Abstract
The heterotrimeric protein phosphatase 2A (PP2A) complex comprises a catalytic subunit and regulatory A and B subunits that modulate enzyme activity and mediate interactions with other proteins. We report here the results of a systematic analysis of the Arabidopsis (Arabidopsis thaliana) regulatory A subunit gene family, which includes the ROOTS CURL IN NAPHTHYLPHTHALAMIC ACID1 (RCN1), PP2AA2, and PP2AA3 genes. All three A subunit isoforms accumulate in the organs of seedlings and adult plants, suggesting extensive overlap in expression domains. We have isolated pp2aa2 and pp2aa3 mutants and found that their phenotypes are largely normal and do not resemble that of rcn1. Whereas rcn1 pp2aa2 and rcn1 pp2aa3 double mutants exhibit striking abnormalities in all stages of development, the pp2aa2 pp2aa3 double mutant shows only modest defects. Together, these data suggest that RCN1 performs a cardinal role in regulation of phosphatase activity and that PP2AA2 and PP2AA3 functions are unmasked only when RCN1 is absent.
INTRODUCTION
Protein phosphatase 2A (PP2A) is a ubiquitous and highly conserved Ser/Thr phosphatase with broad substrate specificity and a diverse repertoire of cellular functions (reviewed in Janssens and Goris, 2001). Association of the PP2A catalytic (C) subunit with regulatory (A and B) subunits produces several species of holoenzymes with distinct properties and functions. The A subunit, a founding member of the HEAT repeat protein family, is required as the scaffold for formation of the heterotrimeric complex (Ruediger et al., 1992; Groves et al., 1999). Binding of the A subunit also alters the enzymatic activity of the catalytic subunit, even in the absence of B subunits (Kamibayashi et al., 1992; Turowski et al., 1997; Price and Mumby, 2000). Whereas C and A subunit sequences show remarkable sequence conservation throughout eukaryotes, regulatory B subunits are more heterogeneous and are thought to play key roles in controlling the localization and specificity of different holoenzymes. Multicellular eukaryotes express at least three different classes of B subunits, typically designated as B55 (or B), B′, and B″. In addition, accessory proteins and posttranslational modifications control PP2A subunit associations and activities.
Although some sequences or motifs required for subunit interactions have been identified in the A and B subunits (Ruediger et al., 1992; Li and Virshup, 2002), the mechanisms governing specific PP2A complex formation are not fully understood. In mammalian systems, two isoforms (α and β) of both the A and C subunits are found, and the two C subunit isoforms are known to play distinct roles. For instance, the early embryonic lethality of a Cα knockout in the mouse is attributed to the lack of a Cα-specific interaction with the cell adhesion molecule E-cadherin. This interaction is required for signaling of the secreted glycoprotein Wnt, an important regulator of animal development, before gastrulation (reviewed in Janssens and Goris, 2001). However, Cα and Cβ appear to bind equally well to Aα and Aβ and to three different B regulatory subunits, and they have the same catalytic activities in defined holoenzyme complexes (Zhou et al., 2003a). By contrast, the A subunit isoforms show very different binding properties. The Aα isoform binds strongly to C and B subunits and to simian virus 40 small T antigen, a PP2A binding tumor antigen, whereas Aβ binds more weakly to C and B and fails to bind small T (Zhou et al., 2003b). Little is known about isoform-specific A subunit functions, but the genes encoding both Aα and Aβ have been identified as potential tumor suppressor loci (Wang et al., 1998; Calin et al., 2000).
As in animal systems, plant PP2A is a multifunctional regulator. Forward genetic analysis in Arabidopsis (Arabidopsis thaliana) has demonstrated roles for PP2A in hormone-mediated growth regulation and in control of cell shape and plant morphology (Rashotte et al., 2001; Camilleri et al., 2002; Kwak et al., 2002; Larsen and Cancel, 2003). Biochemical and pharmacological data implicate PP2A in cold responses and in regulation of metabolic enzyme activity, hormone and pathogen responses, guard cell ion channel activity, cell cycle progression, and root cortical cell elongation in a variety of plant species. The Arabidopsis genome sequence predicts the existence of up to 255 heterotrimeric PP2A isoforms; genes encoding five C subunits, three A subunits, and 17 B subunits have been annotated. RNA gel blot and reverse transcription–PCR analyses suggest broad and overlapping expression patterns for five C, three A, two B55, and eight B' subunits (Haynes et al., 1999; Terol et al., 2002), but reporter gene fusions reveal that promoters for four of these genes exhibit some tissue specificity in seedling, leaf, and floral expression patterns (Deruère et al., 1999; Thakore et al., 1999; Kwak et al., 2002). Thus, the available data leave open the possibility of considerable functional redundancy among PP2A subunits.
The only plant PP2A mutants reported to date are the Arabidopsis rcn1 and tonneau2 (ton2)/fass/gordo mutants; the ROOTS CURL IN NAPHTHYLPHTHALAMIC ACID1 (RCN1) gene encodes the A1 or α isoform of the regulatory A subunit (Garbers et al., 1996), whereas TON2/FASS/GORDO encodes a B″ subunit (Camilleri et al., 2002). The ton2/fass/gordo mutant exhibits abnormal morphogenesis, cortical microtubule disorganization, and increased auxin content and ethylene production (Torres-Ruiz and Jürgens, 1994; Fisher et al., 1996; Camilleri et al., 2002). The rcn1 mutant exhibits defects in differential cell elongation responses including gravitropism, in polar auxin transport, and in abscisic acid responses (reviewed in Muday and DeLong, 2001; Kwak et al., 2002). An rcn1 allele was independently isolated in a screen for ethylene response mutants and shows increased ethylene sensitivity and biosynthesis (Larsen and Chang, 2001; Larsen and Cancel, 2003); another allele was isolated in a screen for altered root susceptibility to agrobacterial transformation (Zhu et al., 2003). These data suggest specific, nonredundant roles for the RCN1 A subunit in these processes. Physiological and biochemical assays show that the wild-type, RCN1-encoded A subunit functions as a positive regulator of the PP2A holoenzyme (Deruère et al., 1999). Treatment of wild-type seedlings with a low dose of phosphatase inhibitor creates a phenocopy of rcn1 in growth curvature, auxin transport, and ethylene response assays, indicating that these phenotypes are caused by reduced PP2A activity (Deruère et al., 1999; Rashotte et al., 2001; Larsen and Cancel, 2003). However, analysis of RCN1 function has shed little light on the biological and biochemical roles of the other two isoforms, PP2AA2 and PP2AA3.
The availability of large collections of sequence-indexed insertion mutants in Arabidopsis (Alonso et al., 2003) allows a functional genomics approach to the question of isoform-specific functions. To characterize the roles of the two other regulatory A subunit gene family members, we have isolated new mutants carrying T-DNA insertions in the genes encoding PP2AA2 and PP2AA3. Immunoblotting shows that each mutant lacks one of the A subunit proteins, suggesting that the mutations are strong loss-of-function alleles or knockouts. The new mutants do not resemble the rcn1 mutant, and neither shows increased sensitivity to the phosphatase inhibitor cantharidin. However, double mutant lines carrying rcn1 and either pp2aa2-1 or pp2aa3-1 exhibit severe phenotypes, including abnormal embryogenesis, radial cell expansion, dwarfing, and sterility. Surprisingly, the pp2aa2 pp2aa3 double mutant line exhibits a relatively normal phenotype, suggesting a weaker effect on regulation of PP2A activity than is observed in rcn1 plants. Together, these data demonstrate that maintenance of PP2A regulation is crucial for normal plant growth. The regulatory A subunit isoforms perform partially overlapping biological tasks, but the RCN1 protein plays a cardinal role in overall PP2A regulation.
