Background: How Tau immunotherapy clears Tau is not well known.
Results: Tau antibody uptake correlates with Tau levels, is primarily via clathrin-dependent FcγII/III endocytosis, and is required for acute Tau clearance.
Conclusion: Following receptor-mediated uptake into neurons, antibodies co-localize with Tau aggregates and promote Tau clearance.
Significance: Results support intracellular clearance as a viable route for immunotherapy and have major implications for future development.
Keywords: Alzheimer Disease, Antibodies, Endocytosis, Immunotherapy, Tau
Abstract
Tau immunotherapy is effective in transgenic mice, but the mechanisms of Tau clearance are not well known. To this end, Tau antibody uptake was analyzed in brain slice cultures and primary neurons. Internalization was rapid (<1 h), saturable, and substantial compared with control mouse IgG. Furthermore, temperature reduction to 4 °C, an excess of unlabeled mouse IgG, or an excess of Tau antibodies reduced uptake in slices by 63, 41, and 62%, respectively (p = 0.002, 0.04, and 0.005). Uptake strongly correlated with total and insoluble Tau levels (r2 = 0.77 and 0.87 and p = 0.002 and 0.0002), suggesting that Tau aggregates influence antibody internalization and/or retention within neurons. Inhibiting phagocytosis did not reduce uptake in slices or neuronal cultures, indicating limited microglial involvement. In contrast, clathrin-specific inhibitors reduced uptake in neurons (≤78%, p < 0.0001) and slices (≤35%, p = 0.03), demonstrating receptor-mediated endocytosis as the primary uptake pathway. Fluid phase endocytosis accounted for the remainder of antibody uptake in primary neurons, based on co-staining with internalized dextran. The receptor-mediated uptake is to a large extent via low affinity FcγII/III receptors and can be blocked in slices (43%, p = 0.04) and neurons (53%, p = 0.008) with an antibody against these receptors. Importantly, antibody internalization appears to be necessary for Tau reduction in primary neurons. Overall, these findings clarify that Tau antibody uptake is primarily receptor-mediated, that these antibodies are mainly found in neurons with Tau aggregates, and that their intracellular interaction leads to clearance of Tau pathology, all of which have major implications for therapeutic development of this approach.
Introduction
A host of neurodegenerative diseases involve deposition of abnormal protein aggregates in the brain, including Alzheimer, Parkinson, Huntington, and prion diseases. In Alzheimer disease (AD),2 the defining lesions are amyloid-β plaques and neurofibrillary tangles composed of the microtubule-associated protein Tau. Active and passive immunotherapies are among the more attractive strategies for clearing these pathological proteins. Immunotherapy was first utilized in the context of AD to clear Aβ plaques and improve cognitive function in animal models (1–6). However, early clinical trials were suspended over concerns regarding encephalitis in a small percentage of participants (7). Although immunization was effective at reducing amyloid burden, there was no beneficial effect on synapses or clinical progression (8). Thus, plaque clearance alone was insufficient to alter the disease course. Following further refinement, additional clinical trials targeting various forms of Aβ are ongoing. Recent findings from phase III trials of bapineuzumab and solanezumab, antibodies recognizing amyloid-β, have been disappointing. No overall cognitive benefits were seen with either antibody, although subgroups showed significant albeit minimal slowing of memory deterioration in mild AD with solanezumab (9).
Besides Aβ, Tau lesions present an attractive target for disease-modifying interventions. Our group initially demonstrated the feasibility of targeting these aggregates by effectively utilizing active and passive Tau immunization in transgenic (Tg) models of tauopathy. Both approaches targeted the phosphoserine 396 and 404 region and consistently reduced pathological Tau and ameliorated related functional impairments (10–12). These findings have been confirmed and extended by other groups as well (13–18).
Recent results from our group have shown that Tau antibodies not only co-localize with intraneuronal pathological Tau but also with endosomal/lysosomal pathway markers (10, 19). Similar findings have been observed by others utilizing antibodies for α-synuclein and Aβ (20–22). However, the mechanism by which antibodies enter cells remains unknown. To clarify this issue, we studied the uptake of labeled Tau antibodies in slice cultures prepared from Tg and wild type mice, as well as in primary neurons prepared from Tg mice. The antibody used in these studies, 4E6G7, was developed by our group and has previously shown promising results in preliminary experiments in transgenic animals (23). Mechanism of uptake was assessed via temperature manipulation, blocking with excess IgG, anti-Tau, or anti-Fc antibodies, and pharmacological interventions. In addition, the effect of antibody treatment on Tau levels in primary neuronal cultures was assessed. Our findings indicate that uptake of Tau antibodies is an energetic process and to a large extent occurs through a clathrin-mediated endocytic pathway via FcγII/III receptors. Importantly, antibody internalization is necessary for acute clearance of Tau in neuronal cultures.
EXPERIMENTAL PROCEDURES
Materials
Radioactive iodine was purchased from PerkinElmer Life Sciences, IODO-BEADs were from Pierce, and iodination reagent and desalting columns were from Fisher. Chlorpromazine, cytochalasin D, filipin III, dansylcadaverine, Superblock, and SX1-4 scintillation liquid were also purchased from Fisher. Neurobasal A, minimum Eagle's medium, GlutaMAXTM, sodium pyruvate, glutamine, HBSS, HEPES, DNase, and B27 vitamins were purchased from Invitrogen. Anti-Fcγ II/III (CD16/CD32) and Fcγ I (both rat anti mouse) were obtained from BD Biosciences; mouse IgG1κ was from eBioscience (San Diego); PHF-1 (mouse monoclonal) antibody was a gift from Dr. Peter Davies. pan-Tau rabbit polyclonal antibody corresponding to residues 243–441 was purchased from Dako (Carpinteria, CA). Rabbit polyclonal anti-Tau Ser(P)199 antibody was purchased from Santa Cruz Biotechnology (Dallas). Complete protease inhibitor mixture tablets were purchased from Roche Applied Science.
Animals
Animals utilized for slice cultures in experiments with 125I-labeled antibodies were aged adults (15–18 months) from three different mouse lines. The hTau/PS1 (12) line was utilized for the majority of the experiments. These animals express all six isoforms of human Tau in the absence of murine Tau (24). In addition, they express human presenilin containing the M146L mutation (25). For experiments correlating pathology with antibody uptake, additional aged animals from the 3×Tg line (26) and their wild type littermates were used. For experiments with fluorescently labeled antibodies, adult animals (3–4 months) from the JNPL3 (27) line were used. These animals express human 0N4R Tau containing the P301L mutation. JNPL3 pups at postnatal day 0 were utilized for primary neuronal cultures. In all lines, animals of both sexes were enrolled. All animals were housed in accordance with IACUC regulations in AAALAC approved facilities, and given free access to food and water ad libitum.
4E6G7 Preparation
Monoclonal antibodies were generated by GenScript Inc. Wild type (WT) BALB/c mice were immunized with a peptide corresponding to the phospho-Ser396/404 region (cTDHGAEIVYK(pS)PVVSGDT(pS)PRHL). The peptide was conjugated to keyhole limpet hemocyanin via the cysteine residue, and mice showing a satisfactory immune response were used in hybridoma production. Monoclonal antibody 4E6G7 was selected and purified from the culture supernatant with a low endotoxin unit of the protein A affinity column. Further characterization of this antibody can be found in Gu et al. (42).
125I Labeling
Uptake studies utilized 4E6G7, an IgG1κ isotype monoclonal antibody developed by this laboratory; 4E6G7 was selected from a panel of antibodies made by subcontractor Genscript Inc. (Piscataway, NJ) against a phospho-epitope encompassing serine 396 and 404 as detailed above. This antibody selectively recognizes this region, primarily the phosphoserine 404, with lesser reactivity toward nonphospho-Tau. See Gu et al. (42) for a further characterization of this antibody. 4E6G7 and control IgG1κ were labeled with carrier-free Na125I using Pierce iodination beads and reagents according to the manufacturer's instructions. Specific activity was determined as 2.04 and 2.12 μCi/μg, respectively.
Fluorescent Labeling
4E6G7 was labeled using the Alexa Fluor 568 labeling kit from Invitrogen. Briefly, the antibody was incubated with reactive dye at room temperature for 1 h with stirring. The elution column was prepared as per the instructions, and the antibody dye mixture was added, followed by antibody collection and verification of labeling.
Slice Cultures
Slice cultures were prepared as described previously (28). Briefly, mice were killed via cervical dislocation, and their brains were removed. The brainstem and cerebellum were discarded, and the two hemispheres were separated. Each hemisphere was cut into 400-μm sections on a tissue chopper from Brinkmann Instruments. Slices were separated in ice-cold culture buffer (124 mm NaCl, 1.5 mm KCl, 0.62 mm KH2PO4, 4.01 mm MgSO4, 1.35 mm CaCl2, 1.74 mm NaHCO3, 5 mm glucose, 1 mm ascorbic acid, 0.02 mm ATP) and distributed among six wells. Slices were left for 30 min at room temperature to recover. Following recovery, slices were placed into a Beion BS3 brain slice chamber with oxygenated culture buffer.
Each apparatus contains six wells allowing each animal to be utilized for multiple conditions as well as serve as its own internal control. Because pathology is regional, slices are distributed among the wells so that each well contains a similar selection of brain regions.
