dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol. 2007;98:2612–21. doi: 10.1152/jn.00599.2007. [DOI] [PubMed] [Google Scholar]
- 72.Cobbett P, Smithson KG, Hatton GI. Immunoreactivity to vasopressin-but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Res. 1986;362:7–16. doi: 10.1016/0006-8993(86)91392-2. [DOI] [PubMed] [Google Scholar]
- 73.Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol. 1994;475:115–28. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–60. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghamari-Langroudi M, Bourque CW. Excitatory role of the hyperpolarization-activated inward current in phasic and tonic firing of rat supraoptic neurons. J Neurosci. 2000;20:4855–63. doi: 10.1523/JNEUROSCI.20-13-04855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang YF, Hatton GI. Milk ejection burst-like electrical activity evoked in supraoptic oxytocin neurons in slices from lactating rats. J Neurophysiol. 2004;91:2312–21. doi: 10.1152/jn.00697.2003. [DOI] [PubMed] [Google Scholar]
- 77.Popescu IR, Morton LA, Franco A, Di S, Ueta Y, Tasker JG. Synchronized bursts of miniature inhibitory postsynaptic currents. J Physiol. 2010;588:939–51. doi: 10.1113/jphysiol.2009.181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Engelmann M, Bull PM, Brown CH, Landgraf R, Horn TF, Singewald N, Ludwig M, Wotjak CT. GABA selectively controls the secretory activity of oxytocin neurons in the rat supraoptic nucleus. Eur J Neurosci. 2004;19:601–8. doi: 10.1111/j.1460-9568.2004.03151.x. [DOI] [PubMed] [Google Scholar]
- 79.Haam J, Popescu IR, Morton LA, Halmos KC, Teruyama R, Ueta Y, Tasker JG. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J Neurosci. 2012;32:572–82. doi: 10.1523/JNEUROSCI.3826-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA. 1996;93:11699–704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Douglas AJ, Leng G, Russell JA. The importance of oxytocin mechanisms in the control of mouse parturition. Reproduction. 2002;123:543–52. doi: 10.1530/rep.0.1230543. [DOI] [PubMed] [Google Scholar]
- 82.Antonijevic IA, Douglas AJ, Dye S, Bicknell RJ, Leng G, Russell JA. Oxytocin antagonists delay the initiation of parturition and prolong its active phase in rats. J Endocrinol. 1995;145:97–103. doi: 10.1677/joe.0.1450097. [DOI] [PubMed] [Google Scholar]
- 83.Chan WY, Chen DL, Manning M. Oxytocin receptor subtypes in the pregnant rat myometrium and decidua: pharmacological differentiations. Endocrinology. 1993;132:1381–6. doi: 10.1210/endo.132.3.8382600. [DOI] [PubMed] [Google Scholar]
- 84.Alexandrova M, Soloff MS. Oxytocin receptors and parturition. I. Control of oxytocin receptor concentration in the rat myometrium at term. Endocrinology. 1980;106:730–5. doi: 10.1210/endo-106-3-730. [DOI] [PubMed] [Google Scholar]
- 85.Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–3. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 86.Summerlee AJ. Extracellular recordings from oxytocin neurones during the expulsive phase of birth in unanaesthetized rats. J Physiol. 1981;321:1–9. doi: 10.1113/jphysiol.1981.sp013967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Summerlee AJ, Lincoln DW. Electrophysiological recordings from oxytocinergic neurones during suckling in the unanaesthetized lactating rat. J Endocrinol. 1981;90:255–65. doi: 10.1677/joe.0.0900255. [DOI] [PubMed] [Google Scholar]
- 88.Wakerley JB, Lincoln DW. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973;57:477–93. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- 89.Lincoln DW, Wakerley JB. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol. 1974;242:533–54. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moos FC. GABA-induced facilitation of the periodic bursting activity of oxytocin neurones in suckled rats. J Physiol. 1995;488:103–14. doi: 10.1113/jphysiol.1995.sp020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sjoquist M, Huang W, Jacobsson E, Skott O, Stricker EM, Sved AF. Sodium excretion and renin secretion after continuous versus pulsatile infusion of oxytocin in rats. Endocrinology. 1999;140:2814–8. doi: 10.1210/endo.140.6.6831. [DOI] [PubMed] [Google Scholar]
- 92.Blackburn-Munro G, Brown CH, Neumann ID, Landgraf R, Russell JA. Verapamil prevents withdrawal excitation of oxytocin neurones in morphine-dependent rats. Neuropharmacology. 2000;39:1596–607. doi: 10.1016/s0028-3908(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 93.Ludwig M, Brown CH, Russell JA, Leng G. Local opioid inhibition and morphine dependence of supraoptic nucleus oxytocin neurones in the rat in vivo. J Physiol. 1997;505:145–52. doi: 10.1111/j.1469-7793.1997.145bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown CH, Munro G, Murphy NP, Leng G, Russell JA. Activation of oxytocin neurones by systemic cholecystokinin is unchanged by morphine dependence or withdrawal excitation in the rat. J Physiol. 1996;496:787–94. doi: 10.1113/jphysiol.1996.sp021727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luckman SM, Antonijevic I, Leng G, Dye S, Douglas AJ, Russell JA, Bicknell RJ. The maintenance of normal parturition in the rat requires neurohypophysial oxytocin. J Neuroendocrinol. 1993;5:7–12. doi: 10.1111/j.1365-2826.1993.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 96.Pumford KM, Russell JA, Leng G. Effects of the selective kappa-opioid agonist U50,488 upon the electrical activity of supraoptic neurones in morphine-tolerant and morphine-naive rats. Exp Brain Res. 1993;94:237–46. doi: 10.1007/BF00230291. [DOI] [PubMed] [Google Scholar]
- 97.Douglas A, Scullion S, Antonijevic I, Brown D, Russell J, Leng G. Uterine contractile activity stimulates supraoptic neurons in term pregnant rats via a noradrenergic pathway. Endocrinology. 2001;142:633–44. doi: 10.1210/endo.142.2.7962. [DOI] [PubMed] [Google Scholar]
- 98.Hatton GI, Wang YF. Neural mechanisms underlying the milk ejection burst and reflex. Prog Brain Res. 2008;170:155–66. doi: 10.1016/S0079-6123(08)00414-7. [DOI] [PubMed] [Google Scholar]
- 99.Lincoln DW, Wakerley JB. Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol. 1975;250:443. doi: 10.1113/jphysiol.1975.sp011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang YF, Hatton GI. Burst firing of oxytocin neurons in male rat hypothalamic slices. Brain Res. 2005;1032:36–43. doi: 10.1016/j.brainres.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 101.Dyball RE, Leng G. Regulation of the milk ejection reflex in the rat. J Physiol. 1986;380:239–56. doi: 10.1113/jphysiol.1986.sp016283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Teruyama R, Armstrong WE. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J Neuroendocrinol. 2002;14:933–44. doi: 10.1046/j.1365-2826.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- 103.Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996;16:4861–71. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol. 2005;566:505–18. doi: 10.1113/jphysiol.2005.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Teruyama R, Lipschitz DL, Wang L, Ramoz GR, Crowley WR, Bealer SL, Armstrong WE. Central blockade of oxytocin receptors during mid-late gestation reduces amplitude of slow afterhyperpolarization in supraoptic oxytocin neurons. Am J Physiol Endocrinol Metab. 2008;295:E1167–71. doi: 10.1152/ajpendo.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J Physiol. 2000;526:109–14. doi: 10.1111/j.1469-7793.2000.t01-1-00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron. 1997;19:1103–14. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 108.Stern JE, Armstrong WE. Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: ionic dependence and pharmacology. J Physiol. 1997;500:497–508. doi: 10.1113/jphysiol.1997.sp022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Belin V, Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986;377:369–90. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown CH, Ludwig M, Leng G. Temporal dissociation of the feedback effects of dendritically co-released peptides on rhythmogenesis in vasopressin cells. Neuroscience. 2004;124:105–11. doi: 10.1016/j.neuroscience.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 111.de Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–34. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans. 2007;35:1236–42. doi: 10.1042/BST0351236. [DOI] [PubMed] [Google Scholar]
- 113.Ludwig M, Bull PM, Tobin VA, Sabatier N, Landgraf R, Dayanithi G, Leng G. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J Physiol. 2005;564:515–22. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shibuya I, Noguchi J, Tanaka K, Harayama N, Inoue U, Kabashima N, Ueta Y, Hattori Y, Yamashita H. PACAP increases the cytosolic Ca2+ concentration and stimulates somatodendritic vasopressin release in rat supraoptic neurons. J Neuroendocrinol. 1998;10:31–42. doi: 10.1046/j.1365-2826.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- 115.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der PL, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–8. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–66. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- 117.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–40. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 118.Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–95. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 119.Kombian SB, Mouginot D, Hirasawa M, Pittman QJ. Vasopressin preferentially depresses excitatory over inhibitory synaptic transmission in the rat supraoptic nucleus in vitro. J Neuroendocrinol. 2000;12:361–7. doi: 10.1046/j.1365-2826.2000.00462.x. [DOI] [PubMed] [Google Scholar]