RESULTS
Isolation of Regulatory A Subunit Mutants
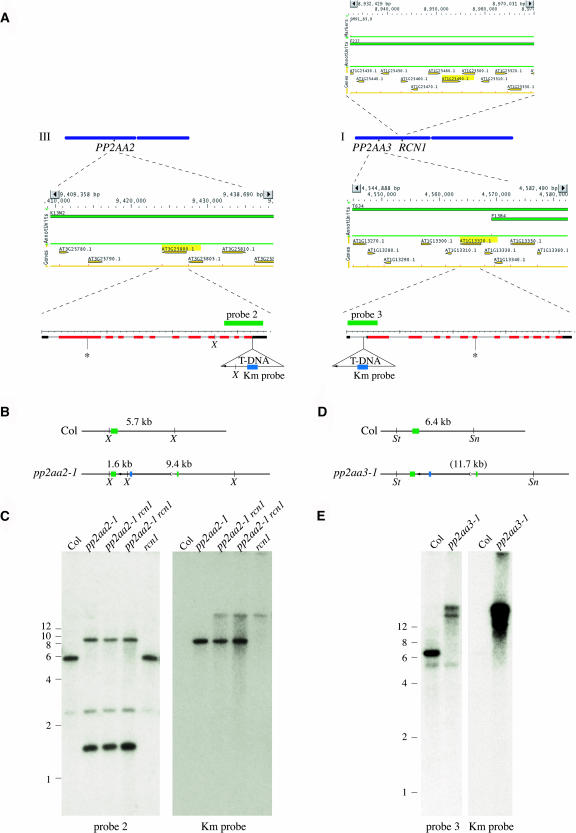
The PP2A regulatory A subunit gene family comprises three members, RCN1 (At1g25490; Garbers et al., 1996) and the genes encoding the PP2AA2 (At3g25800) and PP2AA3 (At1g13320) isoforms (Figure 1A). cDNAs for PP2AA2 and PP2AA3 have been isolated previously and were designated AtAβ/DF1 and AtAγ/DF2, respectively (Slabas et al., 1994, Corum et al., 1996). The predicted PP2AA2 and PP2AA3 proteins show 94% amino acid sequence identity with each other and 86% identity with RCN1. The mammalian Aα and Aβ isoforms also exhibit 86% sequence identity, but there is no direct correspondence between the Arabidopsis and mammalian isoforms, with all three predicted Arabidopsis proteins showing higher sequence similarity to the mammalian Aα isoform (Slabas et al., 1994; Garbers et al., 1996). To gain further insight into the regulation of PP2A activity and the roles of the PP2AA2 and PP2AA3 regulatory subunits, we screened the public databases for insertion alleles of these two genes. Two insertion alleles likely to impair gene function were identified in the T-DNA Express database (Alonso et al., 2003). Transgenic lines carrying the insertion alleles were obtained from the ABRC, and plants homozygous for the T-DNA insertions were identified (see Methods). DNA sequence analysis shows that the T-DNA insertions map quite close to the positions indicated in the T-DNA Express database (Figure 1A). For PP2AA2 (At3g25800) the T-DNA lies in the 3′ untranslated region (UTR), 12 bp downstream from the stop codon, with a direct junction between the T-DNA left border (LB) and the PP2AA2 flanking DNA. Examination of PP2AA2 cDNA sequences (AV817504.1, AV813324.1, AV810034.1, and AV811125.1) shows that most PP2AA2 transcripts continue 100 to 200 bp beyond this insertion point. For PP2AA3 (At1g13320) the T-DNA insertion occurs in the 5′ UTR, 80 bp upstream from the start codon. A 76-bp fragment of nonhomologous DNA lies between the end of the T-DNA LB sequences and the PP2AA3 flanking DNA (data not shown).
Figure 1.
Isolation of T-DNA Insertions in the PP2AA2 and PP2AA3 Genes.
Chromosomes I and III are shown as blue bars (A), with the map positions of the RCN1 (At1g25490), PP2AA2 (At3g25800), and PP2AA3 (At1g13320) genes indicated. Map positions were determined using the Arabidopsis Information Resource chromosome map tool (http://www.arabidopsis.org/jsp/ChromosomeMap/tool.jsp). Above (RCN1) or below (PP2AA2 and PP2AA3) each chromosome is a MapViewer (http://www.arabidopsis.org/servlets/mapper) diagram of the chromosomal region surrounding the A subunit gene, which is highlighted in yellow. The structures of the PP2AA2 and PP2AA3 transcript splicing models, as shown in the Institute for Genomic Research database (http://www.tigr.org), are enlarged below, with exons (red bars), UTRs (black bars), and introns (lines) indicated for each. The gene structures are shown from 5′ (left) to 3′ (right), for example, in the opposite orientation from that shown in the MapViewer diagrams. The positions of the T-DNA insertions in pp2aa2-1 and pp2aa3-1 are shown, with the small arrowheads in each indicating the LB sequences. Asterisks indicate the positions of the pp2aa2-2 and pp2aa3-2 T-DNA insertions. Gene-specific probe fragments used in DNA gel blotting are shown as green bars, and the T-DNA–specific probe fragment (Km) is shown as a blue bar. The restriction map predicted for a single T-DNA insertion in the PP2AA2 locus (B) matches the DNA gel blotting data obtained with gene-specific (probe 2) and T-DNA–specific (Km) probes (C). The restriction map predicted for a single T-DNA insertion in the PP2AA3 locus (D) does not match the DNA gel blotting data (E). Genomic DNA was extracted from plants of the genotypes indicated and was digested with XbaI (C) or with SnaBI plus StuI (E). Weakly hybridizing bands were observed as a result of cross-hybridization of probe 2 with the PP2AA3 gene (C) and cross-hybridization of probe 3 with the PP2AA2 gene (E). The Km probe hybridizes with the rcn1 T-DNA (C). Sn, SnaBI; St, StuI; X, XbaI.
To ascertain the effect of the T-DNA insertions on A subunit protein expression, the new mutants were screened for loss of A subunit expression by immunoblotting using a polyclonal antiserum raised against recombinant RCN1 protein (Deruère et al., 1999). This antiserum recognizes three closely migrating bands in immunoblots, with the middle band corresponding to the RCN1 protein (Figure 2A). Because the predicted PP2AA2 and PP2AA3 amino acid sequences are 86% identical to that of the RCN1 protein, we expected that the closely migrating bands might represent PP2AA2 and PP2AA3 proteins. Plants homozygous for the pp2aa2-1 T-DNA express RCN1 and the lower RCN1-related protein but lack the upper band (Figure 2A). Plants homozygous for the pp2aa3-1 insertion express RCN1 and the upper RCN1-related protein but lack the lower band. These results identify the closely migrating bands as three distinct A subunit proteins: PP2AA2 (uppermost band), RCN1 or PP2AA1 (middle band), and PP2AA3 (lowest band). The different apparent molecular weights of the three A subunits in our gel system are not explained by primary sequence differences, as the predicted molecular weights of the proteins differ by <0.1 kD (65.49 kD for RCN1, 65.51 kD for PP2AA3, and 65.57 kD for PP2AA2).
Figure 2.
PP2A A Subunit Expression in Wild-Type and Mutant Plants.
(A) Each A subunit mutant lacks expression of one A subunit protein. Protein extracts were isolated from dark-grown wild-type and mutant seedlings and subjected to immunoblot analysis using antisera raised against RCN1 protein (see Methods). Ws is the parental wild-type for the rcn1 mutant, whereas Columbia (Col) is the parent of the pp2aa2-1 and pp2aa3-1 mutants.
(B) Independent T-DNA insertion alleles exhibit matching protein expression patterns. Protein extracts were isolated from shoots (H) and roots (R) of dark-grown seedlings of the genotypes indicated and analyzed by immunoblotting with anti-RCN1 (top half) and anti-C subunit (bottom half) antisera.
(C) Seedling roots are enriched for PP2A subunits. Protein extracts were isolated from hypocotyls (H), cotyledons plus leaves (L), and roots (R) of light-grown seedlings (left) and from shoots (H) and roots (R) of dark-grown seedlings (right) of the genotypes indicated and analyzed by immunoblotting as described above. Equal amounts of total protein were loaded in each gel lane.
(D) All three A subunit proteins are detected in different organs of adult plants. Organs were harvested from adult wild-type plants, and protein extracts were isolated and analyzed as described above. Rosette, whole rosettes; silique (e), 5- to 7-mm green siliques; silique (l), 7- to 10-mm green siliques.