Primary Neuronal Cultures
Neuronal cultures were prepared from JNPL3 pups at postnatal day 0. Briefly, plates were coated for 3 h with poly-l-lysine. Brains were harvested, and meninges and brainstem were removed. The remaining brain tissue was washed five times in HBSS+++ (975 ml Hanks' balanced salt solution, 10 ml of 1 m HEPES, 5 ml of penicillin/streptomycin, 10 ml of 100 mm sodium pyruvate) and then incubated with 200 μl of 0.5% trypsin for 15 min. Trypsin was neutralized with an equal volume of plating media (423.5 ml of minimum Eagle's medium, 15 ml of GlutaMAXTM (100×), 5 ml of 200 mm glutamine, 50 ml of FBS, 4 ml of B27, 2.5 ml of penicillin/streptomycin), and 100 μg of DNase was added to further dissociate the cells. Tissue was again washed five times with HBSS+++ and centrifuged for 1 min at 0.5 × g. Tissue was then resuspended in 1 ml of plating media and mechanically dissociated to form a cell suspension. Cells were centrifuged again, and the resuspended cells were evenly distributed among the wells. Plating medium was replaced with culture media (499 ml Neurobasal A, 1 ml of B27 supplement, 17 μl of basal medium Eagle) the following day. Cells were maintained in culture and allowed to develop processes prior to use in experiments.
Antibody Uptake in Primary Neurons and Brain Slices
Cells were prepared as described on glass coverslips. Fluorescently labeled 4E6G7 was added to the culture media, and cells were incubated at 37 °C for 1 h. Cells were then washed and fixed for 10 min. Coverslips were washed again and mounted on slides using Dako Cytomation fluorescent mounting media. Images were collected on a Nikon Eclipse Ti confocal microscope. Additional cells were incubated with fluorescently labeled dextran as well as 4E6G7 for 1 h at 37 °C.
Brain slices were incubated at 37 °C in the presence of fluorescently labeled 4E6G7 for 2 h. Slices were then fixed, sectioned, and stained with anti-Tau antibodies. Sections were mounted using Dako Cytomation fluorescent mounting media. Images were collected using a Zeiss 700 confocal microscope.
Staining of Primary Neurons and Brain Slices
Cells were cultured as described, washed, and fixed for 10 min. Cells were permeabilized with 0.3% Tween in PBS for an additional 10 min. Coverslips were incubated with primary antibody at room temperature for 2 h. Following primary antibody, coverslips were washed three times and then incubated with secondary antibodies for 1 h. Coverslips were washed an additional three times and then mounted on slides.
Sections from acute brain slice cultures were also utilized for staining. Sections were fixed and permeabilized with 0.3% Tween in PBS. Slices were incubated in primary antibodies overnight and secondary antibodies for 1 h at room temperature.
Dose-response and Time Course Experiments
Brain sections prepared as described above from hTau/PS1 mice (n = 3, age 15–18 months) were incubated with increasing concentrations of 125I -labeled 4E6G7 antibody. Concentrations of antibody in culture buffer ranged from 0.01 to 5 μg/ml. Each brain was sectioned at 400 μm, and sections were divided evenly between the treatment groups.
Sections were maintained in oxygenated buffer at 37 °C. At 30, 60, and 120 min, sections were removed, weighed, and rinsed with acidified culture buffer, pH 5. Sections were washed a further three times in ice-cold culture buffer to remove surface-bound antibodies. Following rinsing, sections were placed in plastic vials with 5 ml of scintillation liquid, and radioactivity was measured on a Beckman LS 6500 liquid scintillation counter. Counts/min values were converted to nCi and corrected for differing tissue mass. Average and S.E. were determined from the results, plotted against the concentration of antibody in buffer, and fit to a linear curve.
To determine whether uptake was saturable, an additional group of brain slices from hTau/PS1 animals were incubated with increasing concentrations of 125I-labeled 4E6G7 with an additional 10 μg/ml dose added. For this experiment, all samples were collected after 60 min of incubation. Samples were prepared, and radioactivity was determined as described above. A saturation curve was also prepared using slices from wild type animals. All samples were collected after 60 min of incubation with up to 10 μg/ml 125I-labeled 4E6G7.
Further dose-response experiments were carried out utilizing WT animals and mice from the 3×Tg line (n = 3 per group, age 15–18 months). Brain sections were prepared as described and incubated for 1 h at 37 °C with increasing concentrations of 125I-labeled 4E6G7 (0.01–5 μg). Sections were washed, and radioactivity was measured. As before, values were converted and plotted against the concentration of antibody in the incubation buffer.
With fluorescently labeled 4E6G7, antibody incubation and washing were carried out using sections from JNPL3 mice (n = 3 per group, age 3–4 months) exactly as with radiolabeled antibodies. Following washing, slices were homogenized and centrifuged for 15 min, and the values were corrected for total protein concentration in the samples. A standard curve was generated by adding antibodies to brain homogenate with a known protein concentration.
Temperature Sensitivity
Prior to experiments, the slice chamber was placed at 4 °C for 2 h. Sections from hTau/PS1 mice (n = 3, age 15–18 months) were prepared as described and incubated at 4 °C for 1 h in culture buffer containing 0.5 μg/ml of 4E6G7 antibody. Following treatment, slices were weighed and washed, and radioactivity was measured. Results were compared with those obtained from 37 °C control sections. As before, fluorescence experiments were carried out using the same procedure. For each group, averages and S.E. were determined. Two-tailed t test was used to determine significance.
Effects of Excess IgG and 4E6G7
In addition to temperature, the effects of an excess of mouse IgG on uptake were examined. For each animal (n = 3 hTau/PS1 mice, age 15–18 months), the brain sections were separated into three groups, control without antibody, 0.5 μg/ml 4E6G7, and 0.5 μg/ml 4E6G7 plus an excess of unlabeled mouse IgG1κ (either 5 or 50 μg/ml). Samples were collected and processed as described above. The effect of excess 4E6G7 was tested using the same procedure. Samples were incubated with 0.5 μg/ml 125I-labeled 4E6G7 alone or 125I-labeled 4E6G7 in the presence of 10 or 100× excess unlabeled 4E6G7. Averages and S.E. were determined for each group. Fluorescence experiments were done with an additional group of JNPL3 mice (n = 3, age 3–4 months). As with temperature experiments, a two-tailed t test was used to determine significant differences between groups.
Endocytosis Inhibitors
Slice cultures were prepared from hTau/PS1 mice as described above. To understand the contribution of different types of endocytosis, three different inhibitors were utilized, filipin III (caveolin), chlorpromazine (clathrin), and cytochalasin D (phagocytosis). As with other experiments, slices were incubated with 0.5 μg/ml 4E6G7 for 1 h at 37 °C. Increasing concentrations of inhibitors were added to each sample, and radioactivity was determined. The same protocol was utilized to assess the effect of the drugs on primary neuronal cultures; with the exception that chlorpromazine was replaced with dansylcadaverine due to solubility issues in the cell culture media. Cells were washed in acidified HBSS+++, pH 5, and neutral HBSS+++ and lysed with RIPA. As with slices, final radioactivity was corrected for protein concentration. For both slices and neurons, a one-way ANOVA followed by Tukey's HSD was utilized to determine significance.
Fc Block
To determine the contribution of Fc receptors to antibody uptake, slices were prepared as described above (hTau/PS1 mice, n = 3, age 15–18 months) and incubated at 37 °C for 1 h with 0.5 μg/ml 4E6G7. Increasing concentrations of anti-CD16/CD32 Fc receptor blocking antibody (Fc block anti-CD16/CD32 (BD Biosciences) 0.05–50 μg/ml) were added to the culture buffer. Following incubation, slices were treated as described, and average radioactivity per treatment group was determined. Additional slices were incubated with increasing concentrations of an antibody against FcγI receptor (anti-FcγRI, 0.05–50 μg/ml).
Fc block was also added to primary neuronal cultures prepared from JNPL3 pups (postnatal day 0, n = 6 wells per group). Cells were incubated with 0.5 μg/ml 4E6G7 and increasing concentrations (0.5–10 μg/ml) of Fc block for 1 h at 37 °C. Cells were washed following treatment and processed as described. A second group of neurons was treated with anti-FcγRI (0.5–10 μg/ml) under the same conditions. Final radioactivity for all samples was obtained as described and normalized for total protein concentration. Significance in both slices and neurons was determined using a one-way ANOVA followed by Tukey's honestly significant difference.
Effects of 4E6G7 on Tau
Primary neurons were prepared as described above to determine the effect of 4E6G7 on intraneuronal Tau levels. Cells were incubated for 24 or 72 h in the presence of 4E6G7 alone (1, 10, or 20 μg/ml, n = 6 wells per group) or 4E6G7 and 1 μg/ml dansylcadaverine. After treatment, cells were collected and processed for immunoblotting. Immunoblots for total Tau levels, Tau phosphorylated at Ser199, and actin were carried out. Data were analyzed using a two-way ANOVA and Bonferroni post hoc test.
Immunoblotting
Brain sections not used in uptake experiments and primary neurons were retained for immunoblotting. Each sample was homogenized in modified RIPA buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1 mm NaF, 1 mm Na3VO4, 1 μg/ml complete protease inhibitor mixture (Roche Applied Science)) and subjected to a low speed spin (22 × g) to remove the membrane fraction. The supernatant was retained, and volumes were adjusted for total protein concentration. Samples were diluted in O+ buffer (62.5 mm Tris-HCl, pH 6.8, 5% glycerol, 2-mercaptoethanol, 2.3% SDS, 1 mm EGTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 1 μg/ml Roche Applied Science complete protease inhibitor mixture), boiled for 5 min, and loaded onto a 12% polyacrylamide gel. Gels were transferred at 100 V for 1 h and blots incubated overnight in appropriate dilutions of primary antibody. Blots were washed and incubated with a peroxidase-labeled mouse secondary antibody for 1 h followed by further washing.
