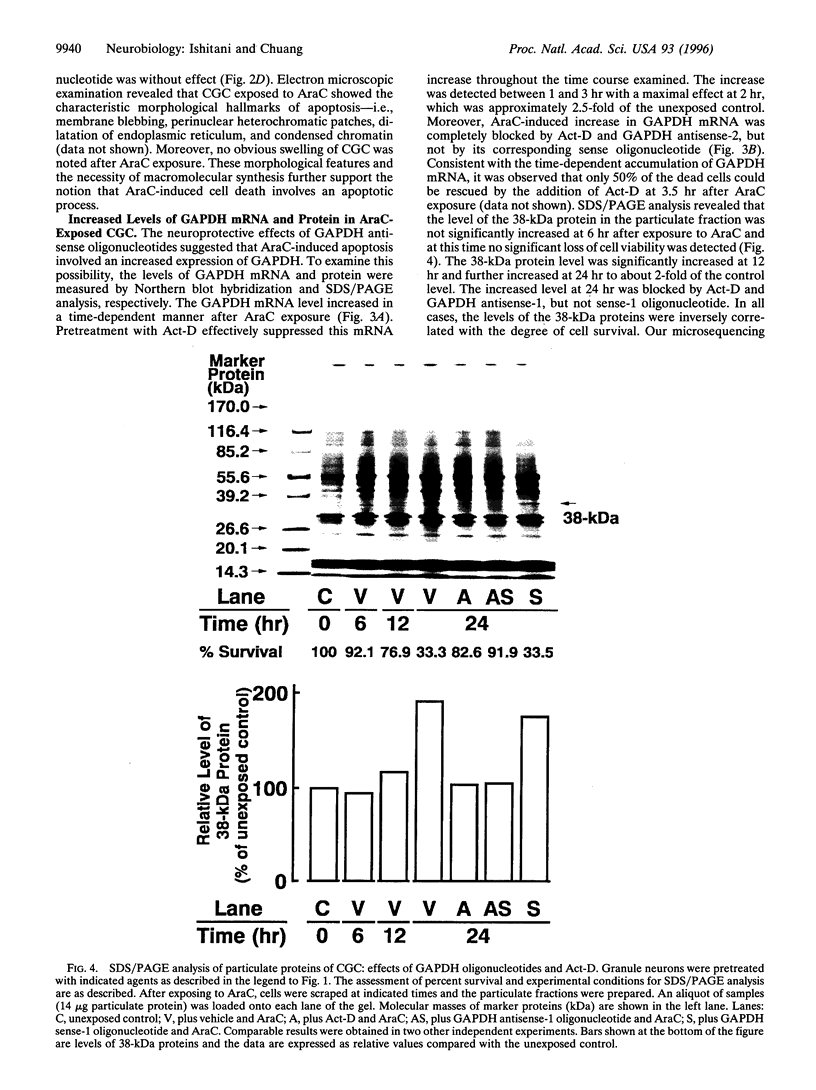
Abstract
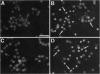
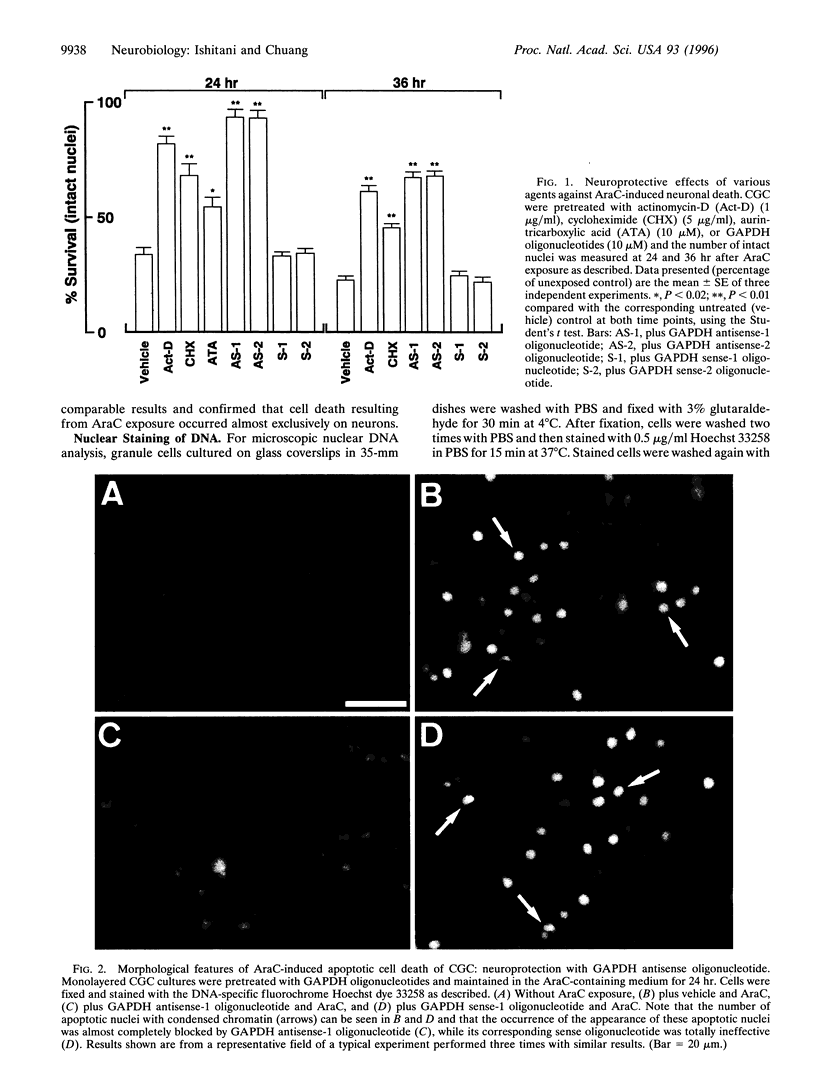
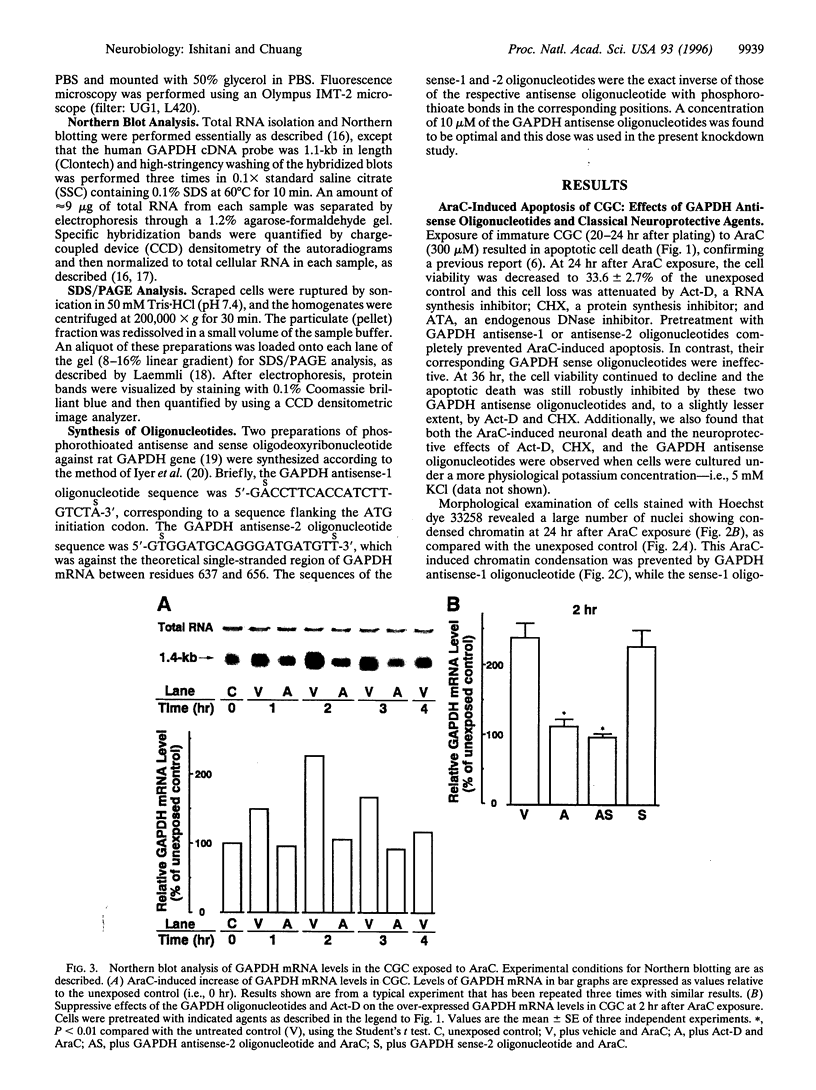
Cytosine arabinonucleoside (AraC) is a pyrimidine antimetabolite that kills proliferating cells by inhibiting DNA synthesis and, importantly, is also an inducer of apoptosis. We recently reported that age-induced apoptotic cell death of cultured cerebellar neurons is directly associated with an over-expression of a particulate 38-kDa protein, identified by us as glyceraldehyde-3-phosphate dehydrogenase (GAPDH; EC 1.2.1.12). We now show that the AraC-induced neuronal death of immature cerebellar granule cells in culture is effectively delayed by actinomycin-D, cycloheximide, or aurintricarboxylic acid (a DNase inhibitor). Furthermore, two GAPDH antisense, but not their corresponding sense, oligodeoxyribonucleotides markedly arrested AraC-induced apoptosis. This protection was more effective than that induced by the above-mentioned classical inhibitors of apoptosis. Prior to AraC-induced neuronal death, GAPDH mRNA levels increased by approximately 2.5-fold, and this mRNA accumulation was blocked by actinomycin-D and the GAPDH antisense (but not sense) oligonucleotide. Like actinomycin-D, a GAPDH antisense oligonucleotide also suppressed the AraC-induced over-expression of the 38-kDa particulate protein (i.e., GAPDH), while the corresponding sense oligonucleotide was totally ineffective. Thus, the present results show that GAPDH over-expression is involved in AraC-induced apoptosis of cultured cerebellar granule cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi J., Hough C., Chuang D. M. Paradoxical increase of 5-hydroxytryptamine2 receptors and 5-hydroxytryptamine2 receptor mRNA in cerebellar granule cells after persistent 5-hydroxytryptamine2 receptor stimulation. Mol Pharmacol. 1993 Mar;43(3):349–355. [PubMed] [Google Scholar]
