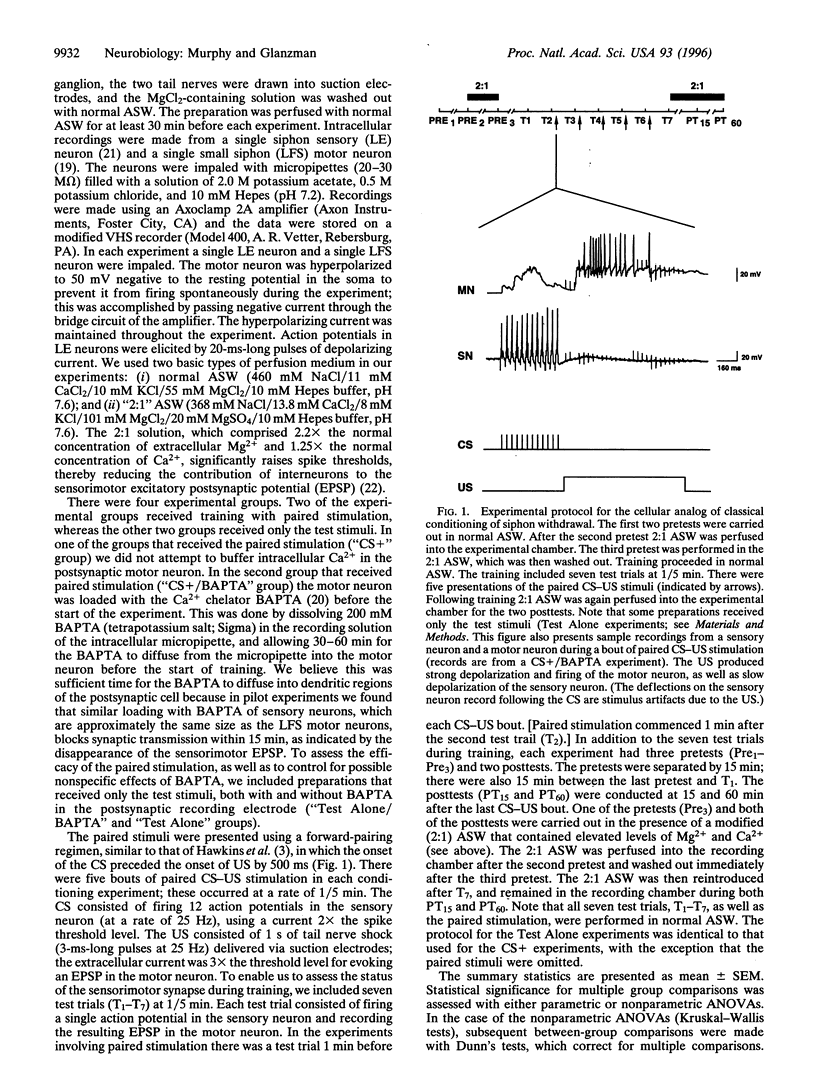
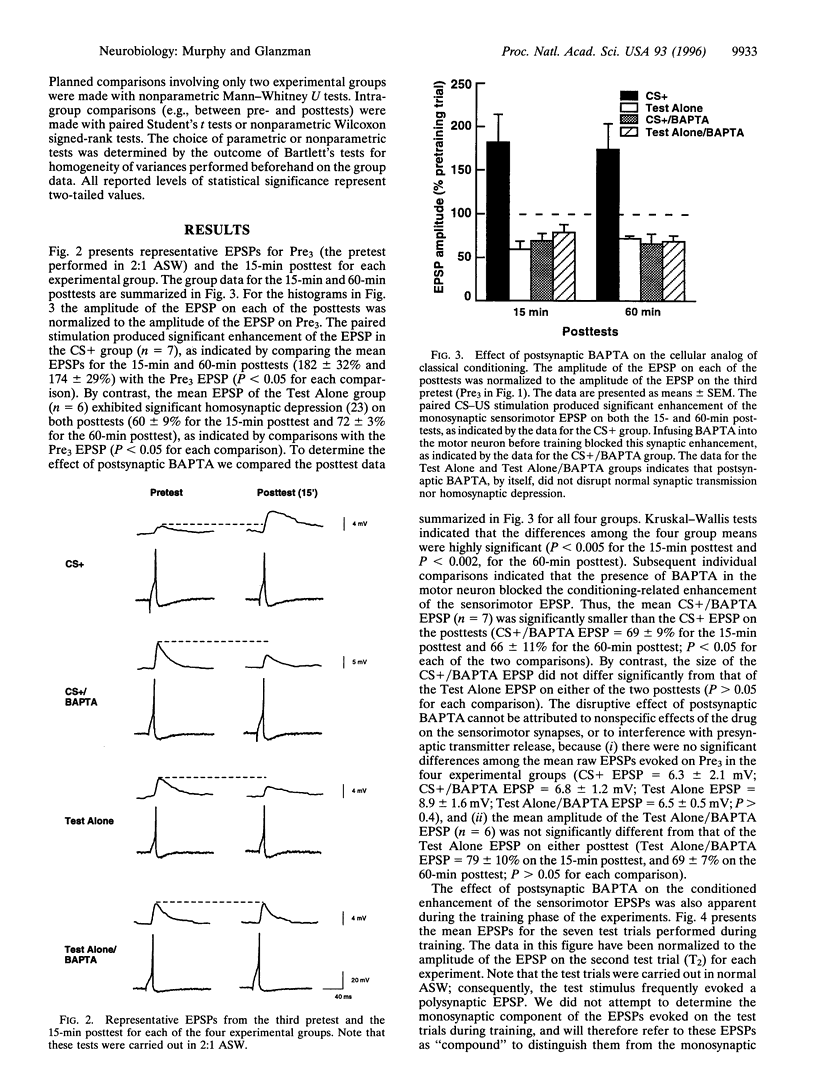
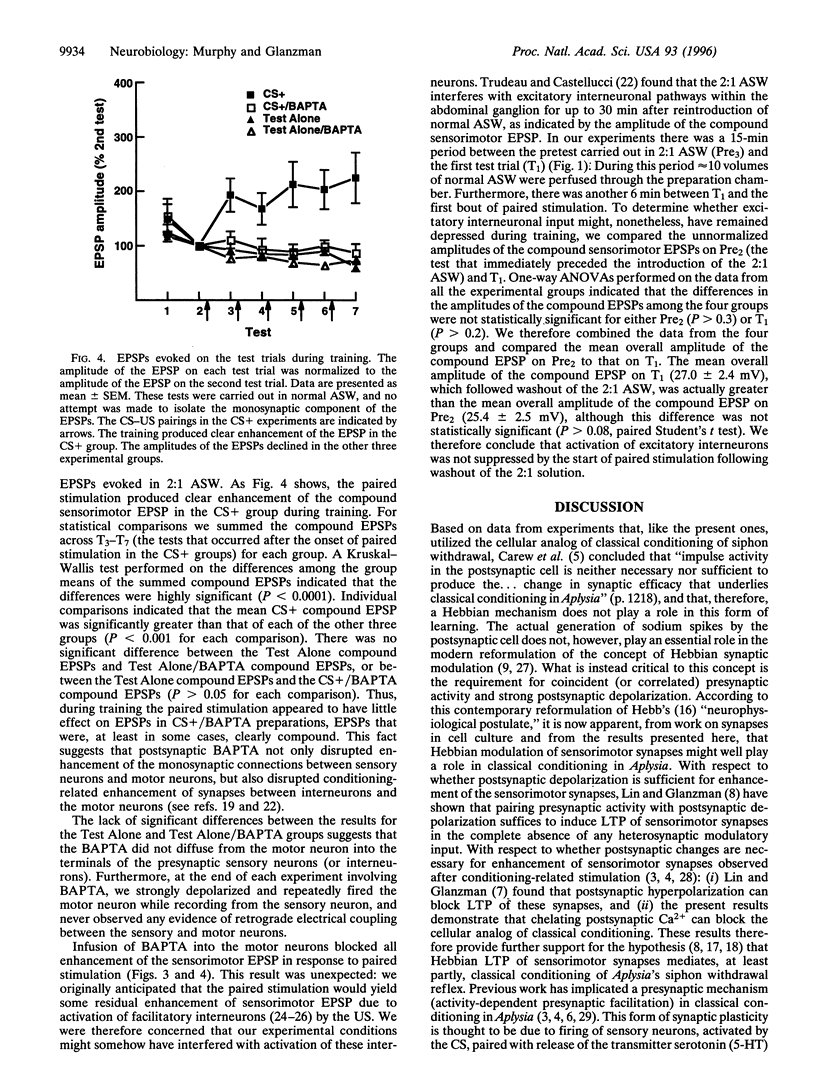
Abstract
Classical conditioning of Aplysia's siphon-withdrawal reflex is thought to be due to a presynaptic mechanism-activity-dependent presynaptic facilitation of sensorimotor connections. Recent experiments with sensorimotor synapses in dissociated cell culture, however, provide an alternative cellular mechanism for classical conditioning-Hebbian long-term potentiation (LTP) of sensorimotor connections. Induction of Hebbian LTP of these connections is mediated by activation of N-methyl-D-aspartate-related receptors and requires the postsynaptic elevation of intracellular Ca2+. To determine whether the enhancement of sensorimotor synapses during classical conditioning in Aplysia-like LTP of sensorimotor synapses in culture-also depends upon the elevation of postsynaptic Ca2+, we carried out experiments involving the cellular analog of classical conditioning of siphon withdrawal. We examined changes in the strength of monosynaptic siphon sensorimotor connections in the abdominal ganglion of Aplysia following paired presentations of sensory neuron activation and tail nerve shock. This training regimen resulted in significant enhancement of the monosynaptic sensorimotor excitatory postsynaptic potential, as compared with the sensorimotor excitatory postsynaptic potential in preparations that received only test stimulation. Infusing the motor neuron with 1,2-bis(2-aminophenoxy)ethane-N,N-N',N'-tetraacetic acid, a specific chelator of intracellular Ca2+, prior to paired stimulation training blocked this synaptic enhancement. Our results implicate a postsynaptic, possibly Hebbian, mechanism in classical conditioning in Aplysia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. H., Kairiss E. W., Keenan C. L. Hebbian synapses: biophysical mechanisms and algorithms. Annu Rev Neurosci. 1990;13:475–511. doi: 10.1146/annurev.ne.13.030190.002355. [DOI] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Bröcher S., Artola A., Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992 Feb 21;573(1):27–36. doi: 10.1016/0006-8993(92)90110-u. [DOI] [PubMed] [Google Scholar]
