Background: Migfilin is a focal adhesion protein implicated in control of integrin-mediated cell adhesion, spreading, and migration.
Results: Migfilin LIM domains are required for focal adhesion targeting and for binding to kindlin-1 and kindlin-2.
Conclusion: Kindlins support migfilin recruitment to focal adhesions and kindlin is important for normal migfilin dynamics in cells.
Significance: Migfilin/kindlin interactions are important for regulating subcellular localization.
Keywords: Adhesion, Cell Adhesion, Cytoskeleton, Fluorescence Resonance Energy Transfer (FRET), Integrin, LIM Domain, Focal Adhesion, Kindlin, Migfilin
Abstract
Focal adhesions (FAs), sites of tight adhesion to the extracellular matrix, are composed of clusters of transmembrane integrin adhesion receptors and intracellular proteins that link integrins to the actin cytoskeleton and signaling pathways. Two integrin-binding proteins present in FAs, kindlin-1 and kindlin-2, are important for integrin activation, FA formation, and signaling. Migfilin, originally identified in a yeast two-hybrid screen for kindlin-2-interacting proteins, is a LIM domain-containing adaptor protein found in FAs and implicated in control of cell adhesion, spreading, and migration. By binding filamin, migfilin provides a link between kindlin and the actin cytoskeleton. Here, using a combination of kindlin knockdown, biochemical pulldown assays, fluorescence microscopy, fluorescence resonance energy transfer (FRET), and fluorescence recovery after photobleaching (FRAP), we have established that the C-terminal LIM domains of migfilin dictate its FA localization, shown that these domains mediate an interaction with kindlin in vitro and in cells, and demonstrated that kindlin is important for normal migfilin dynamics in cells. We also show that when the C-terminal LIM domain region is deleted, then the N-terminal filamin-binding region of the protein, which is capable of targeting migfilin to actin-rich stress fibers, is the predominant driver of migfilin localization. Our work details a correlation between migfilin domains that drive kindlin binding and those that drive FA localization as well as a kindlin dependence on migfilin FA recruitment and mobility. We therefore suggest that the kindlin interaction with migfilin LIM domains drives migfilin FA recruitment, localization, and mobility.
Introduction
Cell adhesion is the process by which cells create and establish a connection to their extracellular substrates. The ability of cells to form and release contact with their surrounding extracellular matrix is essential for systemic processes such as tissue development and organization, immune patrolling, hemostasis, and vascular health (1, 2). It is well known that integrins, a family of transmembrane, heterodimeric receptors, mediate cell adhesion to both the extracellular matrix and to counter receptors on adjacent cells. Integrins fluctuate between an “inactive” form, with relatively low affinity for binding extracellular ligands, and an “active” conformation, with a higher ligand-binding affinity (1, 3). The direct binding of intracellular proteins (specifically talin and kindlin) to the β integrin cytoplasmic tail can dramatically impact integrin conformations (2, 4, 5). Once activated and bound to ligand, integrins can cluster to form a strong link between the cell and the extracellular matrix. Large clusters of integrins engaged with the extracellular matrix and located at the ends of actin stress fibers are called focal adhesions (FA).2 These FAs are signaling hubs, filled with a multitude of signaling and adaptor proteins responsible for regulating integrin activation, integrin-mediated signaling, and the link to the actin cytoskeleton (2, 6, 7). FAs serve as cytoskeletal anchorage points and through this link, cells are capable of transducing chemical and mechanical signals to and from the extracellular environment. Integrin signaling impacts cell adhesion, spreading and migration, all processes that at least in part rely on signaling between integrins and the actin cytoskeleton.
The kindlin family of proteins is an important class of regulators of integrin activation and signaling. Like the integrin-activator talin, kindlins are 4.1 band, ezrin, radixin, moesin (FERM) domain-containing proteins that bind directly to conserved motifs present in the cytoplasmic tail of integrin β subunits (5, 8–10). There are three kindlin isoforms each with a specific tissue distribution (11). Kindlin-1 is generally expressed in epithelial cells. In humans, nonfunctional kindlin-1 leads to a rare skin disease called Kindler syndrome (12). These patients have skin blistering phenotypes and severe ulcerative colitis. Kindlin-2 is very broadly expressed and although no diseases are known to be associated with its deficiency, the kindlin-2 knock-out mouse embryo fails to implant along the uterine wall during early development (5). Kindlin-2 knockdown cells have β1 integrin activation defects as well as deficiencies in adhesion formation, cell spreading, and migration (5, 13). In kindlin-2 heterozygous mouse and zebrafish models, kindlin-2 was also found to play a critical role in angiogenesis (14). Kindlin-3 is largely restricted to cells of hematopoetic origin (although it has been reported in endothelial cells (15)) and kindlin-3 loss in humans leads to leukocyte adhesion deficiency III (9, 16, 17). Leukocyte adhesion deficiency III patients have less active β1, β2, and β3 integrins (18) manifesting in immune problems and severe bleeding symptoms (9). It is therefore clear that kindlins are important regulators of integrin activation. Kindlin-1 and -2 localize to integrin-rich FAs where, in addition to controlling integrin activation, they are implicated in regulating downstream integrin signaling and cytoskeletal dynamics (5, 8, 10, 19, 20). However, how kindlins modulate signaling and the link to the cytoskeleton has not been clearly resolved. Kindlins lack any known enzymatic activity and are therefore thought to function primarily as adaptor proteins.
Migfilin is a previously identified kindlin-interacting protein (21–23), implicated in modulating cell shape and motility (23). Migfilin is thought to positively regulate cell spreading (23) and the phenotype of one migfilin knock-out mouse suggests that migfilin plays a role in regulating bone remodeling by regulating osteoblast progenitors in bone marrow (24). Additionally, increased migfilin expression correlates with the tumor grade of leiomyosarcoma and glioma with a dependence on phospholipase Cγ and STAT3 signaling pathways (25, 26). Migfilin is comprised of an N-terminal unstructured region, followed by a proline-rich segment and three C-terminal LIM domains (Fig. 1A). Migfilin was first identified as a kindlin-interacting protein in yeast two-hybrid screens and was subsequently shown to interact with vasodilator-stimulated phosphoprotein (27) and bind the large actin cross-linking protein filamin (23) (Fig. 1A). Migfilin therefore potentially forms part of an integrin-kindlin-migfilin-filamin-actin linkage. The filamin-binding site is located within the first 15 amino acids of migfilin and has been characterized by NMR and x-ray crystallography (28, 29). In addition to potentially providing a link to F-actin, migfilin binding to filamin inhibits filamin binding to integrin β tails and so impairs filamin-mediated suppression of integrin activation (29–31). In contrast to the filamin-binding site, the kindlin-binding site in migfilin is much less well characterized, although kindlin binding is thought to be required for migfilin targeting to FAs (23, 32).
FIGURE 1.
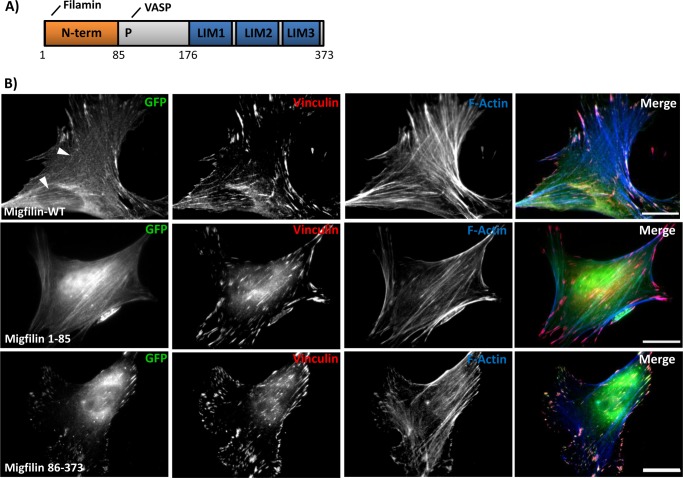
The migfilin C terminus is required and sufficient for focal adhesion targeting. A, schematic of the full-length migfilin protein with amino acid residues noted. Migfilin interacts with filamin and vasodilator-stimulated phosphoprotein via its N terminus and proline-rich region (P), respectively. B, NIH3T3 fibroblasts were transfected with GFP-migfilin full-length (WT), migfilin(1–85), or migfilin(86–373). The cells were replated on fibronectin, fixed, and endogenous vinculin and F-actin were stained to label focal adhesions and stress fibers, respectively. White arrows show weak stress fiber localization. Scale bar = 20 μm.
