Abstract
Objective
Loss of lean body mass (sarcopenia) is associated with increased morbidity and mortality in patients receiving chronic hemodialysis (CHD). Insulin resistance (IR), which is highly prevalent in patients receiving CHD, has been proposed to play a critical role in the development of sarcopenia. The aim of this study was to examine the effect of IR on amino acid metabolism in patients receiving CHD.
Design
This was a cross-sectional study.
Subjects
The study included 12 prevalent (i.e., patients that have been on dialysis for more than 90 days) African American patients receiving CHD.
Methods
IR was measured as glucose disposal rate (GDR) determined from hyperinsulinemic euglycemic clamp (HGEC) studies performed 3 consecutive times. Plasma amino acid (AA) concentrations were measured by real-time high-performance liquid chromatography (HPLC) throughout the clamp study. The primary outcome was percentage change in leucine concentrations during the clamp study. The main predictor was the GDR measured simultaneously during the HGEC studies. Mixed model analysis was used to account for repeated measures.
Results
All individual AA concentrations declined significantly in response to high-dose insulin administration (P < .001). There was a significant direct association between GDR by HECG studies and the percentage change in leucine concentration (P = .02). Although positive correlations were observed between GDR values and concentration changes from baseline for other AAs, these associations did not reach statistical significance.
Conclusions
Our results suggest that the severity of IR of carbohydrate metabolism is associated with a lesser decline in plasma leucine concentrations, suggesting a similar resistance to protein anabolism. Insulin resistance represents a potential mechanism for sarcopenia commonly observed in patients receiving CHD.
Introduction
Patients receiving chronic hemodialysis (CHD) experience multiple metabolic and nutritional derangements, collectively termed protein energy wasting (PEW). One of the central characteristics of PEW is loss of lean body mass, also termed sarcopenia, which associates strongly with increased hospitalization and mortality in this patient population.1-3 In addition to its robust association with clinical outcomes, the cause of PEW in patients receiving CHD is multifactorial, including but not limited to dialysis-related causes, adverse consequences of advanced kidney disease, and certain hormonal and metabolic derangements. Among the latter, the presence of diabetes mellitus has been identified as an independent predictor of loss of lean body mass in incident patients receiving CHD.4 Even lesser degrees of glucose intolerance in the absence of overt diabetes mellitus are associated with both increased whole-body and skeletal muscle protein breakdown4-6 in patients receiving CHD and young obese healthy women,7 respectively. This is relevant because insulin resistance (IR) is highly prevalent in patients with advanced chronic kidney disease (CKD) even without diabetes.8,9 Although the cause of IR in CKD is multifactorial, it has been recognized that abnormalities in insulin metabolism,10 as well as a post–insulin receptor defect primarily affecting skeletal muscle glucose uptake,11,12 are some of the predominant features.
The precise mechanisms regarding how insulin regulates protein turnover are not completely understood. However a variety of investigations have demonstrated that insulin enhances amino acid (AA) uptake and stimulates protein synthesis.13 Conversely, in various animal and human models it has been shown that physiologic increments in serum insulin concentrations blunt protein breakdown in both skeletal muscle bed and liver.14 Overall, these data strongly suggest that insulin plays an important anabolic role in the physiologic regulation of whole-body protein metabolism. Accordingly, it is important to understand whether IR has a role as a mediator of whole-body protein imbalance in patients receiving CHD.
Although insulin sensitivity can be assessed bya numberof methods, the hyperinsulinemic euglycemic clamp (HEGC) technique originally developed by DeFronzo et al15 is the gold standard for the measurement of peripheral and central insulin sensitivity in different disease states, including in patients with CKD.16,17 In this study, we hypothesized that there is a direct correlation between the glucose disposal rate (GDR) measured by the HEGC technique and AA metabolism related to diminished insulin action in patients receiving CHD. To test this hypothesis, we examined the relationship between IR of carbohydrate metabolism and the changes in plasma concentration of leucine, as a marker of protein metabolism, measured simultaneously during HEGC studies in 12 patients receiving CHD. Additionally we examined if indirect indices of IR correlated with the changes in leucine and other AAs measured during the clamp study.
Materials and Methods
Subjects
Twelve prevalent patients 18 years or older who were receiving CHD were recruited from the Vanderbilt University Medical Center outpatient dialysis units between April 2008 and January 2010. Inclusion criteria included having received CHD for more than 6 months, well-functioning hemodialysis vascular access, equilibrated Kt/V greater than 1.2, and no active infectious or chronic inflammatory disease or hospitalization history within 1 month of enrollment. Patients with type 1 diabetes mellitus were excluded from the study, whereas patients with diabetes mellitus type 2 who were not taking insulin sensitizers (thiazolidinediones) were allowed in the study. Following the American Diabetes Association criteria, diabetes mellitus type 2 was defined as having at least 2 fasting plasma glucose measurements greater than or equal to 126 mg/dL or taking oral antidiabetes drugs or insulin. Impaired fasting glucose was defined as fasting glucose levels between 100 and 125 mg/dL on at least 2 occasions. The study was approved by the Vanderbilt University Medical Center Institutional Review Board, and informed consent was obtained from all patients (NCT00656032).
