Background: Little is known about the function of specific phosphatidylinositol 3-kinase (PI3K) isoforms in osteoclasts.
Results: Using a panel of isoform-selective inhibitors, we found that PI3Kδ regulates osteoclast morphology, actin cytoskeletal organization, and resorptive activity.
Conclusion: The PI3Kδ isoform plays a critical role in regulating osteoclast resorptive activity.
Significance: PI3Kδ is an attractive target for anti-resorptive therapeutics.
Keywords: Actin, Bone, Cytoskeleton, Osteoclast, Phosphatidylinositol 3-Kinase, GS-9820, Bone Resorption, Lamellipod
Abstract
Phosphatidylinositol 3-kinases (PI3K) participate in numerous signaling pathways, and control distinct biological functions. Studies using pan-PI3K inhibitors suggest roles for PI3K in osteoclasts, but little is known about specific PI3K isoforms in these cells. Our objective was to determine effects of isoform-selective PI3K inhibitors on osteoclasts. The following inhibitors were investigated (targets in parentheses): wortmannin and LY294002 (pan-p110), PIK75 (α), GDC0941 (α, δ), TGX221 (β), AS252424 (γ), and IC87114 (δ). In addition, we characterized a new potent and selective PI3Kδ inhibitor, GS-9820, and explored roles of PI3K isoforms in regulating osteoclast function. Osteoclasts were isolated from long bones of neonatal rats and rabbits. Wortmannin, LY294002, GDC0941, IC87114, and GS-9820 induced a dramatic retraction of osteoclasts within 15–20 min to 65–75% of the initial area. In contrast, there was no significant retraction in response to vehicle, PIK75, TGX221, or AS252424. Moreover, wortmannin and GS-9820, but not PIK75 or TGX221, disrupted actin belts. We examined effects of PI3K inhibitors on osteoclast survival. Whereas PIK75, TGX221, and GS-9820 had no significant effect on basal survival, all blocked RANKL-stimulated survival. When studied on resorbable substrates, osteoclastic resorption was suppressed by wortmannin and inhibitors of PI3Kβ and PI3Kδ, but not other isoforms. These data are consistent with a critical role for PI3Kδ in regulating osteoclast cytoskeleton and resorptive activity. In contrast, multiple PI3K isoforms contribute to the control of osteoclast survival. Thus, the PI3Kδ isoform, which is predominantly expressed in cells of hematopoietic origin, is an attractive target for anti-resorptive therapeutics.
Introduction
The skeleton is remodeled by the coupled processes of breakdown of old bone by osteoclasts and formation of new bone by osteoblasts (1). Maintenance of bone integrity is dependent on the coordinated activity of osteoclasts and osteoblasts, with perturbations to this balance causing skeletal disease (2). Osteoclasts and osteoclast precursors receive signals from adjacent cells, soluble mediators, and the extracellular matrix to regulate their recruitment, differentiation, resorptive activity, and survival.
Class I phosphatidylinositol 3-kinases (PI3K)3 have critical roles in a variety of cellular processes such as differentiation, metabolism, migration, and survival (3). PI3Ks are further divided into class IA and IB, all of which are heterodimeric enzymes consisting of a regulatory subunit in complex with a 110-kDa catalytic subunit that phosphorylates the primary substrate phosphatidylinositol 4,5-bisphosphate producing phosphatidylinositol 3,4,5-trisphosphate (4). Phosphatidylinositol 3,4,5-trisphosphate is the active product that mediates downstream actions through effectors such as protein kinases with pleckstrin homology domains and GTPase-activating proteins (3, 5). Class IA includes catalytic isoforms p110α (PI3Kα), β (PI3Kβ), and δ (PI3Kδ) and regulatory subunits p85α or β, p55α or γ, and p50α, and signals downstream of receptor tyrosine kinases. In contrast, class IB includes catalytic subunit p110γ (PI3Kγ) and regulatory subunits p101 and p84, and signals downstream of G protein-coupled receptors (GPCR) (3, 6). Recent data indicate that most class I PI3K subunits can be activated by GPCRs either directly (e.g. Gβγ activating PI3Kγ and PI3Kβ (7)) or indirectly (e.g. through Ras or receptor tyrosine kinase activation (4)).
Genetic manipulation as well as pharmacological inhibition approaches have allowed researchers to address overlapping and non-redundant functions of PI3K isoforms. These studies have revealed important roles for specific PI3K isoforms in immunity, metabolism, and cardiac function. Some examples include PI3Kα in insulin signaling and oncogenesis, PI3Kβ in thrombosis, and PI3Kδ and PI3Kγ in immune function and inflammation (4, 5). Generally, PI3Kα and PI3Kβ are thought to be ubiquitously expressed, whereas PI3Kδ and PI3Kγ expression is low in most cells, but high in cells of hematopoietic origin (8). Recently developed isoform-selective PI3K inhibitors show promise for the treatment of inflammatory disease and cancer, and are making their way through clinical development (5, 9, 10).
In osteoclasts, PI3K affects survival, resorptive activity, cytoskeletal organization, and motility (11–13). Investigations on PI3K isoforms in macrophages demonstrate that PI3Kδ is important in control of cell migration and vesicle trafficking (14, 15). In addition, investigations in osteoclasts demonstrate that PI3Kγ modulates osteoclastogenesis (16). Nevertheless, relatively little information is available on the functions of specific PI3K isoforms in osteoclasts, thereby providing a rationale for further investigation and possible therapeutic development. There have been recent breakthroughs using selective PI3K inhibitors to treat B-cell malignancies (9, 10). It is conceivable that new therapeutics could impact malignancies as well as osteoclasts, with benefits for the treatment of metastatic tumors in bone.
EXPERIMENTAL PROCEDURES
Materials
Medium 199 (M199, Earles, 12340) buffered with 25 mm HEPES and 26 mm HCO3−, HCO3−-free M199 (Hanks, 12350) buffered with 25 mm HEPES, heat-inactivated fetal bovine serum (FBS, 12483), and antibiotic-antimycotic stock solution (penicillin, 10,000 units/ml; streptomycin, 10,000 μg/ml; and amphotericin B, 25 μg/ml, 15240) were purchased from Invitrogen. Dulbecco's modified Eagle's medium (DMEM, D7777) with 4500 mg/liter of glucose, l-glutamine, and sodium pyruvate, without sodium bicarbonate was purchased from Sigma. Bovine serum albumin (BSA) (crystallized) was from ICN Biomedicals. Mounting medium (VectaShield) was from Vector Laboratories (Burlingame, CA). Recombinant mouse RANKL was purchased from R&D Systems (Minneapolis, MN). GDC0941 bismesylate (1377), TGX221 (1417), AS252424 (1424), and PIK75 (1334) were purchased from Axon Med Chem (Groningen, Holland). IC87114 and GS-9820 (formerly CAL-120) were provided by Calistoga Pharmaceuticals Inc. (now Gilead Sciences, Foster City, CA). Wortmannin (681675) and LY292004 (440202) were purchased from Calbiochem Merck Chemicals (Darmstadt, Germany). Stock solutions of PI3K inhibitors were prepared in dimethyl sulfoxide (DMSO) from Sigma. Table 1 summarizes the inhibitors used.
TABLE 1.
Target selectivity of inhibitors against class I PI3Ks
| Inhibitor | PI3K isoform (from low to high IC50) | Ref. |
|---|---|---|
| PIK75 | p110α > p110γ > p110δ, p110β | 22, 47 |
| TGX221 | p110β > p110δ ≫ p110α, p110γ | 48 |
| AS252424 | p110 γ ≫ p110α, p110β, p110δ | 24 |
| GS-9820 | p110δ ≫ p110γ, p110β, p110α | Table 2 |
| IC87114 | p110δ ≫ p110γ, p110β, p110α | 25 |
| GDC0491 | p110α ≈ p110δ > p110β ≈ p110γ | 48 |
| Wortmannin | p110β ≈ p110α ≈ p110δ ≈ p110γ | 48 |
| LY294002 | p110α ≈ p110δ ≈ p110β ≈ p110γ | 48 |
Osteoclast Isolation
Osteoclasts were isolated from the long bones of newborn Wistar rats or New Zealand White rabbits, as described previously (17). All procedures were approved by the Council on Animal Care of The University of Western Ontario and were in accordance with the guidelines of the Canadian Council on Animal Care. Briefly, long bones were dissected free of soft tissue and minced with a scalpel in HCO3−-buffered M199 supplemented with 15% FBS and 1% antibiotic solution. The resulting cells were suspended by repeated passage through a glass pipette and plated on FBS-coated 12-mm glass coverslips, MatTek glass bottom culture dishes (MatTek Corporation, Ashland, MA), or calcium phosphate-coated discs (BD BioCoatTM OsteologicTM Discs, BD Biosciences, Bedford, MA). Freshly isolated osteoclasts were incubated at 37 °C in 5% CO2 for 1 h, washed gently with PBS to remove non-adherent cells, and incubated in medium. Osteoclasts were identified by their characteristic morphology and presence of three or more nuclei and, in other studies, have been shown to stain for tartrate-resistant phosphatase, retract in response to calcitonin, and resorb mineralized substrates (18–20).
In Vitro Kinase Activity Profiling
Biochemical in vitro lipid kinase assays were performed by the SelectScreen® biochemical kinase assay service (Invitrogen Ltd.). A stock solution of GS-9820 was prepared in DMSO at a concentration of 10 mm. Ten-point kinase inhibitory activities were measured over a concentration range (5 to 104 nm) with ATP at a concentration consistent with the Km of each of the enzymes.
Kinase Binding Selectivity Profiling
GS-9820 was tested at 10 μm in ATP site-dependent competition binding assays for 393 kinases (358 excluding mutant kinases) by contract with Ambit Biosciences (San Diego, CA) (21). GS-9820 was considered active if <35% of binding to immobilized probes remained compared with DMSO control.
Real-time RT-PCR Analyses
Total RNA was isolated from RAW264.7 cells (American Type Culture Collection, Manasas, VA) cultivated for 5 days with or without 100 ng/ml of RANKL, using TRIzol reagent (Invitrogen) and the RNeasy Mini kit (Qiagen). Primers and probes for murine phosphatidylinositol 3-kinase, catalytic α-polypeptide (Pik3ca, Mm00435673_m1), phosphatidylinositol 3-kinase, catalytic β-polypeptide (Pik3cb, Mm00659576_m1), phosphatidylinositol 3-kinase, catalytic γ-polypeptide (Pik3cg, Mm00445038_m1), phosphatidylinositol 3-kinase, catalytic δ-polypeptide (Pik3cd, Mm00435674_m1), glyceraldehyde 3-phosphate dehydrogenase (Gapdh, product number 4308313), and 18S ribosomal RNA (product number 4308329) were from Applied Biosystems (Gene Expression Assay). Real-time RT-PCR was performed using TaqMan One-step RT-PCR Master Mix Reagents kit (Applied Biosystems) and the ABI Prism 7900HT Sequence Detector (Applied Biosystems), according to the manufacturer's recommendations. Samples were amplified in triplicate. Relative mRNA content was calculated by the ΔΔCt method using 18S as a reference and Gapdh value as a calibrator.