As a first step toward understanding the cellular roles of migfilin, we examined the biochemical and functional relationship between kindlin and migfilin. Here, we use focal adhesion targeting studies, in vitro pulldown assays, and fluorescence resonance energy transfer (FRET) and fluorescent recovery after photobleaching (FRAP) experiments to map regions of migfilin that drive FA targeting and an interaction with kindlin. Our work demonstrates that the C-terminal LIM domains of migfilin: 1) drive FA localization of the protein and 2) mediate an interaction with kindlin, and finally 3) that kindlin is important for normal migfilin adhesion dynamics in cells.
EXPERIMENTAL PROCEDURES
Reagents and Cloning
Monoclonal anti-vinculin (Sigma), secondary Alexa Fluor-568-conjugated anti-mouse (Invitrogen), secondary Alexa Fluor-647-conjugated anti-mouse (Invitrogen), monoclonal anti-FLAG M2 (Sigma), secondary Alexa Fluor-800-conjugated anti-mouse (Licor), polyclonal anti-Kindlin-2 (AbCam), polyclonal anti-Kindlin-2 (Proteintech), secondary Alexa Fluor-680-conjugated anti-rabbit (Licor), or secondary antibodies coupled to fluorescein isothiocyanate and rhodamine red-X (Jackson ImmunoResearch Labs) and phalloidin-Alexa Fluor-647 were purchased. The kindlin-2 antibody used in keratinocyte immunofluorescence was a generous gift from Dr. Cary Wu. The integrin β6 antibody was provided by Sheila Violette from Stromedix and Paul Weinreb from Biogen Idec. The kindlin-1 antibody was a generous gift from Dr. Mary Beckerle. The filamin A (FLNa) antibody was generated as described previously (33). Human FLAG-tagged kindlin-1 was generated as described previously (34) as was GST-kindlin-1 (10). Migfilin fragments were generated as described previously (29). Briefly, migfilin constructs were generated by polymerase chain reaction and subcloned into pEGFP (BD Biosciences) or pFLAG-CMV2 (Sigma).
GST Protein Production and Purification
Production and purification of GST and GST-kindlin-1 was carried out as described previously (10). Briefly, proteins were transformed into Escherichia coli Rosetta cells (Novagen), induced with a final concentration of 0.1 mm isopropyl 1-thio-β-d-galactopyranoside at 16 °C, lysed in PBS, and purified on glutathione-Sepharose 4 Fast Flow medium (GE Healthcare).
GST Pulldown Assays
CHO cells cultured in Dulbecco's modified essential media (DMEM) (Invitrogen) containing 9% fetal bovine serum (FBS) (Atlantica Biological), 1 mm sodium pyruvate (Invitrogen), 1% non-essential amino acids (Invitrogen), and 1% penicillin/streptomycin (Invitrogen) were plated at one million cells/10-cm tissue culture-treated dish. Approximately 24 h later, 2 or 3 μg of specified DNA constructs was transfected into the cells using polyethylenimine (Polysciences). Approximately 24 h later, cells were harvested and lysed in buffer X (1 mm NaVO4, 50 mm NaF, 40 mm NaPPi, 50 mm NaCl, 150 mm sucrose, 10 mm Pipes, pH 6.8) containing 0.5% Triton X-100 and 0.2% deoxycholic acid. Cell lysate was separated from insoluble material by centrifugation. Cell lysates were incubated with glutathione beads coated with GST or GST-kindlin-1 diluted in buffer X-T (buffer X containing 0.05% Triton X-100). Beads were collected, washed, and bound protein was eluted in SDS sample buffer. Released proteins were fractionated by SDS-PAGE and analyzed by Western blotting.
Viral Infection and Knockdown of Kindlin-2 in Keratinocytes and NIH3T3 Cells
TRC library-based lentiviral scramble and kindlin-2 shRNA plasmids were purchased from Sigma. Virus was produced by co-transfection of these plasmids with the packaging construct pCMVD8.9 and the envelope coding plasmid pCMV-VSVG (from Soosan Ghazezadeh, SUNY, Stony Brook, NY) into HEK293T cells (cultured in 9% FBS, 1 mm sodium pyruvate, and 1% penicillin/streptomycin). Supernatant containing the virus was collected after 72 h followed by centrifugation to remove cells. Primary keratinocytes were cultured in high calcium E medium (DMEM/>F-12) in a 3:1 ratio with 15% FBS supplemented with insulin, transferrin, hydrocortisone, cholera toxin, triiodothyronine, and penicillin/streptomycin at 32 °C. NIH3T3 cells were cultured in DMEM containing 9% fetal clone III (HyClone) or bovine calf serum (HyClone), 1 mm sodium pyruvate, and 1% penicillin/streptomycin and plated the day before infection. On the day of infection, Polybrene was added to the viral supernatant (final concentration of 8 μg/ml) and this was incubated with the cells for 4–6 h at 37 °C. The virus-containing medium was replaced with fresh fibroblast or keratinocyte medium and cells were selected in the presence of 4 μg/ml of puromycin. Cells that survived selection and replating were used for Western blotting and immunofluorescence.
Quantification of Kindlin-2 Knockdown in Infected Cells
Cells were lysed in RIPA buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS) and clarified. Lysates were fractionated by SDS-PAGE and probed for kindlin-2 and vinculin or filamin A (loading controls) using Western blotting. Membranes were scanned using the Odyssey infrared imaging system (Licore) and quantified using ImageJ. Kindlin-2 knockdown was calculated based on normalization to loading controls.
Quantification of Co-immunoprecipitation or GST Fusion Protein Binding Assays
The amount of co-precipitated or GST fusion-bound protein was detected using the Odyssey infrared imaging system and quantified using ImageJ. Percent of protein co-precipitated or bound was expressed as a fraction of the total (calculated from input). Co-precipitation was normalized to WT levels in each experiment. For all Western blotting analyzed with the Odyssey system, care was taken to ensure that the detected signal was not saturated.
Kindlin-Migfilin Co-immunoprecipitation
Fibroblasts were lysed in RIPA (10 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 1% sodium deoxycholate, 10 mm sodium fluoride, 1 μm okadaic acid with protease inhibitor complex (Calbiochem)). Cells were centrifuged at 13,000 × g for 5 min, and the lysate protein concentration was determined with the BCA assay (Pierce). Lysates were pre-cleared with protein A/G beads (Santa Cruz Biotechnology) and incubated with control IgG1 (Invitrogen) or anti-kindlin-2 (Novus Biologicals mouse monoclonal clone 14A11) followed by incubation with protein A/G beads for 2 h at 4 °C. Beads were washed 3 times for 10 min in lysis buffer, boiled in SDS-PAGE sample buffer, and analyzed by Western blotting with anti-migfilin antibody (mouse monoclonal clone 43.9).
GFP-Migfilin and Endogenous Filamin A Co-immunoprecipitation
CHO cells were transfected with 2 μg of plasmid and 24 h later trypsinized and lysed in buffer X (described in GST-binding assay methods). Lysates were incubated on ice for 15 min and clarified at 14,000 × g for 10 min. Lysates were diluted in buffer X-T (described earlier) and GFP antibody was added to the mixture. Lysates with antibody were rocked at 4 °C overnight. The next day lysates were incubated with protein G (Santa Cruz) beads for 2 h at 4 °C. Beads were pelleted, washed with buffer X-T, and bound proteins were eluted with sample buffer, fractionated by SDS-PAGE, and detected by Western blotting.
Focal Adhesion Targeting and Immunofluorescence in NIH3T3, Keratinocytes, Kindlin-2-deficient NIH3T3 Cells, and Kindlin-2-deficient Keratinocytes
NIH3T3 fibroblasts were plated at a density of half a million cells/10-cm tissue culture-treated dish. Approximately 24 h later, they were transfected with polyethylenimine or Lipofectamine 2000 (Invitrogen). Approximately 24 h later, cells were replated on coverslips pre-coated with 5 μg/ml of fibronectin and allowed to spread for 6 h. Cells were then fixed in 4% paraformaldehyde and permeabilized in PBS (pH 7.4) containing 0.2% BSA, 50 mm NH4Cl, and 0.3% Triton X-100. After PBS washing, coverslips were incubated with primary antibody or fluorophore-conjugated phalloidin for 1 h, washed in PBS, and incubated with secondary antibody for 1 h. Coverslips were mounted using ProLongGold anti-fade mounting agent (Invitrogen). Images were acquired using a Nikon TE2000, with ×20 or 100 objectives using IPLab (version 3.5.2; Scanalytics) and images were analyzed using ImageJ.
Fibroblasts expressing kindlin-2-targeting shRNA or scrambled shRNA were plated on coverslips pre-coated with 5 μg/ml of fibronectin. The next day, they were transfected with GFP-migfilin constructs using Lipofectamine 2000 and the following day the coverslips were treated as described above.
