Abstract
Background
Glycosylated hemoglobin A1c (HbA1c) has been applied to identify type 2 diabetes (T2DM) in the U.S. and European countries. It has not been used in China mainly due to lack of a standardized approach to measure HbA1c, short of knowledge about racial-specific standard and deficiency of an optimal cut-off point.
Methods
To evaluate combination of HbA1c and fasting plasma glucose (FPG) in diagnosing T2DM in Chinese adults, a multistage sampling cross-sectional study was conducted in Shanghai, China, in 2009. The FPG measurement, HbA1c assay, and oral glucose tolerance test (OGTT) were performed in 6,661 Chinese adults (3057 men, 3604 women) who had no prior history of diabetes to identify the unrecognized T2DM.
Results
A total of 454 participants were identified as T2DM based on the 1999 World Health Organization (WHO) diagnostic criteria. Of these patients, 239 were detected using an FPG ≥ 7.0 mmol/l and 141 were further identified using an HbA1c ≥ 43 mmol/mol (6.1%), achieving a sensitivity of 83.7% and a specificity of 89.3% for combining use of FPG and HbA1c. In subjects at high risk of diabetes, the combining use of FPG and HbA1c produced a higher sensitivity and an improved positive predictive value (PPV), and had a satisfactory specificity and negative predictive value (NPV).
Conclusions
The combining use of FPG and HbA1c is a potential screening and diagnosis approach for T2DM in Chinese adults, especially among those at high risk of the disease.
Keywords: Type 2 diabetes, Diagnosis, Glycosylated hemoglobin A1c, Fasting plasma glucose, Chinese adults
Background
Type 2 diabetes mellitus (T2DM) is a common disease reflecting metabolic disorders characterized with hyperglycemia, which may lead to specific long-term complications affecting heart, brain, eyes, kidneys and nervous system [1]. Currently, diagnosis of T2DM in Chinese adults is principally according to the 1999 World Health Organization (WHO) diagnostic criteria [2], namely, a person can be diagnosed as T2DM when he or she has 1) a random plasma glucose ≥ 11.1 mmol/l accompanied by typical symptoms of diabetes such as thirst and polyuria; or 2) a fasting plasma glucose (FPG) ≥ 7.0 mmol/l; or 3) a 2-hour plasma glucose (2hPG) after an oral glucose tolerance test (OGTT) ≥ 11.1 mmol/l [3]. These diagnostic criteria are determined based on the relationship between glucose levels and the risk of subsequent long-term complications.
The accuracy of glucose measurement, however, is more or less affected by the pre-analytic instability and biologic variability of glucose concentrations within and between days. Moreover, OGTT requires a second blood sample and is therefore more costly and time-consuming, bringing inconvenient in logistics and causing uncomfortable feeling in individuals. Compared with 2hPG in OGTT, FPG measurement is more reproducible and simpler to perform in clinics and communities [4]. Therefore FPG measurement is usually used alone to screen T2DM in China [5]. Due to that a certain proportion of T2DM patients have a normal FPG but an elevated 2hPG (≥ 11.1 mmol/l) [6], almost half diabetes patients remain undiagnosed in China [7]. A more efficient and practical method is needed to identify the individuals with diabetes in general population.
Hemoglobin A1c (HbA1c), a glycosylated form of hemoglobin, reflects one’s plasma glucose levels over past 2–3 months and is less influenced by recent diet and emotional stress [8]. HbA1c level is a convenient and reliable measurement of chronic glycemia [9], and can be tested concurrently with FPG at fasting. HbA1c is associated with the risk of long-term diabetic complications and has been used to monitor glycemic control status in prevalent patients with diabetes for about 30 years. Substantial studies [10-12], including one conducted in Chinese population [12], have proved that HbA1c may be a helpful tool for diagnosing T2DM. The American Diabetes Association (ADA) has used an HbA1c cut-point of 48 mmol/mol (6.5%) as one of diagnostic criteria for T2DM in its 2010 Clinical Practice Recommendations [1].
Although HbA1c has been applied to identify T2DM in the U.S. and European countries, it has not been used in China, mainly due to lack of a standardized approach to measure HbA1c, short of knowledge about racial-specific standard and deficiency of an optimal cut-point for detecting T2DM in the population. HbA1c values between 37 mmol/mol (5.5%) and 48 mmol/mol (6.5%) have been associated with a substantially increased risk for developing T2DM [13]. Due to racial disparity in HbA1c level [14], however, the optimal threshold varies across populations. In Chinese adults at high risk of glucose intolerance, combining use of an FPG ≥ 6.1 mmol/l and an HbA1c ≥ 43 mmol/mol (6.1%) has been shown to predict subsequent T2DM accurately [15]. Bao et al.[12] reported that an HbA1c ≥ 45 mmol/mol (6.3%) can be used as a diagnostic criterion for diabetes in Chinese adults when FPG and OGTT are not available. Given that the HbA1c value reflects average level of glycemia over the preceding 2–3 months and the FPG represents the current glucose concentration, combining use of the two indicators may improve the accuracy of diagnosis.