PI3K Isoforms in RAW264.7 Cells
Whole cell lysates from RAW264.7 cells were prepared in lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin), supplemented with 1× complete mini protease inhibitor (Roche Diagnostics Corp.), and 1× Phosphatase Inhibitor Mixture Set I, II (EMD Millipore, Billerica, MA) for 10 min on ice. Lysates were cleaned by sedimentation at 14,000 × g for 10 min at 4 °C, and the soluble protein was analyzed by Western blotting using anti-PI3Kα (4255, Cell Signaling, Danvers, MA), anti-PI3Kβ (32569, Abcam, Cambridge, MA), anti-PI3Kδ (7176, Santa Cruz Biotechnology, Santa Cruz, CA), and anti-PI3Kγ (ABD-026L, Jena Bioscience, Jena, Germany). Immunoreactive bands were visualized using LI-COR Odyssey (Lincoln, NE).
PI3K Isoform-selective Cell-Based Assays
Murine embryonic fibroblasts (American Type Culture Collection) were used for the analysis of PI3Kα and PI3Kβ signaling. Cells were transferred to serum-free medium for 2 h followed by 2 h incubation in the absence or presence of increasing concentrations of GS-9820, and then stimulated with PDGF (10 ng/ml; Cell Signaling, Danvers, MA) or lysophosphatidic acid (LPA) (10 μm; Echelon, Salt Lake City, UT) for 10 min at 37 °C to activate PI3Kα and PI3Kβ, respectively (5, 9, 10). Cells were trypsinized, washed in cold PBS, and the cell pellet was lysed in lysis buffer for 10 min on ice. Whole cell lysates were cleaned by sedimentation at 14,000 × g for 15 min at 4 °C, and the soluble protein was analyzed by Western blotting for Akt and pAkt levels (anti-total Akt, phospho-Akt Ser473, number 2920, number 9271, respectively, Cell Signaling).
For the analysis of PI3Kδ and PI3Kγ signaling, basophil activation was measured in whole blood (5, 9, 10). Blood samples were obtained after written informed consent was obtained in accordance with the Declaration of Helsinki and with local institutionally approved protocols. Basophil activation was measured using the Flow2 CAST® kit according to the manufacturer's standardized methods (Buhlman Laboratories AG, Switzerland). Briefly, PI3Kδ was activated with anti-FcϵRI and PI3Kγ was activated with N-formyl-methionyl-leucyl-phenylalanine (fMLP) in the absence or presence of increasing concentrations of GS-9820. To monitor the basophil cell population and cellular activation, anti-CD63-FITC and anti-CCR3-PE antibodies were added to each sample. Cells were fixed and analyzed on a FC500MPL flow cytometer.
Assessment of Cell Viability
The effect of inhibitors on RAW264.7 cell survival was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. RAW264.7 cells were seeded in Falcon flat bottom 96-well plates at a density of 2.5–3 × 104 cells/cm2 in 100 μl of DMEM with 10% FBS and 1% antibiotic solution. After seeding, the cells were allowed to attach for 24 h then exposed to control or treatment for 24 h. After incubation at 37 °C in 5% CO2, MTT substrate (Roche Applied Science) was added at a final concentration of 0.5 mg/ml for 4 h. Following a 4-h incubation, 100 μl of solubilization solution was added to each well to dissolve the formazan crystals and samples were analyzed after 24 h. Absorbance of the samples was assessed using a plate reader (Tecan, Mannedorf, Switzerland) using a wavelength of 550 nm and a reference wavelength of 700 nm.
Assessment of Osteoclast Morphology
To perform time-lapse recordings, culture medium was removed and replaced with a HEPES-buffered M199 medium (HCO3−-free) supplemented with 15% FBS and 1% antibiotic solution. Dishes were placed in a heated stage and maintained at ∼35 °C. Osteoclasts were observed using a Nikon Eclipse TE300 phase-contrast microscope and images were captured using Image Master 5 Software (Photon Technology International, Birmingham, NJ). For data analysis, the periphery of each osteoclast was traced periodically to quantify the planar area using Image Master software. The osteoclast area was standardized and expressed as a percentage of the average initial area before time 0. Initial planar area was 5440 ± 3200 μm2 (mean ± S.D., n = 90 osteoclasts).
Assessment of F-actin Organization
Osteoclasts were plated on FBS-coated glass coverslips or BD BioCoatTM OsteologicTM coverslips and fixed with 4% paraformaldehyde and 2% sucrose in PBS for 10 min at room temperature, washed three times with PBS, and permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. Following three washes with PBS, cells were incubated in 1–3% BSA for 1 h at room temperature, stained for filamentous actin (F-actin) using 66 nm Alexa Fluor® 488 phalloidin (Invitrogen) for 20 min at room temperature in the dark, and washed three times with PBS. Following the wash, osteoclasts were visualized for fluorescence. The number of osteoclasts exhibiting a complete F-actin belt or sealing zone present under each condition was counted along with the total number of osteoclasts (per 12-mm coverslip). Osteoclasts observed to have an F-actin belt or sealing zone more than 75% complete were assigned as “complete,” whereas anything below 75% was considered “incomplete.” Images under each condition were captured with Zeiss AxioVision 4.8 imaging software using a Zeiss Observer Z1 microscope (Chester, VA) or Zeiss LSM 5.0 software using a Zeiss LSM 510 META confocal microscope.
Adenoviruses expressing EGFP and human β-actin-EGFP fusion proteins were created using the ViraPowerTM adenoviral expression system, according to the manufacturer's protocols (Invitrogen). pAd/CMV/V5-DEST and pENTR1A Gateway vectors were used. An EcoRI-NotI fragment containing the EGFP coding sequence and a NheI-BamHI fragment containing actin-EGFP were isolated from pEGFP-N1 and pActin-EGFP vectors (Clontech), respectively, and cloned into the pENTR1A vector. HEK293A cells (Invitrogen) were used for adenovirus amplification. Crude viral lysates (50 μl) were applied to rabbit osteoclasts in MatTek glass bottom dishes for 20 min at 37 °C. Thereafter, M199 (Earles) supplemented with 15% FBS and 1% antibiotic solution (2 ml) was added. Cultures were maintained for 1–2 days before performing experiments. Preliminary experiments were performed to optimize transduction conditions for each virus preparation.
Assessment of Osteoclast Survival
Rat osteoclasts were isolated and plated on FBS-coated glass coverslips and incubated at 37 °C in 5% CO2 for 1 h. Coverslips were then washed gently with PBS to remove non-adherent cells and incubated for an additional 0.5–1 h in HCO3−-buffered M199 supplemented with 15% FBS and 1% antibiotic solution. Osteoclasts were then counted using phase-contrast microscopy. Cultures were incubated for an additional 15–18 h at 37 °C in 5% CO2. Following incubation, the number of osteoclasts per coverslip was counted, and survival was expressed as the percentage of the initial osteoclast number on the same coverslip. The number of osteoclasts per coverslip was 75 ± 25 (mean ± S.D., n = 90 coverslips).
Pit-formation Assay
Resorption was assessed as described (19), using elephant ivory (gift from the Canadian Wildlife Service). Ivory slices (∼22 mm2) were plated with osteoclasts from neonatal rabbits in HCO3−-buffered M199 with FBS (15%) and antibiotic solution (1%). After 3 h, slices were washed to remove non-adherent cells, and then transferred to 10% CO2 in the same medium with vehicle (DMSO) or inhibitors: 1 μm wortmannin, 1 μm GS-9820, 1 μm TGX221, 100 nm PIK75, or 1 μm AS252424. Following a 48-h incubation, preparations were fixed in 4% paraformaldehyde and stained for tartrate-resistant acid phosphatase. The number of osteoclasts was determined as the number of multinucleated, tartrate-resistant acid phosphatase-positive cells. Slices were then washed with 5% sodium hypochlorite to remove cells, rinsed with water, and stained with 1% (w/v) toluidine blue in 1% (w/v) sodium borate. Ivory was examined (Nikon SMZ1500 microscope), resorption pits were counted and the area determined using Image Master 5 software. Pit depth was determined using a reflection light microscope (Zeiss Axio imager M2m). Scanning electron microscope images were taken on a Hitachi 3400-N Variable Pressure Scanning Electron Microscope at the Western University Biotron Experimental Climate Change Research Centre.
Statistical Analyses
Unless otherwise indicated, results are presented as mean ± S.E. Differences between two groups were evaluated using a Student's t test and differences among more than two groups were evaluated by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test or two-way ANOVA followed by Bonferroni's multiple comparisons test. Differences were accepted as statistically significant at p < 0.05.