Hyperinsulinemic-Euglycemic Glucose Clamp Study
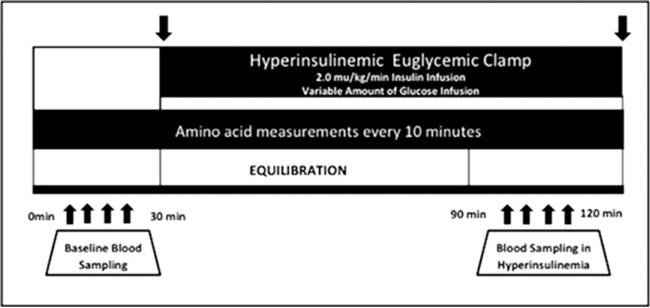
All HEGC studies were performed at the General Clinical Research Center at Vanderbilt University Medical Center (Fig. 1) as part of a prospective study examining the effects of active vitamin D administration in patients receiving CHD (NCT00656032). All assessments were performed after an overnight 10-hour fasting period. On the morning of the clamp study, the dialysis shunt was accessed using 15-gauge fistula needles placed in opposite directions at least 4 fingerbreadths apart. The venous needle was used for the infusions of glucose, insulin, and dextrose. All blood samples were taken through a dialysis needle placed at the arterial side of the dialysis access. Blood samples were drawn at 5-minute intervals for 30 minutes to assess basal levels of glucose at steady state and every 10 minutes for AA basal levels. Subsequently, a primed continuous infusion of human regular insulin (50 U/50 mL of normal saline) was started at a rate of 2.0 mU/kg/minute and maintained at that level through 120 minutes. The insulin dose used in this study was determined by the required dose during a HGEC clamp study in patients with end-stage renal disease (ESRD) to suppress the hepatic gluconeogenesis and allow the measurement of peripheral IR.18 After insulin initiation, the plasma glucose levels were allowed to drop to ± 5 mg/dL of the patient's baseline glucose value and were maintained at that level throughout the study by adjusting a variable infusion of 20% dextrose. Constant monitoring of plasma glucose concentration was performed every 5 minutes. Once steady state was reached and confirmed at 90 minutes, GDR or the “M” value (mg/kg/minute insulin-mediated glucose disposal) was calculated during the last 30 minutes of the clamp study as an index of in vivo insulin sensitivity. Based on literature published in the general population, GDR higher than 7.5 mg/kg/minute was considered to be an insulin-sensitive state, whereas values lower than 4.0 mg/kg/minute were considered to be an insulin-resistant state. Levels between 4.0 and 7.5 mg/kg/minute suggest “impaired glucose tolerance,” an early sign of IR.
Figure 1.
The hyperinsulinemic euglycemic clamp protocol. Blood was drawn every 10 minutes for amino acid measurements during the procedure.
Plasma Amino Acid Measurements
Plasma AA concentrations were determined by reverse-phase high-performance liquid chromatography after derivatization with phenyl isothiocyanate.19 Each AA was also placed into groups for analysis. The groups included branched-chain AAs (BCAAs) (leucine, isoleucine, valine) and total AAs (TAAs) as a sum of all individual AAs. During the clamp protocol, insulin-induced AA changes were calculated as follows: (baseline AA concentration – average concentration of AAs over the last 30 minutes of the clamp)/baseline AA concentration.
Derived Insulin Resistance Indices
We also explored the extent of insulin resistance (IR) by using other IR indices and evaluated the correlation of AA changes. The other IR indices measured were as follows: homeostatic model assessment (HOMA)-IR, insulin (μU/mL) × glucose (mg/dL)/405; HOMA-adinopectin (AD), insulin (μU/mL) × glucose (mg/dL)/AD [μg/mL], leptin to adinopectin ratio (LAR), leptin (ng/mL)/AD (μg/mL); McAuley's index, exponential (exp) (2.63-0.28 Ln (natural logarithm) insulin [μU/mL]–0.31 Ln triglycerides [mM/L]); quantitative insulin check index (QUICKI), 1/(log glucose [mg/dL] + log insulin [μU/mL]). IR state was evaluated according to the reference values as published.9,20,21
Blood Samples
All blood sampling was performed at the General Clinical Research Center and processed at Vanderbilt Cytokine and Hormonal Core facilities. Blood was drawn into Vacutainer (Becton Dickinson, Franklin Lakes, NJ) tubes containing ethylenediaminetetraacetic acid for plasma separation. Samples were transported on ice and immediately centrifuged at 20°C at 3,000 rpm for 15 minutes. Supernatants were stored in aliquots at –80°C until needed for measurement.