(E) Mutations in A subunit genes do not alter expression patterns of the remaining A subunits. Organs were harvested from adult plants of the genotypes indicated, and protein extracts were isolated and analyzed as described above. +, Ws rosette leaf extract (loading/blotting control).
Both mutants carry T-DNA insertions in the expected genomic DNA fragments (Figures 1B to 1E). The pp2aa2-1 line carries a single T-DNA at the PP2AA2 locus, and no additional T-DNAs are detected. The pp2aa3-1 line carries multiple T-DNAs. DNA gel blot mapping indicates that at least two T-DNA copies are inserted in tandem at the PP2AA3 locus. Both gene-specific probes and T-DNA probes hybridize to extra restriction fragments not predicted for a simple insertion, suggesting some rearrangement at the PP2AA3 locus plus additional T-DNA copies. Two rounds of backcrossing to the wild-type parent line are not sufficient to alter this complex hybridization pattern, indicating that the additional T-DNA copies are linked to PP2AA3 (data not shown).
Phenotypes of A Subunit Single Mutants
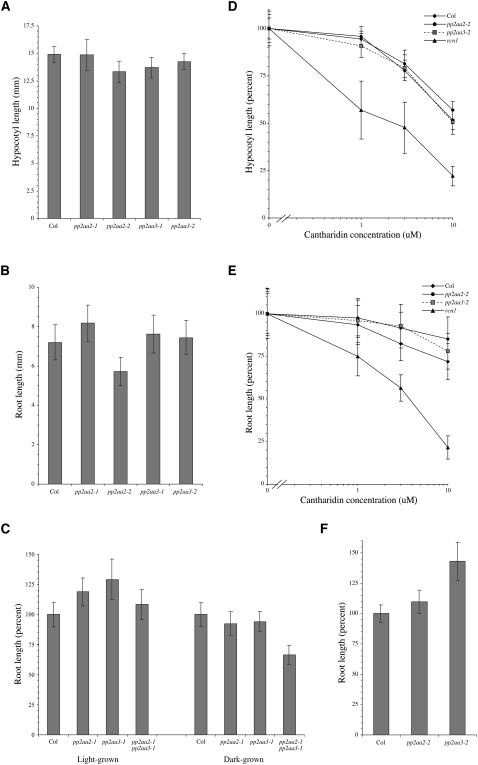
The gross phenotypes of the pp2aa2-1 and pp2aa3-1 mutant lines are normal. Adult plants form normal rosettes and develop inflorescence bolts carrying fertile flowers. Both dark- and light- grown mutant seedlings exhibit normal hypocotyl lengths, and apical hypocotyl hook morphology is normal (Figure 3A and data not shown). Dark-grown pp2aa2-1 and pp2aa3-1 show normal root lengths (Figure 3B), but light-grown seedlings exhibit slightly increased root lengths (Figure 3C). We tested for rcn1-like root phenotypes in root waving and root curling assays. Unlike rcn1, the pp2aa2-1 and pp2aa3-1 mutants exhibit wild-type waving phenotypes and also show wild-type root curling phenotypes in the presence and absence of the auxin transport inhibitor naphthylphthalamic acid (data not shown).
Figure 3.
Organ Elongation and Cantharidin Sensitivity in pp2aa2 and pp2aa3 Mutants.
Wild-type and mutant seedlings were grown for 5 d in the dark ([A] to [E]) or for 7 d in constant light ([C] and [F]) in the absence ([A] to [C] and [F]) or presence ([D] and [E]) of the phosphatase inhibitor cantharidin. The cantharidin sensitivity differences between wild-type and mutant roots at 3 μM and 10 μM cantharidin (E) are statistically significant (Student's t test values <0.001). Values shown represent the average for at least 30 seedlings; error bars represent SD.
To confirm that the normal phenotypes of the pp2aa2-1 and pp2aa3-1 mutants are not allele-specific anomalies, we obtained families carrying independent T-DNA insertions in the PP2AA2 and PP2AA3 genes. The T-DNA insertions in the pp2aa2-2 and pp2aa3-2 alleles occur in coding regions (Figure 1), and immunoblot analysis shows that each mutant lacks the expected A subunit protein (Figure 2B). Like pp2aa2-1 and pp2aa3-1, they exhibit normal hypocotyl lengths as well as normal apical hypocotyl hook, root waving, and adult phenotypes (Figure 3A and data not shown). In some assays, dark-grown pp2aa2-2 seedlings exhibit reduced root lengths (Figure 3B), but this phenotype is not observed in all assays and may be conditional. Although both pp2aa2 alleles have a modest effect on root lengths in light-grown seedlings, both pp2aa3 alleles confer more dramatic increases (Figures 3C and 3F). We conclude that the phenotypes of the mutants carrying coding sequence insertions are very similar to those of the pp2aa2-1 and pp2aa3-1 mutants.
To characterize the effect of A subunit mutations on PP2A enzyme activity, we assayed seedling sensitivity to the phosphatase inhibitor cantharidin in organ elongation assays, and we tested PP2A activity in extracts. Hypocotyl cantharidin sensitivity in pp2aa2-2 and pp2aa3-2 seedlings is normal (Figure 3D), as it is in pp2aa2-1 and pp2aa3-1 seedlings (data not shown). Roots of dark-grown pp2aa2-2 and pp2aa3-2 seedlings are slightly less sensitive to cantharidin than the wild-type parent (Figure 3E), whereas pp2aa2-1 and pp2aa3-1 seedlings exhibit normal root cantharidin sensitivity (data not shown). Enzyme activity assays show that PP2A activity in protein extracts from dark-grown pp2aa2-1 and pp2aa3-1 seedlings is ∼10 to 20% greater than that measured for the wild-type parent (Table 1). Similar results were obtained in phosphatase activity assays using extracts from rosette leaves of adult plants (data not shown). These data contrast sharply with the decreased PP2A activity and increased phosphatase inhibitor sensitivity of the rcn1 mutant (Deruère et al., 1999). Whereas our earlier work demonstrated a positive role for the RCN1 regulatory subunit, the pp2aa2 and pp2aa3 mutant phenotypes suggest that these A subunits have a small negative effect on PP2A activity.
Table 1.
Protein Phosphatase Activity in pp2aa2-1 and pp2aa3-1 Mutants
| MBP Phosphatase Activity (nmol/min/mg Protein)
|
||
|---|---|---|
| PP2A | PP1 | |
| Col | 294 ± 29 | 68 ± 7 |
| pp2aa2-1 | 341 ± 25 | 73 ± 14 |
| pp2aa3-1 | 318 ± 41 | 59 ± 17 |
Protein extracts from dark-grown seedlings were assayed for protein phosphatase activity with radioactively labeled myelin basic protein (MBP) as substrate (see Methods). Col, wild-type Columbia.
Expression of A Subunit Isoforms
To determine whether A subunit protein accumulation exhibits organ specificity, we assayed PP2A protein abundance in organs of wild-type and mutant plants (Figures 2B to 2E). We observe three distinct A subunit bands in light- and dark-grown seedlings and in adult organs, indicating that discrete gel mobilities are characteristic of the A subunit proteins. In light-grown seedlings, the A subunit proteins, as well as the C subunit, appear to be more abundant in root tissue than in hypocotyl and leaf tissues (Figure 2C). In dark-grown seedlings, A subunit protein levels appear nearly equal in roots and shoots, although the C subunit is more abundant in roots (Figures 2B and 2C). The relative abundance of A and C subunit proteins in mutant seedlings appears unchanged.
In wild-type adult plants, we observe no exclusive A subunit expression patterns, although the relative amounts of each subunit vary in different organs. The three A subunits show highest abundance in roots and flowers, followed by stems and young siliques (Figure 2D). Their abundance is lower in rosette leaves, presumably because of the preponderance of photosynthetic proteins there, and is drastically reduced in older siliques. As in seedlings, the pattern of A subunit accumulation in adult organs does not change in the mutant lines (Figure 2E). These data argue against the hypothesis that the pp2aa2 and pp2aa3 mutants exhibit normal phenotypes because of compensatory upregulated expression of the remaining A subunits. C subunit accumulation in wild-type and mutant plants closely parallels A subunit levels (Figure 2D and data not shown). Similar results have been obtained in RNA expression analysis of two rice (Oryza sativa) PP2A catalytic subunit genes (Yu et al., 2001).