RESULTS
GS-9820 Is a Potent and Selective Inhibitor of PI3Kδ
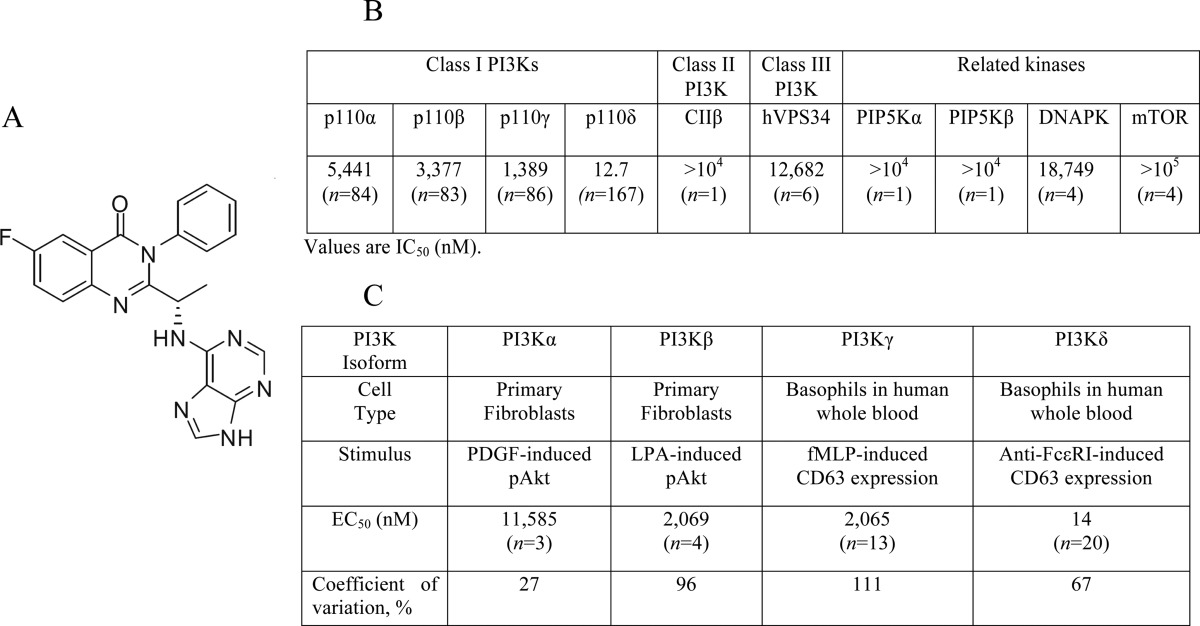
A number of the compounds used in the present study were characterized in previous investigations (i.e. PIK75 (22), TGX221 (23), AS252424 (24), IC87114 (25), GDC0941 (26), wortmannin and LY294002 (27)). The novel PI3Kδ inhibitor GS-9820 (6-fluoro-3-phenyl-2-[(1S)-1-(9H-purin-6-ylamino) ethyl]-4(3H)-quinazolinone) (Table 2A) was characterized using in vitro activity assays. We found that GS-9820 was more selective for PI3Kδ relative to other PI3K class I enzymes (IC50: PI3Kα, 5,441 nm; PI3Kβ, 3,377 nm; PI3Kγ, 1,389 nm; and PI3Kδ, 12.7 nm) (Table 2B). GS-9820 was also 103-fold more selective against PI3Kδ than against related kinases, such as CIIβ, hVPS34, DNAPK, and mammalian target of rapamycin.
TABLE 2.
GS-9820 inhibits PI3Kδ with high selectivity
A, chemical structure of GS-9820. B, GS-9820 in vitro activity profiles (IC50 values, in nm) for recombinant enzymes of PI3K Class I, II, and III and other related kinases. GS-9820 was dissolved in dimethyl sulfoxide at a stock concentration of 10 mm and 10-point kinase inhibitory activities were measured with ATP at a concentration consistent with the Km of each enzyme. C, potency of GS-9820 in cell-based assays evaluating the activity of specific PI3K Class I isoforms (EC50 values in nm). For the analysis of PI3Kα and PI3Kβ signaling, murine embryonic fibroblasts were incubated for 2 h with several concentrations of GS-9820 followed by stimulation with PDGF or LPA. Soluble protein was analyzed by Western blotting for Akt and pAkt473 levels. For the analysis of PI3Kγ and PI3Kδ signaling, basophil activation was measured in human whole blood. PI3Kγ was activated with fMLP and PI3Kδ was activated with anti-FCϵRI. To monitor the basophil cell population and cellular activation, anti-CCR3-PE and anti-CD63-FITC antibodies were added and each sample was analyzed on a FC500MPL flow cytometer. Data are geometric mean of EC50 values. Concentration-dependence curves for PI3K isoforms are shown in supplemental Fig. S2. For in vitro kinase studies, n = 1 to 167 independent experiments and for isoform-selective cell-based assays, n = 3 to 20 independent experiments.
GS-9820 was also assayed for its potential binding interaction with kinases by screening a comprehensive panel of 393 kinases, including mutants, in the Ambit KINOMEscanTM (21). No binding outside of PI3K was observed at 10 μm GS-9820, further demonstrating a high degree of selectivity (supplemental Table S1 and Fig. S1).
EC50 values were characterized using in vitro cell-based assays as described previously (9). In fibroblasts, the PDGF receptor signals through PI3Kα and the GPCR for LPA signals through PI3Kβ (28). GS-9820 reduced PDGF-induced pAkt by only 50% at 11,585 nm, and LPA-induced pAkt by 50% at 2,069 nm (Table 2C). Expression of PI3Kγ and PI3Kδ is largely restricted to cells of hematopoietic origin, including basophils. In basophils, FcϵRI signals through PI3Kδ, whereas fMLP signals through PI3Kγ via GPCRs with either stimulus leading to expression of CD63 (29, 30). GS-9820 suppressed FcϵRI PI3Kδ-mediated CD63 expression with an EC50 of 14 nm, and fMLP PI3Kγ-mediated CD63 expression with an EC50 of 2,065 nm (supplemental Fig. S2). Thus, GS-9820 showed selectivity for PI3Kδ over the other class I PI3K isoforms and virtually no binding to a panel of other kinases.
PI3K Isoforms in Osteoclast-like Cells
We first used quantitative real-time PCR analysis of RAW264.7 cells, a leukemic murine monocyte-macrophage-like cell line that, when treated with RANKL, produces multinucleated, tartrate-resistant acid phosphatase-positive osteoclast-like cells (31). We identified transcripts encoding all class I PI3K isoforms (Table 3). Differentiation with RANKL caused a significant increase in message for PI3Kβ and a significant decrease in mRNA encoding PI3Kγ. Western blotting confirmed the presence of the four isoforms in RAW264.7 cells (Fig. 1), consistent with the PCR data.
TABLE 3.
mRNA for PI3K isoforms in RAW264.7 and RAW264.7-derived osteoclasts
Quantitative PCR analysis of PI3K isoforms in RAW264.7 cells and after differentiation into osteoclast-like cells. Differentiation of osteoclasts with RANKL was accompanied by increase in expression of message for PI3Kβ and decrease in PI3Kγ. Values are mean ± S.E. from four independent experiments. Student's t test was used for statistical analyses.
| Without RANKL | With RANKL | |
|---|---|---|
| Pik3ca, p110-α | 0.0029 ± 0.0007 | 0.0032 ± 0.0006 |
| Pik3cb, p110-β | 0.0109 ± 0.0023 | 0.0223 ± 0.0035a |
| Pik3cg, p110-γ | 0.0065 ± 0.0010 | 0.0022 ± 0.0007a |
| Pik3cd, p110-δ | 0.0027 ± 0.0001 | 0.0016 ± 0.0002 |
a p < 0.05 for the effect of RANKL.
FIGURE 1.
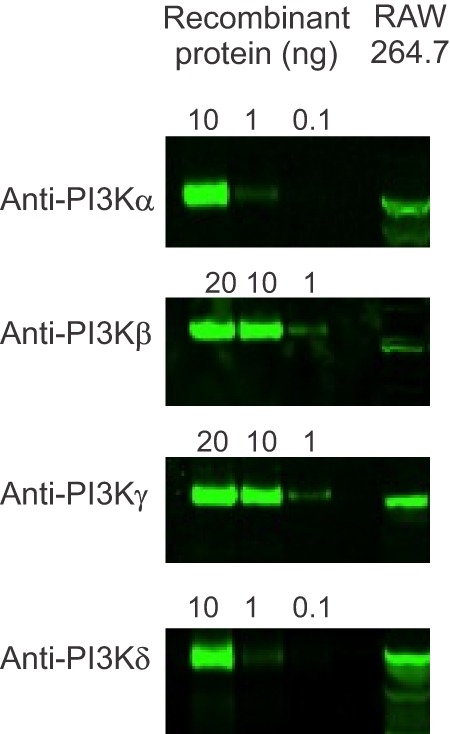
PI3K isoform expression in RAW264.7 cells. First to third lanes illustrate different amounts of recombinant PI3K isoforms, as indicated at the top. Lysates from undifferentiated RAW264.7 cells are shown in the fourth lane, immunoblotted with isoform-specific anti-PI3K antibodies, with evidence for all four isoforms. Immunoblot data are representative of two independent cell preparations.
Effect of Isoform-selective PI3K Inhibitors on the Viability of RAW264.7 Cells
To assess possible toxicity, we incubated undifferentiated RAW264.7 cells with inhibitors at varying concentrations for 24 h, after which viability was assessed using an MTT assay. The inhibitors were: wortmannin (pan-p110), LY294002 (pan-p110), GDC0941 (α and δ inhibitor), PIK75 (α), TGX221 (β), AS252424 (γ), IC87114 (δ), and GS-9820 (δ) (summarized in Table 1). Toxic effects were only observed for PIK75, at concentrations of 1 and 10 μm, and LY294002, at a concentration of 100 μm (p < 0.05) (Fig. 2), concentrations we subsequently avoided for long-term experiments.
FIGURE 2.
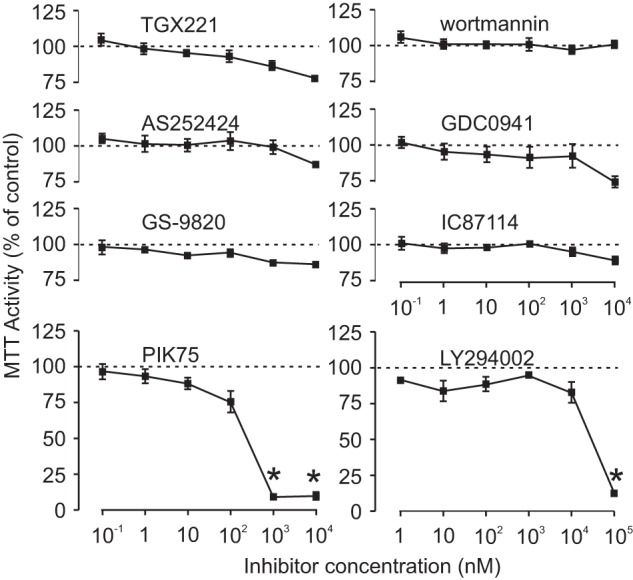
Effects of PI3K isoform-selective inhibitors on viability of RAW264.7. Undifferentiated RAW264.7 cells were treated with inhibitors (100 pm to 10 μm, or 1 nm to 100 μm for LY294002) or vehicle for 24 h, after which viability was assessed using an MTT assay. Data are expressed as a percentage of values for vehicle-treated control samples. Dashed lines indicate 100%. Viability was diminished only by PIK75 at concentrations of 1 and 10 μm and LY294002 at 100 μm. Data are mean ± S.E., n = 4 independent experiments, except for IC87114 and LY294002, where n = 3. Differences were assessed using one-way ANOVA and Tukey's multiple comparisons test. * indicates p < 0.05 compared with vehicle.
