Background: Regulation of TRPV1 by phosphoinositides is controversial.
Results: ATP reactivates TRPV1 after run-down in excised inside-out patches by generating phosphoinositides; many other negatively charged lipids may also support TRPV1 activity.
Conclusion: Despite its promiscuous activation, phosphoinositides are the key endogenous cofactors for TRPV1 activity.
Significance: Our data may reconcile discordant data obtained in different experimental settings.
Keywords: Electrophysiology, Inositol Phospholipid, Ion Channels, Phosphoinositides, TRP Channels, TRPV1
Abstract
The regulation of the heat- and capsaicin-activated transient receptor potential vanilloid 1 (TRPV1) channels by phosphoinositides is controversial. Data in cellular systems support the dependence of TRPV1 activity on phosphoinositides. The purified TRPV1, however, was recently shown to be fully functional in artificial liposomes in the absence of phosphoinositides. Here, we show that several other negatively charged phospholipids, including phosphatidylglycerol, can also support TRPV1 activity in excised patches at high concentrations. When we incorporated TRPV1 into planar lipid bilayers consisting of neutral lipids, capsaicin-induced activity depended on phosphatidylinositol 4,5-bisphosphate. We also found that TRPV1 activity in excised patches ran down and that MgATP reactivated the channel. Inhibition of phosphatidylinositol 4-kinases or enzymatic removal of phosphatidylinositol abolished this effect of MgATP, suggesting that it activated TRPV1 by generating endogenous phosphoinositides. We conclude that endogenous phosphoinositides are positive cofactors for TRPV1 activity. Our data highlight the importance of specificity in lipid regulation of ion channels and may reconcile discordant data obtained in various experimental settings.
Introduction
Transient receptor potential vanilloid 1 (TRPV1)3 is a polymodal cation channel, activated by heat, low tissue pH, capsaicin, and a variety of endogenous mediators of nociception. Proinflammatory agents released upon tissue damage, most of which act through the phospholipase C (PLC) pathway, sensitize these channels to their activators. It was proposed that upon PLC activation, the decrease in the concentration of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) sensitizes the channel by relieving a tonic inhibition by this lipid (1).
Subsequent studies, however, showed that in excised patches, TRPV1 activity is enhanced by the application of various phosphoinositides, including PI(4,5)P2 and PI(4)P (2–5). Data with various inducible lipid phosphatases in intact cells also supported the positive regulatory role of phosphoinositides, though the relative contributions of PI(4,5)P2 and PI(4)P are debated (4, 6–8). Depletion of phosphoinositides via Ca2+-induced activation of PLC has been shown to be a major factor in desensitization of TRPV1 (3, 8, 9). These data argued for phosphoinositides being positive cofactors for TRPV1 activity.
Despite the clear positive regulation of TRPV1 activity by phosphoinositides both in excised patches and intact cells, there are data supporting the existence of a concurrent, partial inhibitory effect of PI(4,5)P2 in intact cells (3, 8, 10, 11). The lack of data demonstrating that PI(4,5)P2 inhibits TRPV1 in excised patches suggested that this effect may be indirect (12).
In a recent study, TRPV1 was purified and functionally reconstituted in artificial liposomes of defined composition (13). Two key findings of the study with respect to phosphoinositide regulation of these channels were as follows: 1) TRPV1 was fully active in the absence of any phosphoinositides; 2) when the phosphoinositides PI, PI(4)P, or PI(4,5)P2 were added to the lipid mix, the capsaicin dose-response curve was shifted to the right, i.e. the channel was partially inhibited by these lipids. These findings suggest direct inhibition by PI(4,5)P2 and no dependence of activity on phosphoinositides.
Can these findings be reconciled with earlier excised patch data? To address this apparent contradiction, we studied TRPV1 behavior in excised patches, taking advantage of the intrinsic lipid kinase and phosphatase activities in the patch membrane that can be stimulated with MgATP (14, 15). This technique studies the effects of endogenous phosphoinositides and avoids application of exogenous lipids, which have been argued to cause non-physiological effects (13, 16).
We also re-examined the lipid specificity of the channel and found that, in addition to phosphoinositides, TRPV1 was activated by a variety of negatively charged lipids, including phospholipids, such as phosphatidylglycerol (PG) and phosphatidylinositol (PI), as well as other negatively charged lipids, such as oleoyl-CoA and the artificial lipid 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (DGS-NTA). This may explain the lack of requirement for phosphoinositides in reconstituted vesicles that contained high concentrations of PG (13). Accordingly, when we reconstituted TRPV1 in planar lipid bilayers consisting of neutral lipids, capsaicin-induced activity depended on the presence of PI(4,5)P2. Our data may reconcile discordant data obtained in various experimental settings, and we conclude that in cellular membranes TRPV1 activity depends on the presence of PI(4,5)P2.
EXPERIMENTAL PROCEDURES
Electrophysiology in Oocytes
Excised inside-out patch clamp measurements were performed as described previously (3). Briefly, Xenopus laevis oocytes were injected with the cRNA of TRPV1 transcribed from the rat TRPV1 clone in the pGEMSH vector. Measurements were performed 5–7 days after injection, using borosilicate glass pipettes (World Precision Instruments) of 0.4–0.7 megohm resistance, filled with a solution containing the following: 96 mm NaCl, 2 mm KCl, 1 mm MgCl2, and 5 mm HEPES (pH 7.4), substituted with 0.2 μm, 0.5 μm, or 4 μm capsaicin (as indicated in figure legends). For experiments with TRPV6, we used a pipette solution devoid of capsaicin, consisting of the following: 96 mm LiCl, 1 mm EGTA, and 5 mm HEPES, (pH 7.4). After establishing gigaohm resistance seals on devitellinized surfaces of oocytes, inside-out configuration was established, and currents were measured using a ramp protocol from −100 to +100 mV applied every second. The main perfusing solution contained the following: 96 mm KCl, 5 mm EGTA, and 10 mm HEPES, with pH adjusted to 7.4. Currents were amplified with an Axopatch 200B unit and analyzed with the pClamp software (version 9.2, Molecular Devices). Measurements were performed at 18–20 °C. Various stimulating solutions were applied to the internal side of the inside-out membrane patch using a custom-made, gravity-driven micro-perfusion system.
Expression and Purification of TRPV1
HEK-293 cells transiently expressing TRPV1 tagged with the Myc epitope on its N terminus were grown to 70–80% confluence, washed, and collected with cold PBS. Cells were harvested and resuspended in NCB buffer containing 500 mm NaCl, 50 mm NaH2PO4, 20 mm HEPES, and 10% glycerol (pH 7.5), with addition of 1 mm of the protease inhibitor PMSF and 5 mm β-mercaptoethanol. Then, the cells were lysed by the freeze-thawing method and centrifuged at 40,000 × g for 2.5 h, and the pellet was resuspended in NCB buffer with addition of a protease inhibitor mixture (Roche Applied Science), 0.1% Nonidet P40 (Roche Applied Science), and 0.5% dodecyl maltoside (CalBiochem, San Diego, CA). The suspension was incubated overnight at 4 °C on a shaker with gentle agitation and then centrifuged for 1 h at 40,000 g. Further, the TRPV1 protein was purified by immunoprecipitation using AG-protein magnetic beads (Thermo Fisher Scientific Inc., Rockford, IL, USA) conjugated with the Myc antibodies. Protein was eluted from the beads with Myc peptide (150 μg/ml; Sigma-Aldrich). All steps of purification were performed at 4 °C. The purified protein ran on SDS gels mainly as a tetramer, see Fig. 6F.