Keratinocytes expressing control or kindlin-2 shRNA were generated as previously described (19) and transfected using FuGENE-6 (Promega) for 48 h and then treated as previously described (19). Briefly, cells were plated on coverslips coated with 10 μg/ml of fibronectin. Cells were fixed with 4% paraformaldehyde, then permeabilized with PBS containing 0.2% Triton X-100 and blocked with 5% donkey serum. Then cells were incubated with primary antibody, washed with PBS, incubated with fluorophore-conjugated secondary antibodies, and stained with 4′,6-diamidino-2-phenylindole (DAPI). Coverslips were mounted and cells were visualized and photographed using an Olympus BX53 fluorescence microscope equipped with an UpanLFN 100 ×1.3 NA oil lens. Olympus DS2 software was used to acquire the images, which were then saved as TIF files and imported into Photoshop. The images were modified for brightness and contrast.
Fluorescence Lifetime Imaging Microscopy (FLIM)
NIH3T3 fibroblasts were cultured in DMEM containing 10% FCS, penicillin/streptomycin, and glutamine. Transfections were performed using FuGENE HD (Roche) according to manufacturer's instructions. FLIM was performed with cells expressing specified constructs. Cells were fixed using 4% paraformaldehyde followed by quenching with 1 mg/ml of sodium borohydride and mounted onto glass slides using Fluorsave (Merck). Details of the time domain FLIM performed with a multiphoton microscope system were described previously (35). Briefly, fluorescence lifetime imaging capability was provided by time-correlated single photon counting electronics (Becker & Hickl, SPC 700). Widefield acceptor (mRFP) images were acquired using a CCD camera (Hammamatsu) at exposure times of <100 ms. Data were analyzed using TRI2 software (developed by Dr. Paul Barber). Average lifetimes were calculated from the mean of all pixels measured within each image/cell and pooled from multiple experiments for statistical analysis. All histogram data are plotted as mean FRET efficiency from >10 cells per sample. Lifetime images of exemplary cells are presented using a pseudocolor scale whereby blue depicts normal GFP lifetime (i.e. no FRET) and red depicts reduced GFP lifetime (areas of high FRET). Each experiment was repeated at least 3 times. Analysis of variance was used to test statistical significance between different populations of data.
FRAP Microscopy and Analysis
FRAP experiments were performed on a confocal microscope (A1R; Nikon) equipped with a CFI Plan-Fluor ×40 oil objective lens and housed in an environmental chamber maintained at 37 °C. GFP was bleached within the region of interest using a 488-nm laser at 100% power for ∼2 s. Images were captured, analyzed, and exported using Nikon software (NIS Elements). Analysis of FRAP data were performed as described in Ref. 36. In brief, raw intensity data were corrected for background fading during imaging and plotted as the percentage recovery over time, and (τ½) values were calculated from the resultant curves. The mobile and immobile fractions were calculated from the plateau values of the final intensity recovery curves. Data were pooled from at least 10 cells over two independent experiments.
RESULTS
Migfilin LIMs Dictate Focal Adhesion Targeting
Prior reports indicate that migfilin localizes to two major sites in adherent cells, integrin-rich FA and F-actin-rich stress fibers (23, 29, 32). Available data suggests that FA targeting is mediated via interactions between migfilin and kindlins, whereas we and others have shown that migfilin targeting to stress fibers requires binding of the actin-binding protein filamin (23, 29). Consistent with these reports, we find that when expressed in NIH3T3 fibroblasts, GFP-tagged migfilin localizes primarily to FAs but also exhibits weak stress fiber localization (Fig. 1B, white arrows).
To study which domains are important for migfilin adhesion targeting we generated expression constructs for various GFP-tagged migfilin fragments based on the predicted migfilin domain architecture (Fig. 1A). Constructs were transfected into NIH3T3 fibroblasts and the following day cells were plated on fibronectin-coated coverslips, allowed to adhere and spread for 24 h, and fixed and stained for endogenous vinculin (a marker of focal adhesions) and phalloidin (to label the F-actin stress fibers). Consistent with our previous findings (29), the N-terminal 85-amino acid fragment of migfilin, which includes the filamin-binding site, exhibits stress fiber localization (Fig. 1B). Conversely, the complementary fragment spanning amino acids 86–373 exhibited strong co-localization with vinculin, a marker of FAs. Hence, the stress fiber- and FA-targeting sites in migfilin are separable.
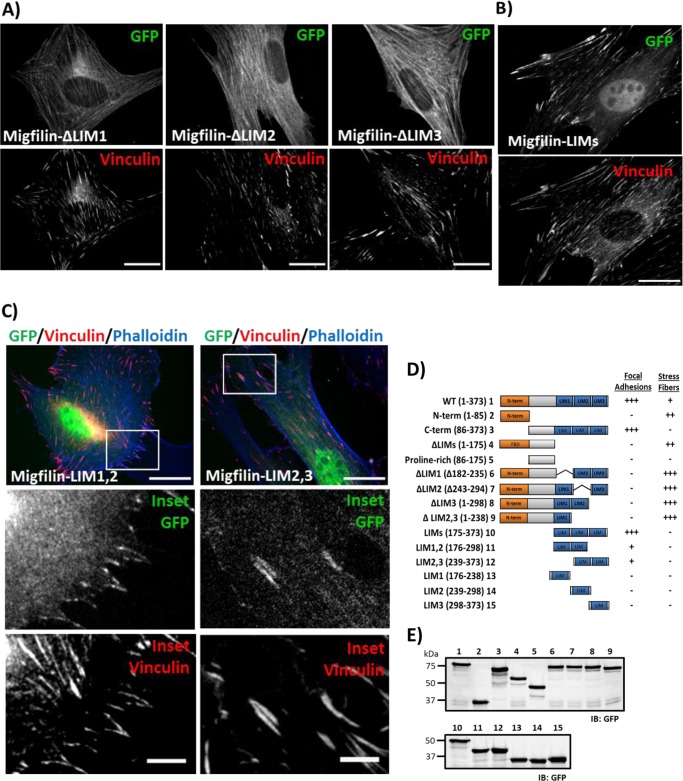
The preceding results demonstrate that FA localizing sites reside within amino acids 86–373. This region contains the three C-terminal LIM domains. To determine whether these domains are important for FA targeting, we generated GFP-migfilin constructs lacking LIM1, LIM2, or LIM3. Deletion of any of the LIM domains dramatically impaired FA targeting (Fig. 2A). Vinculin staining confirmed that the overexpressed migfilin fragments did not impair FA formation but rather that the lack of LIM domains prevented accumulation of mutant protein in existing FAs. However, whereas deletion of any of the three LIM domains prevented FA localization, stress fiber targeting was enhanced, presumably because in the absence of FA targeting filamin can more efficiently recruit migfilin to stress fibers.
FIGURE 2.
Migfilin LIM pair fragments are capable of focal adhesion targeting. A, in fibroblasts plated on fibronectin, single LIM deletions within the context of full-length migfilin are not clearly detected in adhesions. B, C-terminal LIM fragment is sufficient to drive strong FA targeting. C, in the same system, tandem LIM pair 1 and 2 (LIM1,2) or LIM pair 2 and 3 (LIM2,3) are capable of weak focal adhesion targeting. Merged panel shows the GFP channel in green, vinculin channel in red, and phalloidin channel in blue. White box in the merge panel diagrams the magnified inset panels. Scale bar = 20 μm; inset scale bar = 5 μm. D, a summary of qualitatively scored, relative targeting of a panel of GFP-migfilin fragments and deletions. E, Western blot of constructs numbered in D showing that each GFP construct is expressed at the appropriate molecular weight.
To determine whether migfilin LIM domains were able to target to FAs, we expressed the C-terminal three tandem LIM domain fragment tagged to GFP in cells and found that this is sufficient for incorporation into FA (Fig. 2B). We next sought to determine whether less than three LIM domains were sufficient for FA targeting. GFP-tagged tandem LIM1,2 and LIM2,3 domain constructs as well as individual LIM domains were expressed and localization of the constructs analyzed in fixed cells. Whereas results show that the migfilin LIM domain pairs exhibited weak, but consistent colocalization with vinculin (Fig. 2C), individual LIM domains were not detected in FAs or stress fibers (summarized in Fig. 2D). GFP fusion protein expression was checked using Western blotting (Fig. 2E) and all proteins and fragments appear stable.