Blots were developed using a Fuji LAS-4000 and signal quantified with Multigauge. Chemiluminescent signal was measured and, when appropriate, plotted against radioactivity and fit to a linear curve.
RESULTS
4E6G7 Recognizes Pathological Tau in Tissue
In sections from 3×Tg and WT animals aged 22 months, 4E6G7 shows strong neuronal staining in the hippocampus of 3×Tg mice (Fig. 1A). PHF-1 also produced robust staining (Fig. 1B) in transgenic animals, although the pattern is less punctate. However, 4E6G7 did not stain tissue from WT mice of the same strain's background (Fig. 1C). This region has prominent Tau and Aβ lesions in this model. See Gu et al. (42) for additional characterization of this antibody.
FIGURE 1.
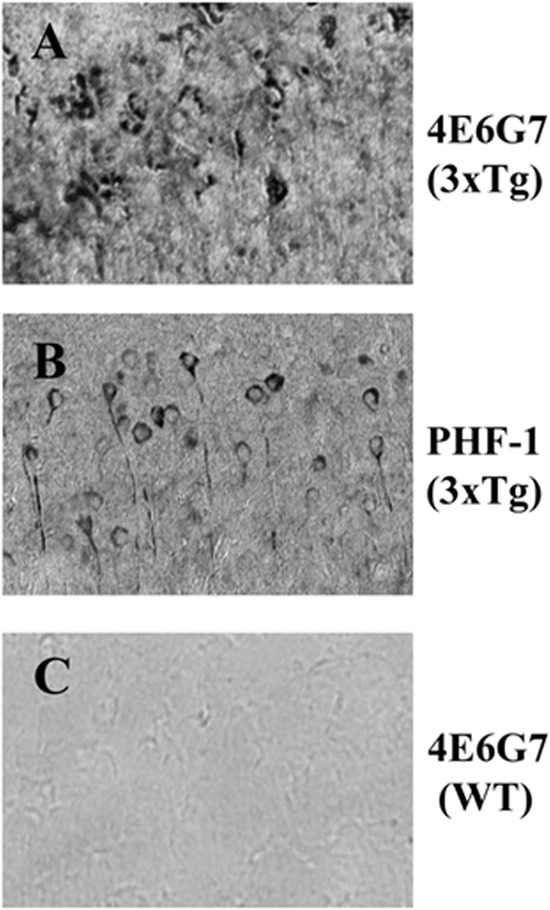
4E6G7 stains pathological Tau in sections from transgenic animals. A, brains from 3×Tg mice (aged 22 months) were sectioned and incubated with a 1:2000 dilution of purified 4E6G7. Biotinylated mouse secondary and 3,3′-diaminobenzidine were used to visualize the antibody. Representative images from coronal sections of the subiculum show extensive staining of pathological Tau in the neuronal cell bodies. B, adjacent sections from the same animal stained with PHF-1 show neuronal Tau aggregates as well. The pattern of staining with PHF-1 is less punctate than that obtained with 4E6G7. C, age-matched wild type animal from the same line was also incubated with 4E6G7 and showed no reactivity. All images were collected at ×100 magnification.
Uptake Is Rapid and Saturable
Slice cultures from hTau/PS1 transgenic mice (n = 3, 15–18 months) were incubated at 37 °C with increasing concentrations (0–10 μg/ml) of 125I-labeled Tau antibody 4E6G7 raised against the Ser(P)396/404 region of the Tau protein. Individual sections were removed at 30, 60, and 120 min. Final intracellular radioactivity at all end points was linear up to antibody concentrations of 5 μg/ml at 1 h of incubation (Fig. 2A), with saturation occurring between 5 and 10 μg/ml (Fig. 2B). Similar values were obtained when utilizing fluorescent antibodies with JNPL3 slices, n = 3, age 3–4 months, with uptake reaching saturation between 5 and 10 μg/ml (Fig. 2C). This pattern appears fairly robust across mouse lines, as similar results were obtained in wild type animals using 125I-labeled 4E6G7 at 1 h of incubation (Fig. 2D). In addition, uptake was rapid and remained steady at each time point. On the basis of these results, other experiments were carried out using a 0.5 μg/ml antibody concentration and an incubation time of 1 h. In control experiments, we have found that slice cultures prepared from wild type animals show reactivity to PHF-1 on Western blots. It appears that the degree of PHF-1 reactivity is increased due to the process by which the slice cultures are made. When compared with sections allowed to recover at room temperature, PHF-1 chemiluminescent signal in flash-frozen hemispheres was reduced by 28% (Fig. 3).
FIGURE 2.
4E6G7 uptake is rapid and saturable. Brain sections prepared from hTau/PS1 mice (n = 3, 15–18 months) were incubated with increasing concentrations of 125I-4E6G7 antibody (Ab). Concentrations of antibody in buffer ranged from 0.01 to 5 μg/ml of buffer. A, sections were removed and assayed for radioactivity at 30, 60, and 120 min. Similar values were obtained for each time point indicating that uptake is rapid, occurring within 30 min of incubation. All points represent an average ± S.E. B, as above, brain slices from hTau/PS1 animals (15–18 months) were incubated with increasing concentrations of 125I-4E6G7. In this experiment, an additional 10 μg/ml dose was added, and all samples were collected after 60 min of incubation. Uptake of the antibody appears linear to 5 μg/ml, but it saturates at higher concentrations. C, similar results are obtained utilizing fluorescently labeled 4E6G7 with slice cultures prepared from JNPL3 mice (n = 3, 3–4 months). D, slices from wild type animals (n = 4, 12–17 months) were incubated with 125I-4E6G7 at increasing concentrations for 60 min. Similar to both transgenic lines, uptake saturated between 5 and 10 μg/ml.
FIGURE 3.

Slice cultures are enriched in phospho-Tau relative to flash-frozen tissue. Tissue from wild type animals (n = 3) was collected to assess the effect of slice culture preparation method on the levels of phosphorylated Tau. Control hemispheres (C) were prepared as normal. The other hemisphere was flash-frozen on dry ice (F). A, immunoblots of both hemispheres were probed with antibodies against total Tau (Dako, rabbit polyclonal) and PHF-1. B, flash freezing did not significantly change total Tau levels relative to control hemispheres. C, PHF-1 signal was reduced in flash-frozen hemispheres by 26% (p = 0.006). D, controlling for total Tau levels, chemiluminescent signal in flash-frozen tissue was 28% lower than for slices allowed to incubate at room temperature (p = 0.01). All bars represent averages ± S.E. **, p ≤ 0.01.
To validate the system, we performed several control experiments. Slices that had been incubated with labeled 4E6G7 were further sectioned at 30 μm. Each section was added to 5 ml of scintillation fluid, and radioactivity was measured. There was no significant difference in radioactivity between sections taken at different levels (data not shown) indicating homogeneous distribution of antibody in tissue. Additionally, we compared sections with unsectioned hippocampus to determine the contribution of broken cell membranes to final radioactivity. There was a 30% decrease in radioactivity in unsectioned tissue, indicating that at least a portion of antibody uptake is via damaged membranes.
Uptake Is Reduced at Low Temperatures
To ascertain the effect of temperature on antibody uptake, the slice chamber was chilled for 2 h at 4 °C prior to the addition of brain slices. Slices were prepared as described above and incubated at 4 °C for 1 h with 0.5 μg/ml 125I-labeled 4E6G7. Samples were collected, weighed, and rinsed, and levels of radioactivity were determined. Lowered temperatures significantly reduced the uptake of antibody from 0.171 ± 0.6 to 0.031 ± 0.006 nCi/mg (p = 0.002; 63% decrease relative to 37 °C control), suggesting that antibodies are internalized mainly via an energy-dependent process. The same pattern of uptake was observed in JNPL3 slices incubated with fluorescently labeled 4E6G7 with 4 °C sections showing a 35% reduction in signal 0.048 ± 0.009 to 0.031 ± 0.004 μg of antibody/mg tissue (p = 0.05).
Excess IgG and Unlabeled 4E6G7 Partially Block Antibody Uptake
In addition to temperature, co-incubation with an excess of mouse IgG1κ also reduced uptake of 4E6G7. Slices from each animal were separated into three groups as follows: control 0.5 μg/ml 4E6G7 and 0.5 μg/ml 4E6G7 plus IgG1κ. The presence of a 10× excess of mouse IgG1κ resulted in a 41% decrease in radioactivity, a significant reduction relative to antibody alone (p = 0.04, Fig. 4A). A second group of slices was prepared the same way and incubated with 100× excess IgG1κ. Again, radioactivity was significantly reduced relative to 0.5 μg/ml 4E6G7 alone (40% decrease, p = 0.02, Fig. 4A), but the additional IgG1κ did not lead to further inhibition of uptake (Fig. 4A). Excess IgG also resulted in a 29% decrease in uptake when using fluorescently labeled antibodies (p = 0.05; Fig. 4B). Additional slices were incubated with 0.5 μg/ml 125I-labeled 4E6G7 and either 5 or 50 μg/ml nonradiolabeled 4E6G7. Addition of a 10- and 100-fold excess of 4E6G7 reduced labeled antibody uptake by 35 and 62%, respectively (p = 0.01 and 0.005; Fig. 4C). Both the mouse IgG and unlabeled 4E6G7 compete with 125I-4E6G7 for binding to the receptors on the cell surface. However, the apparently greater effect of 4E6G7 at the 100× dose (62% versus 40% reduction in radioactivity) may be related to its effect on retention. Unlike the mouse IgG, the unlabeled 4E6G7 can compete with 125I-4E6G7 for binding with intracellular Tau. Thus, the excess of 4E6G7 may affect both uptake and retention of the labeled antibody. In control experiments, we found that as much as 30% of the final radioactivity was the result of antibody uptake through damaged cells. Thus, this may mask the true extent of blockage and prevent complete blockage of uptake.
