Background: Chemokines have been thought to act in a redundant fashion through their shared receptors.
Results: Chemokines can display different efficacies for G proteins and β-arrestins, resulting in different chemotactic profiles.
Conclusion: Chemokines can behave as biased agonists at their receptors, leading to functionally distinct, not redundant, responses.
Significance: Biased agonism plays an important role in biological signaling.
Keywords: Arrestin, Chemokines, G Protein-coupled Receptors (GPCR), G Proteins, Signaling, Biased Agonism
Abstract
Chemokines display considerable promiscuity with multiple ligands and receptors shared in common, a phenomenon that is thought to underlie their biochemical “redundancy.” Their receptors are part of a larger seven-transmembrane receptor superfamily, commonly referred to as G protein-coupled receptors, which have been demonstrated to be able to signal with different efficacies to their multiple downstream signaling pathways, a phenomenon referred to as biased agonism. Biased agonism has been primarily reported as a phenomenon of synthetic ligands, and the biologic prevalence and importance of such signaling are unclear. Here, to assess the presence of biased agonism that may underlie differential signaling by chemokines targeting the same receptor, we performed a detailed pharmacologic analysis of a set of chemokine receptors with multiple endogenous ligands using assays for G protein signaling, β-arrestin recruitment, and receptor internalization. We found that chemokines targeting the same receptor can display marked differences in their efficacies for G protein- or β-arrestin-mediated signaling or receptor internalization. This ligand bias correlates with changes in leukocyte migration, consistent with different mechanisms underlying the signaling downstream of these receptors induced by their ligands. These findings demonstrate that biased agonism is a common and likely evolutionarily conserved biological mechanism for generating qualitatively distinct patterns of signaling via the same receptor in response to different endogenous ligands.
Introduction
Chemokines play central roles in cell chemotaxis and positioning in the immune system and development. Their signaling is central to the inflammatory process and is dysregulated in autoimmune disorders, rheumatologic diseases, and atherosclerosis (1). The chemokine superfamily consists of over 40 chemokines and 15 chemokine receptors that display considerable promiscuity with multiple ligands acting as agonists at the same receptor (2). Although this biochemical “redundancy” was originally proposed to result in some level of physiologic redundancy, studies using genetic models have demonstrated that these chemokines are not functionally redundant (3). This has led to a model for chemokine signaling where different chemokines play distinct roles through their discrete patterns of spatial and temporal expression while acting through the same receptor (1).
However, the lack of functional redundancy could also be explained by different signaling properties of the ligands through a specific receptor. Over the past decade, it has been better appreciated that 7TMRs2 are capable of signaling with different efficacies to their multiple downstream signaling pathways (4), most commonly those mediated by heterotrimeric G proteins or by the multifunctional adapter proteins, the βarrs. This phenomenon has been referred to as “biased agonism” or functional selectivity (5), and ligands that display such behavior are referred to as biased agonists (6). For example, at the angiotensin II type 1A receptor, a modified version of angiotensin II acts as an agonist of βarr-mediated pathways but an antagonist of G protein pathways with clear physiologic differences in response compared with the native ligand (7). We hypothesized that ligand bias serves as a general mechanism in the chemokine superfamily for different ligands to induce qualitatively distinct patterns of signaling via the same receptor, thus explaining the apparent disconnect between signaling redundancy at the biochemical level and phenotypic differences at the genetic level. Therefore, we tested whether different ligands targeting the same chemokine receptor displayed bias between their different signaling pathways and whether this was associated with distinct physiologic responses.
EXPERIMENTAL PROCEDURES
Pharmacologic Assay Systems
PathHunter® GPCR Arrestin, PathHunter GPCR Active Internalization, and cAMP HunterTM cell lines (DiscoveRx Corp., Fremont, CA) were used to test the function of the chemokine GPCR targets.
All of the assays used in this study are based on enzyme fragment complementation technology (8). The basis of enzyme fragment complementation technology centers on two fragments of β-galactosidase (β-gal) enzyme: the enzyme acceptor (EA), which lacks residues 11–41, and the enzyme donor (ED), whose structure includes those missing residues. When EA and ED are mixed, they bind to form an active β-gal enzyme that hydrolyzes substrate to yield a detectable luminescent signal (9). This technology has been described in detail previously (8–10).
To link β-gal activity to cellular cAMP levels, three reagents are used in the HitHunterTM assay (11, 12): an ED conjugated with cAMP (ED-cAMP), a separate antibody that binds to cAMP (cAMP antibody), and the EA fragment. In the absence of cAMP, the cAMP antibody is bound to ED-cAMP, preventing formation of an active enzyme complex. Free cAMP generated upon adenylate cyclase activation by Gs results in competitive displacement of ED-cAMP from the cAMP antibody; this ED-cAMP then complements with EA to form an active enzyme. The greater the concentration of free cAMP, the more antibody that is bound to it, thus leaving ED-cAMP to form an active β-gal enzyme complex and hydrolyze the substrate to produce a luminescent signal. Detailed protocols for this assay result in highly reproducible and quantitative data as described previously (12).
For βarr recruitment, the PathHunter β-gal complementation system from DiscoveRx Corp. was used (13). In this assay, the 7TMR of interest is fused to the ED peptide, termed ProLink (PK), which weakly binds a complementary fragment, EA, which is fused to the C terminus of the arrestin protein. Activation of the PK-tagged 7TMR results in recruitment of the βarr-EA fusion and results in complementation of the enzyme. Again, the enzymatic activity is detected with luminescence. The βarr-EA fusion in PathHunter parental cells is designed to be in excess such that the PK-fused 7TMRs are the limiting factor; this results in an assay with no receptor reserve and where potency will closely track with ligand binding. A detailed protocol for performing this experiment has been published previously (13).
Receptor internalization was quantified using the GPCR Active Internalization assay from DiscoveRx Corp. that is based on previously described technology that monitors total receptor internalization (14). In this assay, a cell line is engineered to coexpress an untagged GPCR, an EA-tagged βarr, and a PK tag linked to endofin, which is localized to endosomes. Activation of the untagged GPCR induces βarr recruitment to and internalization of the receptor·βarr-EA complex in PK-tagged endosomes. Therefore, this assay measures recruitment of receptor·βarr complexes to endosomes.
