Background: Hardening of the insect eggshell is due to peroxidases that promote protein cross-linking via H2O2, from an unknown source.
Results: In Rhodnius prolixus, this H2O2 is produced by Duox, and it also contributes to waterproofing, preventing desiccation.
Conclusion: H2O2 from Duox has a double role in protecting the Rhodnius eggshell.
Significance: Duox activity is essential to insect reproduction.
Keywords: Hydrogen Peroxide, Insect, NADPH Oxidase, Protein Cross-linking, Reproduction, Eggshell, Waterproofing
Abstract
In insects, eggshell hardening involves cross-linking of chorion proteins via their tyrosine residues. This process is catalyzed by peroxidases at the expense of H2O2 and confers physical and biological protection to the developing embryo. Here, working with Rhodnius prolixus, the insect vector of Chagas disease, we show that an ovary dual oxidase (Duox), a NADPH oxidase, is the source of the H2O2 that supports dityrosine-mediated protein cross-linking and eggshell hardening. RNAi silencing of Duox activity decreased H2O2 generation followed by a failure in embryo development caused by a reduced resistance to water loss, which, in turn, caused embryos to dry out following oviposition. Phenotypes of Duox-silenced eggs were reversed by incubation in a water-saturated atmosphere, simultaneous silencing of the Duox and catalase genes, or H2O2 injection into the female hemocoel. Taken together, our results show that Duox-generated H2O2 fuels egg chorion hardening and that this process plays an essential role during eggshell waterproofing.
Introduction
The insect eggshell, or chorion, was described by Beament (1) as “that part of the egg lying outside the oocyte cell membrane, which is secreted by the follicle.” It is a multilayered structure that confers physical and biological protection to the embryo during development. Although chorion hardening of Aedes aegypti eggs takes place only after oviposition (2), in other insects, such as Drosophila melanogaster and Rhodnius prolixus, the hardening of the eggshell occurs while the oocytes are still in the ovary (3). Similar to what happens with the body wall cuticle of all insects, the hardening of the eggshell in D. melanogaster and A. aegypti involves cross-linking of the exochorion proteins, which is accomplished by peroxidases through the oxidation of tyrosine residues and the subsequent formation of di- and trityrosine bonds between the chorion proteins, a process that requires H2O2 (4, 5). Although the source has not been identified, Margaritis (6) hypothesized that the H2O2 that supports chorion hardening in D. melanogaster is likely to be produced by an oxidase present at the apical surfaces of the follicle cells.
Dual oxidase (Duox)2 enzymes are NADPH oxidases that present an amino-terminal peroxidase-like domain facing the extracellular side of the plasma membrane that is not found in other types of NADPH oxidases (7). Duox enzymes generate hydrogen peroxide (8) either to fuel their own peroxidase activity (9–11) or to support the activity of other peroxidases, including tyrosine bond formation (12, 13) and several other biological processes (14). Resistance to water loss is thought to be an essential feature of insect eggs and probably was a key adaptation that allowed the marine ancestors of insects to invade the terrestrial environment. Immediately after oviposition, the eggs of A. aegypti are susceptible to desiccation and become waterproof only after several hours, when the serosal cuticle is formed. This layer, which is secreted below the endochorion by the serosa, surrounds the embryo (15). By contrast, the waterproofing process in D. melanogaster and R. prolixus takes place in the ovaries, simultaneously with the hardening of the chorion, during the final stages of oogenesis (16, 17). In the present study, we used R. prolixus, a hemipteran that is a vector of Chagas disease, to show that a Duox enzyme present in the ovarian follicular epithelium is the source of the H2O2 that fuels the hardening of the chorion, a process that is essential for waterproofing the eggshell.
EXPERIMENTAL PROCEDURES
Insects
The insects used were adult mated females taken from a R. prolixus colony, maintained at 28 °C and 75% relative humidity, and fed on rabbit blood. When mentioned, the insects were kept in a water-saturated atmosphere (relative humidity >96%) at 28 °C. To avoid bacterial and fungal proliferation, 100 units penicillin, 100 μg of streptomycin, and 2.5 μg of fungizone per ml were added to the water that was used to fill the humid chamber.
Ethics Statement
All animal care and experimental protocols were conducted in accordance with the guidelines of the Committee for Evaluation of Animal Use for Research (Comissão de Avaliac̨ão do Uso de Animais em Pesquisa da Universidade Federal do Rio de Janeiro, CAUAP-UFRJ) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocols were approved by the CAUAP-UFRJ under Registry Number IBQM001. Dedicated technicians at the animal facility at the Institute of Medical Biochemistry (UFRJ) carried out all aspects related to rabbit husbandry under strict guidelines to ensure careful and consistent handling of the animals.
Tissues
The salivary glands, heart, Malpighian tubules, anterior midgut, posterior midgut, hindgut, fat body, ovary, and follicular epithelia were dissected from cold-anesthetized insect females in cold Tyrode's solution (137 mm NaCl, 2.68 mm KCl, 1.8 mm CaCl2, 5.56 mm glucose, 0.32 mm NaH2PO4, 1.16 mm NaHCO3, pH 7.4).
Identification of the Duox and Catalase Genes
A local BLAST search using the cDNA sequences of Duox, NOX5, and catalase orthologs as queries was used to identify the Duox and catalase sequences in a R. prolixus 454 transcriptome database. These partial cDNAs were used to identify the Duox (RPTMP03545), NOX5 (RPTMP07634), and catalase (RPTMP07126) full-length transcripts in the R. prolixus genome database available at VectorBase.
RpDuox Structure and Phylogenetic Analysis
Transmembrane α-helices were predicted using the TMHMM server v. 2.0, available from the Center for Biological Sequence Analysis, and the amino acid residue hydrophobicity profile was performed according to Kyte and Doolittle (18) and also by alignment with Duox sequences from other insects. The positions of peroxidase-like and NADPH oxidase (NOX) domains as well as EF-hand calcium-binding sites were predicted according to Marchler-Bauer et al. (19). The Duox sequences of Acyrthosiphon pisum, Anopheles gambiae, and D. melanogaster as well as RpDuox were aligned using the Clustalw2 software, available at the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) website.