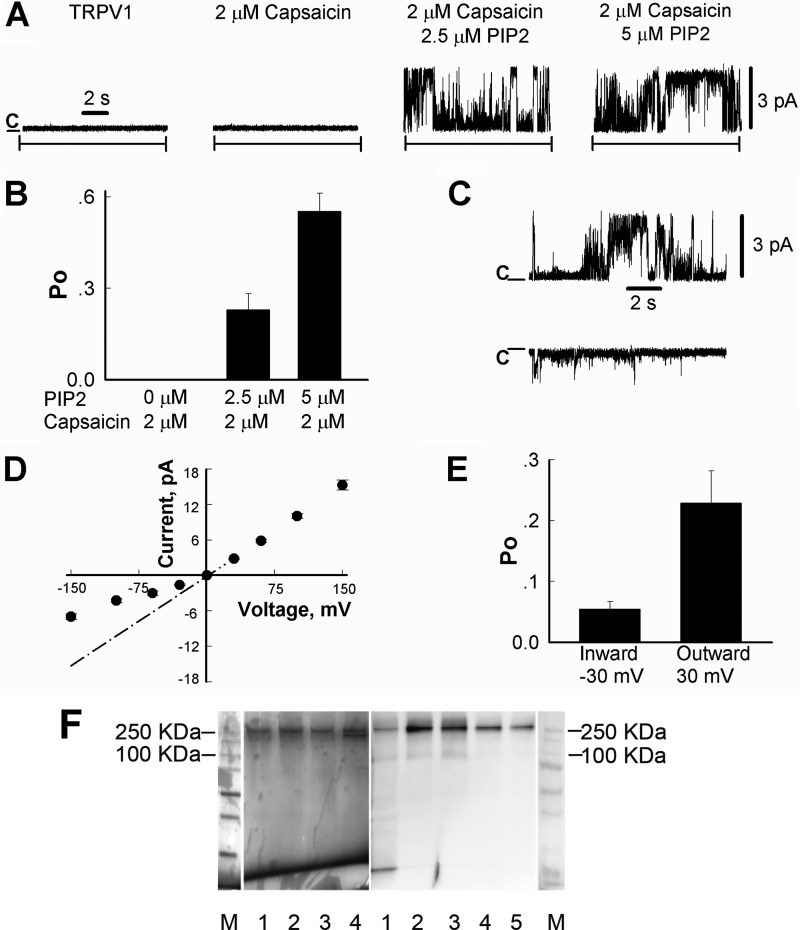
FIGURE 6.
Activation of TRPV1 channels in planar lipid bilayers by capsaicin and PI(4,5)P2. A, representative single-channel current recordings of TRPV1 channels incorporated in planar lipid bilayers at +30 mV, as described under “Experimental Procedures.” Capsaicin was added to both compartments, whereas diC8 PI(4,5)P2 was added only to the cis compartment. B, open probability of TRPV1 channels in the presence of 2.5 μm and 5 μm DiC8 PI(4,5)P2 measured at +30 mV. Data were analyzed from a total of four experiments. C, representative current traces of outward (upper trace) and inward (lower trace) currents of TRPV1 channels in the presence of 2 μm capsaicin and 2.5 μm PI(4,5)P2 with clamping potentials of +30 mV and −30 mV, respectively. D, current-voltage relationship of TRPV1 in the presence of 2 μm capsaicin and 2.5 μm PI(4,5)P2. The dashed line corresponds to the mean conductance of fully open channels, working in an inward direction, this state is rarely observed due to the low open probability of this subconductance level. Data were analyzed from a total of six experiments. E, open probability of TRPV1 channels measured at +30 mV and −30 mV in the presence of 2 μm capsaicin and 2.5 μm PI(4,5)P2. Data were analyzed from a total of four experiments. F, purification of the full-length TRPV1 protein. TRPV1 protein was purified as described under “Experimental Procedures.” Left panel, silver staining results; right panel, Western blot results with an anti-Myc antibody. M, molecular mass standards; left panel, lanes 1–4, purified TRPV1; right panel, lane 1, cell lysate; lanes 2–5, purified TRPV1.
Planar Lipid Bilayer Experiments
Planar lipid bilayer experiments were performed as described earlier (17). Bilayers were formed from a solution of synthetic 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (phosphatidylcholine, PC, or POPC) and 1-palmitoyl-2-oleoyl-glycero-3-phosphoethanolamine (phosphatidylethanolamine, PE or POPE) in a 3:1 ratio in n-decane (Sigma-Aldrich). The solution was used to paint a bilayer in an aperture of ∼150 μm diameter in a Delrin cup (Warner Instruments) between symmetric aqueous bathing solutions of 150 mm KCl, 0.02 mm MgCl2, and 20 mm HEPES (pH 7.2), at 22 °C. Bilayer capacitances were in the range of 50–75 pF. The purified TRPV1 protein derived in dodecyl-maltoside micelles was incorporated into lipid micelles consisting of a mixture of POPC/POPE (3:1, v/v). After the bilayers were formed, 0.2 μl of the TRPV1 micellar solution (0.001 μg/ml) was painted to the aperture with an air bubble glass capillary. Unitary currents were recorded with an Axopatch 200B amplifier. Currents through the voltage-clamped bilayers (background conductance < 3 pS) were filtered at the amplifier output (low pass, −3 dB at 10 kHz, 8-pole Bessel response). Data were secondarily filtered at 100 Hz through an 8-pole Bessel filter (950 TAF; Frequency Devices, Ottawa, IL) and digitized at 1 kHz using an analog-to-digital converter (Digidata 1322A; Molecular Devices) controlled by pClamp (version 9, Molecular Devices). Data were analyzed using the Clampfit software (version 9, Molecular Devices). All experiments were conducted at room temperature (22 °C).
Materials
Dioctanoyl (DiC8) phosphoinositides were purchased from Cayman Chemical (Ann Arbor, MI). Capsaicin, oleoyl-CoA, and phosphatidylinositol-specific phospholipase C (PI-PLC) were purchased from Sigma, the latter in two different preparations, both as a powder, and as a glycerol containing solution. The following were purchased from Avanti Polar Lipids (Alabaster, AL): arachidonyl-stearyl (AASt) phosphoinositides; 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (phosphatidylserine, PS, or POPS); 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-1′-rac-glycerol (phosphatidylglycerol, PG or POPG); POPC; POPE; and DGS-NTA.
Long acyl chain phospholipids that were applied at high concentrations, such as PG, PS, PC and PI are poorly soluble in water and form micelles in aqueous solutions, resulting in visible turbidity at the nominal concentrations applied in our experiments (250–500 μm). Extensive sonication of these solutions was necessary for their effects to be reliable; we used an Ultrasonic Homogenizer model 3000 V/T (Biologics, Inc). During the course of an experimental day, we found that resonication of these solutions approximately every 1–1.5 h resulted in more reproducible effects of these lipids.
Data Analysis
Data are presented as mean ± S.E.; statistical significance is calculated with t test; *, p < 0.05; **, p < 0.01.
