Abstract
Traditional medicines are being focused on as possible treatments for diabetes and its complications because of their negligible toxic and/or side effects. In line with this, our group has reported that Corni Fructus, a traditional medicine considered exhibiting beneficial effects on liver and kidney functions, possessed an antidiabetic effect via ameliorating glucose-mediated metabolic disorders. To add to these findings, we screened the iridoid glycoside fraction containing morroniside and loganin, and low molecular weight polyphenol fraction containing 7-O-galloyl-d-sedoheptulose (GS) from Corni Fructus. To our knowledge, GS is a compound only detected in Corni Fructus, and its biological activity has been poorly understood until now. For these reasons, we examined whether GS has an ameliorative effect on diabetic changes using type 2 diabetic db/db mice. Our findings suggest that GS has a beneficial effect on the pathological state of the serum, kidney, and adipose tissue related to diabetic damage.
1. Background
Diabetes is a metabolic disorder known to cause deleterious changes in various tissues exhibited as diabetic complications triggered by hyperglycemia, dyslipidemia, oxidative stress, inflammation, and advanced glycation [1]. Among these pathogenic factors in diabetes, abnormal lipid metabolism and hyperglycemia-induced oxidative and carbonyl stress (so-called lipotoxicity and glucotoxicity) play a central role in the initiation and progression of diabetes-related disease [2]. Chronic hyperglycemia and dyslipidemia cause oxidative stress and inflammatory responses through the formation of advanced glycation end-products (AGEs) [3, 4], activation of the protein kinase C pathway [5, 6], increased glucose flux through the polyol pathway [7], and the accelerated generation of reactive oxygen species (ROS) [8, 9]. The resulting glycative, glycoxidative, and carbonyl lipotoxicity and oxidative stresses play a key role in the pathogenesis of diabetes [10–13]. Therefore, the attenuation of oxidative stress and regulation of hyperlipidemia have been considered as ways to alleviate diabetes and diabetic complications.
Clinical evidence has suggested that the appropriate use of traditional Chinese medicines with modern Western medicinal, or mainstream antidiabetic drugs, can prevent or ameliorate the development of diabetic complications. Many diabetic patients choose alternative therapeutic approaches such as herbal or traditional Chinese medicine along with mainstream antidiabetic drugs, thus making alternative therapy for diabetes very popular [14]. However, these medicines usually have an insufficient scientific basis, and the exact mechanisms behind their beneficial effects are unknown. Therefore, recently, based on a large number of chemical and pharmacological studies, numerous bioactive compounds have been identified in Chinese medicinal plants for diabetes [15], and we have investigated the mechanism and bioactive constituents of Corni Fructus, the fruit of Cornus officinalis Sieb. et Zucc. (Cornaceous), in diabetic animal models.
Corni Fructus is an important crude herb used in Chinese medicine. It is considered to be one of the 25 plant-based drugs most frequently used in China, Japan, and Korea. It is known to exhibit several biological activities, including hypoglycemic, antineoplastic, and antimicrobial effects, and improve liver and kidney functions [16–18]. We previously reported that treatment with Corni Fructus for 10 days suppressed hyperglycemia, proteinuria, renal AGE formation, and related protein expressions, that is, receptor for AGEs (RAGE), nuclear factor-kappa B (NF-κB), transforming growth factor-β 1 (TGF-β 1), and N ε-(carboxymethyl)lysine (CML), in the same way as with aminoguanidine. However, improvement of the renal function, shown via serum creatinine and creatinine clearance, was superior to aminoguanidine treatment [19]. In addition, the administration of Corni Fructus inhibited the elevation of both systolic and diastolic blood pressures, and lowered serum total cholesterol levels with a decrease in esterified cholesterol in a diet-induced hypercholesterolemia rat model [20]. Moreover, the atherogenic index was decreased in a dose-dependent manner, suggesting its protective role against cardiovascular disease through regulating cholesterol and lipoprotein levels [20]. Therefore, Corni Fructus was suggested to have beneficial effects on diabetes and diabetic complications.
The discovery of efficacious components is essential for clarification of the precise mechanisms of herbal medicines. However, studies on the biological activities of the active components in Corni Fructus are limited. Therefore, we isolated the major active components of Corni Fructus by employing activity-guided fractionation (Figure 1), and the effects of morroniside, loganin, and 7-O-galloyl-d-sedoheptulose (GS) were assessed on glucose metabolism, AGE formation, oxidative stress, and inflammation in type 2 diabetic liver, kidney, pancreas, and adipose tissue to identify their effects and mechanism of action in type 2 diabetes [21–30]. Among the isolated components of Corni Fructus which were suggested to be important contributors to prevent and/or delay the onset of diabetic disease, GS, to our knowledge, is a compound only detected in Corni Fructus [31]. Part of the sugar (sedoheptulose) in GS is ketoheptose, a monosaccharide with seven carbon atoms and a ketone functional group. Sedoheptulose is a seven-carbon ketose sugar originally found in Sedum spectabile, a common perennial garden plant. It is often a part of the human diet. This sugar, d-sedoheptulose (I), is a significant intermediary compound in the cyclic regeneration of d-ribulose. It also plays an important role as a transitory compound in the cyclic regeneration of d-ribulose for carbon dioxide fixation in plant photosynthesis.
Figure 1.
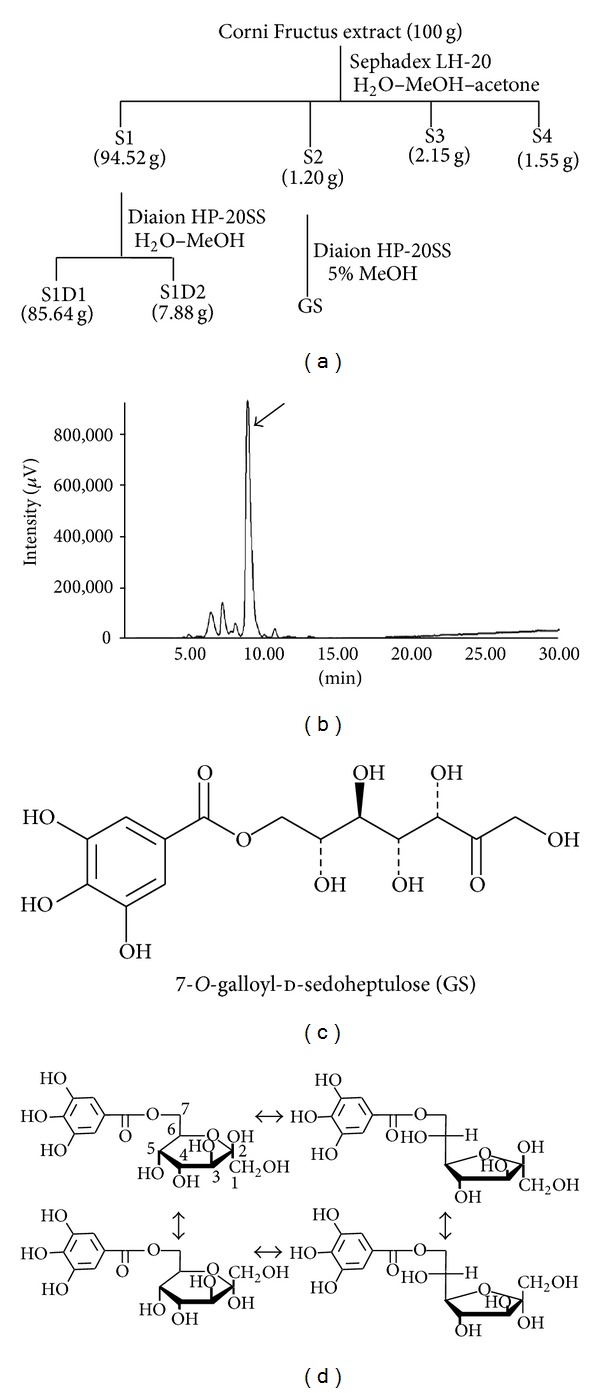
Fractionation of Corni Fructus, HPLC profile of GS, and its structure. (a) Fractionation of Corni Fructus was performed as described in Biological & Pharmaceutical Bulletin, vol. 30, no. 7, pp. 1289–1296, 2007. (b) HPLC profile. The large peak shown by the arrow is the structure of GS, as described in (c), and the other peaks represent its four isomers, as described in (d).