Assay conditions were executed as follows. 20 μl of working cell suspension (5000 cells/well for internalization and arrestin; 10,000 cells/well for cAMP measurements) were added to white, tissue culture treated 384-well assay plates and incubated overnight at 37 °C in 5% CO2. Ligands were reconstituted in PBS + 0.1% BSA prior to use. Chemokine ligands were used from DiscoveRx Corp. or R&D Systems (Minneapolis, MN). Agonists were serially diluted 1:3 in Opti-MEM + 1% FBS to generate 11-point titrations of ligand (plus vehicle-only (−) control). PathHunter Arrestin cell lines were exposed to compound for 1.5 h at 37 °C in 5% CO2; PathHunter Active Internalization cell lines were exposed to compound for 3 h at 37 °C in 5% CO2. PathHunter chemiluminescence detection reagents (DiscoveRx Corp., catalog number 93-0001) were added to cells according to the manufacturer's instructions. For cAMP assays, on the day of the assay, cell plating medium was removed from the plate and replaced with 15 μl of an antibody solution consisting of 2 parts Hanks' balanced salt solution, 10 mm HEPES and 1 part cAMP XS+ antibody reagent. Agonists were serially diluted 1:3 in Hanks' balanced salt solution, 10 mm HEPES + forskolin to generate 11-point titrations of ligand (plus vehicle-only (−) control) with a constant concentration of forskolin. Cells were exposed to compound for 0.5 h at 37 °C in 5% CO2. HitHunter cAMP XS+ detection reagents were added to according to the manufacturer's instructions (DiscoveRx Corp., catalog number 90-0075). The signal for all assays was detected using an EnVision 2100 multilabel reader (PerkinElmer Life Sciences) in luminescence mode. Raw relative luminescence units were plotted using GraphPad Prism (sigmoidal dose response, variable slope) software (San Diego, CA).
Bias Analysis
Equimolar plots (“bias plots”) and bias factors were calculated as described previously (15). The theoretical rationale for the equation used to calculate ligand bias has been described previously (15). The equation used to calculate bias factor is derived from the method of Furchgott (16) for comparison of equiactive signaling and is related to intrinsic relative activities proposed by Ehlert and co-workers (17) (see supplemental materials from Rajagopal et al. (15) for a detailed discussion). Briefly, we first account for different levels of amplification inherent to each pathway downstream of the stimulus of AR* by including an amplification factor apath, resulting in s = apath × [AR*]. The fraction of receptors in a signaling-competent conformation is given by Equation 1.
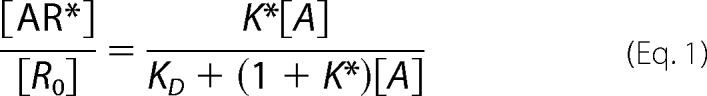 |
When the responses of the two different pathways, e.g. G protein and βarr, are equal, the underlying stimuli are equal.
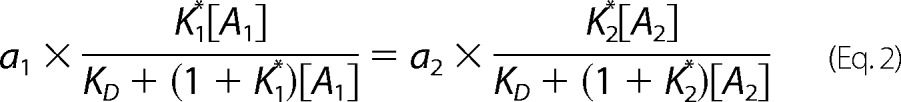 |
After some straightforward manipulations, 1/[A2] can be expressed in terms of 1/[A1] as follows.
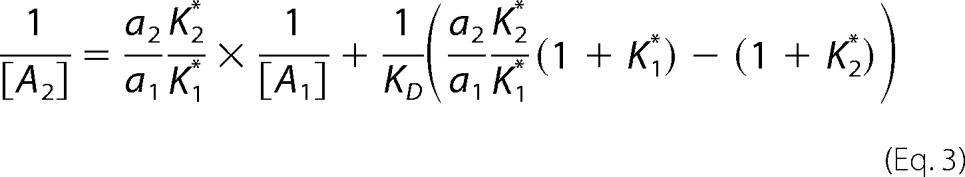 |
In this linear relationship, m = (a2/a1) × (K2*/K1*) is the slope of the line. If we then compare the slopes from one ligand (mlig) with the reference balanced agonist (mref), the amplification terms cancel each other out, and we obtain Equations 4 and 5.
 |
and
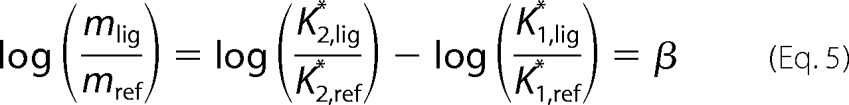 |
where β is the bias factor and estimates the molecular efficacy of pathway 1 versus pathway 2 on a logarithmic scale; e.g. a bias factor of 1 for Gi/βarr means that the ligand is 10 times better at generating the active receptor conformation for Gi signaling compared with βarr signaling (compared with the reference balanced agonist).
If we derive this same relationship in terms of a pharmacologic fit with the Hill coefficient set to 1 with no basal activity, we obtain a similar expression after some straightforward manipulations as shown below.
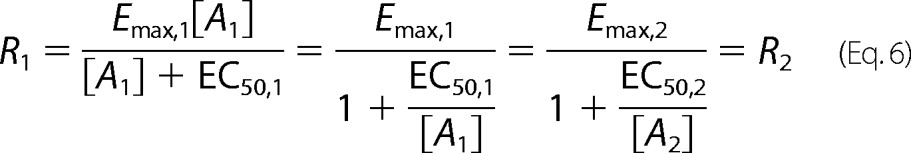 |
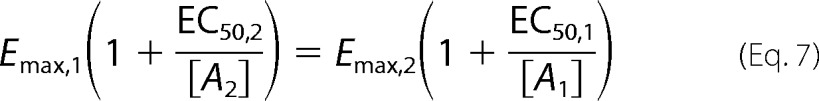 |
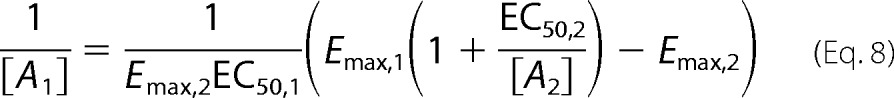 |
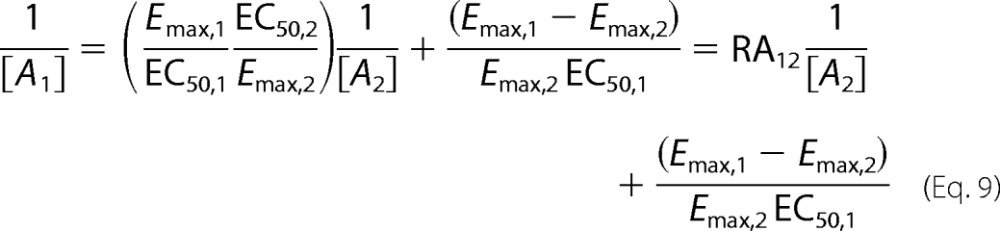 |
where RA12 is the intrinsic relative activity proposed by Ehlert (18). Therefore, if the data can be fit well by a logistic expression with a Hill coefficient equal to 1, then the intrinsic relative activity is equal to the ratio of equilibrium constants for receptor activation. Then we obtain Equation 10.