- 120.Hirasawa M, Mouginot D, Kozoriz MG, Kombian SB, Pittman QJ. Vasopressin differentially modulates non-NMDA receptors in vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci. 2003;23:4270–7. doi: 10.1523/JNEUROSCI.23-10-04270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kombian SB, Hirasawa M, Mouginot D, Pittman QJ. Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus. Prog Brain Res. 2002;139:235–46. doi: 10.1016/s0079-6123(02)39020-4. [DOI] [PubMed] [Google Scholar]
- 122.Hermes ML, Ruijter JM, Klop A, Buijs RM, Renaud LP. Vasopressin increases GABAergic inhibition of rat hypothalamic paraventricular nucleus neurons in vitro. J Neurophysiol. 2000;83:705–11. doi: 10.1152/jn.2000.83.2.705. [DOI] [PubMed] [Google Scholar]
- 123.Sabatier N, Shibuya I, Dayanithi G. Intracellular calcium increase and somatodendritic vasopressin release by vasopressin receptor agonists in the rat supraoptic nucleus: involvement of multiple intracellular transduction signals. J Neuroendocrinol. 2004;16:221–36. doi: 10.1111/j.0953-8194.2004.01155.x. [DOI] [PubMed] [Google Scholar]
- 124.Brown CH, Ruan M, Scott V, Tobin VA, Ludwig M. Multi-factorial somato-dendritic regulation of phasic spike discharge in vasopressin neurons. Prog Brain Res. 2008;170:219–28. doi: 10.1016/S0079-6123(08)00419-6. [DOI] [PubMed] [Google Scholar]
- 125.Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J Neurosci. 1998;18:1879–85. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shibuya I, Kabashima N, Tanaka K, Setiadji VS, Noguchi J, Harayama N, Ueta Y, Yamashita H. Patch-clamp analysis of the mechanism of PACAP-induced excitation in rat supraoptic neurones. J Neuroendocrinol. 1998;10:759–68. doi: 10.1046/j.1365-2826.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- 127.Roper P, Callaway J, Armstrong W. Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study. J Neurosci. 2004;24:4818–31. doi: 10.1523/JNEUROSCI.4203-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dayanithi G, Sabatier N, Widmer H. Intracellular calcium signalling in magnocellular neurones of the rat supraoptic nucleus: understanding the autoregulatory mechanisms. ExpPhysiol. 2000;85(Spec No):75S. doi: 10.1111/j.1469-445x.2000.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 129.Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7:75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- 130.Sabatier N, Richard P, Dayanithi G. Activation of multiple intracellular transduction signals by vasopressin in vasopressin-sensitive neurones of the rat supraoptic nucleus. J Physiol. 1998;513:699–710. doi: 10.1111/j.1469-7793.1998.699ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–61. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 132.Moos F, Gouzenes L, Brown D, Dayanithi G, Sabatier N, Boissin L, Rabie A, Richard P. New aspects of firing pattern autocontrol in oxytocin and vasopressin neurones. Adv Exp Med Biol. 1998;449:153–62. doi: 10.1007/978-1-4615-4871-3_18. [DOI] [PubMed] [Google Scholar]
- 133.Bundzikova J, Pirnik Z, Zelena D, Mikkelsen JD, Kiss A. Response of substances co-expressed in hypothalamic magnocellular neurons to osmotic challenges in normal and Brattleboro rats. Cell Mol Neurobiol. 2008;28:1033–47. doi: 10.1007/s10571-008-9306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–81. [PubMed] [Google Scholar]
- 135.Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. J Neurosci. 1999;19:2658–64. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burazin TC, Larm JA, Gundlach AL. Regulation by osmotic stimuli of galanin-R1 receptor expression in magnocellular neurones of the paraventricular and supraoptic nuclei of the rat. J Neuroendocrinol. 2001;13:358–70. doi: 10.1046/j.1365-2826.2001.00640.x. [DOI] [PubMed] [Google Scholar]
- 137.O’Carroll AM, Lolait SJ. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraopric nuclei by osmotic stimuli. J Neuroendocrinol. 2003;15:661–6. doi: 10.1046/j.1365-2826.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- 138.Tobin VA, Bull PM, Arunachalam S, O’Carroll AM, Ueta Y, Ludwig M. The effects of apelin on the electrical activity of hypothalamic magnocellular vasopressin and oxytocin neurons and somatodendritic peptide release. Endocrinology. 2008;149:6136–45. doi: 10.1210/en.2008-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002;113:653–62. doi: 10.1016/s0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 140.Brailoiu GC, Dun SL, Yang J, Ohsawa M, Chang JK, Dun NJ. Apelin-immunoreactivity in the rat hypothalamus and pituitary. Neurosci Lett. 2002;327:193–7. doi: 10.1016/s0304-3940(02)00411-1. [DOI] [PubMed] [Google Scholar]
- 141.Roberts EM, Pope GR, Newson MJ, Landgraf R, Lolait SJ, O’Carroll AM. Stimulus-specific neuroendocrine responses to osmotic challenges in apelin receptor knockout mice. J Neuroendocrinol. 2010;22:301–8. doi: 10.1111/j.1365-2826.2010.01968.x. [DOI] [PubMed] [Google Scholar]
- 142.Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortes C. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–96. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 143.Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 2004;145:4392–400. doi: 10.1210/en.2004-0384. [DOI] [PubMed] [Google Scholar]
- 144.Poulain DA, Wakerley JB, Dyball RE. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977;196:367–84. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- 145.Quirion R, Finkel MS, Mendelsohn FA, Zamir N. Localization of opiate binding sites in kidney and adrenal gland of the rat. Life Sci. 1983;33(Suppl 1):299–302. doi: 10.1016/0024-3205(83)90502-7. [DOI] [PubMed] [Google Scholar]
- 146.Yamada K, Imai M, Yoshida S. Mechanism of diuretic action of U-62,066E, a kappa opioid receptor agonist. Eur J Pharmacol. 1989;160:229–37. doi: 10.1016/0014-2999(89)90495-0. [DOI] [PubMed] [Google Scholar]
- 147.Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. The kappa opioid receptor and dynorphin co-localize in vasopressin magnocellular neurosecretory neurons in guinea-pig hypothalamus. Neuroscience. 2000;96:373–83. doi: 10.1016/s0306-4522(99)00472-8. [DOI] [PubMed] [Google Scholar]
- 148.Glasgow E, Kusano K, Chin H, Mezey E, Young WS, 3rd, Gainer H. Single cell reverse transcription-polymerase chain reaction analysis of rat supraoptic magnocellular neurons: neuropeptide phenotypes and high voltage-gated calcium channel subtypes. Endocrinology. 1999;140:5391–401. doi: 10.1210/endo.140.11.7136. [DOI] [PubMed] [Google Scholar]
- 149.Inenaga K, Nagatomo T, Nakao K, Yanaihara N, Yamashita H. Kappa-selective agonists decrease postsynaptic potentials and calcium components of action potentials in the supraoptic nucleus of rat hypothalamus in vitro. Neuroscience. 1994;58:331–40. doi: 10.1016/0306-4522(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 150.Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–58. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown CH, Ghamari-Langroudi M, Leng G, Bourque CW. Kappa-opioid receptor activation inhibits post-spike depolarizing after-potentials in rat supraoptic nucleus neurones in vitro. J Neuroendocrinol. 1999;11:825–8. doi: 10.1046/j.1365-2826.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- 152.Scott V, Brown CH. State-dependent plasticity in vasopressin neurones: dehydration-induced changes in activity patterning. J Neuroendocrinol. 2010;22:343–54. doi: 10.1111/j.1365-2826.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- 153.Ciosek J, Cisowska A. Centrally administered galanin modifies vasopressin and oxytocin release from the hypothalamo-neurohypophysial system of euhydrated and dehydrated rats. J Physiol Pharmacol. 2003;54:625–41. [PubMed] [Google Scholar]
- 154.Cisowska-Maciejewska A, Ciosek J. Galanin influences vasopressin and oxytocin release from the hypothalamo-neurohypophysial system of salt loaded rats. J Physiol Pharmacol. 2005;56:673–88. [PubMed] [Google Scholar]
- 155.Kai A, Ono K, Kawano H, Honda E, Nakanishi O, Inenaga K. Galanin inhibits neural activity in the subfornical organ in rat slice preparation. Neuroscience. 2006;143:769–77. doi: 10.1016/j.neuroscience.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 156.Nagyeri G, Galfi M, Radacs M, Molnar AH, Laszlo F, Varga C, Laszlo FA. Effects of galanin-monoaminergic interactions on vasopressin secretion in rat neurohypophyseal cell cultures. Regul Pept. 2009;155:76–80. doi: 10.1016/j.regpep.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 157.Kozoriz MG, Kuzmiski JB, Hirasawa M, Pittman QJ. Galanin modulates neuronal and synaptic properties in the rat supraoptic nucleus in a use and state dependent manner. J Neurophysiol. 2006;96:154–64. doi: 10.1152/jn.01028.2005. [DOI] [PubMed] [Google Scholar]
