Abstract
Voice and speech in Parkinson's disease (PD) patients are classically affected by a hypophonia, dysprosody, and dysarthria. The underlying pathomechanisms of these disabling symptoms are not well understood. To identify functional anomalies related to pathophysiology and compensation we compared speech-related brain activity and effective connectivity in early PD patients who did not yet develop voice or speech symptoms and matched controls. During fMRI 20 PD patients ON and OFF levodopa and 20 control participants read 75 sentences covertly, overtly with neutral, or with happy intonation. A cue-target reading paradigm allowed for dissociating task preparation from execution. We found pathologically reduced striato-prefrontal preparatory effective connectivity in early PD patients associated with subcortical (OFF state) or cortical (ON state) compensatory networks. While speaking, PD patients showed signs of diminished monitoring of external auditory feedback. During generation of affective prosody, a reduced functional coupling between the ventral and dorsal striatum was observed. Our results suggest three pathomechanisms affecting speech in PD: While diminished energization on the basis of striato-prefrontal hypo-connectivity together with dysfunctional self-monitoring mechanisms could underlie hypophonia, dysarthria may result from fading speech motor representations given that they are not sufficiently well updated by external auditory feedback. A pathological interplay between the limbic and sensorimotor striatum could interfere with affective modulation of speech routines, which affects emotional prosody generation. However, early PD patients show compensatory mechanisms that could help improve future speech therapies.
Abbreviations: AC, auditory cortex; CN, caudate nucleus; COMT, catechol-O-methyltransferase; CON, control participant; DAT1, dopamine transporter; DLPFC, dorsolateral prefrontal cortex; dPMC, dorsal premotor cortex; dstriatum, dorsal striatum; EPI, echo-planar imaging; fMRI, functional magnetic response imaging; FWE, family-wise error; GLM, general linear model; HRF, hemodynamic response function; IFG, inferior frontal gyrus; LSVT, Lee Silverman Voice Treatment; mPFC, medial prefrontal cortex; PD, Parkinson's disease; PPI, psycho-physiological interaction; PUT, putamen; ROI, region of interest; SEM, standard error of the mean; SMA, supplementary motor area; SPL, superior parietal lobule; STS, superior temporal sulcus; SVC, small volume correction; T, Tesla; UPDRS, Unified Parkinson's Disease Rating Scale; vstriatum, ventral striatum
Keywords: Hypophonia, Dysarthria, Parkinson's disease, Speech production, Dysarthrophonia, Functional MRI
Highlights
-
•
Speech networks are altered in PD patients before they develop speech symptoms.
-
•
Hypophonia relates to reduced energization due to reduced striato-prefrontal coupling.
-
•
PD patients show signatures of reduced monitoring of auditory feedback.
-
•
Dysarthria may result from imprecise shaping of motor representations by feedback.
-
•
External therapeutic models help normalizing speech-related neural anomalies.
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disease that presents with voice and speech symptoms (Möbes et al., 2008). About 90% of PD patients suffer from voice and/or articulatory symptoms in the course of their disease (Aronson, 1990). Voice symptoms usually precede dysarthria: PD patients classically speak in a soft and breathy voice that lacks modulation in volume (monoloudness) and fundamental frequency (monopitch), resulting in flat speech melody (dysprosody) (Canter, 1963). When voice symptoms like hypophonia are accompanied by speech symptoms like hypokinetic dysarthria in later stages of the disease the patients' speech often becomes incomprehensible (Logemann et al., 1978). Neither hypophonia nor dysarthria responds well to dopaminergic treatment (Rascol et al., 2003; Romito and Albanese, 2010) and both symptoms often worsen upon deep brain stimulation of the subthalamic nucleus (Klostermann et al., 2008). Speech and language therapy regimes that focus on voice symptoms by training patients in speaking more loudly and with more increased vocal effort are a particularly effective therapeutic tool (Fox et al., 2002; Ramig et al., 2004). Revealing the pathomechanisms underlying hypophonia and dysarthria could help improve our therapeutic efforts. Hypophonia has been suggested to be caused by a reduced motor drive on the basis of basal ganglia dysfunction, which reminds pathomechanisms of general hypokinesia (Fox et al., 2002). The reduced motor drive may translate into reduced speech intensity but also diminished intensity modulation. This may affect particularly the speech melody inducing monopitch and monoloudness. Prosody may also be affected by dysfluencies that result in rate changes and inappropriate silences. Indeed, prosody production depends on intact basal ganglia circuits (Cancelliere and Kertesz, 1990). The basal ganglia have been shown to be part of sensorimotor loops controlling prosody production but are also thought to modulate speech melody as a function of affective state (Pichon and Kell, 2013). Given that PD patients' speech symptoms become particularly pronounced during emotional speech (Benke et al., 1998; Caekebeke et al., 1991) generation of affective prosody may be impaired earlier than prosody of emotionally neutral utterances.