RESULTS
MgATP Reactivates TRPV1 Channels after Run-down in a Phosphatidylinositol 4-Kinase (PI4K)-dependent Manner
We studied TRPV1 channels in large excised inside-out patches in Xenopus oocytes with capsaicin in the patch pipette. After excision, we usually observed a small transient increase of TRPV1 currents (presumably due to differences in ionic composition of the bath solution and the intracellular environment of the cell), followed invariably by a decrease in channel activity to 10–15% of the cell-attached levels in ∼2–3 min, termed run-down (Fig. 1, A and C–F). Run-down is characteristic of PI(4,5)P2-dependent ion channels and is generally assumed to reflect dephosphorylation of PI(4,5)P2 by lipid phosphatases associated with the patch membrane (18). These experiments were performed in the presence of 0.2 μm capsaicin in the patch pipette. With 4 μm capsaicin in the patch pipette, run-down was much slower and usually incomplete within 5 min (Fig. 1, F and G). The velocity of run-down has been shown earlier to inversely correlate with the apparent affinity of phosphoinositide-sensitive channels to PI(4,5)P2 (18, 19). This inverse correlation is consistent with our earlier finding that capsaicin increases the apparent affinity of the channel for PI(4,5)P2 (3). In most experiments, we used 0.2 or 0.5 μm capsaicin, a condition under which substantial run-down was observed within 2–3 min (Fig. 1F).
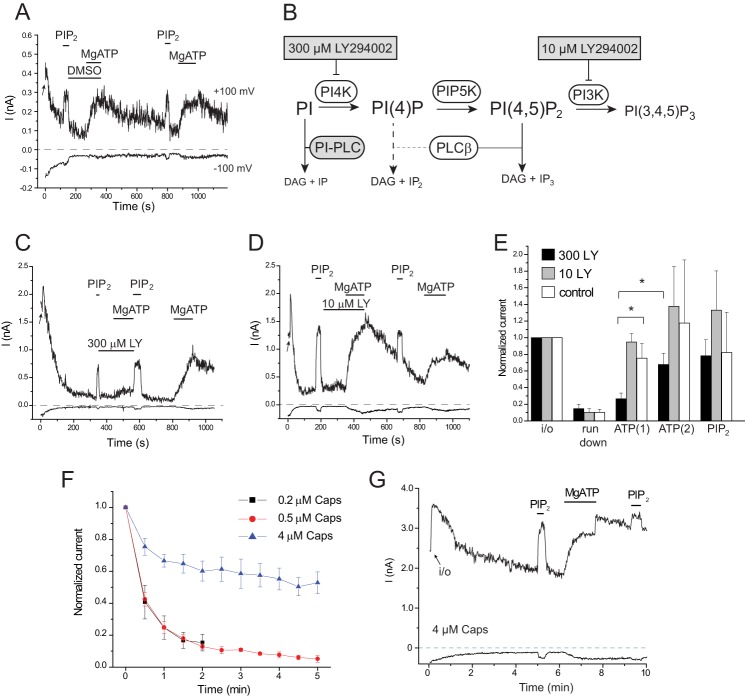
FIGURE 1.
MgATP reactivates TRPV1 after run-down in a PI4K-dependent manner. Excised inside-out patch clamp measurements were performed as described under “Experimental Procedures” on TRPV1 expressing Xenopus oocytes. Capsaicin (0.2 μm) was included in the patch pipette. A, representative current traces at −100 and +100 mV; the establishment of the inside-out configuration is indicated by the arrow; the applications of 25 μm DiC8 PI(4,5)P2, 2 mm Mg2+ and 2 mm ATP (MgATP), and 0.3% dimethyl sulfoxide (DMSO) are indicated with horizontal lines. B, schematic of phosphoinositide metabolism, showing the effects of LY294002 and PI-PLC. DAG, diacylglycerol; PIP5K, phosphatidylinositol 4-phosphate 5-kinase. C and D, similar experiments to that shown in A, with application of 300 μm and 10 μm LY294002 (LY). E, statistical summary normalized to current values at +100 mV immediately after the establishment of the inside-out configuration (n = 5–8). F, velocity of run-down depends on the capsaicin (Caps) concentration. Relative TRPV1 current values, normalized to the amplitude immediately after excision, are plotted for 0.2, 0.5, and 4 μm capsaicin in the patch pipette, mean ± S.E. for n = 6–9 experiments G, representative trace for current run-down in the presence of 4 μm capsaicin in the patch pipette.
Fig. 1A shows that after run-down, channel activity could be restored by the application of MgATP. This, again, is a characteristic of PI(4,5)P2-dependent ion channels (18, 20). To demonstrate that MgATP exerts its effect via lipid kinases, we used LY294002 to inhibit PI4K. This drug selectively inhibits PI3K at low concentrations and also inhibits type-III PI4Ks at high concentrations (Fig. 1B). As shown in Fig. 1, C–E, LY294002 inhibited the effect of MgATP at 300 μm but not at 10 μm, where the drug selectively inhibits PI3Ks. This result suggests that MgATP exerts its effect by generation of phosphoinositides via PI4K.
Intracellular ATP has been described to have a direct positive effect on TRPV1, an effect which does not require Mg2+ (21, 22). We also saw a quickly developing effect of ATP (4 mm) in the absence of Mg2+ (data not shown). Our MgATP solutions contained 2 mm Mg2+ and 2 mm ATP, in which the free ATP and free Mg2+ concentrations were 0.43 mm and 0.31 mm, respectively (MaxChelator). In our study, this concentration of MgATP had a negligible direct effect, judged by the essential absence of quickly developing currents. This could be due to either the low free ATP concentration or the direct inhibition by free Mg2+, see the occasional quick increase in current amplitudes after wash out of MgATP (Figs. 1G and 2A).
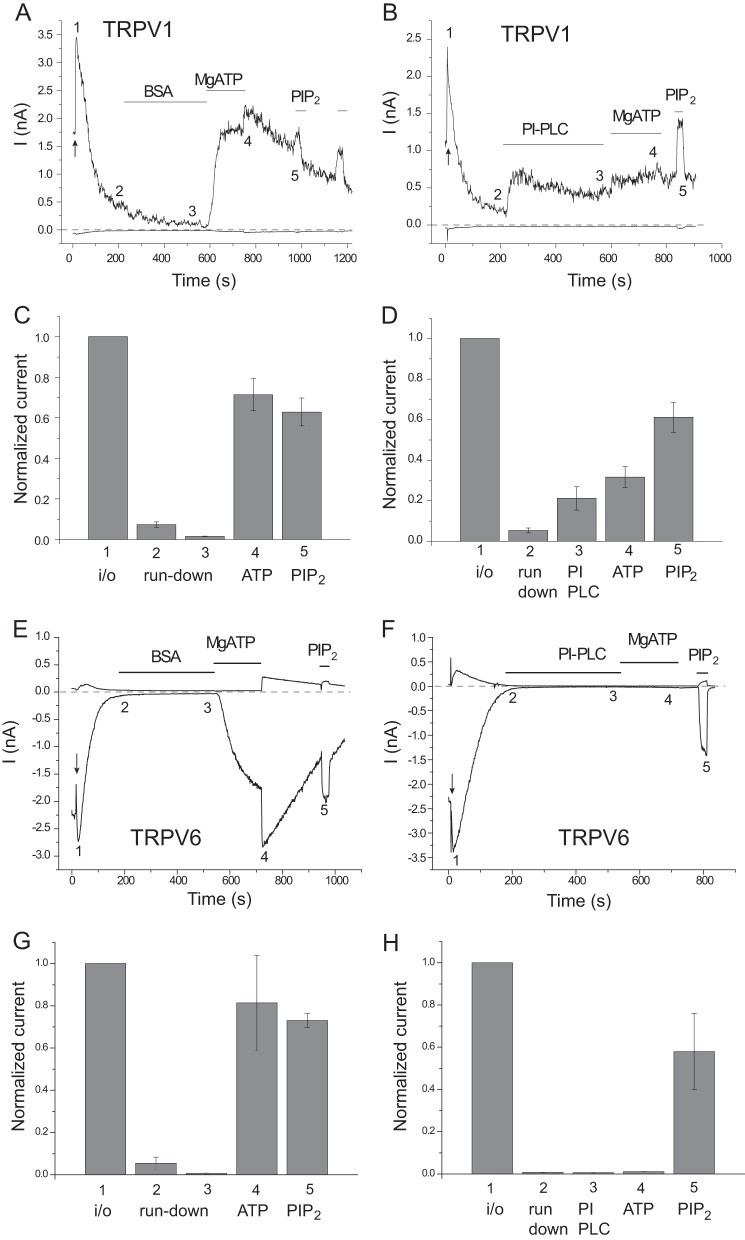
FIGURE 2.