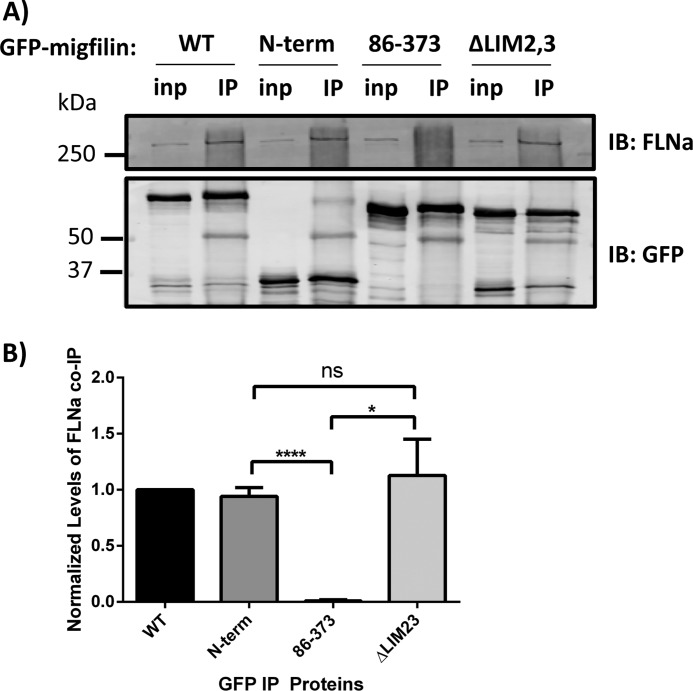
Migfilin targeting to stress fibers requires binding to filamin (29). However, our results show that migfilin ΔLIM2,3 has stronger stress fiber localization than wild-type migfilin even though both contain an intact FLNa-binding site. To test whether a compromised LIM domain region enhances the association of migfilin with filamin (and hence stress fiber localization), we immunoprecipitated GFP-migfilin constructs from CHO cells and assessed co-immunoprecipitated FLNa by Western blotting (Fig. 3A). All migfilin fragments containing the N-terminal filamin-binding site co-precipitated comparable amounts of endogenous FLNa, whereas a migfilin(86–373), which lacks the filamin-binding region, did not (Fig. 3B). Thus, rather than increasing stress fiber targeting by enhancing filamin binding, we suggest that the increased stress fiber localization of migfilin constructs with disrupted LIM domains may be due to a loss of their sequestration in focal adhesions.
FIGURE 3.
Removing the LIM domains of migfilin does not result in increased migfilin association with filamin. A, GFP-migfilin constructs were transfected into CHO cells and immunoprecipitated using a GFP antibody. Endogenous FLNa co-precipitation was detected by Western blotting and quantified (B). inp, 5% input material. IP, immunoprecipitated. n = 4. *, p = 0.01; ****, p < 0.0001 using Student's t test. IB, immunoblot; ns, nonspecific.
In summary, we find that migfilin constructs with all three LIM domains target very well to FAs in fibroblasts. In migfilin fragments containing the N-terminal filamin-binding site, loss of even one LIM domain prevents targeting to FAs and instead results in stress fiber localization, but not increased association with filamin. In the absence of the stress fiber targeting motif we find that LIM domain pairs are sufficient to weakly target to FAs. This means that the N and C termini of migfilin are each responsible for a specific subcellular localization for the protein and suggests that a binding partner of tandem migfilin LIM domains is responsible for FA targeting.
Migfilin LIMs Exhibit FRET with Kindlin-2 in Fibroblasts
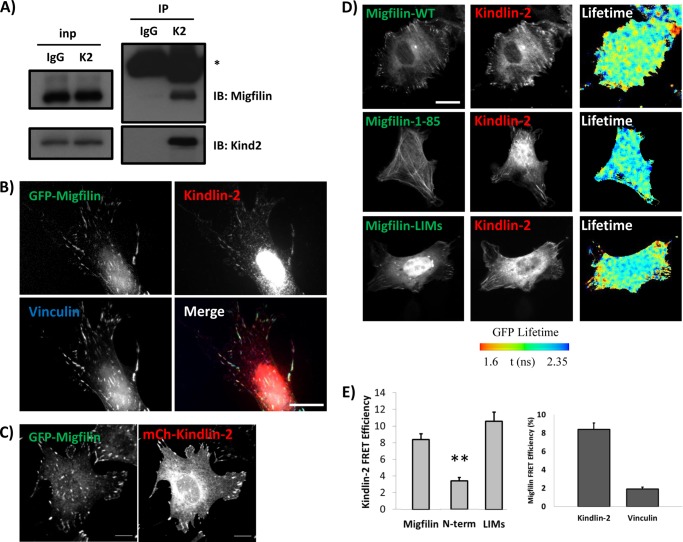
Yeast two-hybrid data suggest that migfilin binds kindlin-2 (23) and this, coupled with analysis of migfilin localization in kindlin-2 knockdown cells, has led to the suggestion that kindlin-2 is responsible for migfilin localization in FAs (23). In fibroblasts, we observe endogenous kindlin-2 and migfilin co-immunoprecipitation (Fig. 4A), confirming the kindlin/migfilin protein interaction. Consistent with the hypothesis that kindlin targets migfilin to FAs, GFP-migfilin co-localizes with endogenous kindlin-2 and vinculin in NIH3T3 fibroblasts (Fig. 4B) and co-expressed GFP-migfilin and mCherry-kindlin-2 co-localize in FAs (Fig. 4C). To test whether kindlin-2 and migfilin interact in intact NIH3T3 fibroblasts we used FLIM to measure FRET between co-expressed GFP-tagged migfilin constructs and mCherry-tagged kindlin-2. FLIM allows for highly sensitive and accurate analysis of FRET and, unlike ratiometric or acceptor photobleaching approaches, is independent of fluorophore concentrations. FLIM therefore represents the best currently available technique to image interactions between two separate proteins co-expressed within a single cell. Moreover, use of the time domain of FLIM employed here offers the additional benefit of spatially mapping FRET efficiencies across individual cells on a pixel-by-pixel basis (37). Consistent with a direct interaction we found that kindlin-2 exhibits FRET with migfilin (Fig. 4, D and E). Furthermore, we obtained similar results with the shorter FA targeting fragment spanning the migfilin LIM domains. However, the N-terminal migfilin fragment that does not target to FAs, also does not exhibit FRET with kindlin-2. Hence migfilin constructs that efficiently localize to FAs also exhibit direct binding to kindlin-2, whereas constructs that fail to target do not.
FIGURE 4.
Migfilin co-localizes and exhibits specific FRET with kindlin-2 in cells. A, endogenous migfilin and kindlin-2 co-immunoprecipitate from NIH3T3 lysates. * = antibody heavy chain. B, GFP-migfilin co-localizes with endogenous kindlin-2 and vinculin in NIH3T3 fibroblasts plated on fibronectin. Scale bar = 20 μm. C, GFP-migfilin co-localizes with mCherry-kindlin-2 in NIH3T3 fibroblasts plated on fibronectin. Scale bar = 10 μm. D, fibroblasts on fibronectin coexpress GFP-migfilin constructs and mCherry-kindlin-2. Individual channels are shown and lifetime of the donor fluorophore is shown in pseudocolor, where cyan indicates less FRET and red/yellow indicates more FRET (see bar). Scale bar, 20 μm. E, quantification of kindlin-migfilin or migfilin-vinculin FRET efficiency. **, p value < 0.001.
FRET requires that the donor and acceptor molecules be in very close proximity (<9 nm) and as such the presence of FRET provides support for a direct interaction between two proteins. However, as migfilin and kindlin both localize in FAs it was important to exclude the possibility that the positive FRET was due to local clustering of proteins rather than direct interactions. We therefore used mCherry-tagged vinculin as an acceptor in place of kindlin-2. As expected, mCherry-vinculin localized to FA but did not exhibit FRET with migfilin, confirming the specificity of the kindlin-migfilin FRET (Fig. 4E).
