Background: We investigated the different modes of calreticulin-substrate binding.
Results: Calreticulin binds glycosylated and nonglycosylated proteins with similar affinities but distinct kinetics and P-domain conformations.
Conclusion: Successful substrate recruitment by calreticulin requires glycan and P-domain-dependent interactions.
Significance: Elucidation of the distinct modes of calreticulin binding to substrate glycan and polypeptide components and their combined contributions to substrate recruitment in cells.
Keywords: Endoplasmic Reticulum (ER), Fluorescence Resonance Energy Transfer (FRET), Glycosylation, Molecular Chaperone, Protein Folding, Calreticulin, ERp57
Abstract
Calreticulin is an endoplasmic reticulum chaperone with specificity for monoglucosylated glycoproteins. Calreticulin also inhibits precipitation of nonglycosylated proteins and thus contains generic protein-binding sites, but their location and contributions to substrate folding are unknown. We show that calreticulin binds glycosylated and nonglycosylated proteins with similar affinities but distinct interaction kinetics. Although both interactions involve the glycan-binding site or its vicinity, the arm-like proline-rich (P-) domain of calreticulin contributes to binding non/deglycosylated proteins. Correspondingly, ensemble FRET spectroscopy measurements indicate that glycosylated and nonglycosylated proteins induce “open” and “closed” P-domain conformations, respectively. The co-chaperone ERp57 influences substrate-binding kinetics and induces a closed P-domain conformation. Together with analysis of the interactions of calreticulin with cellular proteins, these findings indicate that the recruitment of monoglucosylated proteins to calreticulin is kinetically driven, whereas the P-domain and co-chaperone contribute to stable substrate binding. Substrate sequestration in the cleft between the glycan-binding site and P-domain is a likely mechanism for calreticulin-assisted protein folding.
Introduction
Since the pioneering work of Anfinsen (1), it has been known that the amino acid sequence of a protein determines its three-dimensional fold. However, in the crowded cellular milieu, protein chaperones aid in folding nascent proteins by shielding their exposed hydrophobic cores from the solvent, stabilizing folding intermediates, and preventing aggregation, thus allowing the protein to attain its native structural state. Hence, the endoplasmic reticulum (ER),2 a major site for protein folding and assembly, contains many chaperones that guide proteins on the path to attaining their correct fold (reviewed in Ref. 2).
Calreticulin (CRT) is a soluble chaperone that is primarily present within the lumen of ER. Calreticulin is recruited into the MHC class I peptide loading complex where it helps fold MHC class I molecules that mediate immune surveillance by CD8+ T-cells and natural killer cells (reviewed in Ref. 3). Structurally, calreticulin resembles the ER chaperone calnexin (4) and comprises a globular lectin-like domain containing a glycan-binding site (5, 6) and an arm-like proline-rich domain (P-domain), the tip of which interacts with co-chaperones like the oxidoreductase ERp57 (4, 7, 8).
In the ER, α-glucosidases I and II act on nascent glycoproteins (bearing triglucosylated glycans; reviewed in Ref. 9) to generate di-, mono-, and nonglucosylated glycans. UDP-glucose glycoprotein glucosyltransferase 1 acts as a protein-folding sensor and generates monoglucosylated glycoproteins from misfolded nonglucosylated glycoproteins (reviewed in Ref. 10). Calreticulin and calnexin preferentially interact with monoglucosylated glycoproteins via their glycan-binding sites (reviewed in Refs. 11–13). Although glycan-dependent binding is thought to define the primary interaction profiles of calreticulin and calnexin in vivo (reviewed in Ref. 9), a glycan-independent binding mode has been defined based on in vitro measurements of the abilities of calreticulin and calnexin to inhibit aggregation of nonglycosylated proteins (14–18). However, the location of the glycan-independent interaction site of calreticulin is unknown, and it is unclear how glycan-dependent and -independent interactions are integrated into the cellular chaperone cycle of calreticulin.
Recent structural studies have pointed to the presence of a putative protein-protein interaction site in the vicinity of the glycan-binding site of calreticulin (6). However, other studies have indicated that a calreticulin-specific monoglucosylated glycan, Glcα1–3Manα1–2Manα1–2Man (G1M3; a model calreticulin-binding glycan (5, 18)) was unable to inhibit the binding of hydrophobic peptides to calreticulin (19). Therefore, it is unclear whether the vicinity of the glycan-binding site can also participate in glycan-independent interactions. Additionally, calreticulin and calnexin constructs lacking the P-domain show a reduced ability to inhibit aggregation of protein substrates (19, 20), suggesting a role for the flexible arm-like domains of these proteins in mediating glycan-independent interactions. Previous in silico studies using molecular dynamics simulations have suggested that the P-domain of calreticulin is conformationally flexible and that interactions between calreticulin and binding partners such as thrombospondin-1 induce an “open” P-domain conformation (21). However, a structure for full-length calreticulin is unavailable, and there are little data on the relative orientations of the globular and P-domains of calreticulin, orientation changes induced by substrate and co-chaperone binding, and whether the P-domain directly participates in substrate binding.
To address some of these gaps in knowledge, we employed a variety of biophysical approaches to study the kinetics of binding of calreticulin to glycosylated and nonglycosylated proteins, the location of the glycan-independent binding site, and P-domain conformational changes that accompany substrate binding. Together with analyses of the interactions of calreticulin with cellular proteins, the findings of this study allow us to propose a model for the cellular chaperone functions of calreticulin.
EXPERIMENTAL PROCEDURES
Supplies
Unless indicated, all reagents were purchased from Sigma-Aldrich. Normal avian IgY was purchased from Gallus Immunotech (Cary, NC). Glcα1–3Manα1–2Manα1–2Man (G1M3) was purchased from the Alberta Research Council. The Pierce EZ-Link NHS-PEG4-biotin biotinylation kit and biocitin were purchased from Fisher Scientific (Pittsburg, PA). Streptavidin sensors were purchased from FortéBio (Menlo Park, CA). Thiol-reactive maleimide-derivitized fluorescent probes (ATTO 532 and ATTO 647-N) were purchased from AttoTec GmBH (Siegen, Germany).
Calreticulin Mutants
Construction of N-terminally histidine-tagged murine calreticulin (mCRT(WT)), point mutants lacking the ability to bind glycans (mCRT(Y92A)) and ERp57 (mCRT(W244A)) and a truncation mutant lacking the P-domain (residues 187–283; mCRT(ΔP)), were described previously (17). A calreticulin construct with a N-terminal maltose-binding protein (MBP) tag (MBP-CRT) was generated using ligation-independent cloning to insert human CRT(WT) into the pMCSG9 vector (22) as described earlier (17, 23). mCRT(K70C), mCRT(H128C), and the mCRT(E110C/E245C) double mutant were made in the background of mCRT(C146G) using the QuikChange II site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) as described earlier (24) with mCRT in the pMCSG7 vector.
The following primers were used to generate the mCRT(K70C), mCRT(H128C), and mCRT(E110C/E245C) constructs: mCRT(K70C): forward, 5′-GGC ACC AAG AAG GTT TGC GTC ATC TTT AAC TAC AAG GGC-3′, and reverse, 5′-GCC CTT GTA GTT AAA GAT GAC GCA AAC CTT CTT GGT GCC-3′; mCRT(H128C): forward, 5′-GAA CCC TTC AGC AAT TGT GGC CAG ACA CTG GTG GTA CAG-3′, and reverse, 5′-CTG TAC CAC CAG TGT CTG GCC ACA ATT GCT GAA GGG TTC-3′; mCRT(E110C): forward, 5′-GAC ATG CAT GGA GAC TCA TGC TAT AAC ATC ATG TTT GGT CCG-3′, and reverse, 5′-CGG ACC AAA CAT GAT GTT ATA GCA TGA GTC TCC ATG CAT GTC-3′; mCRT(E245C): forward, 5′-GAG ATG GAT GGA GAG TGG TGC CCA CCA GTG ATT CAA AAT CCT GAA TAC-3′, and reverse, 5′-GTA TTC AGG ATT TTG AAT CAC TGG TGG GCA CCA CTC TCC ATC CAT CTC-3′. DNA sequences of all calreticulin constructs were verified.
Protein Purifications
Calreticulin and ERp57 were purified via nickel affinity chromatography as described previously (17, 24). The secondary structure profiles of the calreticulin constructs were assessed via far-UV circular dichroism spectroscopy as described earlier (24). Recombinant human β2-microglobulin (β2M) was purified from Escherichia coli inclusion bodies as described previously (25, 26). Refolded β2M monomers were purified to homogeneity via size-exclusion chromatography in 50 mm Tris (pH 7.5) and 150 mm NaCl using a Superdex 200 column (GE Healthcare). Purified β2M was biotinylated at a 1:1 molar ratio using the Pierce EZ-Link NHS-PEG4-biotin biotinylation kit (Thermo Scientific Pierce) as directed by the manufacturer.
A truncation construct of listeriolysin O (LLO) comprising its first three domains (LLO(d123)) was purified as described earlier (18). Purified LLO(d123) was biotinylated at a 1:1 molar ratio as described above for β2M.
IgY enriched for its monoglucosylated form (IgY-EN) was purified from commercially purchased normal avian IgY as follows: IgY was biotinylated at a 1:1 molar ratio as described above for β2M. Following biotinylation, excess biotin was removed via gel filtration over a Superdex 200 column (GE Healthcare) in PBS (pH 7.4) (Invitrogen). IgY was eluted in two distinct peaks. 0.8 mg of the biotinylated IgY from the second peak was incubated with 0.5 mg of hexahistidine-tagged mCRT(WT) immobilized on Ni-NTA resin in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 5 mm CaCl2 for 1 h at 4 °C. Following elution of any unbound protein, IgY-EN was eluted from the nickel resin via the addition of 350 mm imidazole. Deglycosylated IgY-EN was made via PNGase F (New England BioLabs) digestion of IgY-EN under nondenaturing conditions. For this purpose, 0.25 mg of IgY-EN was incubated with 1000 units of PNGase F (New England BioLabs) in PBS (pH 7.4) (Invitrogen) at 37 °C for 3 h. Deglycosylation efficiency was confirmed via SDS-PAGE analysis of PNGase F-digested and nondigested IgY-EN. All proteins were stored at 4 °C for the short term or aliquoted and frozen at −70 °C for long term storage with the use of 10% glycerol as a cryoprotectant.