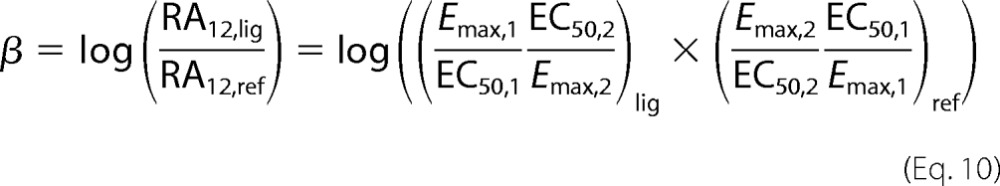 |
This form is equivalent to one that directly compares the effective signaling, or relative efficacy, of a ligand through each pathway relative to a reference balanced agonist. To better account for experiment errors, significance for bias was assessed not on whether the bias factor was statistically different from zero but rather on whether it was statistically different from the “balanced” agonist that had been chosen arbitrarily.
Ligand bias is not an absolute term but rather a relative term in comparing signaling under different conditions or between different ligands. As most receptors have a single endogenous agonist, that ligand can be chosen as the reference balanced agonist. However, in the setting of multiple endogenous agonists, the choice of the reference balanced agonist is necessarily arbitrary. Calculations and assessment of significance were performed in GraphPad Prism 5.1.
Cell Transfection
HEK293 cells were maintained in Eagle's minimum essential medium supplemented with 10% FBS and 1% penicillin/streptomycin. For DNA transfection, FuGENE transfection reagent (Roche Applied Science) was added at a ratio of 5 μl to 1 μg of plasmid DNA to a solution of plasmid DNA in 500 μl of serum-free Eagle's minimum essential medium. This transfection mixture was incubated for 30–45 min prior to addition to HEK293 cells of 50% confluence.
Confocal Microscopy
For confocal microscopy, HEK293 cells were split into 35-mm glass bottom dishes (MatTek, Ashland, MA) and transfected with constructs encoding 1 μg of receptor and 0.2 μg of βarr2-GFP. Forty-eight hours after transfection, cells were starved for at least 5 h in serum-free medium prior to treatment with ligand. For live cell imaging, cells were maintained at 37 °C with a heating plate while confocal microscopy was performed. Chemokines were added to a final concentration of 100 nm, and images were taken from 0 to 30 min. For immunostaining, cells were treated for 30 min with 100 nm ligand followed by aspiration of serum and fixation with 4% formaldehyde for 30 min. Samples were then washed three times with PBS followed by permeabilization and blocking with 0.1% Triton X-100 in 2% BSA in PBS for 1 h. Cells were then incubated overnight with primary antibody targeting the receptor at 1:200 dilution in 2% BSA in PBS. Samples were then washed three times with BSA/PBS prior to 1-h incubation with secondary antibody at a 1:500 dilution and with Hoechst 33258 to stain DNA. Samples were then washed with BSA/PBS and visualized.
Ex Vivo Chemotaxis Assay for Human Peripheral Blood Lymphocytes
Blood was drawn into EDTA tubes, and RBCs were sedimented with dextran T-500. RBC-depleted suspension was overlaid on Ficoll-Paque Plus (GE Healthcare) and spun at 400 × g for 45 min at room temperature. Interface cells (peripheral blood mononuclear cells) were recovered and washed in a large volume of migration medium (RPMI 1640 medium (Invitrogen) with 10% bovine calf serum (Invitrogen) pre-equilibrated overnight in a 37 °C incubator with 5% CO2). Peripheral blood mononuclear cells were resuspended in migration medium and incubated in a T-175 flask for 45 min at 37 C in 5% CO2. Nonadherent cells (primarily lymphocytes) were recovered for the chemotaxis assay.
Corning Costar 24-well Transwell plates were used for ex vivo migration. Chemokine dilutions in 600 μl of migration medium were added to the inner 8 wells of each 24-well plate. Recombinant human CCR10 ligands CCL27 and CCL28 were obtained from R&D Systems, and recombinant human CXCR4 ligands CXCL9, CXCL10, and CXCL11 were obtained from Peptrotech, Inc. (Rocky Hill, NJ). Transwell inserts containing tissue culture treated polycarbonate membranes with 5-μm pore size were gently placed on top of the chemokine dilution, being careful not to trap bubbles. A lymphocyte suspension (100 μl containing 5 × 105 lymphocytes) was added to each Transwell rapidly after the Transwell was placed in the well. Four wells were set up for each concentration of each chemokine. Plates were incubated at 37 °C in 5% CO2 for 90 min, and then Transwells were removed and discarded.
Polystyrene microspheres (15 μm; Polysciences, Warrington, PA) were added to each well (5 × 104/well) for subsequent quantitation of migrated cells, and the contents of each well were transferred to centrifuge tubes for flow cytometry staining. Cells migrated to CCR10 ligands were stained using a three-color protocol consisting of anti-human CD4, CD34, and CD27. Cells migrated to CXCR3 ligands were stained using a four-color protocol consisting of anti-human CD3, CD8, CD27, and CD45RA. Flow cytometry data were collected using a FACSCanto flow cytometer (BD Biosciences) and analyzed using FlowJo 8.6.6 (Tree Star, Inc., Stanford, CA). The percentage of migration of each cell type was determined by calculating the ratio of beads to each cell type in the starting population versus the same cell type in the migrated population.
RESULTS
Signaling by Chemokine Receptors
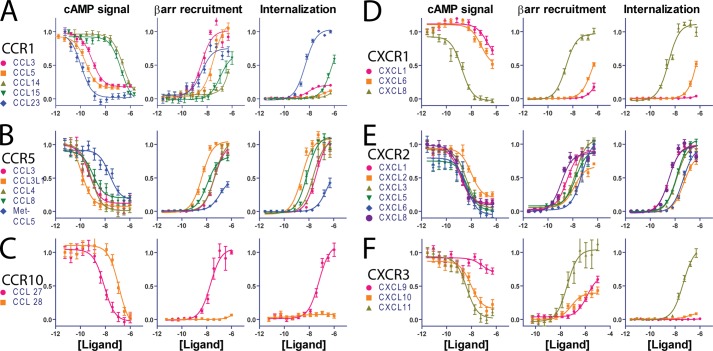
To test the hypothesis that different ligands for the same chemokine receptor were acting as biased ligands, we obtained detailed concentration-response data on G protein signaling, βarr recruitment, and receptor internalization for a set of representative chemokine receptors. There are two major classes of chemokine receptors based on their chemokine ligands. CC ligands (CCL), which bind to CC receptors (CCR), have a motif with two adjacent cysteines, whereas CXC ligands (CXCL), which bind to CXC receptors (CXCR), have a single amino acid in between the two conserved cysteines. We chose three CCRs (1, 5, and 10) and three CXCRs (1–3) and analyzed their responses to a number of their multiple endogenous agonists (Table 1). G protein signaling was assessed by inhibition of forskolin-stimulated cAMP (a Gi/o response) using an antibody-based assay (12), βarr signaling was assessed by a recruitment assay based on enzyme complementation (13), and receptor internalization was quantified by an assay based on complementation in early endosomes (DiscoveRx Corp.). All of these assays display excellent signal to noise, and the data that were obtained were fit well with simple stimulus-response curves with Hill coefficients of 1 (Fig. 1).
