Abstract
Annually thousands of sudden deaths involving young individuals (< 35 years of age) remain unexplained following a complete medicolegal investigation that includes an autopsy. In fact, epidemiological studies have estimated that over half of sudden deaths involving previously healthy young individuals have no morphological abnormalities identifiable at autopsy. Cardiac channelopathies associated with structurally normal hearts such as long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), and Brugada syndrome (BrS), leave no evidence to be found at autopsy, leaving investigators to only speculate that a lethal arrhythmia might lie at the heart of a sudden unexplained death (SUD). In cases of autopsy-negative SUD, continued investigation, through the use of a cardiological and genetic evaluation of first- or second-degree relatives and/or a molecular autopsy, may pinpoint the underlying mechanism attributing to the sudden death and allow for the identification of living family members with the pathogenic substrate that renders them vulnerable to an increased risk for cardiac events, including sudden death.
Keywords: Molecular autopsy, Sudden death, Channelopathies, Long QT syndrome, Genetic testing
Tragically, thousands of individuals younger than 35 years of age die suddenly each year. While many of these deaths are attributed to sudden cardiac death due to an underlying structural pathology evident at autopsy, a significant number remain unexplained and are termed sudden unexplained death (SUD). In this review, we will explore the epidemiology of sudden cardiac death in the young, the relationship between cardiac channelopathies and SUD, and the indispensible steps of a clinical assessment of surviving relatives and the molecular autopsy of the decedent in the evaluation of SUD. Finally, we will examine some of the current recommendations in the evaluation of sudden death in the young as well as provide some indications for a molecular autopsy.
Epidemiology of sudden cardiac death in the young—how common is sudden unexplained death?
It is estimated that 300,000–400,000 individuals die suddenly each year in the United States, with the vast majority being the elderly [40]. In comparison, sudden death in the young is uncommon, with an incidence between 1.3 and 8.5 per 100,000 patient-years [22]. However, annually, thousands of young individuals (<35 years of age) die suddenly. Fortunately, the cause and manner of death can often be identified through postmortem investigations that include an autopsy.
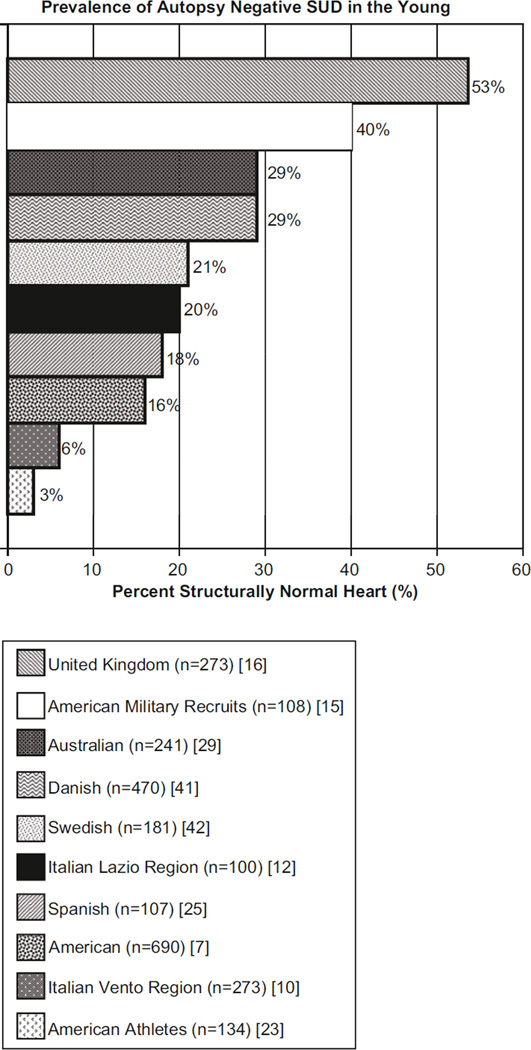
An autopsy may reveal noncardiac forms of death, such as pulmonary embolism, asthma, or epilepsy; however, the most common cause of sudden death is sudden cardiac death (SCD). SCD has been defined by the American Heart Association as the sudden, abrupt loss of heart function in a person who may or may not have been diagnosed with heart disease whereby the time and mode of death are unexpected, and the death occurs either instantly or shortly after symptoms appear [1]. Many SCD cases have structural cardiac abnormalities such as hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), congenital coronary artery anomalies, or myocarditis, which are typically identifiable through autopsy. However, it is estimated that at least 3 % and up to 53 % of cases of youthful (age 1–35 years) sudden deaths have no morphologic abnormalities that are identified through autopsy (Fig. 1, [7,10,12,15, 16, 23, 25, 29, 41, 42]). These cases are referred to as autopsy-negative sudden unexplained death (SUD).
Fig. 1.