Migfilin Targets to Focal Adhesions in Kindlin-2 Knockdown Keratinocytes
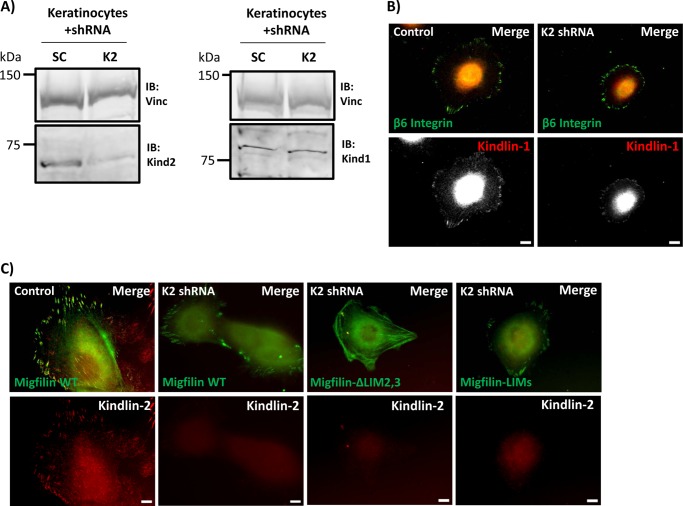
Kindlin-2 is the most widely expressed kindlin and the majority of the available data on kindlin/migfilin interactions relate to kindlin-2. Based on one report (23), the current belief is that kindlin is required for migfilin FA localization. Most migfilin targeting studies have been performed in cells that express mainly kindlin-2 or kindlins-1 and -2 (22, 23, 26, 27, 32, 38). One study utilized Kindler syndrome keratinocytes or kindlin-1 knockdown HaCaT cells to show that migfilin can still localize to FAs in these cells in the absence of endogenous kindlin-1 (22), presumably because these cells contain kindlin-2. Kindlin-1 and kindlin-2 are very similar (∼62%, identity ∼24% similar amino acids) and kindlin-1 has been reported to co-immunoprecipitate with migfilin (21, 22). Therefore, we used previously characterized kindlin-2 knockdown keratinocytes (19) to test whether kindlin-1, in the absence of wild-type levels of kindlin-2, can support normal migfilin localization. The knockdown keratinocytes express only 15–25% of wild-type kindlin-2 levels but have normal levels of kindlin-1 (Fig. 5A). As previously reported (19), kindlin-2-deficient keratinocytes are less well spread than control scrambled shRNA cells, but still form FAs containing endogenous kindlin-1 (Fig. 5B). By expressing GFP-migfilin constructs in these cells we examined the ability of migfilin fragments to target to FAs in the presence of mainly kindlin-1. As observed in kindlin-2 expressing fibroblasts, we found that migfilin targeted to FAs as did the tandem LIM domains. However, migfilin ΔLIM2,3 lacking two LIM domains, did not target FAs and showed more of a fibrous localization, akin to stress fibers (Fig. 5C). These results suggest that if kindlins are required for FA localization of migfilin then either kindlin-1 or -2 can support targeting.
FIGURE 5.
Migfilin targets to focal adhesions in kindlin-2 knockdown keratinocytes. A, primary keratinocytes were infected with scrambled (SC) or kindlin-2 (K2) shRNA. Cells were lysed and lysates were probed for endogenous kindlin-2 and kindlin-1 levels using vinculin as a loading control. B, whereas kindlin-2 knockdown keratinocytes have a spreading defect, immunofluorescence shows that both control and K2 knockdown keratinocytes still have kindlin-1 present in β6-rich focal adhesions. C, control or K2 knockdown keratinocytes were transfected with GFP-migfilin full-length, ΔLIM2,3, or LIMs and co-stained for endogenous kindlin-2. As in fibroblasts, full-length migfilin and LIMs target to adhesions, whereas ΔLIM2,3 does not. Scale bar = 10 μm. IB, immunoblot.
Migfilin LIM Domains Mediate Binding to GST-kindlin-1 in Lysate Pulldown Assays and Exhibit FRET with Kindlin-1
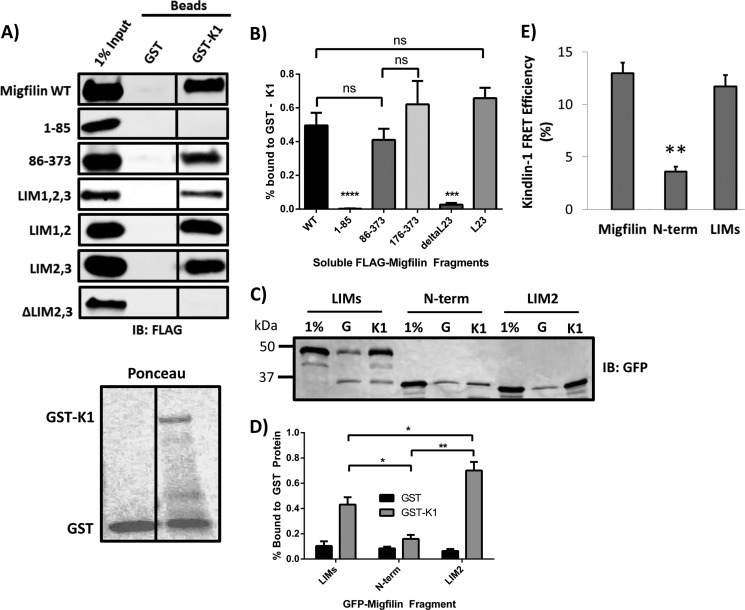
Migfilin localization in NIH3T3 fibroblasts, and wild-type and kindlin-2 knockdown keratinocytes are consistent with a model where the migfilin LIM domains bind kindlin-1 or kindlin-2. To validate this interaction and confirm the localization of the kindlin-binding domain in migfilin we used migfilin fragments to map the domain of migfilin important for a kindlin interaction in lysate pulldown assays.
GST and GST-kindlin-1 were bacterially expressed and purified, then coated onto glutathione beads for use as an affinity matrix. Recombinant full-length kindlins are produced with low efficiency but using GST-kindlin-1 allowed us to obtain a full-length fusion protein where the major contaminant was GST. We therefore used beads coated with comparable levels of GST only as a negative control matrix. Coated washed beads were incubated with lysates of cells transfected with FLAG-migfilin constructs. After incubation, the beads were washed and bound proteins were eluted in SDS sample buffer, fractionated by SDS-PAGE, and visualized by Western blotting. These assays confirmed an interaction between full-length migfilin and GST-kindlin-1, whereas there was negligible migfilin binding to GST-coated beads (Fig. 6A). We then tested additional migfilin fragments and found that migfilin(86-373), migfilin LIMs, migfilin LIM1,2 and migfilin LIM2,3 also bound GST-kindlin-1 but neither migfilin(1–85) nor migfilin ΔLIM2,3 interacted with kindlin-1 (Fig. 6A). We quantified the binding of migfilin fragments that were tested more than five times and saw no significant difference in kindlin-binding capabilities (Fig. 6B). Thus, all kindlin-binding constructs contain at least two LIM domains, whereas the non-binding constructs either completely lack the LIM domain region or have a compromising C-terminal deletion. Furthermore, there is good correlation between kindlin binding and focal adhesion localization, consistent with an important role for kindlin binding in recruiting or stabilizing migfilin at adhesions.
FIGURE 6.
Migfilin interaction with GST-kindlin-1 requires LIM domains. A, lysates of CHO cells overexpressing FLAG-migfilin constructs were incubated with beads coated with either GST or GST-kindlin-1 (GST-K1). Beads were washed, bound proteins eluted with SDS-sample buffer and fractionated by SDS-PAGE. Bound migfilin was detected by Western blotting with anti-FLAG antibodies. Migfilin proteins containing the LIM domains (WT, 86–373, LIMs, LIM1,2, and LIM2,3) pull down with GST-kindlin-1, whereas 1–85 and ΔLIM2,3 do not. Black vertical lines between Western blot lanes denote non-adjacent lanes on the same blot, with the same level of exposure and contrast. Lower panel, Ponceau stain of the membrane shows loading of the GST and GST-kindlin-1 affinity matrices. B, kindlin-1 binding quantification of constructs with n ≥ 5. ****, p < 0.0001; ***, p < 0.001 using Student's t test. C, GFP-migfilin constructs were tested in a GST pulldown assay and quantification. D shows that LIMs and LIM2 bind GST-K1 above that of the migfilin N-terminal negative control; n = 3; *, p < 0.05; **, p < 0.01. E, fibroblasts on fibronectin coexpressed GFP-migfilin constructs and mCherry-kindlin-1 and FRET efficiency was quantified using FLIM; **, p < 0.001 using Student's t test.
As both LIM1,2 and LIM2,3 constructs pull down with GST-kindlin-1, we wanted to test whether the isolated LIM2 domain is sufficient to bind migfilin. Unfortunately FLAG-LIM2 did not express well and was not stable. We could, however, produce GFP-LIM2 and so tested whether this could interact with GST-kindlin-1. Although the use of GFP-tagged protein resulted in higher nonspecific binding to GST, GFP-migfilin LIM2 is clearly capable of pulldown with kindlin-1 at levels above that of the N-terminal negative control (Fig. 6, C and D), suggesting that LIM2 is sufficient for kindlin binding. However, our localization studies show that a single LIM is not enough to support detectable accumulation in focal adhesions. Thus, whereas kindlin binding may be required for localization in adhesions additional interactions may also be required.