In this study, we included 6,661 Chinese adults with no prior history of diabetes to evaluate whether the combination of FPG and HbA1c can be used as a tool for screening and diagnosis of T2DM in Chinese community settings.
Methods
Study design and population
A cross-sectional survey for T2DM was conducted in Chinese adults in Shanghai, China, in 2009. As described in our previous report [7], a multistage sampling process was applied to select a representative sample of the residents of Shanghai who were at ages of 35–74 years old. Briefly, 4 districts and 2 counties were randomly selected from all 12 districts and 7 counties. Then 1–2 sub-districts or towns were randomly selected from each selected district or county. And then, 1–2 communities or villages were randomly selected from each selected sub-district or town. Finally, 1,000-2,000 eligible subjects (permanent residents of Shanghai, 35–74 years old and having been in the city for at least 5 years) were randomly selected from each selected community or village and invited to participate in the survey. Among 11,844 eligible adults recruited, 7,964 participated in the survey, resulting in a response rate of 67.2%. The main reasons for no-response were refusal to participate and absent during the period of enrollment. Pregnant women, individuals with type 1 diabetes, and those physically or mentally disabled were also excluded from participation. After further excluding subjects with incomplete questionnaires and those having a prior history of T2DM or missing values of HbA1c, we finally included 6,661 subjects (3,057 men and 3,604 women) in our analysis (Figure 1).
Figure 1.
Flow diagram of recruitment of participants.
The Institutional Review Board at Shanghai Municipal Center of Disease Control and Prevention approved the study. Informed written consent was obtained from each participant before collecting data and bio-specimen.
Data collection
Information on demographic characteristics and lifestyle factors of the participants was collected by trained interviewers. At the interview, body weight, standing height, waist circumstance (WC) and blood pressure was measured for each participant according to a standard protocol, as described previously [7]. Two measurements were taken and the mean value was used in the analyses. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2) using direct measurements. Hypertension was defined as the systolic/diastolic blood pressure (SBP/DBP) ≥ 140/90 mmHg or using antihypertensive medications due to hypertension.
Laboratory measurements
All participants were asked to maintain their usual physical activities and diets for at least 3 days before having an OGTT. After at least 10 hours of overnight fasting, a 1–1.5 ml venous blood sample was collected for each subject in a vacuum tube containing sodium fluoride for measuring FPG, and a 3–3.5 ml non-anticoagulated venous blood sample was drawn for measuring total cholesterol (TC), triglycerides (TG), high and low density lipoprotein cholesterol (HDL-C and LDL-C). For those with an FPG < 7.0 mmol/l, a standard 75-g glucose load was given and a second blood sample was drawn at 120 minutes after the glucose load to measure 2hPG.
Biochemical assay was conducted in several Community Healthcare Centers according to a standardized protocol. Plasma glucose was tested using glucose oxidase-peroxidase (GOD-PAP) method and serum cholesterol and triglyceride levels were assayed enzymatically using commercial reagents. HbA1c level was assayed using high-performance liquid chromatography (HPLC), which is recommended by the National Glycohemoglobin Standardization Program [16]. The interassay coefficient of variation (CV) was < 1.82% for FPG (SD < 0.23 mmol/l), < 1.38% for TG (SD < 0.02 mmol/l), < 1.54% for TC (SD < 0.08 mmol/l), < 1.60% for HDL-C (SD < 0.01 mmol/l), < 5.30% for LDL-C (SD < 0.21 mmol/l), and < 6.13% for HbA1c (SD < 0.77). An elevated WC was defined as WC ≥ 90 cm for men or ≥ 80 cm for women, and an elevated TG level referred to a fasting serum TG level of ≥ 1.695 mmol/l. Hypertriglyceridemic waist (HW) phenotype was defined as having both an elevated WC and an elevated TG level [17].
Statistical analysis
We used SAS version 9.1 for all statistical analyses. Medians and interquartile ranges of demographic and clinical characteristics of 6,661 participants were presented and compared by sex using Wilcoxon tests. Pearson partial correlation analysis was used to calculate the correlation coefficient of HbA1c level with concentrates of FPG and 2hPG. Restricted cubic splines (RCS) were used to evaluate the potential nonlinear relationship of HbA1c with FPG or 2hPG using the 5th, 25th, 75th and 95th percentiles as fixed knots. ANOVA tests and Spearman rank correlation analyses were applied to compare the differences and the trend of glycemic levels among subgroups with specific demographic and clinical characteristics. We considered P < 0.05 as statistically significant for a two-sided test.