TABLE 1.
Chemokine receptor-ligand combinations tested in this study
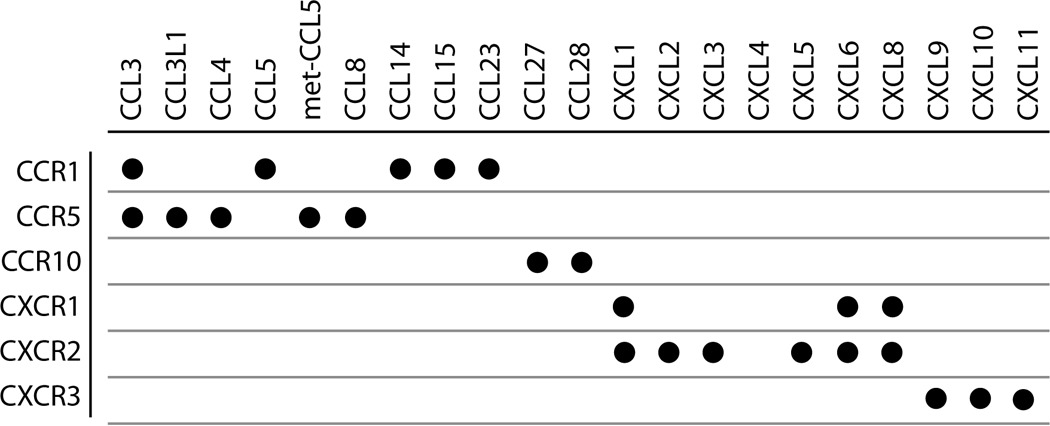
FIGURE 1.
Multiple assays for assessment of chemokine signaling. Concentration-response data for G protein signaling (left column; cAMP signal demonstrating inhibition of forskolin-stimulated cAMP by Gi/o), βarr recruitment (middle column), and receptor internalization (right column) for CCR1 (A), CCR5 (B), CCR10 (C), CXCR1 (D), CXCR2 (E), and CXCR3 (F) are shown. Shown are means ± S.E. from at least three experiments and fits with logistic dose-response functions with Hill coefficient equal to 1 (Table 2). Error bars represent S.E.
From an initial analysis of the simple pharmacologic parameters (Emax and EC50), some degrees of gross bias were appreciated (Table 2). For example, at CCR10 (Fig. 1C), CCL28 is identified as a G protein-biased agonist: CCL28 (orange) signals through G proteins alone, whereas CCL27 (magenta) displays balanced signaling through both G proteins and βarrs. At CXCR1 (Fig. 1D) and CXCR3 (Fig. 1F), there appear to be two agonists that display significantly higher levels of receptor internalization than the other ligands, CXCL8 at CXCR1 (Fig. 1D, brown) and CXCL11 at CXCR3 (Fig. 1F, brown). A more subtle form of bias is present at CXCR3 (Fig. 1F) as judged by a change in the rank order of efficacies for different signaling pathways (4) with CXCL11 (brown) > CXCL10 (yellow) > CXCL9 (magenta) at G protein signaling and CXCL11 (brown) > CXCL9 (magenta) > CXCL10 (yellow) at βarr recruitment. Thus, from an analysis of concentration-response data alone, behavior consistent with biased agonism was identified at three (CCR10, CXCR1, and CXCR3) of the six receptors tested.
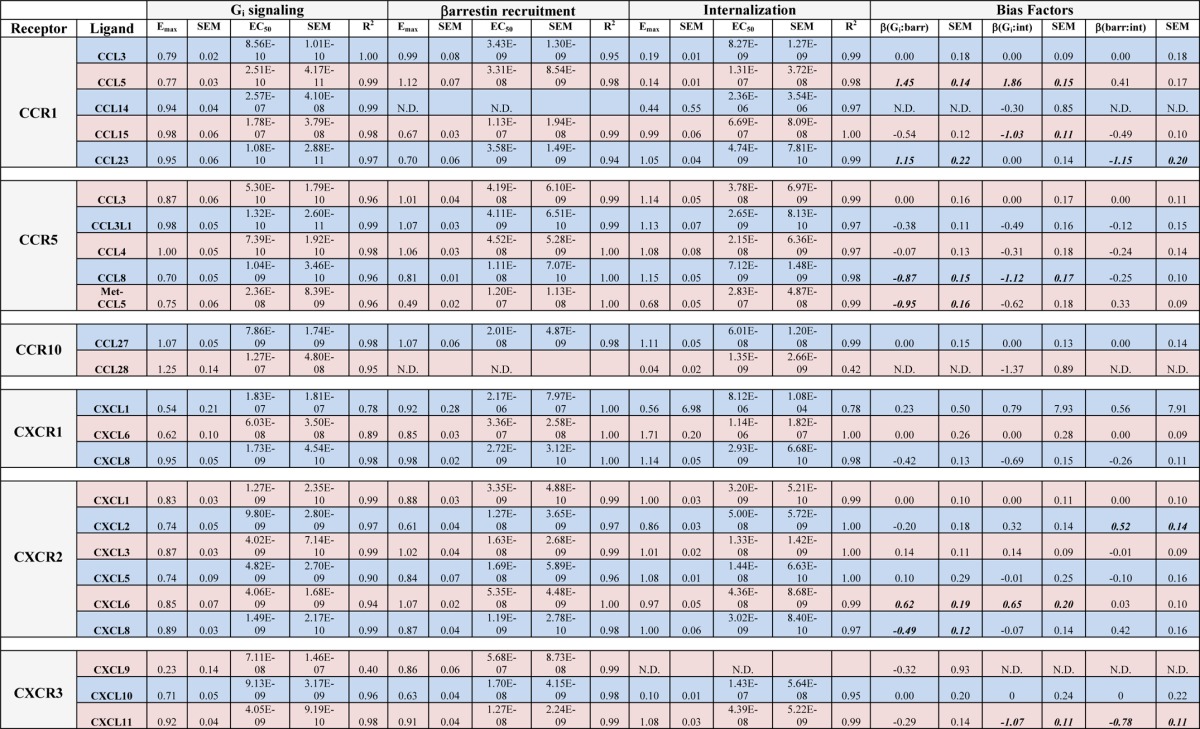
TABLE 2.