The prevalence of autopsy-negative “structurally normal” heart sudden unexplained death in retrospective autopsy series. It is estimated that at least 3 % and perhaps as much as 53 % of sudden deaths involving previously healthy children, adolescents, and young adults have no identifiable morphologic abnormalities found at autopsy (“structurally normal heart”), and the SCD is labeled as autopsy-negative sudden unexplained death (SUD). Shown is a bar graph with the percent of autopsy-negative “structurally normal heart” identified during ten retrospective analyses of large population-based autopsy cohorts [33]
In a 1996 study by Maron and colleagues, HCM was the most common cause of SCD involving young competitive athletes, where 48/134 (36 %) of SCDs were ascribed to HCM and an additional 10 % had “possible HCM.” Only 3 % of cases were deemed to be autopsy-negative SUD [23]. In a study by Corrado and colleagues, only 6 % of their 273 cases of SCD in young people (≤ 35 years of age) in the Veneto region of northeastern Italy were considered autopsy-negative SUD following detailed histological examination of the 28 % of cases that had macroscopically normal hearts. Histological examination revealed concealed pathologic substrates including myocarditis, regional arrhythmogenic right ventricular cardiomyopathy, and conduction system abnormalities [10].
In 2005, Puranik and colleagues examined a population-based cohort of 427 young sudden unexpected death cases (5–35 years old) in eastern Sydney, Australia. SCD was identified in over half of these cases, and contrary to the previous studies, autopsy-negative SUD (29 %) was the leading cause of SCD [29]. Eckart and colleagues examined 6.3 million men and women, American military recruits, aged 18–35 years over a 25-year period. The sudden nontraumatic death rate was 13 per 100,000 recruit years. Approximately half of these deaths could be attributed to an identifiable cardiac abnormality during autopsy, but 35 % of these sudden deaths were autopsy-negative SUD. Several of the cases had a previous family history of sudden death suggesting a potential heritable lethal arrhythmia [15]. Morentin and colleagues analyzed all sudden, non-violent deaths in persons 1–35 years of age occurring in northern Spain from 1991 to 1998. Among the 107 cases of sudden death, 18 % were considered SUD. Interestingly, antecedent symptoms consistent with cardiac arrhythmia manifestations were evident in five SUD cases [25]. Fabre and colleagues reported that over 50 % of their cohort of 453 United Kingdom sudden death cases, 15–81 years of age, had normal hearts both macroscopically and microscopically. Among cases aged 15–35 years, 53.5 % had normal hearts [16]. In a Swedish study involving 15- to 35-year-old subjects with unexplained death, 21 % had a normal heart [42]. An American study involving an autopsy series of 14- to 40–year-olds, found 16 % with structurally normal hearts [7]. In 2011, Winkel and colleagues performed a nationwide study of SCD in young Danish individuals aged 1–35 years and determined that 29 % were autopsy-negative SUD [41] (Fig. 1).
The discordance in “structurally normal heart” rates observed in these epidemiology studies is most likely attributed to the extensive variation and lack of standardization in the process in which the forensic pathologists/medical examiners/ coroners, responsible for defining the exact cause of sudden death, approach this increasingly complex task. This heterogeneity makes interpretation of epidemiological data on sudden death difficult. In 2008, the Association for European Cardiovascular Pathology described a minimum autopsy standard required for the assessment of SCD including protocols on heart examination, toxicology, histological sampling as well as molecular investigations [3].
Although the majority of SCDs can be attributed to structural abnormalities, there are an alarming number of sudden deaths in the young that remain unexplained after autopsy and postmortem investigation.
Cardiac channelopathies and autopsy-negative sudden unexplained death
Potentially lethal and heritable channelopathy disorders such as long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), and Brugada syndrome (BrS) may explain a significant number of these autopsy-negative SUD cases. These disorders lead to electrical disturbances in structurally normal hearts and have the capability of instigating lethal cardiac arrhythmias. The electrical abnormalities are often benign, but can quickly spiral out of control in an unsuspecting individual leading to a sudden, early death in an otherwise healthy young individual. Over the past 15 years, through advances in genetic screening, the underlying genetic basis responsible for many inherited cardiac arrhythmia disorders has been discovered. To date, several studies involving either the cardiological assessment of surviving first-degree relatives or the postmortem genetic analysis (the cardiac channel “molecular autopsy”) of the decedent’s DNA have implicated LQTS, CPVT, and BrS as a clinical/molecular basis for as much as one-third of autopsy-negative SUD [33].