Therefore, this paper presents a review of our recent findings, with emphasis on the therapeutic potential of the polyphenol, GS, of Corni Fructus against diabetic damage in the kidney and adipose tissue.
2. Type 2 Diabetic db/db Mice
To investigate the effect of GS, db/db mice were used. A spontaneous mutant strain, C57BLKS/J db/db mice, has the db mutation, a splicing mutation caused by a point mutation in the downstream intron of the leptin receptor gene, and so it is unresponsive to leptin. Leptin is a peptide hormone secreted by adipocytes and is involved in eating behavior and energy homeostasis. For this reason, after birth, the homozygous diabetic (db/db) mice show unrepressed eating behavior, become obese, and develop severe insulin resistance associated with hyperinsulinemia and hyperglycemia [32]. In this study, db/db mice showed diabetic characteristics, such as hyperglycemia, hyperleptinemia, and hyperinsulinemia, compared with homozygous control (m/m) mice, as presented in Table 1. GS administration significantly reduced serum leptin and insulin levels at a dose of 100 mg/kg, while the serum glucose level was slightly decreased without significance. The serum C-peptide level was compared as an indirect biomarker of insulin secretion. As expected, there was a significant increase in the serum C-peptide level in the vehicle-treated db/db group, which was closely associated with the increased removal of blood glucose (Table 1). Thus, GS treatment prevents diabetes in db/db mice, as evidenced by improved insulin sensitivity through the maintenance of normal insulin and glucose levels and the preservation of insulin and C-peptide levels in the serum, meaning that GS can ameliorate impaired glucose and insulin tolerance in db/db mice.
Table 1.
Glucose, leptin, insulin, and C-peptide in serum.
| Group | Dose (mg/kg body weight/day) | Glucose (mg/dL) | Leptin (ng/dL) | Insulin (ng/mL) | C-peptide (pg/mL) |
|---|---|---|---|---|---|
| m/m | — | 186 ± 25c | 2.30 ± 0.32c | 1.82 ± 0.06b | 177 ± 15c |
| db/db | |||||
| Veh | — | 791 ± 42 | 20.24 ± 0.29 | 3.72 ± 0.45 | 1,983 ± 277 |
| GS | 20 | 745 ± 31 | 18.51 ± 0.75 | 2.68 ± 0.11a | 1,135 ± 139a |
| GS | 100 | 683 ± 41 | 17.57 ± 0.87a | 2.40 ± 0.04b | 970 ± 142b |
m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS20 mg/kg body weight-treated db/db mice; GS100, GS100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01, c P < 0.001 versus vehicle-treated db/db mouse values.
3. GS Ameliorates Renal Damage Triggered by ROS-Sensitive Pathway of Inflammation and Apoptosis
Initial diabetic renal damage is known to involve hyperglycemia-induced oxidative stress. Increased oxygen and peroxy radicals aggravate tissue oxidative stress, which affects the oxidation of important macromolecules including proteins, lipids, carbohydrates, and DNA chains. Moreover, ROS activates the signal transduction cascade and transcription factors and overexpression of genes and proteins in glomerular mesangial and tubular epithelial cells, leading to pathological changes in the kidney [33]. Therefore, in this study, we investigated the effect of GS on the oxidative stress and ROS-related factors involved in the development of diabetic renal damage using type 2 diabetic C57BLKS/J db/db mice.
As shown in Figure 2, GS effectively attenuated oxidative stress via a decrease in ROS and thiobarbituric acid-reactive substance (TBARS) levels as well as an enhanced reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio. In addition, increased serum urea nitrogen and creatinine levels associated with an abnormal renal function were significantly lowered by GS treatment.
Figure 2.
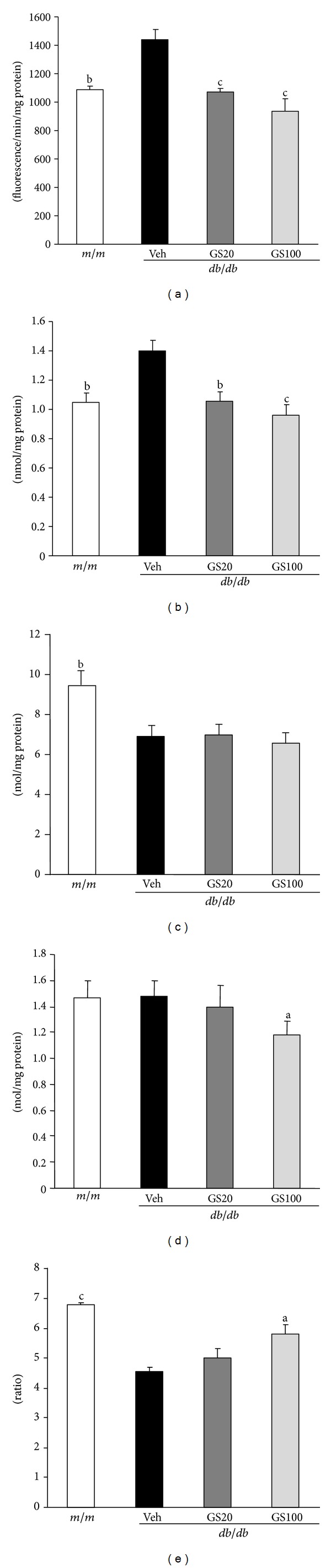
ROS (a), TBARS (b), GSH (c), GSSG (d), and GSH/GSSG (e) levels in the kidney. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01 versus vehicle-treated db/db mouse values.
In the diabetic kidney, enzymatic and nonenzymatic sources of ROS include autoxidation of glucose, transition metal-catalyzed Fenton reactions, advanced glycation, polyol pathway flux, mitochondrial respiratory chain deficiencies, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [34]. Although the origin of increased ROS generation in renal disease is multifactorial, recent studies have focused on the fact that NADPH oxidase mainly participates in the process of ROS generation [35–37]. There is accumulating evidence that nonphagocytic NADPH oxidases are major enzymatic sources of ROS generation in ischemia-reperfusion injury, inflammation, hypertension, and atherosclerosis based on experimental animal and human studies [38, 39]. Also, renal NADPH oxidase expression was reported to be enhanced in glomeruli and distal tubules in the presence of diabetic nephropathy [40]. Structurally, NADPH oxidase comprises the membrane-associated cytochrome b 558, composed of one p22phox and one gp91phox subunit and at least four cytosolic subunits (p47phox, p67phox, p40phox, and the small GTPase rac1 or rac2) [41]. In particular, Nox-4 and p22phox were found to be a major source of ROS production in the kidney and could play a role in pathological conditions [35, 42, 43]. Also, in a rodent model of type 2 diabetes (db/db mouse), the renal expression of Nox-4 and p22phox was increased, and this was associated with ROS-induced renal damage [44]. Therefore, we examined the renal protein expression of Nox-4 and p22phox, subunits of NADPH oxidase, to identify the exact mechanism behind the reduction of renal ROS levels in the GS-treated group. In Western blot analysis, Nox-4 and p22phox protein expressions were significantly upregulated in the type 2 diabetic kidney; however, GS 100 mg/kg administration significantly normalized the increased subunits of NADPH oxidase (Figure 3). These results indicate that the inhibitory effect of GS on ROS generation was due to the downregulated expression of NADPH oxidase in db/db mice.
Figure 3.