Pharmacologic parameters of signaling by chemokine receptors
Concentration-response data were fit with simple logistic equations with Hill coefficients equal to 1, allowing determination of maximal response (Emax) and EC50 for G protein signaling, βarr recruitment, and receptor internalization. Bias factors (β) were calculated as described under “Experimental Procedures.” Bias factors that were statistically significantly different from the arbitrarily defined balanced agonist with a bias factor of 0 (p < 0.05 by unpaired t test) are in bold. Data from at least three experiments were used for the fits. S.E., standard error; N.D., not determined.
Assessment of Chemokine Ligand Bias
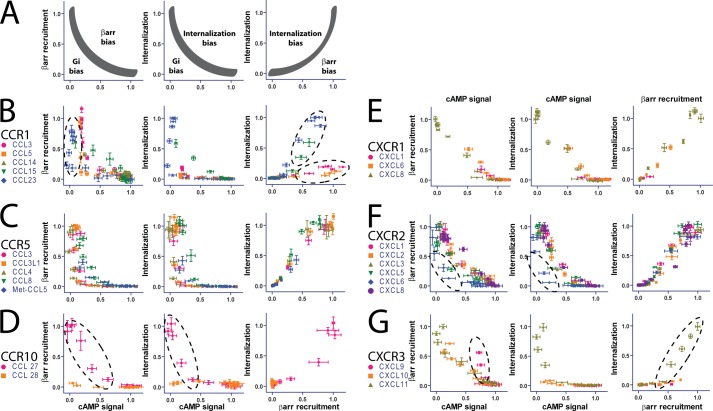
We then performed a detailed analysis of ligand bias using approaches we have described previously (15). We first performed a qualitative analysis of ligand bias by comparing responses through different signaling pathways at the same ligand concentration. This direct comparison, a “bias plot” (19), typically results in a hyperbolic curve due to the differences in amplification for different signaling assays (with G protein assays based on second messengers typically displaying significantly more amplification than assays for βarr recruitment). Ligands that have differently shaped curves from a balanced agonist (typically chosen as the most physiologically relevant endogenous ligand or, in the cases of multiple endogenous ligands, can be chosen arbitrarily) are then identified as either biased or not toward one of the signaling pathways (Fig. 2A). A complementary approach to identifying biased ligands is to calculate the level of ligand bias as encapsulated by a bias factor. Bias factors are a method of quantifying ligand bias on a logarithmic scale; e.g. a bias factor of 1 between G protein- and βarr-mediated signaling signifies 10-fold more effective signaling through G proteins than βarrs (15). A limitation of this calculation is in cases of very low signaling through one pathway, which typically results in a poor fit with relatively high errors and thus a poorly determined bias factor. In such cases, a biased ligand can be identified by a bias plot. These complementary approaches allow a comprehensive analysis of ligand bias and identification of biased agonists.
FIGURE 2.
Biased ligands can be identified qualitatively by an equimolar comparison. A, an equimolar comparison allows a direct visualization of bias between different signaling pathways on the ordinate and abscissa as shown schematically. The gray curve is a hypothetical relationship between two signaling pathways at equal ligand concentrations, the shape of which is determined by the differences in amplification between those pathways. A ligand that falls outside that curve can be classified as biased toward one pathway over another. B–G, equimolar comparison of different chemokines suggests bias at a number of receptors (hashed ellipses). Error bars represent S.E. See text for discussion.
The qualitative bias plot analysis suggested the presence of ligand bias in a number of chemokine receptors (highlighted by dashed black circles): CXCR2 (Fig. 2F), CXCR3 (Fig. 2G), CCR1 (Fig. 2B), and CCR10 (Fig. 2D). At CXCR2 (Fig. 2F), CXCL6 (blue) signals more effectively through G proteins over βarr or internalization compared with the other ligands, consistent with it being a G protein-biased agonist. At CXCR3 (Fig. 2G), CXCL9 (magenta) appears to be relatively βarr-biased, whereas CXCL11 (brown) is biased toward internalization. At CCR1 (Fig. 2B), there is significant spread between a number of agonists in their responses to G proteins and βarrs, consistent with some level of G protein and βarr bias between those ligands. An interesting pattern is present in the comparison of βarr recruitment with internalization with CCL15 (green) and CCL23 (blue), demonstrating increased internalization compared with βarr recruitment, whereas CCL3 (magenta) and CCL5 (orange) demonstrate relatively decreased internalization compared with similar levels of βarr recruitment. At CCR10 (Fig. 2D), CCL27 (magenta) is clearly identified as a G protein-biased agonist compared with CCL28 (orange). Notably, at CXCR1 (Fig. 2E), the suggestion of increased internalization by CXCL8 compared with other signaling responses from simple pharmacologic parameters alone does not hold; rather, CXCL1 (magenta) and CXCL6 (orange) are balanced partial agonists compared with the full, balanced agonist CXCL8.
A quantitative analysis of ligand bias was then performed based on the pharmacologic fits from Table 2, allowing a calculation of bias factors (Table 2 and Fig. 3). Bias factors could be calculated for the majority of ligand-receptor combinations, although in a few situations they could not be determined because of low signaling in one of the pathways (Table 2 and Fig. 3). At half of the receptors tested (CCR1, CCR10, and CXCR3), there were statistically and likely physiologically significant levels of ligand bias observed. At CCR1 (Fig. 3A), CCL5 and CCL23 were identified as G protein-biased compared with CCL3 with CCL5, CCL15, and CCL23 displaying some levels of bias for or against internalization relative to βarr recruitment. At CCR5 (Fig. 3B), CCL8 and met-CCL5 both show statistically significant βarr bias compared with the reference agonist CCL3 although with bias factors less than 1. At CCR10 (Fig. 3C), because of the lack of βarr recruitment by CCL28, most bias factors could not be calculated. At CXCR1 (Fig. 3D), no statistically significant levels of bias were observed, whereas at CXCR2 (Fig. 3E), some ligands displayed statistically significant levels of bias but all less than 1. At CXCR3 (Fig. 3F), there were no significant βarr- or G protein-biased agonists, but CXCL11 displayed significantly higher levels of internalization than CXCL9 or CXCL10.
FIGURE 3.
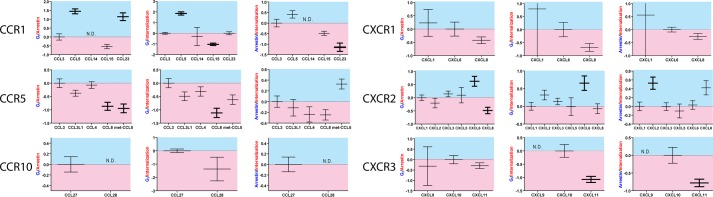
Chemokine bias factors. Bias factors between different pathways for each ligand were calculated as described; shown are mean ± S.E. from Table 2. Bias factors that were statistically significantly different from the balanced agonist are in bold. In the example of the Gi/arrestin bias factor, a positive bias factor denotes bias toward Gi signaling, whereas a negative bias factor denotes bias toward βarr recruitment. N.D., not determined. Error bars represent S.E. See text for discussion.