- Burke J. R., Enghild J. J., Martin M. E., Jou Y. S., Myers R. M., Roses A. D., Vance J. M., Strittmatter W. J. Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH. Nat Med. 1996 Mar;2(3):347–350. doi: 10.1038/nm0396-347. [DOI] [PubMed] [Google Scholar]
- Copani A., Bruno V., Battaglia G., Leanza G., Pellitteri R., Russo A., Stanzani S., Nicoletti F. Activation of metabotropic glutamate receptors protects cultured neurons against apoptosis induced by beta-amyloid peptide. Mol Pharmacol. 1995 May;47(5):890–897. [PubMed] [Google Scholar]
- D'Mello S. R., Galli C., Ciotti T., Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi F., Pollard H., Moreau J., Ben-Ari Y., Charriaut-Marlangue C. Cytosine arabinoside induces apoptosis in cerebellar neurons in culture. J Neurochem. 1995 May;64(5):1980–1987. doi: 10.1046/j.1471-4159.1995.64051980.x. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Yan C. Y., Greene L. A. N-acetylcysteine (D- and L-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci. 1995 Apr;15(4):2857–2866. doi: 10.1523/JNEUROSCI.15-04-02857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C., Meucci O., Scorziello A., Werge T. M., Calissano P., Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. J Neurosci. 1995 Feb;15(2):1172–1179. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P. E., Gross R. W. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry. 1995 Sep 26;34(38):12193–12203. doi: 10.1021/bi00038a013. [DOI] [PubMed] [Google Scholar]
- Ishitani R., Kimura M., Sunaga K., Katsube N., Tanaka M., Chuang D. M. An antisense oligodeoxynucleotide to glyceraldehyde-3-phosphate dehydrogenase blocks age-induced apoptosis of mature cerebrocortical neurons in culture. J Pharmacol Exp Ther. 1996 Jul;278(1):447–454. [PubMed] [Google Scholar]