- 158.Papas S, Bourque CW. Galanin inhibits continuous and phasic firing in rat hypothalamic magnocellular neurosecretory cells. J Neurosci. 1997;17:6048–56. doi: 10.1523/JNEUROSCI.17-16-06048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.D’Amato F, Cocco C, Noli B, Cabras T, Messana I, Ferri GL. VGF peptides upon osmotic stimuli: changes in neuroendocrine regulatory peptides 1 and 2 in the hypothalamic-pituitary-axis and plasma. J Chem Neuroanat. 2012;44:57–65. doi: 10.1016/j.jchemneu.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 160.Chu JY, Chung SC, Lam AK, Tam S, Chung SK, Chow BK. Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol Cell Biol. 2007;27:2499–511. doi: 10.1128/MCB.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chu JY, Cheng CY, Lee VH, Chan YS, Chow BK. Secretin and body fluid homeostasis. Kidney Int. 2011;79:280–7. doi: 10.1038/ki.2010.397. [DOI] [PubMed] [Google Scholar]
- 162.Velmurugan S, Brunton PJ, Leng G, Russell JA. Circulating secretin activates supraoptic nucleus oxytocin and vasopressin neurons via noradrenergic pathways in the rat. Endocrinology. 2010;151:2681–8. doi: 10.1210/en.2009-1440. [DOI] [PubMed] [Google Scholar]
- 163.Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- 164.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- 165.Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- 166.Freund-Mercier MJ, Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984;352:447–66. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moos F, Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brussaard AB, Kits KS, de Vlieger TA. Postsynaptic mechanism of depression of GABAergic synapses by oxytocin in the supraoptic nucleus of immature rat. J Physiol. 1996;497:495–507. doi: 10.1113/jphysiol.1996.sp021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–12. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- 170.Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–24. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol. 2008;4:e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hatton GI. Astroglial modulation of neurotransmitter/peptide release from the neurohypophysis: present status. J Chem Neuroanat. 1999;16:203–21. doi: 10.1016/s0891-0618(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 173.Perlmutter LS, Tweedle CD, Hatton GI. Neuronal/glial plasticity in the supraoptic dendritic zone: dendritic bundling and double synapse formation at parturition. Neuroscience. 1984;13:769–79. doi: 10.1016/0306-4522(84)90095-2. [DOI] [PubMed] [Google Scholar]
- 174.Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol. 1994;6:369–73. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 175.Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478:275–87. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tobin V, Gouty LA, Moos FC, Desarmenien MG. A store-operated current (SOC) mediates oxytocin autocontrol in the developing rat hypothalamus. Eur J Neurosci. 2006;24:400–4. doi: 10.1111/j.1460-9568.2006.04935.x. [DOI] [PubMed] [Google Scholar]
- 177.Tobin VA, Hurst G, Norrie L, Dal Rio FP, Bull PM, Ludwig M. Thapsigargin-induced mobilization of dendritic dense-cored vesicles in rat supraoptic neurons. Eur J Neurosci. 2004;19:2909–12. doi: 10.1111/j.1460-9568.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- 178.Tobin VA, Ludwig M. The role of the actin cytoskeleton in oxytocin and vasopressin release from rat supraoptic nucleus neurons. J Physiol. 2007;582:1337–48. doi: 10.1113/jphysiol.2007.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- 180.Moos F, Marganiec A, Fontanaud P, Guillou-Duvoid A, Alonso G. Synchronization of oxytocin neurons in suckled rats: possible role of bilateral innervation of hypothalamic supraoptic nuclei by single medullary neurons. Eur J Neurosci. 2004;20:66–78. doi: 10.1111/j.0953-816X.2004.03455.x. [DOI] [PubMed] [Google Scholar]
- 181.Sumner BE, Coombes JE, Pumford KM, Russell JA. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience. 1990;37:635–45. doi: 10.1016/0306-4522(90)90095-l. [DOI] [PubMed] [Google Scholar]
- 182.Smith MJ, Wise PM. Localization of kappa opioid receptors in oxytocin magnocellular neurons in the paraventricular and supraoptic nuclei. Brain Res. 2001;898:162–5. doi: 10.1016/s0006-8993(01)02154-0. [DOI] [PubMed] [Google Scholar]
- 183.Brown CH, Munro G, Johnstone LE, Robson AC, Landgraf R, Russell JA. Oxytocin neurone autoexcitation during morphine withdrawal in anaesthetized rats. Neuroreport. 1997;8:951–5. doi: 10.1097/00001756-199703030-00027. [DOI] [PubMed] [Google Scholar]
- 184.Russell JA, Neumann I, Landgraf R. Oxytocin and vasopressin release in discrete brain areas after naloxone in morphine-tolerant and -dependent anesthetized rats: push-pull perfusion study. J Neurosci. 1992;12:1024–32. doi: 10.1523/JNEUROSCI.12-03-01024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brown CH, Murphy NP, Munro G, Ludwig M, Bull PM, Leng G, Russell JA. Interruption of central noradrenergic pathways and morphine withdrawal excitation of oxytocin neurones in the rat. J Physiol. 1998;507:831–42. doi: 10.1111/j.1469-7793.1998.831bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao BG, Chapman C, Bicknell RJ. Opioid-noradrenergic interactions in the neurohypophysis. I. Differential opioid receptor regulation of oxytocin, vasopressin, and noradrenaline release. Neuroendocrinology. 1988;48:16. doi: 10.1159/000124984. [DOI] [PubMed] [Google Scholar]
- 187.Xiang Z, Bo X, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res. 1998;813:390–7. doi: 10.1016/s0006-8993(98)01073-7. [DOI] [PubMed] [Google Scholar]
- 188.Xiang Z, He C, Burnstock G. P2X5 receptors are expressed on neurons containing arginine vasopressin and nitric oxide synthase in the rat hypothalamus. Brain Res. 2006;1099:56–63. doi: 10.1016/j.brainres.2006.04.126. [DOI] [PubMed] [Google Scholar]
- 189.Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol. 1999;514:351–67. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;292:R423–31. doi: 10.1152/ajpregu.00495.2006. [DOI] [PubMed] [Google Scholar]
- 191.Mori M, Tsushima H, Matsuda T. Antidiuretic effects of ATP induced by microinjection into the hypothalamic supraoptic nucleus in water-loaded and ethanol-anesthetized rats. Jpn J Pharmacol. 1994;66:445–50. doi: 10.1254/jjp.66.445. [DOI] [PubMed] [Google Scholar]
- 192.Song Z, Gomes DA, Stevens W, Sladek CD. Multiple alpha1-adrenergic receptor subtypes support synergistic stimulation of vasopressin and oxytocin release by ATP and phenylephrine. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1529–37. doi: 10.1152/ajpregu.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hiruma H, Bourque CW. P2 purinoceptor-mediated depolarization of rat supraoptic neurosecretory cells in vitro. J Physiol. 1995;489(Pt 3):805–11. doi: 10.1113/jphysiol.1995.sp021093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vavra V, Bhattacharya A, Zemkova H. Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience. 2011;188:1–12. doi: 10.1016/j.neuroscience.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 195.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–86. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 196.Day TA, Sibbald JR, Khanna S. ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993;607:341–4. doi: 10.1016/0006-8993(93)91528-z. [DOI] [PubMed] [Google Scholar]
- 197.Song Z, Sladek CD. Does conversion of ATP to adenosine terminate ATP-stimulated vasopressin release from hypothalamo-neurohypophyseal explants? Brain Res. 2005;1047:105–11. doi: 10.1016/j.brainres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 198.Bull PM, Brown CH, Russell JA, Ludwig M. Activity-dependent feedback modulation of spike patterning of supraoptic nucleus neurons by endogenous adenosine. Am J Physiol Regul Integr Comp Physiol. 2006;291:R83–90. doi: 10.1152/ajpregu.00744.2005. [DOI] [PubMed] [Google Scholar]
- 199.Ruan M, Brown CH. Feedback inhibition of action potential discharge by endogenous adenosine enhancement of the medium afterhyperpolarization. J Physiol. 2009;587:1043–56. doi: 10.1113/jphysiol.2008.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oliet SH, Poulain DA. Adenosine-induced presynaptic inhibition of IPSCs and EPSCs in rat hypothalamic supraoptic nucleus neurones. J Physiol. 1999;520:815–25. doi: 10.1111/j.1469-7793.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ponzio TA, Wang YF, Hatton GI. Activation of adenosine A2A receptors alters postsynaptic currents and depolarizes neurons of the supraoptic nucleus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R359–66. doi: 10.1152/ajpregu.00747.2005. [DOI] [PubMed] [Google Scholar]
- 202.Ponzio TA, Hatton GI. Adenosine postsynaptically modulates supraoptic neuronal excitability. J Neurophysiol. 2005;93:535–47. doi: 10.1152/jn.01185.2003. [DOI] [PubMed] [Google Scholar]