Clinical assessment of those left behind
In 2003, Behr and colleagues completed cardiovascular evaluations of 109 first-degree relatives of 32 SUD victims. This study identified that 22 % of the families had evidence suggesting an inherited cardiac disease with the majority having clinical sequelae suggestive of LQTS [6]. In 2005, Tan and colleagues found that 28 % of families had an identifiable cardiac channelopathy [32]. In a 2008 follow-up study by Behr and colleagues, a diagnosis of heritable heart disease was determined in 53 % of first-degree relatives of SUD victims following a more comprehensive clinical evaluation, with 70 % being diagnosed with either LQTS (53 %) or BrS (17 %). Interestingly, 30 % of the families reported a family history of additional unexplained sudden deaths under the age of 45 years, and 20 % of the decedents had a prior history of syncope [5].
In 2010, Van der Werf and colleagues examined a cohort of surviving relatives of 140 SUD cases aged 1–50 years and identified a certain or probable diagnosis in 33 % following a cardiological clinical assessment, with 96 % of the families diagnosed with an inherited cardiac diseases (21 % LQTS, 17 % CPVT, 15 % BrS, and 15 % ARVC). The diagnostic yield depended significantly on the age of the decedent ranging from a high of 70 % when the decedent was aged 1–10 years to a low of 21 % when the decedent was between the ages of 41 and 49 years. While there was no prior clinical diagnosis for the decedent or any family member, many of these sudden death victims had warning signs prior to their lethal event, such as previous personal syncope in 15 % or a family history of young sudden death in 29% [39].
Incomplete penetrance and variable expressivity are challenging clinical features of the various cardiac channelopathies that consequently lead to “concealed” forms of these disorders [28]. Therefore, clinical assessment of surviving family members of SUD victims may not adequately detect LQTS, CPVT, or BrS in some unsuspecting individuals. A molecular autopsy involving postmortem cardiac channel genetic testing may provide the much needed utility for the forensic evaluation of an SUD case.
Molecular autopsy series of sudden unexplained death in the young
Owing to the increasing suspicion that autopsy-negative SUD cases may indeed be caused by lethal arrhythmias, investigators have sought to determine the spectrum and prevalence of mutations in ion channels that have been attributed to LQTS, CPVT and BrS in autopsy-negative SUD cases. To date, there have been ten molecular autopsy studies (Tab. 1) [9,11,13,14,17, 26, 31, 34, 36, 37] examining the major LQTS-, BrS-, and CPVT-susceptibility genes.
Tab. 1.
Summary of molecular autopsy series in autopsy-negative sudden unexplained death (arranged from the smallest to the largest case series published)
| Reference | Year pub lished |
Number of cases |
Age range (years) |
Males/ females |
Genes ana lyzed |
Number of mutations (% yield) |
DNA source |
Mutation analysis technique |
|---|---|---|---|---|---|---|---|---|
| Creighton [11] | 2008 | 9 | 1–43 | 5M/4F | KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, RYR2 (target) | 1 KCNQ1,2RYR2(33%) | Frozen tissue | Direct DNA sequencing |
| Di Paolo [13] | 2004 | 10 | 13–29 | 5M/5F | LQTS genes | 2KCNQ1 (20%) | FF-PET | SSCP |
| Chug [9] | 2004 | 12 | N/A | N/A | KCNQI, KCNH2, SCN5A, KCNE1, KCNE2 | 2KCNH2(17%) | FF-PET | SSCP/direct DNA sequencing |
| Nishio [26] | 2009 | 17 | 12–42 | 13M/4F | KCNQI, KCNH2, SCN5A, RYR2 (target) | 1 KCNQ1,3RYR2(24%) | Autopsy blood | High-resolution melt |
| Gladding [17] | 2010 | 18 | 2–39 | 11 M/7 F | KCNQI, KCNH2, SCN5A, KCNE1, KCNE2 | 2KCNQ1,2KCNH2(22%) | Guthrie (blood spot) card | DHPLC |
| Skinner [31] | 2011 | 33 | 1–40 | 24M/9F | KCNQI, KCNH2, SCN5A, KCNE1, KCNE2 | 1 KCNQ1,1 KCNH2, 1 SCN5A, 2KCNE1(15%) | Frozen tissue or autopsy blood | DHPLC |
| Doolan [14] | 2008 | 59 | 1–35 | 38M/21 F | KCNQI, SCN5A (target) | 0(0%) | FF-PET | DHPLC |
| Tester [34,36,37] | 2004, 2007, 2011 | 173 | 1–43 | 106M/67F | KCNQI, KCNH2, SCN5A, KCNE1, KCNE2, RYR2 (target) | 11 KCNQ1, 6KCNH2, 6 SCN5A, 2KCNE2, 20RYR2 (26%) | Frozen tissue or autopsy blood | DHPLC |
N/A not available, M male, F female, FF-PET formalin-fixed paraffin-embedded tissue, SSCP single-stranded confirmation polymorphism, DHPLC denaturing high-performance liguid chromatography
In 2004, Chugh and colleagues identified 12 cases of SUD following a comprehensive postmortem analysis of a consecutive series of 270 adult (age ≥ 20 years) cases of SCD occurring over a 13-year period. Postmortem genetic analysis of the LQTS-susceptibility genes revealed the identical KCNH2 mutation in two of 12 (17 %) cases of autopsy-negative SUD [9]. Similarly, Di Paolo and colleagues performed LQTS postmortem genetic testing on ten cases of juvenile (ages 13–29 years) SUD and identified KCNQ1 mutations in two individuals [13]. In 2006, Creighton and colleagues performed a study on nine cases of autopsy-negative SUD and identified a 33 % yield of putative pathogenic channelopathy mutations [11]. In 2009, Nishio identified channel mutations in 24 % of their 17 SUD case cohort [26], and in 2010, Gladding, using DNA isolated from Guthrie (newborn blood spot) cards, identified LQTS-associated mutations in four of 18 (22 %) cases of SUD, aged 2–39 years [17]. In 2011, Skinner and colleagues examined 33 cases, and identified putative pathogenic mutations in 15 % of the cases [31]. Although these studies are small, a trend begins to emerge that 15–33 % of autopsy-negative SUD may be due to lethal arrhythmias.