Bio-layer Interferometry (BLI)
BLI studies were undertaken using an Octet Red system (FortéBio) with the biotinylated substrates (0.05 mg/ml IgY, 0.5 mg/ml β2M, or 0.5 mg/ml LLO(d123)) immobilized on hydrated streptavidin sensors (FortéBio) and calreticulin serving as the analyte. Interactions between calreticulin and ERp57 were measured with biotinylated calreticulin (at 1 mg/ml) immobilized on streptavidin sensors and ERp57 serving as the analyte. Following immobilization of the substrate, free streptavidin moieties on the BLI sensors were blocked using 0.6 mg/ml biocitin (Fisher). Measurement of interactions between calreticulin and substrates were undertaken by incubating the immobilized substrate with varying concentrations of calreticulin in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 5 mm CaCl2. Experiments with IgY and β2M were run at 25 °C. Experiments with LLO(d123) were run at 37 °C.
For all BLI studies, calreticulin was allowed to associate with the substrate for 250 s followed by a 500-s dissociation in the binding buffer and regeneration of the substrate in 10 mm glycine (pH 2.0). After subtraction of the signal from a buffer-alone blank sensor, the data were analyzed via nonlinear least squares regression in the FortéBio Analysis module using a one-site specific partial fit model to calculate the associated kinetic and affinity constants.
Binding of β2M and LLO(d123) to Matrix-immobilized Calreticulin
0.5 ml of 11 μm MBP-CRT or MBP alone were immobilized on 0.25 ml of amylose resin (GE Healthcare) for 1 h at 4 °C in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 5 mm CaCl2 with gentle shaking. Following immobilization, 0.25 mg of β2M or LLO(d123) (in 20 mm HEPES, pH 7.5, 10 mm NaCl, and 5 mm CaCl2) were applied to each column and incubated for 1 h at 4 °C (β2M) or 25 °C (LLO(d123)) with gentle shaking. After incubation, the unbound protein fraction was removed, and the amylose-bound proteins was eluted with 0.5 m maltose. The resulting unbound and amylose-bound fractions were analyzed via SDS-PAGE.
Fluorescence Spectroscopy
Fluorescence quenching was undertaken with ATTO-532-labeled calreticulin constructs containing cysteine mutations at positions 70 and 128 (mCRT(K70C) and mCRT(H128C)), corresponding to the glycan-binding surface and hydrophobic face of calreticulin, respectively. All proteins were labeled as follows: 5 μm calreticulin (mCRT(K70C) or mCRT(H128C) in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 5 mm CaCl2) was labeled with 50 μm ATTO-532 dye (1 mm stock solution in N,N-dimethylformamide). Following incubation of the protein-probe complex for 1 h at 21 °C, excess dye was removed via two rounds of buffer exchange using an Amicon Ultra concentrator with a 10,000-Da molecular mass cutoff. This was followed by gel filtration of the protein on a Superdex 75 column (GE Healthcare) that was equilibrated in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 5 mm CaCl2.
Fluorescence measurements of labeled mCRT(WT), mCRT(K70C), and mCRT(H128C) were undertaken using a FluoroMax-3 spectrofluorometer (Horiba Scientific) or a SpectraMax M5 microplate reader (Molecular Devices) with calreticulin at a concentration of 0.2 μm in the presence and absence of 4 μm substrate (IgY, β2M, or LLO(d123)). Emission spectra were measured from 538 to 640 nm following excitation at 532 nm.
FRET Spectroscopy
FRET measurements were undertaken with a calreticulin construct containing cysteine residues at positions 110 and 245 in the background of mCRT(C146G) (mCRT(C146G/E110C/E245C)). For FRET analysis, 5 μm mCRT(C146G/E110C/E245C) (in 20 mm HEPES, pH 7.5, 10 mm NaCl, and 5 mm CaCl2) was labeled simultaneously with a 50 μm solution of ATTO 532 and ATTO 647-N (1 mm stock in N,N-dimethylformamide) in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 5 mm CaCl2, as described previously (27). Following incubation at room temperature for 1 h, excess dyes were removed as described above for the calreticulin single mutants.
Ensemble FRET measurements were undertaken using a FluoroMax-3 spectrofluorometer (Horiba Scientific) or a SpectraMax M5 microplate reader (Molecular Devices) with 0.2 μm labeled calreticulin in the presence or absence of different concentrations of IgY-EN, deglycosylated IgY-EN, β2M, or LLO(d123). Emission spectra were recorded from 538 to 720 nm following excitation at 532 nm.
The donor and acceptor florescence intensities (at 559 and 667 nm, respectively) obtained in the presence of ligands were normalized relative to the corresponding intensities in the ligand-free state. Differences in the normalized intensities were analyzed using unpaired t tests. The acceptor/donor FRET intensity ratios (667/559 nm) were calculated for each tested condition. FRET ratios obtained in the presence of different concentrations of ligand were normalized relative to the corresponding ligand-free condition, and normalized FRET ratios were analyzed via nonlinear least squares regression using a one-site total fit model to calculate KD values for each ligand.
Isothermal Titration Calorimetry (ITC)
ITC measurements (at 25 °C) for calreticulin interacting with G1M3 were undertaken as described earlier (24). ITC runs were performed with calreticulin at a concentration of 50 μm in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 0.5 mm CaCl2. 10-μl injections of 0.5 mm stock G1M3 in the same buffer as calreticulin were titrated into the protein sample, and the changes in enthalpy were measured. Twenty-five total injections were performed, and the final data were analyzed using NanoAnalyze (version 2.2.0) (TA instruments, New Castle, DE). Each ITC run was repeated twice.
Differential Scanning Fluorimetry (DSF)
Thermostabilities of mCRT(WT) and mCRT(Y92A) in the presence or absence of 20 μm G1M3 were undertaken using a Thermofluor instrument (Johnson and Johnson) to measure the intensity of 1-anilinonaphthalene-8-sulfonic acid binding to exposed hydrophobic regions of calreticulin (in the presence or absence of G1M3) as a function of temperature. All DSF scans were run as described earlier (28) with calreticulin at a concentration of 11 μm in 20 mm HEPES (pH 7.5), 10 mm NaCl, and 0.5 mm CaCl2. The resulting thermal melting curves were analyzed in GraphPad Prism (version 6.0c) via nonlinear regression. Each sample was run in triplicate, and each experiment was repeated twice.
Cell Culture
Calreticulin-deficient K42 mouse embryonic fibroblasts (29) were grown and maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% (v/v) fetal bovine serum (Invitrogen) and antibiotic-antimycotic (1× from a 100× stock) (Invitrogen). The cells were cultured in 100-mm plates at 37 °C in 5% CO2. Global inhibition of glucosidase activity was undertaken via the addition of castanospermine to the cell medium (to a final concentration of 100 μg/ml) for 16 h.
Metabolic Labeling
Calreticulin-deficient mouse embryonic fibroblasts (29) were pulsed with 0.15 mCi of 35S trans label (MP Biomedicals) for 20 min and lysed for 1 h on ice in 20 mm HEPES (pH 7.5), 140 mm NaCl, 5 mm CaCl2, and 1% Nonidet P-40 with the addition of 50 μm N-butyl deoxynojirimycin (Sigma) as needed. The lysate was cleared via centrifugation at 14,000 × g for 30 min at 4 °C, and equal volumes of the supernatant were incubated with 250 μl of 20 μm hexahistidine-tagged calreticulin preimmobilized on a Ni-NTA matrix (25 μl) (Qiagen). The cell lysate supernatants were allowed to interact with calreticulin for 1 h at room temperature in the presence or absence of a cross-linker (2 mm dimethyl dithiobispropionimidate (Sigma)). Following elution of any unbound proteins, the beads were washed, and calreticulin-associated proteins were eluted with 350 mm imidazole followed by the addition of 2 mm DTT as needed to dissociate any cross-linked proteins. MHC class I heterodimers were isolated from the Ni-NTA eluate via immunoprecipitation for 16 h at 4 °C using a mouse anti-H2-Kb antibody (Y3 (30)) in 20 mm HEPES (pH 7.5), 140 mm NaCl, 5 mm CaCl2, and 1% Nonidet P-40, with the addition of 2 mm iodoacetamide to neutralize any residual DTT. Isolated MHC class I heterodimers were digested with endoglycosidase Hf (Endo H; New England Biolabs) or jack bean α-mannosidase (JBM; Sigma) for 2 h at 37 °C to determine its glycosylation state. The proteins were separated on 4–20% SDS-PAGE gels (Bio-Rad). Radiographic images were acquired via phosphorimaging using a Typhoon scanner (GE Healthcare). Densitometry and analysis of MHC class I migration patterns were undertaken using ImageJ (31). Differences in the mean distance between the Endo H- and JBM-digested MHC class I molecules associated with the tested calreticulin constructs relative to mCRT(WT) were assessed via one-way ANOVA followed by a Dunnett's post hoc test.
Statistical Analysis
All statistical analyses were undertaken in GraphPad Prism version 6.0c (GraphPad Software, La Jolla, CA). Kinetic constants from BLI analyses were obtained via nonlinear least squares regression in the FortéBio Analysis module (version 7.0) using a one-site specific partial fit model.
DSF thermostability data were analyzed using an asymmetric five-parameter dose-response curve (detailed in Ref. 24) to obtain the melting point (Tm) of each protein. Paired t tests were used to assess significance of the change in Tm associated with the presence of glycans.
The inhibition constants (IC50) for G1M3-dependent inhibition of calreticulin-substrate binding were assessed via nonlinear least squares regression analyses using a one-site IC50 model to fit the binding signal of the calreticulin-substrate interaction as a function of the log[G1M3]. Statistical significance was assessed at an α level of 5%.
