Abstract
Plant respiratory burst oxidase homologs (Rboh) are homologs of the human neutrophil pathogen-related gp91phox. Antisense technology was employed to ascertain the biological function of Lycopersicon esculentum (tomato) Rboh. Lines with diminished Rboh activity showed a reduced level of reactive oxygen species (ROS) in the leaf, implying a role for Rboh in establishing the cellular redox milieu. Surprisingly, the antisense plants acquired a highly branched phenotype, switched from indeterminate to determinate growth habit, and had fasciated reproductive organs. Wound-induced systemic expression of proteinase inhibitor II was compromised in the antisense lines, indicating that ROS intermediates supplied by Rboh are required for this wound response. Extending these observations by transcriptome analysis revealed ectopic leaf expression of homeotic MADS box genes that are normally expressed only in reproductive organs. In addition, both Rboh-dependent and -independent wound-induced gene induction was detected as well as transcript changes related to redox maintenance. The results provide novel insights into how the steady state cellular level of ROS is controlled and portrays the role of Rboh as a signal transducer of stress and developmental responses.
INTRODUCTION
The kinetics and defense functions of superoxide O2− and H2O2 during activation of mammalian neutrophils have served as a model for similar processes in plants. The mammalian NADPH oxidase consists of two plasma membrane proteins, gp91phox and p22phox (phox, phagocyte oxidase), which together form heterodimeric flavocytochrome b558. The three cytosolic regulatory proteins p40phox, p47phox, and p67phox translocate to the plasma membrane after stimulation to form the active complex (Bokoch et al., 1994). In plants, enhanced O2− generation can be observed in microsomal preparations from pathogen-challenged leaf material (Doke and Ohashi, 1988). Diphenylene iodonium, a nonspecific suicide substrate inhibitor of the neutrophil NADPH oxidase and other flavin-containing enzymes (Cross and Jones, 1986), blocks the plant oxidative burst (Doke and Ohashi, 1988).
Homology to the neutrophil gp91phox was the basis for molecular cloning of plant respiratory burst oxidase homologs (Rboh) in Arabidopsis (Arabidopsis thaliana) (Keller et al., 1998; Torres et al., 1998). Plant Rboh defines transcripts encoding a protein of ∼105 to 112 kD, with a C-terminal region that shows pronounced similarity to the gp91phox. Rboh proteins have a cytosolic N-terminal domain containing calcium binding EF hand motifs and a degree of similarity to the human RanGTPase-activating protein (Keller et al., 1998; Simon-Plas et al., 2002). Subsequently, human NADPH oxidase 5 (NOX5), a gene containing a gp91phox core cytochrome and N-terminal EF hand motifs, was identified (Banfi et al., 2001). Direct activation of plant Rboh by calcium may be important for rapid stimulation of the oxidative burst during the hypersensitive response, and plant Rboh, unlike the mammalian gp91phox complex, is active in the absence of additional cytosolic components (Sagi and Fluhr, 2001). Interestingly, human NOX5 displayed calcium-dependent activity as well (Banfi et al., 2001).
Rapid generation of reactive oxygen species (ROS) is considered to be an important component of the resistance response of plants to pathogen challenge. ROS intermediates can serve as direct protective agents by their toxicity or by their ability to contain pathogen ingress by driving the cross-linking of the cell wall (Baker and Orlandi, 1995). The oxidative burst can further trigger the collapse of challenged host cells at the onset of the hypersensitive response and generate apoptopic-like signals (Allan and Fluhr, 1997). The gp91phox homologs AtrbohD and AtrbohF from Arabidopsis, NtrbohD from Nicotiana tabacum, and NbrbohA and NbrbohB from N. benthamiana were shown to be required for ROS accumulation in plant defense responses (Simon-Plas et al., 2002; Torres et al., 2002; Yoshioka et al., 2003).
ROS can function as signaling molecules that mediate responses to various stimuli in both plant and animal cells (Neill et al., 2002a). The wounding response is thought to progress through the release of systemin (an 18–amino acid wound signal) in the wounded leaf, subsequent activation of early-response signal relay genes, such as polygalacturonase, allene oxide synthase, and lipoxygenase, and synthesis of the long-distance signal jasmonic acid (JA). A second wave of gene induction follows, involving synthesis of proteinase inhibitor (PIN) and other defense polypeptides (Ryan, 2000; Lee and Howe, 2003). The wound-induced increase in H2O2 levels is JA dependent and diphenylene iodonium sensitive, suggesting that a NADPH-like oxidase activity is required for the activation of wound/systemin-responsive genes (Orozco-Cardenas and Ryan, 1999; Orozco-Cardenas et al., 2001). Potential sources of ROS include NADPH oxidase, cell wall peroxidase, other flavin-containing oxidases, and oxalate oxidase (Neill et al., 2002a, 2002b). Thus, the exact source of wound-induced ROS remains unknown.
ROS also can play a role in hormonal regulation of plant development, as shown by ROS involvement in auxin-regulated gravitropic responses such as root bending (Joo et al., 2001). NADPH oxidase–mediated H2O2 synthesis has been implicated in abscisic acid (ABA)-induced signaling processes in Arabidopsis (Pei et al., 2000) and likely in maize (Zea mays) (Jiang and Zhang, 2003). The Arabidopsis genes AtrbohD and AtrbohF function in ROS-dependent ABA signaling for stomatal closure (Kwak et al., 2003). Arabidopsis RbohC-deficient mutants were defective in Ca2+ uptake and displayed short root hairs on stunted roots, suggesting that this Rboh species regulates plant cell expansion (Foreman et al., 2003).
We wished to examine the global role that Rboh genes play in plant environmental responses and development. We employed an antisense technique to downregulate Rboh activity in Lycopersicon esculentum (tomato) lines. The results showed a requirement of Rboh for expression of certain wound response genes, whereas other wound-responsive genes were regulated in a Rboh-independent manner. The reduced Rboh levels shifted redox-related metabolism, induced multiple effects on plant development, and resulted in ectopic expression of flower-specific homeotic genes.
RESULTS
Repression of Rboh Sense Transcripts and Polypeptides in Transgenic Plants
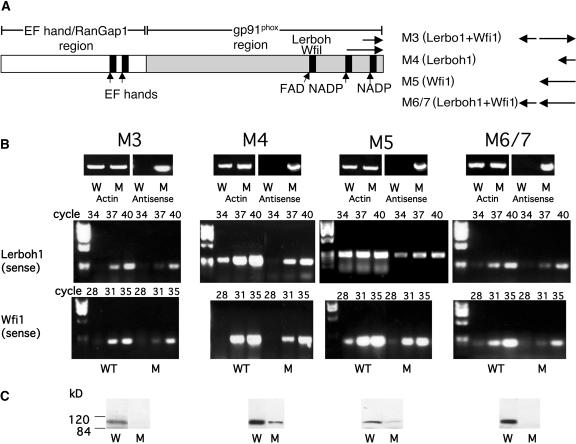
AtrbohD and AtrbohF are the most highly expressed genes among the Arabidopsis Rboh multigene family (Torres et al., 1998). Based on accumulative compilation of ESTs, whitefly-induced (Wfi1) and Lerboh1 are the most highly expressed L. esculentum Rboh homologs (AAF73124 and AAD25300, respectively; The Institute for Genomic Research [TIGR], version 9.0). They belong by homology to the gene families of NbrbohB and AtrbohD and NbrbohA and AtrbohF, respectively (Yoshioka et al., 2003). Based on sequence comparison between Lerboh1 and Wfi1, the largest unbroken stretch of homology in the region employed for antisense regulation was 12 bp. Sequence data for other L. esculentum Rboh genes is not known. Antisense transgenic L. esculentum lines were generated by expression of sequence combinations from the 3′ gene region of the L. esculentum Rboh homologs Wfi1and Lerboh1under the control of the 35S promoter (Methods, Figure 1A). For each antisense construct (M3, M4, M5, and M6/7), at least four independent lines of each type were brought to the homozygous state and analyzed. All of the L. esculentum lines expressed the antisense construct (Figure 1B, Antisense) and were subjected to quantitative reverse transcriptase (RT) PCR analysis specific for each gene type using actin transcript as an expression level control. Lines M4 and M5, which express antisense constructs of Lerboh1 and Wfi1, respectively, repressed both types of transcript when compared with the wild-type levels (cf. cycles, Figure 1B). Similarly, constructs with combinations of antisense elements (M3 and M6/7) showed sense repression in both transcripts (cf. cycles, Figure 1B). We conclude that in all four lines both gene sets are repressed. The lack of specificity is apparently attributable to the residual homology between these two genes.