Glucose concentrations were measured by using the glucose oxidase method (Glucose Analyzer 2; Beckman Coulter, Brea, CA). Insulin was measured by using a double-antibody radioimmunoassay (DA RIA; Millipore, St. Charles, MO). AD and resistin were measured using the MILLIPLEX MAP Human Serum Adipokine Panel A kit (Millipore, Billerica, MA). Interleukin-6 concentrations were determined using cytometric bead arrays (Becton Dickinson, San Jose, CA). Two-color flow cytometric analysis was performed using a BD LSR II flow cytometer (Becton Dickinson). C-reactive protein levels were measured using the high-sensitivity particle-enhanced turbidimetric UniCel Dxl Immunoassay System (Beckman Coulter). All other measurements (including triglycerides, high-density lipoprotein, low-density lipoprotein, and leptin) were performed using routine laboratory tests and certified methods.
Statistical Analysis
All values are expressed as mean ± SD or median with interquartile range (IQR) depending on their distribution. A paired t test was performed to compare the changes of AA levels from baseline to the end of the clamp protocol. The correlation coefficient between GDR and AA change percentages was evaluated using Spearman correlation analysis in all 12 patients. The association between GDR and AA concentrations for all time points (repeated measures) was determined using a linear mixed effect model, both unadjusted and adjusted for age and sex. The association between other indirect IR indices such as HOMA, HOMA-AD, LAR, and AA change percentages was derived from a mixed effect model. Logarithmic transformation was performed for HOMA, HOMA-AD, and LAR levels before the analysis to ensure normality. Sensitivity analyses were performed for the Spearman correlation between GDR and percent changes in AA concentrations for baseline clamp studies and for the mixed model analysis after excluding 2 individuals who, by repeated fasting glucose measurements, met the criteria for diabetes mellitus. Statistical significance was assessed at the 95% confidence interval. Analyses were performed using SPSS, version 19 for Windows (IBM Corp, Armonk, NY).
Results
Demographic Characteristics of Patients
Baseline characteristics of the study subjects are shown in Table 1. The median age of the study population was 50 years (range, 40-73 years) and all of the patients were African American. The median time on dialysis was 46 months, with an IQR of 37 to 94 months. Hypertension was the dominant primary disorder leading to ESRD (83%). Thirty-three percent of patients were women and 58% percent of these women were obese (body mass index >30 kg/m2). The median fasting glucose level was 101 mg/dL (IQR;88, 111). According to the American Diabetes Association criteria, 2 patients had diabetes mellitus and 4 patients had impaired fasting glucose levels based on repeated fasting glucose measurements.
Table 1.
Demographic and Laboratory Characteristics of Study Population
| Variable | N = 12 |
|---|---|
| Age (y) (median, IQR) | 50 (40, 73) |
| Sex (male/female) | 8/4 |
| Body mass index (kg/m2; mean ± SD) | 34.4 ± 7.6 |
| Duration of hemodialysis (months; median, IQR) | 46 (37, 94) |
| Albumin (g/dL; median, IQR) | 3.85 (3.5, 4.2) |
| Carbohydrate metabolism | |
| Fasting glucose (mg/dL; median, IQR) | 101 (88, 111) |
| Fasting insulin (mU/L; median, IQR) | 12.31 (9.32, 17.47) |
| Glycated hemoglobin (median, IQR) | 5.10 (5.0, 6.0) |
| Insulin sensitivity by glucose disposal rate (mg/kg/min; median, IQR) | 5.65 (2.63, 11.07) |
| Fasting amino acid measurements | |
| Leucine (μmol/L; mean ± SD) | 79.2 ± 15 |
| Phenylalanine (μmol/L; mean ± SD) | 45 ± 10 |
| BCAAs (μmol/L; mean ± SD) | 260 ± 43 |
| TAAs (μmol/L; mean ± SD) | 2,291 ± 310 |
BCAA, branched chain amino acids; IQR, interquartile range; TAAs, total amino acids; SD, standard deviation.