Ligand-dependent Differences in βarr Recruitment and Receptor Internalization
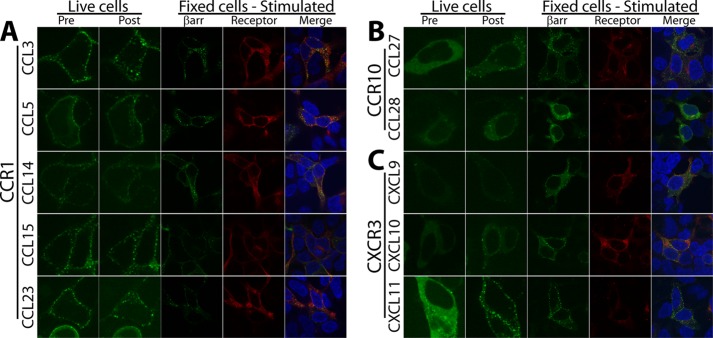
For those receptor-ligand systems (CCR1, CCR10, and CXCR3) that demonstrated evidence of significant ligand bias (i.e. an absolute value of any bias factor >1 was our cutoff for significant ligand bias), confocal microscopy was used to determine βarr redistribution, receptor internalization, and βarr-receptor colocalization in response to ligand stimulation at a single, high concentration of ligand (100 nm) in HEK293 cells that were transiently transfected with βarr2-GFP and receptor. As described below, this orthogonal assay confirmed the results of the luminescence-based βarr redistribution and receptor internalization assays.
Both CCR1 (Fig. 4A and supplemental Fig. 1) and CXCR3 (Fig. 4C and supplemental Fig. 1) displayed constitutive activity with “class A” patterns of βarr distribution with puncta at the membrane in cells transfected with both receptor and βarr2, whereas βarr cotransfected with CCR10 (Fig. 4B and supplemental Fig. 1) was uniformly distributed in the cytoplasm, consistent with minimal constitutive activity. Upon ligand stimulation, these receptors all showed some level of βarr redistribution. At CCR1 (Fig. 4A and supplemental Fig. 1), stimulation with CCL3, -5, and -23 all resulted in βarr redistribution from the class A pattern to a “class B” pattern of βarr localization into endosomes with internalized receptor. Stimulation with CCL14 or CCL15 did not result in significant βarr redistribution or receptor internalization. Notably, only CCL15 and CCL23 treatment resulted in significant receptor internalization with considerably less receptor at the plasma membrane, consistent with the luminescence-based receptor internalization assay. We also confirmed the results of the luminescence-based receptor assay in an alternate clone that again demonstrated higher levels of receptor internalization induced by CCL23 compared with CCL3 (Fig. 5).
FIGURE 4.
Biased ligands display distinct patterns of βarr recruitment and receptor internalization. At CCR1 (A) and CXCR3 (C), ligand stimulation results in a change (Live cells) from a class A pattern prior to ligand stimulation (Pre), consistent with weak constitutive activity, to differential class B patterns with βarr internalization after ligand stimulation (Post). At CCR10 (B), there is little constitutive activity, and stimulation of the receptor results in a class A pattern. These are associated with distinct patterns (Fixed cells) of βarr recruitment, receptor internalization, and colocalization (βarr2-GFP, green; receptor, red; DNA, blue). See text for full discussion.
FIGURE 5.
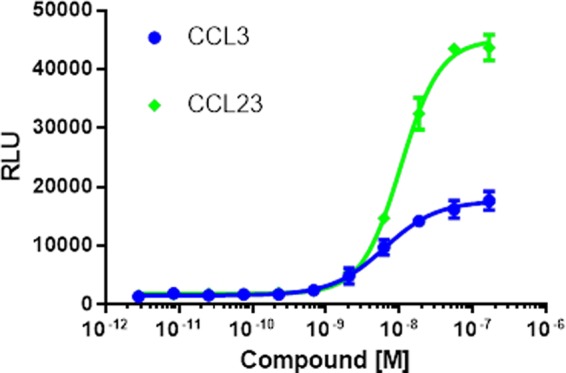
Active internalization dose-response curves for CCL3 (blue) and CCL23 (green). This experiment used an alternate clone expressing untagged CCR1 with an enzyme acceptor-tagged βarr and a PK tag linked to endofin, which is localized to endosomes. The results obtained were consistent with the original clone and with the results of confocal microscopy. RLU, relative luminescence units. Error bars represent S.E.
At CCR10 (Fig. 4B and supplemental Fig. 1), stimulation with CCL27 resulted in a class B pattern, whereas that with CCL28 resulted in a weak class A pattern, consistent with its weak response in the recruitment assay (only a 2-fold-response with an EC50 of 300 nm). This difference in βarr redistribution was also reflected by significant receptor internalization with CCL27 that was absent in cells stimulated with CCL28. These findings were again consistent with the results of the luminescence-based receptor assays.
At CXCR3 (Fig. 4C and supplemental Fig. 1), CXCL9 and CXCL10 stimulation resulted in a class A pattern, whereas CXCL11 stimulation resulted in a class B pattern, consistent with its increased signal in the βarr recruitment assay (Fig. 1F). CXCL9- and CXCL10-treated cells displayed more surface-expressed receptor than CXCL11-treated cells, whereas all samples displayed some internalized receptor, consistent with the known constitutive internalization of the receptor (20). Thus, with biased chemokines targeting the same receptor, we observed distinct patterns of βarr redistribution and receptor internalization.
Ligand Bias Is Associated with Distinct Physiologic Outcomes
We then tested whether differences in bias between different ligands for the same receptor were associated with different chemotactic profiles in human peripheral blood mononuclear cells. Because the ligands tested for CCR1 are shared with a number of other receptors (1), we did not test it any further. CCR10 demonstrated a high degree of ligand bias between its two ligands, CCL27 and CCL28, both of which are capable of reaching maximal G protein signaling as assessed by inhibition of cAMP formation but only one of which, CCL27, is capable of recruiting βarr and internalizing the receptor (Fig. 1C). CCL28 was clearly identified as a G protein-biased agonist in a qualitative assessment (Fig. 2) and was biased to such an extent that its bias factor could not be calculated (Fig. 3). This bias was confirmed in an assessment of βarr redistribution and receptor internalization (Fig. 4). Notably, although CCL28 is a lower potency agonist than CCL27 for G protein signaling, it displays similar potency with higher efficacy for monocyte migration compared with the balanced agonist CCL27 (Fig. 6A). The simplest explanation for this behavior is that the absence of βarr recruitment in response to CCL28 results in unopposed G protein activation without receptor desensitization and internalization and therefore relatively higher potency and efficacy for chemotaxis compared with the balanced agonist CCL27.