Figure 1.
Expression of Lerboh1 and Wfi1 Transcripts and Rboh Polypeptide Levels in Antisense Plants.
(A) Schematic presentation of L. esculentum Rboh showing gp91phox, EF hand, and RanGap homology regions. The location of the antisense fragments of Lerboh1 and Wfi1 are shown in the C-terminal region. The combinations of inserts used in constructs M3 to M6/7 are shown at the right. Fragment types are indicated in parenthesis, and the insert orientations are indicated at the right. Arrows pointing to the left or right represent antisense and sense orientations, respectively.
(B) RT-PCR expression analysis of antisense construct and Lerboh1 and Wfi1 sense expressions in wild-type (W) and antisense (M) plants. Top panels; RT-PCR product of antisense expression transcript showing fragments of 810, 293, 648, and 927 bp for M3, M4, M5, and M6/7, respectively; middle panels, RT-PCR of endogenous Lerboh1 sense transcript showing the expected PCR fragment of 270 bp; bottom panels, RT-PCR of endogenous Wfi1 sense transcript showing the expected PCR products of 213 bp. L. esculentum actin (Tom41 actin gene, U60480) was used as a standard, and the expected PCR product of a 325-bp fragment is shown. The data are representative of results obtained in at least four independent lines.
(C) Immunoblot analysis of L. esculentum Rboh levels in wild-type (W) and transgenic antisense (M) lines. Proteins were extracted from wild-type and antisense leaves and fractionated (100 μg per lane) by denaturating SDS-PAGE and immunoblotted with antisera against the C-terminal portion of the L. esculentum Rboh. The data are representative of results obtained in at least four independent lines.
Rboh protein levels were determined by performing immunoanalysis of plant extracts using antisera raised against L. esculentum Rboh (Sagi and Fluhr, 2001). Polyclonal antibody was raised against 214–amino acid length of the conserved C terminus of Wfi1 and is not expected to differentiate among members of the L. esculentum Rboh family. Immunoblot analysis of extracts of wild-type leaves revealed major immunoreactive polypeptides of ∼112 kD, the expected size of Lerboh and Wfi1 proteins. By contrast, levels were severely reduced in the antisense lines (Figure 1C).
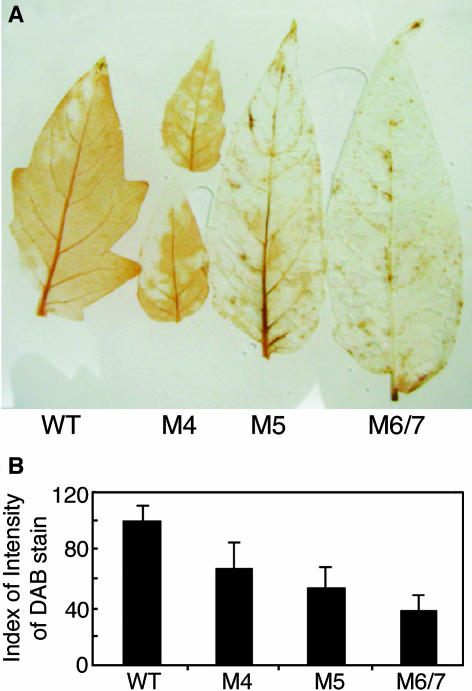
Repression in expression levels of the Rboh polypeptide may imply a reduction in the constitutive level of ROS. Although the L. esculentum Rboh has been shown to produce superoxide radicals (Sagi and Fluhr, 2001), staining for H2O2 produced by endogenous superoxide dismutation of superoxide radicals has been used to quantify Rboh activity (Simon-Plas et al., 2002; Torres et al., 2002; Yoshioka et al., 2003). Excised leaves were allowed to imbibe a 1-mg/mL solution of 3,3′-diaminobenzidine reagent (DAB). DAB polymerizes and turns deep brown in the presence of H2O2, and the intensity of coloration can be qualitatively assessed. The development of the DAB-H2O2 reaction product in wild-type and transgenic L. esculentum leaves is shown in Figures 2A and 2B. M4 leaves showed partial ROS development (∼70%) compared with wild-type leaves, whereas in M5 and M6/7 leaves, the majority of the DAB-H2O2 reaction product was eliminated. Thus, based on RT-PCR and protein immunoblot and activity data, the antisense lines contain reduced levels of total Rboh expression and activity.
Developmental Effects of Reduced Rboh Expression
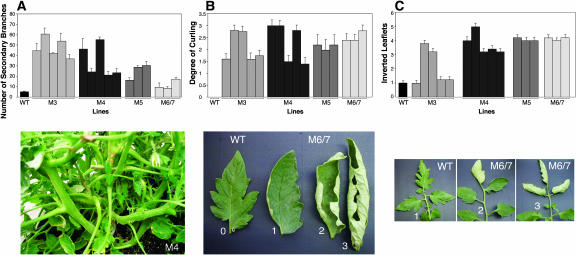
L. esculentum lines that showed reduced Rboh expression were selected for further physiological and growth parameter analysis. Total chlorophyll content in all antisense lines tested was 20 to 30% lower than that of the wild type (data not shown). The parental line used for transformation (L. esculentum cv Motelle) exhibits indeterminate growth habit with minor secondary branching. By contrast, all of the transformed lines displayed decreased apical dominance reflected in enhanced branching ranging from 2- to 10-fold more than the wild type. This yielded a bushy growth style particularly in M3 and M4 lines and to a lesser extent in M5 and M6/7 lines (Figure 3A, 3B, and 3C). In addition, leaflets tended to exhibit curling (Figure 3B). The evaluation of leaf curling in the mutant plants compared with wild-type plants was calculated according to the example shown in Figure 3B: the severity extended from slightly curled at the edges (severity 1) to a complete curled phenotype (severity 3). The tabulation of this phenomenon showed that all lines were affected (Figure 3B). In many instances, leaves contained inverted terminal and preterminal leaflets. The tabulation of this phenomenon shows that it is a general robust feature of Rboh compromised lines (Figure 3C).
Figure 3.
Characterization of Vegetative Growth of Rboh Antisense Plants.
(A) Top panel, quantitative chart of appearance of secondary branches in wild-type and antisense plants. Each column represents an independent antisense line. Bottom panel, antisense plant line M4 showing a bushy phenotype.
(B) Top panel, quantitative chart of appearance of leaf curl in wild-type and antisense plants. Each column in the chart represents an independent antisense line. The analysis was performed using a 0 to 3 severity score as shown in the bottom panel, with 0 representing the wild type and 3 representing the most severe curling.
(C) Top panel, quantitative analysis of inverted leaflets in wild-type and antisense plants. Each column in the chart represents an independent antisense line. The analysis was performed using a 1 to 5 severity score (see Methods). The bottom panel illustrates wild-type leaflets and leaflets from an antisense line.