The 1999 WHO diagnostic criteria for T2DM were used as the gold standard. The sensitivity was calculated as the number of subjects correctly classified as diabetes by a certain diagnosing threshold divided by the total number of diabetes by the gold standard, and specificity as the ratio of true negatives to all negatives. The Youden Index (sensitivity + specificity – 1) was used to identify the optimal cut-off point based on ROC curve [18]. Positive predictive value (PPV) was defined as the number of true positives divided by the total number of people who test positive, while negative predictive value (NPV) referred to the proportion of subjects with a negative test result who were correctly diagnosed.
Results
Table 1 shows the demographic and clinical characteristics of participants of the survey. Compared with the men, the women had a lower average level of BMI, WC, SBP, DBP or TG but a higher concentration of TC, HDL-C, LDL-C or 2hPG (all P values < 0.05). No significant difference was observed for age and average levels of FPG and HbA1c between men and women.
Table 1.
Demographic and clinical characteristics of the participants of the study
|
Characteristics |
Median (25th, 75th percentile) |
P value |
||
|---|---|---|---|---|
| All subjects (n = 6,661) | Men (n = 3,057) | Women (n = 3,604) | ||
| Age (years) |
54 (48, 61) |
54 (48, 61) |
54 (48, 60) |
0.6403 |
| BMI (kg/m2) |
24.0 (21.9, 26.3) |
24.2 (22.1, 26.3) |
23.9 (21.8, 26.3) |
0.0315 |
| WC (cm) |
82 (76, 89) |
85 (79, 90) |
80 (74, 87) |
<0.0001 |
| SBP (mmHg) |
123 (113, 135) |
125 (115, 137) |
121 (111, 134) |
<0.0001 |
| DBP (mmHg) |
79 (72, 85) |
80 (73, 87) |
79 (71, 83) |
<0.0001 |
| TC (mmol/l) |
4.60 (4.00, 5.25) |
4.45 (3.87, 5.03) |
4.80 (4.14, 5.41) |
<0.0001 |
| TG (mmol/l) |
1.35 (0.91, 2.01) |
1.40 (0.92, 2.10) |
1.31 (0.90, 1.95) |
0.0003 |
| HDL-C (mmol/l) |
1.30(1.10, 1.55) |
1.21 (1.03, 1.45) |
1.37 (1.17, 1.60) |
<0.0001 |
| LDL-C (mmol/l) |
2.67 (2.20, 3.09) |
2.60 (2.18, 3.00) |
2.71 (2.29, 3.17) |
<0.0001 |
| FPG (mmol/l) |
5.0 (4.6, 5.4) |
5.0 (4.6, 5.4) |
5.0 (4.7, 5.4) |
0.0486 |
| 2hPG (mmol/l)a |
6.0 (5.0, 7.1) |
5.9 (4.8, 7.1) |
6.0 (5.1, 7.1) |
<0.0001 |
| HbA1c (mmol/mol &%) | 37 (32, 40) & 5.5 (5.1, 5.8) | 37 (32, 41) & 5.5 (5.1, 5.9) | 37 (33, 40) & 5.5 (5.2, 5.8) | 0.4235 |
aFor 6,422 participants who had an OGTT (2,919 men and 3,503 women). All P values were from Wilcoxon rank sum tests.
Presented in Additional file 1: Table S1 are the average levels of FPG, 2hPG and HbA1c by demographic and clinical characteristics of the subjects. In both sexes, the levels of FPG, 2hPG and HbA1c increased with age and increasing BMI, with Spearman correlation coefficients varying from 0.10 to 0.25 (all P < 0.05). The participants with elevated WC, hypertension, elevated TG, reduced HDL-C or HW phenotype had a higher concentration of FPG, 2hPG or HbA1c than those without (P < 0.05), but with the difference in the FPG level between subjects with and without reduced HDL-C reaching significant only among women.