The effect of PI-PLC supplemented with BSA. Excised inside-out patch clamp measurements were performed as described under “Experimental Procedures” on TRPV1 expressing Xenopus oocytes; traces show currents at +100 and −100 mV. Here, we used a PI-PLC preparation supplied as a lyophilized powder; to stabilize PI-PLC in these experiments, we supplemented the experimental solution with 0.05% BSA, as recommended by the supplier, to prevent it from adsorbing to the walls of the perfusion tubes. A and B, representative measurements on TRPV1 with 0.5 μm capsaicin in the patch pipette; the establishment of the inside-out configuration is indicated by the arrow; the applications of 1 unit/ml PI-PLC, 0.05% BSA, 2 mm MgATP, and 50 μm DiC8 PI(4,5)P2 are indicated by the horizontal lines. C and D, statistical summary at the time points indicated in A and B; current values are normalized to the current amplitude immediately after excision (n = 8). E–H, similar measurements on TRPV6.
PI-PLC Prevents Activation by MgATP
Next, we tested a bacterial PI-PLC directly applied to excised patches. PI-PLC selectively hydrolyzes PI without any direct effect on PI(4)P or PI(4,5)P2 (14, 20). If MgATP acted as a substrate for lipid kinases to generate phosphoinositides, cleaving the phosphoinositide precursor PI would eliminate its effect (Fig. 1B), as we showed earlier with TRPV6 channels (15). First, we dissolved the enzyme in bath solution, and even though it inhibited the effect of MgATP on TRPV1, the data were quite variable, with a general tendency to lose activity over time even in control experiments with TRPV6 (data not shown).
To stabilize PI-PLC, we supplemented the solution with 0.05% BSA, which prevents it from adsorbing to the walls of the tubes, as recommended by the supplier. Fig. 2, A–D, shows that pretreatment with PI-PLC almost completely prevented the effect of MgATP. PI-PLC treatment by itself also induced a partial reactivation of TRPV1 after run-down, consistent with earlier results (1). PI-PLC did not increase the activity of TRPV6, but it also prevented the reactivating effect of MgATP (Fig. 2, E–H), consistent with earlier reports (15).
Since PI-PLC did not increase TRPV1 activity substantially when we dissolved it without BSA (data not shown), we also tested a different preparation of PI-PLC, supplied as a glycerol-containing stock solution, see methods, which does not require BSA for stabilization. This preparation of PI-PLC behaved very similarly to the previous one, i.e. it partially activated TRPV1 and eliminated the effect of MgATP (Fig. 3). These data show that the partial activation by PI-PLC is not due to the presence of BSA, but likely requires high activity of the enzyme.
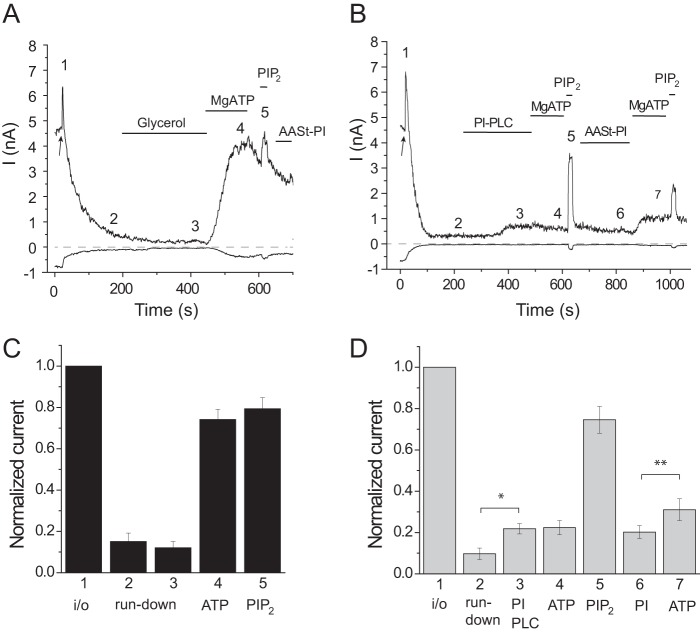
FIGURE 3.
The effects of PI-PLC in excised patches. In these experiments, we used a preparation of PI-PLC that was purchased as a liquid in a glycerol-containing solution. Excised inside-out patch clamp measurements were performed as described under “Experimental Procedures” on TRPV1-expressing Xenopus oocytes; traces show currents at +100 and −100 mV, and the pipette solution contained 0.5 μm capsaicin. A, representative control measurement, the establishment of the inside-out configuration is indicated by the arrow; the patch was perfused with bath solution containing 0.5% glycerol (vehicle) and then 2 mm MgATP, 50 μm DiC8 PI(4,5)P2, and 250 μm AASt PI. B, similar measurement, the patch was treated with 1 unit/ml PI-PLC. C and D, summaries of current amplitudes at the time points indicated in the representative traces normalized to the current amplitude immediately after excision (n = 8).
It could be argued that PI-PLC activated TRPV1 by removing the inhibitory PI. To test this possibility, we applied long acyl chain natural AASt PI after PI-PLC treatment. PI failed to significantly re-inhibit channel activity, arguing that PI-PLC did not increase channel activity by eliminating the putative inhibitory effect of PI. It did, however, partially restore the effect of MgATP (Fig. 3B and D), demonstrating that the lipid indeed incorporated into the patch membrane.
Promiscuous Activation of TRPV1 by Various Negatively Charged Lipids
Our data so far are consistent with the dependence of TRPV1 activity on PI(4)P and PI(4,5)P2 in the context of the plasma membrane. We had previously found that TRPV1 is activated not only by PI(4,5)P2, but also by a variety of other phosphoinositides, such as PI(4)P, PI(3,4)P2, and PI(3,4,5)P3 (3). This lack of specificity is reminiscent of the nonspecific activation of KATP channels (Kir6.2) by phosphoinositides (23). Those channels are activated in excised patches not only by phosphoinositides, but also by other negatively charged lipids, such as phosphatidylserine (PS), when they are applied at high concentrations (24). To test whether TRPV1 behaves similarly to KATP channels in this respect, we tested the effects of various negatively charged lipids on TRPV1.