FIGURE 6.
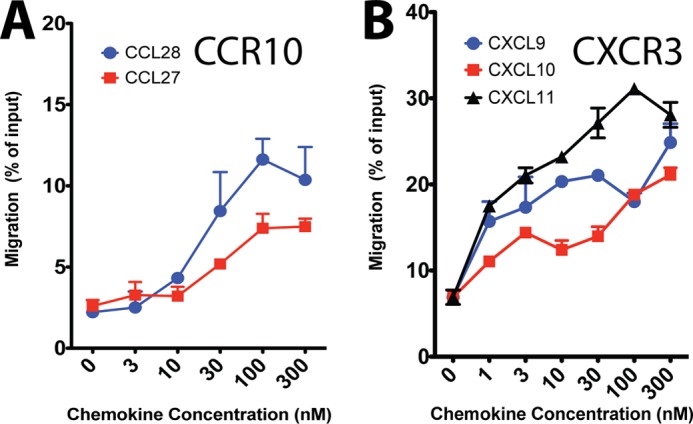
Leukocyte migration in response to biased endogenous ligands targeting CCR10 and CXCR3. Chemotactic responses of peripheral blood monocytes to chemokine biased agonists targeting CCR1 (A) and CXCR3 (B) are shown. Error bars represent S.E. See text for full discussion.
At CXCR3, we tested the effects of its three ligands, CXCL9, CXCL10, and CXCL11, on peripheral blood mononuclear cell migration (Fig. 6B). These ligands did not display the same level of marked bias that CCL28 displayed at CCR10; rather, the ligands for CXCR3 displayed more complex behavior. CXCL11 appeared to be biased toward internalization (Figs. 2 and 3) and was also a more efficacious agonist in all pathways tested compared with CXCL9 and CXCL10 (Fig. 1). This activity is most consistent with the pattern of βarr recruitment by the different ligands, suggesting that at CXCR3 βarrs may play a role in mediating chemotaxis (21) and a smaller role in receptor desensitization because the most potent βarr binder was also the most potent chemotactic stimulus.
DISCUSSION
Biased agonism has been noted as a property of 7TMRs for over two decades (4) with one of the first descriptions being the functionally selective responses of the acetylcholine receptor to pilocarpine and carbachol (22). In general, biased agonists targeting 7TMRs have been generated synthetically and display distinct physiologic profiles from endogenous, balanced agonists, suggesting a role for these compounds as novel drugs (23, 24). However, it has been unclear whether biased agonism is an “accident” of 7TMR complexity that has only been exploited by synthetic drugs or whether it is a property that is utilized by endogenous systems as an added layer for specificity in signaling (25). One of the best characterized examples of endogenous ligand bias is at CCR7, a chemokine receptor with two ligands, CCL19 and CCL21, which both activate G protein signaling (26) but only one of which, CCL19, results in higher levels of βarr recruitment (26), GRK3-dependent βarr internalization, and receptor desensitization (27), which presumably results in a distinct physiological response as these two ligands play different roles in the immune system while acting through the same receptor. At CXCR4, there are distinct signaling patterns associated with monomeric or dimeric CXCL12 that can either promote or inhibit chemotaxis (28). Here we have demonstrated that in systems with multiple endogenous ligands biased agonism is a general, and likely conserved, mechanism for generating qualitatively distinct patterns of signaling via the same receptor.
Viewing previous studies through the paradigm of biased agonism yields a consistent model for receptor activation: different chemokines are capable of binding different sites of the receptor, thereby allosterically activating different signaling pathways. For example, CXCR3 ligands have been shown previously to bind at distinct sites of the receptor (29) that are also associated with signaling that requires different portions of the receptor for chemotaxis, calcium mobilization, and receptor internalization (30). Notably, our finding that βarr recruitment did not correlate with receptor internalization is consistent with the previous observation that CXCR3 internalization can be independent of clathrin and the βarrs (20), suggesting that at CXCR3 βarrs may act primarily as a positive regulator of signaling as opposed to an alternate pathway of constitutive receptor internalization and degradation (20). We did find, unlike an earlier study (31), that CXCL9 did stimulate βarr recruitment, although there was no apparent βarr bias observed at this receptor. The biology and pharmacology of CCR10 (32) are not as well characterized as that of CXCR3, and so the functional significance of the extreme bias between its two ligands is less clear. These findings clearly buttress the evolving model of multiple active states of the receptor associated with distinct signaling profiles (33).
Biased agonism therefore allows a single chemokine receptor to generate distinct physiologic responses in response to different chemokines, thereby adding a layer of complexity to chemokine receptor signaling to augment the diversity attained with spatiotemporal expression. These distinct chemokine signaling profiles likely contribute significantly to the distinct phenotypes of knock-out mice to different ligands for the same receptor (34). Presumed biochemical redundancy has been considered to be a major hurdle in chemokine drug development, and although that may be true for antagonists, our findings suggest that biased agonists targeting these receptors would have distinct physiologic effects. It also suggests that post-translational processing of chemokines, such as cleavage of N-terminal residues (35), may not just change the relative agonism of a molecule but also its relative bias. Thus, the presence of endogenously biased agonists has far ranging impact on our understanding of chemokine pharmacology and biology.
Acknowledgment
We thank Robert J. Lefkowitz for invaluable discussions and guidance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL07101-34, a T32 training grant (to S. R.); R21AI092388 and R21AI097468 (to J. C.); and R01AI039795-14 and 5R01HL51366-15 (to C. G.). D. B. and T. W. are employees of DiscoveRx Corporation, which commercializes the PathHunter technology.

This article contains supplemental Fig. 1.
- 7TMR
- seven-transmembrane receptor
- βarr
- β-arrestin
- GPCR
- G protein-coupled receptor
- EA
- enzyme acceptor
- ED
- enzyme donor
- PK
- ProLink
- CCL
- CC ligand
- CCR
- CC receptor
- CXCL
- CXC ligand
- CXCR
- CXC receptor.