- 203.Aguila FA, Oliveira-Pelegrin GR, Yao ST, Murphy D, Rocha MJ. Anteroventral third ventricle (AV3V) lesion affects hypothalamic neuronal nitric oxide synthase (nNOS) expression following water deprivation. Brain Res Bull. 2011;86:239–45. doi: 10.1016/j.brainresbull.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 204.Ventura RR, Giusti-Paiva A, Gomes DA, Elias LLK, Antunes-Rodrigues J. Neuronal nitric oxide synthase inhibition differentially affects oxytocin and vasopressin secretion in salt loaded rats. Neurosci Lett. 2005;379:75–80. doi: 10.1016/j.neulet.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 205.Bull PM, Ludwig M, Blackburn-Munro GJ, Delgado-Cohen H, Brown CH, Russell JA. The role of nitric oxide in morphine dependence and withdrawal excitation of rat oxytocin neurons. Eur J Neurosci. 2003;18:2545–51. doi: 10.1046/j.1460-9568.2003.03005.x. [DOI] [PubMed] [Google Scholar]
- 206.Liu QS, Jia YS, Ju G. Nitric oxide inhibits neuronal activity in the supraoptic nucleus of the rat hypothalamic slices. Brain Res Bull. 1997;43:121–5. doi: 10.1016/s0361-9230(96)00209-2. [DOI] [PubMed] [Google Scholar]
- 207.Stern JE, Ludwig M. NO inhibits supraoptic oxytocin and vasopressin neurons via activation of GABAergic synaptic inputs. Am J Physiol. 2001;280:R1815–22. doi: 10.1152/ajpregu.2001.280.6.R1815. [DOI] [PubMed] [Google Scholar]
- 208.Ozaki M, Shibuya I, Kabashima N, Isse T, Noguchi J, Ueta Y, Inoue Y, Shigematsu A, Yamashita H. Preferential potentiation by nitric oxide of spontaneous inhibitory postsynaptic currents in rat supraoptic neurones. J Neuroendocrinol. 2000;12:273–81. doi: 10.1046/j.1365-2826.2000.00448.x. [DOI] [PubMed] [Google Scholar]
- 209.Filosa JA, Naskar K, Perfume G, Iddings JA, Biancardi VC, Vatta MS, Stern JE. Endothelin-mediated calcium responses in supraoptic nucleus astrocytes influence magnocellular neurosecretory firing activity. J Neuroendocrinol. 2012;24:378–92. doi: 10.1111/j.1365-2826.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 210.Reis WL, Biancardi VC, Son S, Antunes-Rodrigues J, Stern JE. Enhanced expression of heme oxygenase-1 and carbon monoxide excitatory effects in oxytocin and vasopressin neurones during water deprivation. J Neuroendocrinol. 2012;24:653–63. doi: 10.1111/j.1365-2826.2011.02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 212.Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol. 2012;24:566–76. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kuzmiski JB, Bains JS. Metabotropic glutamate receptors: gatekeepers of homeostasis. J Neuroendocrinol. 2010;22:785–92. doi: 10.1111/j.1365-2826.2010.02020.x. [DOI] [PubMed] [Google Scholar]
- 215.Tweedle CD, Hatton GI. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977;181:59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- 216.Theodosis DT, Poulain DA, Vincent JD. Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience. 1981;6:919–29. doi: 10.1016/0306-4522(81)90173-1. [DOI] [PubMed] [Google Scholar]
- 217.Theodosis DT, Poulain DA. Evidence that oxytocin-secreting neurones are involved in the ultrastructural reorganisation of the rat supraoptic nucleus apparent at lactation. Cell Tissue Res. 1984;235:217–9. doi: 10.1007/BF00213745. [DOI] [PubMed] [Google Scholar]
- 218.Catheline G, Touquet B, Lombard MC, Poulain DA, Theodosis DT. A study of the role of neuro-glial remodeling in the oxytocin system at lactation. Neuroscience. 2006;137:309–16. doi: 10.1016/j.neuroscience.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 219.Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–60. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- 220.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–6. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 221.Boudaba C, Linn DM, Halmos KC, Tasker JG. Increased tonic activation of presynaptic metabotropic glutamate receptors in the rat supraoptic nucleus following chronic dehydration. J Physiol. 2003;551:815–23. doi: 10.1113/jphysiol.2003.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–84. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 223.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101:2151–5. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bonfardin VD, Fossat P, Theodosis DT, Oliet SH. Glia-dependent switch of kainate receptor presynaptic action. J Neurosci. 2010;30:985–95. doi: 10.1523/JNEUROSCI.3389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011;589:3929–41. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Theodosis DT, Poulain DA. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol. 1999;468:175–82. doi: 10.1007/978-1-4615-4685-6_14. [DOI] [PubMed] [Google Scholar]
- 227.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R291–300. doi: 10.1152/ajpregu.00056.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci. 2013;33:631–40. doi: 10.1523/JNEUROSCI.3936-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol. 2009;587:4645–60. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–9. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- 231.Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J, Blackburn-Munro RE, Feng J, Onaka T, Verbalis JG, Russell JA, Ludwig M. Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci. 2001;21:6967–77. doi: 10.1523/JNEUROSCI.21-17-06967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–34. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 233.Huang DY, Boini KM, Lang PA, Grahammer F, Duszenko M, Heller-Stilb B, Warskulat U, Haussinger D, Lang F, Vallon V. Impaired ability to increase water excretion in mice lacking the taurine transporter gene TAUT. Pflugers Arch. 2006;451:668–77. doi: 10.1007/s00424-005-1499-y. [DOI] [PubMed] [Google Scholar]
- 234.Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. [PubMed] [Google Scholar]
- 235.Armstrong WE, Stern JE. Electrophysiological and morphological characteristics of neurons in perinuclear zone of supraoptic nucleus. J Neurophysiol. 1997;78:2427–37. doi: 10.1152/jn.1997.78.5.2427. [DOI] [PubMed] [Google Scholar]
- 236.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332:123–43. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 237.Boudaba C, Tasker JG. Intranuclear coupling of hypothalamic magnocellular nuclei by glutamate synaptic circuits. Am J Physiol Regul Integr Comp Physiol. 2006;291:R102–R11. doi: 10.1152/ajpregu.00795.2005. [DOI] [PubMed] [Google Scholar]
- 238.Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–60. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- 240.Daftary SS, Boudaba C, Szabo K, Tasker JG. Noradrenergic excitation of magnocellular neurons in the rat hypothalamic paraventricular nucleus via intranuclear glutamatergic circuits. J Neurosci. 1998;18:10619–28. doi: 10.1523/JNEUROSCI.18-24-10619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Boudaba C, Di S, Tasker JG. Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J Neuroendocrinol. 2003;15:803–10. doi: 10.1046/j.1365-2826.2003.01063.x. [DOI] [PubMed] [Google Scholar]
- 242.Mason WT, Ho YW, Eckenstein F, Hatton GI. Mapping of cholinergic neurons associated with rat supraoptic nucleus: combined immunocytochemical and histochemical identification. Brain Res Bull. 1983;11:617–26. doi: 10.1016/0361-9230(83)90133-8. [DOI] [PubMed] [Google Scholar]
- 243.Zaninetti M, Blanchet C, Tribollet E, Bertrand D, Raggenbass M. Magnocellular neurons of the rat supraoptic nucleus are endowed with functional nicotinic acetylcholine receptors. Neuroscience. 2000;95:319–23. doi: 10.1016/s0306-4522(99)00477-7. [DOI] [PubMed] [Google Scholar]
- 244.Hatton GI, Yang QZ. Synaptic potentials mediated by alpha 7 nicotinic acetylcholine receptors in supraoptic nucleus. J Neurosci. 2002;22:29–37. doi: 10.1523/JNEUROSCI.22-01-00029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–30. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zaninetti M, Tribollet E, Bertrand D, Raggenbass M. Nicotinic cholinergic activation of magnocellular neurons of the hypothalamic paraventricular nucleus. Neuroscience. 2002;110:287–99. doi: 10.1016/s0306-4522(01)00536-x. [DOI] [PubMed] [Google Scholar]
- 247.Sladek CD, Joynt RJ. Cholinergic involvement in osmotic control of vasopressin release by the organ-cultured rat hypothalamo-neurohypophyseal system. Endocrinology. 1979;105:367–71. doi: 10.1210/endo-105-2-367. [DOI] [PubMed] [Google Scholar]
- 248.McKinley MJ, Pennington GL, Oldfield BJ. Anteroventral wall of the third ventricle and dorsal lamina terminalis: headquarters for control of body fluid homeostasis? Clin Exp Pharmacol Physiol. 1996;23:271–81. doi: 10.1111/j.1440-1681.1996.tb02823.x. [DOI] [PubMed] [Google Scholar]
- 249.Hussy N, Bres V, Rochette M, Duvoid A, Alonso G, Dayanithi G, Moos FC. Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. J Neurosci. 2001;21:7110–6. doi: 10.1523/JNEUROSCI.21-18-07110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Leng G, Blackburn RE, Dyball RE, Russell JA. Role of anterior peri-third ventricular structures in the regulation of supraoptic neuronal activity and neurohypophysial hormone secretion in the rat. J Neuroendocrinol. 1989;1:35–46. doi: 10.1111/j.1365-2826.1989.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 251.Hollis JH, McKinley MJ, D’Souza M, Kampe J, Oldfield BJ. The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex: a neuroanatomical framework for the generation of thirst. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1390–R401. doi: 10.1152/ajpregu.00869.2007. [DOI] [PubMed] [Google Scholar]