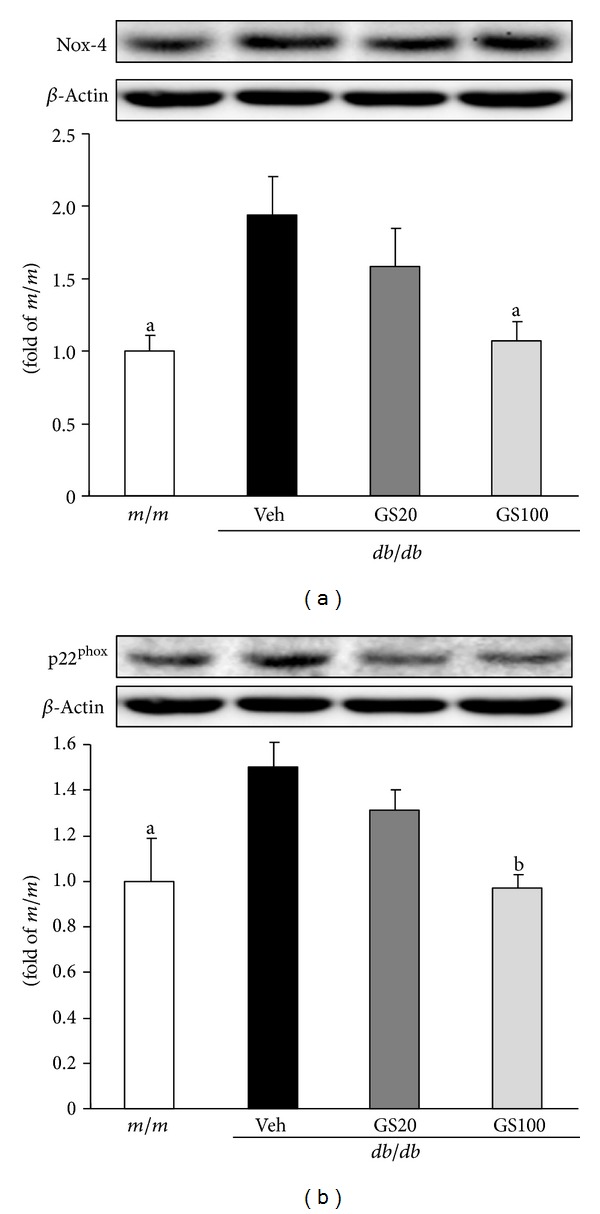
Nox-4 (a) and p22phox (b) protein expressions in the kidney. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01 versus vehicle-treated db/db mouse values.
Furthermore, ROS has been shown to induce apoptosis in the proximal tubular cells of an animal model of unilateral ureteral obstruction [9]. Apoptotic cells have been detected in both proximal and distal tubular epithelia of human and experimental diabetic kidneys [45], suggesting that apoptosis is also involved in the loss of tubular cells in diabetic nephropathy. Increased mitochondrial superoxide production initiates a range of damaging reactions through the production of H2O2, ferrous iron, ∙OH, and ONOO−, which can then damage lipids, proteins, and nucleic acids. A number of functional enzymes within the mitochondria are particularly susceptible to ROS-mediated damage, leading to altered ATP synthesis, cellular calcium dysregulation, and the induction of mitochondrial permeability transition, all of which predispose the cell to necrosis or apoptosis. Podocyte apoptosis has been proposed as a new cellular pathomechanism in diabetic nephropathy [46]. Apoptosis is most likely caused by changing the balance in the expression of the anti- and proapoptotic molecules, Bcl-2 and Bax, respectively. While Bcl-2 expression may account for the maintenance of glomerular hypercellularity, Bax expression might be more important in cell loss leading to glomerulosclerosis. Bax forms oligomers, thereby increasing mitochondrial permeability and facilitating the release of cytochrome c from the mitochondrial intermembrane space. Once released from the mitochondria, cytochrome c further activates apoptosis. In this study, GS administration in db/db mice significantly suppressed renal protein expression of Bax and cytochrome c, although there was no change in Bcl-2 protein levels among all experimental groups (Figure 4). These results suggest that GS prevents apoptosis-induced renal damage, at least in part, through the amelioration of oxidative stress-induced mitochondrial dysfunction.
Figure 4.
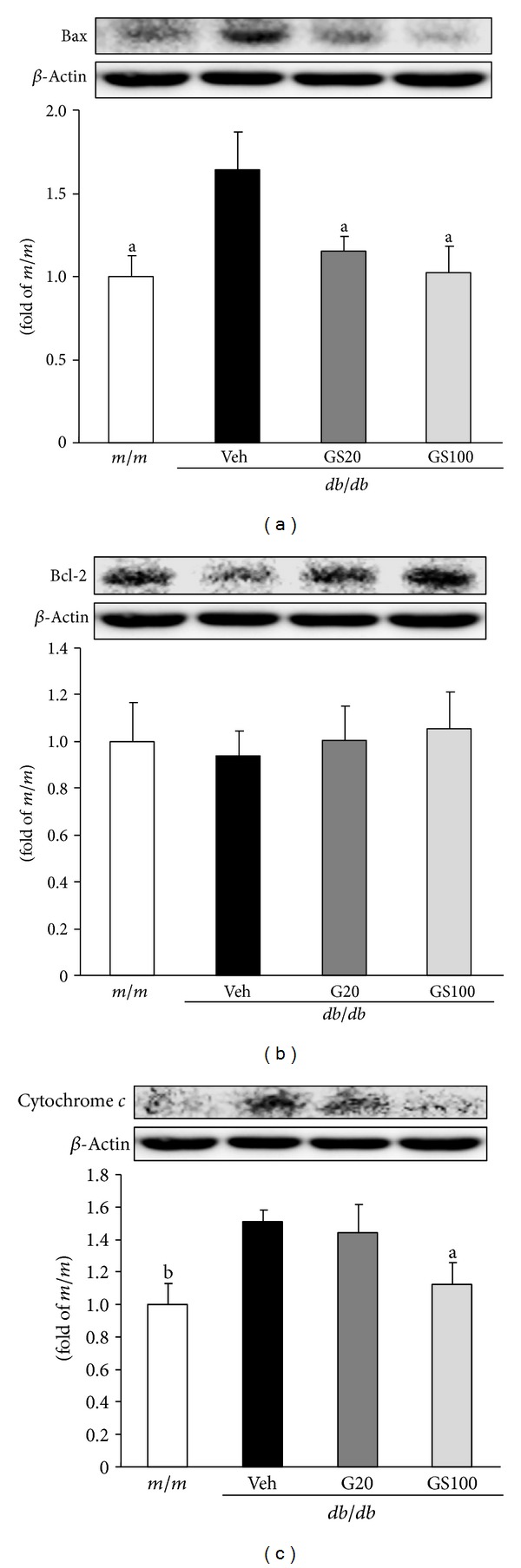
Bax (a), Bcl-2 (b), and cytochrome c (c) protein expressions in the kidney. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01 versus vehicle-treated db/db mouse values.
On the other hand, NF-κB is one of the crosstalk points of multiple signal transduction pathways, and plays a key role in the regulation of transcription and expression of many genes involved in inflammatory responses [47, 48]. For example, enhanced oxidative stress leads to NF-κB transcription and, consequently, induces expressions of its related proinflammatory factors such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) [49]. In humans, COX-2 expression is readily detectable in glomerular podocytes of adults [50, 51], and its expression level has been reported to increase during acute renal allograft rejection [52, 53]. In cultured podocytes, COX-2 overexpression led to more marked cytoskeletal disorganization and apoptosis in response to high-glucose stimulation [54]. These changes were ameliorated by treatment with a specific COX-2 inhibitor, indicating that podocyte COX-2 expression increases susceptibility to the development of diabetic nephropathy [54]. Meanwhile, the rapid induction of iNOS expression can trigger NO-dependent apoptosis in vitro, which appears to result from DNA damage and may be mediated by a p53-dependent apoptotic pathway [55]. iNOS expression is typically absent in unstimulated cells, but is markedly induced by proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6 [56, 57]. For that reason, proinflammatory factors such as NF-κB and its transcriptional factors have been important target genes to prevent further renal damage caused by the inflammatory response and apoptosis. In this study, GS administration to type 2 diabetic db/db mice caused significant renal protein downregulation of NF-κB, COX-2, and iNOS (Figure 5), suggesting that GS efficiently inhibited renal inflammation-related injury in db/db mice.
Figure 5.