FIGURE 4.

Excess unlabeled IgG or 4E6G7 reduces uptake of labeled 4E6G7. A, slices were prepared as described from hTau/PS1 mice. Brain sections were separated into three groups as follows: control (0.5 μg/ml 125I-4E6G7 alone) or 0.5 μg/ml 4E6G7 plus excess unlabeled mouse IgG1 (either 5 or 50 μg/ml). Averages and S.E. were determined for each treatment group. Addition of 10× excess IgG to the culture media significantly reduced the levels of radioactivity in the tissue (41% decrease, p = 0.04). Incubation with 100× IgG also produced a significant decrease relative to control (40% decrease, p = 0.02), but it was not significantly reduced relative to 10× treated slices. B, similar results were obtained using sections from JNPL3 mice and fluorescently labeled 4E6G7 (29% decrease, p = 0.05). C, slices were prepared as described and incubated with 0.05 μg/ml 125I-4E6G7 and either 5 or 50 μg/ml unlabeled 4E6G7. Significant reductions were seen in final radioactivity at both doses (35 and 61%, p = 0.01 and 0.005, respectively). Unlike IgG samples, 50 μg/ml of unlabeled antibody significantly reduced radioactivity relative to the 5 μg/ml concentration (p = 0.001). Bars represent average ± S.E. *, p ≤ 0.05; **, p ≤ 0.01.
Pathology Correlates with Antibody Uptake
Slice cultures were created from animals of three different mouse lines (hTau/PS1, 3×Tg, and wild type (WT), n = 3 animals per group, age 15–18 months) with differing levels of Tau. Brain slices from each animal were incubated at 37 °C for 1 h with increasing concentrations of 125I-labeled 4E6G7 antibody. Average radioactivity was measured and nCi/mg of tissue determined. In addition, brain sections from each animal were washed three times and retained for Tau immunoblotting.
Animals from the hTau/PS1 line showed significantly higher levels of total Tau and stronger PHF-1 immunoreactive bands in the Sarkosyl-insoluble fraction relative to both 3×Tg and WT animals (Fig. 5, A–D). Both transgenic lines (hTau/PS1 and 3×Tg) expressing Tau had significantly higher levels of radioactivity relative to WT mice at 1 μg/ml (49 and 34%, respectively; p = 0.002 and 0.04) and 5 μg/ml 4E6G7 (86 and 30% respectively; p = 0.008 and 0.02). Furthermore, at the 5 μg/ml dose, animals from the hTau/PS1 line had significantly higher levels of uptake than the 3×Tg line (43% increase, p = 0.02; Fig. 5E).
FIGURE 5.
4E6G7 uptake correlates with pathological Tau levels. A and B, immunoblots using a pan-Tau antibody (Dako) and PHF-1 were carried out on the total and Sarkosyl-insoluble (aggregated) fractions, respectively, from three different mouse lines (n = 3 mice per group, age 15–18 months). C, total Tau levels from the low speed supernatant were quantified for each of the different mouse lines used. Bars represent average ± S.E. D, Sarkosyl-insoluble fraction was isolated, and chemiluminescence was determined. Values were corrected for background and the average values obtained. Bars represent average ± S.E. E, slices prepared from the same hTau/PS1, 3×Tg, and WT mice were incubated for 1 h in varying concentrations of 125I-4E6G7 (n = 3 mice per group). Average radioactivity per mg of tissue was determined as described above, and values were fit to a linear curve (squares, hTau/PS1; circles, 3×Tg; triangles, WT). All points represent averages ± S.E. F, chemiluminescent signal was plotted against radioactivity for each sample (total n = 9). A linear regression was fit to the data yielding r2 of 0.77 (p = 0.02). G, chemiluminescent signal from the Sarkosyl-insoluble fraction was plotted against final radioactivity for each animal (total n = 9) and fit to a linear regression. An r2 of 0.87 (p = 0.002) was obtained indicating that levels of pathological Tau and antibody uptake are highly correlated. H, additional sections from hTau/PS1 and WT mice were incubated with 125I-IgG1κ. Samples were processed as described. IgG uptake was greater in slice cultures made from hTau/PS1 relative to WT, but both were substantially less than 4E6G7 uptake.
Within the identified mouse groups, levels of both total Tau and Sarkosyl-insoluble Tau correlated highly with final radioactivity (r2 0.77 and 0.87, p = 0.002 and 0.0002, respectively; Fig. 5, F and G) for pooled samples. These results indicate that higher levels of intracellular Tau and the degree of phosphorylation at Ser396/404 may promote greater uptake or retention of the 4E6G7 antibody in the tissue. As stated above, the method used to make the slices produces an increase (28%) in Tau phosphorylated at Ser396/404 in WT animals (Fig. 3). This may account for the higher than expected degree of 4E6G7 uptake/retention in the WT mice.
IgG1κ, the same isotype as 4E6G7, was also labeled with 125I and incubated with sections from hTau/PS1 and WT animals as described. As with 4E6G7, hTau/PS1 animals had higher levels of final radioactivity at 0.5, 1, and 5 μg/ml IgG concentrations (71, 73, and 48% increase, p = 0.003, 0.025, and 0.020, respectively, relative to WT mice). In both cases, however, the uptake was greatly reduced relative to 4E6G7-treated hTau/PS1 animals at 1 and 5 μg/ml IgG concentrations (84 and 91% decrease, p = 0.008 and 0.00007) (Fig. 5H). The difference in the overall levels of IgG1κ uptake relative to 4E6G7 may be explained by retention of the antibody in the tissue. 4E6G7 will be bound to Tau and thus less subject to recycling out of the cell than the unbound IgG. Additionally, excess IgG may be degraded more rapidly by the lysosomal system.
Antibody Uptake Occurs Mainly via Clathrin-mediated Endocytosis
Endocytosis inhibitors were utilized to further clarify the mechanism of antibody uptake. Brain slices from hTau/PS1 mice were incubated with varying concentrations of either chlorpromazine, filipin III, or cytochalasin D. Phagocytosis inhibitor cytochalasin D did not significantly alter antibody uptake (Fig. 6A). Filipin III did not affect final radioactivity at any of the concentrations used, although the 50 μg/ml dose produced a trend toward reduced uptake (p = 0.07), indicating caveolin-mediated endocytosis may be involved but is not a major route of uptake (Fig. 6A). Chlorpromazine produced a significant reduction in antibody uptake (p = 0.03), and at the 20, 50, and 100 μg/ml doses, the final radioactivity was reduced to 85, 66, and 66% of control values, respectively (Fig. 6A). These results indicate that uptake is occurring to a large extent via clathrin-dependent endocytosis.
FIGURE 6.
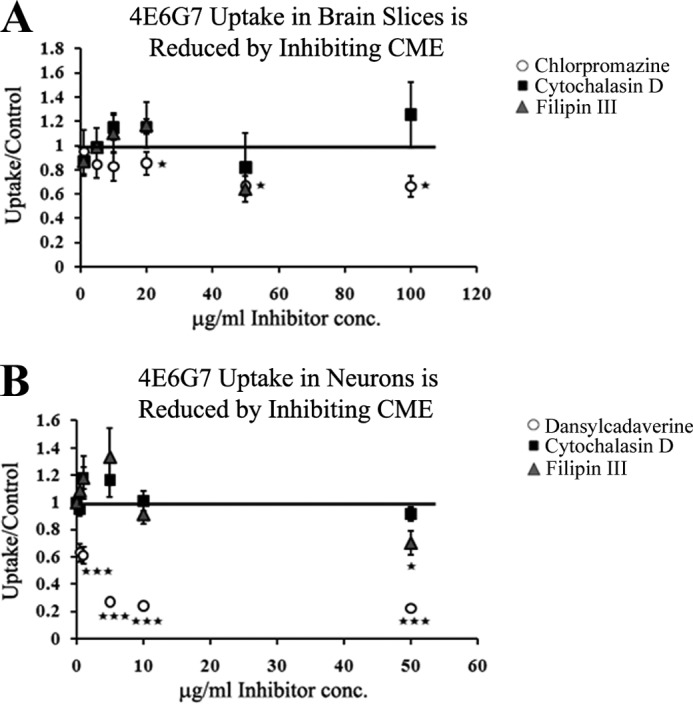
Specific inhibitors of clathrin-mediated endocytosis (CME) reduce 4E6G7 uptake. A, sections from hTau/PS1 mice (n = 3 per group) were incubated for 1 h with 0.5 μg/ml 125I-4E6G7 and increasing concentrations of endocytosis inhibitors. No change in uptake was observed with filipin III (caveolin-specific). Incubation with chlorpromazine (clathrin-specific) significantly reduced final radioactivity at high concentrations (10, 50, and 100 μg/ml) (15–34% reduction, p = 0.03). Phagocytosis inhibitor cytochalasin D did not alter uptake. B, primary neurons (n = 6 wells per group) were cultured from JNPL3 mice and incubated with 125I-4E6G7 and increasing concentrations (0.5–50 μg/ml) of endocytosis inhibitors. Clathrin-specific inhibitor dansylcadaverine significantly inhibited uptake at every dose (36–78% reduction, p = 0.00006–0.000005). Filipin III produced a 30% reduction (p = 0.04) at the highest dose, the only one to significantly alter uptake. Cytochalasin D did not affect radioactivity at any dose. All bars and markers represent average ± S.E. *, p ≤ 0.05; ***, p ≤ 0.001.