In addition, PD patients often fail to increase their speech volume when the environment is noisy (Ho et al., 1999). Importantly, they also fail in reducing their speech volume when they receive loud auditory feedback (Ho et al., 2000). This suggests that PD patients not only have difficulties in speaking loudly but also in scaling their speech volume. Physiologically, speech volume can be evaluated on the basis of motor awareness or by judging auditory or somatosensory feedback. Thus, disturbed volumetric scaling in PD could result either from a primary dysfunctional sensorimotor integration during speech production and/or a secondary perceptual deficit (Ho et al., 2000). Finally, there is ongoing debate whether hypokinetic dysarthria primarily reflects abnormal muscle tone or rather hypokinesia of articulators (Berardelli et al., 2001). Yet, we hypothesize that dysarthria could also be a late consequence of pathological sensorimotor integration. Sensorimotor loops could not only be used to scale speech intensity but are also thought to contribute substantially to phonematic processing: Speech is thought to depend on overlearned feedforward speech motor commands that are represented in the opercular part of the left inferior frontal gyrus (IFG) and ventral premotor cortex (Ghosh et al., 2008). These representations are learned during language acquisition on the basis of sensorimotor mapping and are for the rest of the life shaped and updated by sensory feedback. The sensorimotor cortico-basal loops involved in speaking have been proposed to host an internal model that represents and functionally couples feedforward plans and sensory consequences of articulatory gestures (Hickok et al., 2011). Imprecise sensorimotor mapping resulting from basal ganglia dysfunction could lead to fading speech motor representations and thus induce dysarthria.
So far, functional imaging studies on speech symptoms in PD have focused on symptomatic patients. They have shown that PD patients' dysarthrophonia is associated with functional anomalies in the basal ganglia, orofacial motor cortex, and cerebellum, together with an increased recruitment of premotor and prefrontal cortices during speech production (Liotti et al., 2003; Pinto et al., 2004). However, given that the included patients were symptomatic regarding speech symptoms, these studies cannot dissociate whether the observed functional anomalies are cause or consequence of speech symptoms. In case of overt speech symptoms, the functional anomalies could point to different behavior during scanning which renders interpretation of such results difficult. We thus performed a functional magnetic resonance imaging (fMRI) study in early PD patients who did not yet experience speech difficulties at the time of testing. Nearly all included patients developed hypophonia and/or dysarthria two years after inclusion in this study. Functional anomalies in brain activity observed in these patients could directly point to consequences of PD pathology that will eventually lead to development of symptoms but cannot be explained by different speech behavior at the time of scanning. Yet, these anomalies could also reflect compensatory mechanisms that help maintaining speech normal despite disease activity. In our study, we use the comparison between PD patients ON and OFF medication to separate pathological from compensatory anomalies: Given that the included patients speak normal under both conditions, we interpret anomalies that are levodopa-responsive as compensatory, because the increased dopamine availability ON medication diminishes the need for compensation. Please note that this aspect is not contradictory with the finding that overt speech symptoms are usually not levodopa-responsive (Rascol et al., 2003; Romito and Albanese, 2010) but rather acknowledges that dopamine depletion may play a role in the generation of speech symptoms. Indeed, left-lateralized dopaminergic signaling has been implicated in overt articulation (Simonyan et al., 2013) suggesting that dopamine depletion and replacement may affect cortico-basal speech networks despite the inefficacy of acute levodopa administration on the behavioral level.
Given previous hypotheses on pathomechanisms in hypokinetic dysarthria (see above), we tested whether we could detect correlates of a reduced drive to act prior to articulation by dissociating, as in previous studies (Kell et al., 2011; Pichon and Kell, 2013), a cognitive preparation phase from motor preparation and ongoing articulation. Thus, PD patients (ON and OFF medication) and matched control participants were scanned during a cue-target reading paradigm (see Material and methods section). To reveal different sensorimotor speech processing our analyses focused on the contrast between overt and covert reading. We also investigated happy reading because affective prosody generation requires additional drive and vocal effort, modulation of speech intensity and fundamental frequency and necessitates interactions between the limbic and the sensorimotor striatum (Pichon and Kell, 2013). To target cortico-basal loops, we studied voxel-wise brain activity as well as effective connectivity between selected cortical and subcortical brain regions involved in speech production.