Our pulldown assays demonstrated formation of a complex between migfilin and kindlin-1. To determine whether this was due to direct interactions between migfilin and kindlin-1 we again used FLIM to analyze FRET between these two proteins in intact cells. As we previously observed for kindlin-2, GFP-migfilin and GFP-migfilin LIMs both exhibited FRET with mCherry kindlin-1, whereas the N-terminal migfilin fragment exhibited only background levels of FRET (Fig. 6E). The FRET between kindlin-1 and migfilin show a specific interaction that takes place within the cell and again supports the hypothesis that kindlin and migfilin directly interact.
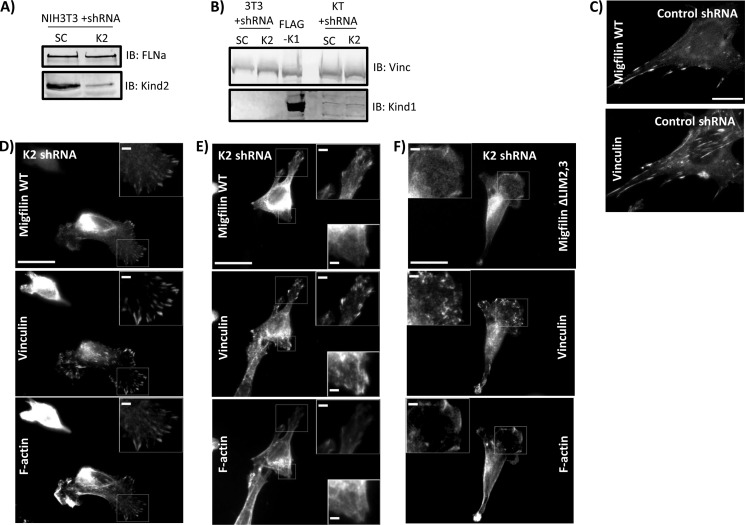
Migfilin Targeting in Kindlin-2-deficient Fibroblasts
Through localization analysis, FRET, and biochemical assays, we have shown a strong correlation between regions of migfilin that drive adhesion localization and migfilin regions that interact with kindlin. Tu et al. (23) suggest that migfilin FA localization relies on the presence of kindlin-2. Using kindlin-2 knockdown keratinocytes we showed that kindlin-1 may also be sufficient to target migfilin to adhesions. Fibroblasts are thought to express mainly kindlin-2, so kindlin-2 knockdown fibroblasts should provide a cellular system containing very little total kindlin. Toward that end, NIH3T3 fibroblasts were infected with control shRNA or shRNA that targets endogenous kindlin-2. Similar to previous reports (19), kindlin-2 knockdown cells have ∼90% kindlin-2 knockdown (Fig. 7A) and exhibit spreading defects. Fibroblasts expressing control or kindlin-2 shRNA did not have detectable endogenous kindlin-1 (Fig. 7B). Nonetheless, all adherent kindlin-2 knockdown cells can slightly spread and make some small vinculin-rich adhesion structures. Kindlin-2 knockdown cells were transfected with GFP-migfilin, plated on fibronectin, and GFP-migfilin co-localization with endogenous vinculin or F-actin was evaluated. Migfilin targeting to FAs was normal in control shRNA-expressing fibroblasts (Fig. 7C). Surprisingly, GFP-migfilin showed partial co-localization with endogenous vinculin in kindlin-2-depleted fibroblasts. Some cells consistently showed migfilin in FA-like structures (Fig. 7D). Others showed mixed targeting where migfilin could target to some but not all vinculin-rich adhesion structures within the same cell (Fig. 7E). GFP-migfilin ΔLIM2,3 did not target in kindlin-2 knockdown cells (Fig. 7F) but instead co-localized more with the weak F-actin filaments, confirming that an intact C-terminal LIM domain is absolutely required for migfilin to be found in adhesions. We were unable to probe for residual kindlin-2 in the adhesions of the knockdown cells because our kindlin-2 antibody shows a very strong nuclear signal (Fig. 4B) obscuring the weak signal from the small adhesions in the poorly spread kindlin knockdown cells. Nonetheless, our data show that wild-type kindlin-2 levels are not absolutely required for GFP-migfilin to localize to adhesions. However, because GFP-migfilin FA targeting was not consistent in all kindlin-2 knockdown cells, and because the cells do have residual kindlin-2, GFP-migfilin adhesion localization may still have some kindlin-2 dependence.
FIGURE 7.
Migfilin localization in kindlin-2 knockdown fibroblasts. NIH3T3 fibroblasts were infected with lentivirus containing control (SC, scrambled) shRNA or kindlin-2 (K2) shRNA. A, these cell lysates were probed for endogenous kindlin-2 using FLNa as a loading control. B, using lysate of CHO cells transfected with FLAG-kindlin-1 and lysates of scramble (SC) or kindlin-2 knockdown keratinocyte (KT) as positive controls, SC or K2 knockdown 3T3 lysates were probed for endogenous kindlin-1. C, scramble control cells were plated on fibronectin and transfected with GFP-migfilin full-length (WT), then fixed and stained for endogenous vinculin. D and E, kindlin-2 knockdown 3T3 cells were transfected with GFP-migfilin WT or migfilin ΔLIM2,3 (F) and stained for endogenous vinculin or F-actin. Scale bar = 20 μm; inset scale bar, 5 μm.
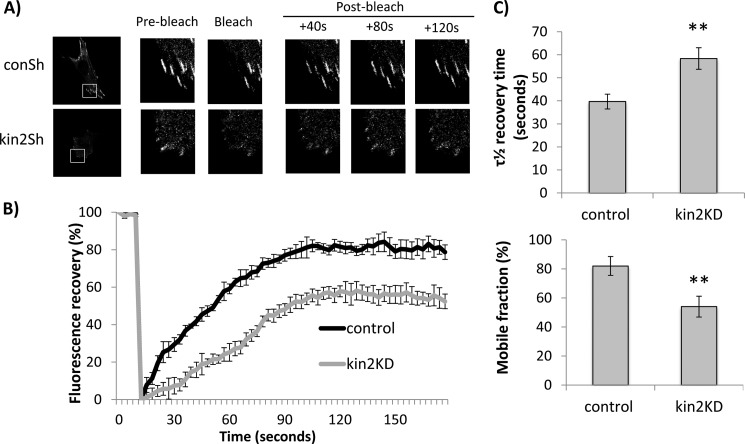
Migfilin Recruitment and Release from FAs in Kindlin-2 Knockdown Cells
The preceding data suggest that whereas the kindlin-binding LIM domains are essential for GFP-migfilin to accumulate in fibroblast adhesions, wild-type levels of kindlin-2 may not be required. To assess the functional importance of the kindlin/migfilin interaction in controlling migfilin dynamics, we tested whether kindlin-2 depletion alters migfilin behavior at FAs. We used FRAP to evaluate turnover of GFP-migfilin within FAs. Fibroblasts stably expressing a control shRNA were transfected with GFP-migfilin. GFP-positive adhesions were bleached using a 488-nm laser for 2 s and the recovery of GFP protein was monitored over 120 s (Fig. 8A, top panels). Short time measurements allow us to mainly measure protein turnover in an identified adhesion rather than the assembly/disassembly of the adhesion. Furthermore, although the adhesions in kindlin-2 knockdown cells are generally smaller than those in wild-type or control knockdown cells, for these experiments we only analyzed small adhesions (of 1–2.5 μm length) in all cells.
FIGURE 8.
Kindlin-2 is required for migfilin recruitment to and release from focal adhesions. A, example still taken from FRAP movies of NIH3T3 cells stably expressing control or kindlin-2 shRNA transfected with GFP-migfilin. Cells were imaged on a confocal microscope, regions of interest were bleached using 488 nm laser at maximum power for 2 s. B, cumulative FRAP recovery traces from control and kindlin-2 shRNA cells (n = 10 cells per condition, and >2 adhesions per cell). Fluorescence recovery is normalized to pre-bleach levels and corrected for any fading of fluorophores over time. C, bar graphs of (top) τ½ recovery times and (bottom) mobile fractions of migfilin-GFP in control or kindlin-2 knockdown cells. **, p < 0.001.
Analysis of recovery curves demonstrated that GFP-migfilin at adhesions showed a half-life (τ½) of ∼40 s (Fig. 8, B and C). However, when GFP-migfilin recovery was assessed in kindlin-2 knockdown cells, the recovery was significantly slower (Fig. 8A, bottom panels). Quantification showed that in addition to slower recovery (Fig. 8C), there was also significantly less mobile GFP-migfilin at adhesions in kindlin-2 knockdown cells than in the control cells (Fig. 8C, lower panel). These FRAP studies are the first demonstration of a kindlin contribution to migfilin dynamics within adhesions. Our results show that in the absence of kindlin-2, migfilin recruitment to adhesions is significantly impaired. Additionally, once localized to an adhesion, migfilin is less mobile in the absence of kindlin-2. Together, these data further support our hypothesis that kindlin plays an important role in regulating adhesion localization of migfilin.