- 252.Buggy J, Johnson AK. Preoptic-hypothalamic periventricular lesions: thirst deficits and hypernatremia. Am J Physiol. 1977;233:R44–R52. doi: 10.1152/ajpregu.1977.233.1.R44. [DOI] [PubMed] [Google Scholar]
- 253.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. Am J Physiol. 1986;250:R267–R75. doi: 10.1152/ajpregu.1986.250.2.R267. [DOI] [PubMed] [Google Scholar]
- 254.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–75. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience. 2000;100:539–47. doi: 10.1016/s0306-4522(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 256.McKinley MJ, Oldfield BJ. The brain as an endocrine target for peptide hormones. Trends Endocrinol Metab. 1998;9:349–54. doi: 10.1016/s1043-2760(98)00092-7. [DOI] [PubMed] [Google Scholar]
- 257.Gehlert DR, Gackenheimer SL, Schober DA. Autoradiographic localization of subtypes of angiotensin II antagonist binding in the rat brain. Neuroscience. 1991;44:501–14. doi: 10.1016/0306-4522(91)90073-w. [DOI] [PubMed] [Google Scholar]
- 258.Knepel W, Nutto D, Meyer DK. Effect of transection of subfornical organ efferent projections on vasopressin release induced by angiotensin or isoprenaline in the rat. Brain Res. 1982;248:180–4. doi: 10.1016/0006-8993(82)91161-1. [DOI] [PubMed] [Google Scholar]
- 259.Richard D, Bourque CW. Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol. 1995;489:567–77. doi: 10.1113/jphysiol.1995.sp021073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nissen R, Renaud LP. GABA receptor mediation of median preoptic nucleus-evoked inhibition of supraoptic neurosecretory neurones in rat. J Physiol. 1994;479:207–16. doi: 10.1113/jphysiol.1994.sp020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jhamandas JH, Lind RW, Renaud LP. Angiotensin II may mediate excitatory neurotransmission from the subfornical organ to the hypothalamic supraoptic nucleus: an anatomical and electrophysiological study in the rat. Brain Res. 1989;487:52–61. doi: 10.1016/0006-8993(89)90939-6. [DOI] [PubMed] [Google Scholar]
- 262.Naeini RS, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–8. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- 263.Voisin DL, Bourque CW. Integration of sodium and osmosensory signals in vasopressin neurons. Trends Neurosci. 2002;25:199–205. doi: 10.1016/s0166-2236(02)02142-2. [DOI] [PubMed] [Google Scholar]
- 264.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–31. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 265.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1172–83. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- 266.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–85. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2279–89. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shi P, Martinez MA, Calderon AS, Chen Q, Cunningham JT, Toney GM. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol. 2008;586:5231–45. doi: 10.1113/jphysiol.2008.159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ciriello J. Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci Lett. 1983;36:37–42. doi: 10.1016/0304-3940(83)90482-2. [DOI] [PubMed] [Google Scholar]
- 270.Murphy NP, Onaka T, Brown CH, Leng G. The role of afferent inputs to supraoptic nucleus oxytocin neurons during naloxone-precipitated morphine withdrawal in the rat. Neuroscience. 1997;80:567–77. doi: 10.1016/s0306-4522(97)00142-5. [DOI] [PubMed] [Google Scholar]
- 271.Shioda S, Nakai Y. Noradrenergic innervation of vasopressin-containing neurons in the rat hypothalamic supraoptic nucleus. Neurosci Lett. 1992;140:215–8. doi: 10.1016/0304-3940(92)90106-h. [DOI] [PubMed] [Google Scholar]
- 272.Raby WN, Renaud LP. Dorsomedial medulla stimulation activates rat supraoptic oxytocin and vasopressin neurones through different pathways. J Physiol. 1989;417:279–94. doi: 10.1113/jphysiol.1989.sp017801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Randle JC, Bourque CW, Renaud LP. Alpha 1-adrenergic receptor activation depolarizes rat supraoptic neurosecretory neurons in vitro. Am J Physiol. 1986;251:R569–74. doi: 10.1152/ajpregu.1986.251.3.R569. [DOI] [PubMed] [Google Scholar]
- 274.Yamashita H, Inenaga K, Kannan H. Depolarizing effect of noradrenaline on neurons of the rat supraoptic nucleus in vitro. Brain Res. 1987;405:348–52. doi: 10.1016/0006-8993(87)90304-0. [DOI] [PubMed] [Google Scholar]
- 275.Day TA, Randle JC, Renaud LP. Opposing alpha- and beta-adrenergic mechanisms mediate dose-dependent actions of noradrenaline on supraoptic vasopressin neurones in vivo. Brain Res. 1985;358:171–9. doi: 10.1016/0006-8993(85)90961-8. [DOI] [PubMed] [Google Scholar]
- 276.Khanna S, Sibbald JR, Day TA. Alpha 2-adrenoceptor modulation of A1 noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993;613:164–7. doi: 10.1016/0006-8993(93)90469-4. [DOI] [PubMed] [Google Scholar]
- 277.Ceccatelli S, Millhorn DE, Hokfelt T, Goldstein M. Evidence for the occurrence of an enkephalin-like peptide in adrenaline and noradrenaline neurons of the rat medulla oblongata. Exp Brain Res. 1989;74:631–40. doi: 10.1007/BF00247366. [DOI] [PubMed] [Google Scholar]
- 278.Levin MC, Sawchenko PE, Howe PR, Bloom SR, Polak JM. Organization of galanin-immunoreactive inputs to the paraventricular nucleus with special reference to their relationship to catecholaminergic afferents. J Comp Neurol. 1987;261:562–82. doi: 10.1002/cne.902610408. [DOI] [PubMed] [Google Scholar]
- 279.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–53. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 280.Bittencourt JC, Benoit R, Sawchenko PE. Distribution and origins of substance P-immunoreactive projections to the paraventricular and supraoptic nuclei: partial overlap with ascending catecholaminergic projections. J Chem Neuroanat. 1991;4:63–78. doi: 10.1016/0891-0618(91)90032-8. [DOI] [PubMed] [Google Scholar]
- 281.Cunningham ET, Jr., Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 282.Sawchenko PE, Arias C, Bittencourt JC. Inhibin beta, somatostatin, and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1990;291:269–80. doi: 10.1002/cne.902910209. [DOI] [PubMed] [Google Scholar]
- 283.Day TA, Renaud LP, Sibbald JR. Excitation of supraoptic vasopressin cells by stimulation of the A1 noradrenaline cell group: failure to demonstrate role for established adrenergic or amino acid receptors. Brain Res. 1990;516:91–8. doi: 10.1016/0006-8993(90)90901-m. [DOI] [PubMed] [Google Scholar]
- 284.Knott TK, Hussy N, Cuadra AE, Lee RH, Ortiz-Miranda S, Custer EE, Lemos JR. Adenosine trisphosphate appears to act via different receptors in terminals versus somata of the hypothalamic neurohypophysial system. J Neuroendocrinol. 2012;24:681–9. doi: 10.1111/j.1365-2826.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Song Z, Vijayaraghavan S, Sladek CD. Simultaneous exposure to ATP and phenylephrine induces a sustained elevation in the intracellular calcium concentration in supraoptic neurons. Am J Physiol Regul Integr Comp Physiol. 2006;291:R37–45. doi: 10.1152/ajpregu.00718.2005. [DOI] [PubMed] [Google Scholar]
- 286.Kapoor JR, Sladek CD. Substance P and NPY differentially potentiate ATP and adrenergic stimulated vasopressin and oxytocin release. Am J Physiol. 2001;280:R69–R78. doi: 10.1152/ajpregu.2001.280.1.R69. [DOI] [PubMed] [Google Scholar]
- 287.Mecawi AS, Vilhena-Franco T, Araujo IG, Reis LC, Elias LL, Antunes-Rodrigues J. Estradiol potentiates hypothalamic vasopressin and oxytocin neuron activation and hormonal secretion induced by hypovolemic shock. Am J Physiol Regul Integr Comp Physiol. 2011;301:R905–15. doi: 10.1152/ajpregu.00800.2010. [DOI] [PubMed] [Google Scholar]
- 288.Harris MC. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocrinol. 1979;82:115–25. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- 289.Kendrick KM, Leng G. Haemorrhage-induced release of noradrenaline, 5-hydroxytryptamine and uric acid in the supraoptic nucleus of the rat, measured by microdialysis. Brain Res. 1988;440:402–6. doi: 10.1016/0006-8993(88)91016-5. [DOI] [PubMed] [Google Scholar]
- 290.Rodovalho GV, Franci CR, Morris M, Anselmo-Franci JA. Locus coeruleus lesions decrease oxytocin and vasopressin release induced by hemorrhage. Neurochem Res. 2006;31:259–66. doi: 10.1007/s11064-005-9015-5. [DOI] [PubMed] [Google Scholar]
- 291.Banks D, Harris MC. Lesions of the locus coeruleus abolish baroreceptor-induced depression of supraoptic neurones in the rat. J Physiol. 1984;355:383–98. doi: 10.1113/jphysiol.1984.sp015425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Day TA, Renaud LP. Electrophysiological evidence that noradrenergic afferents selectively facilitate the activity of supraoptic vasopressin neurons. Brain Res. 1984;303:233–40. doi: 10.1016/0006-8993(84)91209-5. [DOI] [PubMed] [Google Scholar]
- 293.Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:25–53. [PubMed] [Google Scholar]