We found pathologically reduced striato-prefrontal preparatory effective connectivity in early PD patients as a possible cause of a reduced drive to act associated with subcortical (OFF state) or cortical (ON state) compensatory networks. Additionally, PD patients' brain activity and connectivity patterns suggested diminished monitoring of external auditory feedback (defined as hearing one own's voice while speaking) and increased processing in a network involved in feedforward control during speech production. Reduced functional coupling between the limbic ventral and sensorimotor dorsal striatum during generation of affective prosody may point to already disturbed processes underlying emotional modulation of ongoing prosody prior to overt speech symptom onset.
2. Material and methods
2.1. Participants
20 native German PD patients in early stages of their disease (prior to typical Parkinsonian speech symptom onset, without overt speech or voice difficulties, Hoehn and Yahr stage I and II (Hoehn and Yahr, 1967), eight female, mean age 63.9 years, SEM = 1.5 years, average laterality quotient of 66.1 as measured by the Edinburgh handedness preference inventory (Oldfield, 1971)) were recruited from the Neurological outpatient department of Goethe University Hospital, Frankfurt, Germany.
All patients fulfilled the standard UK Brain Bank clinical diagnostic criteria for idiopathic Parkinson's disease (Hughes et al., 1992) and were diagnosed by an experienced movement disorders specialist. Exclusion criteria were any overt speech difficulties, hearing or reading impairments, which were evaluated by two independent speech language pathologists, as well as general MRI exclusion criteria. We further excluded subjects with pronounced head tremor, dementia, neurological, psychiatric and affective disorders, based on an in depth analysis of the past medical history, a clinical neurological, and a neuropsychological exam. The latter was performed by an experienced neuropsychologist using the Geriatric Depression Scale (Yesavage et al., 1983), the Mini-Mental-State-Examination (Folstein et al., 1975), and the dementia detection test (Kalbe et al., 2004).
Patients were studied twice on two different mornings, once with (ON) and once without (OFF) dopaminergic treatment (> 12 h washout). To achieve a standardized ON condition, PD patients received soluble 200 mg levodopa and 50 mg benserazid before testing (Hilker et al., 2005). After 30–60 min all patients reached a sufficient ON stage without hyper-dopaminergic symptoms. To minimize effects of experimental repetition, a time-interval greater than two weeks between the two testings was chosen. Half of the PD patients were scanned first OFF medication and then at the second testing ON medication, whereas the other half were scanned vice versa to counterbalance medication status in the experimental testing.
Disease severity was measured by the Unified Parkinson's Disease Rating Scale (UPDRS) on both occasions (Fahn et al., 1987). Individual scores of the motor part of the UPDRS (UPDRS III, Tables 1 and 2) were used for subsequent correlation analyses with fMRI data (mean 17.4, SEM = 1.3 for the ON; mean 26.1, SEM = 1.7 for the OFF-condition; OFF vs. ON: p < 0.001). The correlation of fMRI activity or effective connectivity data with UPDRS III did not reveal any significant (p < 0.005, uncorrected) results and is thus not further reported. Neither behavior nor imaging results were significantly different between PD patients in stage I and II or between akinetic rigid PD and tremor-dominant PD patients. Consequently, PD patients were treated as one group.
Table 1.
Comparison of the demographic and clinical features of the PD patients and matched controls.
| N | Mean age (SEM) | Sex F:M |
Mean laterality quotient | COMT mm:mv:vv |
DAT1 10 repeat:9 repeat |
Mean UPDRS III (SEM) | Mean disease duration (years) (SEM) |
Mean equivalent levodopa dose (mg/day) (SEM) |
|
|---|---|---|---|---|---|---|---|---|---|
| PD patients | 20 | 63.9 (1.5) | 8:12 | 66.1 | 9:9:2 | 12:8 | ON: 17.4 (1.3) OFF: 26.1 (1.7) |
5.8 (0.8) | 449.9 (71.0) |
| Controls | 20 | 64.2 (1.2) | 8:12 | 70.6 | 9:9:2 | 12:8 | 0.4 (0.2) | – | – |
N = number of participants; SEM = standard error of the mean, written in parenthesis; M = male, F = female; mm = met/met, mv = met/val, vv = val/val.