DISCUSSION
Migfilin is a LIM domain containing focal adhesion protein, which has been implicated in control of cell adhesion, spreading, and migration (23, 25, 26, 39). Using a combination of biochemical pulldown assays, fluorescence microscopy, FRET measured by FLIM, and FRAP analysis, we have established that the C-terminal LIM domains of migfilin dictate its FA localization and mediate an interaction with kindlin in vitro and in cells, and demonstrated that kindlin is important for normal migfilin adhesion dynamics in cells.
Migfilin was originally identified in a yeast two-hybrid screen for kindlin-2 (mig-2)-interacting proteins (23) and was then shown to localize to FAs and actin filaments. Additional yeast two-hybrid screens revealed a migfilin/filamin interaction (23) and we (29) and others (28, 30) subsequently showed that the N terminus of migfilin binds filamin, and that this interaction supports its stress fiber localization and impacts integrin activation. As described here, consistent with previous reports for endogenous migfilin (22, 23, 39), our GFP-migfilin localizes primarily to FAs with occasional stress fiber localization. We also show that a migfilin, which lacks the first 85 residues containing the filamin-binding site, localizes only to vinculin-rich focal adhesions, and that the three tandem LIM domains are sufficient to drive FA targeting. A single LIM domain is capable of kindlin interaction, but it is not sufficient to target to adhesions in a cell. Tandem LIM domain pairs target very weakly to FAs but LIM pairs in the context of a large migfilin fragment are found mainly on stress fibers. This suggests that single LIM deletions compromise the FA targeting capabilities enough such that the N-terminal stress fiber targeting interactions are the main drivers of subcellular localization. Taken together, these data show that it is the LIM domain region that determines subcellular localization of migfilin.
The initial description of migfilin reported that migfilin bound kindlin-2 and that migfilin failed to localize in FAs in kindlin-2 knockdown cells, raising the possibility that kindlin-2 is responsible for migfilin FA targeting (23). Consistent with this, our work detects an interaction between endogenous kindlin and migfilin, shows that GFP-migfilin co-localizes with endogenous kindlin-2 or mCherry-kindlin-2 in adhesions, and that the migfilin LIM domains are sufficient to support this co-localization. Notably the LIM domains were the minimal region shown to bind kindlin-2 in yeast two-hybrid assays (23). We have also used FRET to demonstrate, for the first time, that migfilin directly interacts with kindlin in cells. Furthermore, we observed a correlation between migfilin constructs that targeted to FAs and those that exhibited FRET with kindlin-2. Because FRET requires such close proximity of donor and acceptor fluorophores, our data supports the yeast two-hybrid data that suggests a direct interaction between kindlin and migfilin.
Most prior work on migfilin localization to FAs has used cells that mainly contain endogenous kindlin-2 (22, 23, 26, 27, 30, 32, 38). Some studies used Kindler syndrome keratinocytes or kindlin-1 knockdown HaCaT cells to show that migfilin localized in adhesions in cells expressing mainly kindlin-2 (22). However, migfilin can be co-immunoprecipitated with kindlin-1 (21, 22), suggesting that kindlin-1 also binds migfilin and hence could potentially support migfilin targeting. To test the functional importance of the migfilin/kindlin-1 interaction, we knocked down kindlin-2 in keratinocytes resulting in cells that express wild-type levels of kindlin-1 and ∼10–15% of wild-type kindlin-2 levels. In these cells, we observed GFP-migfilin fragments with an intact LIM domain region targeting to FAs. As expected, migfilin fragments lacking the LIMs were not found in FAs. Additionally, we observed a correlation between migfilin fragments that targeted FAs and those that exhibited FRET with kindlin-1, suggesting that endogenous migfilin can directly interact with kindlin-1 in cells. In addition to providing the first evidence for a direct migfilin/kindlin-1 interaction, our work suggests that if the localization of migfilin is kindlin dependent, then kindlin-2 and kindlin-1 can both target migfilin to adhesions.
To support our cellular interaction assays we used bacterially expressed GST-kindlin-1 in lysate pulldown assays to show that fragments of one, two, or three migfilin LIM domains were sufficient to interact with kindlin-1, and migfilin fragments that lacked at least two LIM domains did not interact with kindlin-1. Assays were only performed using GST-kindlin-1 as we were unsuccessful in producing sufficient quantities of purified GST-kindlin-2. We are therefore unable to compare migfilin binding to kindlin-1 and kindlin-2. Nonetheless, our assays confirm the interaction between kindlin-1 and migfilin, and when taken together with our FRET studies suggest that the migfilin LIM domains directly interact with kindlins in cells.
Having established that migfilin binds kindlin in cells and shown a correlation between migfilin fragments that target to FAs and those that bind kindlin, we next sought to address the significance of this FA localization and kindlin binding. It is reported that kindlin-2 knockdown significantly alters migfilin localization (23). We used kindlin-2 knockdown fibroblasts as a cellular system that lacks most kindlins and expressed GFP-migfilin to investigate this same phenomenon. As previously reported, kindlin-2-depleted fibroblasts are severely impaired in cell spreading and form only a few small FAs (5). We were therefore surprised to find GFP-migfilin still capable of localizing to the remaining adhesions in kindlin-2 knockdown cells. We speculate that the residual kindlin-2 (∼10–15% of wild-type levels remaining) in these cells is capable of targeting migfilin to FAs. Because we are unable to examine migfilin targeting in a completely kindlin-2 null system we cannot definitively assess migfilin FA targeting in the absence of kindlin. We therefore tested whether this kindlin-2 depletion alters migfilin behavior at FAs. Our FRAP studies demonstrated that kindlin-2 depletion leads to significantly slower recovery of GFP-migfilin to FAs. Additionally, once localized to an adhesion, migfilin is less mobile when kindlin-2 is knocked down. Not only does this support the suggestion that migfilin FA recruitment has some kindlin-2 dependence, but this is the first demonstration that migfilin mobility within adhesions also has a kindlin dependence. This also confirms that kindlin plays an important role in regulating adhesion formation and dynamics. Our work provides an additional detail, showing that kindlin impacts the localization and dynamics of migfilin and perhaps other focal adhesion components.
Migfilin was originally implicated in cell adhesion, spreading, and migration based on the phenotype of migfilin knockdown or knock-out cells (23, 25, 39). The ability of migfilin to compete with integrin for binding to filamin (29) led to the hypothesis that migfilin might activate integrins by preventing binding of the activation inhibitor filamin (28–30). Furthermore, as discussed here, migfilin binds to the integrin activator kindlin. In addition to effects on integrin-mediated processes, migfilin has also been reported to localize to cell-cell contacts (32). Given the range of data implicating migfilin in adhesion-dependent processes it was surprising that the first published description of a migfilin knock-out mouse or isolated cells from these animals show no obvious phenotype (39). The authors of this study hypothesized that up-regulation of another LIM domain-containing protein may compensate for the lack of migfilin. However, a more recent study (24) of an independently generated migfilin knock-out mouse reports a key role for migfilin in regulation of bone remodeling. Thus, whereas the exact cellular role of migfilin is not yet clear, our work shows a correlation between FA-targeting domains and kindlin-binding regions of migfilin, suggesting that kindlin binding is very important for migfilin targeting and function. Additionally, we show the first line of evidence for a direct interaction between mammalian migfilin and kindlins using FRET. Finally, our FRAP data is the first description of migfilin dynamics in adhesions and the first demonstration of a kindlin dependence of migfilin adhesion recovery and mobility.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 GM-068600, RO1 GM088240, and T32 GM-007223 and American Cancer Society Grant RSG-12-053-01.
- FA
- focal adhesion
- FRET
- fluorescence resonance energy transfer
- FRAP
- fluorescent recovery after photobleaching
- FLNa
- filamin A
- FLIM
- fluorescence lifetime imaging microscopy.