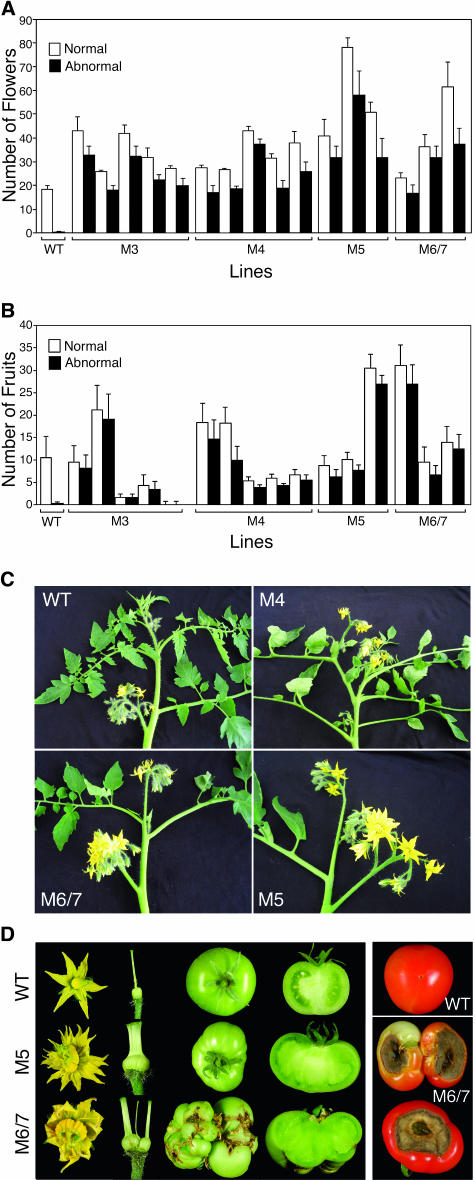
Vegetative and reproductive phases alternate regularly during sympodial growth in L. esculentum. Thus, in the Motelle cultivar, the inflorescences are separated by three vegetative nodes. When in determinate-habit plants homozygous for the recessive allele of the self pruning gene (the Arabidopsis CEN ortholog), sympodial segments develop progressively fewer nodes until the shoot is terminated by two consecutive inflorescences (Pnueli et al., 1998). Instead of the indeterminate nature of the Motelle parent cultivar, antisense plants displayed a determinate sp-like growth habit (Figure 4C). Examination of Rboh antisense L. esculentum plants revealed a two- to threefold increase in number of inflorescences (data not shown) and total flower number (Figure 4A). This is likely the result of decreased apical dominance and the sp-like phenotype. A significant proportion exhibited abnormal flowers resulting in sterility or a high ratio of abnormal fruits in the transgenic lines (Figures 4A to 4D).
Figure 4.
Characterization of Reproductive Organs in Rboh Antisense Plants.
(A) Chart of normal versus abnormal flowers counted in 100-d-old wild-type and antisense plants. Each black-and-white column pair in the chart represents an independent antisense line.
(B) Chart of normal versus abnormal fruits counted in 150-d-old wild-type and antisense plants. Each black-and-white column pair in the chart represents an independent antisense line.
(C) Growth habit in wild-type and M4, M5, and M6/7 lines.
(D) Left panel, flower, styles, ovaries, and whole and sliced green fruit of the wild type (top row), antisense M5 (middle row), and antisense M6/7 plants (bottom row). Right panel, BER illustrated in M6/7 lines (middle and bottom) compared with normal parental fruit (top).
Flower development in the transgenic lines was accompanied by homeotic-like deformations, characterized by abnormal petal number (more than six) and fasciated styles and ovaries (Figure 4D). Fasciated-like stems were observed as well (data not shown). The abnormal flowers yielded parthenocarpic fruits (Figure 4D). The sterile seeds were of reduced size (1.5 to 1.2 mm, 38 to 30% of wild-type seed diameter). M6/7 lines also were characterized by a phenomenon called blossom end rot ([BER]; ∼10% of the fruits), a physiological disorder thought to be related to calcium deficiency (Figure 4D). BER occurrence has been noted in plants exposed to abiotic stresses, such as salinity and drought. It is associated with reduced translocation of calcium to fruit tips because of competition with the leaf or inadequate xylem differentiation because of reduced lignification (Ho et al., 1993). The latter possibility is consistent with the reduced ROS milieu detected in antisense plants.
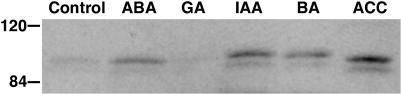
Hormones Modulate Rboh Levels
The developmental effects of Rboh downregulation, including reduced chlorophyll content, loss of apical dominance, and changes in morphology, may reflect hormone-controlled developmental events that are mediated by Rboh activity. It is therefore of interest to look at the direct effect of hormones on Rboh levels in normal plants. Rboh protein levels were monitored by following the immunoreactive 112-kD polypeptide in leaves of plants imbibed with various hormones for 24 h. As shown in Figure 5, the phytohormones ABA, indoleacetic acid (IAA), benzylaminopurine (BA), and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), but not gibberellic acid (GA), induced Rboh accumulation. Thus, hormones may exert long-term effects on Rboh levels.
Figure 5.
Protein Gel Blot Analysis of L. esculentum Rboh Protein in Leaves Exposed to Select Phytohormones.
Plants (28 d) were placed in solutions containing 50 μM ABA, GA, IAA, BA, or the ethylene precursor ACC. After 24 h, proteins were extracted from the second upper leaf and fractionated (100 μg per lane) by denaturating SDS-PAGE and immunoblotted with antiserum against the C-terminal portion of the L. esculentum Rboh (Sagi and Fluhr, 2001).
Reduced Systemic Accumulation of ROS and PIN in Response to Wounding
The general reduction of Rboh activity in the antisense lines represents an opportunity to resolve the source of wound ROS as well as the involvement of Rboh in global management of wound and additional cellular responses. To this end, 3-week-old plants were wounded, and 5 h after wounding, unwounded leaves of wounded plants (systemic leaves) and leaves from unwounded control plants were examined by DAB infiltration. As shown in Figures 6A and 6B, wild-type leaves of unwounded control plants had a basal level of hydrogen peroxide that was enhanced in the systemic leaf in response to wounding. By contrast, in M3, M5, and M6/7 plants, the majority of the DAB-H2O2 reaction product was eliminated and did not increase in systemic leaves after wounding. Leaves of M4 plants showed a weaker phenotype with an ∼30% reduction in ROS development compared with wild-type leaves. Extracts of control and apical systemic leaves were examined 24 h after wounding. Immunoblots using antiserum specific for PIN II revealed suppression of PIN II polypeptide accumulation in the antisense lines (Figure 6C), showing that Rboh functions in wound-signal transmission.
Figure 6.
ROS and PIN II Production in the Systemic Leaf of Wild-Type and Antisense Plants 24 h after Wounding.
(A) ROS accumulation in control and systemic leaves of wild-type and antisense (M) L. esculentum plants 5 h after wounding. Plants were imbibed with DAB for 3 h. Subsequently, lower leaves were wounded. Five hours later, leaves from unwounded control plants and upper systemic leaves of wounded plants were assayed for DAB staining. Brown precipitates correlate with the presence of H2O2.
(B) Quantitative analysis of DAB staining. Quantitative measurements were performed as described in the Methods. Each point represents the mean of four terminal leaflets derived from four different plants. Bars represent se.
(C) Protein gel blot analysis of PIN II protein accumulation in control and systemic leaves of wild-type and antisense L. esculentum plants 24 h after wounding. Leaves of the same size and position in unwounded plants served as controls. Proteins were extracted and fractionated (100 μg per lane) by denaturating SDS-PAGE and immunoblotted with antiserum against PIN II.