Doolan and colleagues performed a molecular autopsy study on 59 cases of SUD and did not identify any mutations following a limited mutational analysis of only KCNQ1 and a targeted analysis of SCN5A using genomic DNA derived from formalin-fixed paraffin-embedded tissue (FF-PET) [14]. The authors concluded that the “hit-rate” of a molecular autopsy in young SUD is low. However, because of their limited mutational analysis and the use of a largely unreliable source (FF-PET) of high-quality DNA, it is not too surprising that their mutation detection yield was low. Important to note, in order to successfully perform postmortem genetic testing, coroners, medical examiners, and forensic pathologist must secure “DNA-friendly” blood or tissue samples at autopsy.
In 2007, we completed a cardiac channel molecular autopsy on 49 cases of autopsy-negative SUD [34, 37]. Since then, we have extended this cohort to now include 173 cases of SUD to provide a more extensive analysis to better define the expected yield of mutation detection and offer possible genotype/phenotype correlations that may assist in guiding phenotype-directed mutation detection efforts in future cases of SUD. In this expanded molecular analysis, 26 % of the 173 SUD cases had a putative pathogenic mutation, with 14.5 % having mutations in the LQTS-associated genes and 11.5 % with mutations in the CPVT-associated RYR2 gene. Sudden death was the sentinel event in 67 % of the mutation-positive SUD cases in this series. Tragically, however, despite no premortem diagnosis of a suspected cardiac channelopathy in the decedent or family member, there was either a personal or family history of cardiac events indicative of an underlying disorder in nearly 60 % of the mutation-positive SUD cases that went unheeded prior to the unfortunate early demise of the decedent [36].
Together, these studies provide clinical and molecular evidence suggesting that a significant portion of autopsy-negative SUD indeed stems from an underlying cardiac channelopathy. Continued investigation through cardiological assessment and genetic evaluation of first-degree relatives and a molecular autopsy may be beneficial in elucidating the cause of the unexplained death. Because these potentially lethal arrhythmia syndromes are often familial inherited, determining the exact underlying basis for the SUD can have a great impact on the surveillance and treatment of surviving relatives.
Evaluation of sudden unexplained death in the young
Currently, although there is consensus of the necessity to perform thorough evaluations on SUD in the young, there is a general lack of standardization of this process. However, several societies have published specific guidelines for autopsy investigation of sudden unexpected death in the young. In 2008, Basso and colleagues, on behalf of the Association for European Cardiovascular Pathology, recommended strongly for postmortem genetic analysis in both structural and non-structural genetically determined heart disease, to fulfill the requirements for an adequate postmortem assessment of SCD [3]. The Trans-Tasman Response Against Sudden Death in the Young (TRAGADY), endorsed by the Royal College of Pathologists of Australasia and the National Heart Foundation of New Zealand, have proposed guidelines to standardize autopsy practice in young sudden unexpected deaths, ancillary testing, and procurement of materials for postmortem genetic testing (Tab. 2). TRAGADY emphasizes the importance of skilled postmortem autopsies, especially because there are instances when families will suffer the tragic loss of multiple family members owing to insufficient investigations [38].
Tab. 2.