Inhibition of PI3Kδ Induces Osteoclast Retraction
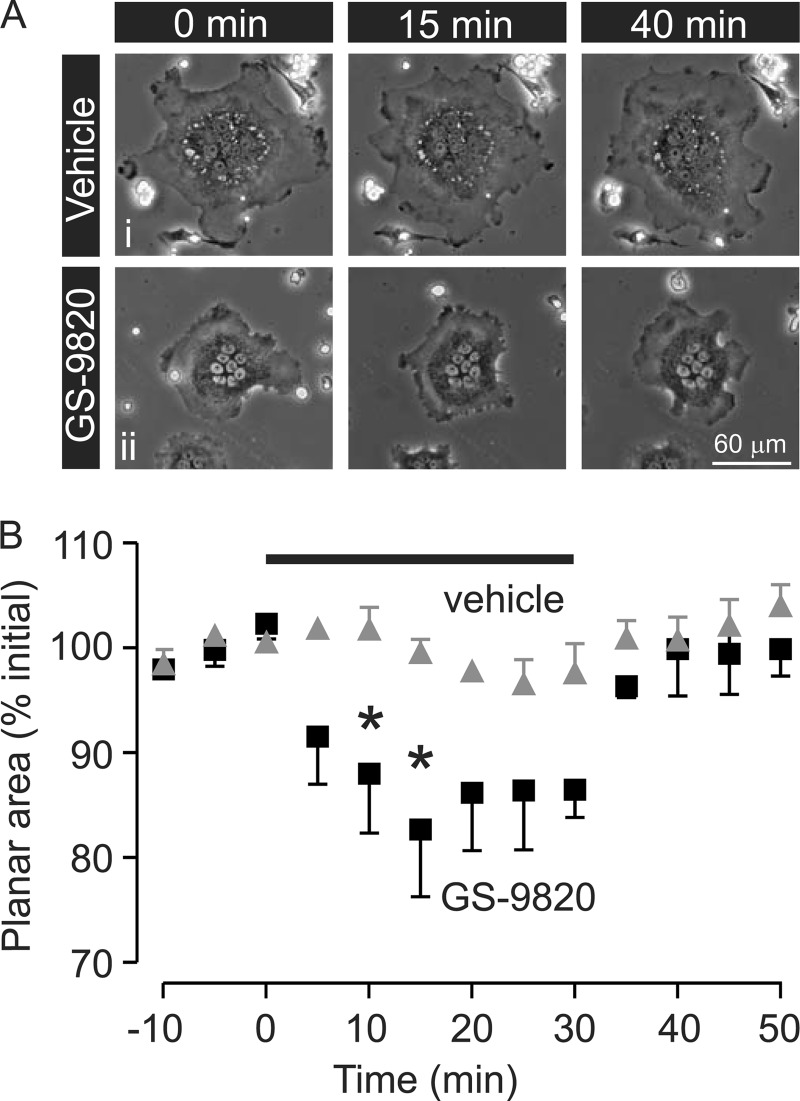
Conventional PI3K inhibitors such as wortmannin and LY294002 have been shown to induce osteoclast retraction and inhibit motility (13). However, these inhibitors are not selective for particular PI3K p110 isoforms, prompting us to characterize the effects of selective inhibitors. Freshly isolated rat osteoclasts were monitored using time-lapse phase-contrast microscopy. Under control conditions, osteoclast lamellipodia were well spread. Basal morphology and motility were recorded for 25 min before addition of vehicle or inhibitors. Representative images are shown in Fig. 3, where wortmannin and GS-9820, but not vehicle, PIK75, or TGX221, caused prompt retraction of lamellipodia, indicated by the arrows.
FIGURE 3.
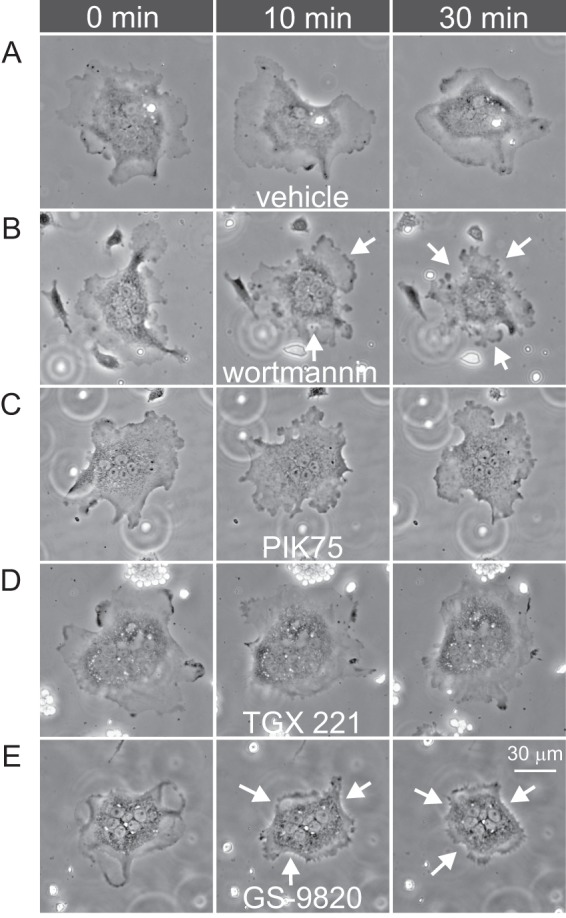
Wortmannin and GS-9820 induce osteoclast retraction. Rat osteoclasts were bathed in HEPES-buffered M199 medium with 15% FBS and antibiotics and imaged using time-lapse phase-contrast microscopy. A–E illustrates 5 different osteoclasts, each shown at 3 times. Images labeled time 0 min illustrate the appearance of cells immediately prior to addition of vehicle, wortmannin (1 μm), PIK75 (1 μm), TGX221 (1 μm), or GS-9820 (1 μm) to the bath. Prior to treatment, osteoclast lamellipodia were well spread and motile. Wortmannin and GS-9820 induced prompt retraction of lamellipodia, which was sustained for at least 30 min. Images are representative of the responses of 9–15 osteoclasts from four to eight independent preparations. Scale bar represents 30 μm for all panels. Images displayed were γ adjusted for clarity of the osteoclast periphery.
To quantify the change in morphology, the planar area of osteoclasts was measured periodically and expressed as a percentage of the initial area before treatment. Within 10–15 min, GDC0941, LY294002, wortmannin, IC87114, and GS-9820 (Fig. 4) induced a significant retraction to 65–75% of the initial area. In contrast, there was no significant response to PIK75, AS252424 at 1 μm, or vehicle. We note that the PI3Kβ inhibitor TGX221 caused minor retraction. These data are consistent with the involvement of specific PI3K isoforms in regulating the osteoclast cytoskeleton.
FIGURE 4.
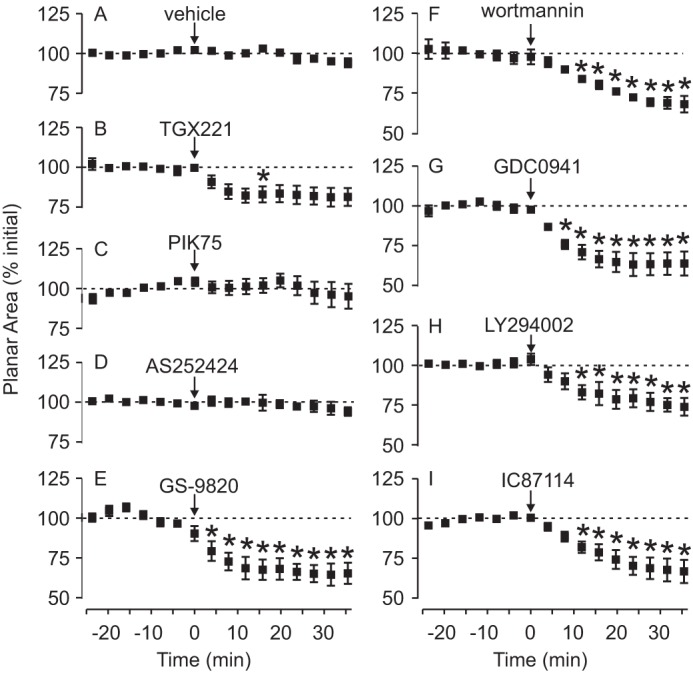
PI3Kδ is important in osteoclast lamellipodia spreading. Rat osteoclasts were imaged by time-lapse phase-contrast microscopy and treated with the indicated test substance at time 0, as described in the legend to Fig. 3. Image analysis software was used to calculate the planar area of osteoclasts at 4-min intervals. Data are expressed as a percentage of the mean initial area before time 0 (from −24 to 0 min) and are mean ± S.E. A, there was no marked change in osteoclast area in vehicle-treated cells (n = 4 independent preparations, a total of 9 osteoclasts). B–D, the following inhibitors had no consistent effect on osteoclast area: TGX221 (1 μm, n = 8 independent preparations, a total of 10 osteoclasts), PIK75 (1 μm, n = 5 independent preparations, a total of 10 osteoclasts), and AS252424 (1 μm, n = 5 independent preparations, a total of 9 osteoclasts). E–I, in contrast, the following inhibitors caused significant, sustained decreases in osteoclast planar area: GS-9820 (1 μm, n = 5 independent preparations, a total of 15 osteoclasts), wortmannin (1 μm, n = 4 independent preparations, a total of 8 osteoclasts), GDC0941 (1 μm, n = 5 independent preparations, a total of 8 osteoclasts), LY294002 (50 μm, n = 3 independent preparations, a total of 7 osteoclasts), and IC87114 (5 μm, n = 4 independent preparations, a total of 12 osteoclasts). Differences were assessed using a two-way ANOVA and Bonferroni's multiple comparisons test. * indicates p < 0.05 compared with vehicle at the corresponding times.
Some PI3K inhibitors are irreversible (e.g. wortmannin), so we examined the reversibility of the PI3Kδ-selective inhibitor, GS-9820 (Fig. 5A). Planar area of rat osteoclasts was measured periodically over 60 min. Following a control period of 10 min, vehicle or GS-9820 (1 μm) was applied to the bath at 0 min and washed out at 30 min (Fig. 5B). Vehicle caused no change in osteoclast planar area before or after the wash (Fig. 5A, i). In contrast, lamellipodia were retracted in the presence of GS-9820 with apparent re-spreading following the wash (Fig. 5A, ii). GS-9820 caused a significant retraction of osteoclasts at 10 and 15 min, followed by a return to baseline after washout (Fig. 5B). These data reflect washout from the bath, and not degradation of GS-9820, which is stable, with a half-life of 11.7 days at pH 2 and 50 °C. This demonstrates that the retraction is reversible and reveals an important feature of GS-9820. It should be noted that these studies involved lengthy time-lapse recordings of single osteoclasts, precluding us from carrying out full concentration-dependence studies.