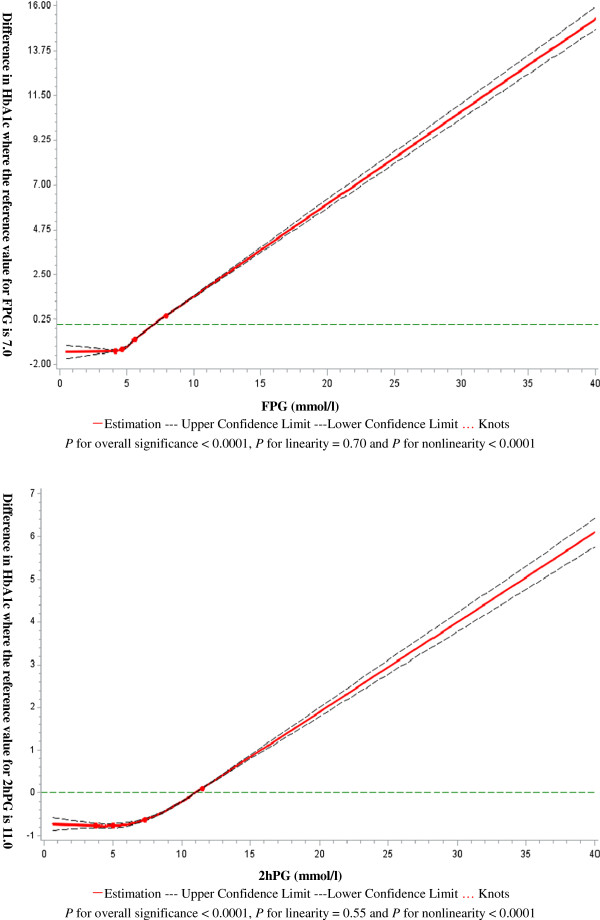
In this population, HbA1c was closely correlated with FPG (r = 0.613, P < 0.0001) and 2hPG (r = 0.299, P < 0.0001) after adjusting for age and sex. As shown in Figure 2, however, nonlinear does-response relationship was observed for HbA1c level with the levels of FPG and 2hPG after accounting for age and sex (P values for non-linear association < 0.0001).
Figure 2.
Nonlinear relationship of HbA1c with FPG and 2hPG using RCS with 4 knots.
Using the 1999 WHO diagnostic criteria as the gold standard, the sensitivity of FPG ≥ 7.0 mmol/l in diagnosing T2DM was 52.6%, while the sensitivity and specificity of HbA1c of 43 mmol/mol (6.1%), the optimal cut-point of the index in this population, reached 75.6% and 89.3%, respectively (Table 2). The optimal threshold of HbA1c for detecting T2DM ranged from 40 to 45 mmol/mol (5.8-6.3%) in the subgroups stratified by sex, age, BMI, WC, TG, HDL-C or presence of hypertension or HW phenotype. These cut-points increased with age and appeared higher in subjects with an elevated TG level or HW phenotype. All the optimal cut-points performed better in sensitivity than did the FPG ≥ 7.0 mmol/l. It is of note that the PPV of the optimal threshold increased with age and increasing BMI, and was higher in the subjects with elevated WC, hypertension, elevated TG, reduced HDL-C or HW phenotype (Table 2).
Table 2.
Performance of the optimal HbA1c cut-points in participants by demographic and clinical characteristics
|
Characteristics |
Sensitivity of FPG ≥ 7.0 mmol/l (%) |
Optimal HbA1c mmol/mol (%) |
Performance of optimal HbA1c value (%) |
|||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |||
| All subjects |
52.6 |
43 (6.1) |
75.6 |
89.3 |
34.1 |
98.0 |
| Gender | ||||||
| Male |
59.0 |
43 (6.1) |
78.2 |
88.8 |
36.7 |
98.0 |
| Female |
45.9 |
43 (6.1) |
72.7 |
89.8 |
31.6 |
98.1 |
| Age | ||||||
| 35-44 |
68.8 |
40 (5.8) |
84.4 |
84.9 |
15.1 |
99.4 |
| 45-54 |
59.9 |
41 (5.9) |
85.9 |
82.5 |
23.5 |
98.9 |
| 55-64 |
51.1 |
42 (6.0) |
82.2 |
81.3 |
27.3 |
98.2 |
| 65-74 |
40.0 |
45 (6.3) |
70.0 |
91.9 |
51.1 |
96.2 |
| BMI | ||||||
| ≤23.0 |
52.9 |
43 (6.1) |
73.6 |
92.7 |
27.0 |
99.0 |
| 23.1-24.9 |
58.0 |
43 (6.1) |
75.0 |
90.1 |
32.9 |
98.2 |
| ≥25.0 |
50.6 |
43 (6.1) |
76.4 |
85.3 |
37.8 |
96.9 |
| Elevated WCa | ||||||
| No |
56.6 |
43 (6.1) |
78.9 |
91.8 |
30.2 |
99.0 |
| Yes |
50.4 |
43 (6.1) |
73.6 |
85.8 |
37.1 |
96.6 |
| Hypertensionb | ||||||
| No |
52.6 |
43 (6.1) |
76.3 |
90.6 |
29.7 |
98.7 |
| Yes |
52.7 |
43 (6.1) |
74.6 |
84.5 |
42.1 |
95.7 |
| Elevated TGc | ||||||
| No |
50.8 |
41 (5.9) |
83.2 |
83.1 |
18.3 |
99.1 |
| Yes |
53.9 |
43 (6.1) |
78.1 |
85.1 |
39.9 |
96.8 |
| Reduced HDL-Cd | ||||||
| No |
56.7 |
43 (6.1) |
72.7 |
89.6 |
31.6 |
98.0 |
| Yes |
46.4 |
43 (6.1) |
79.9 |
88.7 |
38.4 |
98.0 |
| HW phenotypee | ||||||
| No |
53.2 |
43 (6.1) |
75.3 |
90.8 |