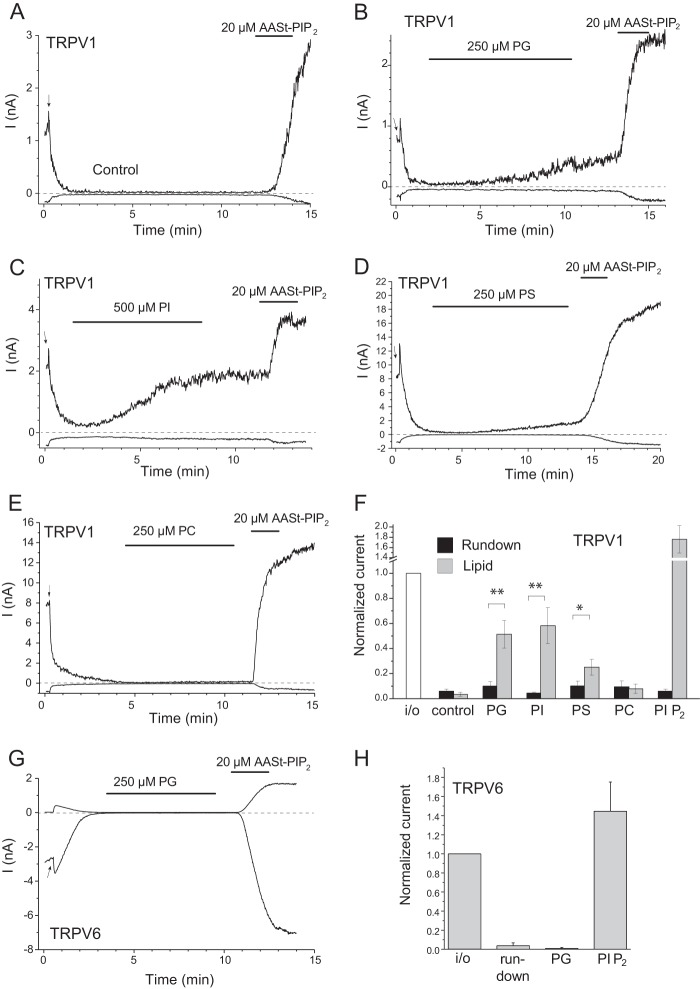
Fig. 4B shows that 250 μm phosphatidylglycerol (PG) slowly but consistently reactivated TRPV1 after run-down in the presence of 0.5 μm capsaicin in the patch pipette. The slow onset of the effect is consistent with the necessity of incorporation of PG micelles into the patch membrane. Lower concentrations of PG were less efficient, and at this high concentration, the lipid needed to be repeatedly sonicated shortly before the experiment to obtain reproducible effects, see “Experimental Procedures.” We retested the effect of PI at a higher concentration (500 μm) and, after more thorough sonication, we found that this lipid could also reactivate TRPV1 (Fig. 4C). PS, another negatively charged phospholipid, also reactivated TRPV1 at 250 μm (Fig. 4D), but the effect was smaller than that of PI or PG. In contrast, the neutral lipid phosphatidylcholine (PC) did not activate TRPV1 (Fig. 4E). Importantly, PG did not activate TRPV6 (Fig. 4, G and H), a channel showing higher phosphoinositide specificity and activation by PI(4,5)P2, but not by PI(4)P (25).
FIGURE 4.
PG, PS, and PI activate TRPV1 at high concentrations. Excised inside-out patch clamp measurements were performed as described under “Experimental Procedures” on TRPV1-expressing (A–F) and TRPV6-expressing (G and H) Xenopus oocytes. Traces show currents at +100 and −100 mV; the patch pipette solution contained 0.5 μm capsaicin, and the establishment of the inside-out configuration is indicated by the arrow in each trace. A, control experiment on TRPV1; B, the effect of 250 μm PG; C, the effect of 500 μm AASt PI; D, the effect of 250 μm PS; E, the effect of 250 μm PC on TRPV1. F, summary of the data (n = 4–11), current amplitudes normalized to the amplitude immediately after excision are shown for run-down and reactivation for each compound. For the control, the 3- and 12-min values are shown. G, the effect of 250 μm PG on TRPV6; H, summary of the effect of PG and AASt PI(4,5)P2 on TRPV6 (n = 3).
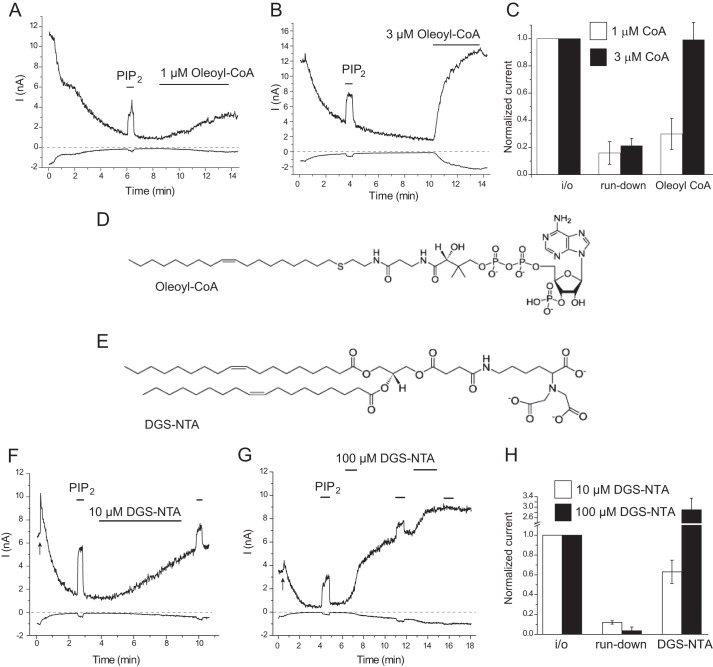
We also tested the effects of two other negatively charged amphipathic lipids, oleoyl-CoA and DGS-NTA, both of which could activate KATP channels in excised patches (23, 26). Fig. 5A shows that oleoyl-CoA induced a robust activation of TRPV1 at 3 μm; we observed a slowly developing partial activation at 1 μm (Fig. 5B). The artificial lipid DGS-NTA also activated TRPV1; the effect was slower and incomplete at 10 μm and faster and larger at 100 μm (Fig. 5, F–H). DiC8 PI(4,5)P2 induced either a smaller effect or no effect after DGS-NTA, depending on the level of activation, suggesting a common mechanism of activation by the two lipids. Both oleoyl-CoA and DGS-NTA have three negative charges (Fig. 5, D and E), and both induced a higher level of activation of TRPV1 at lower concentrations than either PG or PI, each of which has only one negative charge.
FIGURE 5.
Oleoyl-CoA and DGS-NTA activates TRPV1 in excised inside-out patches. A and B, representative traces for the effects of 1 and 3 μm oleoyl-CoA, with 0.2 μm capsaicin in the patch pipette. C, summary of the effects of oleoyl-CoA (n = 4 each); the data are pooled from experiments with 0.2 and 0.5 μm capsaicin in the patch pipette. D and E, chemical structures of oleoyl-CoA and DGS-NTA. F, representative traces for the effect of 50 μm DiC8 PI(4,5)P2 and 10 μm DGS-NTA. G, representative traces for the effect of 50 μm DiC8 PI(4,5)P2 and 100 μm DGS-NTA. H, summary of the effect of 10 μm DGS-NTA. Experiments were performed with 0.5 μm capsaicin in the patch pipette.