REFERENCES
- 1. Schall T. J., Proudfoot A. E. (2011) Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat. Rev. Immunol. 11, 355–363 [DOI] [PubMed] [Google Scholar]
- 2. Zlotnik A., Yoshie O. (2012) The chemokine superfamily revisited. Immunity 36, 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Power C. A. (2003) Knock out models to dissect chemokine receptor function in vivo. J. Immunol. Methods 273, 73–82 [DOI] [PubMed] [Google Scholar]
- 4. Kenakin T. (1995) Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 16, 232–238 [DOI] [PubMed] [Google Scholar]
- 5. Kenakin T. (2007) Functional selectivity through protean and biased agonism: who steers the ship? Mol. Pharmacol. 72, 1393–1401 [DOI] [PubMed] [Google Scholar]
- 6. Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boerrigter G., Lark M. W., Whalen E. J., Soergel D. G., Violin J. D., Burnett J. C., Jr. (2011) Cardiorenal actions of TRV120027, a novel β-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ. Heart Fail. 4, 770–778 [DOI] [PubMed] [Google Scholar]
- 8. Eglen R. M. (2002) Enzyme fragment complementation: a flexible high throughput screening assay technology. Assay Drug Dev. Technol. 1, 97–104 [DOI] [PubMed] [Google Scholar]
- 9. Wehrman T. S., Raab W. J., Casipit C. L., Doyonnas R., Pomerantz J. H., Blau H. M. (2006) A system for quantifying dynamic protein interactions defines a role for Herceptin in modulating ErbB2 interactions. Proc. Natl. Acad. Sci. U.S.A. 103, 19063–19068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olson K. R., Eglen R. M. (2007) β-Galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev. Technol. 5, 137–144 [DOI] [PubMed] [Google Scholar]
- 11. Bradley J., McLoughlin D. (2009) Use of the DiscoveRx Hit hunter cAMPII assay for direct measurement of cAMP in Gs and Gi GPCRs. Methods Mol. Biol. 552, 171–179 [DOI] [PubMed] [Google Scholar]
- 12. Bassoni D. L., Jafri Q., Sastry S., Mathrubutham M., Wehrman T. S. (2012) Characterization of G-protein coupled receptor modulators using homogeneous cAMP assays. Methods Mol. Biol. 897, 171–180 [DOI] [PubMed] [Google Scholar]
- 13. Bassoni D. L., Raab W. J., Achacoso P. L., Loh C. Y., Wehrman T. S. (2012) Measurements of β-arrestin recruitment to activated seven transmembrane receptors using enzyme complementation. Methods Mol. Biol. 897, 181–203 [DOI] [PubMed] [Google Scholar]
- 14. Hammer M. M., Wehrman T. S., Blau H. M. (2007) A novel enzyme complementation-based assay for monitoring G-protein-coupled receptor internalization. FASEB J. 21, 3827–3834 [DOI] [PubMed] [Google Scholar]
- 15. Rajagopal S., Ahn S., Rominger D. H., Gowen-MacDonald W., Lam C. M., Dewire S. M., Violin J. D., Lefkowitz R. J. (2011) Quantifying ligand bias at seven-transmembrane receptors. Mol. Pharmacol. 80, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furchgott R. F. (1966) in Advances in Drug Research (Harper N. J., Simmonds A. B., eds) pp 21–55, Academic Press, New York [Google Scholar]
- 17. Griffin M. T., Figueroa K. W., Liller S., Ehlert F. J. (2007) Estimation of agonist activity at G protein-coupled receptors: analysis of M2 muscarinic receptor signaling through Gi/o, Gs, and G15. J. Pharmacol. Exp. Ther. 321, 1193–1207 [DOI] [PubMed] [Google Scholar]
- 18. Ehlert F. J. (2008) On the analysis of ligand-directed signaling at G protein-coupled receptors. Naunyn Schmiedebergs Arch. Pharmacol. 377, 549–577 [DOI] [PubMed] [Google Scholar]
- 19. Gregory K. J., Hall N. E., Tobin A. B., Sexton P. M., Christopoulos A. (2010) Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 285, 7459–7474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meiser A., Mueller A., Wise E. L., McDonagh E. M., Petit S. J., Saran N., Clark P. C., Williams T. J., Pease J. E. (2008) The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. J. Immunol. 180, 6713–6724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fong A. M., Premont R. T., Richardson R. M., Yu Y. R., Lefkowitz R. J., Patel D. D. (2002) Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 99, 7478–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gurwitz D., Haring R., Heldman E., Fraser C. M., Manor D., Fisher A. (1994) Discrete activation of transduction pathways associated with acetylcholine m1 receptor by several muscarinic ligands. Eur. J. Pharmacol. 267, 21–31 [DOI] [PubMed] [Google Scholar]
- 23. Boerrigter G., Soergel D. G., Violin J. D., Lark M. W., Burnett J. C., Jr. (2012) TRV120027, a novel β-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ. Heart Fail. 5, 627–634 [DOI] [PubMed] [Google Scholar]
- 24. Gesty-Palmer D., Flannery P., Yuan L., Corsino L., Spurney R., Lefkowitz R. J., Luttrell L. M. (2009) A β-arrestin biased agonist of a parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci. Transl. Med. 1, 1ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whalen E. J., Rajagopal S., Lefkowitz R. J. (2011) Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol. Med. 17, 126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohout T. A., Nicholas S. L., Perry S. J., Reinhart G., Junger S., Struthers R. S. (2004) Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem. 279, 23214–23222 [DOI] [PubMed] [Google Scholar]
- 27. Zidar D. A., Violin J. D., Whalen E. J., Lefkowitz R. J. (2009) Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. U.S.A. 106, 9649–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drury L. J., Ziarek J. J., Gravel S., Veldkamp C. T., Takekoshi T., Hwang S. T., Heveker N., Volkman B. F., Dwinell M. B. (2011) Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 108, 17655–17660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cox M. A., Jenh C. H., Gonsiorek W., Fine J., Narula S. K., Zavodny P. J., Hipkin R. W. (2001) Human interferon-inducible 10-kDa protein and human interferon-inducible T cell α chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol. Pharmacol. 59, 707–715 [DOI] [PubMed] [Google Scholar]
- 30. Colvin R. A., Campanella G. S., Sun J., Luster A. D. (2004) Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J. Biol. Chem. 279, 30219–30227 [DOI] [PubMed] [Google Scholar]
- 31. Watts A. O., Scholten D. J., Heitman L. H., Vischer H. F., Leurs R. (2012) Label-free impedance responses of endogenous and synthetic chemokine receptor CXCR3 agonists correlate with Gi-protein pathway activation. Biochem. Biophys. Res. Commun. 419, 412–418 [DOI] [PubMed] [Google Scholar]
- 32. Hu S., Yang K., Yang J., Li M., Xiong N. (2011) Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc. Natl. Acad. Sci. U.S.A. 108, E1035–E1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kahsai A. W., Xiao K., Rajagopal S., Ahn S., Shukla A. K., Sun J., Oas T. G., Lefkowitz R. J. (2011) Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat. Chem. Biol. 7, 692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsou C. L., Peters W., Si Y., Slaymaker S., Aslanian A. M., Weisberg S. P., Mack M., Charo I. F. (2007) Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 117, 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mortier A., Gouwy M., Van Damme J., Proost P. (2011) Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp. Cell Res. 317, 642–654 [DOI] [PubMed] [Google Scholar]