30.0 |
98.6 |
| Yes | 51.9 | 43 (6.1) | 75.9 | 82.9 | 42.3 | 95.4 |
aWC ≥ 90 cm in men and ≥ 80 cm in women;
bSBP/DBP ≥ 140/90 mmHg or being on antihypertensive medications;
cTG ≥ 1.695 mmol/l;
dHDL-C < 1.036 mmol/l (men) and < 1.295 mmol/l (women);
ehypertriglyceridemic waist phenotype, defined as having both a high WC ( ≥ 90 cm for men, ≥ 80 cm for women) and an elevated TG level ( ≥ 1.695 mmol/l).
As shown in Additional file 2: Table S2, 239 T2DM were identified by elevated FPG (≥ 7.0 mmol/l) and 215 by increased 2hPG (≥ 11.0 mmol/l). Of the two groups of diabetes patients, 74.1% (177 of 239) and 45.6% (98 of 215) were identified as patients using an HbA1c cut-point of 48 mmol/mol (6.5%) recommended by ADA, producing a sensitivity of 60.6% and a specificity of 96.2%. By using an HbA1c of 45 mmol/mol (6.3%) recommended by Bao et al. for Chinese adults [12], the sensitivity increased to 67.2% and the specificity decreased to 94.0%. HbA1c of 43 mmol/mol (6.1%) achieved a sensitivity of 75.6%, a specificity of 89.3%, a PPV of 34.1% and an NPV of 98.0% in diagnosis of T2DM.
As presented in Table 3, combining use of an FPG ≥ 7.0 mmol/l and an HbA1c ≥ 43 mmol/mol (6.1%) had a higher sensitivity (83.7%) and PPV (36.4%) than using either one of the indicators alone in identify T2DM, together with a specificity of 89.3% and an NPV of 98.0%. Combining use of FPG and HbA1c performed much better among the subjects with an elevated TG level or HW phenotype in the sensitivity (87.4% and 86.6% versus 83.7%) and PPV (42.7% and 44.9% versus 36.4%) than in all subjects, but with a certain decrease in the specificity (85.1% and 82.9% versus 89.3%) and NPV (98.2% and 97.4% versus 98.7%). Combining use of the two indicators was observed to classify 26.9% (272/1011) of pre-diabetes defined by 1999 WHO criteria as diabetes (Additional file 3: Table S3).
Table 3.
Performance of FPG, 2hPG and HbA1c alone and combining use of FPG (mmol/l) and HbA1c (mmol/mol) in diagnosing diabetes
| Population | Subjects | Diabetes | Se (%) | Sp (%) | PPV | NPV |
|---|---|---|---|---|---|---|
| Individual test |
|
|
|
|
|
|
| By FPG alonea |
6,661 |
239 |
52.6 |
100.0 |
100.0 |
96.7 |
| By 2hPG aloneb |
6,422 |
215 |
100.0 |
100.0 |
100.0 |
100.0 |
| By HbA1c alonec |
6,661 |
343 |
75.6 |
89.3 |
34.1 |
98.0 |
| Combining use of FPG and HbA1c | ||||||
| All subjects |
6,661 |
380 |
83.7 |
89.3 |
36.4 |
98.0 |
| Subjects with elevated WCd |
2,810 |
239 |
83.0 |
85.8 |
39.8 |
97.8 |
| Subjects with elevated TGe |
2,385 |
269 |
87.4 |
85.1 |
42.7 |
98.2 |
| Subjects with HWf | 1,320 | 162 | 86.6 | 82.9 | 45.5 | 97.4 |
aFPG ≥ 7.0 mmol/l; b2 hPG ≥ 11.1 mmol/l; cHbA1c ≥ 43 mmol/mol (6.1%); dWC ≥ 90 cm in men, ≥ 80 cm in women; eTG ≥ 1.695 mmol/l; fhypertriglyceridemic waist phenotype, defined as having both a high WC (≥ 90 cm for men, ≥ 80 cm for women) and an elevated TG level (≥ 1.695 mmol/l).