The predicted amino acid sequences of these proteins were aligned using the program Muscle (20). Phylogenetic analysis, performed using only the NOX domain, which is found in all members of the NOX family, was based on maximum likelihood analysis, which was carried out with the PhyML program (21) using the JTT model of substitution, with the frequencies estimated from the data and a discrete γ distribution with four categories and 500 bootstrap replicates. The following sequences were used in sequence alignments and phylogenetic analyses. The Duox sequences were: RPR, R. prolixus (RPTMP03545); HSAD1, Homo sapiens Duox1 (gi 20149640); HSAD2, H. sapiens Duox2 (gi 132566532); CEL, Caenorhabditis elegans (gi 351063525); DRE, Danio rerio (gi 115391856); API, A. pisum (gi 193650217); DME, D. melanogaster (gi 281364292); AME, Apis mellifera (gi 328779750); DPU, Daphnia pulex (gi 321466984); ISC, Ixodes scapularis (gi 241624918); and AGA, A. gambiae (gi 158298988) and PHU, Pediculus humanus corporis (gi 242018811). The NOX5 sequences were: RPR, R. prolixus (RPTMP07634); HSA, H. sapiens (gi 115527743); DRE, D. rerio (gi 189537273); API, A. pisum (gi 328705704); TCA, Tribolium castaneum (gi 189239162); DME, D. melanogaster (gi 161077140); AME, A. mellifera (gi 151427582); and PHU, P. humanus corporis (gi 242015786) and AGA, A. gambiae (gi 151427580). The NOX1–4 sequences were: HSA1, H. sapiens NOX1 (gi 148536873); HSA2, H. sapiens NOX2 (gi 6996021); and HSA3, H. sapiens NOX3 (gi 11136626) and HSA4, H. sapiens NOX4 (gi 8393843).
RNA Extraction, Conventional PCR, and qPCR
Total RNA was extracted from tissues using TRIzol (Invitrogen) according to the manufacturer's protocol. RNA was treated with RNase-free DNase I (Fermentas International Inc., Burlington, Canada), and cDNA was synthesized using the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). cDNA from salivary glands, heart, Malpighian tubules, anterior midgut, posterior midgut, hindgut, fat body, and ovary were PCR-amplified using the PCR master mix (Fermentas International Inc.), and the same primers were used for qPCR (described below). The fragments were separated by agarose gel electrophoresis (2% w/v), and their sizes were compared with GeneRulerTM 100 bp Plus DNA ladder fragments (Fermentas International Inc.). qPCR was performed on a StepOnePlus real-time PCR system (Applied Biosystems) using the Power SYBR Green PCR master mix (Applied Biosystems). The comparative Ct method (22) was used to compare gene expression levels. The R. prolixus 18 S rRNA gene was used as an endogenous control (23). The primer pairs used for the amplification of Duox and 18 S rRNA cDNA fragments for both conventional and real-time PCR, named DuoxRt and 18sRt, respectively, were DuoxRt, forward 5′-TTGTGTTCGCACATCCAACT-3′ and reverse 5′-GGTCCAACGAAAAATATCCAAA-3′; 18SRt, forward, 5′-TGTCGGTGTAACTGGCATGT-3 and reverse, 5′-TCGGCCAACAAAAGTACACA-3′.
Hydrogen Peroxide Measurement
Measurement of H2O2 production by whole ovaries or by dissected follicular epithelia was performed by incubating tissues for 60 min at 25 °C in Tyrode's solution with 40 μm Amplex Red and 0.08 units/μl of horseradish peroxidase (24). After incubation, the ovaries or epithelia were spun, and the supernatant was collected. Fluorescence (excitation, 530 nm; emission, 590 nm) was measured with a microplate reader, the SpectraMax M5 (Molecular Devices). Because Duox activity depends on calcium, Ca2+-dependent H2O2 generation was evaluated by preincubation of the ovaries and follicular epithelia in the presence or absence of 1 μm ionomycin, 10 μm BAPTA/AM, or both for 10 min at 28 °C. Inhibition of Duox activity was achieved via preincubation with 1 μm diphenyleneiodonium. The fluorescence of nonspecific (H2O2-independent) oxidation of Amplex Red was evaluated in the absence of HRP and used as a blank.
RNAi Experiments
A 404-bp fragment from the Duox gene and a 453-bp fragment from the catalase gene were amplified from reverse-transcribed RNAs extracted from R. prolixus ovaries using the primer pairs DuoxDs1 and CatDs1, respectively. The amplification products were subjected to nested PCR with an additional pair of primers (DuoxDs2 and CatDs2) that included the T7 promoter sequence in each fragment. The primers mentioned above were DuoxDs1, forward, 5′-ATGGGTAATCCTGCGTTGAG-3′ and reverse, 5′-CCGTCTTTTGATTCCAGCAT-3′; CatDs1, forward, 5′-GGAGCGTTCGGTTACTTTGA-3′ and reverse, 5′-GCAAGTTTCACCTCGGTCAT-3′; DuoxDs2, forward, 5′-TAATACGACTCACTATAGGGATGGGTAATCCTGCGTTGAG-3′ and reverse, 5′-TAATACGACTCACTATAGGGCCGTCTTTTGATTCCAGCAT-3′; CatDs2, forward, 5′-TAATACGACTCACTATAGGGGGAGCGTTCGGTTACTTTGA-3′ and reverse, 5′-TAATACGACTCACTATAGGGGCAAGTTTCACCTCGGTCAT-3′. The nested PCRs generated 444- and 493-bp fragments of Duox and catalase, respectively. These fragments were used as a template to synthesize double-stranded RNA (dsRNA) specific for Duox (dsDuox) and catalase (dsCat) using the MEGAscript RNAi kit (Ambion, Austin, TX) according to the manufacturer's protocol. An unrelated dsRNA (dsMal) specific for the Escherichia coli MalE gene (Gene ID: 948538) was used as a control for the off-target effects of dsRNA. The Mal fragment was amplified from the Litmus 28i-mal plasmid (New England Biolabs) with a single primer (T7, 5′-TAATACGACTCACTATAGGG-3′) specific for the T7 promoter sequence that is on both sides of the MalE sequence. For gene silencing experiments, cold-anesthetized adult females were injected in the hemocoel with 1 μl of sterile distilled water containing 1 mg/ml dsRNA using a 5-μl Hamilton syringe. Six days after dsRNA injection, the insects were fed with rabbit blood.
Oviposition and Eclosion Ratios
After a blood meal, the females were individually separated into vials and kept at 28 °C and 75% relative humidity. After completion of oviposition, the number of eggs laid by each female was counted. The eclosion ratios were calculated by dividing the number of hatched first instar nymphs by the number of eggs laid by each female.
Eggshell Fluorescence
Eggshells were photographed after eclosion with an Olympus MVX10 macroview fluorescence microscope equipped with a Olympus DP-72 color CCD camera without filters, an external LED white light source for bright field imaging, and a filter set for dityrosine fluorescence (4900 ET - DAPI - EX D350/50X; BS 400DCL; EM ET460/50Chroma). Comparison of fluorescence levels between the distinct systems was performed using the same objectives and exposure times. For fluorescence quantification, pictures of three groups of 10 eggs from control and silenced females were analyzed with ImageJ software (24). To calculate the corrected total egg fluorescence (CTEF), we used the following formula: CTEF = integrated density − (area of selected egg × mean fluorescence of background readings).