Transcriptome Profile of Rboh Antisense Plants
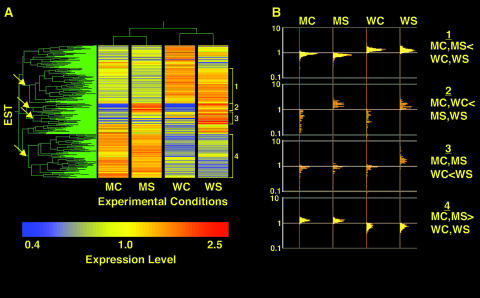
Line M6/7 was chosen for in-depth microarray analysis. The mRNA of wild-type and M6/7 nonwounded control and nonwounded systemic leaves of wounded plants was sampled using a 12K L. esculentum EST slide as a probe. To improve statistical veracity, all samples were compared with a reference sample composed of equally mixed RNA extracts from all treatments (Oleksiak et al., 2002). Transcripts that are differentially expressed in at least one of the conditions (1473 of total 12,000 ESTs; one-way analysis of variance [ANOVA] equal variance, P ≤ 0.05) were selected and subjected to two-way hierarchical clustering of genes and experimental conditions. As shown in Figure 7A, M6/7 antisense control and wounding treatments clustered separately from wild-type control and wounding treatments. Furthermore, the ESTs clustered into a variety of activity groups. Questions that can be asked are as follows. Which genes are constitutively influenced by the reduced level of Rboh protein? How does Rboh affect gene functions related to development? More specifically, how does Rboh impact the wound response? We focused on four clusters showing distinct patterns of behavior (Figure 7A, arrows and brackets). The EST populations chosen are shown in Figure 7B, and select ESTs were verified by direct quantitative PCR (Figure 8).
Figure 7.
Microarray Analysis of Leaves of Nonwounded Plants and the Systemic Leaves of Wounded Plants from Wild-Type and Transgenic M6/7 Lines.
(A) Double clustering analysis of transcripts showing a change in expression level (one-way ANOVA equal variance, P ≤ 0.05) in either control or systemic leaves of wild-type or antisense plants in the four experimental conditions. Each condition is the averaged result of two to three independent biological replicates. The conditions are as follows: MC, mutant control leaf; MS, mutant systemic leaf; WC, wild-type control leaf; WS, wild-type systemic leaf. The arrows point to selected clusters that are assigned numbers (brackets) corresponding to four groups (described in [B]).
(B) Log scale distribution of individual transcript activity in the selected groups.
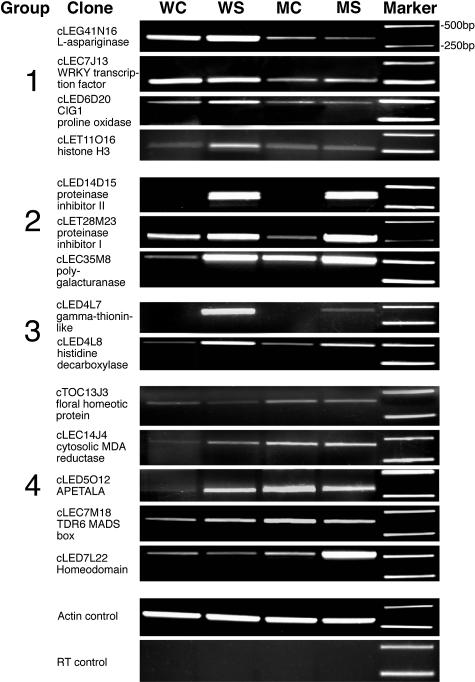
Figure 8.
RT-PCR Analysis of Transcript Levels of Select ESTs.
Primers were synthesized for select ESTs in Table 1, and quantitative PCR was performed as described in the Methods. All PCR products were confirmed by sequence. WC, WS, MC, and MS are as in Figure 7.
Groups 1 and 4 represent genes that are constitutively upregulated or downregulated because of the change in Rboh levels in the wild type compared with the transgenic plants. Group 1 contains 384 ESTs in which the median fold change of the wild type to mutant is 1.5, irrespective of wounding treatment. Conversely, in group 4, the 485 ESTs are always expressed at a lower level in the wild-type line than the antisense line with a median fold change of 0.59. Within these groups may reside transcripts that reflect the different constitutive redox status of the mutant and wild-type plants.
Groups 2 and 3 together represent 169 wound upregulated transcripts. The transcripts in group 3 are upregulated only in systemic leaves of wild-type wounded plants but not systemic leaves of the antisense plants, whereas the transcripts in group 2 show varying degrees of systemic leaf induction in the mutant line as well. We can consider that Rboh plays a role when the ratio of (wild-type wound induction):(mutant wound induction) is >1. In this case, 54 of the 94 transcripts of group 2 are influenced to a smaller or larger extent by perturbation of Rboh levels. Hence, 129 (54 of group 2 and 75 of group 3) of 169 transcripts exhibit a Rboh requirement. The remaining 40 ESTs are induced by wounding to the same extent in wild-type and antisense plants, despite the compromised Rboh levels. Thus, they represent an Rboh-independent pathway. Data for the activity distribution of all of the EST groups are available in Supplemental Table 1 online.
Table 1.
Expression Levels of Select Transcripts in EST Groups 1 to 4
| Groupa | AvWt/AvMtb | Pc | Descriptiond | |||
|---|---|---|---|---|---|---|
| Nuclear Factors | ||||||
| 1* | cLEC7J13(1)e | 2.45 | 3.7E-03 | WRKY transcription factor | ||
| 1* | cLED4B14(1) | 1.97 | 1.5E-02 | WRKY transcription factor | ||
| 1* | cLEC7B1(1) | 1.77 | 7.0E-03 | WRKY transcription factor | ||
| 1 | cLED7J4 | 2.97 | 3.4E-03 | Histone H3 variant H3R-21 | ||
| 1 | cLET11O16 | 3.46 | 1.0E-02 | Histone H3 variant H3R-21 | ||
| 1 | cLED5G7 | 1.97 | 1.6E-02 | Histone H3 variant H3.3 | ||
| 1 | cLEM6P24 | 1.67 | 7.6E-04 | Histone H3 variant H3.3 | ||
| Metabolism | ||||||
| 1 | cLED6D20 | 5.97 | 6.8E-04 | CIG1 Pro oxidase | ||
| 1 | cLEG41N16 | 4.71 | 6.5E-04 | l-Asparaginase | ||
| Defense | Mf | Wg | W/Mh | |||
| 2 | cLED14D15(1) | 10.5 | 51.4 | 4.87 | 4.8E-04 | PIN II |
| 2 | cLET21I5(1) | 14.7 | 50.2 | 3.42 | 1.5E-03 | PIN II |
| 2 | cLEC40H22 | 9.60 | 32.8 | 3.41 | 2.0E-03 | PIN II |
| 2 | cLED4O3 | 8.39 | 24.5 | 2.92 | 4.5E-03 | PIN I |
| 2 | cLET23O14(1) | 8.74 | 25.4 | 2.91 | 6.0E-03 | PIN II |
| 2 | cLED6E24(1) | 4.46 | 7.23 | 1.62 | 4.8E-03 | PIN II |
| 2 | cLED5P11 | 2.59 | 4.16 | 1.61 | 8.9E-04 | PIN II |
| 2 | cLED4N20 | 15.6 | 22.0 | 1.41 | 2.1E-03 | PIN I |
| 2 | cLED6L3 | 5.18 | 6.48 | 1.25 | 1.6E-02 | PIN PID |
| 2 | cLED19A9 | 6.33 | 7.38 | 1.17 | 6.7E-03 | PIN I |
| 2 | cLED6N13 | 7.16 | 7.84 | 1.10 | 4.8E-03 | PIN I |
| 2 | cLED5K5 | 2.70 | 2.59 | 0.96 | 8.3E-03 | PIN (auxin) |
| 2 | cLET22A6 (2) | 8.09 | 7.19 | 0.89 | 8.4E-03 | PIN I |
| 2 | cLET28M23 (2) | 5.60 | 4.31 | 0.77 | 1.2E-02 | PIN I |
| 2 | TC116378 | 8.65 | 25.3 | 2.92 | 5.4E-03 | Cytochrome c |
| 3 | cLED4L8 | 1.60 | 13.2 | 8.24 | 4.3E-04 | His decarboxylase |
| 3 | cLED4L7(1) | 2.77 | 61.9 | 22.4 | 1.4E-03 | γ-Thionin–like defensin |
| 3 | cLED25O16(1) | 2.06 | 10.9 | 5.29 | 2.0E-03 | γ-Thionin–like defensin |
| Nuclear Factors | AvWt/AvMt | |||||
| 4 | cLEX1O3 | 0.57 | 1.0E-03 | Histone H3 variant H3.3 | ||
| 4 | cLED29P24 | 0.60 | 7.7E-03 | Histone H3 | ||
| 4 | cLED7L22(1) | 0.56 | 4.5E-03 | Homeodomain HDZ2 homolog | ||
| 4 | cLED8G3(1) | 0.52 | 2.8E-03 | Homeodomain HDZ2 homolog | ||
| 4 | cTOC13J3 | 0.54 | 6.7E-04 | Floral homeotic protein PMADS 2 | ||
| 4 | cLEC7M18 | 0.39 | 3.2E-03 | TDR6 MADS box protein | ||
| 4 | cLED5O12 | 0.38 | 2.9E-03 | APETALA3 LEAP3 | ||
| 4 | cLED5P12 | 0.32 | 2.7E-05 | APETALA3 LEAP3 | ||
| Metabolism | ||||||
| 4 | cLEC14J4 | 0.34 | 1.4E-02 | Cytosolic MDA reductase |
Cluster groups (1 to 4) refer to the clustering of expression patterns as shown in Figure 7, in which twofold or more induction is observed in an EST or in an assigned tentative consensus group. Asterisks indicate ESTs from related genes not selected in the assigned clusters.