RESULTS
Glycan-dependent and Independent Binding of Calreticulin to Protein Substrates Display Distinct Kinetic Profiles
To investigate the molecular basis for the in vitro chaperone activity of calreticulin toward nonglycosylated proteins, we compared interactions of calreticulin with glycosylated and nonglycosylated proteins using a number of biophysical assays. IgY, the avian homolog of mammalian IgG, was used as a substrate protein because it is one of the few mature secreted proteins that contains an appreciable level of monoglucosylated glycans (∼25%) (32). Gel filtration purified IgY was enriched on a calreticulin column (IgY-EN) and used in BLI assays, which revealed the rapid association and dissociation kinetics of the calreticulin-IgY-EN interaction (Fig. 1A and Table 1). Tyr-92 of calreticulin participates in binding to glycans (5, 17, 33), and the rapid kinetics of interaction between mCRT(WT) and IgY-EN are dependent upon this residue (Fig. 1A and Table 1), suggesting that this interaction profile is glycan-dependent. The interaction of calreticulin with IgY-EN is relatively independent of the P-domain (Fig. 1A and Table 1).
FIGURE 1.
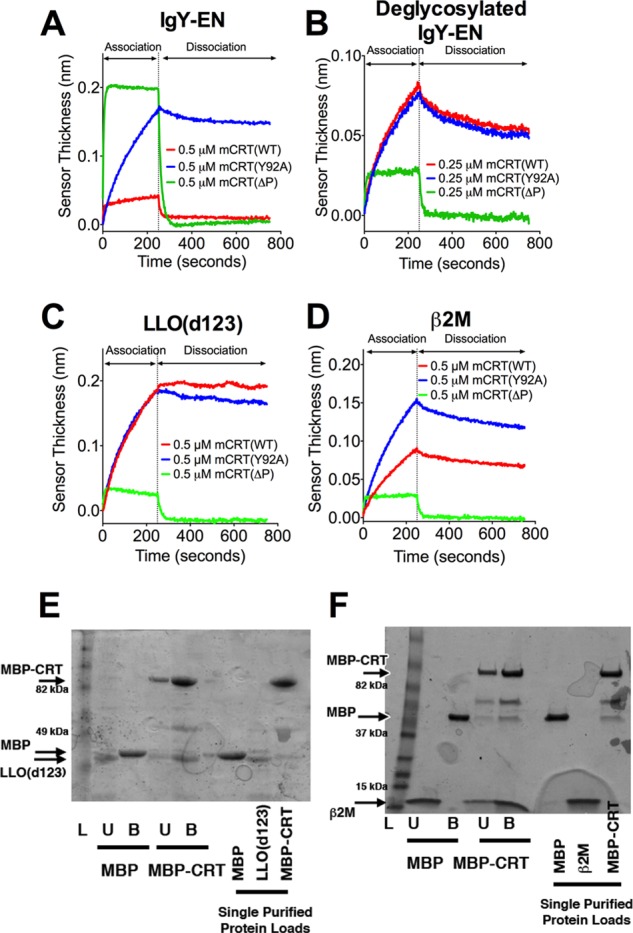
Distinct kinetic profiles characterize the interaction of calreticulin with glycosylated and nonglycosylated proteins. Representative BLI sensorgrams for the interaction between mCRT(WT), mCRT(ΔP), and mCRT(Y92A) with IgY enriched on a calreticulin affinity column (IgY-EN) (A), deglycosylated IgY-EN (B), LLO(d123) (C), or β2M (D). The associated kinetic rate constants and data replicates are detailed in Table 1. E and F, representative SDS-PAGE gels (of three independent experiments) depicting the binding of LLO(d123) (E) or β2M (F) to MBP or MBP-calreticulin (MBP-CRT) immobilized on an amylose resin. Lanes L, ladder; lanes U, amylose-resin unbound fraction; lanes B, amylose-resin bound fraction. Arrows depict migration positions of β2M, LLO(d123), MBP, and MBP-CRT. Interactions between MBP-CRT and LLO(d123) (E) were assessed using a 10% SDS-PAGE gel, whereas MBP-CRT binding to β2M (F) was assessed using a 4–20% gradient gel (Bio-Rad). MBP was found to migrate at a slightly reduced speed when analyzed on a gradient gel.
TABLE 1.
Calreticulin binds glycosylated and nonglycosylated substrates with similar affinities but distinct kinetic profiles, and these interactions can be inhibited by the calreticulin-specific glycan Glcα1–3Manα1–2Manα1–2Man (G1M3)
Biolayer interferometry-based measurements of the kinetics of interaction between calreticulin and macromolecular substrates and their inhibition by the synthetic glycan G1M3. The kinetics of interaction between calreticulin and IgY-EN and deglycosylated IgY-EN, as well as between mCRT(ΔP) and all substrates, were measured over the first 50 seconds of the association phase and first 100 seconds of the dissociation phase. The interaction kinetics for mCRT(WT) and mCRT(Y92A) binding to LLO(d123) and β2M were calculated over the entire 250 seconds of the association phase and 500 seconds of the dissociation phase. Interaction kinetics were measured over a calreticulin concentration range of 0.25–4 μm. Inhibition of calreticulin-substrate interactions by G1M3 was measured over G1M3 concentration ranges of 0–14 μm and analyzed via nonlinear regression by fitting individual response signals as a function of log[G1M3]. The n values indicate the number of experimental replicates used to derive the indicated binding or inhibition constants.
| Calreticulin construct | Substratea | Biolayer interferometry binding datab |
G1M3 inhibition data |
||||
|---|---|---|---|---|---|---|---|
| n | KD | kon | koff | n | IC50c | ||
| μm | m−1 s−1 | s−1 | μm | ||||
| mCRT(WT) | IgY-EN | 31 | 0.76 ± 0.08 | 196582.5 ± 17423.7 | 0.08 ± 0.004 | 4 | 0.82 ± 0.18 |
| mCRT(ΔP) | IgY-EN | 9 | 0.41 ± 0.05 | 224056.0 ± 17199.6 | 0.08 ± 0.002 | 4 | 1.79 ± 0.58 |
| mCRT(Y92A) | IgY-EN | 9 | 1.99 ± 0.62 | 9804.1 ± 2003.0 | 0.01 ± 0.001 | 4 | 1.16 ± 0.22 |
| mCRT(WT) | IgY-EN (deglycosylated) | 12 | 1.53 ± 0.49 | 17395.7 ± 4274.5 | 0.01 ± 0.002 | 3 | 1.11 ± 0.27 |
| mCRT(ΔP) | IgY-EN (deglycosylated) | 3 | 0.24 ± 0.05 | 401985.7 ± 88190.7 | 0.07 ± 0.006 | 3 | 1.69 ± 0.12 |
| mCRT(Y92A) | IgY-EN (deglycosylated) | 4 | 0.9 ± 0.22 | 15177.3 ± 2948.4 | 0.01 ± 0.001 | 3 | 2.3 ± 0.74 |
| mCRT(WT) | LLO(d123) | 4 | 0.54 ± 0.24 | 6746.3 ± 1452.8 | 0.002 ± 0.0007 | 2 | 0.9 ± 0.04 |
| mCRT(ΔP) | LLO(d123) | 4 | 0.26 ± 0.04 | 455236.4 ± 156276 | 0.06 ± 0.003 | ||
| mCRT(Y92A) | LLO(d123) | 2 | 1.14 ± 0.33 | 4301.4 ± 892.3 | 0.004 ± 0.0003 | ||
| mCRT(WT) | β2M | 4 | 3.11 ± 1.33 | 4015.9 ± 756.6 | 0.005 ± 0.0004 | 3 | 1.06 ± 0.4 |
| mCRT(ΔP) | β2M | 3 | 3.27 ± 0.82 | 110324.0 ± 47865.6 | 0.08 ± 0.006 | ||
| mCRT(Y92A) | β2M | 3 | 2.1 ± 0.57 | 4768.3 ± 1495.1 | 0.005 ± 0.0002 | ||
a The substrates used are: immunoglobulin-Y enriched on a calreticulin column (IgY-EN), enzymatically deglycosylated IgY-EN (IgY-EN (deglycosylated)), β2-microglobulin (β2M), and a listeriolysin-O construct containing its first three domains (LLO(d123)).
b Data represent the mean kinetic or affinity constants ± S.E.
c Data represent the mean IC50 values ± S.E.
Calreticulin also bound to enzymatically (PNGase F)-deglycosylated IgY-EN (Fig. 1B and Table 1). The binding of mCRT(WT) to deglycosylated IgY-EN displayed markedly slower (≥8-fold) interaction kinetics compared with those observed with IgY-EN (Fig. 1, A and B, and Table 1). Additionally, the interaction between calreticulin and deglycosylated IgY-EN is dependent upon the armlike P-domain of calreticulin, as indicated by the significant increase in the kinetic rate constants for the binding of deglycosylated IgY-EN to a calreticulin construct lacking the P-domain (mCRT(ΔP)), relative to mCRT(WT). Furthermore, the interaction between calreticulin and deglycosylated IgY-EN is independent of Tyr-92 (Fig. 1B and Table 1). Interestingly, despite an order of magnitude difference in the kinetic rate constants of association (kon) and dissociation (koff) for the interaction between mCRT(WT) and IgY-EN and deglycosylated IgY-EN, the calculated equilibrium affinities were similar (Table 1). These results are consistent with previous findings from steady-state assays that indicated a similar pattern of interaction of calreticulin with IgY and IgG, despite a lack of monoglucosylated glycans on IgG (34).