Discussion
In this study including 6,661 Chinese adults randomly selected from a community setting, we find that an HbA1c threshold of 43 mmol/mol (6.1%) performs best in diagnosing T2DM when combining with an FPG ≥ 7.0 mmol/l, particularly among those at a high risk of T2DM. Our results provide additional evidence on the value of HbA1c measurement in identifying diabetes patients in Chinese population.
For diabetes patients, it is diabetic complications occurred subsequently that damage their health and quality of life. The value of OGTT in diagnosing diabetes lies in the close relationship of 2hPG with the risk of diabetic complications. Elevated HbA1c level has also been linked to a higher risk of diabetic retinopathy [19], nephropathy [20], cardiovascular diseases [21,22] and premature death [23,24]. Moreover, HbA1c possesses much lower intra-individual CV (3.6%) than 2hPG (16.7%) and FPG (5.7%), and has been used as the most reproducible and repeatable measurement of glycemic status [25]. Therefore, HbA1c has great potential as a screening or diagnosing tool for T2DM.
Several cross-sectional [11,12] and prospective [10,26] studies have provided evidence on the superior performance of HbA1c to FPG in screening or diagnosing T2DM. Consistent with these reports, we find that the optimal HbA1c threshold of 43 mmol/mol (6.1%) had a higher sensitivity (75.6%) than did the FPG ≥7.0 mmol/l (52.6%) in our population, and produced a specificity of 89.3%, a PPV of 34.1% and an NPV of 98.0%. It is of note that, in this population, the HbA1c cut-point of 45 mmol/mol (6.3%) achieved a slightly higher sensitivity (67.2% vs. 62.8%) and a slightly lower specificity (94.0% vs. 96.1%) than in Bao et al’s report [12]. The older average age of our population may partly explain the difference.
Interestingly, when combining the cut-point of 43 mmol/mol (6.1%) for HbA1c and 7.0 mmol/l for FPG, the sensitivity and the PPV increased to 83.7% and 36.4%, respectively, with the specificity and the NPV as high as by using HbA1c alone. Based on these results and the fact that HbA1c is more stable and convenient than OGTT in practice [12], we recommend that the combination of an HbA1c ≥ 43 mmol/mol (6.1%) and an FPG ≥ 7.0 mmol/l can be used as screening and diagnostic criteria for T2DM in Chinese population. We also find that, among the subjects with an elevated TG level or with HW phenotype, the combination of an HbA1c ≥ 43 mmol/mol (6.1%) and an FPG ≥ 7.0 mmol/l achieved a satisfactory sensitivity and a greatly improved PPV, implicating that the criteria are more efficient in detecting T2DM among subgroups at high risk of T2DM.
Although it has been suggested that HbA1c alone has potential to be used as a diagnostic criterion for T2DM in Chinese adults [12], combining use of the measurement with FPG has two additional advantages. Firstly, genetic variants of hemoglobin (e.g. sickle cell trait, HbS trait, HbC trait, etc.) and some medical conditions that could shorten or conversely prolong the survival of erythrocytes may affect the accuracy of HbA1c measurements [27]. Combining use of HbA1c and FPG can reduce the misdiagnosis of T2DM caused by using HbA1c alone. Secondly, as HbA1c value reflects average level of blood glucose over the preceding 2–3 months, subjects having diabetes in 3 months cannot be detected by testing HbA1c alone. Combining use of HbA1c with FPG can compensate for this weak point.
The main strength of the study is its large sample size. However, the response rate of 67.2% arouses a concern on the representation of the sample, and the lack of characteristics information on the non-participants limits our ability to evaluate the potential selection bias. In this population, age appeared to be the most important characteristic factor linked to different optimal HbA1c cut-off points (Table 2). The older average age of our sample population due to non-response of the subjects who refused to participate or were absent during the enrolment period may lead to a higher cut-off point of HbA1c for diagnosis. However, the non-inclusion of these individuals is unlikely matter in the potential application of the public health tool. Additionally, the biochemical assays were conducted in several Community Healthcare Centers, which may have led to inter-lab bias in measurements of HbA1c. The unified protocol and strict quality control process may help to release our concerns. Moreover, although the sensitivity and specificity of combining use of HbA1c and FPG were as high as 83.7% and 89.3%, respectively, false positive and negative cannot be neglected. Finally, the high cost of HbA1c test may limit its widespread use in China. Compared with FPG assay, HbA1c test is much more expensive. Considering that the cost for HbA1c assay almost equals to that for the OGTT [12,28], however, combining use of HbA1c and FPG will not lead to an extra economic burden comparing with the current application of 1999 WHO diagnostic criteria.