Double Mutants with rcn1 Exhibit Severe Phenotypes
The similar amino acid sequences of the A subunits and the broad expression patterns described above suggest that there may be functional redundancy among these gene family members. To uncover redundant A subunit functions, we constructed double mutant lines by crossing rcn1 plants with pp2aa2-1 and pp2aa3-1 mutant plants. Double mutants were identified in the F2 and F3 generations by screening for rcn1-like (or more severe) phenotypes in the F2 and testing for novel phenotypes in F3 progeny. Genotypes were confirmed by PCR analysis, DNA gel blotting, and immunoblotting (e.g., Figures 1B and 4). Extracts from rcn1 pp2aa2 plants lack the two upper A subunit proteins, whereas rcn1 pp2aa3 plants lack the two lower bands.
Figure 4.
Immunoblot Analysis of Double Mutants.
Proteins were extracted from parental wild-type, single, and double mutant seedlings after 7 d of growth in the dark and subjected to immunoblot analysis for A and C subunits (A) or for A subunits (B) as described in Figure 2.
We used a similar strategy to construct a pp2aa2-1 pp2aa3-1 mutant line, but no severe phenotypes were observed in the F2 and F3 progeny of the cross. We used PCR screening to identify an F2 individual homozygous for the pp2aa2-1 T-DNA and heterozygous for the pp2aa3-1 T-DNA. Self-progeny of this individual appeared normal as seedlings, therefore we identified homozygous double mutants by PCR analysis. These plants were of normal stature and fertility. Protein extracts from progeny pp2aa2-1 pp2aa3-1 seedlings contain the RCN1 protein but lack the PP2AA2 and PP2AA3 proteins (Figure 4B). Root lengths in light-grown seedlings are normal but in dark-grown seedlings are decreased by ∼30% in the pp2aa2-1 pp2aa3-1 double mutant line (Figure 3C).
Seedling Development Is Abnormal in rcn1 pp2aa2 and rcn1 pp2aa3 Plants
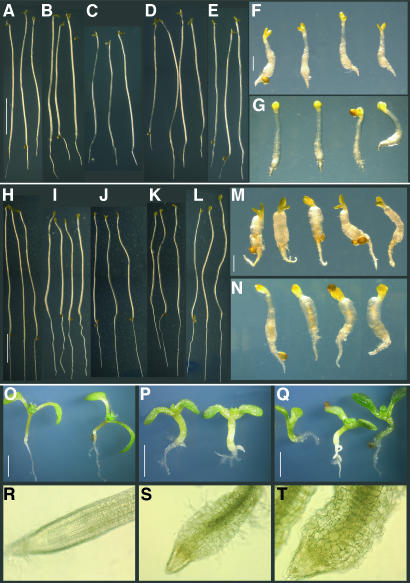
Seedlings carrying the rcn1 pp2aa2-1 and rcn1 pp2aa3-1 double mutant combinations exhibit striking phenotypes affecting cotyledons, hypocotyls, and roots (Figures 5A to 5Q). Both dark- and light-grown seedlings exhibit reduced elongation and dramatic radial expansion of the hypocotyl. Roots also show reduced elongation and a severe radial expansion phenotype. Microscopic examination of rcn1 pp2aa2-1 and rcn1 pp2aa3-1 double mutant root tips reveals severe morphological abnormalities (Figures 5R to 5T). Epidermal and cortical cells are irregular in size and shape, producing disorganized cell layers and an uneven epidermal surface. Because radial expansion in some root morphology mutants is growth rate dependent (Benfey et al., 1993), we asked whether growth in the absence of sucrose would suppress the radial expansion phenotype of rcn1 pp2aa2-1 and rcn1 pp2aa3-1 double mutant roots. These roots still exhibit radial expansion on medium lacking sucrose, indicating that this phenotype is not growth rate dependent. However, the severe inhibition of hypocotyl elongation in dark-grown seedlings is weakly suppressed by growth on medium lacking sucrose (Figures 5 and 6A).
Figure 5.
Seedling Phenotypes of rcn1 pp2aa2-1 and rcn1 pp2aa3-1 Mutants.
Seedlings were grown for 7 d in the dark on standard medium containing no sucrose ([A] to [G]) or 1% sucrose ([H] to [N]), or for 7 d ([O] to [Q]) or 5 weeks ([R] to [T]) in the light on medium containing 1% sucrose. Wild-type Ws ([A] and [H]), Columbia ([E], [L], and [O]), and single mutant rcn1 ([B] and [I]), pp2aa2-1 ([C] and [J]), and pp2aa3-1 ([D] and [K]) seedlings show elongated hypocotyls, whereas rcn1 pp2aa2-1 ([F], [M], and [P]) and rcn1 pp2aa3-1 ([G], [N], and [Q]) show radial expansion and reduced hypocotyl and root elongation. Light microscopy reveals regular cell layers in rcn1 root tips (R) and dramatic cell expansion in rcn1 pp2aa2-1 (S) and rcn1 pp2aa3-1 (T) root tips. Scale bars = 5 mm in (A) to (E) and (H) to (L); 1 mm in (F), (G), (M), and (N); and 2 mm in (O) to (Q).
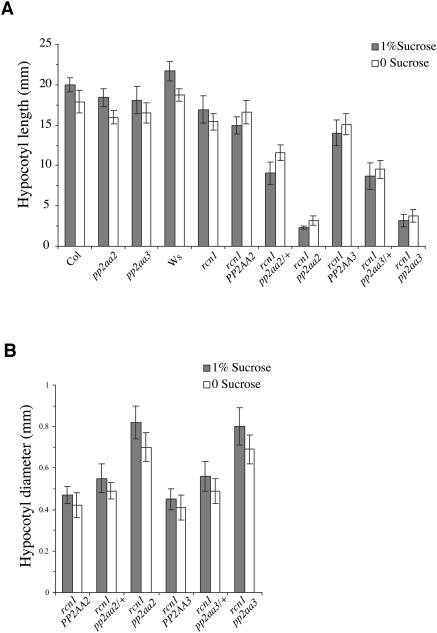
Figure 6.
Gene Dosage Effects on Hypocotyl Growth.
Self-progeny of rcn1 pp2aa2-1/+ and rcn1 pp2aa3-1/+ plants were grown in the dark for 7 d on medium with or without 1% sucrose, then divided into phenotypic classes. Hypocotyl lengths were measured and compared with those of parental wild-type (Ws and Columbia [Col]) and single mutant (pp2aa2-1, pp2aa3-1, and rcn1) seedlings (A). Hypocotyl diameters also were measured (B). The genotypes indicated were confirmed by PCR analysis of a randomly chosen subset (n = 24) of each segregating family. Values shown represent the averages for at least 10 seedlings; error bars indicate SD. In segregating families, differences between phenotypic classes are statistically significant (P ≤ 0.002 for the rcn1 pp2aa2 family; P < 0.001 for the rcn1 pp2aa3 family). Attenuation of the rcn1 pp2aa2 double mutant phenotype in the absence of sucrose also is significant (P < 0.0005 for hypocotyl length and diameter). Similar results were obtained in an independent experiment.
Self-progeny of plants that are homozygous for rcn1 and heterozygous for pp2aa2-1 or pp2aa31 exhibit a clear gene dosage effect, with doubly homozygous progeny showing the severe phenotypes described above, whereas rcn1 pp2aa2/+ and rcn1 pp2aa3/+ progeny show intermediate phenotypes with less severe radial expansion and inhibition of hypocotyl elongation (Figures 6A and 6B). These data indicate that reduced PP2AA2 or PP2AA3 function, although phenotypically silent in a wild-type background, impairs growth when PP2A regulation is compromised by the rcn1 lesion.