Previously, LLO (a nonglycosylated Listeria monocytogenes-derived protein that is thermally unstable at temperatures above 33 °C (35)) and a LLO truncation mutant (LLO(d123)) have been used as model nonglycosylated substrates to measure the in vitro aggregation-suppression activity of calreticulin at physiological temperatures (37 °C) (17, 18). The interaction of LLO(d123) with mCRT(WT) displayed a kinetic profile similar to that observed with deglycosylated IgY-EN and a similar P-domain dependence (Fig. 1C and Table 1). Similar results were obtained for the interaction between calreticulin and β2M, the nonglycosylated light chain of the MHC class I heterodimer (Fig. 1D and Table 1). Taken together, these results show that calreticulin contains a generic polypeptide-binding site that interacts with a variety of nonglycosylated proteins and that these interactions are dependent on the armlike P-domain of calreticulin. Additionally, an amylose resin-immobilized MBP-calreticulin fusion, but not MBP alone, is able to bind both LLO(d123) and β2M (Fig. 1, E and F), findings that further support the presence of a generic protein-binding site on calreticulin.
Identification of a Polypeptide-binding Site in Proximity to the Glycan-binding Site of Calreticulin
Previously, the crystal structure of the globular domain of human calreticulin (Protein Data Bank code 3pos) had suggested the presence of a polypeptide-binding site located on the edge of the lectin-binding site of calreticulin (6). To further examine this possibility, we asked whether occupancy of the glycan-binding site of calreticulin with the calreticulin-specific glycan Glcα1–3Manα1–2Manα1–2Man G1M3 could limit interactions between calreticulin and protein substrates.
G1M3 reduced the binding of mCRT(WT) to IgY-EN as well as deglycosylated IgY-EN, β2M and LLO(d123) in dose-dependent manners (Fig. 2, A–D). The derived IC50 values were similar to the published affinity constant for the interaction of murine calreticulin with G1M3 (0.77 μm) (5) (Table 1), suggesting that residues in the vicinity of the glycan-binding site are involved in binding glycosylated and nonglycosylated substrates.
FIGURE 2.
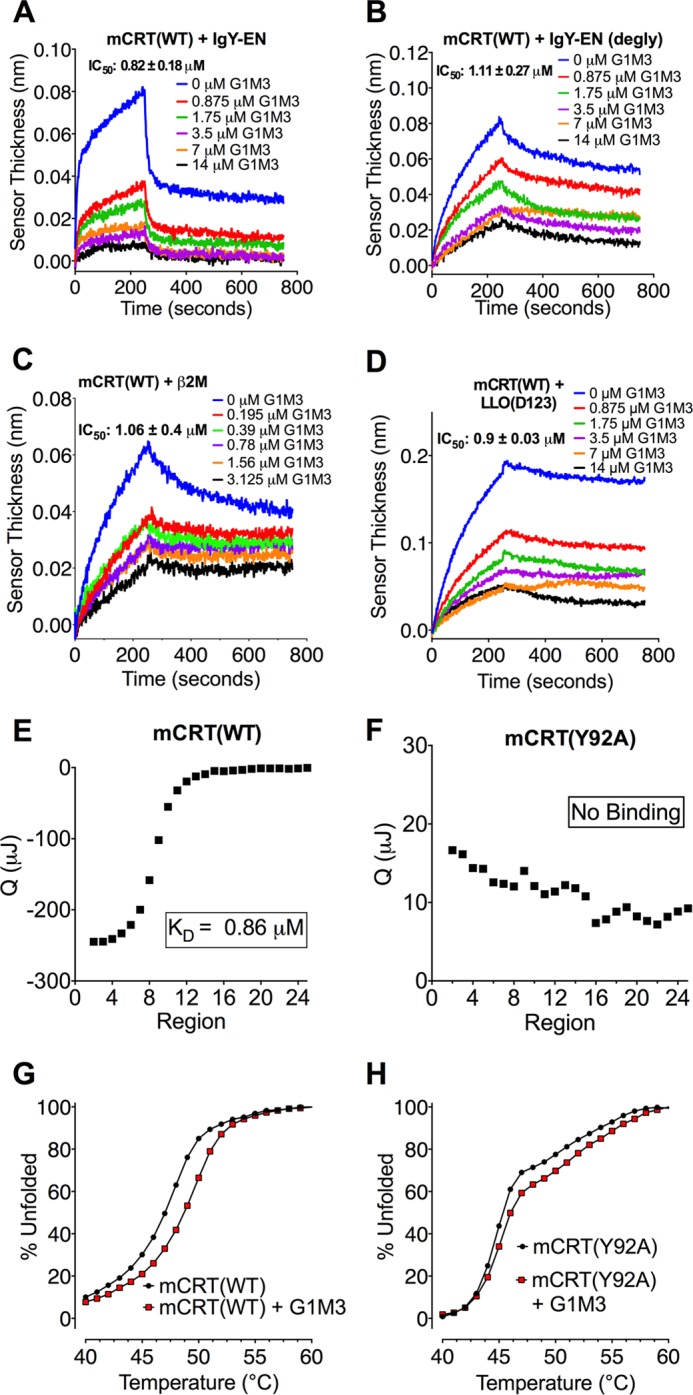
The synthetic glycan G1M3 inhibits glycan-dependent and independent calreticulin-substrate interactions. A–D, representative BLI sensorgrams depicting inhibition of the interactions between 0.5 μm mCRT(WT) and IgY-EN (A), deglycosylated IgY-EN (B), β2M (C), and LLO(d123) (D) following preincubation of calreticulin with varying concentrations of the glycan G1M3. The calculated IC50 values and data replicates are detailed in Table 1. E and F, representative ITC profiles for mCRT(WT) (E) and mCRT(Y92A) (F) binding G1M3. The figures show representative titration curve fits of two independent experiments. G and H, representative DSF curves (of two independent experiments) measuring the change in the thermostability of mCRT(WT) (G) and mCRT(Y92A) (H) in the presence or absence of G1M3.
Somewhat unexpectedly, G1M3 inhibited interactions between mCRT(Y92A) and IgY-EN, as well as deglycosylated IgY-EN with IC50 values similar to those observed for mCRT(WT) (Table 1). Previous findings based on thermostability analyses had indicated that mCRT(Y92A) is impaired in its ability to interact with G1M3 compared with mCRT(WT) (17). ITC also indicated impaired enthalpy-driven binding of G1M3 to mCRT(Y92A) relative to mCRT(WT) (Fig. 2, E and F). However, differential scanning fluorimetry revealed a G1M3-induced increase in the thermostability of mCRT(Y92A) (ΔTm = 0.63 ± 0.42 °C), albeit smaller than that observed for mCRT(WT) (ΔTm = 1.94 ± 0.1 °C) (Fig. 2, G and H). Together, these findings show that mCRT(Y92A) retains some capability to interact with glycans, although changes in enthalpy associated with this interaction may be masked by the heat of dilution of G1M3 into the protein solution. As such, the residual glycan binding ability of mCRT(Y92A) may explain the ability of G1M3 to inhibit its interactions with protein substrates. Alternatively, noncompetitive modes of inhibition could explain the inhibitory influences of G1M3.
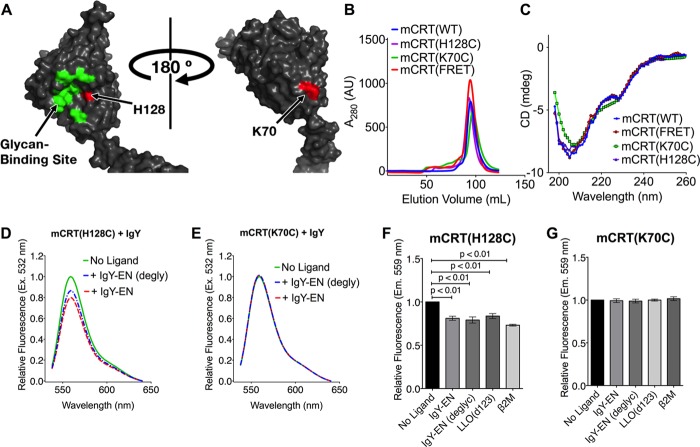
To further examine the presence of a polypeptide-binding site in the vicinity of the glycan-binding site of calreticulin, fluorophores were attached to the concave surface of the globular domain of calreticulin in the vicinity of its glycan-binding site (at position 128; Fig. 3A), as well as to the opposing, predominantly hydrophobic face of the calreticulin globular domain (at position 70; Fig. 3A). Because Cys-146 of calreticulin is a free thiol (5), cysteine mutants were engineered at positions His-128 and Lys-70 on the mCRT(C146G) background. The resulting mCRT(K70C) and mCRT(H128C) constructs migrated as monomers via gel filtration chromatography (Fig. 3B), and their monomeric nature was confirmed via native-PAGE (data not shown). The mCRT(K70C) and mCRT(H128C) constructs also possessed a similar secondary structure content to mCRT(WT) as assessed via far-UV CD spectroscopy (undertaken as detailed earlier (24)) (Fig. 3C) and interacted with IgY-EN with kinetic profiles similar to mCRT(WT) (kon = 195396.7 ± 123447.7 m−1 s−1 and koff = 0.05 ± 0.03 s−1 for mCRT(K70C) and kon = 396766.7 ± 146308.0 m−1 s−1 and koff = 0.09 ± 0.02 s−1 for mCRT(H128C)). mCRT(H128C) and mCRT(K70C) were labeled with a thiol-reactive fluorophore and fluorescence spectra were obtained in the presence and absence of substrates (glycosylated or deglycosylated IgY-EN, β2M, and LLO(d123)).
FIGURE 3.
Glycan-dependent and independent substrate interactions involve the glycan-binding face of calreticulin. A, structure of calreticulin (Protein Data Bank code 3rg0 (19)) highlighting His-128 and Lys-70, which are located in the vicinity of the glycan-binding site and on the opposing hydrophobic face of the globular domain, respectively. B and C, representative gel filtration profiles (B) of the calreticulin constructs used in this investigation (mCRT(K70C), mCRT(H128C), and mCRT(C146G/E110C/E245C) (mCRT(FRET))) together with representative far-UV CD spectra (C) (of two independent experiments) demonstrating the calreticulin constructs possess a similar secondary structure content. CD spectra were collected with calreticulin at a concentration of 0.1 mg/ml in 50 mm NaHPO4 (pH 7.5), 500 mm NaF, and 0.5 mm CaCO3. In the gel filtration analyses, protein elution volumes were variable between different protein preparations (± 5% of column volume) based on the protein yields, column equilibration time, and system pressure. The monomeric nature of the proteins was further confirmed via native-PAGE (data not shown). D and E, quenching of 0.2 μm Atto 532-labeled mCRT(H128C) (D) or mCRT(K70C) (E) by 4 μm IgY-EN or deglycosylated IgY-EN. Normalized fluorescence quenching data show representative spectra from four independent experiments. F and G, quantification of the quenching of 0.2 μm Atto 532-labeled mCRT(H128C) (F) or mCRT(K70C) (G) by the indicated substrates. The data represent the means ± S.E. of three or four independent experiments with statistical significance assessed via a one-way ANOVA followed by a Dunnett's post hoc test.