Conclusions
In conclusion, a threshold of HbA1c ≥ 43 mmol/mol (6.1%) in combination with an FPG ≥ 7.0 mmol/l produce a satisfactory sensitivity and specificity for diagnosis of T2DM in our population. Our findings support the combining use of FPG and HbA1c as a screening and diagnosis tool of T2DM in Chinese adults, especially among those at a high risk of the disease. Further epidemiological and clinical studies are warranted to validate our results.
Abbreviations
2hPG: 2 hour plasma glucose; ADA: American Diabetes Association; BMI: Body mass index; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; HbA1c: Glycosylated hemoglobin A1c; HDL-C: High density lipoprotein cholesterol; HW: Hypertriglyceridemic waist (phenotype); IFG: Impaired fasting glucose; IGT: Impaired glucose tolerance; LDL-C: Low density lipoprotein cholesterol; NFG: Normal fasting glucose; NGT: Normal glucose tolerance; OGTT: Oral glucose tolerance test; SBP: Systolic blood pressure; T2DM: Type 2 diabetes; TC: Total cholesterol; TG: Triglycerides; WC: Waist circumference; WHO: World Health Organization.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MM drafted the manuscript. RL and WHX coordinated the study and contributed to study design, statistical analysis, and revision of the manuscript. HZ, GZ and QJ contributed to revision of the manuscript. WZ, YR, LS, DL, QY and YL contributed to data acquisition. WHX and RL are the guarantors of this work and have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Glycemic levels (mean ± SD) in male and female subjects by demographic and clinical characteristics.
Distribution of participants with different glycemic status stratified by WHO recommended criteria and several HbA1c thresholds.
Performance of combining use of FPG (mmol/l) and HbA1c (mmol/mol) in detecting glycemic status defined by WHO criteria.
Contributor Information
Miao Mo, Email: 10211020008@fudan.edu.cn.
Weijian Zhong, Email: wjzhong@scdc.sh.cn.
Genming Zhao, Email: gmzhao@shmu.edu.cn.
Ye Ruan, Email: yruan@scdc.sh.cn.
Hua Zhang, Email: 11211020013@fudan.edu.cn.
Liang Shi, Email: lshi@scdc.sh.cn.
Dajiang Lu, Email: ludajiang2000@yahoo.com.cn.
Qundi Yang, Email: qdyang@scdc.sh.cn.
Yanyun Li, Email: yyli@scdc.sh.cn.
Qingwu Jiang, Email: jiangqw@fudan.edu.cn.
Rui Li, Email: rli@scdc.sh.cn.
Wang-Hong Xu, Email: wanghong.xu@fudan.edu.cn.
Acknowledgements
This study was funded by the Key Program of Shanghai Municipal Committee of Science and Technology (04DZ19502), the Shanghai Medical Development Program (01ZD001) and the Shanghai Municipal Health Bureau (GWDTR201204). The authors thank the study participants of the cross-sectional survey and the healthcare workers in each community involved.
References
- International Expert Committee. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Report of a WHO Consultation. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation 1999. Part 1: Diagnosis and classification of diabetes mellitus. In.; 1999.