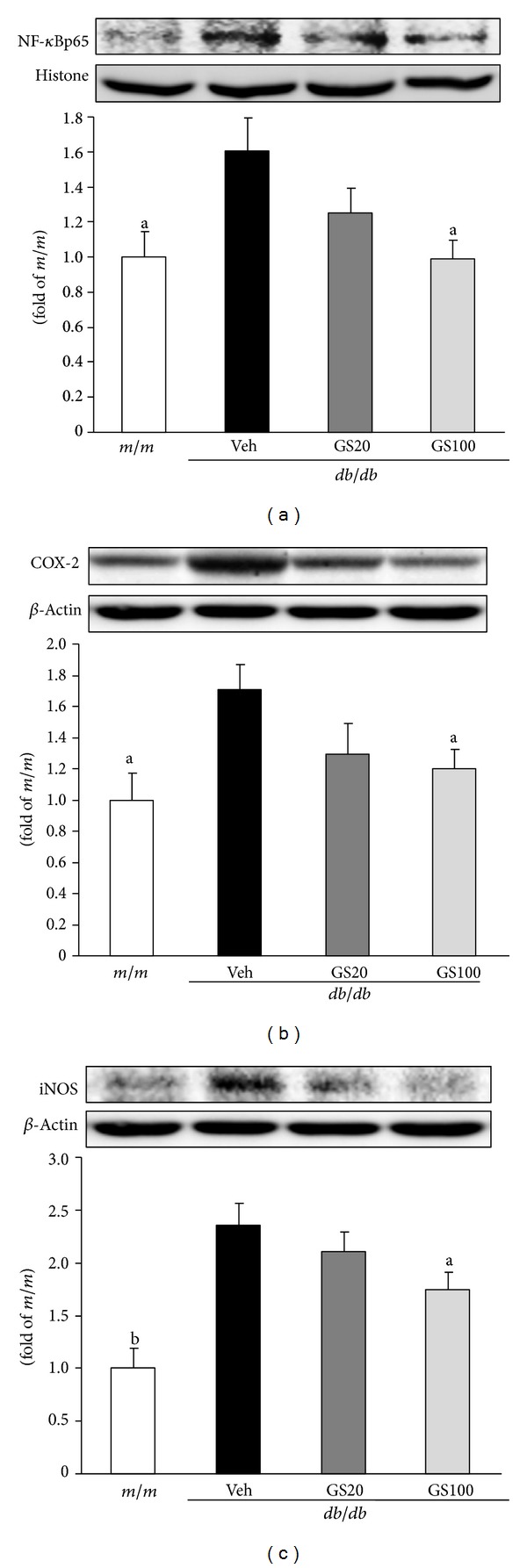
NF-κBp65 (a), COX-2 (b), and iNOS (c) protein expressions in the kidney. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01 versus vehicle-treated db/db mouse values.
This study supports the concept that, in hyperglycemia, enhanced oxidative stress, upregulation of NADPH oxidase and apoptosis, and NF-κB-related inflammation are associated with renal damage in type 2 diabetes. GS administration effectively alleviated these unfavorable responses in the presence of diabetic injury of kidney, as shown in Figure 6. Therefore, this study suggests that GS exerts its renal protective potential through the inhibition of oxidative stress-sensitive mechanisms of apoptosis and the proinflammatory response in the kidney of type 2 diabetics.
Figure 6.
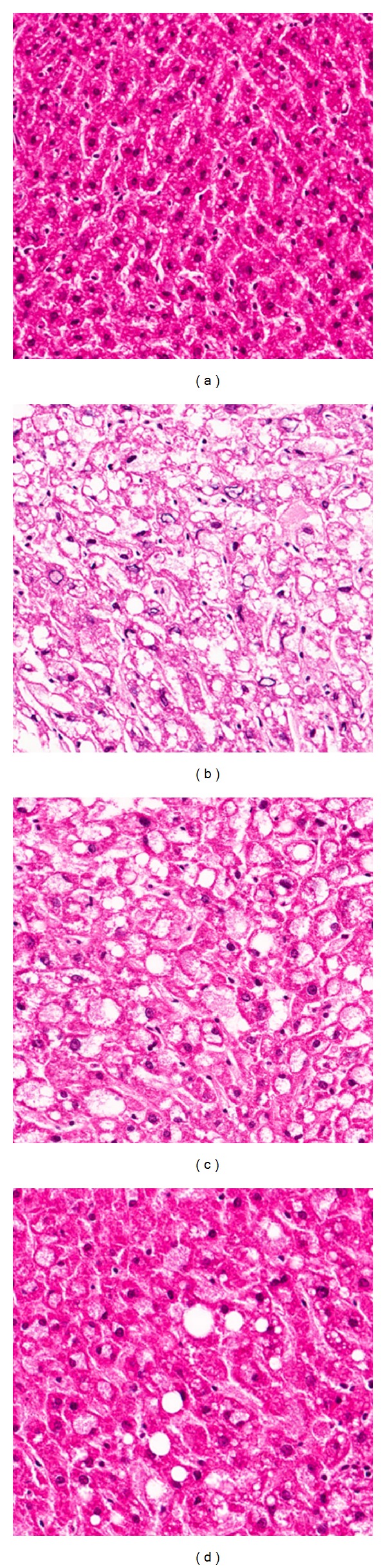
HE staining of the kidney. (a) Misty, (b) vehicle-treated db/db mice, (c) GS 20 mg/kg body weight-treated db/db mice, and (d) GS 100 mg/kg body weight-treated db/db mice. ×200.
4. GS Acts as a Regulator of Oxidative Stress, Inflammation, and Fibrosis in Adipose Tissue
Adipose tissue stores energy in the form of lipids and releases fatty acids in response to nutritional signals or energy insufficiency. In addition, adipocytes have endocrine functions, secreting hormones and factors that regulate physiological functions such as the immune response, insulin sensitivity, and food intake [58]. Excessive fat accumulation in the body and white adipose tissue causes obesity and results in an increased risk of many serious diseases, including type 2 diabetes, hypertension, and heart disease. In the present study, we examined whether GS could prevent the gluco- and lipotoxicity of adipose tissue triggered by the ROS-sensitive pathway of inflammation and fibrosis in type 2 diabetic db/db mice.
The major biochemical alterations in diabetes are hyperglycemia and dyslipidemia, leading to gluco- and lipotoxicity, which directly or indirectly account for diabetic complications in various organs [59–62]. Longitudinal hyperlipidemia, which is associated with the abnormal expression of transcriptional factors such as peroxisome proliferator activated receptor (PPAR) α or sterol regulatory element binding proteins (SREBPs) in the nucleus, increases nonesterified fatty acids (NEFA) uptake and accumulations of triglycerides and cholesterol in tissues. Critical toxicity caused by dyslipidemia is also oxidative stress due to impaired antioxidant defense systems and increased ROS generated by the mitochondrial respiratory chain reaction and glucose autoxidation [63–66]. In this study, the concentrations of triglycerides, total cholesterol, NEFA, high-density lipoprotein (HDL) cholesterol, and very low-density lipoprotein (VLDL)/low-density lipoprotein (LDL) cholesterol in the serum, and triglycerides, total cholesterol, and NEFA in the adipose tissue were significantly elevated in db/db compared to those in m/m mice. The oral administration of GS affected its favorable influences on the lipid profile of serum and adipose tissue (Table 2, Figure 7). Besides its beneficial effects on lipid metabolism, GS administration promoted antioxidant activity. The elevated ROS and TBARS levels in the serum and adipose tissue were ameliorated nearly to those of m/m mice (Table 2, Figure 8). On the other hand, the serum adiponectin level increased on GS treatment, which may be correlated with the decreased serum NEFA level (Table 2). Moreover, lipid metabolism-related protein expressions in the adipose tissue were measured. As shown in Figure 9, protein expressions of transcriptional factors related to lipid regulation, PPARα and PPARγ, were lower in vehicle-treated db/db than m/m mice, but these decreased expressions were significantly elevated by the 20 or 100 mg GS administration. Also, the elevated SREBP-1 expression in vehicle-treated db/db mice was recovered nearly to that of m/m mice on 100 mg/kg GS treatment, suggesting that GS modified lipid metabolism, especially triglyceride synthesis.
Table 2.