FIGURE 5.
Reversibility of the effects of the PI3Kδ-selective inhibitor GS-9820 on osteoclast retraction. Rat osteoclasts were imaged by time-lapse phase-contrast microscopy and exposed to vehicle or GS-9820 (1 μm) from 0 to 30 min, as indicated by the horizontal bar above the data in B. A illustrates 2 different osteoclasts, each shown at 3 times. i, lamellipodia remain well spread in vehicle-treated osteoclast. ii, GS-9820 induces retraction of lamellipodia (evident at 15 min). Following washout of GS-9820 at 30 min, lamellipodia respread (evident at 40 min). B, planar area of osteoclasts was calculated periodically and expressed as a percentage of the mean initial area before time 0 (from −10 to 0 min). Data are mean ± S.E. There was no marked change in osteoclast area in vehicle-treated cells (n = 3 independent preparations, a total of 8 osteoclasts). In contrast, the planar area exhibited a significant decrease in the presence of GS-9820 and returned promptly to control values following its washout (n = 3 independent preparations, a total of 5 osteoclasts). Differences were assessed using a two-way ANOVA and Bonferroni's multiple comparisons test. * indicates p < 0.05 compared with vehicle at the corresponding times.
Inhibition of PI3Kδ Disrupts Actin Organization
We next investigated whether retraction of lamellipodia was associated with reorganization of the actin cytoskeleton. On non-mineralized substrates such as glass or plastic, osteoclasts form clusters of podosomes, or a band of podosomes at the periphery of the cell, called an F-actin belt. On mineralized substrates, such as bone or calcium phosphate matrices, podosomal units condense, forming a ring in the interior of the cell, called a sealing zone (32). We quantified the proportion of cells with complete or disrupted F-actin belts and sealing zones. The effects of selected class IA PI3K isoform inhibitors were examined. PIK75, TGX221, GS-9820, or wortmannin were applied to osteoclasts for 10 min and then cells were analyzed using fluorescence microscopy by an individual blinded to the treatments (Fig. 6A). Wortmannin and GS-9820 caused a significant reduction in the incidence of F-actin belts in comparison to vehicle, whereas TGX221 and PIK75 had no significant effect (Fig. 6B). Disruption of the F-actin belt was sustained in the continued presence of GS-9820 (Fig. 6C).
FIGURE 6.
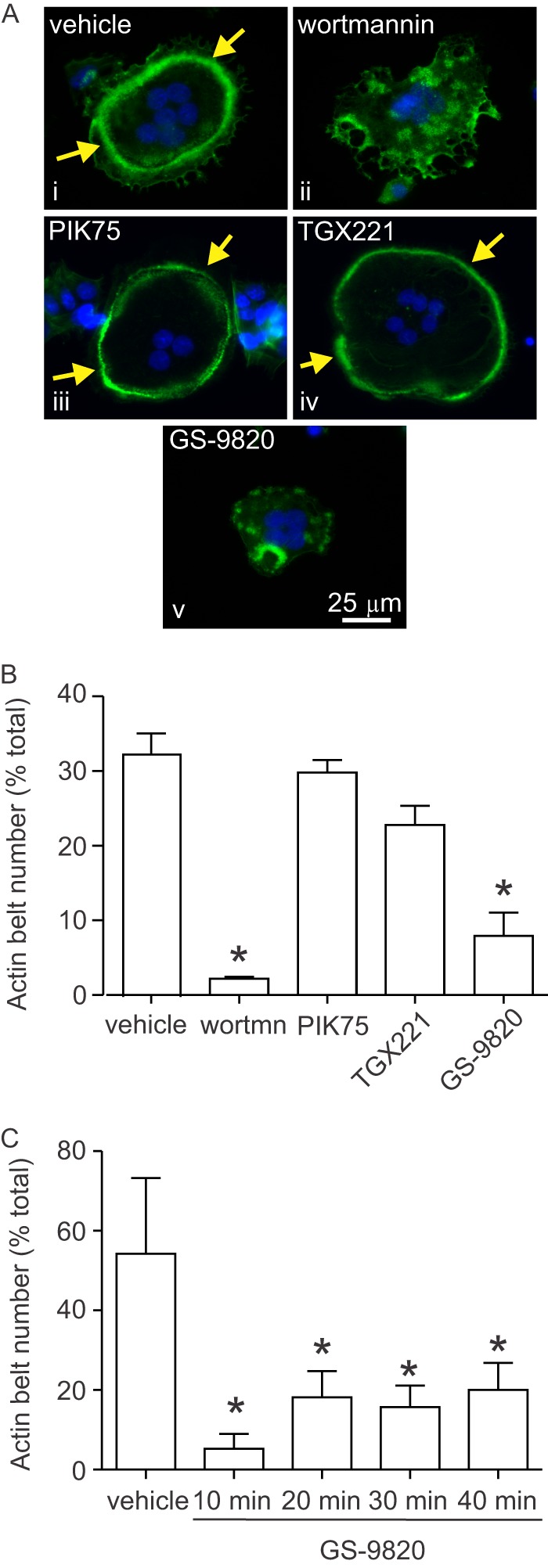
Effects of PI3K inhibitors on actin belt organization in osteoclasts. Rat osteoclasts were plated on FBS-coated glass coverslips in HCO3−-buffered M199 with FBS and antibiotics. Samples were treated with vehicle or inhibitor for 10 min and then fixed. F-actin was labeled using Alexa Fluor 488-conjugated phalloidin (green), nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue), and cells were examined by fluorescence microscopy (×40 objective, Zeiss Axio Observer Z1). Inhibitors were wortmannin (wortmn), PIK75, TGX221, and GS-9820 (all 1 μm). A, i, image shows a single untreated rat osteoclast exhibiting a prominent F-actin belt at the cell periphery. A, iii and iv, F-actin belts were also observed in osteoclasts treated with PIK75 and TGX221. A, ii and v, in contrast, osteoclasts treated with wortmannin or GS-9820 for 10 min displayed a disorganized pattern of F-actin staining with clusters of punctate structures. B, we quantified the number of osteoclasts exhibiting actin belts (encompassing at least 75% of the cell periphery). Osteoclasts treated with wortmannin or GS-9820 displayed significantly fewer actin belts than vehicle-treated cells, whereas PIK75 and TGX221 had no significant effect. Data are the percentage of osteoclasts exhibiting actin belts and are mean ± S.E., n = 3 independent experiments with a total of 1,537 osteoclasts. C, effects of PI3K inhibition were persistent. Osteoclasts treated with GS-9820 for up to 40 min displayed significantly fewer actin belts than vehicle-treated cells. Data are the percentage of osteoclasts exhibiting actin belts and are mean ± S.E., n = 4 independent experiments with a total of 1,546 osteoclasts. Data were analyzed by one-way ANOVA followed by Tukey's multiple comparisons test. * indicates p < 0.05 compared with vehicle.
To complement these studies, we examined the effect of GS-9820 on F-actin belts in live cells using rabbit osteoclasts virally transduced with actin-EGFP. When viewed using confocal microscopy, vehicle alone caused no change in F-actin belt organization (Fig. 7A, i). In contrast, GS-9820 disrupted F-actin belt organization, starting within 10 min (Fig. 7A, ii).
FIGURE 7.
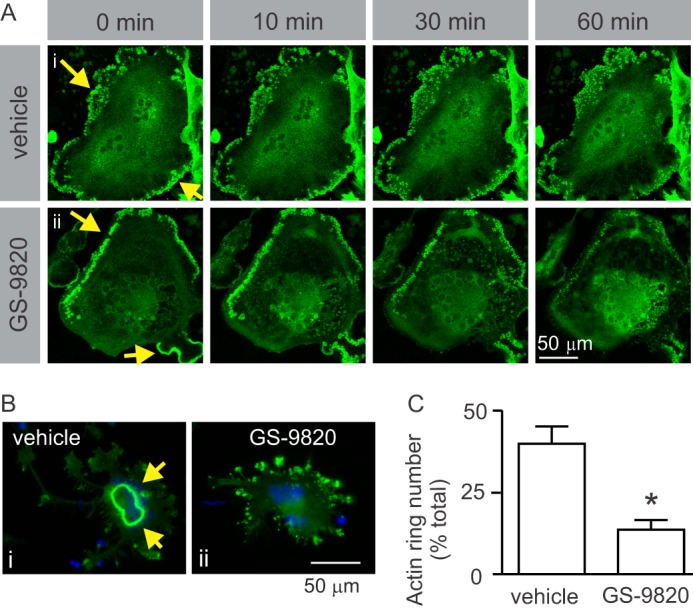
Inhibition of PI3Kδ causes disruption of actin cytoskeleton. A, live-cell imaging of the effects of GS-9820 on F-actin belt dynamics in rabbit osteoclast expressing actin-EGFP. To directly observe the effects of GS-9820 on F-actin belts, rabbit osteoclasts were plated on FBS-coated MatTek glass-bottom culture dishes and transduced with adenoviruses expressing actin-EGFP fusion or EGFP proteins. Cells were then bathed in HEPES-buffered M199 medium with 15% FBS at ∼26 °C and imaged using confocal microscopy (×40 objective, Zeiss LSM 510 META confocal microscope). Images labeled 0 min illustrate the appearance of osteoclasts immediately prior to addition of vehicle or GS-9820 (1 μm) to the bath. Yellow arrows indicate actin belt. A, i, F-actin belt remained intact in vehicle-treated osteoclast. A, ii, GS-9820 induced gradual disappearance of the peripheral actin structures and an increase in actin fluorescence more centrally. Images are representative of a total of 4 osteoclasts from 2 independent preparations. Control samples transduced with EGFP protein alone showed uniform distribution of fluorescence (not shown). B, to examine effects of PI3K on sealing zone formation, rat osteoclasts were plated on resorbable calcium phosphate-coated discs. Samples were treated with vehicle or GS-9820 (1 μm) for 10 min and then fixed. F-actin was labeled using Alexa Fluor 488-conjugated phalloidin (green), nuclei were stained with DAPI (blue), and cells were examined by fluorescence microscopy. B, i, vehicle-treated osteoclast exhibits a prominent F-actin-rich sealing zone, characteristic of active osteoclasts on resorbable substrates. Note that sealing zones are more centrally located than the peripheral actin belts illustrated in A. B, ii, osteoclasts treated with GS-9820 exhibited fewer sealing zones and disorganized clusters of F-actin. C, osteoclasts treated with GS-9820 displayed significantly fewer sealing zones (actin rings) than vehicle-treated cells. Data are the percentage of osteoclasts exhibiting sealing zones and are mean ± S.E., n = 3 independent experiments with a total of 592 osteoclasts. Data were analyzed by unpaired Student's t test. * indicates p < 0.05 compared with vehicle.