Measurement of Insulin Resistance
Insulin sensitivity was assessed by the HEGC procedure. The median GDR was 5.65 (IQR, 2.63-11.07) with 83% (n = 10) of subjects being insulin resistant or glucose intolerant based on the aforementioned criteria (i.e., GDR <7.5 mg/kg/minute). These data were previously published as part of the study, which compared the performance of these commonly used IR indices relative to the HEGC technique.21
Plasma Amino Acid Concentrations During the Hyperinsulinemic Euglycemic Clamp Study
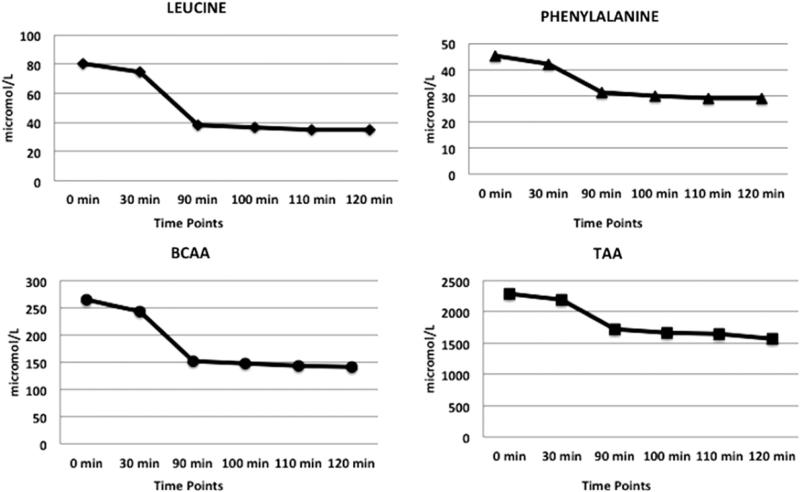
Basal levels of plasma leucine, phenylalanine, BCAAs, and TAAs (mean ± SD) were 79.2 ± 15 μmol/L, 45 ± 10 μmol/L, 260 ± 43 μmol/L, and 2291 ± 310 μmol/L, respectively. Pre–insulin administration levels of plasma leucine, phenylalanine, BCAAs, and TAAs (mean ± SD) were 73.8 ± 18 μmol/L, 42 ± 9 μmol/L, 240 ± 52 μmol/L, and 2,150 ± 363 μmol/L, respectively. All AA levels declined significantly during the hyperinsulinemic period of the clamp procedure (Table 2). The total plasma AA concentration decreased from 2,299 ± 521 μmol/L to 1,656 ± 336 μmol/L (P < .001). The plasma phenylalanine concentration dropped from 45 ± 17 μmol/L to 30 ± 11 μmol/L (P < .001). BCAA concentrations declined from 264 ± 64 μmol/L to 148 ± 48 μmol/L (P < .001); in particular the plasma leucine concentration fell from 80 ± 22 μmol/L to 37 ± 16 μmol/L (P < .001), (Fig. 2).
Table 2.
Arterial Concentrations of Individual, Branched Chain, and Total Amino Acids During Basal and Hyperinsulinemic State
| Variable | Basal | Insulin Clamp (90 to 120 min) |
|---|---|---|
| Leucine | 79.2 ± 15 | 35.3 ± 13* |
| Phenylalanine | 45 ± 10 | 30 ± 8* |
| Alanine | 214 ± 50 | 152 ± 24* |
| Arginine | 60 ± 16 | 40 ± 13* |
| Asparagine | 25 ± 5 | 16 ± 3* |
| Citrulline | 29 ± 5 | 20 ± 5* |
| Cysteine | 66 ± 18 | 64 ± 18† |
| Glutamine | 472 ± 73 | 373 ± 55 |
| Glutamic acid | 63 ± 29 | 44 ± 21* |
| Glycine | 206 ± 77 | 155 ± 53* |
| Histidine | 58 ± 9 | 45 ± 6* |
| Isoleucine | 46 ± 10 | 18 ± 9* |
| Lysine | 107 ± 24 | 80 ± 19* |
| Methionine | 17 ± 9 | 10 ± 11* |
| Proline | 190 ± 47 | 127 ± 30* |
| Serine | 55 ± 11 | 35 ± 8* |
| Taurine | 38 ± 17 | 29 ± 19* |
| Threonine | 117 ± 19 | 79 ± 19* |
| Tryptophan | 10 ± 3 | 8 ± 4† |
| Tyrosine | 33 ± 11 | 18 ± 8* |
| Valine | 135 ± 22 | 90 ± 22* |
| BCAAs | 260 ± 43 | 144 ± 40* |
| TAAs | 2291 ± 310 | 1643 ± 245* |
BCAAs, branched chain amino acids; TAAs, total amino acids.
Values (in μmol/L) are presented mean ± SD.
Significance from basal values at P < .01.
Significance from basal values P < .05.
Figure 2.