Biomarkers associated with lipids, oxidative stress, and inflammation in serum.
| Group | Dose (mg/kg body weight/day) | Triglycerides (mg/dL) | Total cholesterol (mg/dL) | NEFA (mEq/L) | HDL-C (mg/dL) | LDL/VLDL-C (mg/dL) | |
|---|---|---|---|---|---|---|---|
| m/m | — | 114 ± 9c | 110 ± 8c | 0.62 ± 0.02c | 51.01 ± 3.55c | 522 ± 4b | |
| db/db | |||||||
| Veh | — | 263 ± 21 | 186 ± 8 | 2.55 ± 0.06 | 80.95 ± 2.49 | 570 ± 17 | |
| GS | 20 | 198 ± 16a | 179 ± 14 | 1.98 ± 0.04c | 90.88 ± 7.68 | 394 ± 21a | |
| GS | 100 | 175 ± 8b | 163 ± 11 | 1.56 ± 0.15c | 95.88 ± 3.16b | 355 ± 13c | |
|
| |||||||
| Group | Dose (mg/kg body weight/day) | ROS (fluorescence/min/mL) | TBARS (nmol/mL) | Adiponectin (ng/mL) | Resistin (pg/mL) | TNF-α (pg/mL) | IL-6 (pg/mL) |
|
| |||||||
| m/m | — | 790 ± 175a | 18.33 ± 0.46c | 6.32 ± 0.27c | 522 ± 4b | 117 ± 17a | 11.08 ± 0.33c |
| db/db | |||||||
| Veh | — | 1,563 ± 144 | 22.48 ± 0.51 | 3.18 ± 0.09 | 570 ± 17 | 261 ± 28 | 21.27 ± 2.07 |
| GS | 20 | 950 ± 112a | 11.88 ± 1.45 | 3.68 ± 0.12b | 394 ± 21c | 162 ± 30a | 14.90 ± 2.07 |
| GS | 100 | 840 ± 70b | 9.06 ± 1.19c | 4.63 ± 0.19c | 355 ± 13c | 133 ± 13b | 12.45 ± 1.19b |
m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS20 mg/kg body weight-treated db/db mice; GS100, GS100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01, c P < 0.001 versus vehicle-treated db/db mouse values.
Figure 7.
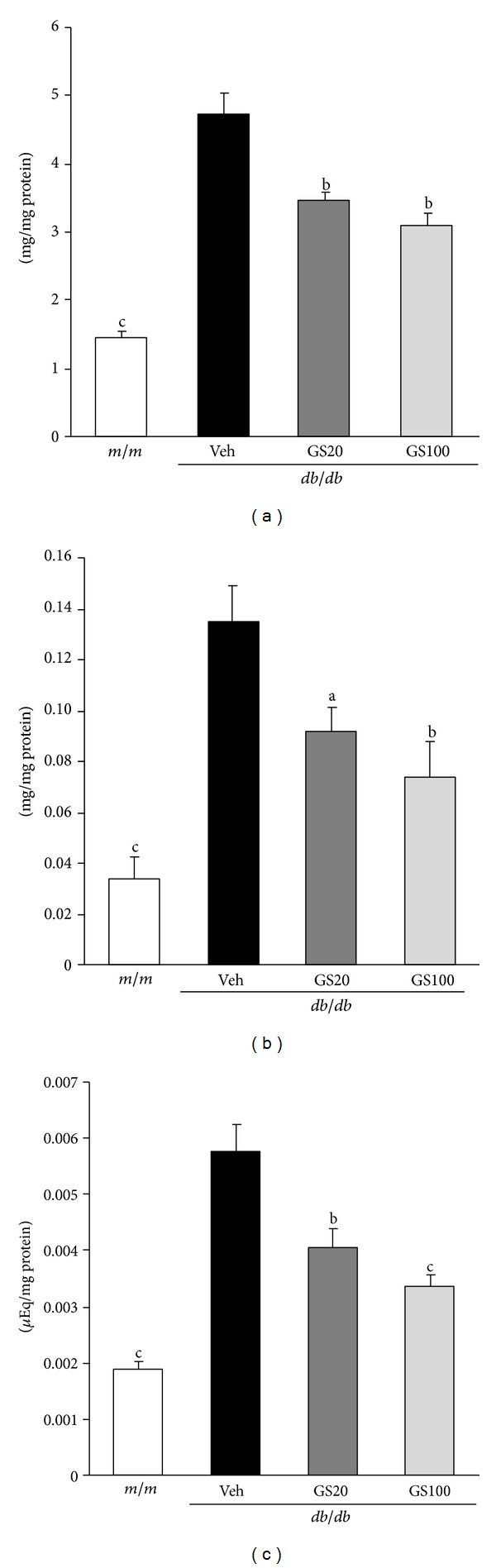
Triglycerides (a), total cholesterol (b), and NEFA (c) contents in the adipose tissue. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01, c P < 0.001 versus vehicle-treated db/db mouse values.
Figure 8.
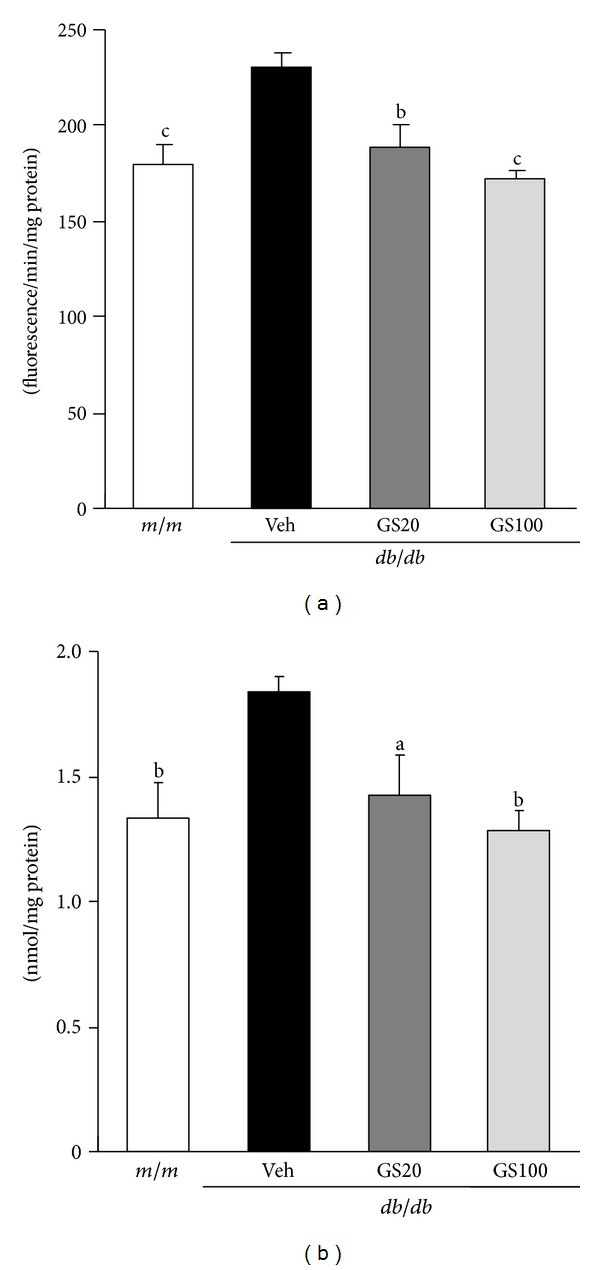
ROS (a) and TBARS (b) levels in the adipose tissue. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01, c P < 0.001 versus vehicle-treated db/db mouse values.
Figure 9.