In the presence of all tested substrates, the fluorophore immobilized in the vicinity of the glycan-binding site (position 128) was quenched, whereas no change in the fluorescence signal was observed for the fluorophore immobilized to the opposing hydrophobic face of calreticulin (position 70) (Fig. 3, D–G). Together with the G1M3 inhibition studies (Table 1 and Fig. 2), these findings show that both glycosylated and nonglycosylated substrates interact with residues within or proximal to the glycan-binding site of calreticulin and support the presence of a generic polypeptide-binding site on the glycan-binding face of calreticulin.
Glycan-dependent and Independent Interactions Involve Distinct P-domain Conformations
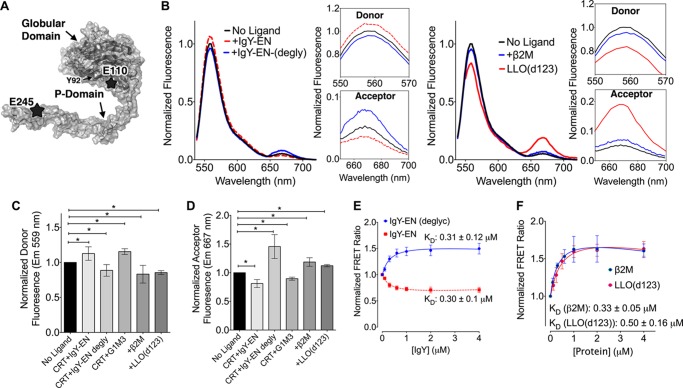
Thus far, the kinetic BLI data suggest a role for the P-domain in mediating interactions between calreticulin and nonglycosylated substrates (Fig. 1 and Table 1). To further investigate conformational changes within the P-domain of calreticulin and their role in facilitating calreticulin-protein interactions, we undertook ensemble FRET spectroscopy with fluorescent probes attached to the globular and P-domains of calreticulin. Double cysteine mutants were engineered onto mCRT(C146G) at positions Glu-110 and Glu-245, corresponding to the vicinity of the glycan-binding site and the tip of the P-domain, respectively (Fig. 4A). The resulting protein was simultaneously labeled with the thiol-reactive ATTO-532 and ATTO-647N fluorescent probes as described previously for BiP (27). The mCRT(E110C/E245C/C146G) construct migrated as a monomer via gel filtration chromatography (Fig. 3B; indicated as FRET mutant), and its monomeric nature was confirmed via native-PAGE (data not shown). The mCRT(E110C/E245C/C146G) construct possessed a similar secondary structure content to mCRT(WT) (assessed via far-UV CD spectroscopy) (Fig. 3C; indicated as FRET mutant) and interacted with IgY-EN with a kinetic profile similar to that observed for mCRT(WT) (kon = 341827.5 ± 88771.0 m−1 s−1; koff = 0.06 ± 0.01 s−1 for mCRT(E110C/E245C/C146G)).
FIGURE 4.
Calreticulin adopts distinct P-domain conformations in the presence of glycosylated and nonglycosylated substrates. A, model for calreticulin based on the crystal structure of the globular domain of calreticulin (Protein Data Bank code 3o0v (5)) and the P-domain of calnexin (Protein Data Bank code 1jhn (4)). The overall shapes of the globular and P-domains of the protein are depicted. FRET labeling positions on the globular and P-domains of calreticulin (110 and 245) are shown as stars, and the glycan-binding residue Tyr-92 is indicated. B, representative FRET spectra of Atto 532- and Atto 647N-labeled mCRT(C146G/E110C/E245C) (0.2 μm) following excitation at 532 nm in the presence and absence of the indicated substrates (4 μm IgY-EN or deglycosylated IgY-EN (left panel) and β2M or LLO(D123) (right panel)). Insets show changes in the emission maxima of the donor and acceptor fluorophore peaks. Fluorescence spectra for the interaction of calreticulin with IgY-EN, deglycosylated IgY-EN, and β2M were collected on a Horiba Scientific spectrophotometer, whereas spectra for the interaction of calreticulin with LLO(d123) were collected on a SpectraMax M5 plate reader. C and D, changes in the normalized donor (C) and acceptor (D) fluorescence peaks following interaction of indicated substrates with calreticulin (data represent the means ± S.E.). Significance was assessed via unpaired t tests. Statistically significant mean differences (p < 0.05) as assessed via unpaired t tests are denoted by asterisks (all p values are < 0.01). The data represent the averages of 14 (ligand-free condition), 7 (+deglycosylated IgY-EN condition), 11 (+IgY-EN conditions), and 3 (+G1M3, +β2M and +LLO(d123)) independent experiments. E and F, dose-dependent changes in the FRET ratios following interaction of 0.2 μm calreticulin with varying concentrations of the indicated proteins. The data represent the means ± S.E. of 4 (+IgY-EN), 5 (+deglycosylated IgY-EN), or 3 (+β2M and +LLO(d123)) independent experiments.
Significant decreases in the fluorescence of the donor fluorophore and corresponding increases in the acceptor fluorescence signals were observed for calreticulin in the presence of nonglycosylated substrates (deglycosylated IgY-EN, β2M and LLO(d123)) compared with the ligand-free state (p < 0.05) (Fig. 4, B–D). These findings indicate a reduction in the average distance between the fluorophore pair and the adoption of a “closed” conformation of the P-domain. On the other hand, a significant increase in the fluorescence of the donor fluorophore and a corresponding decrease in the acceptor fluorescence signal was observed in the presence of the IgY-EN relative to the ligand-free condition (Fig. 4, B–D), suggesting an increase in the average distance between the fluorophores and the adoption of an open P-domain conformation. Remarkably, the presence of G1M3 alone resulted in a significant (p < 0.05) increase in the average distance between the globular and P-domains of calreticulin (Fig. 4, C and D), suggesting that glycan binding alone (in the absence of any tethering polypeptide) is sufficient to stabilize the open conformation of the P-domain.
Furthermore, dose-dependent decreases or increases in the FRET ratios (667/559-nm intensity ratios) were observed in the presence of varying concentrations of IgY-EN and deglycosylated IgY-EN, respectively (Fig. 4E). The calculated steady-state affinities for IgY-EN (KD = 0.30 ± 0.10 μm) and deglycosylated IgY-EN (KD = 0.31 ± 0.12 μm) differ 3–5-fold from the values obtained via BLI (Table 1), which could reflect the different techniques used (steady-state ensemble FRET versus kinetic BLI). Similarly, dose-dependent increases in the FRET signals were observed in the presence of β2M and LLO(d123), with calculated steady-state affinities of 0.33 ± 0.05 μm (β2M) and 0.50 ± 0.16 μm (LLO(d123)) (Fig. 4F). The derived steady-state affinity for LLO(d123) is similar to that obtained via BLI (Table 1). However, the calculated affinity for the β2M-calreticulin interaction differs 9-fold from that obtained via BLI (Table 1), the reason for which is unclear but could reflect measurements of different rate-limiting steps. Nonetheless, both assays demonstrate the ability of calreticulin to bind different nonglycosylated protein as well as IgY-EN. Additionally, the FRET results show that glycan-dependent interactions induce an open conformation of the P-domain of calreticulin, whereas glycan-independent interactions induce a closed P-domain conformation. Together with the findings from Figs. 1–3 and Table 1, the data indicate that although the glycan and polypeptide-binding sites localize to the same face of the globular domain of calreticulin, closure of the arm-like P-domain is likely to be important for stabilizing glycan-independent interactions and could contribute to the chaperone (as opposed to the lectin) activity of calreticulin.
Influences of ERp57 on Calreticulin-IgY-EN Interactions and P-domain Conformation
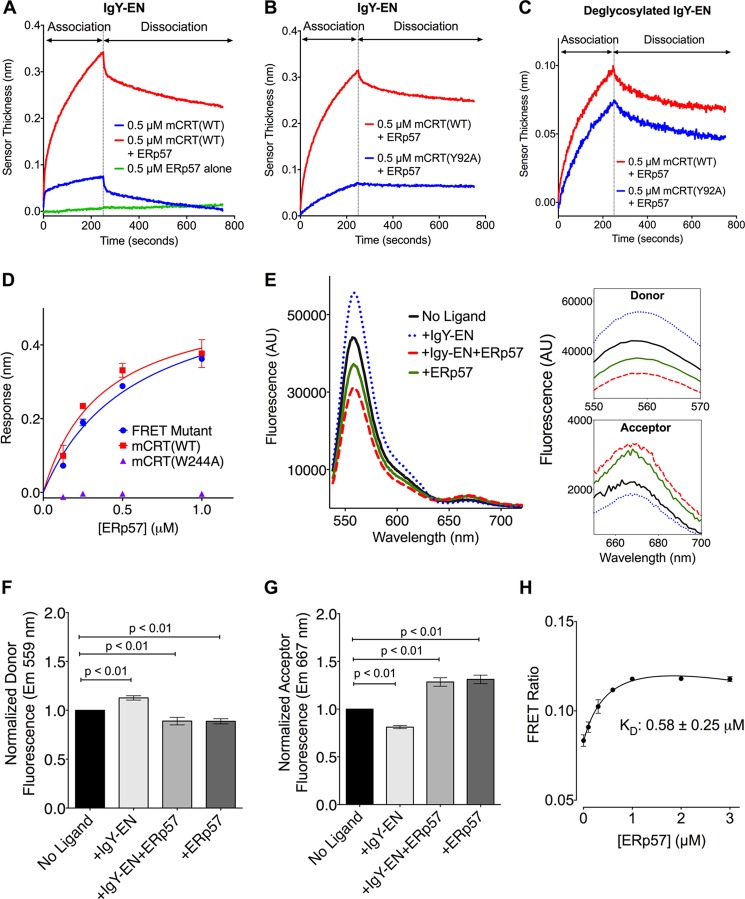
In the ER, the rapid kinetics of interaction associated with glycan-dependent binding (Fig. 1 and Table 1) is expected to preferentially recruit monoglucosylated substrates to the chaperone cycle of calreticulin. However, the kinetic data also suggest that glycan-dependent interactions alone may be insufficient to mediate stable substrate binding and retention. Using BLI and FRET, we asked whether binding of the co-chaperone ERp57 to the P-domain of calreticulin influenced the calreticulin-IgY-EN interaction. The presence of ERp57 significantly altered the kinetics of binding between calreticulin and IgY-EN (Fig. 5A). Additionally, in the presence of ERp57, more significant differences between mCRT(WT) and mCRT(Y92A) binding to IgY-EN were observed, compared with the differences between mCRT(WT) and mCRT(Y92A) binding to deglycosylated IgY-EN (Fig. 5, B and C) (note that kinetic constants are not derived for binding in the presence of ERp57 because the observed rate constants reflect the sum of multiple equilibria). Together, these findings are consistent with the possibility that the interaction between calreticulin and IgY-EN remains glycan-dependent in the presence of ERp57.