The changes of leucine, phenylalanine, branched-chain amino acid (BCAA), and total amino acid (TAA) concentrations during the hyperinsulinemic euglycemic clamp study. Plasma amino acid concentrations were measured in basal state and during a 90-120 minute time interval of the hyperinsulinemic euglycemic clamp technique. All AA levels significantly decreased during the insulin infusion (P < .001). Results are presented as mean ± SD.
Association Between Glucose Disposal Rate Measured by Hyperinsulinemic Euglycemic Clamp Technique and Amino Acid Uptake During the Clamp Protocol
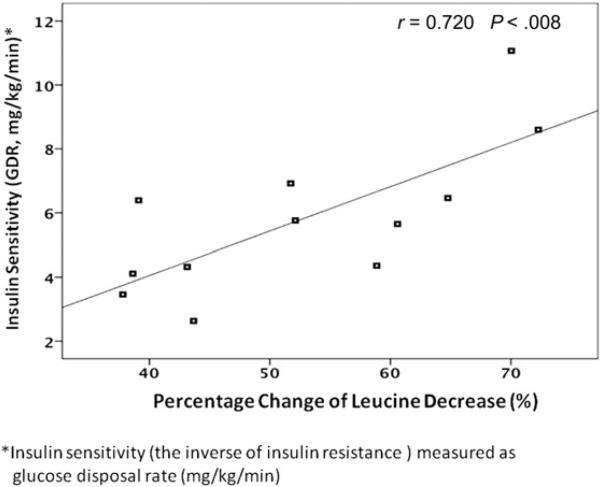
The percentage decreases of plasma AA concentrations during the hyperinsulinemic clamp period were 55% for leucine, 33% for phenylalanine, 44% for BCAAs, and 27% for TAAs. There was a statistically significant positive correlation between GDR and the percentage change of leucine concentration (r = 0.720; P < .008) (Fig. 3). Although positive correlations were observed between GDR levels and other AA groups, these associations were not statistically significant (r = 0.063, P = .846 for phenylalanine; r = 0.538, P = .071 for BCAA; and r = 0.105; P = .746 for TAA) (Table 3).
Figure 3.
The correlation between glucose disposal rate and percentage change of plasma leucine decrease. GDR, glucose disposal rate.
Table 3.
Correlations Between the Percentage Change of Plasma Amino Acid Concentrations and Insulin Resistance Measured by GDR During the Baseline Hyperinsulinemic Euglycemic Clamp Study
| Amino Acids | r Values | P Values |
|---|---|---|
| Leucine | 0.720 | < .008 |
| Phenylalanine | 0.063 | .846 |
| BCAAs | 0.538 | .071 |
| TAAs | 0.105 | .746 |
BCAA, branched chain amino acids; GDR, glucose disposal rate; TAA, total amino acids.
The correlation coefficients were performed by Spearman correlation analysis (r).
Mixed model analysis using data from all study points similarly showed statistically significant association only with percentage changes in leucine concentrations (P = .025), whereas no significant association was observed between GDR and other AA groups. After adjusting the analysis for age and sex, GDR was still statistically significantly associated with percentage change of leucine concentration (P = .026).
Association Between Other Insulin Resistance Indices and Amino Acid Changes
Among the other commonly used indirect indices of IR, none were associated with changes in plasma leucine concentration except HOMA-AD (P = .054). However after adjusting for age and sex, this slight association disappeared. The summary of the results including GDR is depicted in Table 4.
Table 4.
Association Between Insulin Resistance Indices and Percentage Change of Plasma Amino Acid Concentrations During Repeated Clamp Studies Using a Mixed Model Analysis
| Insulin Indices | Plasma Amino Acid Changes |
|||
|---|---|---|---|---|
| Leucine P Values | Phenylalanine P Values | BCAA P Values | TAA P Values | |
| GDR by HECG | .025 | .465 | .388 | .663 |
| LnHOMA-IR | .667 | .308 | .243 | .123 |
| LnHOMA-AD | .054 | .885 | .850 | .584 |
| QUICKI | .538 | .406 | .286 | .141 |
| LnLAR | .245 | .959 | .610 | .900 |
| McAuley's index | .291 | .999 | .888 | .967 |
BCAA, branched-chain amino acid; GDR, glucose disposal rate; HEGC, hyperinsulinemic euglycemic clamp test; LnHOMA-AD, logarithmic homeostatic model assessment by adiponectin; LnHOMA-IR, logarithmic homeostatic model assessment-insulin resistance; LnLAR, logarithmic leptin to adiponectin ratio; QUICKI, quantitative insulin sensitivity check index; TAA, total amino acid.
Significance of association was tested by a generalized linear mixed model with all 12 patients; repeated measures were included. Logarithmic transformation was performed for HOMA-IR, HOMA-AD, and LAR levels to yield normal distribution before the analysis.