The average induction level in wild-type control and systemic leaves relative to the average induction level of control and systemic leaves in the M6/7 line.
The P-values test by sum of squares simultaneous test procedure for null hypothesis. The hypotheses are as follows: group 1, (MC, MS) versus (WC, WS); group 2, (MC, WC) versus (MS, WS); group 3, (MC, MS, WC) versus (WS); and group 4, (MC, MS) versus (WC, WS).
Descriptions are based on the TIGR database (version 9.0; http://www.tigr.org/). The Center for Gene Expression Profiling resequence update was incorporated only when both sequence ends were consistent.
The numbers in parenthesis refer to ESTs that belong to a common contig (tentative consensus) group as described in TIGR databases. A contig unit may represent the same transcript.
Ratio of expression of wounded systemic leaf to control leaf in the M6/7 line.
Ratio of expression of wounded systemic leaf to control leaf in the wild type.
Ratio of induction in wild-type systemic wounded leaves to M6/7 systemic wounded leaves.
Wound Response Genes Are Differentially Regulated
The impact of Rboh on defense gene transcript levels has been confirmed for select ESTs by RT-PCR as shown in Figure 8. Table 1 includes confirmed ESTs and their transcript families as revealed in the microarray analysis. All of the PIN-type ESTs fall into the group 2–type expression pattern (Table 1). PIN II and PIN I ESTs are massively induced in the systemic leaf of both M6/7 and wild-type lines (e.g., cLED14D15 by 10- and 51-fold, respectively). However, the induction of all representatives of PIN II (e.g., Table 1, contig 1; Figure 8, cLED14D15) and some of the PIN I ESTs (e.g., EST cLED4O3) are significantly more highly induced (approximately threefold) in wild-type lines (Table 1, column W/M). This result is consistent with the requirement of Rboh for PIN expression in response to wounding. By contrast, some PIN I representatives (e.g., Table 1, cLET22A6 and cLET28M23 [contig 2]; Figure 8) are at least as highly induced in the systemic leaf of M6/7 lines, suggesting that wound induction of some PIN I gene members has no Rboh requirement. On the other side of the spectrum, inspection of group 3 ESTs (transcripts that require Rboh) reveals massive wound induction of a novel γ-thionin–like polypeptide of putative defensin function (Table 1, group 3; Figure 8, cLED4L7). This transcript is normally constitutively expressed in flowers (Brandstadter et al., 1996).
The WRKY defense-related transcription factors, showed constitutive downregulation in M7 plants (Table 1, group 1; Figure 8, cLEC7J13). Their constitutive downregulation in the M6/7 line relative to the wild type may be related to the inability of these plants to mount a full defense posture.
Growth Anomalies Are Reflected in Modulation of Transcription Factors
Here, the lowered Rboh levels in the antisense lines were shown to have a profound influence on plant growth. The transcriptome profile of the group 4 cluster features homeotic-type transcripts that are overexpressed in leaves of the antisense lines as compared with wild-type lines (Table 1, AvWt/AvMt below 1; Figure 8). Among them are the floral homeotic APETALA3 transcription factor homolog that specifies petal and stamen identities in Arabidopsis and the floral homeotic protein PMADS 2 homolog that is expressed specifically in flowers of Petunia hybrida (petunia) (Immink et al., 2003). Their functions in L. esculentum are unknown; however, inspection of current TIGR databases confirms their recovery exclusively from flower or fruit cDNA libraries. Hence, their upregulation in the transgenic leaf indicates loss of tissue-specific regulation.
Constitutive and Wound-Induced Redox-Related and Amino Acid Metabolism-Related Transcript Changes
The ascorbate–dehydroascorbate redox pair is an important indicator of cellular redox maintenance. Inspection of the transcriptome profile reveals changes in the redox-associated expression level of cytosolic monodehydroascorbate reductase and L. esculentum CIG1 (cytokine induced gene), an L. esculentum Pro oxidase homolog (Table 1, Figure 8). Novel transcripts related to amino acid metabolism are changed. His decarboxylase is upregulated (Table 1, Figure 8). Because this enzymatic step would produce elevated histamine levels, its possible role could be antiherbivore activity. Asparaginase (Table 1, Figure 8) is downregulated in transgenic leaves relative to the wild type. Thus, Rboh repression induced changes in nitrogen assimilation-related transcripts. It is of interest that ectopic expression of prosystemin was shown to substantially increase nitrogen accumulation in Solanum tuberosum (potato) tubers (Narvaez-Vasquez and Ryan, 2002).
DISCUSSION
Rboh as Signal Transponder
We have applied an antisense strategy to deregulate the expression of the L. esculentum Rboh multigene family. Simultaneous silencing of at least two genes was achieved, and the global reduction in Rboh levels facilitated elimination of potential functional redundancy. In this respect, both Arabidopsis AtrbohD and AtrbohF null lines displayed functional overlap with regard to their disease response and imposed partial impairment of the stomatal response to ABA (Torres et al., 2002; Kwak et al., 2003). Interestingly, enhanced water loss as measured by transpiration rates and loss of fresh weight in detached leaves was not detected in the antisense L. esculentum; however, germinating seeds were less sensitive to the presence of 2 μM ABA (data not shown). The elimination of AtrbohC impaired root hair formation in Arabidopsis (Foreman et al., 2003). This phenomenon was not detected here and may be because of the lack of repression of the particular L. esculentum Rboh homolog or differences between L. esculentum and Arabidopsis biology.
What is the molecular basis of the pleiotropic developmental effects of reduction of Rboh levels? Rboh could function as a signal transponder for hormone action. Direct evidence for this can be garnered from the Rboh dependency of the guard cell response to ABA (Kwak et al., 2003). However, as shown here, it is likely that hormonal effects on Rboh are more general and extend beyond ABA action alone, as implied by the results in Figure 5. For example, impairment in the auxin response partially mimics some of the phenotypes described here, and upwardly curled and inverted leaves were described for auxin IAA Arabidopsis mutants as well (Tian and Reed, 1999; Liscum and Reed, 2002). The observations made here extend Rboh function to a plethora of developmental effects in many plant organs and imply that a range of hormones are involved. Thus, hormones might regulate Rboh in two ways. In the short term, hormones might affect Rboh by promoting a ROS burst that may be mediated by functions in the N-terminal segment containing calcium binding EF hands and other unknown regulatory regions. A more lasting and long-term effect of hormones may be achieved by the upregulation of Rboh levels as shown in Figure 5, a result that corresponds with the report of Kwak et al. (2003), showing that application of ABA leads to accumulation of Rboh transcript.