Table 2.
The patients' characteristics.
| Patient | Age | Sex | Laterality quotient | COMT | DAT1 | UPDRS III ON | UPDRS III OFF | H & Y | Disease duration (years) |
Medication | Equivalent levodopa dosea (mg/day) |
After two years follow up |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPDRS III ON | Dysarthriab | Hypophoniab | Speech therapyc | ||||||||||||
| 1 | 57 | F | 80 | mm | 9/10 | 11 | 25 | I | 11 | l-dopa, selegeline | 500 | 28 | + + | + | − |
| 2 | 75 | M | 100 | mv | 9/10 | 23 | 29 | II | 7 | l-dopa, COMT-inhibitor, pramipexole | 650 | 33 | + + | + + | − |
| 3 | 58 | M | − 66 | mm | 10/10 | 25 | 32 | II | 5 | l-dopa, pramipexole | 163 | 23 | + + | + + | + |
| 4 | 58 | F | 100 | mv | 10/10 | 16 | 14 | I | 8 | pramipexole | 200 | ||||
| 5 | 61 | F | 100 | mv | 10/10 | 18 | 23 | I | 1 | none | 0 | ||||
| 6 | 67 | M | 67 | mm | 10/10 | 16 | 20 | II | 4 | l-dopa, selegeline, pramipexole | 400 | ||||
| 7 | 63 | F | 100 | mv | 10/10 | 12 | 22 | II | 3 | l-dopa, COMT-inhibitor, selegeline | 600 | 22 | + | + | + |
| 8 | 52 | F | 80 | mm | 9/10 | 23 | 36 | II | 3 | l-dopa, COMT-inhibitor, selegeline | 1140 | ||||
| 9 | 64 | M | − 100 | mm | 9/10 | 10 | 14 | I | 2 | l-dopa, ropinirole | 460 | 26 | + + | + | + |
| 10 | 66 | M | 0 | mm | 9/9 | 20 | 31 | II | 8 | piribedile | 200 | ||||
| 11 | 72 | M | 100 | vv | 10/10 | 10 | 18 | II | 3 | selegeline, ropinirole | 480 | 22 | + + | + + | + |
| 12 | 72 | M | 67 | mm | 10/10 | 20 | 30 | II | 4 | none | 0 | + | + | − | |
| 13 | 69 | M | 82 | mv | 10/10 | 14 | 23 | I | 7 | l-dopa | 300 | 13 | − | − | − |
| 14 | 64 | F | 82 | mm | 9/9 | 13 | 18 | II | 3 | none | 0 | 15 | + | + | − |
| 15 | 73 | F | 100 | mv | 9/9 | 23 | 30 | II | 10 | l-dopa, pramipexole | 900 | 30 | + + | + | − |
| 16 | 54 | F | 100 | mv | 10/10 | 29 | 39 | II | 3 | selegeline, ropinirole | 480 | 33 | + + | + | + |
| 17 | 63 | M | 80 | mm | 10/10 | 21 | 31 | I | 12 | l-dopa, pramipexole, cabergoline | 750 | + | + | + | |
| 18 | 61 | M | 82 | mv | 10/10 | 11 | 28 | II | 5 | l-dopa, selegeline, ropinirole | 900 | + | + | − | |
| 19 | 70 | M | 67 | vv | 10/10 | 13 | 18 | I | 5 | l-dopa, ropinirole | 575 | 15 | − | + + | − |
| 20 | 58 | M | 100 | mv | 9/9 | 20 | 40 | II | 12 | l-dopa, selegeline | 300 | 23 | + + | + + | + |
M = male, F = female; H & Y = Hoehn and Yahr stage; mm = met/met, mv = met/val, vv = val/val.
1 mg of pramipexole = 5 mg of ropinirole = 60 mg of piribedile = 2 mg of cabergoline = 100 mg of levodopa (= l-dopa) (Hilker et al., 2005).
Speech symptoms: ++ severe, + mild, − none .
Speech therapy: + yes, − no.
All PD patients were at early stages of their disease (disease duration since diagnosis 5.8 years, SEM = 0.8) and did not yet report or showed speech difficulties as objectified by two independent experienced speech language pathologists, who were otherwise not involved in this study. Most PD patients were on anti-Parkinson-medication (mean equivalent levodopa dose 449.9, SEM = 71.0) (Hilker et al., 2005). Three patients were drug-naive.