Chorion Acid Hydrolysis
Egg chorion obtained from newly laid eggs was subjected to acid hydrolysis to evaluate its dityrosine content using the procedure described by Malencik et al. (25), with some modifications. In three independent experiments, pools of 20 eggs laid by 10 dsMal- or dsDuox-injected females were transferred to 1-ml polypropylene tubes containing 1% Triton X-100 in distilled water, homogenized, sonicated for 10 min, and washed 10 times with distilled water. After sedimentation, chorion fragments were collected, dried under a vacuum, and added to 6 n HCl. The samples were hydrolyzed for 24 h at 110 °C under a vacuum and then dried under a vacuum, resuspended in distilled water, and filtered on Amicon filters (10-kDa cutoff). After being dried under a vacuum, the samples were resuspended in 50 mm sodium phosphate buffer, pH 9.6. For standard dityrosine preparation, 2 mg of tyrosine and 80 μg of horseradish peroxidase (Sigma) were added to 2 ml of 100 mm Tris-HCl buffer, pH 9.2, containing 0.005% H2O2. After 24 h of incubation at 37 °C, the reaction medium was filtered on Amicon filters (10-kDa cutoff) to remove horseradish peroxidase and dried under a vacuum. The samples were then resuspended in 50 mm sodium phosphate buffer, pH 9.6. Fluorescence excitation spectra (excitation, 280–360 nm; emission, 410 nm) were measured with a Cary Eclipse 100 spectrofluorometer (Varian, Palo Alto, CA). Crude eggshell hydrolysate is a complex mixture of substances. To circumvent this problem and gain specificity, the spectra of crude hydrolysate from dsMal samples were subtracted from the spectra of dsDuox samples, a procedure that resulted in a spectral profile that reflected only the differences between the two samples.
Statistical Analysis
All experiments were repeated at least twice. Statistical analysis was performed using Student's t test with a 95% confidence interval or a one-way analysis of variance followed by Tukey's multiple comparisons post test (GraphPad Prism).
RESULTS
Structural Features, Domains, and Polygenetic Analysis of RpDuox
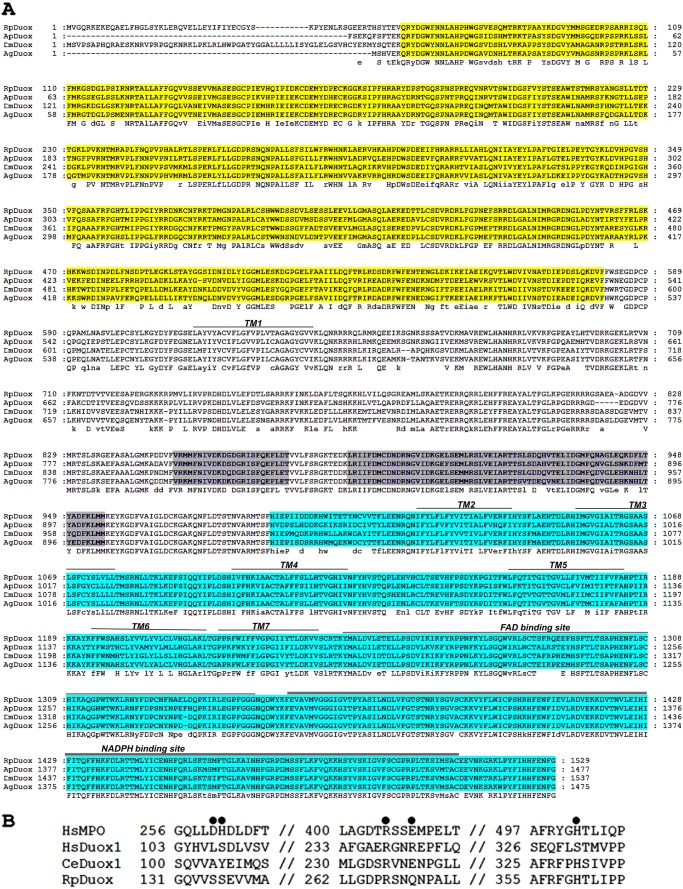
The presence of a Duox-type NADPH oxidase, initially found in a transcriptome, was confirmed by identification of a complete predicted transcript in the R. prolixus genome, hereafter called RpDuox. Fig. 1A shows the characteristic peroxidase-like, EF-hand, and NOX domains of RpDuox, which are conserved among all Duox orthologs. A multiple sequence alignment of the RpDuox protein sequence against A. pisum, A. gambiae, and D. melanogaster Duox sequences showed a remarkable conservation (see Fig. 6). Phylogenetic analysis provided further support for the identification of RpDuox, which clustered with other members of the Duox group (Fig. 1B). Additionally, the Duox and NOX5 branches that were obtained were consistent with known arthropod evolutionary relationships (Fig. 1B), including insects arising from Crustacea (26, 27).
FIGURE 1.
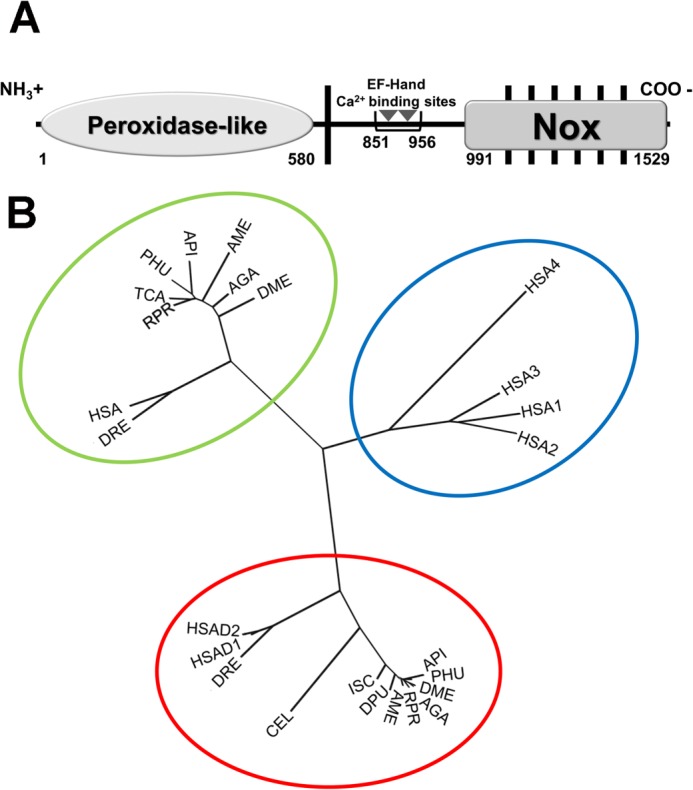
Structure and phylogeny relationships of R. prolixus dual oxidase. A, the scheme shows the positions of the predicted peroxidase-like and NOX domains, transmembrane α-helices (black bars), and cytosolic EF-hand calcium-binding sites (gray triangles) of R. prolixus dual oxidase (RpDuox). B, phylogenetic comparisons between NOX (1–5) sequences and the sequences of Duox NOX domains. The following species were used for tree construction. The Duox sequences were: RPR, R. prolixus; HSAD1, H. sapiens Duox1; HSAD2, H. sapiens Duox2; CEL, C. elegans; DRE, D. rerio; API, A. pisum; DME, D. melanogaster; AME, A. mellifera; DPU, D. pulex; ISC, I. scapularis; AGA, A. gambiae (gi 158298988) and PHU, P. humanus corporis. The NOX5 sequences were: RPR, R. prolixus; HSA, H. sapiens; DRE, D. rerio; API, A. pisum; TCA, T. castaneum; DME, D. melanogaster; AME, A. mellifera; PHU, P. humanus corporis and AGA, A. gambiae. The NOX1–4 sequences were: HSA1, H. sapiens NOX1; HSA2, H. sapiens NOX2; HSA3, H. sapiens NOX3 and HSA4, H. sapiens NOX4. Colored ellipses indicate the three NOX clusters, vertebrate specific NOX1–4 (blue), NOX5 (green), and Duox (red).