FIGURE 5.
ERp57 alters the P-domain conformation of calreticulin and the kinetics of binding of a glycosylated protein. A–C, representative BLI sensorgrams depicting the interaction of mCRT(WT) and mCRT(Y92A) with IgY-EN or deglycosylated IgY-EN in the presence or absence of ERp57. D, representative steady-state analysis of mCRT(WT) and the FRET construct (immobilized on the biosensor) interacting with ERp57. The FRET construct can interact with ERp57 with an affinity similar to that observed for mCRT(WT) (KD = 0.58 ± 0.13 and 0.37 ± 0.09 μm, respectively, for the FRET construct and mCRT(WT) binding to ERp57). This is in contrast to mCRT(W244A), which is deficient in binding ERp57. Data represent the averages of two or three independent experiments. E, representative FRET spectra measured as described in Fig. 4, but in the presence of IgY-EN, ERp57, or ERp57+IgY-EN (4 μm each and 0.2 μm Atto 532- and Atto 647N-labeled mCRT(C146G/E110C/E245C)). Insets show changes in the emission maxima of the donor and acceptor fluorophore peaks. F and G, changes in the normalized donor (F) and acceptor (G) fluorescence peaks for the conditions described in E. FRET data represent the means ± S.E. of 3 (+ERp57 condition), 5 (+IgY-EN+ERp57 condition), or 11 (ligand-free and +IgY-EN conditions) independent experiments. Statistical significance was assessed via unpaired t tests. H, dose-dependent changes in the acceptor/donor FRET ratio following the interaction of 0.2 μm calreticulin with varying concentrations of ERp57. The data represent the means ± S.E. of 2 independent experiments.
We also undertook ensemble FRET spectroscopy to further investigate P-domain conformational changes associated with ERp57 binding. Although Glu-245 is contained within the region of the P-domain of calreticulin implicated in binding ERp57 (8), mCRT(E110C/E245C/C146G) is able to bind ERp57 with a steady-state affinity similar to that of mCRT(WT) as assessed via BLI (Fig. 5D; KD = 0.58 ± 0.13 and 0.37 ± 0.09 μm, respectively, for mCRT(E110C/E245C/C146G) and mCRT(WT) binding to ERp57). This is in contrast to mCRT(W244A), which is impaired in mediating calreticulin-ERp57 interactions (Ref. 17 and Fig. 5D). Thus, mCRT(E110C/E245C/C146G) was useable for FRET measurements of calreticulin P-domain conformations in the presence or absence of ERp57.
Using FRET, we observed that ERp57 binding decreases the average distance between the globular and P-domains of calreticulin (Fig. 5, E–G). Furthermore, the change in the FRET signal is dose-dependent (Fig. 5H), with a derived affinity (KD = 0.58 ± 0.25 μm) similar to that observed for the calreticulin-ERp57 interaction via BLI (Fig. 5D). It is noteworthy that the ERp57-induced enhancement of the FRET signal is maintained in the presence of IgY-EN (Fig. 5, E–G). Taken together with the BLI data (Fig. 5, A–C), these findings support the possibility that the ERp57-bound form of calreticulin engages in a hybrid substrate-binding mode, combining elements of glycan-dependent binding with a closed P-domain conformation.
Modes of Calreticulin Binding to Cellular Substrates
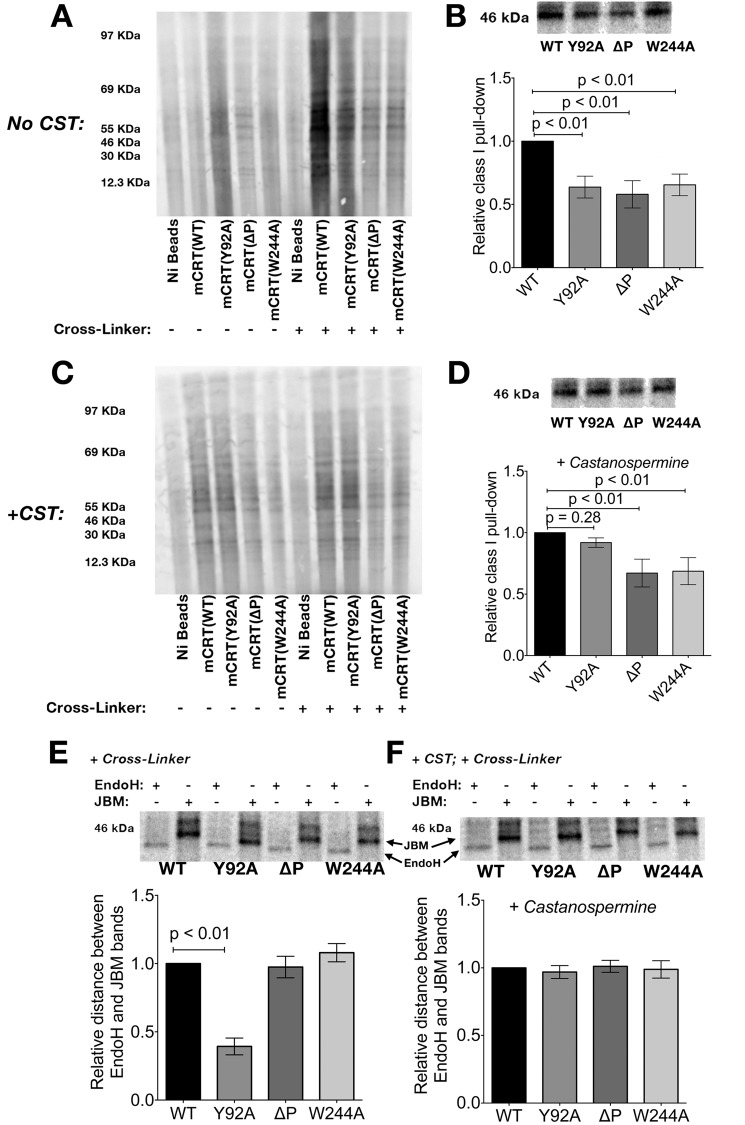
To study the mechanism of cellular protein binding to calreticulin, calreticulin-deficient mouse embryonic fibroblasts (29) were labeled with [35S]methionine and lysed in the presence of N-butyl deoxynojirimycin (an inhibitor of glucosidase II) to trap proteins in their monoglucosylated states. The cell lysates were incubated with histidine-tagged mCRT(WT), mCRT(Y92A), mCRT(ΔP), or mCRT(W244A) immobilized on nickel beads. In a subset of analyses (Fig. 6A, right lanes), a reversible cross-linking agent (dimethyl dithiobispropionimidate) was added to the lysates prior to washing and eluting proteins from the beads. The extent of total calreticulin-substrate associations was analyzed via SDS-PAGE and phosphorimaging (Fig. 6A). In the presence of a cross-linker, mCRT(WT) preferentially bound proteins compared with mCRT(Y92A), indicating that the binding of cellular proteins to calreticulin is glycan-dependent (Fig. 6A, right lanes). Additionally, calreticulin-protein interactions were significantly impacted by the mCRT(ΔP) truncation, as well as the mCRT(W244A) mutation, which impairs calreticulin-ERp57 binding ((17) and Fig. 5D) (Fig. 6A, right lanes). These results suggest a role for the P-domain and ERp57 associations in stabilizing calreticulin-substrate binding. The preferential binding of cellular proteins to mCRT(WT) relative to other calreticulin constructs was dependent on the use of a cross-linker prior to the wash step (Fig. 6A, left lanes compared with right lanes), confirming the transient nature of glycan-dependent calreticulin-substrate interactions.
FIGURE 6.
Glycan-dependent interactions predominate in the interaction of calreticulin with cellular substrates. A, association of the indicated Ni-resin-immobilized calreticulin constructs with [35S]methionine-pulsed cellular proteins in the presence or absence of a chemical cross-linker. B, immunoisolation of MHC class I heterodimers from eluates in A in the presence of a cross-linker and normalized densitometric quantification (mean ± S.E.) of the isolated MHC class I levels (from 5–6 independent experiments). Statistical significance was assessed via a one-way ANOVA followed by a Dunnett's post hoc test. C and D, similar to A and B, except showing the association of calreticulin with labeled proteins from cells pretreated with castanospermine (CST) (n = 4–5 independent experiments). E and F, representative Endo H and JBM digestion profiles of calreticulin-associated MHC class I molecules derived from cells not treated (E) or treated (F) with castanospermine and associated quantification of the relative distances between the Endo H- and JBM-digested MHC class I bands from 4–5 independent experiments (data represent the mean ± S.E.). Distances were normalized relative to the distance between the Endo H- and JBM-digested bands for mCRT(WT)-associated MHC class I. Two independent investigators scored the distances between the Endo H- and JBM-digested bands. Statistical significance was assessed via a one-way ANOVA followed by a Dunnett's post hoc test. The location of the Endo H- and JBM-digested bands are indicated (as EndoH and JBM, respectively).