14 out of 15 patients who we managed to contact again two years after inclusion in the study reported the development of typical Parkinsonian speech symptoms (either hypophonia or dysarthria or both), suggesting that our patient sample was representative and indeed pre-symptomatic regarding speech symptoms.
20 healthy control participants with the same inclusion and exclusion criteria as the patients were matched to the PD patients regarding gender, age, handedness and polymorphisms in genes relevant for synaptic dopamine concentration (Tables 1–3). There were no statistically significant between-group differences (patients versus control participants) regarding age, handedness and dopaminergic polymorphisms. Controls were only scanned once in the morning and were recruited from the Frankfurt Senior Citizens University, an education institution for older adults without specific education qualification.
Table 3.
The controls' characteristics.
| Control participant | Age | Sex | Laterality quotient | COMT | DAT1 | UPDRS III |
|---|---|---|---|---|---|---|
| 1 | 71 | M | 100 | mm | 10/10 | 0 |
| 2 | 62 | F | 80 | mv | 9/10 | 0 |
| 3 | 62 | M | 100 | mm | 10/10 | 0 |
| 4 | 68 | F | 82 | mv | 9/10 | 0 |
| 5 | 66 | F | 100 | mm | 9/10 | 0 |
| 6 | 63 | F | − 50 | mm | 10/10 | 0 |
| 7 | 62 | M | 64 | mm | 9/10 | 0 |
| 8 | 69 | M | 100 | mv | 10/10 | 3 |
| 9 | 70 | M | 80 | mm | 10/10 | 3 |
| 10 | 63 | F | 100 | mm | 10/10 | 0 |
| 11 | 71 | M | 82 | vv | 9/10 | 0 |
| 12 | 70 | M | − 45 | vv | 10/10 | 2 |
| 13 | 60 | M | 100 | mv | 9/10 | 0 |
| 14 | 63 | M | − 23 | mm | 10/10 | 0 |
| 15 | 61 | F | 100 | mv | 9/10 | 0 |
| 16 | 66 | M | 82 | mv | 10/10 | 0 |
| 17 | 71 | M | 100 | mv | 10/10 | 0 |
| 18 | 61 | F | 100 | mv | 10/10 | 0 |
| 19 | 55 | F | 100 | mv | 9/10 | 0 |
| 20 | 51 | M | 60 | mm | 10/10 | 0 |
M = male, F = female; mm = met/met, mv = met/val, vv = val/val.
All participants were native German speakers, gave informed consent and were paid for their participation. The study was approved by the local research ethics committee.
2.2. Genetic analyses
Neurotransmission of dopamine is physiologically influenced by two functional polymorphisms in genes relevant for synaptic dopamine clearance: catechol-O-methyltransferase (COMT) is a dopamine degrading enzyme that is mainly expressed in prefrontal cortex (Karoum et al., 1994; Matsumoto et al., 2003a,b; Reuter et al., 2009), while synaptic dopamine clearance in subcortical regions is primarily controlled by the dopamine transporter (DAT1) (Ciliax et al., 1995; Dreher et al., 2009; Sesack et al., 1998). Because these polymorphisms have been shown to influence brain activity and behavior (Colzato et al., 2010; de Frias et al., 2010; Garcia-Garcia et al., 2010), we balanced these polymorphisms in our groups (Tables 1–3).
Whole blood samples were collected from all participants. DNA was extracted from peripheral blood leukocytes using DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). The polymerase chain reaction (PCR)-based polymorphism-genotyping was carried out in 20 μl with 25 ng genomic DNA, 0.5 U AmpliTaq Polymerase (Life Technologies, Darmstadt, Germany), 5 pmol from each primer (COMT F: GGGGGCCTACTGTGGCTACTC, COMT R: CCCTTTTTCCAGGTCTGACA, DAT1 F: gtagggaacggcctgagAG, DAT1 R: CCAGGCAGAGtgtggtctg) and 0.2 mM dNTPs. PCR conditions were 3 min for initial denaturation at 94 °C, 35 cycles at 94 °C for 30 s for denaturation, 30 s at 60 °C (DAT1) and 57 °C (COMT) for annealing, and 50 s at 72 °C for extension, followed by 7 min at 72 °C for final extension. The PCR products were separated in Agarose 2% DAT1 or NuSieve 4% COMT and visualized with Ethidium Bromide.