Rboh Activity Impinges on Fundamental Cellular Processes
Rboh activity and plant development can be related in additional ways. The ectopic expression of MADS box genes that was detected in mutant leaves (Table 1, group 4) and its correlation with the leaf curling phenotype (Figure 3B) is of special interest. The Arabidopsis curly leaf mutation appears superficially similar to the L. esculentum leaf curl phenotype. curly leaf was shown to be encoded by a polycomb factor. Repression of polycomb group gene activity contributes to destabilization of heterochromatin structure and ectopic expression of flower-specific MADS box genes in the leaf. Their expression is accompanied by the development of curled leaves and fruit abnormality (Krizek and Meyerowitz, 1996; Goodrich et al., 1997). Thus, attenuation of Rboh activity may influence chromosome structure, and in this respect, it is of interest that a number of histone 3.3 variants (belonging to groups 1 and 4 expression patterns; Table 1, Figure 8) also are modulated. The histone H3.3 variant is deposited throughout the cell cycle and is thought to modulate gene expression (Ahmad and Henikoff, 2002). Redox-regulated chromatin remodeling may be executed by an NAD+-dependent protein and/or histone deacetylases (Denu, 2003). Alternatively, proteins active in remodeling can be redox sensitive (e.g., NPR1, the regulator of systemic resistance, undergoes redox-dependent oligomerization that is essential for its signaling attributes) (Mou et al., 2003).
ROS-Dependent and -Independent Gene Induction
The microarray methodology employed to measure gene induction enables the distinction of processes that require Rboh and those that do not (Table 1, Figure 7). Group 2 transcripts, which contain the PIN multigene family, show varying degrees of Rboh dependency. Thus, not all PIN members are coordinately regulated, extending the observations made by Orozco-Cardenas et al. (2001). Comparison of local and systemic transcriptome activity after wounding shows differential responses. Profiling by an 8.2K Arabidopsis array was performed on RNA extracted directly from the wounded tissue (Cheong et al., 2002). Prominent transcripts induced within the wounded leaves were pathogenesis-related (PR) proteins, EREBP and WRKY transcription factors, enzymes for phenylpropanoid metabolism, and components of the JA and ethylene pathways. Thus, it appears that the transcriptome profiles of the wounded leaf and its cognate systemic leaf are distinct. PIN transcripts, but not EREBP or PR protein transcripts, are present in the systemic leaf. The results presented here are consistent with restriction of JA synthesis to the wounded leaf proper and JA functioning as the long-distance wound signal (Strassner et al., 2002; Lee and Howe, 2003).
Rboh Activity Influences Specific Redox-Related Cellular Activity
ROS bursts can be observed by many physiological effectors, such as pathogen-related effectors or wounding; however, it is important to differentiate the transcriptional programs implemented by each burst. The treatment of Arabidopsis cells with hydrogen peroxide affected transcripts of heat-shock proteins and senescence-related transcripts (Desikan et al., 2001). These types of transcripts were not detected as being significantly changed in the Rboh antisense plants. When N. tabacum discs were subjected to oxidative stress by application of ROS-generating methyl-viologen, PR proteins, phytoalexin biosynthetic genes, and genes of oxylipin metabolism were induced (Vranova et al., 2002). These gene types were not found to be upregulated in systemic leaves of wounded plants. Apparently, bursts that originate as a superoxide as compared with hydrogen peroxide might have different spatial, temporal, and target specificities. In this respect, the visualization of rapid and specific subcellular ROS bursts has been reported (Allan and Fluhr, 1997; Coelho et al., 2002).
Comparison of control tissue from wild-type and mutant leaves enables us to ascertain the long-term effects of impaired Rboh activity. Rboh downregulation resulted in reduced hydrogen peroxide accumulation in leaves (Figure 2). The parental line features a compound leaf structure. Examination of the transformed lines showed that the leaflets generally tended to have smoother edges and appeared less lobed (Figures 2). Such changes are reflected in upregulation of the cytosolic monodehydroascorbate reductase transcript (Table 1). The ascorbate–dehydroascorbate redox pair is a measure of the cellular redox state and equilibrates with reduced and oxidized forms of glutathione in plants and animals (Mittler, 2002). In transgenic tissue, the elevated transcript level may facilitate ascorbate regulation in plants with a lower level of reactive oxygen production. The reciprocal downregulation of the L. esculentum Pro oxidase homolog is consistent with the lessened need for NADPH. Pro oxidase catalyzes the oxidation of Pro to pyrroline-5-carboxylate with the concomitant transfer of electrons to cytochrome c (Donald et al., 2001). In the case of transgenic Rboh antisense leaves, a potential source of more reducing power through Pro apparently is not necessary.
Figure 2.
H2O2 Production in Wild-Type and Antisense Leaves.
(A) Constitutive levels of H2O2 in leaves were visualized by DAB staining of the terminal leaflet of the first fully expanded leaves sampled from wild-type and Rboh transgenic 45-d-old plants. Leaflets were collected and vacuum-infiltrated with the DAB solution. The sampled leaves were placed in a plastic box under high humidity for DAB-H2O2 staining development.
(B) Quantitative analysis of DAB staining. Quantitative measurements were performed as described in the Methods. Each point represents the mean of four terminal leaflets derived from four different plants. Bars represent se.
Thus, it is likely that in vivo Rboh produces a continuous flux of ROS, consistent with the fact that a basal activity level for Rboh was present in isolated membranes (Sagi and Fluhr, 2001). Hence, plant Rboh is a quantitative player in dictating the cellular milieu of ROS flux, and it is anticipated that their modulation would demand metabolic adjustment as measured by compensatory fine-tuning in transcriptome profiles. Rboh appears to be a highly regulated, sensitive, and versatile mediator of developmental and environmental signals. Depending on the incoming signals from the plant, pathogen, or environment, the redox state might be altered such that it governs a transcriptional response aimed at maximizing plant fitness in a changing environment. Future work should delineate the molecular components that control Rboh activity and the primary downstream targets modulated by ROS bursts.
METHODS
Plant Material
L. esculentum cv Motelle wild-type and transgenic plants were grown in pots filled with a peat and vermiculite (4:1 [v/v]) mixture containing slow release High N multicote 4 with microelements (0.3% [w/w]; Haifa Chemicals, Haifa Bay, Israel). Greenhouse average temperatures during the growth period fluctuated from 18 to 25°C. Midday photosynthetic photon flux density in the greenhouse was 300 to 500 μmol·m−2·s−1.
Plasmid Constructs
L. esculentum EST 244804 and 243389 that span the C terminus of the two L. esculentum gp91phox NADPH oxidase plant homologs Lerboh1 (AAD25300) and Wfil (AF73124), respectively, were used as a source. T-DNA constructs were made that express subclones of the EST together or separately in sense/antisense orientation downstream of the 35S promoter of Cauliflower mosaic virus using the E9 terminator as described (Savaldi-Goldstein et al., 2003). M4 expresses a Lerboh fragment in antisense orientation. It was constructed with a 345-bp Asp718/Asp718 fragment of the EST. M5 expresses a Wfil fragment in antisense orientation and was constructed with a 665-bp EcoRI/EcoRI fragment of the EST. M3 expresses Lerboh and Wfil fragments in antisense and sense orientation, respectively, whereas M6/7 expresses the Lerboh and Wfil fragments in the antisense orientation. Both, M3 and M6/7 were constructed by insertion of the 665-bp EcoRI/EcoRI fragment of the L. esculentum EST 243389 into EcoRI/EcoRI sites downstream to the 345-bp insert of M4 construct. The resulting constructs were transferred by electroporation into Agrobacterium tumefaciens EHA105. Stable transformation into L. esculentum (cv Motelle) was performed as described (Ori et al., 1997). T2 plants were tested for segregation on kanamycin, and independent lines were selected for further analysis and grown to T3 homozygous plants. Purity of parental wild-type and derived antisense var Motelle lines was confirmed by restriction fragment polymorphism length using SL8 clone as a probe (Ori et al., 1997).