PI(4,5)P2 Activates TRPV1 in Planar Lipid Bilayers
To test the effects of PI(4,5)P2 in a pure system, without the presence of other negatively charged lipids, we purified the TRPV1 protein and incorporated it into planar lipid bilayers consisting of the neutral lipids PC and phosphatidylethanolamine in a 3:1 ratio, and we measured single channel TRPV1 currents. No channel activity was observed when TRPV1 alone was incorporated in the lipid bilayers, and capsaicin (2 μm) failed to activate the channel in the absence of PI(4,5)P2 (Fig. 6A, n = 6). Even after several hours with capsaicin alone, no channel openings were detected. However, the application of 2.5 μm PI(4,5)P2 in the continuous presence of capsaicin resulted in burst openings of TRPV1 (Po = 0.23 ± 0.05, n = 4), and 5 μm PI(4,5)P2 induced a further increase in channel open probability (Po = 0.55 ± 0.06, n = 3) (Fig. 6, A and B). No TRPV1 activity was observed when PI(4,5)P2 was applied alone, but the subsequent application of capsaicin resulted in robust channel activation (data not shown). Currents showed characteristic outward rectification; both single channel conductance and open probability were lower at negative voltages (Fig. 6, C–E). In the presence of 2.5 μm PI(4,5)P2 and 2 μm capsaicin, outward currents exhibited mean slope conductance values of 98.4 ± 1.8 pS, and Po of 0.23 ± 0.05 (at 30 mV) (n = 4), and inward currents were observed with conductance levels of 48.4 ± 2.4 pS and Po of 0.05 ± 0.01 (at −30 mV) (n = 4). The single channel conductance of the purified channel was similar to that reported previously (27, 28).
DISCUSSION
Phosphoinositides are emerging as general regulators of ion channels (29, 30). The best characterized PI(4,5)P2-sensitive ion channels are inwardly rectifying K+ (Kir) channels, for which PI(4,5)P2 is necessary for the activity of all members, exerting its effect via direct binding to the channel (31). High-resolution crystal structures are now available for representative Kir channels in the absence and presence of PI(4,5)P2 (32). TRP channels are also regulated by phosphoinositides, but their regulation is more complex, as both activating and inhibitory effects have been described for some channels (12, 33). Nevertheless, the vast majority of TRP channels display a dependence of activity on PI(4,5)P2 (34–37) (see Ref. 38 for more detail). This conserved activation is likely to be direct, because PI(4,5)P2 activates the purified TRPM8 (17, 39, 40), TRPV6 (15), and TRPV1 (Fig. 6) in planar lipid bilayers.
TRPV1 was first suggested to be inhibited by PI(4,5)P2 (1), but the effects of phosphoinositides had not then been tested in excised patches. A number of subsequent reports demonstrated that, in excised patches, the channels were activated, rather than inhibited by phosphoinositides, including PI(4)P and PI(4,5)P2 (2–4). Experiments with various rapidly inducible lipid phosphatases in intact cells also supported a positive regulatory role of phosphoinositides (4, 6–8).
In a recent study, TRPV1 was purified and reconstituted in artificial liposomes. The channels were activated by capsaicin, heat, low pH, and a variety of other activators, including endocannabinoids, in the absence of phosphoinositides. When either PI, PI(4)P, or PI(4,5)P2 were incorporated in the membranes, all of these lipids shifted the capsaicin dose-response to the right, i.e. inhibited channel activity; PI and PI(4)P were more effective than PI(4,5)P2 (13). To reconcile those data with earlier excised patch experiments, here we studied the effects of endogenous phosphoinositides on TRPV1 in excised patches and revisited the lipid specificity of TRPV1.
First, we took advantage of endogenous lipid kinases and phosphatases in the patch membrane by studying the effects of MgATP. PI(4,5)P2 is generated by sequential phosphorylation of PI into PI(4)P by PI4Ks and then to PI(4,5)P2 by phosphatidylinositol 4-phosphate 5-kinases (Fig. 1B). None of these enzymes currently have specific inhibitors, but type III PI4Ks can be efficiently inhibited by a number of PI3K inhibitors at higher concentrations. Our data show that TRPV1 channel activity runs down in ATP-free solutions, characteristic of PI(4,5)P2-dependent ion channels (14, 15, 20). When MgATP was applied to the patch, channel activity was recovered. This recovery was strongly inhibited by 300 μm LY294002, a compound that inhibits PI4K. Given the effectiveness of LY294002, it is likely that PI(4,5)P2 was dephosphorylated into PI(4)P then PI (Fig. 1B). We obtained similar results earlier with two other TRP channels TRPM8, and TRPV6 (15, 41). Given that both of those channels require PI(4,5)P2 selectively over PI(4)P for activity, it is likely that MgATP stimulated the activity of both PI4K and phosphatidylinositol 4-phosphate 5-kinase, resulting in the synthesis of both PI(4)P and PI(4,5)P2.
Next, we studied the effects of a bacterial PI-PLC enzyme applied directly to excised patches. This enzyme selectively hydrolyzes PI, but not PI(4,5)P2 or PI(4)P. We found that treating patches with PI-PLC eliminated the effect of MgATP on TRPV1 (Figs. 2 and 3) and TRPV6 (Fig. 2), as expected if MgATP worked by activating lipid kinases. When exogenous PI(4,5)P2 was applied after PI-PLC treatment, it consistently activated the channels (Figs. 2B and Fig. 3B), suggesting that PI(4,5)P2 is not simply less inhibitory than PI, but rather that it is itself a positive cofactor for TRPV1.
Consistent with earlier reports (1), PI-PLC also increased TRPV1 activity after run-down, but the effect was much smaller than that induced by MgATP and developed after a substantial delay (Figs. 2 and Fig. 3). This positive effect of PI-PLC may argue for the inhibitory role for phosphoinositides. Taking a closer look at these data, however, our findings are not compatible with the view of phosphoinositides being purely inhibitory. If the activating effect of PI-PLC is due to the removal of PI, then supplying this lipid after PI-PLC treatment should inhibit channel activity. This was not the case, despite the fact that PI partially restored the effect of MgATP, demonstrating its incorporation into the patch membrane (Fig. 3, B and D). Overall, it is likely that the partial activation by PI-PLC is not due to the reduction of PI levels, especially given that very high concentrations of PI also activated the channels (Fig. 4C).
A possible explanation for the partial activation by PI-PLC is that this enzyme also generates diacylglycerol (Fig. 1B). This lipid (42) and several of its breakdown products, 2-arachidonyl-glycerol for example (43, 44) have also been reported to potentiate TRPV1 activity. It is also possible that the PI-PLC preparation has some additional activity, accounting for TRPV1 stimulation.
Overall, our MgATP data argue that endogenous PI(4,5)P2 is a positive regulator of TRPV1 required for its activity. What is the explanation then for the full activity of TRPV1 in artificial vesicles devoid of this lipid? It was demonstrated earlier that TRPV1 is activated not only by PI(4,5)P2, but also by many other phosphoinositides, including PI(4)P and PI(3,4,5)P3 (3–5). This low specificity is reminiscent of that observed with Kir6.2 (KATP) channels (23). Those channels are not only activated by phosphoinositides, but by many other negatively charged lipids, such as long acyl chain coenzyme A (23), phosphatidylserine (24), and the artificial lipid DGS-NTA (26).