- Buonomano D. V., Byrne J. H. Long-term synaptic changes produced by a cellular analog of classical conditioning in Aplysia. Science. 1990 Jul 27;249(4967):420–423. doi: 10.1126/science.2165631. [DOI] [PubMed] [Google Scholar]
- Byrne J. H., Kandel E. R. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996 Jan 15;16(2):425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J., Castellucci V., Kandel E. R. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf in Aplysia. J Neurophysiol. 1974 Sep;37(5):1041–1064. doi: 10.1152/jn.1974.37.5.1041. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Hawkins R. D., Abrams T. W., Kandel E. R. A test of Hebb's postulate at identified synapses which mediate classical conditioning in Aplysia. J Neurosci. 1984 May;4(5):1217–1224. doi: 10.1523/JNEUROSCI.04-05-01217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T. J., Hawkins R. D., Kandel E. R. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983 Jan 28;219(4583):397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Walters E. T., Kandel E. R. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981 Dec;1(12):1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. A., Hawkins R. D., Kandel E. R. Activity-dependent enhancement of presynaptic facilitation provides a cellular mechanism for the temporal specificity of classical conditioning in Aplysia. Learn Mem. 1994 Nov-Dec;1(4):243–257. [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Kandel E. R. L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot L. S., Hawkins R. D., Kandel E. R., Schacher S. Pairing-specific, activity-dependent presynaptic facilitation at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1994 Jan;14(1):368–383. doi: 10.1523/JNEUROSCI.14-01-00368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost W. N., Clark G. A., Kandel E. R. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988 Jun;19(4):297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Glanzman D. L., Mackey S. L., Hawkins R. D., Dyke A. M., Lloyd P. E., Kandel E. R. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989 Dec;9(12):4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman D. L. Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture. J Neurobiol. 1994 Jun;25(6):666–693. doi: 10.1002/neu.480250608. [DOI] [PubMed] [Google Scholar]
- Glanzman D. L. The cellular basis of classical conditioning in Aplysia californica--it's less simple than you think. Trends Neurosci. 1995 Jan;18(1):30–36. doi: 10.1016/0166-2236(95)93947-v. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Castellucci V. F., Kandel E. R. Interneurons involved in mediation and modulation of gill-withdrawal reflex in Aplysia. II. Identified neurons produce heterosynaptic facilitation contributing to behavioral sensitization. J Neurophysiol. 1981 Feb;45(2):315–328. doi: 10.1152/jn.1981.45.2.315. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Schacher S. Identified facilitator neurons L29 and L28 are excited by cutaneous stimuli used in dishabituation, sensitization, and classical conditioning of Aplysia. J Neurosci. 1989 Dec;9(12):4236–4245. doi: 10.1523/JNEUROSCI.09-12-04236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. F., Johnston D. Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science. 1984 Oct 19;226(4672):350–352. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Clifford D. B., Zorumski C. F. Norepinephrine reverses N-methyl-D-aspartate-mediated inhibition of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992 Aug 17;142(2):163–166. doi: 10.1016/0304-3940(92)90364-d. [DOI] [PubMed] [Google Scholar]