- Chinese Diabetes Society. Guidelines for prevention and control of type 2 diabetes in China, 2010 Edition. (In Chinese) Chin J Front Med Sci. 2011;3(6):54–109. [Google Scholar]
- Schneider H, Shaw J, Zimmet P. Guidelines for the detection of diabetes mellitus–diagnostic criteria and rationale for screening. Clin Biochem Rev. 2003;24(3):77–80. [PMC free article] [PubMed] [Google Scholar]
- Hu D, Sun L, Fu P, Xie J, Lu J, Zhou J, Yu D, Whelton PK, He J, Gu D. Prevalence and risk factors for type 2 diabetes mellitus in the Chinese adult population: the InterASIA Study. Diabetes Res Clin Pract. 2009;84(3):288–295. doi: 10.1016/j.diabres.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Lu Q, Tong N, Liu Y, Li N, Tang X, Zhao J, Cao H, Li D, Gou L, Zhang Y. et al. Community-based population data indicates the significant alterations of insulin resistance, chronic inflammation and urine ACR in IFG combined IGT group among prediabetic population. Diabetes Res Clin Pract. 2009;84(3):319–324. doi: 10.1016/j.diabres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Li R, Lu W, Jiang QW, Li YY, Zhao GM, Shi L, Yang QD, Ruan Y, Jiang J, Zhang SN. et al. Increasing prevalence of type 2 diabetes in Chinese adults in Shanghai. Diabetes Care. 2012;35(5):1028–1030. doi: 10.2337/dc11-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed IA. Glycated haemoglobin; past, present, and future are we ready for the change. J Pak Med Assoc. 2011;61(4):383–388. [PubMed] [Google Scholar]
- Gomez-Perez FJ, Aguilar-Salinas CA, Almeda-Valdes P, Cuevas-Ramos D, Lerman GI, Rull JA. HbA1c for the diagnosis of diabetes mellitus in a developing country. A position article. Arch Med Res. 2010;41(4):302–308. doi: 10.1016/j.arcmed.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Perry RC, Shankar RR, Fineberg N, McGill J, Baron AD. HbA1c measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levels of fasting plasma glucose: the Early Diabetes Intervention Program (EDIP) Diabetes Care. 2001;24(3):465–471. doi: 10.2337/diacare.24.3.465. [DOI] [PubMed] [Google Scholar]
- Kumar PR, Bhansali A, Ravikiran M, Bhansali S, Dutta P, Thakur JS, Sachdeva N, Bhadada SK, Walia R. Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: a community-based study. J Clin Endocrinol Metab. 2010;95(6):2832–2835. doi: 10.1210/jc.2009-2433. [DOI] [PubMed] [Google Scholar]
- Bao YQ, Ma X, Li H, Zhou M, Hu C, Wu H, Tang J, Hou X, Xiang K, Jia W. Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: cross sectional epidemiological survey. BMJ. 2010;340:c2249. doi: 10.1136/bmj.c2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, Imperatore G, Williams DE, Albright AL. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33(7):1665–1673. doi: 10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GT, Chan JC, Tsang LW, Cockram CS. Combined use of fasting plasma glucose and HbA1c predicts the progression to diabetes in Chinese subjects. Diabetes Care. 2000;23(12):1770–1773. doi: 10.2337/diacare.23.12.1770. [DOI] [PubMed] [Google Scholar]
- Little RR. Glycated hemoglobin standardization–National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41(9):1191–1198. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gao Y, Chang H, Wang X, Liu D, Zhu Z, Huang G. Hypertriglyceridemic-waist phenotype predicts diabetes: a cohort study in Chinese urban adults. BMC Public Health. 2012;12:1081. doi: 10.1186/1471-2458-12-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Faraggi D, Reiser B, Hu J. Youden Index and the optimal threshold for markers with mass at zero. Stat Med. 2008;27(2):297–315. doi: 10.1002/sim.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Yuan MX, Li HX, Hua L, Feng JP, Shi J, Zhu XR, Cao X, Yang JK. Evaluation for fasting and 2-hour glucose and HbA1c for diagnosing diabetes based on prevalence of retinopathy in a Chinese population. PLoS One. 2012;7(7):e40610. doi: 10.1371/journal.pone.0040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27(6):1259–1264. doi: 10.2337/diacare.27.6.1259. [DOI] [PubMed] [Google Scholar]
- Xu L, Chan WM, Hui YF, Lam TH. Association between HbA1c and cardiovascular disease mortality in older Hong Kong Chinese with diabetes. Diabet Med. 2012;29(3):393–398. doi: 10.1111/j.1464-5491.2011.03456.x. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- Kerr D, Partridge H, Knott J, Thomas PW. HbA1c 3 months after diagnosis predicts premature mortality in patients with new onset type 2 diabetes. Diabet Med. 2011;28(12):1520–1524. doi: 10.1111/j.1464-5491.2011.03443.x. [DOI] [PubMed] [Google Scholar]
- Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, Fulcher G, De Galan BE, Harrap S, Hamet P. et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55(3):636–643. doi: 10.1007/s00125-011-2404-1. [DOI] [PubMed] [Google Scholar]
- Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- Wang WY, Lee ET, Howard BV, Fabsitz RR, Devereux RB, Welty TK. Fasting plasma glucose and hemoglobin A1c in identifying and predicting diabetes: the strong heart study. Diabetes Care. 2011;34(2):363–368. doi: 10.2337/dc10-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med. 2007;24(4):333–343. doi: 10.1111/j.1464-5491.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- Colagiuri S. Glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus–practical implications. Diabetes Res Clin Pract. 2011;93(3):312–313. doi: 10.1016/j.diabres.2011.06.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glycemic levels (mean ± SD) in male and female subjects by demographic and clinical characteristics.
Distribution of participants with different glycemic status stratified by WHO recommended criteria and several HbA1c thresholds.
Performance of combining use of FPG (mmol/l) and HbA1c (mmol/mol) in detecting glycemic status defined by WHO criteria.