Primary neuronal cultures prepared from JNPL3 mice were also treated with the endocytosis inhibitors utilized above with chlorpromazine replaced by dansylcadaverine as the former was not completely soluble in cell culture media. In each case, the cells were incubated with inhibitors for 1 h at 37 °C in culture media containing 0.5 μg/ml 125I-labeled 4E6G7 and increasing concentrations (0.5–50 μg/ml) of drug (Fig. 6B). Addition of dansylcadaverine produced significant reductions in final radioactivity at every dose used (p = 0.00006–0.000005) and up to 78% reduction in signal relative to control. In contrast, filipin III produced a significant decrease in radioactivity at only the highest dose (p = 0.04, 30% reduction). Cytochalasin D had no significant effect. These data indicate that the majority of antibody uptake in neurons occurs via clathrin-mediated endocytosis, but that a smaller fraction is internalized by caveolin-dependent pathways. Other mechanisms may contribute fractionally to antibody uptake. Data are less clear in the slice cultures, where multiple cells types are present and drugs may not have fully penetrated the tissue. Longer incubation times with the inhibitors, or higher concentrations, may be necessary to observe the full effect in that model.
To ensure that observed uptake is receptor-mediated, as hypothesized, rather than fluid phase endocytosis, JNPL3 primary neurons were incubated with both 4E6G7 and fluorescently labeled dextran for 1 h at 37 °C. Images were collected and merged (Fig. 7, A–C). The numbers of red, green, and co-localized puncta were determined (∼1700 puncta counted in total). Only 17 ± 1.2% of the total puncta counted were positive for both dextran and 4E6G7. Of those puncta positive for 4E6G7, 27 ± 1.4% were yellow, indicating that about one-fourth of antibody uptake occurs via fluid phase endocytosis (Fig. 8D).
FIGURE 7.

Fluid phase endocytosis is not the major route of uptake. A–C, primary neuronal cultures from postnatal day 0 JNPL3 mice were incubated for 1 h with both fluorescently labeled dextran (488 nm) and 4E6G7 (568 nm). Coverslips were fixed and mounted, and images were collected using a Nikon Eclipse Ti confocal microscope. D, number of puncta positive for dextran, 4E6G7, or both were counted (∼1700 puncta). Only 17 ± 1.2% of all the puncta were positive for both. Of those positive for 4E6G7 (red or yellow) 27 ± 1.4% were co-localized.
FIGURE 8.

Primary neuronal cultures and brain slices take up 4E6G7 and stain positive for Fcγ II/III receptors. A, representative images of cells positive for Fcγ II/III receptors. Receptors are found throughout the cells. B, primary neurons stained with Dako rabbit polyclonal pan-Tau antibody showing strong staining in neuronal cultures. C, merged image. D, representative images of sections from JNPL3 mice stained for Fcγ II/III showing similar results to those seen in neuronal cultures. E, pan-Tau antibody staining showing cell body and neuropil staining. Tau aggregates are also visible on the inner face of the plasma membrane in neurons. F, merged image. G, fluorescently labeled 4E6G7 uptake in primary neurons. 4E6G7 is seen as bright puncta within the neuron. H, pan-Tau antibody showing strong staining in cultured neurons similar to B. I, merged image. J, fluorescently labeled 4E6G7 uptake in brain slice cultures showing a similar pattern of 4E6G7 distribution to that in G. K, pan-Tau antibody staining in brain slices showing results similar to E. L, merged image.
Incubation with FcγRII/III Antibody, but Not FcγRI, Reduces 4E6G7 Uptake
Primary neurons cultured from JNPL3 mice were stained with Dako pan-Tau polyclonal antibody and FcγII/III antibody (Fig. 8, A–C). Images show that the receptors are distributed throughout the cells. Additionally, sections from JNPL3 mice were also stained with total Tau and FcγII/III antibodies showing a similar pattern to that seen in the primary neurons (Fig. 8, D–F). To visualize antibody uptake, additional cultured neurons were incubated with fluorescently labeled 4E6G7 for 1 h. Images show that the majority of the 4E6G7 antibody is visible as bright puncta bound to Tau within the neurons (Fig. 8, G–I). Similar results were seen in sections from JNPL3 mice incubated with fluorescently labeled 4E6G7 (Fig. 8, J–L).
To ascertain the contribution of Fc receptors, slices from adult (15–18 months) hTau/PS1 animals (n = 3) were incubated with 0.5 μg/ml 4E6G7 for 1 h at 37 °C and increasing concentrations of FcγII/III receptor block anti-CD16/CD32 monoclonal antibody (Fc block). Relative to control, incubation with 0.05 and 0.5 μg/ml Fc block resulted in a trend toward reduced uptake (28 and 36% decrease) (p = 0.11 and 0.06, respectively). Significantly reduced uptake was achieved with 5 and 50 μg/ml concentrations of Fc block with a 41 and 43% reduction in final radioactivity (p = 0.05 and 0.04; Fig. 9A). Incubation with FcγRI (CD64) antibody, however, did not produce a significant change in final radioactivity at any of the concentrations used. There was a trend toward reduced uptake at the 50 μg/ml dose (p = 0.1) indicating that there may be some minor involvement of microglial uptake.
FIGURE 9.

Blocking Fcγ II/III receptors reduces uptake. A, slices from hTau/PS1 (n = 3, 15–18 months) were incubated with 0.5 μg/ml 125I-4E6G7 and increasing concentrations of Fc block (anti-CD16/CD32, FcγII/III). Fc block at 0.05 and 0.5 μg/ml produced a trend toward reduced radioactivity. Both 5 and 50 μg/ml concentrations of Fc block resulted in significantly reduced uptake (41 and 43% reduction, p = 0.05 and 0.04, respectively). The same protocol was used with an Fcγ I antibody (anti-CD64) with no significant reduction in final radioactivity. B, primary neurons (n = 6 wells per group) from JNPL3 pups were also incubated with Fc block and 125I-4E6G7. The 1, 5, and 10 μg/ml antibody concentrations significantly reduced uptake (19, 43, and 53%, respectively, p = 0.01, 0.006, and 0.008). As with slice cultures, incubation with Fcγ I antibodies did not significantly affect uptake at any dose. Bars and markers represent average ± S.E. *, p ≤ 0.05; **, p ≤ 0.01.
As with the slices, a significant decrease in 4E6G7 uptake was observed in primary JNPL3 neurons at the 0.5, 5, and 10 μg/ml (n = 6 wells per group) doses of Fc block (19, 43, and 53% decreases, respectively, p = 0.01, 0.006, and 0.008) (Fig. 9B), whereas anti-FcγRI (0.05–10 μg/ml) did not affect uptake at any dose (Fig. 9B).
Incubation with 4E6G7 Reduces Intracellular Tau, and This Effect Can Be Blocked with Dansylcadaverine
To determine whether antibody entry into neurons is a prerequisite for Tau clearance, primary neurons cultured from P0 JNPL3 pups were incubated with 1, 10, or 20 μg/ml 4E6G7 with or without 1 μg/ml dansylcadaverine to block antibody uptake (n = 6 wells per group). The presence of intracellular antibodies was visible by immunoblot using HRP-conjugated mouse secondary antibody alone. Uptake was linear up to 20 μg/ml for samples incubated for 24 h (data not shown). In contrast, at 72 h, the difference between values observed for 10 and 20 μg/ml was reduced indicating that uptake may be saturating (Fig. 10A). Similar to findings with radiolabeled antibody, incubation with 1 μg/ml dansylcadaverine significantly reduced uptake by 24% (p = 0.008; Fig. 10B). To assess Tau clearance, immunoblots for total Tau and Ser199-phosphorylated Tau were performed. None of the treatments significantly reduced actin levels. In addition, a lactate dehydrogenase assay was performed on media taken from cultured cells treated with 4E6G7 alone or 4E6G7 and 1 μg/ml dansylcadaverine for up to 7 days, again with no significant changes. These data suggest that at short time points antibody-related cell death is minimal. No change in Tau signal was observed in any of the treatment groups at 24 h (data not shown). However, at 72 h, 4E6G7 decreased the total Tau/actin ratio, and this effect was blocked by dansylcadaverine (two-way ANOVA dose, p < 0.0001; treatment, p < 0.0001; interaction, p < 0.0001). Post hoc analysis revealed that all doses of 4E6G7 (1, 10, and 20 μg/ml) reduced total Tau/actin (49, 61, and 58%, respectively, p < 0.001) (Fig. 11, A and C). In contrast, samples incubated with both antibody and dansylcadaverine showed no change in total Tau/actin ratio (Fig. 11, B and C). These data show that neuronal uptake of 4E6G7 is a prerequisite for Tau clearance within these primary neurons.