Double Mutant Plants Show Dwarfing and Defects in Floral Development
Double mutant seedlings grow slowly and require extended culture on sterile medium to promote development of a root system, but on transfer to potting medium in the greenhouse they develop to flowering, producing small rosettes and dwarfed inflorescence bolts (Figure 7A). The rcn1 pp2aa3 mutant is self-fertile, although seed set is low (Figure 7B). Dwarfing is more severe in the rcn1 pp2aa2 double mutant, which exhibits a thickened inflorescence stem and sterile flowers (Figure 7C). The dwarfing and infertility phenotypes of the rcn1 pp2aa2 line are similar to those of the fass/ton2 mutant (Figures 7C and 7D). FASS/TON2 encodes a B″ regulatory PP2A subunit (Camilleri et al., 2002), and strong mutant alleles cause abnormal morphogenesis, cortical microtubule disorganization, and increased auxin content and ethylene production (Torres-Ruiz and Jürgens, 1994; Traas et al., 1995; Fisher et al., 1996). However, direct comparison reveals differences between mature floral apices of rcn1 pp2aa2 and ton2 plants; plants carrying even a weak ton2 allele produce flowers showing drastically reduced development of pedicels and flowers and a dramatic failure of ovary development (Figure 7E).
Figure 7.
Adult and Floral Phenotypes of rcn1 pp2aa2-1 and rcn1 pp2aa3-1 Double Mutants.
Wild-type, single, and double mutant plants were germinated on sterile medium and transferred to potting mix in the greenhouse. Both rcn1 pp2aa2-1 and rcn1 pp2aa3-1 show dwarfing (A), but fertility and less severe dwarfing of rcn1 pp2aa3-1 (B) distinguish it from rcn1 pp2aa2-1 (C) and from plants carrying a weak ton2 allele (D). Mature floral apices (E) of rcn1 pp2aa2-1 (left) show more pistil development than those of ton2 (right). Flowers (F) of single mutants are normal, whereas double mutants show reduced size, shortened sepals, and some abnormalities in petals and stamens. Anthers (G) of wild-type (top) and double mutant rcn1 pp2aa2-1 (bottom) flowers were stained for mature pollen grains (see Methods). Scale bars = 5 cm in (A), 1 cm in (B) to (D), and 1 mm in (F).
Anthers in rcn1 pp2aa2 flowers shed little or no pollen, whereas stigmas are short (Figure 7F). Staining of pollen grains in double mutant anthers indicates that pollen development is not aborted, and accumulation of apparently mature pollen inside anthers suggests a defect in anther splitting, or dehiscence (Figure 7G). Consistent with this hypothesis, outcrosses using intact rcn1 pp2aa2 anthers and wild-type stigmas fail to yield progeny (0/15 stigmas pollinated), whereas outcrossing with manually ruptured anthers produces viable progeny (3/5 stigmas pollinated). To determine whether female reproductive defects also contribute to infertility, we tested rcn1 pp2aa2 stigmas as recipients for wild-type pollen. These pollinations yielded no progeny (0/5 stigmas pollinated). These data show that infertility in rcn1 pp2aa2 plants is a result of defects in both pistil receptivity and anther dehiscence.
Symmetry Defects in Regulatory A Subunit Mutants
Both rcn1 pp2aa2 and rcn1 pp2aa3 double mutant seedlings frequently exhibit abnormal cotyledon numbers and morphologies. These double mutant combinations give rise to a similar spectrum of defects, including collar cotyledons, fused or severely asymmetric cotyledons, and extra cotyledons (Table 2). Because cotyledon number and symmetry are established embryonically, these abnormalities indicate defects in embryogenesis. Two well-characterized mutants that exhibit similar defects are pin1 and pid, which produce collar and fused cotyledons (pin1) or tricots (pid). Mutations in pin1 and pid are thought to affect symmetry by altering auxin transport and/or sensitivity during early embryogenesis (Okada et al., 1991; Liu et al., 1993; Bennett et al., 1995; DeLong et al., 2002).
Table 2.
Cotyledon Abnormalities in A Subunit Mutants
| Parental Genotype | Monocot | Cot Fusion | Tricot | Asymmetric | Other Abnorm. | Total Abnorm. | % Mutant (Observed)a | n (Total) |
|---|---|---|---|---|---|---|---|---|
| Ws | 0.4 | 0.4 | − | 472 | ||||
| rcn1 | 0.2 | 0.2 | 0.4 | 100 | 506 | |||
| Col | 0.4 | 0.4 | − | 498 | ||||
| pp2aa2-1 | <0.2 | 100 | 481 | |||||
| pp2aa3-1 | 0.2 | 0.2 | 100 | 485 | ||||
| rcn1 pp2aa2-1/+ | 0.7 | 3.7 | 0.5 | 1.5 | 6.3 | 25 (18) | 410 | |
| rcn1 pp2aa3-1/+ | 0.3 | 0.3 | 4.8 | 0.8 | 1.1 | 7.3 | 25 (23) | 357 |
| rcn1 pp2aa3-1 | 2.1 | 1.4 | 13.4 | 6.2 | 6.2 | 28.9 | 100 | 97 |
| pin1-6/+ | 4.0 | 4.5 | 4.3 | 0.3 | 13.1 | 25 | 398 | |
| rcn1 pin1-6/+ | 12.9 | 5.0 | 0.5 | 2.1 | 1.6 | 22.0 | 25 | 381 |
| pid-9/+ | 0.3 | 8.8 | 0.3 | 9.3 | 25 | 387 | ||
| rcn1 pid-9/+ | 0.7 | 0.7 | 13.7 | 0.4 | 0.4 | 16 | 25 | 278 |
Self-progeny of plants with the genotypes indicated were assayed for symmetry defects. For double mutant combinations that produce sterile plants, cotyledon phenotypes were scored in segregating families. Seedlings were grown for 7 d in the light on sterile medium and then scored for cotyledon abnormalities using a dissecting microscope. Numbers shown represent percentages (except those shown in italics). Blank spaces indicate that no seedlings exhibited the abnormal cotyledon phenotype specified. In all cases, a blank space indicates a value <0.26%.
In segregating families, 25% of the progeny are expected to be homozygous mutants. Numbers shown in parentheses indicate the observed percentage of progeny exhibiting the homozygous mutant phenotype.
Wild-type seedlings and the single mutant parents exhibit symmetry defects only rarely (<0.4% of all seedlings), but approximately one-third of all rcn1 pp2aa2 and rcn1 pp2aa3 mutant seedlings are abnormal (Table 2). In a family segregating the rcn1 pp2aa2 double mutant, ∼6% of all seedlings carry abnormal cotyledons. Although the expected mutant class is 25% of the total, only 18% were scored as double mutants, based on radial expansion of hypocotyls and roots. Similarly, 7% of seedlings in a family segregating the rcn1 pp2aa3 double mutant exhibit cotyledon defects, with 23% of the seedlings scoring as double mutants. In homozygous rcn1 pp2aa3 families, nearly 30% of the seedlings have abnormal cotyledons. Thus, in each family, about one-third of the double mutant embryos develop with symmetry defects.
To determine whether misregulation of PP2A activity enhances symmetry defects caused by pin1 and pid mutations, we constructed rcn1 pin1 and rcn1 pid double mutants. We used pin1-6 and pid-9, strong mutant alleles in the Wassilewskija (Ws) background (Christensen et al., 2000; Vernoux et al., 2000). Because strong pin1 and pid mutations cause sterility by preventing normal floral development in homozygotes, these mutations are propagated through heterozygotes. The inflorescence phenotypes of pin1-6 and pid-9 are fully penetrant, but the symmetry defects of pin1 and pid mutants are incompletely penetrant. In the self-progeny of a pin1-6 heterozygote, 13% of the seedlings are abnormal, with ∼4% scoring as monocots, 4% exhibiting less severe cotyledon fusion, and 4% scoring as asymmetric dicots. Similarly, 9% of the self-progeny of a pid-9 heterozygote are abnormal, and nearly all of these are tricots. The rcn1 mutation increases both the penetrance and the severity of these phenotypes (Table 2). In the rcn1 mutant background, 22% of pin1-6/+ self-progeny exhibit abnormal phenotypes, with 13% scoring as monocots and another 7% showing cotyledon fusion or other severe defects. The rcn1 effect on pid-9 is similar, with nearly 14% of the seedlings developing as tricots. Monocot and cotyledon fusion phenotypes, which are rare in the pid-9 single mutant, increase in prevalence in the rcn1 background. These data show that loss of RCN1 function doubles the error rate in the embryonic establishment of symmetry in the pin1 and pid mutants.