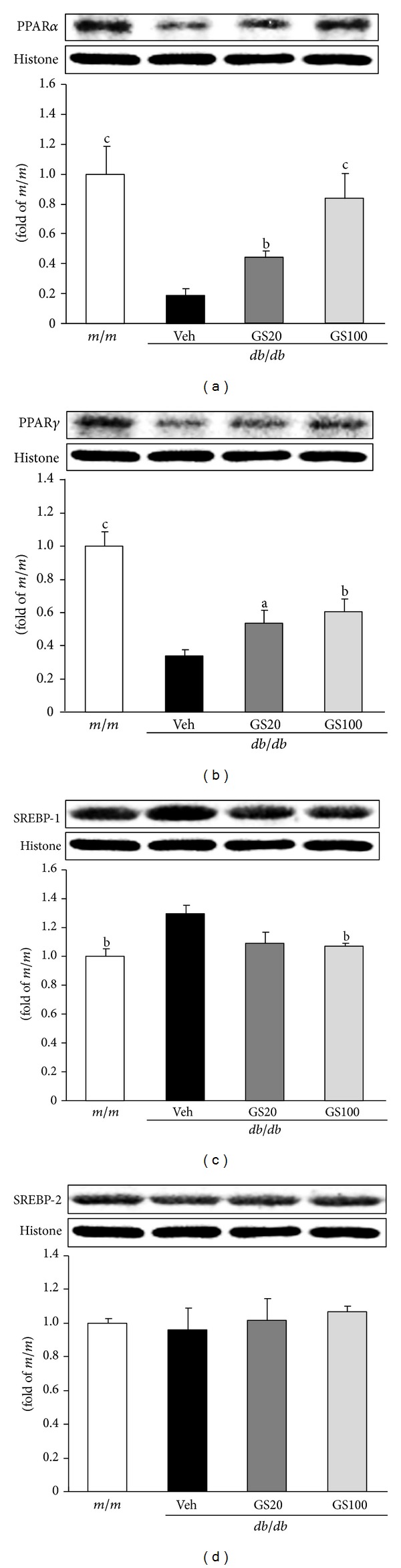
PPARα (a), PPARγ (b), SREBP-1 (c), and SREBP-2 (d) protein expressions in the adipose tissue. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01, c P < 0.001 versus vehicle-treated db/db mouse values.
Previously, we proposed that the suppression of inflammation is possibly linked to antidiabetic effects [67], and other studies have reported that type 2 diabetes can occur through mechanisms related to the inflammatory state [68]. As inflammation is considered to be a major factor contributing to type 2 diabetes [68], we examined proinflammatory markers including TNF-α and IL-6 in the serum, and found that GS treatment inhibited serum TNF-α and IL-6 (Table 2), indicating that the anti-inflammatory properties of GS result in protection against insulin resistance, consistent with a previous report [69], revealing that the suppression of inflammation via the modulation of adiponectin, IL-6, and TNF-α is an important protective factor against insulin resistance. It was reported that NF-κB results in insulin resistance by activating proinflammatory cytokines like TNF-α, IL-6, IL-1β, and resistin, which consequently activates the c-Jun N-terminal kinase (JNK) and NF-κB pathways to create a vicious cycle that exacerbates tissue damage [70].
We further examined proinflammatory NF-κBp65, COX-2, and iNOS protein levels in the adipose tissue of db/db mice, and found that GS treatment downregulated levels of these proteins (Figure 10), suggesting that GS treatment had antidiabetic effects due to its anti-inflammatory actions. These results showing the amelioration of proinflammatory markers, that is, NF-κBp65 and COX-2 protein expressions, are in parallel with a recent report showing enhanced iNOS protein expression due to NF-κB activation [71]. In addition, it has also been shown that polyphenolic compounds can modulate inflammatory responses via the inhibition of COX-2 protein expression through the suppression of JNK activation and inhibition of proinflammatory mediators, like TNF-α, by the attenuation of NF-κB and JNK pathways [72]. GS modulated the activation of JNK pathway (JNK→phosphor (p)-JNK→activator protein (AP)-1→TGF-β 1) (Figure 11). These data are consistent with a previous report [73] showing that not only the modulation of oxidative stress and consequent activation of the JNK pathway, but also the suppression of inflammation are involved in the development of dysfunction found in adipose tissue in the presence of diabetes, which, therefore, would make these useful therapeutic targets against adipose tissue in diabetes.
Figure 10.
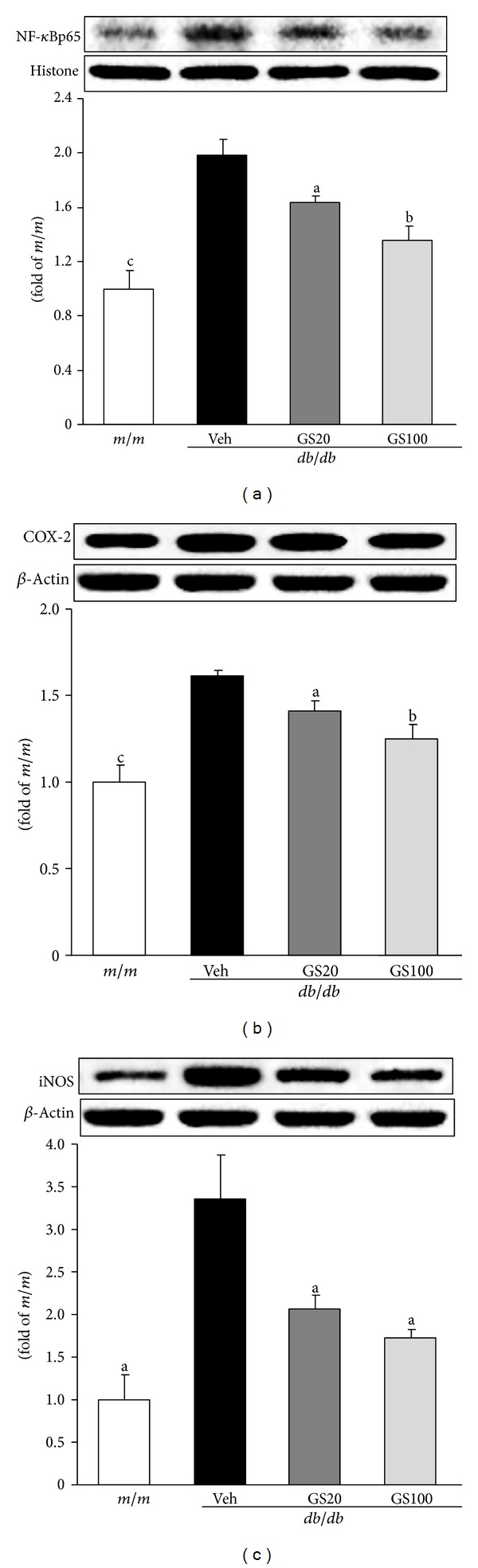
NF-κBp65 (a), COX-2 (b), and iNOS (c) protein expressions in the adipose tissue. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01, c P < 0.001 versus vehicle-treated db/db mouse values.
Figure 11.
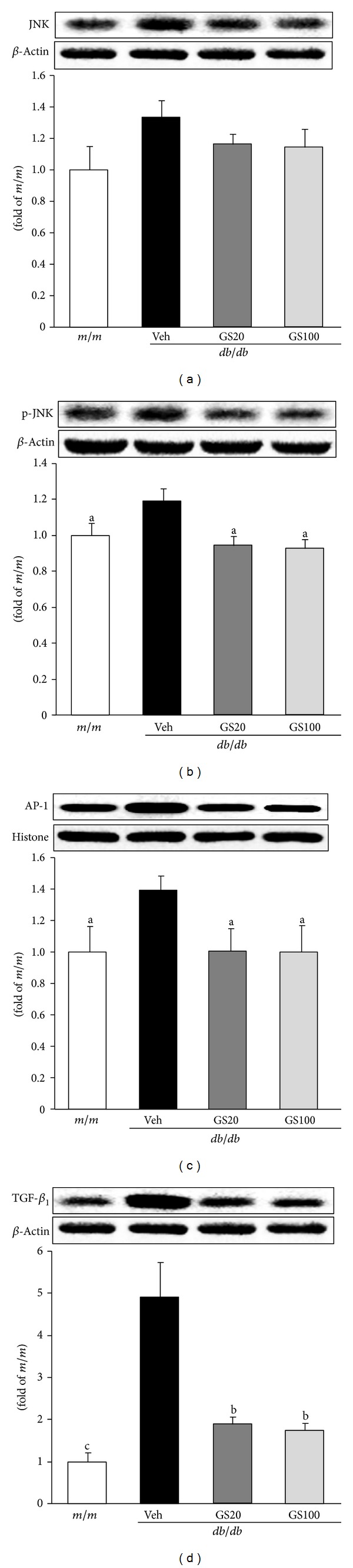
JNK (a), p-JNK (b), AP-1 (c), and TGF-β 1 (d) protein expressions in the adipose tissue. m/m, Misty; Veh, vehicle-treated db/db mice; GS20, GS 20 mg/kg body weight-treated db/db mice; GS100, GS 100 mg/kg body weight-treated db/db mice. The results are presented as the means ± S.E.M. a P < 0.05, b P < 0.01, c P < 0.001 versus vehicle-treated db/db mouse values.