Key principles of postmortem investigations of sudden unexpected death in the young (adapted from “Post-mortem in sudden unexpected death in the young: guidelines on autopsy practice,”devised by TRAGADY—Trans-Tasman Response Against Sudden Death in the Young—and endorsed bythe Royal College of Pathologists of Australasia [38])
| 1. All cases of sudden unexpected or unexplained death in the young (age group of 0–40 years) should have an autopsy |
| 2. A full postmortem examination should be completed |
3. The investigation, ideally lead by a pathologist, should involve a team approach
|
4. A detailed antecedent clinical history must be obtained
|
5. A detailed and relevant family history must be obtained
|
| 6. Skilled macroscopic and microscopic examination of the organs is required, particularly of the heart (especially right ventricular muscle) and the brain. This may require some specimens to be examined by other specialists |
| 7. Adequate histological material be obtained for review or, if necessary, referral |
| 8. Tissue or blood suitable for DNA extraction must be obtained |
| 9. Results, including photography, must be documented clearly |
| 10. Results must be described and annotated in a standard fashion which will allow epidemiological data gathering |
In 2011, the Hearth Rhythm Society and the European Heart Rhythm Association (HRS/EHRA) gave a consensus statement regarding genetic testing, providing their expert consensus recommendation for postmortem genetic testing in SUD: “In the setting of autopsy-negative SUD, comprehensive or targeted ion channel genetic testing may be considered in an attempt to establish probable cause and manner of death and to facilitate the identification of potentially at-risk relatives and is recommended if circumstantial evidence points toward a clinical diagnosis of LQTS or CPVT specifically.” HRS/EHRA also recommends that for all SUD cases, “DNA-friendly samples” should be acquired to enable subsequent genetic testing. Finally, HRS/EHRA recommends mutation-specific genetic testing for family members following the identification of an SUD-causative mutation in the decedent [2].
The Canadian Cardiovascular Society and Canadian Heart Rhythm Society gave a similar recommendation, including procurement and storage of tissue and/or DNA after SCD with negative autopsy findings for potential future genetic testing. In the case of an autopsy-negative SCD, “genetic testing of retained tissue is recommended only when there is evidence of a clinical phenotype in family members.” If a clinical history of events or clinical evaluation of family members is suggestive of a lethal arrhythmia, genetic screening on the proband and all identified affected family members was recommended [18].
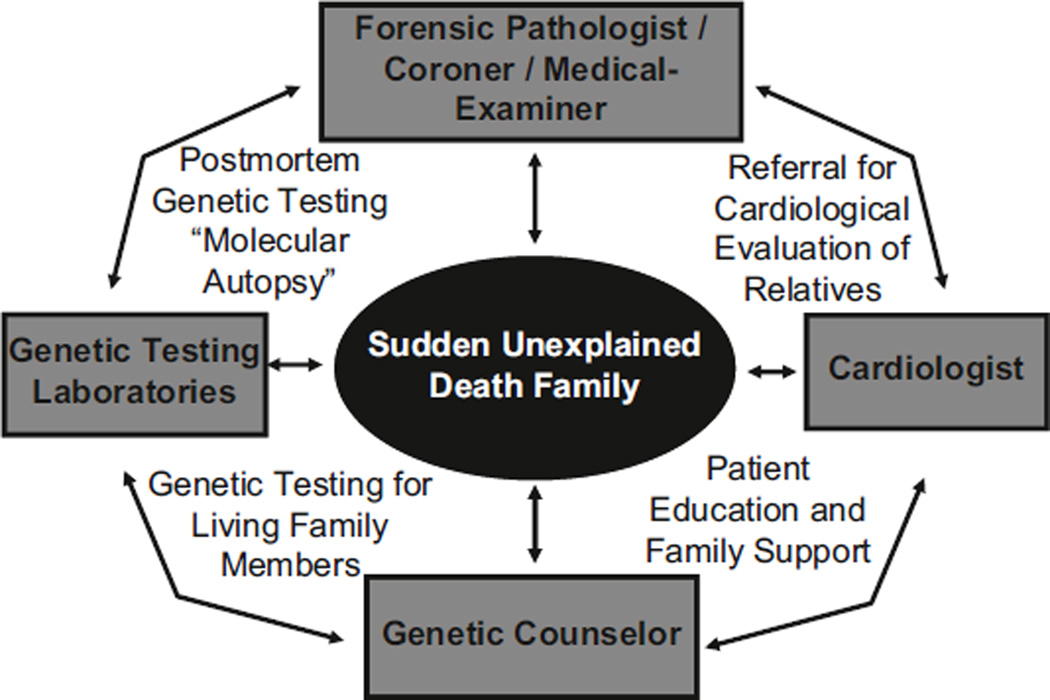
When an SUD case is evaluated, an interdisciplinary collaboration between a pathologist/medical examiner/coroner, cardiologist, and colleagues with experience in genetic counseling is necessary [20, 24, 30]. Hopefully, such a multidisciplinary approach will limit misinterpretation of pathology findings, genetic test results, and borderline cardiac clinical test results [31]. Partnering with a genetic counselor will be beneficial in gathering appropriate family history, counseling the family about clinical cardiological assessments, genetic testing in relatives, as well as completion of a molecular autopsy in the decedent (Fig. 2). The genetic counselor will also be helpful in interpreting the genetic testing results and discussing the modes of inheritance and risk-stratification within the families. Finally, the genetic counselor can help living relatives of SUD cases discuss some of the psychosocial impacts of such information [8,19,21].