Sensitivity Analysis
Spearman correlation analysis between GDR and the percent change in AAs for the baseline clamp studies and the mixed model analysis between GDR and AA concentration changes for all studies were repeated after excluding the 2 individuals who met criteria for diabetes based on fasting glucose values. Results for both analyses were consistent with the primary results.
Discussion
In the present study, we evaluated the association between GDR as a sensitive measure of IR in the peripheral muscle tissue and changes in plasma leucine concentrations in response to high-dose insulin administration in 12 clinically stable African American patients receiving CHD. Our results showed that administration of high-dose insulin resulted in decrements in all plasma AA concentrations, and was most evident in leucine concentrations, consistent with insulin's protein anabolic response. We also demonstrated that the changes in AA concentrations, which is reflective of whole-body protein metabolism, were directly associated with the extent of IR measured as GDR. This relationship was observed most profoundly in plasma leucine concentrations. These data suggested that patients receiving CHD who show evidence of resistance to the metabolic effects of insulin on carbohydrate metabolism also display comparable resistance to the protein anabolic actions of insulin. Hence, IR can be considered as a therapeutic target to prevent the loss of lean body mass in the setting of advanced CKD.
Simultaneous comparison of insulin action on both carbohydrate and AA metabolism has been performed previously in different populations with conflicting results. In studies in which the lack of association between carbohydrate and AA metabolism was observed, possible substrate competition between glucose and AAs, such as decreased glucose uptake allowing enhanced AA uptake, has been proposed as an explanation.22 Conversely, several studies in healthy individuals and patients with ESRD receiving peritoneal dialysis have demonstrated that resistance to insulin action affected both carbohydrate and protein metabolism to the same degree.23-25 To our knowledge, this particular study is the first to demonstrate such a relationship in patients receiving CHD and corroborates the findings by Castellino et al25 in patients receiving peritoneal dialysis. Taken together, by determining the physiologic relevance of impaired glucose and protein metabolism in the setting of diminished insulin activity in patients with advanced CKD, these studies provide a strong rationale to indicate that IR may represent a novel therapeutic target for CKD-associated PEW syndrome.
In our study, we observed that all plasma individual AA levels decreased when insulin was administered to reach supraphysiologic levels. In the postabsorptive state, endogenous proteins are the only source for the appearance of plasma AAs, especially essential AAs such as leucine. Thus the decrease of AA levels in response to a significant increment in plasma insulin concentrations suggests either insulin-mediated AA uptake into the muscle tissue or a pronounced suppression of muscle protein breakdown, or a combination thereof.22,24-29 However it should be emphasized that decreases in plasma AA concentrations do not fully reflect net AA use in muscle. The inhibition of protein breakdown also decreases endogenous AA availability for protein synthesis; consequently hypoaminoacidemia might blunt the further anabolic effects of insulin. Therefore further studies that combine the variable infusion of AA solution with the conventional clamp technique may provide a more appropriate setting to evaluate IR and protein turnover under steady state conditions when all plasma AA concentrations are essentially constant.
In this study, noticeable changes in specific types of AA concentrations were also noted. The most pronounced decrement was observed in BCAAs, primarily driven by leucine concentrations (55%). BCAAs are essential AAs, which are neither synthesized nor degraded in the muscle. In the absence of exogenous intake, their rates of disappearance and appearance reflect the index of protein synthesis and proteolysis, respectively.27 As 1 of the BCAAs, leucine is primarily metabolized in the muscle and the utilization process is modulated mainly by insulin. Previously, Anthony et al reported that leucine administration directly stimulates protein synthesis by promoting initiating factors.30 Further animal experiments have demonstrated that somatostatin, an insulin inhibitor, inhibits leucine's effects on ribosomal protein S6, which is responsible for enhancing the protein synthetic capacity of the cells, suggesting that insulin actions may be responsible.31 Furthermore, administration of insulin antibodies or diazoxide to block insulin action suggest that leucine and insulin have to be in a stable interaction to optimize protein synthesis.32 Several other previous studies, which compared the impact of phenylalanine as another essential AA and leucine as substrates for protein metabolism demonstrated that the rate of incorporation of leucine into the protein was of a greater magnitude than that of phenylalanine.33-35 It has been suggested that phenylalanine is metabolized more in the liver and converted to tyrosine; in contrast, leucine use is more pronounced in the muscle and in the adipose tissue, being 7 times greater than its use in liver.33,34,36 Hence, it is not surprising to observe a larger drop in the concentration of leucine during the hyperinsulinemic state of the clamp procedure compared with the other AAs and confirms the reliability of our experiments.