One of our significant findings in this study was GS's suppression of diverse proinflammatory cytokines such as TNF-α, IL-6, resistin, and TGF-β 1 that activate the JNK and NF-κB pathways and proinflammatory COX-2 protein expression (Table 2, Figures 10 and 11). In particular, our data showing the suppression of both oxidative stress and inflammation by GS treatment are consistent with our previous report [67], revealing a close relationship between antioxidative and anti-inflammatory actions in diabetes. Thus, based on the results from both our previous and current studies, we suggest a possible mechanism by which the antidiabetic action of GS mediates type 2 diabetes through its dual suppression of oxidative stress and inflammation, as shown in our experiments with db/db mice. Consecutively, GS could reduce the increased level of TGF-β 1 in the adipose tissue, showing a reduction in fibrosis. These findings suggest that the hyperglycemic control of GS may, at least in part, be derived from the amelioration of disorders such as fibrosis in adipose tissue.
Although the mechanistic details of GS need to be clarified in future studies, our findings support the therapeutic evidence for GS ameliorating the development of diabetic damage in adipose tissue. An important mechanism of GS's antidiabetic effect is its capacity to lower oxidative stress by reducing ROS generation and lipid peroxidation in adipose tissue. Our data further suggest that another critical mechanism of GS's antidiabetic property is its ability to ameliorate inflammation and fibrosis through modulation of the serum TNF-α and IL-6 levels, and oxidative-, inflammation-, and fibrosis-related protein expressions.
5. Conclusion
For patients with type 2 diabetes, hyperlipidemia, and insulin resistance, thiazolidinediones and fibrate drugs, both of which activate PPARs, have been widely used [74], but side effects such as body weight gain with an excess increase of the fat mass have been reported in diabetes patients [75]. Alternatively, traditional Chinese medicines with negligible toxic and/or side effects have been used in East Asia, and, among them, medicines containing Corni Fructus as the main ingredient have been used to treat diabetes. Among the bioactive compounds of Corni Fructus, there is therapeutic evidence for GS ameliorating the development of diabetic damage in the serum, kidney, and adipose tissues. In conclusion, GS, a bioactive compound of Corni Fructus, ameliorates the development of diabetic damage in the serum, kidney, and adipose tissues.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Stumvoll M, Goldstein BJ, Van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. The Lancet. 2005;365(9467):1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 2.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocrine Reviews. 2008;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. New England Journal of Medicine. 1988;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 5.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform βII and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(22):11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47(6):859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 7.Yabe-Nishimura C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacological Reviews. 1998;50(1):21–33. [PubMed] [Google Scholar]
- 8.Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochemical Journal. 1988;256(1):205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 10.Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes and Metabolism. 2000;26(3):163–176. [PubMed] [Google Scholar]
- 11.Jakus V. The role of free radicals, oxidative stress and antioxidant systems in diabetic vascular disease. Bratislavské Lekárske Listy. 2000;101(10):541–551. [PubMed] [Google Scholar]
- 12.West IC. Radicals and oxidative stress in diabetes. Diabetic Medicine. 2000;17(3):171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 13.Miyata T. Alterations of non-enzymatic biochemistry in uremia, diabetes, and atherosclerosis (“carbonyl stress”) Bulletin et Mémoires de l'Académie Royale de Médecine de Belgique. 2002;157(3-4):189–196. [PubMed] [Google Scholar]
- 14.Ceylan-Isik AF, Fliethman RM, Wold LE, Ren J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Current Diabetes Reviews. 2008;4(4):320–328. doi: 10.2174/157339908786241142. [DOI] [PubMed] [Google Scholar]
- 15.Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. Journal of Ethnopharmacology. 2004;92(1):1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Chang JS, Chiang LC, Hsu FF, Lin CC. Chemoprevention against hepatocellular carcinoma of Cornus officinalis in vitro. American Journal of Chinese Medicine. 2004;32(5):717–725. doi: 10.1142/S0192415X04002296. [DOI] [PubMed] [Google Scholar]
- 17.Liou SS, Liu IM, Hsu SF, Cheng JT. Corni Fructus as the major herb of Die-Huang-Wan for lowering plasma glucose in Wistar rats. Journal of Pharmacy and Pharmacology. 2004;56(11):1443–1447. doi: 10.1211/0022357044670. [DOI] [PubMed] [Google Scholar]
- 18.Vareed SK, Reddy MK, Schutzki RE, Nair MG. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sciences. 2006;78(7):777–784. doi: 10.1016/j.lfs.2005.05.094. [DOI] [PubMed] [Google Scholar]
- 19.Yamabe N, Kang KS, Goto E, Tanaka T, Yokozawa T. Beneficial effect of Corni Fructus, a constituent of Hachimi-jio-gan, on advanced glycation end-product-mediated renal injury in streptozotocin-treated diabetic rats. Biological & Pharmaceutical Bulletin. 2007;30(3):520–526. doi: 10.1248/bpb.30.520. [DOI] [PubMed] [Google Scholar]
- 20.Park CH, Cho EJ, Yokozawa T. Protection against hypercholesterolemia by Corni Fructus extract and its related protective mechanism. Journal of Medicinal Food. 2009;12(5):973–981. doi: 10.1089/jmf.2009.0037. [DOI] [PubMed] [Google Scholar]
- 21.Park CH, Yamabe N, Noh JS, Kang KS, Tanaka T, Yokozawa T. The beneficial effects of morroniside on the inflammatory response and lipid metabolism in the liver of db/db mice. Biological & Pharmaceutical Bulletin. 2009;32(10):1734–1740. doi: 10.1248/bpb.32.1734. [DOI] [PubMed] [Google Scholar]
- 22.Park CH, Noh JS, Tanaka T, Yokozawa T. Effects of morroniside isolated from Corni Fructus on renal lipids and inflammation in type 2 diabetic mice. Journal of Pharmacy and Pharmacology. 2010;62(3):374–380. doi: 10.1211/jpp.62.03.0013. [DOI] [PubMed] [Google Scholar]
- 23.Park CH, Noh JS, Yamabe N, Kang KS, Tanaka T, Yokozawa T. Beneficial effect of 7-O-galloyl-d-sedoheptulose on oxidative stress and hepatic and renal changes in type 2 diabetic db/db mice. European Journal of Pharmacology. 2010;640(1–3):233–242. doi: 10.1016/j.ejphar.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Yamabe N, Noh JS, Park CH, et al. Evaluation of loganin, iridoid glycoside from Corni Fructus, on hepatic and renal glucolipotoxicity and inflammation in type 2 diabetic db/db mice. European Journal of Pharmacology. 2010;648(1–3):179–187. doi: 10.1016/j.ejphar.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 25.Park CH, Noh JS, Kim JH, et al. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biological & Pharmaceutical Bulletin. 2011;34(10):1559–1565. doi: 10.1248/bpb.34.1559. [DOI] [PubMed] [Google Scholar]
- 26.Park CH, Tanaka T, Kim JH, et al. Hepato-protective effects of loganin, iridoid glycoside from Corni Fructus, against hyperglycemia-activated signaling pathway in liver of type 2 diabetic db/db mice. Toxicology. 2011;290(1):14–21. doi: 10.1016/j.tox.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Noh JS, Park CH, Tanaka T, Yokozawa T. 7-O-Galloyl-d-sedoheptulose attenuates oxidative stress-induced diabetic injury via decreasing expression of nuclear factor-kB- and apoptosis-related protein in the liver. Biological & Pharmaceutical Bulletin. 2012;35(6):950–956. doi: 10.1248/bpb.35.950. [DOI] [PubMed] [Google Scholar]
- 28.Park CH, Noh JS, Tanaka T, Yokozawa T. 7-O-Galloyl-d-sedoheptulose ameliorates renal damage triggered by reactive oxygen species-sensitive pathway of inflammation and apoptosis. Journal of Pharmacy and Pharmacology. 2012;64(12):1730–1740. doi: 10.1111/j.2042-7158.2012.01559.x. [DOI] [PubMed] [Google Scholar]
- 29.Park CH, Tanaka T, Yokozawa T. Anti-diabetic action of 7-O-galloyl-d-sedoheptulose, a polyphenol from Corni Fructus, through ameliorating inflammation and inflammation-related oxidative stress in the pancreas of type 2 diabetics. Biological & Pharmaceutical Bulletin. 2013;36(5):723–732. doi: 10.1248/bpb.b12-00543. [DOI] [PubMed] [Google Scholar]