The COMT polymorphism (SNP g/t) creates a new NlaIII restriction site. The 178 base pairs (bp) PCR products were subjected to restriction digestion with Nla III (NEB, Frankfurt, Germany) following the manufacturer's instructions. The COMT val/val genotype was represented by 114, 34, 30 bp, met/met by 96, 34, 30, 18 bp, and met/val by 114, 96, 34, 30 and 18 bp.
The DAT1 polymorphism is a VNTR with a 40 bp repeat (Sano et al., 1993). A 450 bp PCR fragment represents the allele with 10 repeats, whereas the 410 bp fragment represents the allele with 9 repeats.
2.3. Behavioral data acquisition and analysis
2.3.1. Speech sampling and training of affective prosody
Five German semantically neutral declarative sentences (e.g. ‘Alte Mühlen mahlen schnell viel Korn’, translated: ‘Old mills grind rapidly lots of corn’) were presented visually one after the other. First, participants read the sentences out loud with their habitual voice and neutral intonation. Then, they were asked to read the same sentences again in a happy intonation. Given that all participants hesitated reading a neutral sentence happily, all subjects were trained to utter a neutral sentence with happy intonation, thus producing convincing affective prosody (Pichon and Kell, 2013). Training consisted in instructing participants to imagine being happy (emotion induction) but also in model learning by imitating increased effort, speech intensity, modulation and affective nuance. Speech samples were audio-recorded and stored for further analysis.
2.3.2. Acoustic speech analyses
Non-dysfluent speech recordings of overt reading were analyzed regarding speech intensity, intensity variation, spectral range, spectral change, fundamental frequency variation, and reading time (Rosen et al., 2006) using PRAAT (http://www.fon.hum.uva.nl/praat/) (Boersma and Weenik, 1996). For speech intensity analyses, sound pressure level in dB was transformed into linear sound pressure values. After calculating the mean intensity for all sentences separately for groups and conditions, values were retransformed into sound pressure level in dB. Intensity variation was defined as the standard deviation of the root mean square intensity curve contour (5-point averaged). Spectral range corresponded to the range between the minimum and maximum intensity in dB. Spectral change as defined by the intensity's first derivate over time in dB/ms describes the median intensity change of the whole spectrum (Rosen et al., 2006). We used the time that participants required to read the five sentences as a measure of speaking rate. The standard deviation of fundamental frequency was calculated and reflects how much a speaker modulates his voice in terms of frequency (Pichon and Kell, 2013). Altogether, these acoustic parameters reflect acoustic contrastivity during overt reading that is supposed to be decreased in patients with hypokinetic dysarthria (Rosen et al., 2006).
Condition-specific group means of all these acoustic parameters as dependent variables were compared using mixed model analysis in SPSS (SPSS Inc., Chicago, IL, USA) including the following factors: condition (neutral prosody, untrained and trained happy prosody), group (20 PD patients and 20 control participants for the two between group analyses or 20 PD patients ON medication and 20 PD patients OFF medication for the within group analyses). We tested for condition-dependent group differences (interaction of condition × group), significant at p < 0.05. Post-hoc t-tests were performed in case of significant interactions. We were able to acquire acoustic data in 12 PD patients 2 years after inclusion in the study (ON medication) using the same protocol and analyses. A mixed model including data acquisition time as additional factor was explored for a significant effect of time using an F-test, significant at p < 0.05.
We correlated individual acoustic parameters with fMRI results but did not find any significant correlation (all p > 0.05).
2.3.3. Perceptual speech analyses
Two independent experienced speech language pathologists, who were otherwise not involved in this study, judged the recordings of overt reading for pitch, loudness, voice quality, comprehensibility and speech tempo with a standardized Speech Characteristics Rating Scale (http://www.speech-language-therapy.com/~speech/pdf/scr.pdf) (Skinder-Meredith, 2009) and were asked to categorize speech samples as belonging to patients (ON or OFF) or controls. Alike the acoustic speech analyses (see above), condition-specific group means were compared using mixed model analysis in SPSS and judged significant at p < 0.05. We additionally tested for differences in perceptual ratings between patients' data acquired at the timepoint of inclusion in the study and at the follow up two years later (p < 0.05).
We correlated individual perceptual rating parameters with fMRI results but did not find any significant correlation (all p > 0.05).