To examine sealing zones, rat osteoclasts were plated on coverslips coated with resorbable calcium phosphate (BD BioCoatTM OsteologicTM). Cells were treated with vehicle, or GS-9820 (1 μm) for 10 min, fixed, permeabilized, and incubated with fluorescently tagged phalloidin (Fig. 7B). Sealing zones generally had a smaller diameter than F-actin belts. With vehicle treatment ∼40% of cells exhibited actin sealing zones, whereas GS-9820 reduced the prevalence to 10% of cells (Fig. 7C).
Inhibition of PI3K Suppresses the Effects of RANKL on Osteoclast Survival
Resorption is proportional to the number of osteoclasts present at any given time and, thus, osteoclast survival is a key factor that regulates bone loss in vivo (2). Accordingly, we examined the effect of isoform-selective PI3K inhibitors on osteoclast survival. Rat osteoclasts were placed on FBS-coated coverslips and incubated in the absence (control) or presence of RANKL (100 ng/ml), along with vehicle, 1 μm GS-9820, 1 μm TGX221, or 100 nm PIK75. Survival was quantified by counting the number of osteoclasts before and after an 18-h incubation period and the proportion of surviving cells was calculated. Osteoclasts were identified by phase-contrast microscopy as multinucleated cells (≥3 nuclei). There were no significant effects of inhibitors on survival under control conditions (Fig. 8). However, RANKL significantly enhanced osteoclast survival, and GS-9820, TGX221, and PIK75 all suppressed the stimulatory effect of RANKL on survival. These data illustrate vital roles for class I PI3Ks in mediating the effects of RANKL on osteoclast survival.
FIGURE 8.
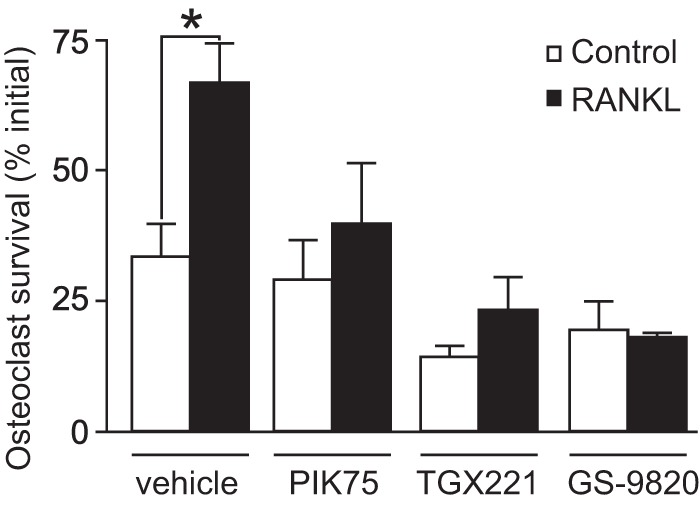
The effects of isoform-selective PI3K inhibitors on osteoclast survival. Rat osteoclasts were plated on coverslips in HCO3−-buffered M199 with 15% FBS and antibiotics. Where indicated, samples were treated with RANKL (100 ng/ml) or its vehicle (Control). In addition, samples were treated with vehicle, PIK75 (100 nm), TGX221 (1 μm), or GS-9820 (1 μm). Survival was assessed by counting (using phase-contrast microscopy) the number of osteoclasts before and after 18 h of culture. As expected, RANKL increased osteoclast survival compared with vehicle. In contrast to their selective effects on cytoskeletal organization, all inhibitors tested (PIK75, TGX221, and GS-9820) suppressed RANKL-induced survival, with no significant effect on basal survival. Data are the number of surviving osteoclasts on each coverslip at 18 h expressed as a percentage of the initial number of osteoclasts on the same coverslip (mean ± S.E., n = 3 independent experiments). Data were analyzed by a two-way ANOVA followed by a Bonferroni's multiple comparisons test. * indicates p < 0.05 for the effect of RANKL.
Effect of Isoform-selective PI3K Inhibitors on Resorptive Activity
We next examined effects of PI3K blockade on osteoclast activity by measuring the resorption of ivory (Fig. 9A). Total area resorbed, pit number, and pit depth were quantified (Fig. 9, B–D). The pan-PI3K inhibitor wortmannin significantly inhibited resorption, measured as total area resorbed and number of pits, consistent with earlier studies (11, 33). Notably, p110δ and -β inhibitors caused a significant reduction in total resorbed area, as well as pit number and depth; whereas α or γ inhibitors were without marked effect.
FIGURE 9.
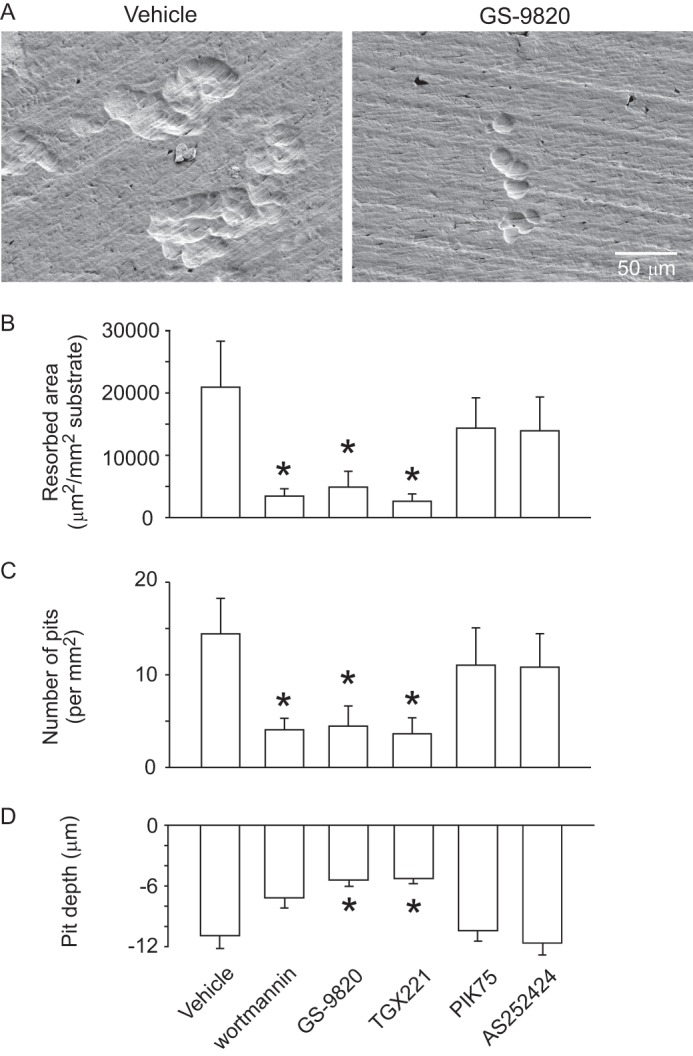
Effects of isoform-selective PI3K inhibitors on osteoclastic resorption. Rabbit osteoclasts were plated on elephant ivory slices and incubated for 48 h in HCO3−-buffered M199 with specified inhibitors. Samples were treated with vehicle (DMSO), wortmannin (1 μm), GS-9820 (1 μm), TGX221 (1 μm), PIK75 (100 nm), or AS252424 (1 μm). A, scanning electron micrographs show representative fields from control (at left) and from sample treated with GS-9820 (at right), illustrating marked reduction in resorptive activity on blockade of PI3K. B and C, ivory was stained with toluidine blue, after which the number of pits and resorbed area were measured. The inhibitors wortmannin, GS-9820, and TGX221 caused a significant decrease of resorbed area (panel B) as well as pit density (panel C). Data are mean ± S.E., n = 3, independent experiments. Results were analyzed by one-way repeated measures ANOVA followed by a Bonferroni's multiple comparisons test. D, resorption pits were significantly more shallow following treatment with GS-9820 and TGX221. Data are maximum pit depths, mean ± S.E., 10 or more pits measured for each condition. * indicates p < 0.05 compared with vehicle.
DISCUSSION
In the present study, we characterized a novel PI3Kδ inhibitor and provided insights into the roles of PI3K isoforms in regulating osteoclast function. Findings include the potency and specificity of the PI3Kδ inhibitor, GS-9820, as well as the effects of isoform-selective PI3K inhibitors on 1) the viability of RAW264.7 cells, 2) osteoclast cytoskeletal organization, 3) osteoclast survival, and 4) resorptive activity. For the first time, we demonstrate that PI3Kδ plays an important role in regulating osteoclast morphology and actin cytoskeletal organization. In contrast, PI3Kα, PI3Kβ, and PI3Kδ isoforms all contribute to RANKL-enhanced survival, and both PI3Kβ and PI3Kδ activity are required for resorption.
We found evidence for all of the Class I PI3K isoforms in RAW264.7-derived osteoclasts, with the highest levels of mRNA encoding PI3Kβ and PI3Kγ compared with PI3Kα and PI3Kδ. These relative levels differ from a previous report of RAW264.7 cells, where mRNA for PI3Kα and PI3Kδ was more abundant than PI3Kβ (PI3Kγ not examined) (34). Expression of all four isoforms was confirmed with Western blotting. Moreover, we demonstrate that RANKL-induced differentiation into osteoclast-like cells was accompanied by a significant increase in PI3Kβ but decrease in PI3Kγ mRNA expression levels. Since osteoclasts are isolated with a number of other cell types, it is difficult to accurately determine the expression levels of PI3K isoforms in authentic osteoclasts.
Genetically modified mice, generated either by deletion of PI3Kδ or by mutation of the kinase domain, exhibit altered immune and inflammatory responses compared with their wild-type controls. For instance, studies of mice with a loss of function in the PI3Kδ p110 subunit exhibit defects in B- and T-cell signaling, including improper maturation, defective antigen receptor signaling, and impaired humoral immune responses (35), although no bone phenotype was noted. Recent studies of macrophages reveal that PI3Kδ is localized around the Golgi membrane and seems to be important in vesicle trafficking (14). Moreover, inhibition of PI3Kδ in neutrophils leads to defects in directed migration (25, 36). Whereas previous studies have shown that broad-spectrum PI3K blockers reduce osteoclast survival (37), the present study is the first to reveal a role specifically for PI3Kδ in osteoclast survival.