RT-PCR Probes
For quantitative RT-PCR, total RNA was extracted with the RNeasy plant mini kit (Qiagen, Valencia, CA). RNA (1.5 μg) was subjected to first-strand synthesis using SuperScript II reverse transcriptase (Gibco BRL, Cleveland, OH) according to the manufacturer's procedure using oligo(dT) as a primer. Parallel reactions in the absence of the enzyme served as control. PCR amplification was conducted on one-tenth of the reaction. Primers used were as follows: for Lerboh, forward 5′-GTCAGGCTTCTACAGAAAAC-3′ and reverse 5′-GTTGATTACAGTAGCCGGTTC-3′, resulting in a 270-bp PCR product; for Wfil, forward 5′-CTGCTTGGAAGAAGAAATC-3′ and reverse 5′-GAATTTTGCATCGCTACAATAG-3′, resulting in a 213-bp PCR product; and for Tom41 actin gene (U60480), forward 5′-ATGCCATTCTCCGTCTTGACTTG-3′ and reverse 5′-GAGTTGTATGTAGTCTCGTGGATT-3′, resulting in a 325-bp PCR product.
The antisense/sense-specific fragments were amplified using the forward primer 5′-CAAGATCTATCGATTCCCG-3′ (complementary to the polylinker cloning site of the binary vector) and the reverse primers for the cDNA inserts, which were as follows: for M3, 5′-GCGTTTGTAGACGTTTCT-3′ (PCR product of 810 bp); for M4, 5′-GGGGTTGATATTGTATCAG-3′ (PCR product of 293 bp); and for M5 and M6/7, 5′-GAGGTGGTTTTATTGGTGG-3′ (PCR products of 648 and 927 bp, respectively). PCR products were separated on a 1 to 2% agarose gel containing ethidium bromide (10 μg/mL) and visualized by the Bio-Imaging system (model 202D; DNR-Imaging Systems, Kiryat Anavim, Israel). PCR products were excised from the gel and sequenced for verification.
Plant Growth and Developmental Characteristics
Number of inflorescences, flower type, and number (fused flowers and number of petals other than six were designated as abnormal) analysis of primary and secondary branches (>5 cm in length) were measured in 100-d-old plants. Total chlorophyll content was measured in extracts of the first fully expanded leaf as described previously (Graan and Ort, 1984). Curling level was measured in 100-d-old plants and designated as follows: 0, no curling; 1, >50% of the leaves are slightly curled; 2, >50% of the leaves are half-way curled; and 3, >50% of the leaves are completely curled. Inverted leaflet levels were designated as follows: 1, no inverted leaflets; 2, in one leaf at least one or more terminal leaflets were inverted; 3, two to three leaves had inverted leaflets; 4, four to five leaves had inverted leaflets; and 5, more than six leaves had inverted leaflets. Abnormal fruits were designated as irregular fruits that showed asymmetric shape, fasciation, catface, corky epidermis, or clausa-like fruits. The number of abnormal and normal fruits with a diameter of >2 cm was measured in 150-d-old plants.
Treatments, Protein, and RNA Extraction
Rboh immunoblotting was performed on wild-type and antisense plants as described previously (Sagi and Fluhr, 2001). For hormonal treatment, extraction and immunoblotting was conducted on the second upper leaf of 28-d-old plants cut at the stem and placed in a solution containing 50 μM ABA, GA, IAA, BA, or the ethylene precursor ACC for 24 h. For wound induction, leaves of 21- to 28-d-old plants (containing three leaves) were crushed with a hemostat, three times perpendicular to the midvein on the distal end of the terminal leaflet of the lower leaf, and 24 h after wounding, the upper unwounded leaves (systemic signal) were sampled for RNA or protein extraction. Leaves of the same size and position in unwounded plants served as controls. Protein extracts were fractionated by SDS-PAGE, 12.5% (w/v) polyacrylamide separating gel and 4% (w/v) stacking gel, and were immunoblotted and developed with antibodies against L. esculentum PIN II (gift of Clarence A. Ryan, Institute of Biological Chemistry, Washington State University, Pullman, WA).
Wounding and Detection of ROS
H2O2 was detected in situ by DAB staining as described (Thordal-Christensen et al., 1997). The terminal leaflet of the first fully expanded leaf was sampled from wild-type and Rboh transgenic 45-d-old plants. Leaflets were collected and vacuum infiltrated with the DAB solution (1 mg/mL, pH 3.8; Sigma, St. Louis, MO). The sampled leaves were placed in a plastic box under high humidity until brown precipitate was observed (5 to 6 h) and then fixed with a solution of 3:1:1 ethanol:lactic acid:glycerol and photographed. Quantitative analyses of leaves stained with DAB were made by scanning the leaves with a computing laser densitometer using Image Quant version 3.19.4 software (Molecular Dynamics, Sunnyvale, CA). To determine H2O2 levels after wounding, leaves of 21- to 28-d-old plants containing three to four leaves were excised at the base with a razor blade and soaked in a solution containing DAB for 3 h before wounding. Wounding was accomplished by crushing the leaves with a hemostat as described above. Five hours after wounding, the upper unwounded leaves (systemic leaves) were sampled as above. Leaves of the same size and position in unwounded plants served as controls.
Quantitative analysis was performed by scanning the leaves with a computing laser densitometer using the Image Quant version 3.19.4 software (Molecular Dynamics).
Microarray Analysis and RT-PCR
Wounding was accomplished in wild-type and antisense plants as described above, and 24 h after wounding, the upper unwounded leaves (systemic leaves) were sampled, whereas leaves on the same position in unwounded wild-type and antisense plants served as controls. Each condition is the averaged result of two to three independent biological replicates as follows: mutant control leaf, two replicates; mutant systemic leaf, three replicates; wild-type control leaf, three replicates; and wild-type systemic leaf, two replicates. Total RNA was extracted using the RNeasy mini kit (Qiagen) and subjected to reverse transcription and amplified by in vitro transcription with T7 polymerase (Ambion, Austin, TX). Amplified RNA products were subjected to reverse transcription and then labeled with Cy3 and Cy5 by the indirect amino-allyl method. A 12K L. esculentum EST slide was used as a probe (Boyce Thompson Institute Center for Gene Expression Profiling, http://bti.cornell.edu/CGEP/CGEP.html). For each biological repetition, two hybridizations with swapped dye labeling were performed. Separate images for each fluorescence were acquired using ScanArray 4000 software (Packard BioScience, Meridan, CT) at a resolution of 10 μm per pixel, adjusting the photomultiplier and laser power to achieve an optimal distribution of signals without minimal saturation. Initial image analysis was performed using QuantArray version 3 software using the histogram method (Packard BioScience). Data analysis was performed applying per-spot and per-chip normalization (GeneSpring 5.1; Silicon Genetics, Redwood City, CA). Two-way hierarchical clustering was performed on differentially expressed genes (one-way ANOVA equal variance, P ≤ 0.05) using Pearson correlation similarity measure. Clusters were tested for a given null hypothesis using the sum of square simultaneous test procedure. RT-PCR analysis was performed on the aRNA as described by Savaldi-Goldstein et al. (2003). Primer pairs used are in Supplemental Table 2 online. The PCR products were excised and separated from 1 to 2% gels and sequenced for verification.
The complete expression data set is available as accession numbers GPL788, GSM13872 to GSM13881, and GSE917 in Gene Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo.