FIGURE 6.
Sequence and structural features of RpDuox. A, amino acid sequence alignment of insect Duox orthologs. From top to bottom, Duox sequences of R. prolixus (RpDuox), A. pisum (ApDuox), D. melanogaster (DmDuox), and A. gambiae (AgDuox) are shown. The consensus sequence is indicated below the alignment. The peroxidase-like domain, EF-hand calcium binding sites, and NOX domain are indicated by the colors yellow, gray, and blue, respectively. The superscripted single, double, and triple bars indicate residues comprising the transmembrane hydrophobic regions (TM1–7), FAD binding sites, and NADPH binding sites, respectively. The sequences were aligned using the Clustalw2 software. Dashes represent gaps introduced to preserve alignment. B, sequence alignment of typical and Duox peroxidase-like domains. From the top to bottom, sequences of H. sapiens myeloperoxidase (HsMPO), H. sapiens Duox1 (HsDuox1), C. elegans Duox1 (CeDuox), and RpDuox are shown. Filled circles indicate the distal and proximal histidines (His261 and His502, respectively), catalytic arginine (Arg405), and covalent heme-binding site residues aspartate (Asp260) and glutamate (Glu408), which are critical for the activity of the typical peroxidase HsMPO. Accession numbers are: RpDuox, RPTMP03545; ApDuox, XP_001951113; DmDuox, NP_608715; AgDuox, XP_319115.4; HsMPO, NP_000241.1; HsDuox, gb AAI14939.1; CeDuox, gb AAF71303.1.
Duox Is Expressed in the Ovaries during Choriogenesis
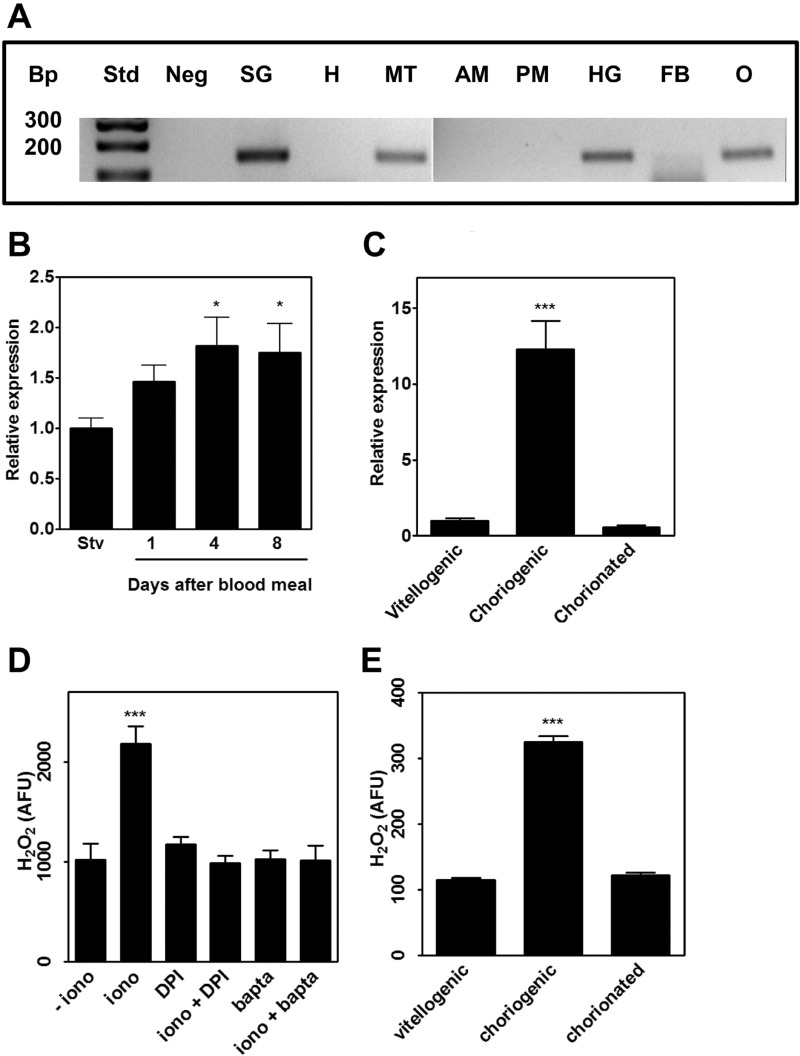
RpDuox mRNA was expressed in several of the tissues tested, including ovaries, salivary glands, Malpighian tubules, hindgut, and fat body (Fig. 2A). RpDuox expression in the ovary increased after a blood meal (Fig. 2B), paralleling the time course of oocyte growth in this insect (28). Evaluation by qPCR of RpDuox mRNA in ovarian follicles at distinct developmental stages revealed that the highest expression levels were found in the epithelium of choriogenic follicles and were almost 10 times higher than those in the epithelia from follicles that either were at the vitellogenic growth stage or had already completed chorion formation (Fig. 2C).
FIGURE 2.