PI3K has been shown to participate in lamellipodia spreading of osteoclasts and other cell types (38). The results of our study show that blocking PI3Kδ pharmacologically causes retraction of osteoclast lamellipodia; at the same time, peripheral pseudopod ruffling is maintained. PI3K signaling might initiate osteoclast spreading through interactions with other signaling pathways, including Rho, Rac, and Cdc42. In keeping with this idea, RhoA and Rac have been shown to be regulated negatively and positively by PI3Kδ, respectively (15, 39). Consistent with our findings, others have shown acute morphological contraction in macrophages upon PI3Kδ inactivation observed in parallel with decreased Rac activity and increased Rho activity (15). Therefore, it would be of interest in future studies to examine the possible role of Rho and Rac in mediating the lamellipod retraction elicited by blocking PI3Kδ in osteoclasts.
Osteoclast retraction is also caused by the hormone calcitonin, and by the bioactive lipid mediators LPA and platelet-activating factor, all of which signal through GPCRs. Specifically, calcitonin-induced retraction of lamellipodia is sustained and osteoclasts remain quiescent (18); LPA-induced retraction of lamellipodia is sustained but peripheral pseudopod ruffling is maintained (19). Furthermore, platelet-activating factor-induced retraction of osteoclast lamellipodia is not sustained, resulting in re-spreading of lamellipodia (40). Interestingly, the morphological responses observed during inactivation of PI3Kδ are similar to LPA-induced retraction and distinct from the responses elicited by calcitonin and platelet-activating factor. Further studies are required to determine if there is interaction between LPA signaling and PI3Kδ. In the present study, we found that TGX221 induced partial retraction of osteoclasts. TGX221 is a potent and selective cell permeable inhibitor of PI3Kβ relative to all other PI3K isoforms, except PI3Kδ (IC50 values: p110α, 1000 nm; p110β, 9 nm; p110δ, 210 nm) (23). Likely, the concentration of TGX221 used in our study (1 μm) fully inhibits PI3Kβ and partially blocks PI3Kδ, accounting for its minor effect on retraction.
Activation of the αvβ3 integrin in osteoclasts initiates a signaling cascade that involves a c-Src, Pyk2, and c-Cbl complex and PI3K, resulting in actin polymerization and cytoskeletal reorganization (32). Since osteoclasts lacking β3 have an abnormal cytoskeleton, fail to spread, and do not have sealing zones in vitro (41), it is possible that αvβ3 integrins activate PI3Kδ to mediate effects on the cytoskeleton. Further study is needed to elucidate the possible association of PI3K isoforms and αvβ3 integrins.
Mature osteoclasts can exhibit two types of specialized actin structures, F-actin belts when adherent to non-mineralized substrata, and actin rings or sealing zones, when adherent to mineralized substrata. It is suggested that osteoclasts only degrade substrate within the area defined by specialized actin structures and, therefore, bone resorption by osteoclasts is dependent on the integrity of the actin cytoskeleton (1). The results from our study show that the prevalence of F-actin belts and sealing zones in osteoclasts is markedly lower when PI3Kδ is inhibited. Live cell imaging of osteoclasts showed gradual disruption of F-actin belts into puncta, along with breakdown of actin structures. This finding is in accordance with the observation that inhibition of PI3K disrupts ring-like F-actin structures in murine osteoclast-like cells (42). Our results indicate that PI3Kδ inhibition results in lamellipodial retraction as well as disruption of F-actin belts and sealing zones. However, as discussed previously, the exact mechanisms by which PI3K affects the actin cytoskeleton are currently unknown. Rho and Rac have an antagonistic relationship affecting cell spreading, and both have been implicated in regulating the formation of F-actin belts and sealing zones (43). F-actin belts and sealing zones have been shown to regulate contractility of osteoclasts in part through myosin II (44). Furthermore, compounds that affect osteoclast retraction also disrupt sealing zones and the ability of osteoclasts to form pits on dentin slices, such as the hormone calcitonin (1).
The lifespan of osteoclast precursors and mature osteoclasts, in conjunction with their recruitment, differentiation, activation, and motility all contribute to the control of bone resorption (2). Several ligands may activate PI3K and regulate osteoclast survival. For example, in previous studies, RANKL has been shown to activate the PI3K/Akt/mammalian target of rapamycin signaling pathway, thereby enhancing osteoclast survival (1). RANKL also activates NF-κB and nuclear factor of activated T cells c1 signaling, promoting osteoclastogenesis and enhancing cell survival (1). According to our results in osteoclasts, all class IA PI3K isoforms play a role in mediating RANKL-induced cell survival, but do not significantly affect basal levels of survival. Our results differ from those reported in leukocytes and fibroblasts, where activity of any class IA PI3K isoform can sustain survival (45), suggesting non-redundant roles for PI3K isoforms in controlling osteoclast survival.
Specific roles for PI3K isoforms are apparent when assessing osteoclastic resorption. Inhibition of PI3Kδ and -β isoforms significantly reduced resorption, whereas inhibitors of α and γ isoforms were without significant effect. Osteoclastic resorptive activity reflects the integration of multiple functions, including cell adhesion, cytoskeletal organization, and cell motility. The importance of orchestrating this range of activities may account for the finding that PI3Kβ inhibition did not cause significant disruption of actin belts, whereas it did inhibit resorption.
Overall, our findings provide evidence for diverse functions of different PI3K isoforms and show for the first time an important role for PI3Kδ in regulating osteoclast morphology and cytoskeletal function. Knowledge of the important roles of each class I PI3K family member in various physiological and pathophysiological processes may allow isoform-selective PI3K inhibitors to be used therapeutically. In particular, the PI3Kδ isoform, which has more limited tissue distribution than PI3Kα and PI3Kβ, is an attractive target for novel anti-resorptive therapeutics. Of relevance to these findings, recent studies reveal beneficial effects of PI3Kδ inhibition in lymphocytic leukemia (9, 10). Interestingly, inhibition of PI3Kδ in chronic lymphocytic leukemic cells suppresses cell survival and migration (10). Morover, inhibition of PI3Kδ reduces macrophage spreading and invasive capacity (46). Similar effects are apparent in osteoclasts, suggesting that selective inhibition of PI3K isoforms could offer new approaches for the treatment of inflammatory bone diseases and skeletal metastases.
Acknowledgments
We thank Tom Chrones and Elizabeth Pruski for expert technical assistance, Latesh Lad for assistance with PI3K biochemical assays, and Christophe Queva, Matt Grol and Roger Ulrich for helpful comments on the manuscript.
This work was supported in part by Canadian Institutes of Health Research Grant 64453 and Alberta Cancer Research Institute Grant 24428.

This article contains supplemental Figs. S1-S2 and Table S1.
- PI3K
- phosphatidylinositol 3-kinase
- ANOVA
- analysis of variance
- DAPI
- 4′,6-diamidino-2-phenylindole
- F-actin
- filamentous actin
- fMLP
- N-formyl-methionyl-leucyl-phenylalanine
- GS-9820
- (S)-2-(1-(9H-purin-6-ylamino)ethyl)-6-fluoro-3-phenylquinazolin-4(3H)-one
- LPA
- lysophosphatidic acid
- M199
- medium 199
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NFAT
- nuclear factor of activated T cells
- NF-κB
- nuclear factor κB
- RANKL
- receptor activator of nuclear factor κB ligand
- DMSO
- dimethyl sulfoxide
- EGFP
- enhanced green fluorescent protein
- GPCR
- G protein-coupled receptor.