- Ishitani R., Sunaga K., Hirano A., Saunders P., Katsube N., Chuang D. M. Evidence that glyceraldehyde-3-phosphate dehydrogenase is involved in age-induced apoptosis in mature cerebellar neurons in culture. J Neurochem. 1996 Mar;66(3):928–935. doi: 10.1046/j.1471-4159.1996.66030928.x. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Chuang D. M., Post R. M. Programmed cell death: implications for neuropsychiatric disorders. Biol Psychiatry. 1994 Jun 15;35(12):946–956. doi: 10.1016/0006-3223(94)91241-6. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Wallace T. L., Johnson E. M., Jr Cytosine arabinoside kills postmitotic neurons in a fashion resembling trophic factor deprivation: evidence that a deoxycytidine-dependent process may be required for nerve growth factor signal transduction. J Neurosci. 1990 Jan;10(1):184–193. doi: 10.1523/JNEUROSCI.10-01-00184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean C., Pons F., Benyamin Y., Roustan C. Antigenic probes locate binding sites for the glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase, aldolase and phosphofructokinase on the actin monomer in microfilaments. Biochem J. 1989 Dec 15;264(3):671–677. doi: 10.1042/bj2640671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Rogalski A. A., Steck T. L., Waseem A. Association of glyceraldehyde-3-phosphate dehydrogenase with the plasma membrane of the intact human red blood cell. J Biol Chem. 1989 Apr 15;264(11):6438–6446. [PubMed] [Google Scholar]
- Schläfer M., Volknandt W., Zimmermann H. Putative synaptic vesicle nucleotide transporter identified as glyceraldehyde-3-phosphate dehydrogenase. J Neurochem. 1994 Nov;63(5):1924–1931. doi: 10.1046/j.1471-4159.1994.63051924.x. [DOI] [PubMed] [Google Scholar]
- Schulze H., Schuler A., Stüber D., Döbeli H., Langen H., Huber G. Rat brain glyceraldehyde-3-phosphate dehydrogenase interacts with the recombinant cytoplasmic domain of Alzheimer's beta-amyloid precursor protein. J Neurochem. 1993 May;60(5):1915–1922. doi: 10.1111/j.1471-4159.1993.tb13420.x. [DOI] [PubMed] [Google Scholar]