Despite the increased use of the clamp technique in evaluating insulin sensitivity on peripheral tissues, the high costs and complexity of the method restricts its use in routine clinical practice. Therefore indirect but more practical methods of determining insulin sensitivity are often applied. Based on our previous work demonstrating that indirect indices of IR correlated to GDR by HEGC in the CHD population,21 we also evaluated the relationship of these indices and the percentage change of AAs. However none of the indirect indices were associated with the AA concentration changes with the exception of HOMAAD. The indirect IR indices provide information based on the interaction between fasting insulin and glucose concentrations under physiologic states. In this study, plasma AA concentrations were obtained during insulin infusion. Thus the decline of plasma AA concentrations may reflect the alterations under high insulin concentrations, which may not accurately reflect basal insulin action during fasting on AA dynamics, which reflects an unstimulated state. However in the absence of a significant association between AA changes and the available indirect indices, it can be speculated that newer practical indices may need to be developed for the purpose of evaluating resistance to the action of insulin in protein anabolism because clamp studies are cumbersome and impractical to perform in larger or population-based studies.
There are a number of strengths in our study. First, we used the gold standard method to assess IR—i.e., HEGC, which is highly precise and accurate. Second, at least to our knowledge, our study is the largest of its kind with 12 HEGC studies in which repeated measurements of AA concentrations were obtained throughout the clamp studies without concomitant administration of any exogenous AAs. There are also some limitations. All study subjects were African-American, limiting its generalizability to other populations. As mentioned earlier, measurement of changes in plasma AA concentrations does not fully represent protein turnover, especially particular contributions of protein synthesis and breakdown. Combining HEGC techniques with labeled isotope procedures may provide more mechanistic information about IR and its consequences on protein metabolism at the physiologic level.
In conclusion, the results of this study indicate that IR might play a significant role in the dysregulated protein metabolism in patients receiving CHD. Interventions targeted at improving IR might be attractive strategies for improving clinical outcomes in patients receiving CHD who are at risk of a progressive decline in lean body mass within the context of PEW.
Practical Application
Insulin resistance is evident in patients receiving CHD. Our results suggest that the severity of IR of carbohydrate metabolism is associated with a lesser decline in plasma leucine concentrations, suggesting a similar IR of protein anabolism. IR represents a potential mechanism for the loss of muscle mass (sarcopenia) commonly observed in patients receiving CHD and may represent a target for intervention.
Acknowledgment
This study was supported in part by grants Clinical and Translational Science Award 1UL-1RR024975 from the National Center for Research Resources, K24 DK 62849 from the National Institute of Diabetes and Digestive and Kidney Diseases, Veterans Administration Merit Award 1I01CX000414 and the Center for D-Receptor Activation Research.
S.M.D. is supported by an International Society of Nephrology/Turkish Society of Nephrology fellowship award. A.H. is supported by a Career Development Award (2-031-09S) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Clinical Sciences Research. The sponsors had no influence on the design, execution, and analysis of the results of the study.
Footnotes
Financial Disclosure: T.A.I. is a consultant to Abbott Nutrition, Renal Advantage Inc, and Fresenius Kabi. Research support was received from Fresenius Medical Care – North America. The remaining authors have no relevant financial disclosures.
References
- 1.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int. 1999;55:1945–1951. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 2.Owen WF, Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329:1001–1006. doi: 10.1056/NEJM199309303291404. [DOI] [PubMed] [Google Scholar]
- 3.Ikizler TA, Hakim RM. Nutrition in end-stage renal disease. Kidney Int. 1996;50:343–357. doi: 10.1038/ki.1996.323. [DOI] [PubMed] [Google Scholar]
- 4.Pupim LB, Heimburger O, Qureshi AR, Ikizler TA, Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005;68:2368–2374. doi: 10.1111/j.1523-1755.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 5.O'Sullivan AJ, Kelly JJ. Insulin resistance and protein catabolism in non-diabetic hemodialysis patients. Kidney Int. 2007;71:98–100. doi: 10.1038/sj.ki.5002045. [DOI] [PubMed] [Google Scholar]
- 6.Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int. 2007;71:146–152. doi: 10.1038/sj.ki.5001984. [DOI] [PubMed] [Google Scholar]
- 7.Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am J Clin Nutr. 2005;82:355–365. doi: 10.1093/ajcn.82.2.355. [DOI] [PubMed] [Google Scholar]