FIGURE 10.

4E6G7 uptake into cells can be visualized by immunoblot and is inhibited by dansylcadaverine. Primary JNPL3 neurons (n = 6 wells per group) were incubated with 4E6G7 at three different concentrations of 1, 10, or 20 μg/ml. Samples were collected and processed for immunoblotting as described. Blots were incubated with mouse secondary alone, and chemiluminescence signal was determined for each. A, antibody uptake is dose-dependent with antibody accumulating in the cells with increased dose. B, samples incubated with 10 μg/ml 4E6G7 in the presence or absence of dansylcadaverine (DC) were compared to assess the effects of blocking clathrin-mediated endocytosis on antibody uptake. Uptake was reduced by 24% with dansylcadaverine present (p = 0.008; all samples run on the same gel). Bars represent average ± S.E. **, p < 0.01.
FIGURE 11.
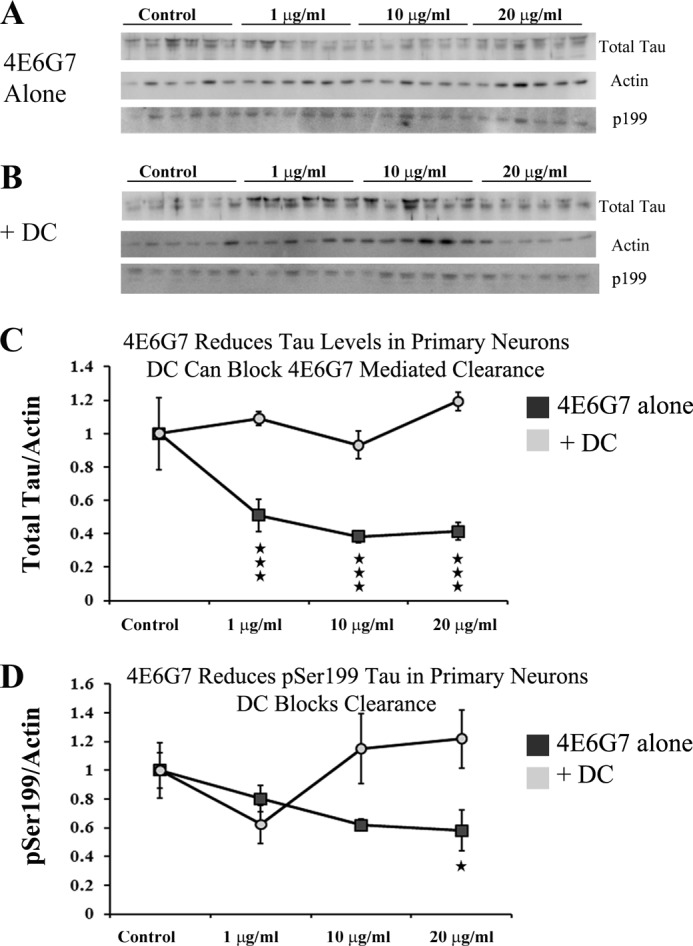
4E6G7 reduces intracellular Tau, and blocking antibody uptake eliminates its effectiveness. Primary JNPL3 neurons (n = 6 wells per group) were incubated with 1, 10, or 20 μg/ml 4E6G7 for 72 h in the presence or absence of 1 μg/ml dansylcadaverine (DC). A and B, immunoblots against total Tau, Ser(P)199, and actin were carried out, and two-way ANOVAs were performed. C, analysis with two-way ANOVA revealed significant effects of dose, presence of dansylcadaverine, as well as interaction effects (p < 0.0001 for all). 4E6G7 alone produced significant reductions in intracellular Tau levels relative to actin levels at all of the doses used (49, 61, and 58%, respectively, p < 0.001). Addition of 1 μg/ml dansylcadaverine blocked Tau reduction. D, two-way ANOVA testing of Ser199 data revealed significant effects of dansylcadaverine and interaction effects (p = 0.03). Phospho-Tau levels were significantly reduced at 20 μg/ml 4E6G7 (p < 0.05). All points represent average ± S.E. *, p ≤ 0.05; ***, p ≤ 0.001.
To assess if phosphorylated Tau was preferentially targeted for clearance, additional immunoblots were performed with a polyclonal antibody recognizing Tau phosphorylated at Ser199. Previously, we have shown that treatment with antibodies can reduce levels of phospho-Tau, including epitopes other than the one targeted (11). A similar pattern emerged as for total Tau, albeit less pronounced (two-way ANOVA treatment, p = 0.03; interaction, p = 0.03). Post hoc analysis showed significant 42% decrease in Ser(P)199/actin at the highest dose of 4E6G7 (p < 0.05), and this effect was blocked by dansylcadaverine (Fig. 11D). Further immunoblotting was performed with antibodies recognizing Tau phosphorylated at Ser396, and Tau phosphorylated at Ser404. However, signal was not detectable indicating that the presence of 4E6G7 may be masking the epitope. These data suggest that although incubation with 4E6G7 reduces Tau levels, it is not specifically targeting phosphorylated Tau for clearance at least in primary tauopathy neurons.
DISCUSSION
Our results show that neuronal uptake of an anti-Tau IgG antibody, 4E6G7, is required for Tau clearance. It is dose-dependent, saturable, and significantly reduced by temperature reduction and excess unlabeled IgG or 4E6G7. Furthermore, internalization strongly correlates with levels of pathological Tau species. Because effects of temperature on endocytosis are well established (29–31), our results indicated that uptake was likely an energy-dependent process. Treatment with clathrin-mediated endocytosis inhibitors, chlorpromazine and dansylcadaverine, significantly reduced uptake in slice cultures and primary neurons. Inhibition of phagocytosis had no effect, indicating limited microglial uptake. We further clarified the receptor type involved by determining that uptake was partially blocked by co-incubation with an Fcγ receptor antibody (CD16/CD32), corresponding to FcγII/III, in brain slices and primary neurons but not with anti-FcγI. These results are consistent with previous findings indicating that FcγII/III receptor-mediated endocytosis occurs via the clathrin-dependent pathway (32–34). No treatment completely blocked uptake, however. This may be due to the nature of the slice cultures; sectioning brains causes disruption of some cell membranes. Other mechanisms may also be involved such as fluid phase endocytosis, although our data indicate that this represents a minority of uptake. Similar results were seen in endothelial cells transfected with neonatal Fc receptors (FcRn), in which fluorescently labeled Fc was taken up via FcRn and found in compartments containing receptors and not co-localized with dextran (35).
Interestingly, there was a higher degree of uptake in WT tissue than expected. In our prior study, antibodies were only detected in brains of Tg tauopathy mice and not WT animals when injected into the carotid artery (10). However, this is likely due to an intact blood brain barrier in WT mice preventing antibodies from crossing into the brain, which is removed in slice cultures. Additionally, slices are left to recover at room temperature, which has been shown to increase Tau phosphorylation in animals (36), and in our experiments this results in a 28% increase in PHF-1 signal by immunoblot. Thus, phospho-Tau levels in slice cultures are higher than in the brains of WT animals. Furthermore, 4E6G7 binds to nonphosphorylated Tau as well, at least on ELISA plates.
Involvement of FcRs in antibody uptake and protein clearance has been examined utilizing Aβ antibodies in AD models with conflicting reports. Early reports demonstrated that Aβ plaques and activated microglia in AD patients were immunoreactive for antibodies against Fcγ I, II, and III (37) with similar results also seen in Parkinson disease (38, 39). Deane et al. (41) indicated that FcR knock-out animals showed minimal clearance following administration of Aβ antibodies relative to FcR-positive animals, and additional data suggest that FcR binding is important for amyloid clearance (40). In contrast, other researchers found that in amyloid precursor protein transgenic animals, knocking out FcγR did not affect antibody clearance of plaques relative to animals expressing FcγR (1). It could be argued that microglial uptake may be important in the experiments described in this work. For example, antibody uptake highly correlates with the degree of Tau lesions. Such pathology may result in greater activation of microglia, which may phagocytose the antibody. Microglial activation may then be a factor in increased 4E6G7 uptake. However, blocking phagocytosis did not significantly affect uptake in either model system utilized, indicating minimal involvement of microglia, which should be abundant in slices and present to some extent in primary cultures. Indeed, we observe much less microglial activation in various tauopathy mouse models than in Aβ plaque models because most Tau lesions are intracellular. Additionally, in brain slice cultures, the majority of fluorescently labeled 4E6G7 was found in neurons, with some uptake in microglia but none in other glial cell types (42). Furthermore, data from primary neuronal cultures showed that uptake is primarily occurring via receptor-mediated clathrin-dependent endocytosis and to a large extent by FCγII/III receptors.
In addition to expression on glial cells, neurons also express Fcγ receptors. Earlier findings indicated the presence of FcγI receptors on sensory neurons but not II or III (43). However, more recent studies have found expression of FcγIIb on Purkinje cells and parvalbumin neurons and expression of I, II, III, and IV in primary neuronal cultures (44–46). Additionally, neurons are responsive to extracellular IgG by up-regulating FcγR (44). These results, and data showing that antibodies against pathological proteins are taken up by neurons, suggest a mechanism by which the FcR contributes to protein clearance in immunotherapy (10, 19–22). Our results show that anti-Tau antibody uptake can be blocked to a large extent using FcγII/III antibodies suggesting that low affinity Fc receptors are the major route into neurons.