DISCUSSION
Systematic Analysis of a PP2A Subunit Gene Family
The regulatory A subunit of PP2A performs a scaffolding function in the canonical heterotrimeric holoenzyme complex (Ruediger et al., 1992; Groves et al., 1999). Three genes encode isoforms of the A subunit in Arabidopsis. We and others have previously shown that the A subunit isoform encoded by the RCN1 gene is involved in an array of processes that control plant growth and responses to environmental stimuli (Garbers et al., 1996; Rashotte et al., 2001; Kwak et al., 2002; Larsen and Cancel, 2003, Zhu et al., 2003). Here, we have presented the results of loss-of-function analysis of the two remaining A subunit gene family members, PP2AA2 and PP2AA3. The lack of one A subunit isoform in each mutant indicates that the new mutants carry null or strong loss-of-function alleles. We have characterized the phenotypes of mutants lacking PP2AA2 and PP2AA3 protein expression, as well as the phenotypes of rcn1 pp2aa2, rcn1 pp2aa3, and pp2aa2 pp2aa3 double mutants. Despite the high amino acid sequence similarity of RCN1, PP2AA2, and PP2AA3 and the apparent abundance of the PP2AA2 and PP2AA3 proteins, the phenotypes of the pp2aa2 and pp2aa3 mutants bear little similarity to that of rcn1.
Overlapping but Nonequivalent Roles for A Subunit Isoforms
The pleiotropic rcn1 mutant phenotype contrasts sharply with the nearly silent phenotypes of the pp2aa2 and pp2aa3 single mutants. In the presence of a functional RCN1 gene, loss of PP2AA2 or PP2AA3 function causes only a slight change in PP2A activity as measured by enzyme activity in vitro. Neither mutation significantly alters the seedling phosphatase inhibitor sensitivity, a physiological indicator of total PP2A activity. Even the pp2aa2 pp2aa3 double mutant exhibits a more normal phenotype than rcn1 and shows a weaker effect on phosphatase inhibitor sensitivity (C. Nussbaumer and A. DeLong, unpublished data). Clearly, it is possible that the pp2aa2 and pp2aa3 mutants express conditional phenotypes that we have not yet identified, but it is also clear that loss of RCN1 function has a profound effect on PP2A function under our standard growth conditions. Phenotypes of rcn1 mutants include altered auxin transport, reduced elongation of seedling organs, increased ethylene production and sensitivity, and reduced abscisic acid sensitivity. Phosphatase inhibitor treatment produces a phenocopy of rcn1, demonstrating that rcn1 defects in these processes are caused by reduced phosphatase activity. This rules out the hypothesis that unique nonphosphatase functions of RCN1 distinguish it from PP2AA2 and PP2AA3 and argues for nonredundant roles of RCN1-containing PP2A in each process. Immunoblotting experiments yield no evidence for exclusive expression patterns among the A subunits, suggesting extensive overlap in the expression domains of the three isoforms. Together, these data suggest that the RCN1 A subunit performs a cardinal role in regulation of phosphatase activity and that PP2AA2 and PP2AA3 functions are unmasked only when RCN1 is absent. Protein expression analysis for each mutant line clearly shows that expression patterns of the remaining A subunits are unchanged by loss of one isoform, arguing against a model of compensatory upregulation.
Although a comparable mutational analysis has not been undertaken in other species, our results present intriguing parallels with a recent study of the mammalian Aα and Aβ isoforms (Zhou et al., 2003b). Direct comparison of in vivo subunit interactions demonstrated striking differences in the B and C subunit binding characteristics of the two isoforms, with Aβ showing lower binding of both C subunit isoforms and the Bα subunit. In addition, the authors observed considerable variability of Aβ expression levels in normal tissues and tumor cell lines, whereas Aα expression levels were uniformly high. These data are consistent with a model in which Aα plays a predominant role in PP2A regulation, with Aβ fulfilling more specialized functions (Zhou et al., 2003b). According to a similar model, the masking of PP2AA2 and PP2AA3 functions may indicate that RCN1 outcompetes PP2AA2 and PP2AA3 for binding of C and B subunits.
Analysis of PP2A complex formation has been complicated by questions of stoichiometry. Both A and C subunit expression levels are tightly regulated in mammalian cells (Wera et al., 1995; Baharians and Schönthal, 1998; D. Lizotte and A. DeLong, unpublished data). Immunodepletion studies indicate that mammalian cells contain a core AC heterodimeric form of PP2A in addition to the heterotrimeric enzyme population (Kremmer et al., 1997). Data from RNA-interference experiments suggest that decreased expression of the A or C subunit reduces accumulation of all PP2A subunits in cultured Drosophila melanogaster cells, arguing for coordinate regulation of A, B, and C subunit levels (Li et al., 2002; Silverstein et al., 2002). However, quantitative analysis of PP2A subunit levels in Saccharomyces cerevisiae shows that the A subunit is threefold to sevenfold less abundant than B and C subunits are, and accumulation of one subunit is not dependent on the presence of another (Gentry and Hallberg, 2002). It is unclear whether excess PP2A subunits are sequestered or complexed with other binding partners. Our data are not consistent with a coordinate regulation model because loss of A subunit expression in single mutants is not correlated with decreased C subunit expression, nor with upregulation of the remaining A subunits.
PP2A Roles in Morphogenesis and Reproduction
Combination of the rcn1 lesion with a pp2aa2 or pp2aa3 mutation reveals important roles of PP2A regulation in all phases of growth and development. The symmetry defects of double mutant seedlings demonstrate a role in embryogenesis, and genetic analysis suggests that PP2A works in concert with PIN1 and PID to establish a symmetrical dicot embryo. The seedling phenotypes of double mutants clearly indicate that normal root and hypocotyl elongation require A subunit function, and adult phenotypes show roles in rosette leaf growth, stem elongation, and reproductive development. The radial expansion phenotypes observed in cells and tissues of double mutant seedlings are consistent with the hypothesis that cortical microtubule arrays may be compromised, as they are in fass/ton2 B″ subunit mutants (Traas et al., 1995; Camilleri et al., 2002). However, fass/ton2 phenotypes are not identical to the A subunit double mutant phenotypes described here; the extreme morphological abnormalities of seedlings carrying strong fass/ton2 alleles distinguish ton2 from the double mutant lines we have generated (Torres-Ruiz and Jürgens, 1994).
The dwarfing phenotypes of the rcn1 pp2aa2 and rcn1 pp2aa3 double mutant plants also show some similarities to those of brassinosteroid signaling mutants (Kauschmann et al., 1996), but several features distinguish them. First, although hypocotyls of dark-grown seedlings are short, double mutant seedlings lack de-etiolation phenotypes, such as cotyledon opening and anthocyanin accumulation. Furthermore, the dramatic inhibition of root elongation observed in double mutant seedlings is not characteristic of brassinosteroid-insensitive or -deficient mutants. Finally, although brassinosteroid defects generally cause male sterility, the rcn1 pp2aa2 double mutant exhibits both male and female sterility.
The failure of anther dehiscence and the lack of stigmatic pollen receptivity in rcn1 pp2aa2 flowers suggest specific roles for PP2AA2 in reproductive development. Anthers of rcn1 pp2aa2 plants contain but do not shed viable pollen, indicating a requirement for PP2A function in achieving dehiscence. Because anther morphology is abnormal, the nondehiscent phenotype may be caused by a structural defect, but we have not ruled out other causes. Dehiscence requires proper regulation of cell wall biosynthesis and local turgor (Stadler et al., 1999; Lane et al., 2001), and a flower-specific MYB family transcription factor also is required, but the critical target genes for dehiscence are not yet known (Steiner-Lange et al., 2003). Jasmonic acid and ethylene signaling play roles in regulating the timing of anther dehiscence (Sanders et al., 2000; Ishiguro et al., 2001; Rieu et al., 2003). Our data also indicate a role for PP2A in development of stigmatic receptivity because rcn1 pp2aa2 mutant plants are infertile as pollen recipients in cross-pollinations. Interestingly, phosphatase inhibitor treatment of excised Brassica flowers reduces the receptivity of stigmas to untreated pollen; however, similar effects were not observed in Arabidopsis (Kandasamy et al., 1993; Rundle et al., 1993). The discrepancy between the results obtained with the different species was attributed to differing requirements for phosphorylation/dephosphorylation events during pollination in the two plants, however it is also possible that technical differences in the treatment protocols might contribute to the disparity. Our data do not distinguish between an early developmental role in establishment of receptivity and a later signaling role in the stigmatic response to pollination.