- 294.Cunningham JT, Grindstaff RJ, Grindstaff RR, Sullivan MJ. Fos immunoreactivity in the diagonal band and the perinuclear zone of the supraoptic nucleus after hypertension and hypervolaemia in unanaesthetized rats. J Neuroendocrinol. 2002;14:219–27. doi: 10.1046/j.0007-1331.2001.00765.x. [DOI] [PubMed] [Google Scholar]
- 295.Grindstaff RJ, Grindstaff RR, Cunningham JT. Baroreceptor sensitivity of rat supraoptic vasopressin neurons involves noncholinergic neurons in the DBB. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1934–43. doi: 10.1152/ajpregu.2000.279.5.R1934. [DOI] [PubMed] [Google Scholar]
- 296.Cunningham JT, Nissen R, Renaud LP. Norepinephrine injections in diagonal band of Broca selectively reduced the activity of vasopressin supraoptic neurons in the rat. Brain Res. 1993;610:152–5. doi: 10.1016/0006-8993(93)91229-l. [DOI] [PubMed] [Google Scholar]
- 297.Jhamandas JH, Renaud LP. Diagonal band neurons may mediate arterial baroreceptor input to hypothalamic vasopressin-secreting neurons. Neurosci Lett. 1986;65:214–8. doi: 10.1016/0304-3940(86)90307-1. [DOI] [PubMed] [Google Scholar]
- 298.Jhamandas JH, Renaud LP. Bicuculline blocks an inhibitory baroreflex input to supraoptic vasopressin neurons. Am J Physiol. 1987;252:R947–R52. doi: 10.1152/ajpregu.1987.252.5.R947. [DOI] [PubMed] [Google Scholar]
- 299.Jhamandas JH, Raby W, Rogers J, Buijs RM, Renaud LP. Diagonal band projection towards the hypothalamic supraoptic nucleus: light and electron microscopic observations in the rat. J Comp Neurol. 1989;282:15–23. doi: 10.1002/cne.902820103. [DOI] [PubMed] [Google Scholar]
- 300.Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17:471–503. doi: 10.1016/s1521-690x(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 301.Kim JS, Kim WB, Kim YB, Lee Y, Kim YS, Shen FY, Lee SW, Park D, Choi HJ, Hur J, Park JJ, Han HC, Colwell CS, Cho YW, Kim YI. Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J Neurosci. 2011;31:13312–22. doi: 10.1523/JNEUROSCI.1440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Michaloudi HC, El Majdoubi M, Poulain DA, Papadopoulos GC, Theodosis DT. The noradrenergic innervation of identified hypothalamic magnocellular somata and its contribution to lactation-induced synaptic plasticity. J Neuroendocrinol. 1997;9:17–23. doi: 10.1046/j.1365-2826.1997.00583.x. [DOI] [PubMed] [Google Scholar]
- 303.Ortega-Villalobos M, Garcia-Bazan M, Solano-Flores LP, Ninomiya-Alarcon JG, Guevara-Guzman R, Wayner MJ. Vagus nerve afferent and efferent innervation of the rat uterus: an electrophysiological and HRP study. Brain Res Bull. 1990;25:365–71. doi: 10.1016/0361-9230(90)90221-k. [DOI] [PubMed] [Google Scholar]
- 304.Douglas AJ, Neumann I, Meeren HK, Leng G, Johnstone LE, Munro G, Russell JA. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J Neurosci. 1995;15:5049–57. doi: 10.1523/JNEUROSCI.15-07-05049.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Meddle SL, Leng G, Selvarajah JR, Bicknell RJ, Russell JA. Direct pathways to the supraoptic nucleus from the brainstem and the main olfactory bulb are activated at parturition in the rat. Neuroscience. 2000;101:1013–21. doi: 10.1016/s0306-4522(00)00300-6. [DOI] [PubMed] [Google Scholar]
- 306.Herbison AE, Voisin DL, Douglas AJ, Chapman C. Profile of monoamine and excitatory amino acid release in rat supraoptic nucleus over parturition. Endocrinology. 1997;138:33–40. doi: 10.1210/endo.138.1.4859. [DOI] [PubMed] [Google Scholar]
- 307.Li C, Chen P, Smith MS. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience. 1999;94:117–29. doi: 10.1016/s0306-4522(99)00236-5. [DOI] [PubMed] [Google Scholar]
- 308.Tobin VA, Leng G, Ludwig M, Douglas AJ. Increased sensitivity of monoamine release in the supraoptic nucleus in late pregnancy: region- and stimulus-dependent responses. J Neuroendocrinol. 2010;22:430–7. doi: 10.1111/j.1365-2826.2010.01957.x. [DOI] [PubMed] [Google Scholar]
- 309.Lipschitz DL, Crowley WR, Bealer SL. Differential sensitivity of intranuclear and systemic oxytocin release to central noradrenergic receptor stimulation during mid- and late gestation in rats. Am J Physiol Endocrinol Metab. 2004;287:E523–8. doi: 10.1152/ajpendo.00572.2003. [DOI] [PubMed] [Google Scholar]
- 310.Bealer SL, Crowley WR. Noradrenergic control of central oxytocin release during lactation in rats. Am J Physiol. 1998;274:E453–8. doi: 10.1152/ajpendo.1998.274.3.E453. [DOI] [PubMed] [Google Scholar]
- 311.Onaka T, Ikeda K, Yamashita T, Honda K. Facilitative role of endogenous oxytocin in noradrenaline release in the rat supraoptic nucleus. Eur J Neurosci. 2003;18:3018–26. doi: 10.1046/j.1460-9568.2003.03037.x. [DOI] [PubMed] [Google Scholar]
- 312.Bealer SL, Armstrong WE, Crowley WR. Oxytocin release in magnocellular nuclei: neurochemical mediators and functional significance during gestation. Am J Physiol Regul Integr Comp Physiol. 2010;299:R452–28. doi: 10.1152/ajpregu.00217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 314.Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res. 1998;788:287–93. doi: 10.1016/s0006-8993(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 315.Moos F, Ingram CD, Wakerley JB, Guerne Y, Freund-Mercier MJ, Richard P. Oxytocin in the bed nucleus of the stria terminalis and lateral septum facilitates bursting of hypothalamic oxytocin neurons in suckled rats. J Neuroendocrinol. 1991;3:163–71. doi: 10.1111/j.1365-2826.1991.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 316.Lambert RC, Moos FC, Ingram CD, Wakerley JB, Kremarik P, Guerne Y, Richard P. Electrical activity of neurons in the ventrolateral septum and bed nuclei of the stria terminalis in suckled rats: statistical analysis gives evidence for sensitivity to oxytocin and for relation to the milk-ejection reflex. Neuroscience. 1993;54:361–76. doi: 10.1016/0306-4522(93)90258-h. [DOI] [PubMed] [Google Scholar]
- 317.Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–9. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- 318.Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987;253:R661–R5. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- 319.Chakfe Y, Bourque CW. Excitatory peptides and osmotic pressure modulate mechanosensitive cation channels in concert. Nat Neurosci. 2000;3:572–9. doi: 10.1038/75744. [DOI] [PubMed] [Google Scholar]
- 320.McCann MJ, Verbalis JG, Stricker EM. Capsaicin pretreatment attenuates multiple responses to cholecystokinin in rats. J Auton Nerv Syst. 1988;23:265–72. doi: 10.1016/0165-1838(88)90101-4. [DOI] [PubMed] [Google Scholar]
- 321.Scott V, Brown CH. Kisspeptin activation of supraoptic nucleus neurons in vivo. Endocrinology. 2011;152:3862–70. doi: 10.1210/en.2011-1181. [DOI] [PubMed] [Google Scholar]
- 322.Luckman SM, Hamamura M, Antonijevic I, Dye S, Leng G. Involvement of cholecystokinin receptor types in pathways controlling oxytocin secretion. Br J Pharmacol. 1993;110:378–84. doi: 10.1111/j.1476-5381.1993.tb13820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kendrick K, Leng G, Higuchi T. Noradrenaline, dopamine and serotonin release in the paraventricular and supraoptic nuclei of the rat in response to intravenous cholecystokinin injections. J Neuroendocrinol. 1991;3:139–44. doi: 10.1111/j.1365-2826.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 324.Ueta Y, Kannan H, Higuchi T, Negoro H, Yamashita H. CCK-8 excites oxytocin-secreting neurons in the paraventricular nucleus in rats--possible involvement of noradrenergic pathway. Brain Res Bull. 1993;32:453–9. doi: 10.1016/0361-9230(93)90290-r. [DOI] [PubMed] [Google Scholar]
- 325.Onaka T, Luckman SM, Antonijevic I, Palmer JR, Leng G. Involvement of the noradrenergic afferents from the nucleus tractus solitarii to the supraoptic nucleus in oxytocin release after peripheral cholecystokinin octapeptide in the rat. Neuroscience. 1995;66:403–12. doi: 10.1016/0306-4522(94)00609-9. [DOI] [PubMed] [Google Scholar]
- 326.McCann MJ, Verbalis JG, Stricker EM. LiCl and CCK inhibit gastric emptying and feeding and stimulate OT secretion in rats. Am J Physiol. 1989;256:R463–R8. doi: 10.1152/ajpregu.1989.256.2.R463. [DOI] [PubMed] [Google Scholar]
- 327.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–8. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- 328.Holder JL, Jr., Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–8. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- 329.Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12:3264–75. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal sim1 deficiency causes hyperphagic obesity and reduced mc4r and oxytocin expression. J Neurosci. 2010;30:3803–12. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–60. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- 332.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–68. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 333.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–82. [PubMed] [Google Scholar]