Tissue distribution, expression levels, and activity of RpDuox. A, tissue distribution. Duox fragments were PCR-amplified from cDNA samples from salivary glands (SG), heart (H), Malpighian tubules (MT), anterior midgut (AM), posterior midgut (PM), hindgut (HG), fat body (FB), and ovary (O) and separated by 2% agarose gel electrophoresis. (Neg, negative control; Bp, base pair). B, RpDuox expression levels in ovaries. qPCR assays were performed with ovaries dissected immediately before (starved) or at different days ABM. Eight pairs of ovaries were used for each data set. The levels of Duox expression were normalized by the level found in the ovaries of starved (Stv) females. C, RpDuox expression levels during developmental stages of ovarian follicle epithelia. Epithelia were dissected 7 days ABM from vitellogenic, choriogenic, or chorionated follicles. Nine pools of 10 epithelia were used for each experimental condition. The levels of Duox expression were normalized by the level found in vitellogenic follicles. D, Duox activity in the whole ovary. Generation of H2O2 was assayed with HRP/Amplex Red in ovaries from insects dissected 3 days ABM, and fluorescence intensity was plotted as arbitrary fluorescence units (AFU). Five pools of two ovaries were used in each data point. Tissues were preincubated in the presence or absence of ionomycin (iono), diphenyleneiodonium (DPI), or BAPTA/AM. E, Duox activity in follicular epithelia from vitellogenic, choriogenic, or chorionated follicles. Experimental conditions were the same as used in D, except that five pools of 10 follicular epithelia were used for each data point. Data shown are of ionomycin-activated H2O2 production. Data shown in all graphics are mean ± S.E. * p < 0.05, *** p < 0.0001 (analysis of variance followed by Tukey's multiple comparison test).
Duox enzymes are calcium-dependent NADPH oxidases due to the presence of the EF-hand domain (14). When ovaries dissected from blood-fed females were incubated in the presence of ionomycin, a calcium ionophore that increases intracellular levels of Ca2+, H2O2 production by this tissue was markedly stimulated (Fig. 2D). Preincubation of ovaries with diphenyleneiodonium (an NADPH oxidase inhibitor) or BAPTA/AM (an intracellular Ca2+ chelator) prevented this ionomycin-induced increase in H2O2 production, consistent with the presence of a Duox-type enzyme in this tissue (29). As expected from the increased expression of RpDuox mRNA during chorion formation (Fig. 2C), we observed increased ionomycin-stimulated H2O2 production in the follicular epithelium during this developmental stage (Fig. 2E), suggesting that this enzyme participates in choriogenesis.
RpDuox Silencing Impairs Egg Development
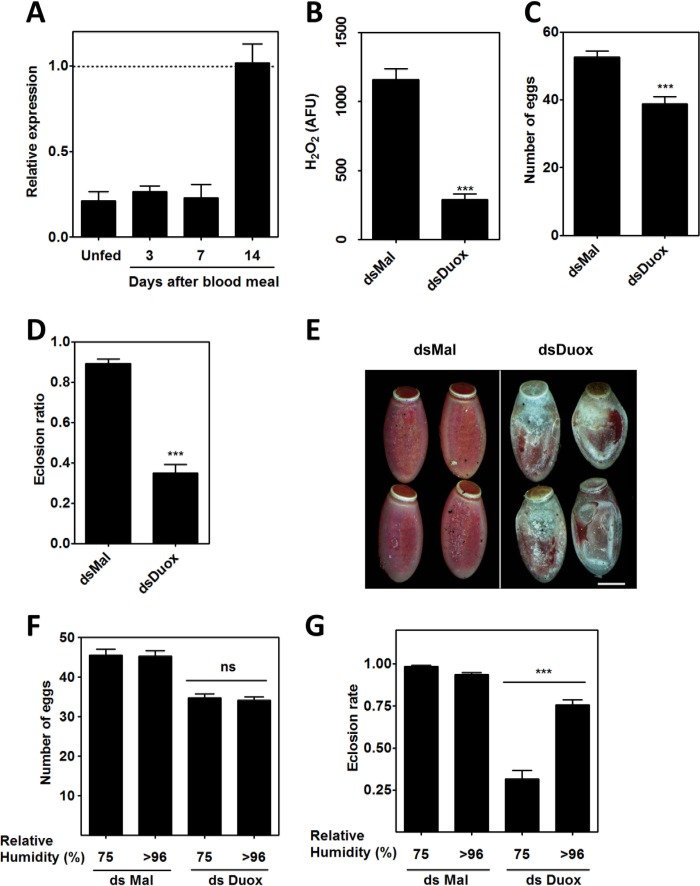
To investigate the function of RpDuox in the ovaries of R. prolixus, we silenced its expression by injecting dsRNA for this gene into the hemocoel of females prior to the blood meal. dsRNA injection reduced Duox mRNA levels in the ovaries by ∼70% in relation to control females. Efficient silencing was achieved before the blood meal and was maintained until 1 week after the blood meal (ABM), with RpDuox expression returning to levels similar to the control near the end of oogenesis, 2 weeks ABM (Fig. 3A). RpDuox silencing led to a significant reduction of Duox activity in the ovaries (Fig. 3B), modestly reduced the number of eggs laid, and markedly decreased the first instar nymph eclosion ratio (Fig. 3, C and D). Upon RpDuox silencing, a major fraction of the eggs dried out in the first 48 h after oviposition and did not develop (Fig. 3E), suggesting that the permeability barrier of the eggshell was compromised. This conclusion was confirmed by incubating RpDuox-silenced eggs in a high humidity atmosphere, which resulted in eclosion rates similar to those of control eggs (Fig. 3, F and G).
FIGURE 3.
RpDuox silencing. R. prolixus adult females were injected with dsMal or dsDuox 6 days before blood meal. A, RpDuox expression inhibition in whole ovaries. Ovaries were dissected immediately before (Unfed) or at the indicated times after blood meal for qPCR analysis. The dashed line represents the expression level shown in the ovaries of control females (dsMal-injected). Five pools of two ovaries were used for each condition. B, inhibition of H2O2 generation in whole ovaries. Three days after the meal, ovaries were dissected and assayed with HRP/Amplex Red to measure H2O2 generation. The data shown represent ionomycin-stimulated H2O2 generation plotted as arbitrary fluorescence units (AFU). Five pools of two ovaries were used for each condition. C and D, effect of Duox RNAi on the number of eggs laid (C) and eclosion ratios (D). Adult females were injected with dsMal (24) and dsDuox (19), respectively, and the number of eggs laid and first instar nymph eclosion ratios were calculated for each female individually. E, RpDuox silencing impairs embryo development. Bright field representative images of eggs laid by control (DsMal) and RpDuox-silenced females at 48 h after oviposition are shown. Bar scale = 0.5 mm. F and G, impairment of embryo development after RpDuox silencing is prevented by incubation in an atmosphere of high relative humidity. Eggs were incubated under high humidity (relative humidity >96%). At least nine females were used per condition. In all graphics, the data shown are the mean ± S.E., and asterisks indicate significantly different values (p < 0.0001, Student's t test). ns, not significant.