METHODS
Plant Growth
Seedlings were grown on sterile media in constant light or in the dark at 22°C as described previously (Garbers et al., 1996; Deruère et al., 1999) or in potting medium at 24°C with a 16-h-light/8-h-dark cycle. To improve the growth of double mutant plants in potting medium, rcn1 pp2aa2-1 and rcn1 pp2aa3-1 seedlings were allowed to grow on sterile medium for 4 weeks with a 16-h-light/8-h-dark cycle. Cantharidin sensitivity of dark-grown seedlings was assayed as described previously (Deruère et al., 1999). At the conclusion of organ elongation assays, each Petri dish was scanned on a flatbed scanner, and hypocotyl and root lengths were measured using NIH Image (National Institutes of Health, http://rsb.info.nih.gov/nih-image/). For cotyledon number assays, surface-sterilized and stratified seeds were gridded onto standard agar medium in Petri dishes and grown under constant light. Cotyledon number and morphology were scored 6 d after germination using a dissecting microscope. Each dish was scored by two people, and labels were coded to eliminate bias.
Molecular Biology Techniques
The SALK T-DNA lines (Alonso et al., 2003) carrying the A subunit mutations used in this study were SALK042724 (pp2aa2-1), SALK037095 (pp2aa2-2), SALK014113 (pp2aa3-1), and SALK099550 (pp2aa3-2), and all were obtained from the ABRC. Gene-specific PCR primers used for screening were oA2RAV (5′-GCGTGCGGTGTCTCTTCTTGCACC-3′), oA2new3′ (5′-CACATTAGTAGCAAGACAATGGACAAAACCCG-3′), oAUPS (5′-TCGTTGAGGAGAAAATTGAGCCGTG-3′), and oA2LYT (5′-CATATCATCCTGACAAAGCTGAGTATACAACG-3′) for PP2AA2, and oA3leader (5′-GATCGCTCGGAACTTGGAAAGCAGC-3′), oA3DDEV (5′-GCCAAAAGCACCTCATCGTCATCGTC-3′), oA3PAYA (5′-GGTGCCTGCATATGCTCGTCTACTTTG-3′), and oA3I8 (5′-CAATGTCGTACAAAGAGATGAGTAACTTGGTCA-3′) for PP2AA3. RCN1 primers were as described previously (Garbers et al., 1996), and primer LBb1 was as described on the T-DNA Express Web site (http://signal.salk.edu/tdna_FAQs.html). PCR screening employed a three-primer PCR strategy that identified wild-type, heterozygous, and homozygous individuals in a single step using two gene-specific primers and LBb1. T-DNA insertion sites were determined by DNA sequence analysis of PCR-amplified T-DNA junction fragments. DNA gel blotting was as described previously (DeLong et al., 1993), using probe fragments amplified from genomic DNA with the primers described above. The kanamycin-resistance (Km) probe used was a 1-kb BglII-XmaI fragment containing the transposon Tn5 Km gene. The maps predicted for single T-DNA insertions in Figure 1 were derived by pasting the pROK-2 T-DNA sequence (http://signal.salk.edu/tdna_FAQs.html) into the PP2AA2 and PP2AA3 genomic DNA sequences.
Immunoblotting and Phosphatase Activity Assays
Protein extraction, SDS-PAGE, and immunoblotting techniques were as described previously (Deruère et al., 1999), using a semidry transfer procedure onto PVDF (Millipore, Bedford, MA) membrane. The anti-C subunit antibody was monoclonal 1D6 (Upstate USA, Charlottesville, VA). Protein phosphatase activity was assayed in protein extracts isolated from dark-grown seedlings 5 d after germination, using the Protein Ser/Thr Phosphatase Assay System (New England Biolabs, Beverly, MA).
Microscopy
Seedlings and flowers were photographed using a SPOT camera on a Leica MZFLIII dissecting microscope (Wetzlar, Germany). Pollen staining followed the procedure of Alexander (1969). Root tips and stained anthers were photographed on a Nikon Microphot-FXA microscope (Tokyo, Japan).
Acknowledgments
We thank Elsbeth Walker, Jean Deruère, and John Sedivy for comments on the manuscript and Estelle Hrabak, Donna Lizotte, and Su-Yang Liu for helpful discussions. We thank Fred Jackson for expert greenhouse assistance. This work was supported by National Science Foundation Grant IBN 0135458 and the National Institutes of Health/National Center for Research Resources Grant 1 P20 RR15578-01.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Alison DeLong (alison_delong@brown.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.018994.
References
- Alexander, M.P. (1969). Differential staining of aborted and non-aborted pollen. Stain Technol. 44, 117–121. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657. [DOI] [PubMed] [Google Scholar]
- Baharians, Z., and Schönthal, A.H. (1998). Autoregulation of protein phosphatase type 2A expression. J. Biol. Chem. 273, 19019–19024. [DOI] [PubMed] [Google Scholar]
- Benfey, P.N., Linstead, P.J., Roberts, K., Schiefelbein, J.W., Hauser, M.T., and Aeschbacher, R.A. (1993). Root development in Arabidopsis: Four mutants with dramatically altered root morphogenesis. Development 119, 57–70. [DOI] [PubMed] [Google Scholar]
- Bennett, S.R.M., Alvarez, J., Bossinger, G., and Smythe, D. (1995). Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 8, 505–520. [Google Scholar]
- Calin, G.A., di Iasio, M.G., Caprini, E., Vorechovsky, I., Natali, P.G., Sozzi, G., Croce, C.M., Barbanti-Brodano, G., Russo, G., and Negrini, M. (2000). Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene 19, 1191–1195. [DOI] [PubMed] [Google Scholar]
- Camilleri, C., Azimzadeh, J., Pastuglia, M., Bellini, C., Grandjean, O., and Bouchez, D. (2002). The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 14, 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.K., Dagenais, N., Chory, J., and Weigel, D. (2000). Regulation of auxin response by the protein kinase PINOID. Cell 100, 469–478. [DOI] [PubMed] [Google Scholar]
- Corum III,, J.W., Hartung, A.J., Stamey, R.T., and Rundle, S.J. (1996). Characterization of DNA sequences encoding a novel isoform of the 55 kDa B regulatory subunit of the type 2A protein serine/threonine phosphatase of Arabidopsis thaliana. Plant Mol. Biol. 31, 419–427. [DOI] [PubMed] [Google Scholar]
- DeLong, A., Calderon-Urrea, A., and Dellaporta, S.L. (1993). Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol dehydrogenase required for stage-specific floral organ abortion. Cell 74, 757–768. [DOI] [PubMed] [Google Scholar]
- DeLong, A., Mockaitis, K., and Christensen, S. (2002). Protein phosphorylation in the delivery of and response to auxin signals. Plant Mol. Biol. 49, 285–303. [PubMed] [Google Scholar]
- Deruère, J., Jackson, K., Garbers, C., Söll, D., and DeLong, A. (1999). The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J. 20, 389–399. [DOI] [PubMed] [Google Scholar]
- Fisher, R.H., Barton, M.K., Cohen, J.D., and Cooke, T.J. (1996). Hormonal studies of fass, an Arabidopsis mutant that is altered in organ elongation. Plant Physiol. 110, 1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers, C., DeLong, A., Deruére, J., Bernasconi, P., and Söll, D. (1996). A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 15, 2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Gentry, M.S., and Hallberg, R.L. (2002). Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol. Biol. Cell 13, 3477–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves, M.R., Hanlon, N., Turowski, P., Hemmings, B.A., and Barford, D. (1999). The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell 96, 99–110. [DOI] [PubMed] [Google Scholar]
- Haynes, J.G., Hartung, A.J., Hendershot iii, J.D., Passingham, R.S., and Rundle, S.J. (1999). Molecular characterization of the B' regulatory subunit gene family of Arabidopsis protein phosphatase 2A. Eur. J. Biochem. 260, 127–136. [DOI] [PubMed] [Google Scholar]