- 30.Park CH, Tanaka T, Yokozawa T. Evaluation of 7-O-galloyl-d-sedoheptulose, isolated from Corni Fructus, in the adipose tissue of type 2 diabetic db/db mice. Fitoterapia. 2013;89:131–142. doi: 10.1016/j.fitote.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YW, Chen YW, Zhao SP. A sedoheptulose gallate from the fruits of Cornus officinalis . Acta Pharmaceutica Sinica. 1999;34(2):153–155. [Google Scholar]
- 32.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. American Journal of Physiology: Renal Physiology. 2003;284(6):F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 33.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. Journal of the American Society of Nephrology. 2003;14(3, supplement):S241–S245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 34.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 35.Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. American Journal of Medicine. 2000;109(8):665–678. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 37.Shiose A, Kuroda J, Tsuruya K, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. Journal of Biological Chemistry. 2001;276(2):1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 38.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circulation Research. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 39.Zalba G, José GS, Moreno MU, et al. Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension. 2001;38(6):1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
- 40.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxidants & Redox Signaling. 2006;8(9-10):1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 41.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Archives of Biochemistry and Biophysics. 2002;397(2):342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 42.Etoh T, Inoguchi T, Kakimoto M, et al. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia. 2003;46(10):1428–1437. doi: 10.1007/s00125-003-1205-6. [DOI] [PubMed] [Google Scholar]
- 43.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. Journal of Biological Chemistry. 2005;280(47):39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 44.Sedeek M, Callera G, Montezano A, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. American Journal of Physiology: Renal Physiology. 2010;299(6):F1348–F1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 45.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Molecular and Cellular Biochemistry. 2004;259(1-2):67–70. doi: 10.1023/b:mcbi.0000021346.03260.7e. [DOI] [PubMed] [Google Scholar]
- 46.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 47.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 48.Viedt C, Hänsch GM, Brandes RP, Kübler W, Kreuzer J. The terminal complement complex C5b-9 stimulates interleukin-6 production in human smooth muscle cells through activation of transcription factors NF-κ B and AP-1. The FASEB Journal. 2000;14(15):2370–2372. doi: 10.1096/fj.00-0468fje. [DOI] [PubMed] [Google Scholar]
- 49.Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutation Research. 2001;480-481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 50.Kömhoff M, Grone HJ, Klein T, Seyberth HW, Nüsing RM. Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: implication for renal function. American Journal of Physiology: Renal Physiology. 1997;272(4):F460–F468. doi: 10.1152/ajprenal.1997.272.4.F460. [DOI] [PubMed] [Google Scholar]
- 51.Adegboyega PA, Ololade O. Immunohistochemical expression of cyclooxygenase-2 in normal kidneys. Applied Immunohistochemistry & Molecular Morphology. 2004;12(1):71–74. doi: 10.1097/00129039-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Rangel EB, Moura LA, Franco M, Pacheco-Silva A. Expression of cyclooxygenases during renal allograft rejection. Transplantation Proceedings. 2004;36(4):838–839. doi: 10.1016/j.transproceed.2004.03.106. [DOI] [PubMed] [Google Scholar]
- 53.Rangel ÉB, Moura LA, Franco MF, Pacheco-Silva Á. Up-regulation of cyclooxygenase-2 during acute human renal allograft rejection. Clinical Transplantation. 2005;19(4):543–550. doi: 10.1111/j.1399-0012.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 54.Cheng H, Fan X, Moeckel GW, Harris RC. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. Journal of the American Society of Nephrology. 2011;22(7):1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian B, Liu J, Bitterman PB, Bache RJ. Mechanisms of cytokine induced NO-mediated cardiac fibroblast apoptosis. American Journal of Physiology: Heart and Circulatory Physiology. 2002;283(5):H1958–H1967. doi: 10.1152/ajpheart.01070.2001. [DOI] [PubMed] [Google Scholar]
- 56.Corbett JA, Kwon G, Misko TP, Rodi CP, McDaniel ML. Tyrosine kinase involvement in IL-1β-induced expression of iNOS by β-cells purified from islets of Langerhans. American Journal of Physiology: Cell Physiology. 1994;267(1):C48–C54. doi: 10.1152/ajpcell.1994.267.1.C48. [DOI] [PubMed] [Google Scholar]
- 57.Medeiros R, Prediger RD, Passos GF, et al. Connecting TNF-α signaling pathways to iNOS expression in a mouse model of Alzheimer’s disease: relevance for the behavioral and synaptic deficits induced by amyloid β protein. Journal of Neuroscience. 2007;27(20):5394–5404. doi: 10.1523/JNEUROSCI.5047-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. American Journal of Physiology: Endocrinology and Metabolism. 2001;280(6):E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- 59.Srikanthan P, Hsueh W. Preventing heart failure in patients with diabetes. Medical Clinics of North America. 2004;88(5):1237–1256. doi: 10.1016/j.mcna.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clinical Gastroenterology and Hepatology. 2004;2(3):262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 61.Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. β-Cell failure as a complication of diabetes. Reviews in Endocrine & Metabolic Disorders. 2008;9(4):329–343. doi: 10.1007/s11154-008-9101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanwar YS, Wada J, Sun L, et al. Diabetic nephropathy: mechanisms of renal disease progression. Experimental Biology and Medicine. 2008;233(1):4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 63.Maritim AC, Sanders RA, Watkins JB., III Diabetes, oxidative stress, and antioxidants: a review. Journal of Biochemical and Molecular Toxicology. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 64.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicology and Applied Pharmacology. 2006;212(2):167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxidants & Redox Signaling. 2007;9(3):343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 66.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signaling. Cardiovascular Research. 2009;82(1):9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 67.Lee YA, Kim YJ, Cho EJ, Yokozawa T. Ameliorative effects of proanthocyanidin on oxidative stress and inflammation in streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry. 2007;55(23):9395–9400. doi: 10.1021/jf071523u. [DOI] [PubMed] [Google Scholar]
- 68.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Letters. 2008;582(1):97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guest CB, Park MJ, Johnson DR, Freund GG. The implication of proinflammatory cytokines in type 2 diabetes. Frontiers in Bioscience. 2008;13(13):5187–5194. doi: 10.2741/3074. [DOI] [PubMed] [Google Scholar]
- 70.Ndisang JF. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators of Inflammation. 2010;2010:18 pages. doi: 10.1155/2010/359732.359732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar NB, Kazi A, Smith T, et al. Cancer cachexia: traditional therapies and novel molecular mechanism-based approaches to treatment. Current Treatment Options in Oncology. 2010;11(3-4):107–117. doi: 10.1007/s11864-010-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shan J, Fu J, Zhao Z, et al. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-κB and JNK/AP-1 activation. International Immunopharmacology. 2009;9(9):1042–1048. doi: 10.1016/j.intimp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Kaneto H, Matsuoka TA, Katakami N, et al. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Current Molecular Medicine. 2007;7(7):674–686. doi: 10.2174/156652407782564408. [DOI] [PubMed] [Google Scholar]
- 74.Michalik L, Auwerx J, Berger JP, et al. International union of pharmacology—LXI. Peroxisome proliferator-activated receptors. Pharmacological Reviews. 2006;58(4):726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 75.Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. American Journal of Medicine. 2003;115(8, supplement):42S–48S. doi: 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]