Fig 2.
Multidisciplinary approach to the evaluation of sudden unexplained death (SUD). The evaluation of SUD should be an interdisciplinary collaboration between an expert pathologist/medical examiner/coroner, cardiologist, and colleagues equipped with expertise in genetic counseling, ideally a cardiogenetic counselor
Despite the previously published guidelines, there is not a clear consensus statement pertaining to the extent and breadth of the clinical evaluation of the living relatives to an SUD victim. Based on the previously described studies, it seems reasonable to advise first-degree relatives of the decedent to undergo cardiovascular evaluation, which would include (at minimum) an extensive personal and family history, a physical examination, a 12-lead electrocardiogram, treadmill stress test, and an echocardiogram. Alternatively, or simultaneously, a molecular autopsy (genetic testing) on the major genes associated with LQTS, CPVT, and BrS should be considered as standard of care in the evaluation of an SUD case, especially when the victim is less than 40 years of age.
Indications for molecular autopsy
Many of the published guidelines have recommended postmortem cardiac channel genetic testing, specifically in cases where there is evidence to suggest that a cardiac channelopathy may be responsible for the sudden death. Unfortunately, given the expensive and time-consuming nature of postmortem genetic testing, it is currently necessary for the medical examiner, coroner, or forensic pathologist to be case selective in pursuing a molecular autopsy [27].
Through our recent molecular autopsy investigations of SUD cases, we have garnered some interesting correlations that may help in selecting cases with the greatest potential for mutation discovery and directing genetic testing efforts [35]. For example, females (39 % yield overall) were significantly (p < 0.005) more likely to have a mutation than males (18 % yield overall), especially if the death was during adolescence (48 % yield females vs. 18 % in males, aged 11–20 years). For those cases that were mutation positive, females were more likely to have mutations in LQTS-susceptibility genes while males more often had mutations in the CPVT-associated RYR2 gene. Decedents whose death was associated with exercise had a greater detection rate (35 % overall, 50 % in females, 27 % in males) than those with a nonspecific trigger/circumstance (27 % overall, 36 % in females, 19 % in males), and those who died during a period of sleep (19 % overall, 32 % in females, 13 % in males) [35, 36].
Interestingly, for those decedents who were aged 1–10 years with an exercise-associated death, the mutation detection yield was 71 % for females and 60 % for males, with mutations usually associated with CPVT1 or LQT1. However, the yield dropped significantly to about 15 % for both male and female decedents aged 11–20 years with an exercise-associated death. Conversely, those aged 11–20 years that died during sleep had a much higher yield (75 % in females and 18 % in males) than when the sleep-associated death occurred in a decedent aged 1–10 years (0 % in females and 6 % in males). Decedents with a positive personal or family history of cardiac events had a significantly greater mutation detection yield (40 %) than those with no history (19 %) of events. Mutations were identified in 45 % of SUD cases with a family history of a prior sudden death [35, 36].
Thus, one might a priori expect a higher yield of LQTS-associated mutation detection in an adolescent or young adult female compared to a higher expected yield of CPVT-associated mutations among male children. Because young male and female children with exercise-associated death and adolescent females with death during a period of sleep have the highest mutation detection rate ranging from 60 to 75 %, these types of SUD cases should undergo molecular autopsy [35, 36]. Understanding the effect of sex, age, death circumstance, and/or personal or family history of cardiac events, on the overall mutation detection yield, may assist in guiding both the clinical assessment of surviving relatives and the molecular autopsy for cases of SUD, thereby creating a more cost-effective approach to the evaluation of SUD.
Biological material used in a molecular autopsy
Owing to its ease of storage and transportation, archived FF-PET is the only source of DNA that is typically collected at autopsy. Unfortunately, DNA from FF-PET is often error prone and may be unreliable for comprehensive genetic testing. The best samples to procure high-quality DNA for genetic testing include at least 5–10 ml of autopsy blood collected in EDTA tubes, and/or 5 g of fresh heart, liver, or spleen tissue [4]. These materials should be stored at − 80 °C until DNA can be extracted. If available, 50–100 µl of whole blood on filter paper can also be utilized to extract DNA. However, this tends to provide a very small amount of DNA; therefore, while it is a viable option, it is suboptimal because of the limited amount of genetic analysis that can be performed [33]. It is very important that guidelines for the procurement of tissue suitable for DNA extraction and analysis be implemented in the standard of care for the postmortem analysis of SUD.