- 334.Jorgensen H, Riis M, Knigge U, Kjaer A, Warberg J. Serotonin receptors involved in vasopressin and oxytocin secretion. J Neuroendocrinol. 2003;15:242–9. doi: 10.1046/j.1365-2826.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- 335.Onaka T, Luckman SM, Guevara-Guzman R, Ueta Y, Kendrick K, Leng G. Presynaptic actions of morphine: blockade of cholecystokinin-induced noradrenaline release in the rat supraoptic nucleus. J Physiol. 1995;482:69–79. doi: 10.1113/jphysiol.1995.sp020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Weinstein D, Pfeifer Y, Sadovsky E, Polishuk WZ, Sulman FG. Effect of indomethacin and cyproheptadine on onset of labour in rats. Arch Int Pharmacodyn Ther. 1977;226:172–6. [PubMed] [Google Scholar]
- 337.van Vulpen EH, Yang CR, Nissen R, Renaud LP. Hypothalamic A14 and A15 catecholamine cells provide the dopaminergic innervation to the supraoptic nucleus in rat: a combined retrograde tracer and immunohistochemical study. Neuroscience. 1999;93:675–80. doi: 10.1016/s0306-4522(99)00173-6. [DOI] [PubMed] [Google Scholar]
- 338.Cheung S, Ballew JR, Moore KE, Lookingland KJ. Contribution of dopamine neurons in the medial zona incerta to the innervation of the central nucleus of the amygdala, horizontal diagonal band of Broca and hypothalamic paraventricular nucleus. Brain Res. 1998;808:174–81. doi: 10.1016/s0006-8993(98)00809-9. [DOI] [PubMed] [Google Scholar]
- 339.Bridges TE, Hillhouse EW, Jones MT. The effect of dopamine on neurohypophysial hormone release in vivo and from the rat neural lobe and hypothalamus in vitro. J Physiol. 1976;260:647–66. doi: 10.1113/jphysiol.1976.sp011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Moos F, Richard P. Excitatory effect of dopamine on oxytocin and vasopressin reflex releases in the rat. Brain Res. 1982;241:249–60. doi: 10.1016/0006-8993(82)91061-7. [DOI] [PubMed] [Google Scholar]
- 341.Buijs RM, Geffard M, Pool CW, Hoorneman EM. The dopaminergic innervation of the supraoptic and paraventricular nucleus. A light and electron microscopical study. Brain Res. 1984;323:65–72. doi: 10.1016/0006-8993(84)90265-8. [DOI] [PubMed] [Google Scholar]
- 342.Yang CR, Bourque CW, Renaud LP. Dopamine D2 receptor activation depolarizes rat supraoptic neurones in hypothalamic explants. J Physiol. 1991;443:405–19. doi: 10.1113/jphysiol.1991.sp018840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Azdad K, Piet R, Poulain DA, Oliet SHR. Dopamine D4 receptor-mediated presynaptic inhibition of GABAergic transmission in the rat supraoptic nucleus. J Neurophysiol. 2003;90:559–65. doi: 10.1152/jn.00226.2003. [DOI] [PubMed] [Google Scholar]
- 344.Baimoukhametova DV, Hewitt SA, Sank CA, Bains JS. Dopamine modulates use-dependent plasticity of inhibitory synapses. J Neurosci. 2004;24:5162–71. doi: 10.1523/JNEUROSCI.4979-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Price CJ, Pittman QJ. Dopamine D4 receptor activation inhibits presynaptically glutamatergic neurotransmission in the rat supraoptic nucleus. J Neurophysiol. 2001;86:1149–55. doi: 10.1152/jn.2001.86.3.1149. [DOI] [PubMed] [Google Scholar]
- 346.Weiss ML, Yang QZ, Hatton GI. Magnocellular tuberomammillary nucleus input to the supraoptic nucleus in the rat: anatomical and in vitro electrophysiological investigations. Neuroscience. 1989;31:299–311. doi: 10.1016/0306-4522(89)90375-8. [DOI] [PubMed] [Google Scholar]
- 347.Luckman SM, Larsen PJ. Evidence for the involvement of histaminergic neurones in the regulation of the rat oxytocinergic system during pregnancy and parturition. J Physiol. 1997;501:649–57. doi: 10.1111/j.1469-7793.1997.649bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ericson H, Blomqvist A, Kohler C. Origin of neuronal inputs to the region of the tuberomammillary nucleus of the rat brain. J Comp Neurol. 1991;311:45–64. doi: 10.1002/cne.903110105. [DOI] [PubMed] [Google Scholar]
- 349.Ericson H, Blomqvist A, Kohler C. Brainstem afferents to the tuberomammillary nucleus in the rat brain with special reference to monoaminergic innervation. J Comp Neurol. 1989;281:169–92. doi: 10.1002/cne.902810203. [DOI] [PubMed] [Google Scholar]
- 350.Li Z, Hatton GI. Histamine-induced prolonged depolarization in rat supraoptic neurons: G-protein-mediated, Ca2+-independent suppression of K+ leakage conductance. Neuroscience. 1996;70:145–58. doi: 10.1016/0306-4522(95)00373-q. [DOI] [PubMed] [Google Scholar]
- 351.Li Z, Hatton GI. Histamine suppresses non-NMDA excitatory synaptic currents in rat supraoptic nucleus neurons. J Neurophysiol. 2000;83:2616–25. doi: 10.1152/jn.2000.83.5.2616. [DOI] [PubMed] [Google Scholar]
- 352.Smith BN, Armstrong WE. Histamine enhances the depolarizing afterpotential of immunohistochemically identified vasopressin neurons in the rat supraoptic nucleus via H1-receptor activation. Neuroscience. 1993;53:855–64. doi: 10.1016/0306-4522(93)90630-x. [DOI] [PubMed] [Google Scholar]
- 353.Smith BN, Armstrong WE. The ionic dependence of the histamine-induced depolarization of vasopressin neurones in the rat supraoptic nucleus. J Physiol. 1996;495:465–78. doi: 10.1113/jphysiol.1996.sp021607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yang QZ, Hatton GI. Histamine mediates fast synaptic inhibition of rat supraoptic oxytocin neurons via chloride conductance activation. Neuroscience. 1994;61:955–64. doi: 10.1016/0306-4522(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 355.Bealer SL, Crowley WR. Stimulation of central and systemic oxytocin release by histamine in the paraventricular hypothalamic nucleus: evidence for an interaction with norepinephrine. Endocrinology. 1999;140:1158–64. doi: 10.1210/endo.140.3.6601. [DOI] [PubMed] [Google Scholar]
- 356.Wang YF, Negoro H, Higuchi T. Lesions of hypothalamic mammillary body desynchronise milk-ejection bursts of rat bilateral supraoptic oxytocin neurones. J Neuroendocrinol. 2012:67–75. doi: 10.1111/j.1365-2826.2012.02368.x. [DOI] [PubMed] [Google Scholar]
- 357.Sawchenko PE, Swanson LW, Joseph SA. The distribution and cells of origin of ACTH(1-39)-stained varicosities in the paraventricular and supraoptic nuclei. Brain Res. 1982;232:365–74. doi: 10.1016/0006-8993(82)90280-3. [DOI] [PubMed] [Google Scholar]
- 358.Brunton PJ, Sabatier N, Leng G, Russell JA. Suppressed oxytocin neuron responses to immune challenge in late pregnant rats: a role for endogenous opioids. Eur J Neurosci. 2006;23:1241–7. doi: 10.1111/j.1460-9568.2006.04614.x. [DOI] [PubMed] [Google Scholar]
- 359.Brunton PJ, Russell JA. Keeping oxytocin neurons under control during stress in pregnancy. Prog Brain Res. 2008;170:365–77. doi: 10.1016/S0079-6123(08)00430-5. [DOI] [PubMed] [Google Scholar]
- 360.Ruan M, Russell JA, Brown CH. Acute morphine administration and withdrawal from chronic morphine increase afterdepolarization amplitude in rat supraoptic nucleus neurons in hypothalamic explants. Neuropharmacology. 2011;61:789–97. doi: 10.1016/j.neuropharm.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 361.Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405:450–71. [PubMed] [Google Scholar]
- 362.Doi N, Brown CH, Cohen HD, Leng G, Russell JA. Effects of the endogenous opioid peptide, endomorphin 1, on supraoptic nucleus oxytocin and vasopressin neurones in vivo and in vitro. Br J Pharmacol. 2001;132:1136–44. doi: 10.1038/sj.bjp.0703911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Russell JA, Leng G, Bicknell RJ. Opioid tolerance and dependence in the magnocellular oxytocin system: a physiological mechanism? Exp Physiol. 1995;80:307–40. doi: 10.1113/expphysiol.1995.sp003850. [DOI] [PubMed] [Google Scholar]
- 364.Brown CH, Russell JA. Cellular mechanisms underlying neuronal excitability during morphine withdrawal in physical dependence: lessons from the magnocellular oxytocin system. Stress. 2004;7:97–107. doi: 10.1080/10253890410001727776. [DOI] [PubMed] [Google Scholar]
- 365.Brown CH, Stern JE, Jackson KL, Bull PM, Leng G, Russell JA. Morphine withdrawal increases intrinsic excitability of oxytocin neurons in morphine-dependent rats. Eur J Neurosci. 2005;21:501–12. doi: 10.1111/j.1460-9568.2005.03885.x. [DOI] [PubMed] [Google Scholar]
- 366.Bailey AR, Giles M, Brown CH, Bull PM, Macdonald LP, Smith LC, Smith RG, Leng G, Dickson SL. Chronic central infusion of growth hormone secretagogues: effects on Fos expression and peptide gene expression in the rat arcuate nucleus. Neuroendocrinology. 1999;70:83–92. doi: 10.1159/000054462. [DOI] [PubMed] [Google Scholar]
- 367.Cui LN, Saeb-Parsy K, Dyball RE. Neurones in the supraoptic nucleus of the rat are regulated by a projection from the suprachiasmatic nucleus. J Physiol. 1997;502:149–59. doi: 10.1111/j.1469-7793.1997.149bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Cui LN, Jolley CJ, Dyball RE. Electrophysiological evidence for retinal projections to the hypothalamic supraoptic nucleus and its perinuclear zone. J Neuroendocrinol. 1997;9:347–53. doi: 10.1046/j.1365-2826.1997.00588.x. [DOI] [PubMed] [Google Scholar]
- 369.Smithson KG, Weiss ML, Hatton GI. Supraoptic nucleus afferents from the main olfactory bulb--I. Anatomical evidence from anterograde and retrograde tracers in rat. Neuroscience. 1989;31:277–87. doi: 10.1016/0306-4522(89)90373-4. [DOI] [PubMed] [Google Scholar]
- 370.Smithson KG, Weiss ML, Hatton GI. Supraoptic nucleus afferents from the accessory olfactory bulb: evidence from anterograde and retrograde tract tracing in the rat. Brain Res Bull. 1992;29:209–20. doi: 10.1016/0361-9230(92)90028-v. [DOI] [PubMed] [Google Scholar]
