Abstract
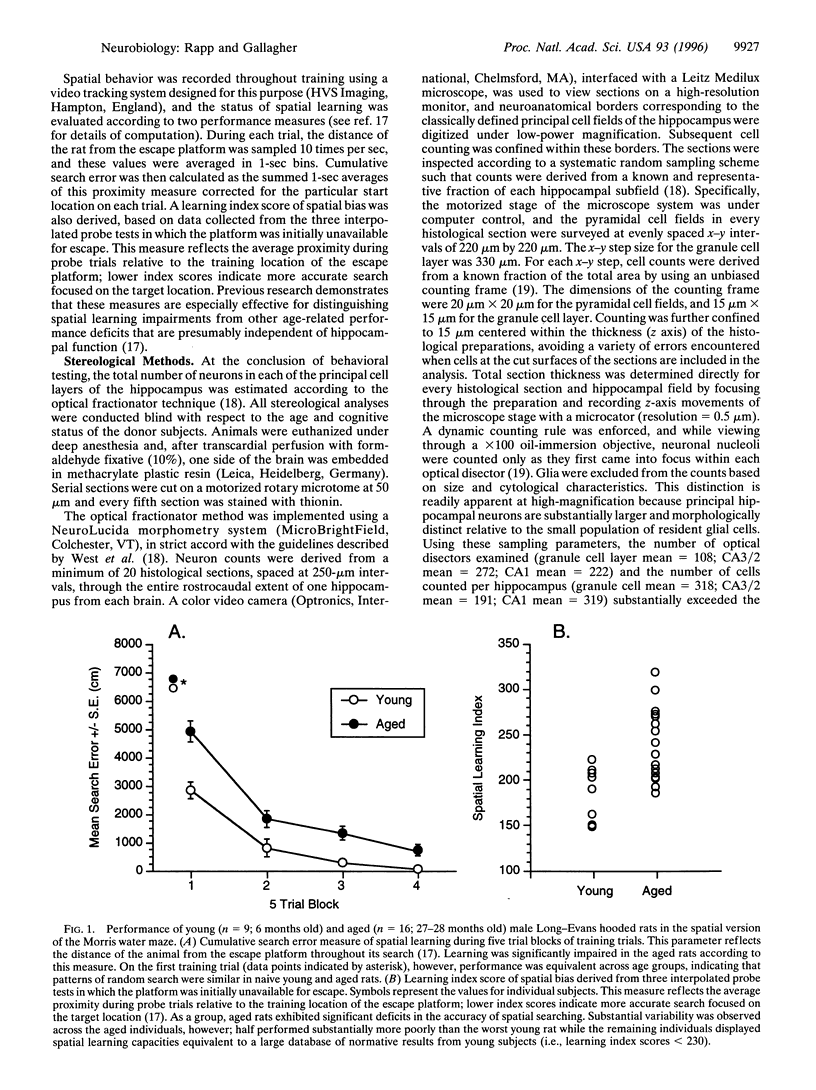
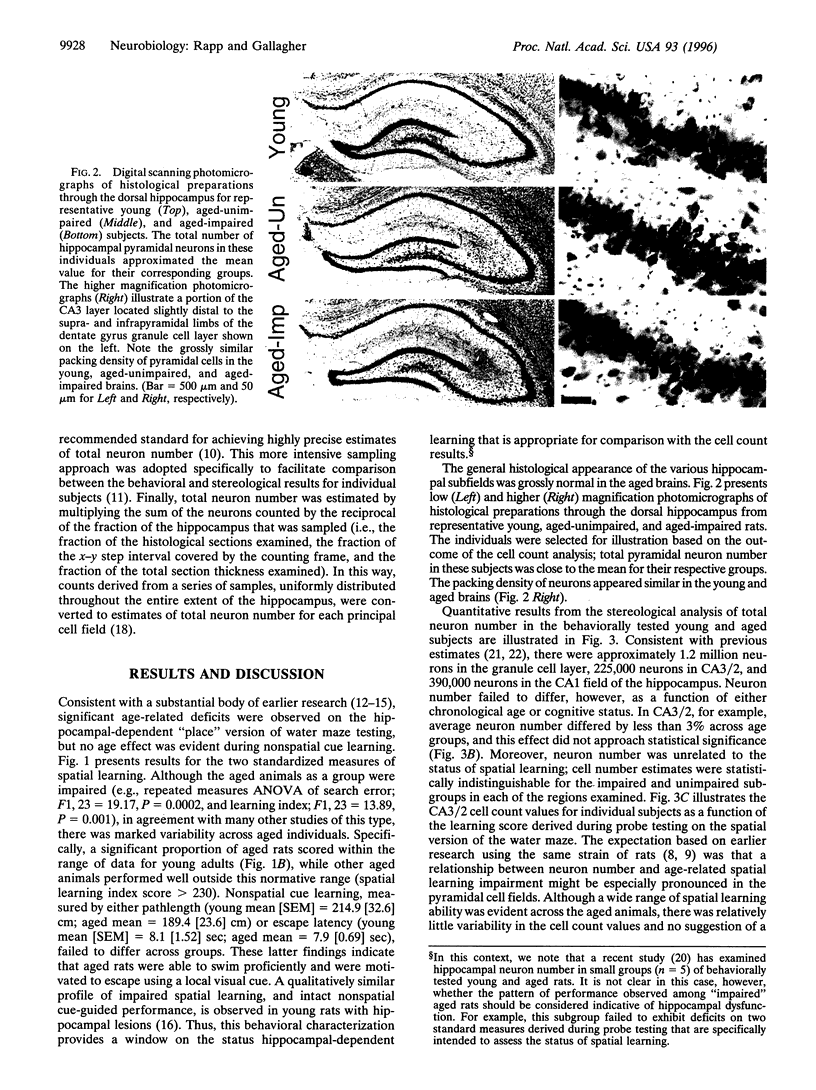
Hippocampal neuron loss is widely viewed as a hallmark of normal aging. Moreover, neuronal degeneration is thought to contribute directly to age-related deficits in learning and memory supported by the hippocampus. By taking advantage of improved methods for quantifying neuron number, the present study reports evidence challenging these long-standing concepts. The status of hippocampal-dependent spatial learning was evaluated in young and aged Long-Evans rats using the Morris water maze, and the total number of neurons in the principal cell layers of the dentate gyrus and hippocampus was quantified according to the optical fractionator technique. For each of the hippocampal fields, neuron number was preserved in the aged subjects as a group and in aged individuals with documented learning and memory deficits indicative of hippocampal dysfunction. The findings demonstrate that hippocampal neuronal degeneration is not an inevitable consequence of normal aging and that a loss of principal neurons in the hippocampus fails to account for age-related learning and memory impairment. The observed preservation of neuron number represents an essential foundation for identifying the neurobiological effects of hippocampal aging that account for cognitive decline.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Ishizuka N., Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- Coleman P. D., Flood D. G. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987 Nov-Dec;8(6):521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Disterhoft J. F., Moyer J. R., Jr, Thompson L. T. The calcium rationale in aging and Alzheimer's disease. Evidence from an animal model of normal aging. Ann N Y Acad Sci. 1994 Dec 15;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Chen K. S., Buzsaki G., Armstrong D. Experimental approaches to age-related cognitive impairments. Neurobiol Aging. 1988 Sep-Dec;9(5-6):645–655. doi: 10.1016/s0197-4580(88)80129-5. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Burwell R., Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993 Aug;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Grady C. L., McIntosh A. R., Horwitz B., Maisog J. M., Ungerleider L. G., Mentis M. J., Pietrini P., Schapiro M. B., Haxby J. V. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995 Jul 14;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987 Sep;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Issa A. M., Rowe W., Gauthier S., Meaney M. J. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990 Oct;10(10):3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. S., Campbell L. W., Applegate M. D., Brodish A., Landfield P. W. Chronic stress-induced acceleration of electrophysiologic and morphometric biomarkers of hippocampal aging. J Neurosci. 1991 May;11(5):1316–1324. doi: 10.1523/JNEUROSCI.11-05-01316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield P. W., Baskin R. K., Pitler T. A. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981 Oct 30;214(4520):581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Eldridge J. C. The glucocorticoid hypothesis of age-related hippocampal neurodegeneration: role of dysregulated intraneuronal calcium. Ann N Y Acad Sci. 1994 Nov 30;746:308–326. doi: 10.1111/j.1749-6632.1994.tb39249.x. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Re-examination of the glucocorticoid hypothesis of stress and aging. Prog Brain Res. 1992;93:365–383. doi: 10.1016/s0079-6123(08)64585-9. [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Aitken D. H., van Berkel C., Bhatnagar S., Sapolsky R. M. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988 Feb 12;239(4841 Pt 1):766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Patton P. E., McNaughton B. Connection matrix of the hippocampal formation: I. The dentate gyrus. Hippocampus. 1995;5(4):245–286. doi: 10.1002/hipo.450050402. [DOI] [PubMed] [Google Scholar]
- Rapp P. R., Amaral D. G. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci. 1992 Sep;15(9):340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- Rapp P. R., Burwell R. D., West M. J. Individual differences in aging: implications for stereological studies of neuron loss. Neurobiol Aging. 1996 May-Jun;17(3):495–500. doi: 10.1016/0197-4580(96)00012-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen T., Schliemann T., Sørensen J. C., Zimmer J., West M. J. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996 Jan-Feb;17(1):143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- West M. J. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993 Jul-Aug;14(4):287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West M. J., Slomianka L., Gundersen H. J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991 Dec;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]