- Ishiguro, S., Kawai-Oda, A., Ueda, J., Nishida, I., and Okada, K. (2001). The DEFECTIVE IN ANTHER DEHISCENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13, 2191–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens, V., and Goris, J. (2001). Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353, 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamibayashi, C., Lickteig, R.L., Estes, R., Walter, G., and Mumby, M.C. (1992). Expression of the A subunit of protein phosphatase 2A and characterization of its interactions with the catalytic and regulatory subunits. J. Biol. Chem. 267, 21864–21872. [PubMed] [Google Scholar]
- Kandasamy, M.K., Thorsness, M.K., Rundle, S.J., Goldberg, M.L., Nasrallah, J.B., and Nasrallah, M.E. (1993). Ablation of papillar cell function in Brassica flowers results in the loss of stigma receptivity to pollination. Plant Cell 5, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauschmann, A., Jessop, A., Koncz, C., Szekeres, M., Willmitzer, L., and Altmann, T. (1996). Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 9, 701–713. [Google Scholar]
- Kremmer, E., Ohst, K., Kiefer, J., Brewis, N., and Walter, G. (1997). Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: Abundant expression of both forms in cells. Mol. Cell. Biol. 17, 1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak, J.M., Moon, J.H., Murata, Y., Kuchitsu, K., Leonhardt, N., DeLong, A., and Schroeder, J.I. (2002). Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis. Plant Cell 14, 2849–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, D.R., et al. (2001). Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-beta-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol. 126, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, P.B., and Cancel, J.D. (2003). Enhanced ethylene responsiveness in the Arabidopsis eer1 mutant results from a loss-of-function mutation in the protein phosphatase 2A A regulatory subunit, RCN1. Plant J. 34, 709–718. [DOI] [PubMed] [Google Scholar]
- Larsen, P.B., and Chang, C. (2001). The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol. 125, 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Scuderi, A., Letsou, A., and Virshup, D.M. (2002). B56-associated protein phosphatase 2A is required for survival and protects from apoptosis in Drosophila melanogaster. Mol. Cell. Biol. 22, 3674–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., and Virshup, D.M. (2002). Two conserved domains in regulatory B subunits mediate binding to the A subunit of protein phosphatase 2A. Eur. J. Biochem. 269, 546–552. [DOI] [PubMed] [Google Scholar]
- Liu, C., Xu, Z., and Chua, N.H. (1993). Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday, G.K., and DeLong, A. (2001). Polar auxin transport: Controlling where and how much. Trends Plant Sci. 6, 535–542. [DOI] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, N.E., and Mumby, M.C. (2000). Effects of regulatory subunits on the kinetics of protein phosphatase 2A. Biochemistry 39, 11312–11318. [DOI] [PubMed] [Google Scholar]
- Rashotte, A.M., DeLong, A., and Muday, G.K. (2001). Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13, 1683–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu, I., Wolters-Arts, M., Derksen, J., Mariani, C., and Weterings, K. (2003). Ethylene regulates the timing of anther dehiscence in tobacco. Planta 217, 131–137. [DOI] [PubMed] [Google Scholar]
- Ruediger, R., Roeckel, D., Fait, J., Berqvist, A., Magnusson, F., and Walter, G. (1992). Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol. Cell. Biol. 12, 4872–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle, S.J., Nasrallah, M.E., and Nasrallah, J.B. (1993). Effects of inhibitors of protein serine/threonine phosphatases on pollination in Brassica. Plant Physiol. 103, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, P.M., Lee, P.Y., Biesgen, C., Boone, J.D., Beals, T.P., Weiler, E.W., and Goldberg, R.B. (2000). The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12, 1041–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein, A.M., Barrow, C.A., Davis, A.J., and Mumby, M.C. (2002). Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc. Natl. Acad. Sci. USA 99, 4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabas, A.R., Fordham-Skelton, A.P., Fletcher, D., Martinez-Rivas, J.M., Swinhoe, R., Croy, R.R.D., and Evans, I.M. (1994). Characterisation of cDNA and genomic clones encoding homologues of the 65 kDa regulatory subunit of protein phosphatase 2A in Arabidopsis thaliana. Plant Mol. Biol. 26, 1125–1138. [DOI] [PubMed] [Google Scholar]
- Stadler, R., Truernit, E., Gahrtz, M., and Sauer, N. (1999). The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis. Plant J. 19, 269–278. [DOI] [PubMed] [Google Scholar]
- Steiner-Lange, S., Unte, U.S., Eckstein, L., Yang, C., Wilson, Z.A., Schmelzer, E., Dekker, K., and Saedler, H. (2003). Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J. 34, 519–528. [DOI] [PubMed] [Google Scholar]
- Terol, J., Bargues, M., Carrasco, P., Perez-Alonso, M., and Paricio, N. (2002). Molecular characterization and evolution of the protein phosphatase 2A B′ regulatory subunit family in plants. Plant Physiol. 129, 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore, C.U., Livengood, A.J., Hendershot, J.D.I., Corum, J.W., Latorre, K.A., and Rundle, S.J. (1999). Characterization of the promoter region and expression pattern of three Arabidopsis protein phosphatase type 2A subunit genes. Plant Sci. 147, 165–176. [Google Scholar]
- Torres-Ruiz, R.A., and Jürgens, G. (1994). Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development 120, 2967–2978. [DOI] [PubMed] [Google Scholar]
- Traas, J., Bellini, C., Nacry, P., Kronenberger, J., Bouchez, D., and Caboche, M. (1995). Normal differentiation patterns in plants lacking microtubular preprophase bands. Nature 375, 676–677. [Google Scholar]
- Turowski, P., Favre, B., Campbell, K.S., Lamb, N.J., and Hemmings, B.A. (1997). Modulation of the enzymatic properties of protein phosphatase 2A catalytic subunit by the recombinant 65-kDa regulatory subunit PR65 alpha. Eur. J. Biochem. 248, 200–208. [DOI] [PubMed] [Google Scholar]
- Vernoux, T., Kronenberger, J., Grandjean, O., Laufs, P., and Traas, J. (2000). PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127, 5157–5165. [DOI] [PubMed] [Google Scholar]
- Wang, S.S., Esplin, E.D., Li, J.L., Huang, L., Gazdar, A., Minna, J., and Evans, G.A. (1998). Alterations of the PPP2R1B gene in human lung and colon cancer. Science 282, 284–287. [DOI] [PubMed] [Google Scholar]
- Wera, S., Fernandez, A., Lamb, N.J., Turowski, P., Hemmings-Mieszczak, M., Mayer-Jaekel, R.E., and Hemmings, B.A. (1995). Deregulation of translational control of the 65-kDa regulatory subunit (PR65 alpha) of protein phosphatase 2A leads to multinucleated cells. J. Biol. Chem. 270, 21374–21381. [DOI] [PubMed] [Google Scholar]
- Yu, S., Lei, H., Chang, W., Söll, D., and Hong, G. (2001). Protein phosphatase 2A: Identification in Oryza sativa of the gene encoding the regulatory A subunit. Plant Mol. Biol. 45, 107–112. [DOI] [PubMed] [Google Scholar]
- Zhou, J., Pham, H.T., Ruediger, R., and Walter, G. (2003. b). Characterization of the Aalpha and Abeta subunit isoforms of protein phosphatase 2A: Differences in expression, subunit interaction, and evolution. Biochem. J. 369, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J., Pham, H.T., and Walter, G. (2003. a). The formation and activity of PP2A holoenzymes do not depend on the isoform of the catalytic subunit. J. Biol. Chem. 278, 8617–8622. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., et al. (2003). Identification of Arabidopsis rat mutants. Plant Physiol. 132, 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]