- 8.Nerpin E, Riserus U, Ingelsson E, et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care. 2008;31:1550–1555. doi: 10.2337/dc08-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoji T, Emoto M, Nishizawa Y. HOMA index to assess insulin resistance in renal failure patients. Nephron. 2001;89:348–349. doi: 10.1159/000046098. [DOI] [PubMed] [Google Scholar]
- 10.Rabkin R, Simon NM, Steiner S, Colwell JA. Effect of renal disease on renal uptake and excretion of insulin in man. N Engl J Med. 1970;282:182–187. doi: 10.1056/NEJM197001222820402. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. 1981;67:563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Smith D, Alvestrand A. Insulin action in uremia. Kidney Int Suppl. 1983;16:S102–S114. [PubMed] [Google Scholar]
- 13.Jefferson LS. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980;29:487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- 14.Flakoll PJ, Kulaylat M, Frexes-Steed M, et al. Amino acids augment insulin's suppression of whole body proteolysis. Am J Physiol. 1989;257:E839–E847. doi: 10.1152/ajpendo.1989.257.6.E839. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Tobin JD, Rowe JW, Andres R. Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest. 1978;62:425–435. doi: 10.1172/JCI109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–534. doi: 10.1046/j.1464-5491.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith D, DeFronzo RA. Insulin resistance in uremia mediated by post-binding defects. Kidney Int. 1982;22:54–62. doi: 10.1038/ki.1982.132. [DOI] [PubMed] [Google Scholar]
- 19.Heinrikson RI, Meredith SC. Amino acid analysis by reverse-phase high pressure liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984;136:65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- 20.Teta D, Maillard M, Halabi G, Burnier M. The leptin/adiponectin ratio: potential implications for peritoneal dialysis. Kidney Int Suppl. 2008;108:S112–S118. doi: 10.1038/sj.ki.5002611. [DOI] [PubMed] [Google Scholar]
- 21.Hung AM, Sundell MB, Egbert P, et al. A comparison of novel and commonly-used indices of insulin sensitivity in African American chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:767–774. doi: 10.2215/CJN.08070910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caballero B, Wurtman RJ. Differential effects of insulin resistance on leucine and glucose kinetics in obesity. Metab Clin Exp. 1991;40:51–58. doi: 10.1016/0026-0495(91)90192-y. [DOI] [PubMed] [Google Scholar]
- 23.Fukagawa NK, Minaker KL, Rowe JW, et al. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985;76:2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellino P, Solini A, Luzi L, et al. Glucose and amino acid metabolism in chronic renal failure: effect of insulin and amino acids. Am J Physiol. 1992;262:F168–F176. doi: 10.1152/ajprenal.1992.262.2.F168. [DOI] [PubMed] [Google Scholar]
- 25.Castellino P, Luzi L, Giordano M, Defronzo RA. Effects of insulin and amino acids on glucose and leucine metabolism in CAPD patients. J Am Soc Nephrol. 1999;10:1050–1058. doi: 10.1681/ASN.V1051050. [DOI] [PubMed] [Google Scholar]
- 26.Luzi L, Giordano M, Caloni M, Castellino P. Effects of insulin and amino acids on leucine metabolism in young and middle-aged humans. Eur J Nutr. 2001;40:106–112. doi: 10.1007/s003940170010. [DOI] [PubMed] [Google Scholar]
- 27.Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest. 1987;80:1784–1793. doi: 10.1172/JCI113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hourani H, Williams P, Morris JA, May ME, Abumrad NN. Effect of insulin-induced hypoglycemia on protein metabolism in vivo. Am J Physiol. 1990;259:E342–E350. doi: 10.1152/ajpendo.1990.259.3.E342. [DOI] [PubMed] [Google Scholar]
- 29.Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57:56–63. doi: 10.2337/db07-0887. [DOI] [PubMed] [Google Scholar]
- 30.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 31.Anthony JC, Lang CH, Crozier SJ, et al. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–E1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- 32.Svanberg E, Zachrisson H, Ohlsson C, Iresjo BM, Lundholm KG. Role of insulin and IGF-I in activation of muscle protein synthesis after oral feeding. Am J Physiol. 1996;270:E614–E620. doi: 10.1152/ajpendo.1996.270.4.E614. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of glutamine and glutamic acid in humans. Am J Physiol. 1993;264:E848–E854. doi: 10.1152/ajpendo.1993.264.6.E848. [DOI] [PubMed] [Google Scholar]
- 34.Tessari P, Barazzoni R, Zanetti M. Differences in estimates of forearm protein synthesis between leucine and phenylalanine tracers following unbalanced amino acid infusion. Metabolism. 1999;48:1564–1569. doi: 10.1016/s0026-0495(99)90246-9. [DOI] [PubMed] [Google Scholar]
- 35.Balage M, Sinaud S, Prod'homme M, et al. Amino acids and insulin are both required to regulate assembly of the eIF4E. eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E565–E574. doi: 10.1152/ajpendo.2001.281.3.E565. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal J, Angel A, Farkas J. Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle. Am J Physiol. 1974;226:411–418. doi: 10.1152/ajplegacy.1974.226.2.411. [DOI] [PubMed] [Google Scholar]