RpDuox Silencing Inhibits Chorion Protein Cross-linking
The hardening of the eggshell is known to involve protein cross-linking through peroxidase-mediated oxidation of tyrosine residues (5), which led us to speculate that RpDuox might be the source of the H2O2 used in eggshell formation. Consistent with this hypothesis, silencing of RpDuox resulted in the formation of eggshells that showed markedly reduced fluorescence emission under ultraviolet excitation (Fig. 4, A and B), a property of dityrosine formed by oxidative protein cross-linking (30, 31). To confirm that this difference in fluorescence was due to a distinct dityrosine content, we subjected eggshells from silenced or control eggs to acid hydrolysis. The fluorescence excitation wavelength spectrum obtained from dsMal sample was subtracted from the dsDuox sample, showing a differential spectrum close to that of standard dityrosine (Fig. 4C).
FIGURE 4.
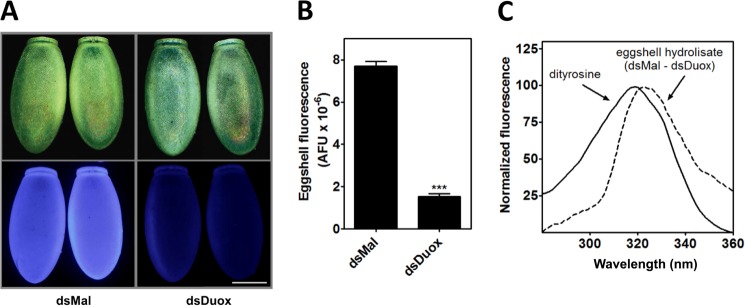
The egg chorion of RpDuox-silenced females shows lower levels of dityrosine. A, dityrosine fluorescence of egg chorion. Representative images are shown of the eggshells of eggs laid by control (dsMal) and RpDuox-silenced females kept until the hatching at high humidity (described in the previous figure). The bright field images (on the top) were taken with a stereomicroscope using an external white light source, and the fluorescence images were taken with a filter set for intrinsic dityrosine fluorescence (on the bottom). Bar scale = 0.5 mm. B, fluorescence emitted by eggshells of dsMal- and dsDuox-injected females. The total eggshell fluorescence was quantified using ImageJ software. Data shown are the mean ± S.E. of total eggshell fluorescence calculated for 10 eggshells selected randomly among ∼400 eggs of each condition. *** p < 0.0001, Student's t test. AFU, arbitrary fluorescence units. C, dityrosine fluorescence excitation spectra of chorion hydrolysates. Eggshells were subjected to acid hydrolysis. After being dried under a vacuum, the samples were resuspended in 50 mm sodium phosphate buffer, pH 9.6. For hydrolysates, the data shown are the normalized spectrum of a dsMal chorion hydrolysate that were subtracted from the spectrum obtained with a dsDuox chorion hydrolysate sample. The preparation of the dityrosine standard is described under “Experimental Procedures.”
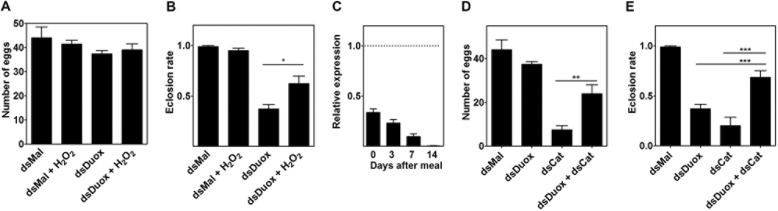
The role of RpDuox in generating H2O2 during chorion formation was further confirmed by reversing the effect of RpDuox silencing by manipulating H2O2 levels in the insect, either by injecting H2O2 or by injecting dsRNA for catalase together with dsRNA for Duox (Fig. 5). Injection of H2O2 did not affect oviposition or the eclosion rate of eggs laid by control females but was able to partially reverse the detrimental effects of RpDuox silencing. Knockdown of catalase, conversely, had a marked effect on oviposition that was partially rescued by simultaneously silencing RpDuox. Interestingly, although silencing either RpDuox or catalase resulted in a pronounced reduction in eclosion, simultaneously silencing both genes resulted in attenuation of this phenotype (Fig. 5).
FIGURE 5.
H2O2 regulates oogenesis and choriogenesis. A and B, H2O2 injection. dsMal- and dsRpDuox-treated females were injected with 5 nmol of H2O2 into the hemocoel 24 h ABM, and the number of eggs laid (A) and the first instar nymph eclosion rates (B) were evaluated. C and D, catalase knockdown. 6 days before the blood meal, adult females were injected with dsMal, dsDuox, dsCat, or dsDuox plus dsCat. C, catalase silencing was evaluated by qPCR in whole ovary extracts. D and E, the number of eggs laid (D) and eclosion ratios (E) were calculated individually for five females in each condition. In all graphic data shown are the mean ± S.E., and the asterisks indicate significantly different values between the conditions (B, p = 0.0205; D, p = 0.0032; Student's t test).
DISCUSSION
Recently, several research groups have looked for biological functions of Duox in insects. In D. melanogaster and A. aegypti, Duox has an immunological role in producing H2O2 that controls the levels of intestinal microbiota (32, 33). In A. gambiae, Duox appears to cross-link peritrophic layer proteins by forming dityrosine bonds on the luminal surface of midgut epithelial cells, a process that limits the diffusion and activity of immune elicitors derived from microbiota (13). In D. melanogaster, Duox plays an essential role in the stabilization of the wing cuticle structure by producing the H2O2 required during sclerotization, melanization, and tyrosine cross-linking (32). Several studies performed with D. melanogaster and A. aegypti (2, 5, 6, 33, 34) have demonstrated the importance of chorion protein cross-linking through the oxidation of tyrosine residues and formation of dityrosine bonds during insolubilization of the eggshell protein moiety. In the present work, we show that a Duox enzyme in the R. prolixus ovary is the source of the H2O2 that is used to cross-link chorion proteins by dityrosine bonding.
The mechanical rigidity of the chorion was recognized a long time ago (1), and it has been attributed to protein cross-linking by peroxidases (2, 4, 5). In the present work, we identified the Duox gene from R. prolixus on the basis of genomic and transcriptomic data, characterized its expression profile, and showed that its expression and activity in the ovary increase after a blood meal. In contrast to mosquitoes, in which the hardening of the chorion takes place in the open environment, after the eggs are laid, in triatomine insects, protein oxidation occurs before oviposition, still in the ovariole. Consistent with this, the highest Duox activities are found in the follicular epithelium during chorion formation. The eggs of Duox-silenced females that hatched on humidity chambers were more fragile than control eggs when pressed with tweezers. Although control eggs broke into pieces when mechanically damaged, dsDuox eggs typically tore. In addition, although control eggshell samples took several hours to solubilize during acid hydrolysis, dsDuox eggshells dissolved completely after only a few minutes of incubation (not shown). As observed originally by Beament (1), this resistance of the eggshell to the harsh conditions used in acid hydrolysis is attributed to the most external layer of the chorion.