Of particular note is the finding that, in cultured neurons, partial blockage of receptor-mediated endocytosis with dansylcadaverine is sufficient to prevent Tau clearance. Importantly, this indicates that antibody-mediated Tau clearance takes place primarily within neurons. Previous data from our laboratory showed that antibodies were present in neurons of treated animals and that the treatment resulted in clearance of phospho-Tau but not total Tau (10), which we subsequently confirmed in other studies (11, 12, 19). Together, these prior studies suggest involvement of intracellular Tau antibodies in Tau clearance, and we now show in the primary Tg JNPL3 neuronal model that this pathway is necessary for removal of Tau. The main difference between those prior animal studies and this experiment is an observed Tau antibody-mediated decrease in total Tau in culture and not in animals. This discrepancy may be explained by numerous differences in these models and study design, including antibodies and their concentration per tissue weight, which is likely much greater in the culture. Our findings that blocking Tau antibody uptake prevented Tau clearance indicate that the intracellular clearance pathway is the primary one, at least in this model and under relatively acute conditions. Additionally, this treatment does not appear toxic at the concentrations used. Lactate dehydrogenase assays revealed no change in neurons treated with 4E6G7 alone or 4E6G7 and dansylcadaverine relative to control cells at up to 7 days of incubation. Actin levels were also not significantly altered at any of the conditions used.
The involvement of FcR, coupled with our data showing uptake-dependent intracellular Tau clearance, also suggests a pathway for improving efficacy of immunotherapy. In Aβ-targeting experiments, antibodies with isotypes having higher affinity for phagocytic FcRs led to better clearance (40). Indeed, affinity for Fc receptors was more important for clearance than affinity for Aβ. This suggests selection of antibodies could be improved by focusing on specific isotypes or modifications that would improve receptor binding. For example, mutations in antibody Fc region are capable of increasing affinity for neonatal FcRs (47), whereas other mutations can shift the ratio of FcγRIIa/FcγRIIb affinity leading activation of macrophages (48). Thus, it is possible that antibodies containing mutations that enhance binding to the desired receptor may provide greater efficacy in clearing protein aggregates. It is uncertain how or if such mutations would affect neuronal uptake, but further studies are clearly warranted.
Besides intracellular aggregates, extracellular Tau may play an important role in disease progression. Recent findings have demonstrated that Tau pathology can spread across synapses and that pathological Tau can be taken up by cells (17, 49–58). Cultured cells, even healthy ones, have also been found to secrete phosphorylated Tau (48), and extracellular Tau increases intracellular calcium in cultured neurons (59). This presents an additional mechanism by which treatment with antibodies might alter disease progression. Antibodies may prevent secretion by targeting intracellular Tau into protein degradation pathways. Extracellularly, antibodies may bind to Tau and promote its uptake into microglia or neurons for clearance and thereby prevent spread of Tau pathology to neighboring neurons.
Previously our lab has shown that Tau antibodies clear Tau aggregates and improve cognition and other Tau-related deficits in Tauopathy mouse models. Also, these antibodies not only cross the blood brain barrier but enter neurons. Herein, we have identified the mechanism of this neuronal uptake showing it to be primarily receptor-mediated via FcγII/III receptors, to correlate very well with the degree of Tau pathology, and to be a prerequisite for Tau clearance. These important findings identify a neuronal pathway that may be activated in response to intraneuronal Tau aggregation and that can be manipulated to improve the efficacy of immunotherapy.
Acknowledgments
Drs. Frank LaFerla and Salvatore Oddo provided the breeding pair of 3×Tg mice, and Dr. Peter Davies supplied the PHF-1 antibody. Dr. Allal Boutajangout was responsible for initiating the hTau/PS1 mouse line. New York University technology on Tau immunotherapy is licensed to and is being co-developed with H. Lundbeck A/S.
This work was supported, in whole or in part, by National Institutes of Health Grants NS077239, AG032611, and AG020197. E. M. Sigurdsson is an inventor on NYU patents on Tau immunotherapy licensed to H. Lundbeck A/S.
- AD
- Alzheimer disease
- Aβ
- amyloid-β
- ANOVA
- analysis of variance
- Tg
- transgenic
- hTau
- human Tau.
REFERENCES
- 1. Das P., Howard V., Loosbrock N., Dickson D., Murphy M. P., Golde T. E. (2003) Amyloid-β immunization effectively reduces amyloid deposition in FcRγ−/− knock-out mice. J. Neurosci. 23, 8532–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janus C., Pearson J., McLaurin J., Mathews P. M., Jiang Y., Schmidt S. D., Chishti M. A., Horne P., Heslin D., French J., Mount H. T., Nixon R. A., Mercken M., Bergeron C., Fraser P. E., St George-Hyslop P., Westaway D. (2000) Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer disease. Nature 408, 979–982 [DOI] [PubMed] [Google Scholar]
- 3. Lemere C. A., Maron R., Spooner E. T., Grenfell T. J., Mori C., Desai R., Hancock W. W., Weiner H. L., Selkoe D. J. (2000) Nasal Aβ treatment induces anti-Aβ antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann. N.Y. Acad. Sci. 920, 328–331 [DOI] [PubMed] [Google Scholar]
- 4. Morgan D., Diamond D. M., Gottschall P. E., Ugen K. E., Dickey C., Hardy J., Duff K., Jantzen P., DiCarlo G., Wilcock D., Connor K., Hatcher J., Hope C., Gordon M., Arendash G. W. (2000) Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer disease. Nature 408, 982–985 [DOI] [PubMed] [Google Scholar]
- 5. Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Liao Z., Lieberburg I., Motter R., Mutter L., Soriano F., Shopp G., Vasquez N., Vandevert C., Walker S., Wogulis M., Yednock T., Games D., Seubert P. (1999) Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 [DOI] [PubMed] [Google Scholar]
- 6. Sigurdsson E. M., Scholtzova H., Mehta P. D., Frangione B., Wisniewski T. (2001) Immunization with a nontoxic/nonfibrillar amyloid-β homologous peptide reduces Alzheimer disease-associated pathology in transgenic mice. Am. J. Pathol. 159, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schenk D. (2002) Amyloid-β immunotherapy for Alzheimer disease: the end of the beginning. Nat. Rev. Neurosci. 3, 824–828 [DOI] [PubMed] [Google Scholar]
- 8. Boche D., Denham N., Holmes C., Nicoll J. A. (2010) Neuropathology after active Aβ42 immunotherapy: implications for Alzheimer disease pathogenesis. Acta Neuropathol. 120, 369–384 [DOI] [PubMed] [Google Scholar]
- 9. Callaway E. (2012) Alzheimer drugs take a new tack. Nature 489, 13–14 [DOI] [PubMed] [Google Scholar]
- 10. Asuni A. A., Boutajangout A., Quartermain D., Sigurdsson E. M. (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 27, 9115–9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boutajangout A., Ingadottir J., Davies P., Sigurdsson E. M. (2011) Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem. 118, 658–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boutajangout A., Quartermain D., Sigurdsson E. M. (2010) Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J. Neurosci. 30, 16559–16566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bi M., Ittner A., Ke Y. D., Götz J., Ittner L. M. (2011) Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One 6, e26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boimel M., Grigoriadis N., Lourbopoulos A., Haber E., Abramsky O., Rosenmann H. (2010) Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol. 224, 472–485 [DOI] [PubMed] [Google Scholar]
- 15. Chai X., Wu S., Murray T. K., Kinley R., Cella C. V., Sims H., Buckner N., Hanmer J., Davies P., O'Neill M. J., Hutton M. L., Citron M. (2011) Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J. Biol. Chem. 286, 34457–34467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. d'Abramo C., Acker C. M., Jimenez H. T., Davies P. (2013) Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS One 8, e62402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kfoury N., Holmes B. B., Jiang H., Holtzman D. M., Diamond M. I. (2012) Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 287, 19440–19451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Troquier L., Caillierez R., Burnouf S., Fernandez-Gomez F. J., Grosjean M. E., Zommer N., Sergeant N., Schraen-Maschke S., Blum D., Buee L. (2012) Targeting phospho-Ser422 by active Tau immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr. Alzheimer Res. 9, 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krishnamurthy P. K., Deng Y., Sigurdsson E. M. (2011) Mechanistic studies of antibody-mediated clearance of Tau aggregates using an ex vivo brain slice model. Front. Psychiatry 2, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masliah E., Rockenstein E., Adame A., Alford M., Crews L., Hashimoto M., Seubert P., Lee M., Goldstein J., Chilcote T., Games D., Schenk D. (2005) Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron 46, 857–868 [DOI] [PubMed] [Google Scholar]