REFERENCES
- 1. Hynes R. O. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2. Harburger D. S., Calderwood D. A. (2009) Integrin signalling at a glance. J. Cell Sci. 122, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calderwood D. A. (2004) Integrin activation. J. Cell Sci. 117, 657–666 [DOI] [PubMed] [Google Scholar]
- 4. Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. (2003) Talin binding to integrin β tails. A final common step in integrin activation. Science 302, 103–106 [DOI] [PubMed] [Google Scholar]
- 5. Montanez E., Ussar S., Schifferer M., Bösl M., Zent R., Moser M., Fässler R. (2008) Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaidel-Bar R., Itzkovitz S., Ma'ayan A., Iyengar R., Geiger B. (2007) Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wehrle-Haller B. (2012) Structure and function of focal adhesions. Curr. Opin. Cell Biol. 24, 116–124 [DOI] [PubMed] [Google Scholar]
- 8. Ma Y. Q., Qin J., Wu C., Plow E. F. (2008) Kindlin-2 (Mig-2). A co-activator of β3 integrins. J. Cell Biol. 181, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. (2008) Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325–330 [DOI] [PubMed] [Google Scholar]
- 10. Harburger D. S., Bouaouina M., Calderwood D. A. (2009) Kindlin-1 and -2 directly bind the C-terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 284, 11485–11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ussar S., Wang H. V., Linder S., Fässler R., Moser M. (2006) The Kindlins. Subcellular localization and expression during murine development. Exp. Cell Res. 312, 3142–3151 [DOI] [PubMed] [Google Scholar]
- 12. Siegel D. H., Ashton G. H., Penagos H. G., Lee J. V., Feiler H. S., Wilhelmsen K. C., South A. P., Smith F. J., Prescott A. R., Wessagowit V., Oyama N., Akiyama M., Al Aboud D., Al Aboud K., Al Githami A., Al Hawsawi K., Al Ismaily A., Al-Suwaid R., Atherton D. J., Caputo R., Fine J. D., Frieden I. J., Fuchs E., Haber R. M., Harada T., Kitajima Y., Mallory S. B., Ogawa H., Sahin S., Shimizu H., Suga Y., Tadini G., Tsuchiya K., Wiebe C. B., Wojnarowska F., Zaghloul A. B., Hamada T., Mallipeddi R., Eady R. A., McLean W. H., McGrath J. A., Epstein E. H. (2003) Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am. J. Hum. Genet. 73, 174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi X., Ma Y. Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F., Wu C. (2007) The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455–20466 [DOI] [PubMed] [Google Scholar]
- 14. Pluskota E., Dowling J. J., Gordon N., Golden J. A., Szpak D., West X. Z., Nestor C., Ma Y. Q., Bialkowska K., Byzova T., Plow E. F. (2011) The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood 117, 4978–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bialkowska K., Ma Y. Q., Bledzka K., Sossey-Alaoui K., Izem L., Zhang X., Malinin N., Qin J., Byzova T., Plow E. F. (2010) The integrin co-activator kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. J. Biol. Chem. 285, 18640–18649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuijpers T. W., van de Vijver E., Weterman M. A., de Boer M., Tool A. T., van den Berg T. K., Moser M., Jakobs M. E., Seeger K., Sanal O., Unal S., Cetin M., Roos D., Verhoeven A. J., Baas F. (2009) LAD-1/variant syndrome is caused by mutations in FERMT3. Blood 113, 4740–4746 [DOI] [PubMed] [Google Scholar]
- 17. Moser M., Bauer M., Schmid S., Ruppert R., Schmidt S., Sixt M., Wang H. V., Sperandio M., Fässler R. (2009) Kindlin-3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 15, 300–305 [DOI] [PubMed] [Google Scholar]
- 18. Malinin N. L., Zhang L., Choi J., Ciocea A., Razorenova O., Ma Y. Q., Podrez E. A., Tosi M., Lennon D. P., Caplan A. I., Shurin S. B., Plow E. F., Byzova T. V. (2009) A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat. Med. 15, 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bandyopadhyay A., Rothschild G., Kim S., Calderwood D. A., Raghavan S. (2012) Functional differences between kindlin-1 and kindlin-2 in keratinocytes. J. Cell Sci. 125, 2172–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bouaouina M., Goult B. T., Huet-Calderwood C., Bate N., Brahme N. N., Barsukov I. L., Critchley D. R., Calderwood D. A. (2012) A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated αIIbβ3 integrin coactivation. J. Biol. Chem. 287, 6979–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Has C., Herz C., Zimina E., Qu H. Y., He Y., Zhang Z. G., Wen T. T., Gache Y., Aumailley M., Bruckner-Tuderman L. (2009) Kindlin-1 Is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am J. Pathol. 175, 1442–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai-Cheong J. E., Ussar S., Arita K., Hart I. R., McGrath J. A. (2008) Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J. Invest. Dermatol. 128, 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tu Y., Wu S., Shi X., Chen K., Wu C. (2003) Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113, 37–47 [DOI] [PubMed] [Google Scholar]
- 24. Xiao G., Cheng H., Cao H., Chen K., Tu Y., Yu S., Jiao H., Yang S., Im H. J., Chen D., Chen J., Wu C. (2012) Critical role of filamin-binding LIM protein 1 (FBLP-1)/migfilin in regulation of bone remodeling. J. Biol. Chem. 287, 21450–21460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ou Y., Ma L., Dong L., Ma L., Zhao Z., Ma L., Zhou W., Fan J., Wu C., Yu C., Zhan Q., Song Y. (2012) Migfilin protein promotes migration and invasion in human glioma through epidermal growth factor receptor-mediated phospholipase C-γ and STAT3 protein signaling pathways. J. Biol. Chem. 287, 32394–32405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papachristou D. J., Gkretsi V., Tu Y., Shi X., Chen K., Larjava H., Rao U. N., Wu C. (2007) Increased cytoplasmic level of migfilin is associated with higher grades of human leiomyosarcoma. Histopathology 51, 499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y., Tu Y., Gkretsi V., Wu C. (2006) Migfilin interacts with vasodilator-stimulated phosphoprotein (VASP) and regulates VASP localization to cell-matrix adhesions and migration. J. Biol. Chem. 281, 12397–12407 [DOI] [PubMed] [Google Scholar]
- 28. Ithychanda S. S., Das M., Ma Y. Q., Ding K., Wang X., Gupta S., Wu C., Plow E. F., Qin J. (2009) Migfilin, a molecular switch in regulation of integrin activation. J. Biol. Chem. 284, 4713–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lad Y., Jiang P., Ruskamo S., Harburger D. S., Ylänne J., Campbell I. D., Calderwood D. A. (2008) Structural basis of the migfilin/filamin interaction and competition with integrin β tails. J. Biol. Chem. 283, 35154–35163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Das M., Ithychanda S. S., Qin J., Plow E. F. (2011) Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils. PloS One 6, e26355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiema T., Lad Y., Jiang P., Oxley C. L., Baldassarre M., Wegener K. L., Campbell I. D., Ylänne J., Calderwood D. A. (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337–347 [DOI] [PubMed] [Google Scholar]
- 32. Gkretsi V., Zhang Y., Tu Y., Chen K., Stolz D. B., Yang Y., Watkins S. C., Wu C. (2005) Physical and functional association of migfilin with cell-cell adhesions. J. Cell Sci. 118, 697–710 [DOI] [PubMed] [Google Scholar]
- 33. Heuzé M. L., Lamsoul I., Baldassarre M., Lad Y., Lévêque S., Razinia Z., Moog-Lutz C., Calderwood D. A., Lutz P. G. (2008) ASB2 targets filamins A and B to proteasomal degradation. Blood 112, 5130–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kloeker S., Major M. B., Calderwood D. A., Ginsberg M. H., Jones D. A., Beckerle M. C. (2004) The Kindler syndrome protein is regulated by transforming growth factor-β and involved in integrin-mediated adhesion. J. Biol. Chem. 279, 6824–6833 [DOI] [PubMed] [Google Scholar]
- 35. Parsons M., Messent A. J., Humphries J. D., Deakin N. O., Humphries M. J. (2008) Quantification of integrin receptor agonism by fluorescence lifetime imaging. J. Cell Sci. 121, 265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Worth D. C., Hodivala-Dilke K., Robinson S. D., King S. J., Morton P. E., Gertler F. B., Humphries M. J., Parsons M. (2010) αvβ3 integrin spatially regulates VASP and RIAM to control adhesion dynamics and migration. J. Cell Biol. 189, 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morton P. E., Parsons M. (2011) Measuring FRET using time-resolved FLIM. Methods Mol. Biol. 769, 403–413 [DOI] [PubMed] [Google Scholar]
- 38. Petricca G., Leppilampi M., Jiang G., Owen G. R., Wiebe C., Tu Y., Koivisto L., Häkkinen L., Wu C., Larjava H. (2009) Localization and potential function of kindlin-1 in periodontal tissues. Eur. J. Oral Sci. 117, 518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moik D. V., Janbandhu V. C., Fässler R. (2011) Loss of migfilin expression has no overt consequences on murine development and homeostasis. J. Cell Sci. 124, 414–421 [DOI] [PubMed] [Google Scholar]