If the mechanical strength of the chorion is clearly explained by protein cross-linking, the same does not apply to the protection against water loss. Beament (35) concluded that the wax layer that covers the inner side of the chorion confers water impermeability to the eggs of R. prolixus. Moussian et al. (36) showed that chitin, the major component of the procuticle of D. melanogaster, is not responsible for the protection from dehydration that is conferred by the cuticle. However, Shaik et al. (37) suggested that the resistance to water loss presented by the cuticle of D. melanogaster larvae is principally due to the dityrosine bonding between cuticular proteins. The results presented here support the conclusion that, similar to the study on insect cuticle, protein cross-linking in the egg chorion is also essential to prevent water loss.
Duox proteins (RpDuox included) are characterized by the presence of a peroxidase-like domain, which is not enzymatically active in some genes described, such as the human Duox1 (10). The catalytic distal histidine (His261) and the covalent heme-binding site residues, aspartate (Asp260) and glutamate (Glu408), which have been implicated as critical for activity in typical peroxidases (38, 39), are missing in RpDuox (Fig. 6B), similar to what was described for human Duox1 (10). Additionally, we were not able to detect any differences between the peroxidase activities of follicular epithelia from control and Duox-silenced insects using l-tyrosine ethyl ester or 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as substrates (data not shown). Some authors, however, support the concept that Duox works as a multiple active site enzyme that generates H2O2 while simultaneously catalyzing the formation of dityrosine bonds through its peroxidase domain (9, 10).
H2O2 injection and silencing of catalase were both able to partially reverse the reduction in the eclosion rate induced by dsDuox, providing additional support for the role of RpDuox in chorion protein insolubilization and waterproofing. However, catalase silencing alone severely reduced the number of eggs that were formed, an effect that was also partially reverted by simultaneously silencing RpDuox. This effect of dsCat on oogenesis revealed an unanticipated role of redox balance in the regulation of vitellogenesis, suggesting that the fine-tuning of reactive oxygen species levels has an essential role in the regulation of egg maturation, a possibility that deserves further investigation. A role for a Duox enzyme has also been reported in sea urchin eggs, where it acts as a source of H2O2 that supports protein cross-linking through dityrosine formation during egg cortex transformation. This process, which starts at the moment of sperm fusion, alters extracellular matrix characteristics, which prevents polyspermy (40) and confers resistance against mechanical distortion and chemical attack, allowing the formation of an appropriate environment for embryo development (41). Because they are aquatic organisms, sea urchins do not have waterproofed eggs. The recognition that these two types of egg extracellular layers, the insect chorion and the sea urchin fertilization barrier, also share the same mechanism of formation leads us to speculate that they may have the same evolutionary origin despite the large phylogenetic distance. If true, this hypothesis would imply an ancestral role for Duox in reproduction.
In summary, the present study shows for the first time that in R. prolixus, Duox is the enzyme that generates H2O2 in the follicular epithelium and supports chorion peroxidase activity during the hardening of eggshell proteins, which is essential in the acquisition of resistance to water loss.
Acknowledgments
We thank all the members of the Laboratory of Biochemistry of Hematophagous Arthropods, especially Dr. Jose Henrique M. Oliveira for help with the H2O2 measurement assays, Dr. Rafael Dias Mesquita (Department of Biochemistry, Institute of Chemistry, Federal University of Rio de Janeiro) for help with the in silico analysis, and Dr. Andre Elias R. Soares (Department of Genetics, Institute of Biology, Federal University of Rio de Janeiro) for help with the phylogeny analysis. We also thank José de S. Lima, Jr., Gustavo Ali, and Litiane M. Rodrigues for insect care. In addition, we express our gratitude to S. R. Cássia for all assistance.
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo a Pesquisa de Estado do Rio de Janeiro (FAPERJ).
This paper is dedicated to the memory of Doctor Alexandre A. Peixoto.
- Duox
- dual oxidase
- RpDuox
- R. prolixus Duox
- NOX
- NADPH oxidase
- BAPTA/AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- qPCR
- quantitative PCR
- ABM
- after the blood meal.
REFERENCES
- 1. Beament J. W. (1946) The formation and structure of the chorion of the egg in an hemipteran, Rhodnius prolixus. Q. J. Microsc. Sci. 87, 393–439 [PubMed] [Google Scholar]
- 2. Li J. S., Li J. (2006) Major chorion proteins and their crosslinking during chorion hardening in Aedes aegypti mosquitoes. Insect. Biochem. Mol. Biol. 36, 954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mindrinos M. N., Petri W. H., Galanopoulos V. K., Lombard M. F., Margaritis L. H. (1980) Crosslinking of the Drosophila chorion involves a peroxidase. Roux Arch. Dev. Biol. 189, 187–196 [DOI] [PubMed] [Google Scholar]
- 4. Konstandi O. A., Papassideri I. S., Stravopodis D. J., Kenoutis C. A., Hasan Z., Katsorchis T., Wever R., Margaritis L. H. (2005) The enzymatic component of Drosophila melanogaster chorion is the Pxd peroxidase. Insect. Biochem. Mol. Biol. 35, 1043–1057 [DOI] [PubMed] [Google Scholar]
- 5. Li J., Hodgeman B. A., Christensen B. M. (1996) Involvement of peroxidase in chorion hardening in Aedes aegypti. Insect. Biochem. Mol. Biol. 26, 309–317 [DOI] [PubMed] [Google Scholar]
- 6. Margaritis L. H. (1985) The egg-shell of Drosophila melanogaster III. Covalent crosslinking of the chorion proteins involves endogenous hydrogen peroxide. Tissue Cell 17, 553–559 [DOI] [PubMed] [Google Scholar]
- 7. Lambeth J. D. (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 [DOI] [PubMed] [Google Scholar]
- 8. Geiszt M., Witta J., Baffi J., Lekstrom K., Leto T. L. (2003) Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 17, 1502–1504 [DOI] [PubMed] [Google Scholar]