REFERENCES
- 1. Novack D. V., Teitelbaum S. L. (2008) The osteoclast. Friend or foe? Annu. Rev. Pathol. 3, 457–484 [DOI] [PubMed] [Google Scholar]
- 2. Manolagas S. C. (2000) Birth and death of bone cells. Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137 [DOI] [PubMed] [Google Scholar]
- 3. Vivanco I., Sawyers C. L. (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 [DOI] [PubMed] [Google Scholar]
- 4. Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11, 329–341 [DOI] [PubMed] [Google Scholar]
- 5. Liu P., Cheng H., Roberts T. M., Zhao J. J. (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 8, 627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vanhaesebroeck B., Ali K., Bilancio A., Geering B., Foukas L. C. (2005) Signalling by PI3K isoforms. Insights from gene-targeted mice. Trends Biochem. Sci 30, 194–204 [DOI] [PubMed] [Google Scholar]
- 7. Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A. J., Okkenhaug K., Vanhaesebroeck B. (2008) The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc. Natl. Acad. Sci. U.S.A. 105, 8292–8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kok K., Geering B., Vanhaesebroeck B. (2009) Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem. Sci 34, 115–127 [DOI] [PubMed] [Google Scholar]
- 9. Lannutti B. J., Meadows S. A., Herman S. E., Kashishian A., Steiner B., Johnson A. J., Byrd J. C., Tyner J. W., Loriaux M. M., Deininger M., Druker B. J., Puri K. D., Ulrich R. G., Giese N. A. (2011) CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 117, 591–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoellenriegel J., Meadows S. A., Sivina M., Wierda W. G., Kantarjian H., Keating M. J., Giese N., O'Brien S., Yu A., Miller L. L., Lannutti B. J., Burger J. A. (2011) The phosphoinositide 3′-kinase δ inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 118, 3603–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golden L. H., Insogna K. L. (2004) The expanding role of PI3-kinase in bone. Bone 34, 3–12 [DOI] [PubMed] [Google Scholar]
- 12. Chellaiah M. A. (2006) Regulation of podosomes by integrin αvβ3 and Rho GTPase-facilitated phosphoinositide signaling. Eur. J. Cell Biol. 85, 311–317 [DOI] [PubMed] [Google Scholar]
- 13. Pilkington M. F., Sims S. M., Dixon S. J. (1998) Wortmannin inhibits spreading and chemotaxis of rat osteoclasts in vitro. J. Bone Miner Res. 13, 688–694 [DOI] [PubMed] [Google Scholar]
- 14. Low P. C., Misaki R., Schroder K., Stanley A. C., Sweet M. J., Teasdale R. D., Vanhaesebroeck B., Meunier F. A., Taguchi T., Stow J. L. (2010) Phosphoinositide 3-kinase δ regulates membrane fission of Golgi carriers for selective cytokine secretion. J. Cell Biol. 190, 1053–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Papakonstanti E. A., Zwaenepoel O., Bilancio A., Burns E., Nock G. E., Houseman B., Shokat K., Ridley A. J., Vanhaesebroeck B. (2008) Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. J. Cell Sci. 121, 4124–4133 [DOI] [PubMed] [Google Scholar]
- 16. Kang H., Chang W., Hurley M., Vignery A., Wu D. (2010) Important roles of PI3Kγ in osteoclastogenesis and bone homeostasis. Proc. Natl. Acad. Sci. U.S.A. 107, 12901–12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naemsch L. N., Dixon S. J., Sims S. M. (2001) Activity-dependent development of P2X7 current and Ca2+ entry in rabbit osteoclasts. J. Biol. Chem. 276, 39107–39114 [DOI] [PubMed] [Google Scholar]
- 18. Komarova S. V., Shum J. B., Paige L. A., Sims S. M., Dixon S. J. (2003) Regulation of osteoclasts by calcitonin and amphiphilic calcitonin conjugates. Role of cytosolic calcium. Calcif. Tissue Int. 73, 265–273 [DOI] [PubMed] [Google Scholar]
- 19. Lapierre D. M., Tanabe N., Pereverzev A., Spencer M., Shugg R. P., Dixon S. J., Sims S. M. (2010) Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival. J. Biol. Chem. 285, 25792–25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naemsch L. N., Weidema A. F., Sims S. M., Underhill T. M., Dixon S. J. (1999) P2X4 purinoceptors mediate an ATP-activated, non-selective cation current in rabbit osteoclasts. J. Cell Sci. 112, 4425–4435 [DOI] [PubMed] [Google Scholar]
- 21. Fabian M. A., Biggs W. H., 3rd, Treiber D. K., Atteridge C. E., Azimioara M. D., Benedetti M. G., Carter T. A., Ciceri P., Edeen P. T., Floyd M., Ford J. M., Galvin M., Gerlach J. L., Grotzfeld R. M., Herrgard S., Insko D. E., Insko M. A., Lai A. G., Lélias J. M., Mehta S. A., Milanov Z. V., Velasco A. M., Wodicka L. M., Patel H. K., Zarrinkar P. P., Lockhart D. J. (2005) A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 [DOI] [PubMed] [Google Scholar]
- 22. Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., Balla T., Weiss W. A., Williams R. L., Shokat K. M. (2006) A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 125, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson S. P., Schoenwaelder S. M., Goncalves I., Nesbitt W. S., Yap C. L., Wright C. E., Kenche V., Anderson K. E., Dopheide S. M., Yuan Y., Sturgeon S. A., Prabaharan H., Thompson P. E., Smith G. D., Shepherd P. R., Daniele N., Kulkarni S., Abbott B., Saylik D., Jones C., Lu L., Giuliano S., Hughan S. C., Angus J. A., Robertson A. D., Salem H. H. (2005) PI 3-kinase p110β. A new target for antithrombotic therapy. Nat. Med. 11, 507–514 [DOI] [PubMed] [Google Scholar]
- 24. Pomel V., Klicic J., Covini D., Church D. D., Shaw J. P., Roulin K., Burgat-Charvillon F., Valognes D., Camps M., Chabert C., Gillieron C., Françon B., Perrin D., Leroy D., Gretener D., Nichols A., Vitte P. A., Carboni S., Rommel C., Schwarz M. K., Rückle T. (2006) Furan-2-yl-methylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase γ. J. Med. Chem. 49, 3857–3871 [DOI] [PubMed] [Google Scholar]
- 25. Sadhu C., Masinovsky B., Dick K., Sowell C. G., Staunton D. E. (2003) Essential role of phosphoinositide 3-kinase δ in neutrophil directional movement. J. Immunol. 170, 2647–2654 [DOI] [PubMed] [Google Scholar]
- 26. Folkes A. J., Ahmadi K., Alderton W. K., Alix S., Baker S. J., Box G., Chuckowree I. S., Clarke P. A., Depledge P., Eccles S. A., Friedman L. S., Hayes A., Hancox T. C., Kugendradas A., Lensun L., Moore P., Olivero A. G., Pang J., Patel S., Pergl-Wilson G. H., Raynaud F. I., Robson A., Saghir N., Salphati L., Sohal S., Ultsch M. H., Valenti M., Wallweber H. J., Wan N. C., Wiesmann C., Workman P., Zhyvoloup A., Zvelebil M. J., Shuttleworth S. J. (2008) The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin −4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 51, 5522–5532 [DOI] [PubMed] [Google Scholar]
- 27. Marone R., Cmiljanovic V., Giese B., Wymann M. P. (2008) Targeting phosphoinositide 3-kinase. Moving towards therapy. Biochim. Biophys. Acta 1784, 159–185 [DOI] [PubMed] [Google Scholar]
- 28. Jia S., Liu Z., Zhang S., Liu P., Zhang L., Lee S. H., Zhang J., Signoretti S., Loda M., Roberts T. M., Zhao J. J. (2008) Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis. Nature 454, 776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ali K., Bilancio A., Thomas M., Pearce W., Gilfillan A. M., Tkaczyk C., Kuehn N., Gray A., Giddings J., Peskett E., Fox R., Bruce I., Walker C., Sawyer C., Okkenhaug K., Finan P., Vanhaesebroeck B. (2004) Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature 431, 1007–1011 [DOI] [PubMed] [Google Scholar]
- 30. Laffargue M., Calvez R., Finan P., Trifilieff A., Barbier M., Altruda F., Hirsch E., Wymann M. P. (2002) Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. Immunity 16, 441–451 [DOI] [PubMed] [Google Scholar]
- 31. Armstrong S., Pereverzev A., Dixon S. J., Sims S. M. (2009) Activation of P2X7 receptors causes isoform-specific translocation of protein kinase C in osteoclasts. J. Cell Sci. 122, 136–144 [DOI] [PubMed] [Google Scholar]
- 32. Saltel F., Chabadel A., Bonnelye E., Jurdic P. (2008) Actin cytoskeletal organisation in osteoclasts. A model to decipher transmigration and matrix degradation. Eur. J. Cell Biol. 87, 459–468 [DOI] [PubMed] [Google Scholar]
- 33. Chellaiah M. A., Soga N., Swanson S., McAllister S., Alvarez U., Wang D., Dowdy S. F., Hruska K. A. (2000) Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275, 11993–12002 [DOI] [PubMed] [Google Scholar]
- 34. Grey A., Chaussade C., Empson V., Lin J. M., Watson M., O'Sullivan S., Rewcastle G., Naot D., Cornish J., Shepherd P. (2010) Evidence for a role for the p110-α isoform of PI3K in skeletal function. Biochem. Biophys. Res. Commun. 391, 564–569 [DOI] [PubMed] [Google Scholar]
- 35. Ramadani F., Bolland D. J., Garcon F., Emery J. L., Vanhaesebroeck B., Corcoran A. E., Okkenhaug K. (2010) The PI3K isoforms p110α and p110δ are essential for pre-B cell receptor signaling and B cell development. Sci. Signal. 3, ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Afonso P. V., Parent C. A. (2011) PI3K and chemotaxis. A priming issue? Sci. Signal. 4, pe22. [DOI] [PubMed] [Google Scholar]
- 37. Adapala N. S., Barbe M. F., Langdon W. Y., Nakamura M. C., Tsygankov A. Y., Sanjay A. (2010) The loss of Cbl-phosphatidylinositol 3-kinase interaction perturbs RANKL-mediated signaling, inhibiting bone resorption and promoting osteoclast survival. J. Biol. Chem. 285, 36745–36758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palacio S., Felix R. (2001) The role of phosphoinositide 3-kinase in spreading osteoclasts induced by colony-stimulating factor-1. Eur. J. Endocrinol. 144, 431–440 [DOI] [PubMed] [Google Scholar]
- 39. Eickholt B. J., Ahmed A. I., Davies M., Papakonstanti E. A., Pearce W., Starkey M. L., Bilancio A., Need A. C., Smith A. J., Hall S. M., Hamers F. P., Giese K. P., Bradbury E. J., Vanhaesebroeck B. (2007) Control of axonal growth and regeneration of sensory neurons by the p110δ PI 3-kinase. PLoS One 2, e869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wood D. A., Hapak L. K., Sims S. M., Dixon S. J. (1991) Direct effects of platelet-activating factor on isolated rat osteoclasts. Rapid elevation of intracellular free calcium and transient retraction of pseudopods. J. Biol. Chem. 266, 15369–15376 [PubMed] [Google Scholar]
- 41. McHugh K. P., Hodivala-Dilke K., Zheng M. H., Namba N., Lam J., Novack D., Feng X., Ross F. P., Hynes R. O., Teitelbaum S. L. (2000) Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 105, 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lakkakorpi P. T., Wesolowski G., Zimolo Z., Rodan G. A., Rodan S. B. (1997) Phosphatidylinositol 3-kinase association with the osteoclast cytoskeleton, and its involvement in osteoclast attachment and spreading. Exp. Cell Res. 237, 296–306 [DOI] [PubMed] [Google Scholar]
- 43. Ory S., Brazier H., Pawlak G., Blangy A. (2008) Rho GTPases in osteoclasts. Orchestrators of podosome arrangement. Eur. J. Cell Biol. 87, 469–477 [DOI] [PubMed] [Google Scholar]
- 44. Chabadel A., Bañon-Rodríguez I., Cluet D., Rudkin B. B., Wehrle-Haller B., Genot E., Jurdic P., Anton I. M., Saltel F. (2007) CD44 and β3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol. Biol. Cell 18, 4899–4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foukas L. C., Berenjeno I. M., Gray A., Khwaja A., Vanhaesebroeck B. (2010) Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc. Natl. Acad. Sci. U.S.A. 107, 11381–11386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mouchemore K. A., Sampaio N. G., Murrey M. W., Stanley E. R., Lannutti B. J., Pixley F. J. (2013) Specific inhibition of PI3K p110δ inhibits CSF-1-induced macrophage spreading and invasive capacity. FEBS J. 280, 5228–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayakawa M., Kaizawa H., Kawaguchi K., Ishikawa N., Koizumi T., Ohishi T., Yamano M., Okada M., Ohta M., Tsukamoto S., Raynaud F. I., Waterfield M. D., Parker P., Workman P. (2007) Synthesis and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3 kinase p110α inhibitors. Bioorg. Med. Chem. 15, 403–412 [DOI] [PubMed] [Google Scholar]
- 48. Shuttleworth S. J., Silva F. A., Cecil A. R., Tomassi C. D., Hill T. J., Raynaud F. I., Clarke P. A., Workman P. (2011) Progress in the preclinical discovery and clinical development of class I and dual class I/IV phosphoinositide 3-kinase (PI3K) inhibitors. Curr. Med. Chem. 18, 2686–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]