- 21. Masliah E., Rockenstein E., Mante M., Crews L., Spencer B., Adame A., Patrick C., Trejo M., Ubhi K., Rohn T. T., Mueller-Steiner S., Seubert P., Barbour R., McConlogue L., Buttini M., Games D., Schenk D. (2011) Passive immunization reduces behavioral and neuropathological deficits in an α-synuclein transgenic model of Lewy body disease. PLoS One 6, e19338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Tampellini D., Magrané J., Takahashi R. H., Li F., Lin M. T., Almeida C. G., Gouras G. K. (2007) Internalized antibodies to the Aβ domain of APP reduce neuronal Aβ and protect against synaptic alterations. J. Biol. Chem. 282, 18895–18906 [DOI] [PubMed] [Google Scholar]
- 23. Boutajangout A., Sait H. B., Gonzalez V., Sigurdsson E. M. (2011) Targeting hyperphosphorylated tau protein with a monoclonal antibody at an advanced stage of tau pathology improves cognition in a mouse model. Alzheimer Dement. 7, S480–S481 [Google Scholar]
- 24. Andorfer C., Kress Y., Espinoza M., de Silva R., Tucker K. L., Barde Y. A., Duff K., Davies P. (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 86, 582–590 [DOI] [PubMed] [Google Scholar]
- 25. Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Perez-tur J., Hutton M., Buee L., Harigaya Y., Yager D., Morgan D., Gordon M. N., Holcomb L., Refolo L., Zenk B., Hardy J., Younkin S. (1996) Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature 383, 710–713 [DOI] [PubMed] [Google Scholar]
- 26. Oddo S., Caccamo A., Shepherd J. D., Murphy M. P., Golde T. E., Kayed R., Metherate R., Mattson M. P., Akbari Y., LaFerla F. M. (2003) Triple-transgenic model of Alzheimer disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 [DOI] [PubMed] [Google Scholar]
- 27. Lewis J., McGowan E., Rockwood J., Melrose H., Nacharaju P., Van Slegtenhorst M., Gwinn-Hardy K., Paul Murphy M., Baker M., Yu X., Duff K., Hardy J., Corral A., Lin W. L., Yen S. H., Dickson D. W., Davies P., Hutton M. (2000) Neurofibrillary tangles, amyotrophy, and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25, 402–405 [DOI] [PubMed] [Google Scholar]
- 28. Gong C. X., Lidsky T., Wegiel J., Grundke-Iqbal I., Iqbal K. (2001) Metabolically active rat brain slices as a model to study the regulation of protein phosphorylation in mammalian brain. Brain Res. Brain Res. Protoc. 6, 134–140 [DOI] [PubMed] [Google Scholar]
- 29. Punnonen E. L., Ryhänen K., Marjomäki V. S. (1998) At reduced temperature, endocytic membrane traffic is blocked in multivesicular carrier endosomes in rat cardiac myocytes. Eur. J. Cell Biol. 75, 344–352 [DOI] [PubMed] [Google Scholar]
- 30. Silverstein S. C., Steinman R. M., Cohn Z. A. (1977) Endocytosis. Annu. Rev. Biochem. 46, 669–722 [DOI] [PubMed] [Google Scholar]
- 31. Weigel P. H., Oka J. A. (1981) Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J. Biol. Chem. 256, 2615–2617 [PubMed] [Google Scholar]
- 32. Mero P., Zhang C. Y., Huang Z. Y., Kim M. K., Schreiber A. D., Grinstein S., Booth J. W. (2006) Phosphorylation-independent ubiquitylation and endocytosis of FcγRIIA. J. Biol. Chem. 281, 33242–33249 [DOI] [PubMed] [Google Scholar]
- 33. Mousavi S. A., Sporstøl M., Fladeby C., Kjeken R., Barois N., Berg T. (2007) Receptor-mediated endocytosis of immune complexes in rat liver sinusoidal endothelial cells is mediated by FcγRIIb2. Hepatology 46, 871–884 [DOI] [PubMed] [Google Scholar]
- 34. Tse S. M., Furuya W., Gold E., Schreiber A. D., Sandvig K., Inman R. D., Grinstein S. (2003) Differential role of actin, clathrin, and dynamin in Fcγ receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 278, 3331–3338 [DOI] [PubMed] [Google Scholar]
- 35. Goebl N. A., Babbey C. M., Datta-Mannan A., Witcher D. R., Wroblewski V. J., Dunn K. W. (2008) Neonatal Fc receptor mediates internalization of Fc in transfected human endothelial cells. Mol. Biol. Cell 19, 5490–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Planel E., Richter K. E., Nolan C. E., Finley J. E., Liu L., Wen Y., Krishnamurthy P., Herman M., Wang L., Schachter J. B., Nelson R. B., Lau L. F., Duff K. E. (2007) Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 27, 3090–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peress N. S., Fleit H. B., Perillo E., Kuljis R., Pezzullo C. (1993) Identification of Fcγ RI, II and III on normal human brain ramified microglia and on microglia in senile plaques in Alzheimer disease. J. Neuroimmunol. 48, 71–79 [DOI] [PubMed] [Google Scholar]
- 38. Cao S., Theodore S., Standaert D. G. (2010) Fcγ receptors are required for NF-κB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson's disease. Mol. Neurodegener. 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orr C. F., Rowe D. B., Mizuno Y., Mori H., Halliday G. M. (2005) A possible role for humoral immunity in the pathogenesis of Parkinson's disease. Brain 128, 2665–2674 [DOI] [PubMed] [Google Scholar]
- 40. Bard F., Barbour R., Cannon C., Carretto R., Fox M., Games D., Guido T., Hoenow K., Hu K., Johnson-Wood K., Khan K., Kholodenko D., Lee C., Lee M., Motter R., Nguyen M., Reed A., Schenk D., Tang P., Vasquez N., Seubert P., Yednock T. (2003) Epitope and isotype specificities of antibodies to β-amyloid peptide for protection against Alzheimer disease-like neuropathology. Proc. Natl. Acad. Sci. U.S.A. 100, 2023–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deane R., Sagare A., Hamm K., Parisi M., LaRue B., Guo H., Wu Z., Holtzman D. M., Zlokovic B. V. (2005) IgG-assisted age-dependent clearance of Alzheimer amyloid β peptide by the blood-brain barrier neonatal Fc receptor. J. Neurosci. 25, 11495–11503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gu J., Congdon E. E., Sigurdsson E. M. (2013) Two novel tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce tau pathology. J. Biol. Chem. 288, 33081–33095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andoh T., Kuraishi Y. (2004) Direct action of immunoglobulin G on primary sensory neurons through Fcγ receptor I. FASEB J. 18, 182–184 [DOI] [PubMed] [Google Scholar]
- 44. Fernandez-Vizarra P., Lopez-Franco O., Mallavia B., Higuera-Matas A., Lopez-Parra V., Ortiz-Muñoz G., Ambrosio E., Egido J., Almeida O. F., Gomez-Guerrero C. (2012) Immunoglobulin G Fc receptor deficiency prevents Alzheimer-like pathology and cognitive impairment in mice. Brain 135, 2826–2837 [DOI] [PubMed] [Google Scholar]
- 45. Suemitsu S., Watanabe M., Yokobayashi E., Usui S., Ishikawa T., Matsumoto Y., Yamada N., Okamoto M., Kuroda S. (2010) Fcγ receptors contribute to pyramidal cell death in the mouse hippocampus following local kainic acid injection. Neuroscience 166, 819–831 [DOI] [PubMed] [Google Scholar]
- 46. van der Kleij H., Charles N., Karimi K., Mao Y. K., Foster J., Janssen L., Chang Yang P., Kunze W., Rivera J., Bienenstock J. (2010) Evidence for neuronal expression of functional Fc (ϵ and γ) receptors. J. Allergy Clin. Immunol. 125, 757–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaccaro C., Zhou J., Ober R. J., Ward E. S. (2005) Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat. Biotechnol. 23, 1283–1288 [DOI] [PubMed] [Google Scholar]
- 48. Richards J. O., Karki S., Lazar G. A., Chen H., Dang W., Desjarlais J. R. (2008) Optimization of antibody binding to FcγRIIa enhances macrophage phagocytosis of tumor cells. Mol. Cancer Ther. 7, 2517–2527 [DOI] [PubMed] [Google Scholar]
- 49. Chai X., Dage J. L., Citron M. (2012) Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol. Dis. 48, 356–366 [DOI] [PubMed] [Google Scholar]
- 50. Clavaguera F., Bolmont T., Crowther R. A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A. K., Beibel M., Staufenbiel M., Jucker M., Goedert M., Tolnay M. (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clavaguera F., Goedert M., Tolnay M. (2010) Induction and spreading of tau pathology in a mouse model of Alzheimer disease. Med. Sci. 26, 121–124 [DOI] [PubMed] [Google Scholar]
- 52. de Calignon A., Polydoro M., Suárez-Calvet M., William C., Adamowicz D. H., Kopeikina K. J., Pitstick R., Sahara N., Ashe K. H., Carlson G. A., Spires-Jones T. L., Hyman B. T. (2012) Propagation of tau pathology in a model of early Alzheimer disease. Neuron 73, 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frost B., Diamond M. I. (2010) Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 11, 155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frost B., Jacks R. L., Diamond M. I. (2009) Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284, 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim W., Lee S., Jung C., Ahmed A., Lee G., Hall G. F. (2010) Interneuronal transfer of human tau between Lamprey central neurons in situ. J. Alzheimers Dis. 19, 647–664 [DOI] [PubMed] [Google Scholar]
- 56. Liu L., Drouet V., Wu J. W., Witter M. P., Small S. A., Clelland C., Duff K. (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7, e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., Jackson B., McKee A. C., Alvarez V. E., Lee N. C., Hall G. F. (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soto C. (2012) In vivo spreading of tau pathology. Neuron 73, 621–623 [DOI] [PubMed] [Google Scholar]
- 59. Gómez-Ramos A., Díaz-Hernández M., Rubio A., Miras-Portugal M. T., Avila J. (2008) Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol. Cell. Neurosci. 37, 673–681 [DOI] [PubMed] [Google Scholar]