- 371.Bader A, Klein B, Breer H, Strotmann J. Connectivity from OR37 expressing olfactory sensory neurons to distinct cell types in the hypothalamus. Front Neural Circuits. 2012;6:84. doi: 10.3389/fncir.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Yang QZ, Smithson KG, Hatton GI. NMDA and non-NMDA receptors on rat supraoptic nucleus neurons activated monosynaptically by olfactory afferents. Brain Res. 1995;680:207–16. doi: 10.1016/0006-8993(95)00153-h. [DOI] [PubMed] [Google Scholar]
- 373.Hatton GI, Yang QZ, Cobbett P. Dye coupling among immunocytochemically identified neurons in the supraoptic nucleus: increased incidence in lactating rats. Neuroscience. 1987;21:923–30. doi: 10.1016/0306-4522(87)90047-9. [DOI] [PubMed] [Google Scholar]
- 374.Modney BK, Yang QZ, Hatton GI. Activation of excitatory amino acid inputs to supraoptic neurons. II. Increased dye-coupling in maternally behaving virgin rats. Brain Res. 1990;513:270–3. doi: 10.1016/0006-8993(90)90466-o. [DOI] [PubMed] [Google Scholar]
- 375.Peter J, Burbach H, Adan RA, Tol HH, Verbeeck MA, Axelson JF, Leeuwen FW, Beekman JM, Ab G. Regulation of the rat oxytocin gene by estradiol. J Neuroendocrinol. 1990;2:633–9. doi: 10.1111/j.1365-2826.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 376.Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Prog Brain Res. 2002;139:15–29. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- 377.Koohi MK, Ivell R, Walther N. Transcriptional activation of the oxytocin promoter by oestrogens uses a novel non-classical mechanism of oestrogen receptor action. J Neuroendocrinol. 2005;17:197–207. doi: 10.1111/j.1365-2826.2005.01298.x. [DOI] [PubMed] [Google Scholar]
- 378.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 379.Brown CH, Brunton PJ, Russell JA. Rapid estradiol-17beta modulation of opioid actions on the electrical and secretory activity of rat oxytocin neurons in vivo. Neurochem Res. 2008;33:614–23. doi: 10.1007/s11064-007-9506-7. [DOI] [PubMed] [Google Scholar]
- 380.Wang H, Ward AR, Morris JF. Oestradiol acutely stimulates exocytosis of oxytocin and vasopressin from dendrites and somata of hypothalamic magnocellular neurons. Neuroscience. 1995;68:1179–88. doi: 10.1016/0306-4522(95)00186-m. [DOI] [PubMed] [Google Scholar]
- 381.Israel JM, Poulain DA. 17-Oestradiol modulates in vitro electrical properties and responses to kainate of oxytocin neurones in lactating rats. J Physiol. 2000;524:457–70. doi: 10.1111/j.1469-7793.2000.t01-2-00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Voisin DL, Simonian SX, Herbison AE. Identification of estrogen receptor-containing neurons projecting to the rat supraoptic nucleus. Neuroscience. 1997;78:215–28. doi: 10.1016/s0306-4522(96)00551-9. [DOI] [PubMed] [Google Scholar]
- 383.Vilhena-Franco T, Mecawi AS, Elias LL, Antunes-Rodrigues J. Oestradiol potentiates hormone secretion and neuronal activation in response to hypertonic extracellular volume expansion in ovariectomised rats. J Neuroendocrinol. 2011;23:481–9. doi: 10.1111/j.1365-2826.2011.02133.x. [DOI] [PubMed] [Google Scholar]
- 384.Brunton PJ, Arunachalam S, Russel JA. Control of neurohypophysial hormone secretion, blood osmolality and volume in pregnancy. J Physiol Pharmacol. 2008;59(Suppl 8):27–45. [PubMed] [Google Scholar]
- 385.Negoro H, Visessuwan S, Holland RC. Unit activity in the paraventricular nucleus of female rats at different stages of the reproductive cycle and after ovariectomy, with or without oestrogen or progesterone treatment. J Endocrinol. 1973;59:545–58. doi: 10.1677/joe.0.0590545. [DOI] [PubMed] [Google Scholar]
- 386.Negoro H, Visessuwan S, Holland RC. Reflex activation of paraventricular nucleus units during the reproductive cycle and in ovariectomized rats treated with oestrogen or progesterone. J Endocrinol. 1973;59:559–67. doi: 10.1677/joe.0.0590559. [DOI] [PubMed] [Google Scholar]
- 387.Antonijevic IA, Russell JA, Bicknell RJ, Leng G, Douglas AJ. Effect of progesterone on the activation of neurones of the supraoptic nucleus during parturition. J Reprod Fertil. 2000;120:367–76. doi: 10.1530/jrf.0.1200367. [DOI] [PubMed] [Google Scholar]
- 388.Francis K, Meddle SL, Bishop VR, Russell JA. Progesterone receptor expression in the pregnant and parturient rat hypothalamus and brainstem. Brain Res. 2002;927:18–26. doi: 10.1016/s0006-8993(01)03318-2. [DOI] [PubMed] [Google Scholar]
- 389.Fenelon VS, Herbison AE. Plasticity in GABAA receptor subunit mRNA expression by hypothalamic magnocellular neurons in the adult rat. J Neurosci. 1996;16:4872–80. doi: 10.1523/JNEUROSCI.16-16-04872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Fenelon VS, Herbison AE. Progesterone regulation of GABAA receptor plasticity in adult rat supraoptic nucleus. Eur J Neurosci. 2000;12:1617–23. doi: 10.1046/j.1460-9568.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 391.Widmer H, Ludwig M, Bancel F, Leng G, Dayanithi G. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. J Physiol. 2003;548:233–44. doi: 10.1113/jphysiol.2002.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Sherwood OD, Crnekovic VE, Gordon WL, Rutherford JE. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980;107:691–8. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- 393.Way SA, Leng G. Relaxin increases the firing rate of supraoptic neurones and increases oxytocin secretion in the rat. JEndocrinol. 1992;132:149. doi: 10.1677/joe.0.1320149. [DOI] [PubMed] [Google Scholar]
- 394.Parry LJ, Poterski RS, Summerlee AJ. Effects of relaxin on blood pressure and the release of vasopressin and oxytocin in anesthetized rats during pregnancy and lactation. Biology of reproduction. 1994;50:622–8. doi: 10.1095/biolreprod50.3.622. [DOI] [PubMed] [Google Scholar]
- 395.Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, Beck F. Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology. 1999;140:445–53. doi: 10.1210/endo.140.1.6404. [DOI] [PubMed] [Google Scholar]
- 396.Burazin TC, Johnson KJ, Ma S, Bathgate RA, Tregear GW, Gundlach AL. Localization of LGR7 (relaxin receptor) mRNA and protein in rat forebrain: correlation with relaxin binding site distribution. Ann N Y Acad Sci. 2005;1041:205–10. doi: 10.1196/annals.1282.031. [DOI] [PubMed] [Google Scholar]
- 397.Kokay IC, Bull PM, Davis RL, Ludwig M, Grattan DR. Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1216–25. doi: 10.1152/ajpregu.00730.2005. [DOI] [PubMed] [Google Scholar]
- 398.Kokay IC, Grattan DR. Expression of mRNA for prolactin receptor (long form) in dopamine and pro-opiomelanocortin neurones in the arcuate nucleus of non-pregnant and lactating rats. J Neuroendocrinol. 2005;17:827–35. doi: 10.1111/j.1365-2826.2005.01374.x. [DOI] [PubMed] [Google Scholar]
- 399.Townsend J, Cave BJ, Norman MR, Flynn A, Uney JB, Tortonese DJ, Wakerley JB. Effects of prolactin on hypothalamic supraoptic neurones: evidence for modulation of STAT5 expression and electrical activity. Neuro Endocrinol Lett. 2005;26:125–30. [PubMed] [Google Scholar]
- 400.Sirzen-Zelenskaya A, Gonzalez-Iglesias AE, Boutet de Monvel J, Bertram R, Freeman ME, Gerber U, Egli M. Prolactin induces a hyperpolarising current in rat paraventricular oxytocinergic neurones. J Neuroendocrinol. 2011;23:883–993. doi: 10.1111/j.1365-2826.2011.02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Parker SL, Armstrong WE, Sladek CD, Grosvenor CE, Crowley WR. Prolactin stimulates the release of oxytocin in lactating rats: evidence for a physiological role via an action at the neural lobe. Neuroendocrinology. 1991;53:503–10. doi: 10.1159/000125764. [DOI] [PubMed] [Google Scholar]
- 402.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–6. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 403.Scott V, Brown CH. Beyond the GnRH axis: Kisspeptin regulation of the oxytocin system in pregnancy and lactation. Advances in Experimental Medicine and Biology. 2013;784 doi: 10.1007/978-1-4614-6199-9_10. In press. [DOI] [PubMed] [Google Scholar]
- 404.Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–9. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 405.Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Bull PM, Douglas AJ, Russell JA. Opioids and coupling of the anterior peri-third ventricular input to oxytocin neurones in anaesthetized pregnant rats. J Neuroendocrinol. 1994;6:267–74. doi: 10.1111/j.1365-2826.1994.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 407.Brunton PJ, Bales J, Russell JA. Allopregnanolone and induction of endogenous opioid inhibition of oxytocin responses to immune stress in pregnant rats. J Neuroendocrinol. 2012;24:690–700. doi: 10.1111/j.1365-2826.2012.02295.x. [DOI] [PubMed] [Google Scholar]
- 408.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 410.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61:340–50. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–9. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 412.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22:1723–34. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]