Conclusion
The combination of a clinical cardiological assessment of surviving relatives and a molecular autopsy of the decedent are indispensible steps for the accurate diagnosis of an SUD. Such a combined, multidisciplinary approach should enable informed genetic counseling for families and should direct the commencement of appropriate preemptive strategies targeted toward averting another tragedy among those left behind. Because autopsy-negative SUD accounts for such a significant number of sudden deaths in the young and considering that cardiac channelopathies contribute to a large portion of these deaths, clinical cardiological assessment of surviving family members and a cardiac channel molecular autopsy should be viewed as the new standard of care for the postmortem evaluation of SUD.
Footnotes
Conflict of interest. On behalf of all authors, the corresponding author states the following: M.J.A. is a consultant for Biotronik, Boston Scientific, Medtronic, St. Jude Medical, Inc., and Transgenomic. Intellectual property derived from M.J.A.’s research program resulted in license agreements in 2004 between Mayo Clinic Health Solutions (formerly Mayo Medical Ventures) and PGxHealth (formerly Genaissance Pharmaceuticals, now recently acquired by Transgenomic). N.J.B. and D.J.T. have no conflicts to disclose.
References
- 1.Ackerman M. State of postmortem genetic testing known as the cardiac channel molecular autopsy in the forensic evaluation of unexplained sudden cardiac death in the young. Pacing Clinical Electrophysiol. 2009;32(Suppl 2):S86–S89. doi: 10.1111/j.1540-8159.2009.02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerman M, Priori S, Willems S, et al. HRS/ EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Burke M, Fornes P, et al. Guidelines for autopsy investigation of sudden cardiac death. Virchows Archiv. 2008;452:11–18. doi: 10.1007/s00428-007-0505-5. [DOI] [PubMed] [Google Scholar]
- 4.Basso C, Carturan E, Pilichou K, et al. Sudden cardiac death with normal heart: molecular autopsy. Cardiovasc Pathol. 2010;19:321–325. doi: 10.1016/j.carpath.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Behr E, Dalageorgou C, Christiansen M, et al. Sudden arrhythmic death syndrome: familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J. 2008;29:1670–1680. doi: 10.1093/eurheartj/ehn219. [DOI] [PubMed] [Google Scholar]
- 6.Behr E, Wood D, Wright M, et al. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet. 2003;362:1457. doi: 10.1016/s0140-6736(03)14692-2. [DOI] [PubMed] [Google Scholar]
- 7.Burke A, Farb A, Virmani R, et al. Sports-related and non-sports-related sudden cardiac death in young adults. Am Heart J. 1991;121:568–575. doi: 10.1016/0002-8703(91)90727-y. [DOI] [PubMed] [Google Scholar]
- 8.Charron P. Clinical genetics in cardiology. Heart. 2006;92:1172–1176. doi: 10.1136/hrt.2005.071308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chugh S, Senashova O, Watts A, et al. Postmortem molecular screening in unexplained sudden death. J Am Coll Cardiol. 2004;43:1625–1629. doi: 10.1016/j.jacc.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 10.Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. doi: 10.1016/s0008-6363(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 11.Creighton W, Virmani R, Kutys R, et al. Identification of novel missense mutations of cardiac ryanodine receptor gene in exercise-induced sudden death at autopsy. J Mol Diagn. 2006;8:62–67. doi: 10.2353/jmoldx.2006.050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Gioia C, Autore C, Romeo Dm, et al. Sudden cardiac death in younger adults: autopsy diagnosis as a tool for preventive medicine. Hum Pathol. 2006;37:794–801. doi: 10.1016/j.humpath.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Di Paolo M, Luchini D, Bloise R, et al. Postmortem molecular analysis in victims of sudden unexplained death. Am J Forensic Med Pathol. 2004;25:182–184. doi: 10.1097/01.paf.0000127406.20447.8a. [DOI] [PubMed] [Google Scholar]
- 14.Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110–112. doi: 10.5694/j.1326-5377.2004.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 15.Eckart R, Scoville S, Campbell C, et al. Sudden death in young adults: A 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 16.Fabre A, Sheppard M. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92:316–320. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladding P, Evans C, Crawford J, et al. Posthumous diagnosis of long QT syndrome from neonatal screening cards. Heart Rhythm. 2010;7:481–486. doi: 10.1016/j.hrthm.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Gollob M, Blier L, Brugada R, et al. Recommendations for the use of genetic testing in the clinical evaluation of inherited cardiac arrhythmias associated with sudden cardiac death: Canadian Cardiovascular Society/Canadian Heart Rhythm Society joint position paper. Can J Cardiol. 2011;27:232–245. doi: 10.1016/j.cjca.2010.12.078. [DOI] [PubMed] [Google Scholar]