- 9. Edens W. A., Sharling L., Cheng G., Shapira R., Kinkade J. M., Lee T., Edens H. A., Tang X., Sullards C., Flaherty D. B., Benian G. M., Lambeth J. D. (2001) Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 154, 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meitzler J. L., Ortiz de Montellano P. R. (2009) Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains: insights into heme binding and catalytic activity. J. Biol. Chem. 284, 18634–18643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chávez V., Mohri-Shiomi A., Garsin D. A. (2009) Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect. Immun. 77, 4983–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong J. L., Créton R., Wessel G. M. (2004) The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Dev. Cell 7, 801–814 [DOI] [PubMed] [Google Scholar]
- 13. Kumar S., Molina-Cruz A., Gupta L., Rodrigues J., Barillas-Mury C. (2010) A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327, 1644–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donkó A., Péterfi Z., Sum A., Leto T., Geiszt M. (2005) Dual oxidases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 2301–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rezende G. L., Martins A. J., Gentile C., Farnesi L. C., Pelajo-Machado M., Peixoto A. A., Valle D. (2008) Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Dev. Biol. 8, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beament J. W. L. (1946) The waterproofing process in eggs of Rhodnius prolixus Stahl. Proc. R. Soc. B Biol. Sci. 133, 407–418 [PubMed] [Google Scholar]
- 17. Papassideri I. S., Margaritis L. H., Gulik-Krzywicki T. (1993) The eggshell of Drosophila melanogaster. VIII. Morphogenesis of the wax layer during oogenesis. Tissue Cell 25, 929–936 [DOI] [PubMed] [Google Scholar]
- 18. Kyte J., Doolittle R. F. (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 [DOI] [PubMed] [Google Scholar]
- 19. Marchler-Bauer A., Lu S., Anderson J. B., Chitsaz F., Derbyshire M. K., DeWeese-Scott C., Fong J. H., Geer L. Y., Geer R. C., Gonzales N. R., Gwadz M., Hurwitz D. I., Jackson J. D., Ke Z., Lanczycki C. J., Lu F., Marchler G. H., Mullokandov M., Omelchenko M. V., Robertson C. L., Song J. S., Thanki N., Yamashita R. A., Zhang D., Zhang N., Zheng C., Bryant S. H. (2011) CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39, D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guindon S., Gascuel O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 [DOI] [PubMed] [Google Scholar]
- 22. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 23. Majerowicz D., Alves-Bezerra M., Logullo R., Fonseca-de-Souza A. L., Meyer-Fernandes J. R., Braz G. R., Gondim K. C. (2011) Looking for reference genes for real-time quantitative PCR experiments in Rhodnius prolixus (Hemiptera: Reduviidae). Insect Mol. Biol. 20, 713–722 [DOI] [PubMed] [Google Scholar]
- 24. Mohanty J. G., Jaffe J. S., Schulman E. S., Raible D. G. (1997) A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 202, 133–141 [DOI] [PubMed] [Google Scholar]
- 25. Malencik D. A., Sprouse J. F., Swanson C. A., Anderson S. R. (1996) Dityrosine: preparation, isolation, and analysis. Anal. Biochem. 242, 202–213 [DOI] [PubMed] [Google Scholar]
- 26. Regier J. C., Shultz J. W., Zwick A., Hussey A., Ball B., Wetzer R., Martin J. W., Cunningham C. W. (2010) Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463, 1079–1083 [DOI] [PubMed] [Google Scholar]
- 27. Trautwein M. D., Wiegmann B. M., Beutel R., Kjer K. M., Yeates D. K. (2012) Advances in insect phylogeny at the dawn of the postgenomic era. Annu. Rev. Entomol. 57, 449–468 [DOI] [PubMed] [Google Scholar]
- 28. Oliveira P. L., Gondim K. C., Guedes D. M., Masuda H. (1986) Uptake of yolk proteins in Rhodnius prolixus. J. Insect. Physiol. 32, 859–866 [Google Scholar]
- 29. Ellis J. A., Mayer S. J., Jones O. T. (1988) The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem. J. 251, 887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neff D., Frazier S. F., Quimby L., Wang R. T., Zill S. (2000) Identification of resilin in the leg of cockroach, Periplaneta americana: confirmation by a simple method using pH dependence of UV fluorescence. Arthropod. Struct. Dev. 29, 75–83 [DOI] [PubMed] [Google Scholar]
- 31. Andersen S. O., Roepstorff P. (2005) The extensible alloscutal cuticle of the tick, Ixodes ricinus. Insect Biochem. Mol. Biol. 35, 1181–1188 [DOI] [PubMed] [Google Scholar]
- 32. Anh N. T., Nishitani M., Harada S., Yamaguchi M., Kamei K. (2011) Essential role of Duox in stabilization of Drosophila wing. J. Biol. Chem. 286, 33244–33251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Margaritis L. H. (1985) Structure and physiology of the eggshell. in Comprehensive Insect Physiology, Biochemistry, and Pharmacology (Kerkut G. A., Gilbert L. I., eds.), 1st Ed., pp. 151–230, Pergamon Press, Oxford, UK [Google Scholar]
- 34. Papassideri I. S., Margaritis L. H. (1996) The eggshell of Drosophila melanogaster: IX. Synthesis and morphogenesis of the innermost chorionic layer. Tissue Cell 28, 401–409 [DOI] [PubMed] [Google Scholar]
- 35. Beament J. W. (1946) Waterproofing mechanism of an insect egg. Nature 157, 370. [DOI] [PubMed] [Google Scholar]
- 36. Moussian B., Schwarz H., Bartoszewski S., Nüsslein-Volhard C. (2005) Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J. Morphol. 264, 117–130 [DOI] [PubMed] [Google Scholar]
- 37. Shaik K. S., Meyer F., Vázquez A. V., Flötenmeyer M., Cerdán M. E., Moussian B. (2012) δ-Aminolevulinate synthase is required for apical transcellular barrier formation in the skin of the Drosophila larva. Eur. J. Cell Biol. 91, 204–215 [DOI] [PubMed] [Google Scholar]
- 38. Zederbauer M., Jantschko W., Neugschwandtner K., Jakopitsch C., Moguilevsky N., Obinger C., Furtmüller P. G. (2005) Role of the covalent glutamic acid 242-heme linkage in the formation and reactivity of redox intermediates of human myeloperoxidase. Biochemistry 44, 6482–6491 [DOI] [PubMed] [Google Scholar]
- 39. Zederbauer M., Furtmüller P. G., Bellei M., Stampler J., Jakopitsch C., Battistuzzi G., Moguilevsky N., Obinger C. (2007) Disruption of the aspartate to heme ester linkage in human myeloperoxidase: impact on ligand binding, redox chemistry, and interconversion of redox intermediates. J. Biol. Chem. 282, 17041–17052 [DOI] [PubMed] [Google Scholar]
- 40. Wong J. L., Wessel G. M. (2005) Reactive oxygen species and Udx1 during early sea urchin development. Dev. Biol. 288, 317–333 [DOI] [PubMed] [Google Scholar]
- 41. Wong J. L., Wessel G. M. (2008) Free-radical crosslinking of specific proteins alters the function of the egg extracellular matrix at fertilization. Development 135, 431–440 [DOI] [PubMed] [Google Scholar]