- Kelso S. R., Ganong A. H., Brown T. H. Hebbian synapses in hippocampus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. Y., Glanzman D. L. Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: partial requirement for activation of an NMDA-related receptor. Proc Biol Sci. 1994 Mar 22;255(1344):215–221. doi: 10.1098/rspb.1994.0031. [DOI] [PubMed] [Google Scholar]
- Lin X. Y., Glanzman D. L. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proc Biol Sci. 1994 Feb 22;255(1343):113–118. doi: 10.1098/rspb.1994.0016. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Mackey S. L., Kandel E. R., Hawkins R. D. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci. 1989 Dec;9(12):4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Malinow R., Miller J. P. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986 Apr 10;320(6062):529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- Sahley C. L. Serotonin depletion impairs but does not eliminate classical conditioning in the leech Hirudo medicinalis. Behav Neurosci. 1994 Dec;108(6):1043–1052. doi: 10.1037//0735-7044.108.6.1043. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull. 1987 Jan;18(1):115–119. doi: 10.1016/0361-9230(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sejnowski T. J. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989 May 18;339(6221):215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Trudeau L. E., Castellucci V. F. Contribution of polysynaptic pathways in the mediation and plasticity of Aplysia gill and siphon withdrawal reflex: evidence for differential modulation. J Neurosci. 1992 Oct;12(10):3838–3848. doi: 10.1523/JNEUROSCI.12-10-03838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci. 1985 Mar;5(3):662–672. doi: 10.1523/JNEUROSCI.05-03-00662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B., Huang Y. Y., Abraham W. C. Hippocampal long-term potentiation is induced by pairing single afferent volleys with intracellularly injected depolarizing current pulses. Acta Physiol Scand. 1986 Feb;126(2):317–319. doi: 10.1111/j.1748-1716.1986.tb07822.x. [DOI] [PubMed] [Google Scholar]