Can the low specificity of phosphoinositide activation of TRPV1 explain the lack of dependence of the purified reconstituted TRPV1 on these lipids? We found that at high concentrations, three different phospholipids with single negative charges, PI, PS, and PG, reactivated TRPV1 channels in excised patches after run-down (Fig. 4). This may explain the lack of dependence of the activity of the purified TRPV1 on phosphoinositides in lipid vesicles that included ∼25% PG (13). In agreement with this idea, when we reconstituted TRPV1 in planar lipid bilayers consisting of neutral lipids, its capsaicin-induced activity depended on PI(4,5)P2 (Fig. 6).
The two most abundant negatively charged phospholipids in mammalian cells are PI, which is found at concentrations of up to 10%, and PS (up to 5%), whereas PG was found at 1% or less in most tissues (45–47). PI(4)P and PI(4,5)P2 are found at concentrations of up to 1% each. Given the 90–95% run-down at submaximal capsaicin concentrations in most excised patch experiments (Figs. 1–4), it is likely that PI(4,5)P2 and PI(4)P are the major lipids supporting channel activity in a cellular context. Based on the 5–10% residual activity after run-down, we conclude that the less highly charged PI, PS, and PG, which are not subject to dephosphorylation, contribute to channel activity up to this extent. At higher capsaicin concentrations, run-down was slower and less complete. Nevertheless, MgATP still stimulated channel activity (Fig. 1, F and G), arguing for the role of phosphoinositides under those conditions. The slower and less complete run-down at higher capsaicin concentrations is likely due to increased apparent affinity for PI(4,5)P2 (3), even though an increased contribution of other negative lipids is also possible.
Our data demonstrate that phosphoinositides are obligate cofactors for TRPV1 activity in a cellular context. Despite the lack of data in excised inside-out patches showing inhibition by PI(4,5)P2, measurements in intact cells support an additional partial inhibition by this phosphoinositide (3, 10). This combination of data suggested that any inhibitory effect of PI(4,5)P2 is likely to be indirect (3, 48). The finding that phosphoinositides inhibit the purified TRPV1 seen in the reconstituted system (13), on the other hand, strongly suggests a direct effect.
Where is the binding site for PI(4,5)P2 on the channel? The cold sensor TRPM8 was proposed to be activated by PI(4,5)P2 through the highly conserved proximal C-terminal TRP domain (36). Brauchi et al. (49) found that the TRP domain is also important for PI(4,5)P2 regulation of TRPV1. Another article found a region close to, but distinct from, the TRP domain to be important for PI(4,5)P2 binding to TRPV1 (5). The inhibitory effect of PI(4,5)P2, however, was proposed to be mediated by a very distal C-terminal region of TRPV1 (13, 50). The picture is further complicated by the potential indirect effects of PI(4,5)P2 through other PI(4,5)P2 binding proteins, such as Pirt (51) and AKAP150 (A-kinase anchoring protein 150) (10). Elucidating the mechanism of the effects of PI(4,5)P2 on TRPV1 will require further work.
What are the biological roles of the general dependence on phosphoinositides and the concurrent partial inhibition? We have recently proposed a model (8) in which the activity of TRPV1 depends on the presence of either PI(4,5)P2 or PI(4)P, and concurrently, PI(4,5)P2 specifically and partially inhibits either the channel itself or the potentiating effect of PKC. According to our model, the regulation of the channel upon activation of different PLC isoforms is determined by the extent and specificity of changes in phosphoinositide levels. During desensitization in response to saturating capsaicin concentrations, a robust influx of Ca2+ activates a highly Ca2+-sensitive PLCδ isoform, leading to the depletion of both PI(4,5)P2 and PI(4)P, limiting channel activity. Upon PLCβ activation by bradykinin, a selective moderate reduction in PI(4,5)P2 levels relieves the inhibition and potentiates the effect of PKC, leading to enhanced activity (sensitization) (8).
The complex regulation of TRPV1 by phosphoinositides is somewhat analogous to that seen in TRPC channels, which are generally activated downstream of PLC. Even though diacylglycerol may serve as a physiological activation mechanism for the TRPC3/C6/C7 subgroup, their activation mechanism has not been fully elucidated (52). Relief of inhibition by PI(4,5)P2 may contribute to activation of some TRPCs (53), but when phosphoinositides or MgATP were tested in excised patches, they activated most of those channels (53, 54). Similarly, data with inducible phosphatases supported the dependence of activity of several TRPC channels on PI(4,5)P2 (53, 55). We refer to our recent reviews for further discussion on this topic (38, 52).
In conclusion, our data show that, in a cellular context, TRPV1 activity depends on the presence of phosphoinositides, most likely PI(4,5)P2 and PI(4)P. We propose that, under certain circumstances, other negatively charged phospholipids may substitute for phosphoinositides. This may reconcile discordant data gathered in different experimental settings.
Acknowledgment
The cDNA clone for the rat TRPV1 was generously provided by Dr. David Julius (University of California, San Francisco).
This work was supported, in whole or in part, by National Institutes of Health Grants R01NS055159 and R01GM093290 (to T. R.) and R01GM098052 (to E. Z.).
- TRPV1
- transient receptor potential vanilloid 1
- PLC
- phospholipase C
- PI
- phosphatidylinositol
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PG
- phosphatidylglycerol
- DGS-NTA
- 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl]
- AASt
- arachidonyl-stearyl
- PI4K
- phosphatidylinositol 4-kinase
- PS
- phosphatidylserine
- PC
- phosphatidylcholine
- DiC8
- dioctanoyl.