- 19.Hershberger R, Cowan J, Morales A, et al. Progress with genetic cardiomyopathies: screening, counseling, and testing in dilated, hypertrophic, and arrhythmogenic right ventricular dysplasia/cardiomyopathy Circ Heart Fail. 2009;2:253–261. doi: 10.1161/CIRCHEARTFAILURE.108.817346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingles J, Semsarian C. Sudden cardiac death in the young: a clinical genetic approach. Int Med J. 2007;37:32–37. doi: 10.1111/j.1445-5994.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 21.Ingles J, Yeates L, Semsarian C. The emerging role of the cardiac genetic counselor. Heart Rhythm. 2011;8:1958–1962. doi: 10.1016/j.hrthm.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Liberthson R. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 23.Maron B, Shirani J, Poliac L, et al. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 24.Michaud K, Fellmann F, Abriel H, et al. Molecular autopsy in sudden cardiac death and its implication for families: discussion of the practical, legal and ethical aspects of the multidisciplinary collaboration. Swiss Med Wkly. 2009;139:712–718. doi: 10.4414/smw.2009.12837. [DOI] [PubMed] [Google Scholar]
- 25.Morentin B, Suarez-Mier M, Aguilera B. Sudden unexplained death among persons 1–35 years old. Forensic Sci Int. 2003;135:213–217. doi: 10.1016/s0379-0738(03)00212-3. [DOI] [PubMed] [Google Scholar]
- 26.Nishio H, Iwata M, Suzuki K. Postmortem molecular screening for cardiac ryanodine receptor type 2 mutations in sudden unexplained death: R420W mutated case with characteristics of status thymico-lymphatics. Circ J. 2006;70:1402–1406. doi: 10.1253/circj.70.1402. [DOI] [PubMed] [Google Scholar]
- 27.Oliva A, Brugada R, D’aloja E, et al. State of the art in forensic investigation of sudden cardiac death. Am J Forensic Med Pathol. 2011;32:1–16. doi: 10.1097/PAF.0b013e3181c2dc96. [DOI] [PubMed] [Google Scholar]
- 28.Priori S, Napolitano C, Schwartz P. Low penetrance in the long-QT syndrome: clinical impact. Circulation. 1999;99:529–533. doi: 10.1161/01.cir.99.4.529. [DOI] [PubMed] [Google Scholar]
- 29.Puranik R, Chow C, Duflou J, et al. Sudden death in the young. Heart Rhythm. 2005;2:1277–1282. doi: 10.1016/j.hrthm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Semsarian C, Hamilton R. Key role of the molecular autopsy in sudden unexpected death. Heart Rhythm. 2012;9:145–150. doi: 10.1016/j.hrthm.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 31.Skinner J, Crawford J, Smith W, et al. Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm. 2011;8:412–419. doi: 10.1016/j.hrthm.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Tan H, Hofman N, Van Langen I, et al. Sudden unexplained death: heritability and diagnostic yield of cardiological and genetic examination in surviving relatives. Circulation. 2005;112:207–213. doi: 10.1161/CIRCULATIONAHA.104.522581. [DOI] [PubMed] [Google Scholar]
- 33.Tester D, Ackerman M. The molecular autopsy—should their evaluation continue after the fu-neral? Pediatr Cardiol. 2012;33:461–470. doi: 10.1007/s00246-012-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tester D, Ackerman M. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young, (see comment) J Am Coll Cardiol. 2007;49:240–246. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Tester D, Medeiros-Domingo A, Will M, et al. The cardiac channel molecular autopsy for structurally normal heart sudden unexplained death. Circulation. 2011;124:A17278. [Google Scholar]
- 36.Tester D, Medeiros-Domingo A, Will M, et al. Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc. 2012;87:524–539. doi: 10.1016/j.mayocp.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tester D, Spoon D, Valdivia H, et al. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner’s cases. Mayo Clin Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 38.Tragady. [Accessed June 18, 2012];Post-mortem in sudden unexpected death in the young: guidelines on autopsy practice Sydney: Royal College of Pathologists of Australasia. 2008 http:/www.rcpa.edu.au/applications/DocumentLibraryManager2/upload/SUDYbestpracticedocumentendorsedMay2708%20(2).pdf.
- 39.Van Der Werf C, Hofman N, Tan H, et al. Diagnostic yield in sudden unexplained death and aborted cardiac arrest in the young: the experience of a tertiary referral center in The Netherlands. Heart Rhythm. 2010;7:1383–1389. doi: 10.1016/j.hrthm.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 40.Virmani R, Burke A, Farb A. Sudden cardiac death. Cardiovasc Pathol. 2001;10:275–282. doi: 10.1016/s1054-8807(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 41.Winkel B, Holst A, Theilade J, et al. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J. 2011;32:983–990. doi: 10.1093/eurheartj/ehq428. [DOI] [PubMed] [Google Scholar]
- 42.Wisten A, Forsberg H, Krantz P, et al. Sudden cardiac death in 15–35-year olds in Sweden during 1992–1999. J Int Med. 2002;252:529–536. doi: 10.1046/j.1365-2796.2002.01038.x. [DOI] [PubMed] [Google Scholar]