- Somers M., Engelborghs Y., Baert J. Analysis of the binding of glyceraldehyde-3-phosphate dehydrogenase to microtubules, the mechanism of bundle formation and the linkage effect. Eur J Biochem. 1990 Oct 24;193(2):437–444. doi: 10.1111/j.1432-1033.1990.tb19357.x. [DOI] [PubMed] [Google Scholar]
- Sunaga K., Chuang D. M., Ishitani R. Tetrahydroaminoacridine increases m3-, but not m2-, muscarinic acetylcholine receptor mRNA levels in differentiating cerebellar granule cells. Neurosci Lett. 1993 Nov 26;163(1):27–30. doi: 10.1016/0304-3940(93)90221-6. [DOI] [PubMed] [Google Scholar]
- Sunaga K., Chuang D. M., Ishitani R. Tetrahydroaminoacridine is neurotrophic and promotes the expression of muscarinic receptor-coupled phosphoinositide turnover in differentiating cerebellar granule cells. J Pharmacol Exp Ther. 1993 Jan;264(1):463–468. [PubMed] [Google Scholar]
- Sunaga K., Takahashi H., Chuang D. M., Ishitani R. Glyceraldehyde-3-phosphate dehydrogenase is over-expressed during apoptotic death of neuronal cultures and is recognized by a monoclonal antibody against amyloid plaques from Alzheimer's brain. Neurosci Lett. 1995 Nov 17;200(2):133–136. doi: 10.1016/0304-3940(95)12098-o. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. W., Rakic P. Elimination of neurons from the rhesus monkey's lateral geniculate nucleus during development. J Comp Neurol. 1988 Jun 15;272(3):424–436. doi: 10.1002/cne.902720310. [DOI] [PubMed] [Google Scholar]
- Yan G. M., Ni B., Weller M., Wood K. A., Paul S. M. Depolarization or glutamate receptor activation blocks apoptotic cell death of cultured cerebellar granule neurons. Brain Res. 1994 Sep 5;656(1):43–51. doi: 10.1016/0006-8993(94)91364-1. [DOI] [PubMed] [Google Scholar]