REFERENCES
- 1. Chuang H. H., Prescott E. D., Kong H., Shields S., Jordt S. E., Basbaum A. I., Chao M. V., Julius D. (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962 [DOI] [PubMed] [Google Scholar]
- 2. Stein A. T., Ufret-Vincenty C. A., Hua L., Santana L. F., Gordon S. E. (2006) Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 128, 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lukacs V., Thyagarajan B., Varnai P., Balla A., Balla T., Rohacs T. (2007) Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 27, 7070–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klein R. M., Ufret-Vincenty C. A., Hua L., Gordon S. E. (2008) Determinants of molecular specificity in phosphoinositide regulation. Phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) is the endogenous lipid regulating TRPV1. J. Biol. Chem. 283, 26208–26216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ufret-Vincenty C. A., Klein R. M., Hua L., Angueyra J., Gordon S. E. (2011) Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 286, 9688–9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao J., Qin F. (2009) Interaction with phosphoinositides confers adaptation onto the TRPV1 pain receptor. PLoS Biol. 7, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammond G. R., Fischer M. J., Anderson K. E., Holdich J., Koteci A., Balla T., Irvine R. F. (2012) PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lukacs V., Yudin Y., Hammond G. R., Sharma E., Fukami K., Rohacs T. (2013) Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J. Neurosci. 33, 11451–11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu B., Zhang C., Qin F. (2005) Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25, 4835–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeske N. A., Por E. D., Belugin S., Chaudhury S., Berg K. A., Akopian A. N., Henry M. A., Gomez R. (2011) A-kinase anchoring protein 150 mediates transient receptor potential family V type 1 sensitivity to phosphatidylinositol-4,5-bisphosphate. J. Neurosci. 31, 8681–8688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patil M. J., Belugin S., Akopian A. N. (2011) Chronic alteration in phosphatidylinositol 4,5-biphosphate levels regulates capsaicin and mustard oil responses. J. Neurosci. Res. 89, 945–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gamper N., Rohacs T. (2012) Phosphoinositide sensitivity of ion channels, a functional perspective. Subcell Biochem. 59, 289–333 [DOI] [PubMed] [Google Scholar]
- 13. Cao E., Cordero-Morales J. F., Liu B., Qin F., Julius D. (2013) TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77, 667–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hilgemann D. W., Ball R. (1996) Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959 [DOI] [PubMed] [Google Scholar]
- 15. Zakharian E., Cao C., Rohacs T. (2011) Intracellular ATP supports TRPV6 activity via lipid kinases and the generation of PtdIns(4,5)P2. FASEB J. 25, 3915–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilgemann D. W. (2012) Fitting KV potassium channels into the PIP2 puzzle: Hille group connects dots between illustrious HH groups. J. Gen. Physiol. 140, 245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zakharian E., Cao C., Rohacs T. (2010) Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 30, 12526–12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C. L., Feng S., Hilgemann D. W. (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391, 803–806 [DOI] [PubMed] [Google Scholar]
- 19. Du X., Zhang H., Lopes C., Mirshahi T., Rohacs T., Logothetis D. E. (2004) Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J. Biol. Chem. 279, 37271–37281 [DOI] [PubMed] [Google Scholar]
- 20. Sui J. L., Petit-Jacques J., Logothetis D. E. (1998) Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. U.S.A. 95, 1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwak J., Wang M. H., Hwang S. W., Kim T. Y., Lee S. Y., Oh U. (2000) Intracellular ATP increases capsaicin-activated channel activity by interacting with nucleotide-binding domains. J. Neurosci. 20, 8298–8304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lishko P. V., Procko E., Jin X., Phelps C. B., Gaudet R. (2007) The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54, 905–918 [DOI] [PubMed] [Google Scholar]
- 23. Rohács T., Lopes C. M., Jin T., Ramdya P. P., Molnár Z., Logothetis D. E. (2003) Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. U.S.A. 100, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan Z., Makielski J. C. (1997) Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 272, 5388–5395 [DOI] [PubMed] [Google Scholar]
- 25. Thyagarajan B., Lukacs V., Rohacs T. (2008) Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J. Biol. Chem. 283, 14980–14987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krauter T., Ruppersberg J. P., Baukrowitz T. (2001) Phospholipids as modulators of KATP channels: distinct mechanisms for control of sensitivity to sulphonylureas, K+ channel openers, and ATP. Mol. Pharmacol. 59, 1086–1093 [PubMed] [Google Scholar]
- 27. Hui K., Liu B., Qin F. (2003) Capsaicin activation of the pain receptor, VR1: multiple open states from both partial and full binding. Biophys. J. 84, 2957–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raisinghani M., Pabbidi R. M., Premkumar L. S. (2005) Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J. Physiol. 567, 771–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gamper N., Shapiro M. S. (2007) Regulation of ion transport proteins by membrane phosphoinositides. Nat. Rev. Neurosci. 8, 921–934 [DOI] [PubMed] [Google Scholar]
- 30. Suh B. C., Hille B. (2008) PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 37, 175–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Logothetis D. E., Jin T., Lupyan D., Rosenhouse-Dantsker A. (2007) Phosphoinositide-mediated gating of inwardly rectifying K+ channels. Pflugers Arch. 455, 83–95 [DOI] [PubMed] [Google Scholar]
- 32. Hansen S. B., Tao X., MacKinnon R. (2011) Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nilius B., Owsianik G., Voets T. (2008) Transient receptor potential channels meet phosphoinositides. EMBO J. 27, 2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Runnels L. W., Yue L., Clapham D. E. (2002) The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat. Cell Biol. 4, 329–336 [DOI] [PubMed] [Google Scholar]
- 35. Liu D., Liman E. R. (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. U.S.A. 100, 15160–15165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rohács T., Lopes C. M., Michailidis I., Logothetis D. E. (2005) PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8, 626–634 [DOI] [PubMed] [Google Scholar]
- 37. Nilius B., Mahieu F., Prenen J., Janssens A., Owsianik G., Vennekens R., Voets T. (2006) The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 25, 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rohacs T. (2013) Phosphoinositide regulation of TRP channels. Handb. Exp. Pharmacol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zakharian E., Thyagarajan B., French R. J., Pavlov E., Rohacs T. (2009) Inorganic polyphosphate modulates TRPM8 channels. PLoS One 4, e5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El-Arabi A. M., Salazar C. S., Schmidt J. J. (2012) Ion channel drug potency assay with an artificial bilayer chip. Lab. Chip 12, 2409–2413 [DOI] [PubMed] [Google Scholar]
- 41. Yudin Y., Lukacs V., Cao C., Rohacs T. (2011) Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J. Physiol. 589, 6007–6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woo D. H., Jung S. J., Zhu M. H., Park C. K., Kim Y. H., Oh S. B., Lee C. J. (2008) Direct activation of transient receptor potential vanilloid 1 (TRPV1) by diacylglycerol (DAG). Mol. Pain 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E. D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 [DOI] [PubMed] [Google Scholar]
- 44. De Petrocellis L., Bisogno T., Davis J. B., Pertwee R. G., Di Marzo V. (2000) Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 483, 52–56 [DOI] [PubMed] [Google Scholar]
- 45. White D. A. (1973) The phospholipid composition of mammalian tissues in Form and Function of Phospholipids (Ansell G. B., Hawthorne J. N., Dawson R. M., eds), pp. 441–482, Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 46. Ohtsuka T., Nishijima M., Akamatsu Y. (1993) A somatic cell mutant defective in phosphatidylglycerophosphate synthase, with impaired phosphatidylglycerol and cardiolipin biosynthesis. J. Biol. Chem. 268, 22908–22913 [PubMed] [Google Scholar]
- 47. Vance J. E., Steenbergen R. (2005) Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 44, 207–234 [DOI] [PubMed] [Google Scholar]
- 48. Rohacs T., Thyagarajan B., Lukacs V. (2008) Phospholipase C mediated modulation of TRPV1 channels. Mol. Neurobiol. 37, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brauchi S., Orta G., Mascayano C., Salazar M., Raddatz N., Urbina H., Rosenmann E., Gonzalez-Nilo F., Latorre R. (2007) Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc. Natl. Acad. Sci. U.S.A. 104, 10246–10251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prescott E. D., Julius D. (2003) A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300, 1284–1288 [DOI] [PubMed] [Google Scholar]
- 51. Kim A. Y., Tang Z., Liu Q., Patel K. N., Maag D., Geng Y., Dong X. (2008) Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 133, 475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rohacs T. (2013) Regulation of Transient Receptor Potential channels by the phospholipase C pathway. Adv. Biol. Regul. 53, 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trebak M., Lemonnier L., DeHaven W. I., Wedel B. J., Bird G. S., Putney J. W., Jr. (2009) Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 457, 757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lemonnier L., Trebak M., Putney J. W., Jr. (2008) Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium 43, 506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Imai Y., Itsuki K., Okamura Y., Inoue R., Mori M. X. (2012) A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P2-diacylglycerol signalling. J. Physiol. 590, 1101–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]