Supplementary Material
Acknowledgments
This work was supported in part by the Peres Center for Peace; the Israel Science Foundation Grant 417/03; the Minerva Foundation, Germany; the Weizmann-Argentina Fundacion Antorchas; and the Raymond Burton Fund for Plant Genomic Research. We are thankful to Neta Rines, Dinah Miller, Moshe Ventura, and Akalu Pascha for their technical assistance.
On-line version contains Web-only data.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) are: Moshe Sagi (gizi@bgumail.bgu.ac.il) and Robert Fluhr (robert.fluhr@weizmann.ac.il).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.019398.
References
- Ahmad, K., and Henikoff, S. (2002). The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200. [DOI] [PubMed] [Google Scholar]
- Allan, A.C., and Fluhr, R. (1997). Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9, 1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C.J., and Orlandi, E.W. (1995). Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33, 299–321. [DOI] [PubMed] [Google Scholar]
- Banfi, B., Molnar, G., Maturana, A., Steger, K., Hegedus, B., Demaurex, N., and Krause, K.H. (2001). A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276, 37594–37601. [DOI] [PubMed] [Google Scholar]
- Bokoch, G.M., Bohl, B.P., and Chuang, T.H. (1994). Guanine-nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J. Biol. Chem. 269, 31674–31679. [PubMed] [Google Scholar]
- Brandstadter, J., Rossbach, C., and Theres, K. (1996). Expression of genes for a defensin and a proteinase inhibitor in specific areas of the shoot apex and the developing flower in tomato. Mol. Gen. Genet. 252, 146–154. [DOI] [PubMed] [Google Scholar]
- Cheong, Y.H., Chang, H.S., Gupta, R., Wang, X., Zhu, T., and Luan, S. (2002). Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 129, 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, S.M., Taylor, A.R., Ryan, K.P., Sousa-Pinto, I., Brown, M.T., and Brownlee, C. (2002). Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in fucus rhizoid cells. Plant Cell 14, 2369–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, A.R., and Jones, O.T. (1986). The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu, J.M. (2003). Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem. Sci. 28, 41–48. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Mackerness, S.A.H., Hancock, J.T., and Neill, S.J. (2001). Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 127, 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke, N., and Ohashi, Y. (1988). Involvement of an O2-generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiol. Mol. Plant Pathol. 32, 163–175. [Google Scholar]
- Donald, S.P., Sun, X.Y., Hu, C.A.A., Yu, J., Mei, J.M., Valle, D., and Phang, J.M. (2001). Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 61, 1810–1815. [PubMed] [Google Scholar]
- Foreman, J., Demidchik, V., Bothwell, J.H., Mylona, P., Miedema, H., Torres, M.A., Linstead, P., Costa, S., Brownlee, C., Jones, J.D., Davies, J.M., and Dolan, L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. [DOI] [PubMed] [Google Scholar]
- Goodrich, J., Puangsomlee, P., Martin, M., Long, D., Meyerowitz, E.M., and Coupland, G. (1997). A polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386, 44–51. [DOI] [PubMed] [Google Scholar]
- Graan, T., and Ort, D.R. (1984). Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J. Biol. Chem. 259, 14003–14010. [PubMed] [Google Scholar]
- Ho, L.C., Belda, R., Brown, M., Andrews, J., and Adams, P. (1993). Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. J. Exp. Bot. 44, 509–518. [Google Scholar]
- Immink, R.G.H., Ferrario, S., Busscher-Lange, J., Kooiker, M., Busscher, M., and Angenent, G.C. (2003). Analysis of the petunia MADS-box transcription factor family. Mol. Genet. Genomics 268, 598–606. [DOI] [PubMed] [Google Scholar]
- Jiang, M., and Zhang, J. (2003). Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedlings. Plant Cell Environ. 26, 929–939. [DOI] [PubMed] [Google Scholar]
- Joo, J.H., Bae, Y.S., and Lee, J.S. (2001). Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 126, 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, T., Damude, H.G., Werner, D., Doerner, P., Dixon, R.A., and Lamb, C. (1998). A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek, B.A., and Meyerowitz, E.M. (1996). The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122, 11–22. [DOI] [PubMed] [Google Scholar]
- Kwak, J.M., Mori, I.C., Pei, Z.M., Leonhardt, N., Torres, M.A., Dangl, J.L., Bloom, R.E., Bodde, S., Jones, J.D.G., and Schroeder, J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G.I., and Howe, G.A. (2003). The tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression. Plant J. 33, 567–576. [DOI] [PubMed] [Google Scholar]
- Liscum, E., and Reed, J.W. (2002). Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 49, 387–400. [PubMed] [Google Scholar]
- Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Mou, Z., Fan, W., and Dong, X. (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935–944. [DOI] [PubMed] [Google Scholar]
- Narvaez-Vasquez, J., and Ryan, C.A. (2002). The systemin precursor gene regulates both defensive and developmental genes in Solanum tuberosum. Proc. Natl. Acad. Sci. USA 99, 15818–15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill, S., Desikan, R., and Hancock, J. (2002. a). Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 5, 388–395. [DOI] [PubMed] [Google Scholar]
- Neill, S.J., Desikan, R., Clarke, A., Hurst, R.D., and Hancock, J.T. (2002. b). Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53, 1237–1247. [PubMed] [Google Scholar]
- Oleksiak, M.F., Churchill, G.A., and Crawford, D.L. (2002). Variation in gene expression within and among natural populations. Nat. Genet. 32, 261–266. [DOI] [PubMed] [Google Scholar]
- Ori, N., Eshed, Y., Paran, I., Presting, G., Aviv, D., Tanksley, S., Zamir, D., and Fluhr, R. (1997). The I2C family from the wilt disease resistance locus I2 belongs to the nucleotide binding, leucine-rich repeat superfamily of plant resistance genes. Plant Cell 9, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas, M., and Ryan, C.A. (1999). Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 96, 6553–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas, M.L., Narvaez-Vasquez, J., and Ryan, C.A. (2001). Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13, 179–191. [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Pnueli, L., Carmel-Goren, L., Hareven, D., Gutfinger, T., Alvarez, J., Ganal, M., Zamir, D., and Lifschitz, E. (1998). The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Ryan, C.A. (2000). The systemin signaling pathway: Differential activation of plant defensive genes. Biochim. Biophys. Acta 1477, 112–121. [DOI] [PubMed] [Google Scholar]
- Sagi, M., and Fluhr, R. (2001). Superoxide production by plant homologues of the gp91(phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 126, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein, S., Aviv, D., Davydov, O., and Fluhr, R. (2003). Alternative splicing modulation by a LAMMER kinase impinges on developmental and transcriptome expression. Plant Cell 15, 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Plas, F., Elmayan, T., and Blein, J.P. (2002). The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J. 31, 137–147. [DOI] [PubMed] [Google Scholar]
- Strassner, J., Schaller, F., Frick, U.B., Howe, G.A., Weiler, E.W., Amrhein, N., Macheroux, P., and Schaller, A. (2002). Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J. 32, 585–601. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen, H., Zhang, Z.G., Wei, Y.D., and Collinge, D.B. (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Tian, Q., and Reed, J.W. (1999). Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126, 711–721. [DOI] [PubMed] [Google Scholar]
- Torres, M.A., Dangl, J.L., and Jones, J.D.G. (2002). Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, M.A., Onouchi, H., Hamada, S., Machida, C., Hammond-Kosack, K.E., and Jones, J.D.G. (1998). Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 14, 365–370. [DOI] [PubMed] [Google Scholar]
- Vranova, E., Atichartpongkul, S., Villarroel, R., Van Montagu, M., Inze, D., and Van Camp, W. (2002). Comprehensive analysis of gene expression in Nicotiana tabacum leaves acclimated to oxidative stress. Proc. Natl. Acad. Sci. USA 99, 10870–10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka, H., Numata, N., Nakajima, K., Katou, S., Kawakita, K., Rowland, O., Jones, J.D., and Doke, N. (2003). Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.