MHC class I heterodimers present in the eluates were further quantified via immunoprecipitation using a H2-Kb antibody (Y3 (30)) following DTT treatment of the eluates (to release the cross-linked calreticulin-protein complexes) and neutralization of DTT. Consistent with the findings in Fig. 6A, preferential binding of mCRT(WT) to MHC class I was observed relative to other calreticulin constructs (Fig. 6B).
Castanospermine is an inhibitor of glucose trimming (36) that is required for the generation of monoglucosylated glycans from their triglucosylated precursors. Pretreatment of cells with castanospermine prior to the methionine pulse abrogated preferential binding of cellular proteins to mCRT(WT) relative to mCRT(Y92A) (Fig. 6, C, right lanes, and D). However, several protein interactions are detectable with both mCRT(WT) and mCRT(Y92A), indicating that calreticulin is capable of glycan-independent binding when monoglucosylation is blocked. Additionally, these glycan-independent interactions are partly dependent on both the P-domain and Trp-244, because protein binding efficiency is reduced with mCRT(ΔP) and mCRT(W244A) (Fig. 6, C, right lanes, and D).
JBM catalyzes the cleavage of eight mannose residues from glycans lacking glucose (GlcNAc2Man8) but only five mannose residues from glycans containing glucose residues (GlcNAc2Man8Glc1–3) (37), whereas Endo H catalyzes a more complete cleavage of high mannose glycans, regardless of their glucose content. MHC class I molecules associated with mCRT(WT), mCRT(ΔP), and mCRT(W244A) displayed a more rapid migration following Endo H digestion compared with that following JBM digestion (Fig. 6E). Additionally, following JBM digestion, there were no significant differences in the migration patterns of MHC class I molecules associated with mCRT(WT), mCRT(ΔP), and mCRT(W244A), whereas mCRT(Y92A)-associated MHC class I migrated more rapidly (Fig. 6E). The migration difference between mCRT(WT) and mCRT(Y92A) was abrogated in castanospermine-treated cells (Fig. 6F). Together, these findings are consistent with the possibility that mCRT(Y92A)-associated MHC class I molecules are differentially glucosylated relative to their mCRT(WT)-associated counterparts.
Furthermore, in the absence of castanospermine treatment, the differences in the migration patterns between the JBM- and Endo H-digested forms of mCRT(ΔP)- and mCRT(W244A)-associated MHC class I molecules were similar to that seen with mCRT(WT) (Fig. 6E). This finding is consistent with the possibility that mCRT(ΔP) and mCRT(W244A) mutants are able to interact with MHC class I in a manner that is at least partially glycan-dependent. Nonetheless, as noted above, the efficiency of the overall isolation of MHC class I is reduced with mCRT(ΔP) and mCRT(W244A) compared with mCRT(WT), indicative of contributions from both the P-domain and ERp57 in stabilizing the association between calreticulin and MHC class I.
DISCUSSION
In this investigation, we show that glycan-independent substrate binding is characterized by slow interaction kinetics (Fig. 1 and Table 1) and involves residues in the vicinity of the glycan-binding site (Figs. 2 and 3). In the glycan-independent binding mode, the P-domain restricts substrate association and dissociation by adopting a closed conformation (Figs. 1 and 4). These findings support a model where the cleft between the glycan-binding site and the P-domain participates in glycan-independent interactions, with the globular domain containing the binding site and the P-domain restricting dissociation (Fig. 7A). Such a binding mode could shield exposed hydrophobic residues and allow for maintenance of the soluble forms of proteins. Thus, the glycan-independent binding mode may define the previously observed chaperone function of calreticulin toward nonglycosylated proteins, such as LLO(d123) (14, 16–18).
FIGURE 7.
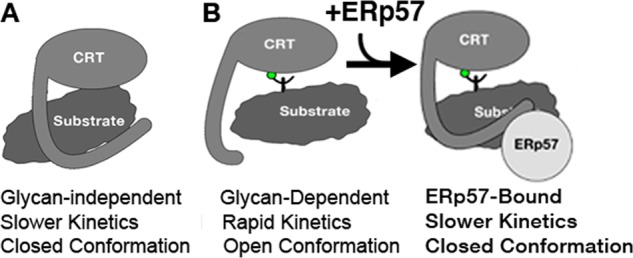
Model for the proposed glycan-independent (A) and glycan-dependent (B) modes of calreticulin-substrate interactions. Glycan-dependent binding is predicted to induce an open P-domain conformation (B, left panel), with subsequent ERp57 binding inducing a closed conformation (B, right panel).
The identification of a polypeptide-binding site in the vicinity of the glycan-binding site of calreticulin (Figs. 2 and 3) is consistent with observations from the crystal structure of the globular domain of human calreticulin (Protein Data Bank code 3pos). A N-terminal affinity tag was found to interact with the edge of the glycan-binding site, suggesting the presence of a polypeptide-binding site in this region (6). Additionally, previous findings indicate that the protein aggregation suppression activity of calreticulin resides within the globular domain of calreticulin, with the P-domain contributing to, but being nonessential for, protein aggregation suppression (19). Although these results are consistent with the P-domain dependence of the glycan-independent binding mode identified in our study (Figs. 1 and 4 and Table 1), the same study suggested that G1M3 does not inhibit the interaction of hydrophobic peptides with calreticulin (19). It is possible that differences in size between a protein and a small peptide could account for the observed discrepancy. A peptide, but not a protein, may be able to interact with other residues on the concave glycan-binding surface of calreticulin that is largely hydrophobic (5).
We also show that glycan-dependent calreticulin-protein interactions are characterized by rapid interaction kinetics (Fig. 1 and Table 1), along with a movement of the P-domain away from the globular domain (Figs. 4 and 7B, left panel). The BLI data (Table 1) support a model in which the rapid kinetics of association between calreticulin and monoglucosylated proteins plays a key role in substrate recruitment to the calreticulin cycle in the ER. Indeed, the glycan-dependent mode of binding predominates in the interaction of calreticulin with IgY-EN (Figs. 1 and 4 and Table 1) and in the interaction between calreticulin and cellular proteins (Fig. 6). However, the findings that both mCRT(ΔP) and a calreticulin mutant that is deficient in binding ERp57 (mCRT(W244A)) are impaired in substrate recruitment (Fig. 6) suggest that glycan-dependent interactions alone are insufficient to mediate stable binding between calreticulin and protein substrates. The association of calreticulin with co-chaperones such as ERp57 appears to be a critical step in stabilizing calreticulin-substrate interactions (Figs. 5 and 6). Currently, ERp57 recruitment is thought to be relevant mainly for substrate oxidation (reviewed in Ref. 38). However, our findings indicate that calreticulin-ERp57 interactions influence substrate binding to calreticulin and alters the P-domain conformation to a closed state (Figs. 5 and 6). These findings are consistent with the possibility that ERp57 induces a hybrid substrate-binding mode, combining elements of glycan-dependent and glycan-independent interactions (Fig. 7B, right panel).
To address whether calreticulin-associated glycans in the ERp57-binding permissive context are exposed or engaged within the glycan-binding site of calreticulin, we analyzed the nature of glycans in a calreticulin-bound glycoprotein (MHC class I) isolated with mCRT(WT) or mCRT(Y92A). Following JBM digest, the migration pattern of mCRT(WT)-associated MHC class I was distinct from that of mCRT(Y92A)-associated MHC class I (Fig. 6E), consistent with the possibility of enhanced glucosylation of mCRT(WT)-linked MHC class I. Taken together with the findings that the P-domain and Trp244 contribute to more stable substrate interactions (Fig. 6, A and B), our data support a model where the glycan is engaged with the glycan-binding site of calreticulin, whereas the P-domain contributes to protein-protein binding in an interaction that is directly or indirectly stabilized by the co-chaperone (Fig. 7B, right panel). Thus, for the interaction of calreticulin with cellular substrates, elements of the glycan-dependent and glycan-independent binding modes appear to be combined into a hybrid mode of interaction.
In summary, the results from this study provide a framework for integrating the known abilities of calreticulin to bind glycosylated and nonglycosylated proteins into a model for its cellular chaperone functions. We show that calreticulin can interact with both glycosylated and nonglycosylated proteins with similar affinities but distinct binding kinetics. The rapid association kinetics (Table 1) for the interaction of calreticulin with monoglucosylated glycoproteins are expected to drive the specific recruitment of nascent cellular proteins or those that are tagged as misfolded by UDP-glucose glycoprotein glucosyltransferase 1 to the chaperone cycle of calreticulin. The P-domain of calreticulin and its interaction with co-chaperones contribute to the stabilization of substrate proteins via direct or indirect protein-protein interactions. The in vitro and cellular chaperone activities of calreticulin are likely to be mediated by substrate sequestration within the cleft between the glycan-binding site and P-domain. Indeed, mutations within the glycan-binding site, ERp57 interaction site, as well as P-domain truncations negatively impact the ability of calreticulin to induce MHC class I assembly and cell surface expression (17). It is also noteworthy that two of the substrates used in this study (IgY and β2M) are members of the immunoglobulin superfamily. Therefore, it is possible that the glycan-independent interactions described here could also mediate interactions between calreticulin and proteins in cellular compartments outside the ER (reviewed in Ref. 3).
Acknowledgments
We thank Dr. Jeanne Stuckey for use of the Octet Red BLI instrument, Dr. Jason Getswicki and Dr. Mi Lim for use of the spectrofluorometer and Dr. Ari Gafni for use of the circular dichroism spectrophotometer. We thank Julia Bourg for early contributions to the FRET studies, William Clay Brown for generating the MBP-CRT construct, and James Delproposto and Leah Makley for their technical assistance with the BLI and DSF measurements respectively. We also thank Dr. Karunesh Arora, Dr. Charles L. Brooks III, Jessica Gagnon, Dr. Ruma Banerjee, Dr. Sivaraj Sivaramakrishnan, and Dr. Nils Walter for many helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health grants (AI066131 and AI044115 to M. R.). This work was also supported by an American Heart Association grant (12POST8810006 to S. J. W.) and utilized the DNA sequencing cores of the Michigan Diabetes Research and Training Center funded by a National Institutes of Health grant (DK020572).
- ER
- endoplasmic reticulum
- β2M
- β2-microglobulin
- BLI
- biolayer interferometry
- CRT
- calreticulin
- DSF
- differential scanning fluorimetry
- ITC
- isothermal titration calorimetry
- LLO
- listeriolysin O
- MBP
- maltose-binding protein
- Ni-NTA
- nickel-nitrilotriacetic acid
- Endo H
- endoglycosidase Hf
- JBM
- jack bean α-mannosidase
- ANOVA
- analysis of variance.
REFERENCES
- 1. Anfinsen C. B. (1973) Principles that govern the folding of protein chains. Science 181, 223–230 [DOI] [PubMed] [Google Scholar]
- 2. Braakman I., Bulleid N. J. (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 80, 71–99 [DOI] [PubMed] [Google Scholar]
- 3. Raghavan M., Wijeyesakere S. J., Peters L. R., Del Cid N. (2013) Calreticulin in the immune system. Ins and outs. Trends Immunol. 34, 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrag J. D., Bergeron J. J., Li Y., Borisova S., Hahn M., Thomas D. Y., Cygler M. (2001) The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell 8, 633–644 [DOI] [PubMed] [Google Scholar]
- 5. Kozlov G., Pocanschi C. L., Rosenauer A., Bastos-Aristizabal S., Gorelik A., Williams D. B., Gehring K. (2010) Structural basis of carbohydrate recognition by calreticulin. J. Biol. Chem. 285, 38612–38620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chouquet A., Païdassi H., Ling W. L., Frachet P., Houen G., Arlaud G. J., Gaboriaud C. (2011) X-ray structure of the human calreticulin globular domain reveals a peptide-binding area and suggests a multi-molecular mechanism. PLoS One 6, e17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellgaard L., Riek R., Herrmann T., Güntert P., Braun D., Helenius A., Wüthrich K. (2001) NMR structure of the calreticulin P-domain. Proc. Natl. Acad. Sci. U.S.A. 98, 3133–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frickel E. M., Riek R., Jelesarov I., Helenius A., Wuthrich K., Ellgaard L. (2002) TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc. Natl. Acad. Sci. U.S.A. 99, 1954–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helenius A., Aebi M. (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049 [DOI] [PubMed] [Google Scholar]
- 10. D'Alessio C., Caramelo J. J., Parodi A. J. (2010) UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin. Cell Dev. Biol. 21, 491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zapun A., Jakob C. A., Thomas D. Y., Bergeron J. J. (1999) Protein folding in a specialized compartment. The endoplasmic reticulum. Structure 7, R173–R182 [DOI] [PubMed] [Google Scholar]
- 12. Coe H., Michalak M. (2009) Calcium binding chaperones of the endoplasmic reticulum. Gen. Physiol. Biophys. 28, F96–F103 [PubMed] [Google Scholar]
- 13. Rutkevich L. A., Williams D. B. (2011) Participation of lectin chaperones and thiol oxidoreductases in protein folding within the endoplasmic reticulum. Curr. Opin. Cell Biol. 23, 157–166 [DOI] [PubMed] [Google Scholar]
- 14. Saito Y., Ihara Y., Leach M. R., Cohen-Doyle M. F., Williams D. B. (1999) Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 18, 6718–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ihara Y., Cohen-Doyle M. F., Saito Y., Williams D. B. (1999) Calnexin discriminates between protein conformational states and functions as a molecular chaperone in vitro. Mol. Cell 4, 331–341 [DOI] [PubMed] [Google Scholar]
- 16. Martin V., Groenendyk J., Steiner S. S., Guo L., Dabrowska M., Parker J. M., Müller-Esterl W., Opas M., Michalak M. (2006) Identification by mutational analysis of amino acid residues essential in the chaperone function of calreticulin. J. Biol. Chem. 281, 2338–2346 [DOI] [PubMed] [Google Scholar]
- 17. Del Cid N., Jeffery E., Rizvi S. M., Stamper E., Peters L. R., Brown W. C., Provoda C., Raghavan M. (2010) Modes of calreticulin recruitment to the major histocompatibility complex class I assembly pathway. J. Biol. Chem. 285, 4520–4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeffery E., Peters L. R., Raghavan M. (2011) The polypeptide binding conformation of calreticulin facilitates its cell-surface expression under conditions of endoplasmic reticulum stress. J. Biol. Chem. 286, 2402–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pocanschi C. L., Kozlov G., Brockmeier U., Brockmeier A., Williams D. B., Gehring K. (2011) Structural and functional relationships between the lectin and arm domains of calreticulin. J. Biol. Chem. 286, 27266–27277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brockmeier A., Brockmeier U., Williams D. B. (2009) Distinct contributions of the lectin and arm domains of calnexin to its molecular chaperone function. J. Biol. Chem. 284, 3433–3444 [DOI] [PubMed] [Google Scholar]
- 21. Yan Q., Murphy-Ullrich J. E., Song Y. (2010) Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly. Biochemistry 49, 3685–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donnelly M. I., Zhou M., Millard C. S., Clancy S., Stols L., Eschenfeldt W. H., Collart F. R., Joachimiak A. (2006) An expression vector tailored for large-scale, high-throughput purification of recombinant proteins. Protein Expr. Purif. 47, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DelProposto J., Majmudar C. Y., Smith J. L., Brown W. C. (2009) Mocr. A novel fusion tag for enhancing solubility that is compatible with structural biology applications. Protein Expr. Purif. 63, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wijeyesakere S. J., Gafni A. A., Raghavan M. (2011) Calreticulin is a thermostable protein with distinct structural responses to different divalent cation environments. J. Biol. Chem. 286, 8771–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garboczi D. N., Utz U., Ghosh P., Seth A., Kim J., VanTienhoven E. A., Biddison W. E., Wiley D. C. (1996) Assembly, specific binding, and crystallization of a human TCR-αβ with an antigenic Tax peptide from human T lymphotropic virus type 1 and the class I MHC molecule HLA-A2. J. Immunol. 157, 5403–5410 [PubMed] [Google Scholar]
- 26. Rizvi S. M., Mancino L., Thammavongsa V., Cantley R. L., Raghavan M. (2004) A polypeptide binding conformation of calreticulin is induced by heat shock, calcium depletion, or by deletion of the C-terminal acidic region. Mol. Cell 15, 913–923 [DOI] [PubMed] [Google Scholar]
- 27. Marcinowski M., Höller M., Feige M. J., Baerend D., Lamb D. C., Buchner J. (2011) Substrate discrimination of the chaperone BiP by autonomous and cochaperone-regulated conformational transitions. Nat. Struct. Mol. Biol. 18, 150–158 [DOI] [PubMed] [Google Scholar]
- 28. Matulis D., Kranz J. K., Salemme F. R., Todd M. J. (2005) Thermodynamic stability of carbonic anhydrase. Measurements of binding affinity and stoichiometry using ThermoFluor. Biochemistry 44, 5258–5266 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura K., Zuppini A., Arnaudeau S., Lynch J., Ahsan I., Krause R., Papp S., De Smedt H., Parys J. B., Muller-Esterl W., Lew D. P., Krause K. H., Demaurex N., Opas M., Michalak M. (2001) Functional specialization of calreticulin domains. J. Cell Biol. 154, 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. (1982) Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 79, 4737–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ. 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohta M., Hamako J., Yamamoto S., Hatta H., Kim M., Yamamoto T., Oka S., Mizuochi T., Matsuura F. (1991) Structures of asparagine-linked oligosaccharides from hen egg-yolk antibody (IgY). Occurrence of unusual glucosylated oligo-mannose type oligosaccharides in a mature glycoprotein. Glycoconj. J. 8, 400–413 [DOI] [PubMed] [Google Scholar]
- 33. Kapoor M., Ellgaard L., Gopalakrishnapai J., Schirra C., Gemma E., Oscarson S., Helenius A., Surolia A. (2004) Mutational analysis provides molecular insight into the carbohydrate-binding region of calreticulin. Pivotal roles of tyrosine-109 and aspartate-135 in carbohydrate recognition. Biochemistry 43, 97–106 [DOI] [PubMed] [Google Scholar]
- 34. Møllegaard K. M., Duus K., Traeholt S. D., Thaysen-Andersen M., Liu Y., Palma A. S., Feizi T., Hansen P. R., Højrup P., Houen G. (2011) The interactions of calreticulin with immunoglobulin G and immunoglobulin Y. Biochim. Biophys. Acta 1814, 889–899 [DOI] [PubMed] [Google Scholar]
- 35. Schuerch D. W., Wilson-Kubalek E. M., Tweten R. K. (2005) Molecular basis of listeriolysin O pH dependence. Proc. Natl. Acad. Sci. U.S.A. 102, 12537–12542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saul R., Ghidoni J. J., Molyneux R. J., Elbein A. D. (1985) Castanospermine inhibits α-glucosidase activities and alters glycogen distribution in animals. Proc. Natl. Acad. Sci. U.S.A. 82, 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hebert D. N., Foellmer B., Helenius A. (1995) Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell 81, 425–433 [DOI] [PubMed] [Google Scholar]
- 38. Ellgaard L., Frickel E. M. (2003) Calnexin, calreticulin, and ERp57. Teammates in glycoprotein folding. Cell Biochem. Biophys. 39, 223–247 [DOI] [PubMed] [Google Scholar]