2.3.4. Speech initiation study
To study whether PD patients initiated speech more slowly than control participants, we recorded speech reaction times while parametrically decreasing instruction delays during a cue-target reading task outside the scanner. Participants performed a reading task in which sentence presentation was preceded by a visual cue (covert, neutral, or happy), indicating whether the upcoming sentence had to be read covertly (without orofacial movement, neutral internal intonation), or overtly with neutral or happy intonation. We varied the instruction delays between 0.33, 0.67, and 1.00 s (Sakai and Passingham, 2006) and measured speech reaction times (defined by the duration between onset of visual sentence presentation as indicated by an auditory trigger and speech intensity exceeding 20 dB). Condition-specific group means for reading with neutral or happy intonation as dependent variables were compared using mixed model analysis in SPSS including the following factors: task, instruction delay (0.33, 0.67, 1.00 s), group (20 PD patients and 20 control participants for the two between group analyses or 20 PD patients ON medication and 20 PD patients OFF medication for the within group analyses). We tested for task- and instruction-delay dependent group differences, significant at p < 0.05.
2.3.5. Executive function test
All subjects performed a complex button press task programmed in Presentation software (Neurobehavioral Systems, Albany, CA, USA) to test for significant differences in executive function. A visually presented cue indicated with which hand the participants had to press a button (right hand, left hand or both hands). After an instruction delay, randomly jittered between 0.28, 0.38, 0.60 and 0.93 s, a visually presented number (target) exactly informed participants about the finger(s) to use for the button press. Condition-specific group means of manual reaction times were compared using a mixed model analysis in SPSS including the factors instruction delay (0.28, 0.38, 0.60 and 0.93 s) and group (20 PD patients and 20 control participants for the two between group analyses or 20 PD patients ON medication and 20 PD patients OFF medication for the within group analyses). We tested for task-dependent group differences, significant at p < 0.05.
2.4. fMRI data acquisition
Data were collected using a 3 Tesla (T) magnetic resonance scanner (Siemens Trio, Erlangen, Germany). We acquired two sessions of a gradient-echo T2*-weighted transverse echo-planar imaging sequence (EPI) (456 volumes each). Each volume included 33 axial slices with a repetition time (TR) of 2000 ms, echo time (TE) of 30 ms, flip angle of 90°, isotropic voxel size of 3 × 3 × 3 mm3, resolution 8 × 8 mm2, distance factor 25%, slice thickness of 3 mm. This sequence was optimized for continuous functional MRI during 3 s of speaking (Preibisch et al., 2003). A high-resolution T1-weighted anatomical scan (144 sagittal slices, 1 slab, TR 2250 ms, TI 900 ms, TE 2.6 ms, flip angle 9°, voxel size 1 × 1 × 1 mm3, resolution 16 × 16 mm2, distance factor 50%, slice thickness 1 mm) was obtained to identify potential structural brain anomalies.
Subjects lay supine and wore headphones for delivery of the acoustic cues (see below) and noise protection. The heads were immobilized with foam cushions. A coil-mounted mirror allowed viewing the projection screen.
2.5. fMRI experimental procedure
Participants performed a sentence reading task during fMRI at 3 T. After having been familiarized with the experimental setting, 75 semantically neutral declarative German sentences not previously used for training (e.g. ‘Große Regentropfen fallen rasch zur Erde’, translated: ‘Large raindrops quickly fall on the ground’) were presented for 3 s each using Presentation software (Neurobehavioral Systems, Albany, CA, USA). Their visual presentation was preceded 2–4 s earlier by an auditory instruction (covert, neutral, or happy), indicating whether the upcoming sentence had to be read covertly (without orofacial movement, neutral internal intonation), or overtly with neutral or happy intonation. The 2–4 s in which subjects knew about the task rules and could prepare the relevant brain networks were termed preparation phase (Kell et al., 2011). The auditory cue did not inform about the content of the upcoming utterance, so that the preparation phase only allowed for cognitive preparation and not for any linguistic processing or motor preparation. In every trial, each preparation phase was followed by a corresponding execution phase that included specific stimulus processing upon sentence presentation (Fig. 1). Jittering the instruction delays allowed for temporal de-correlation of variance related to preparation and execution for analyses using SPM (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/). This was important to specifically target effects related to cognitive preparation (reflecting the set up of task-relevant networks) separately from stimulus-related computation during linguistic and motor processing (Kell et al., 2011). Given that a cue-target paradigm necessitates the use of externally generated linguistic items (written sentences), we could not study spontaneous speaking. Speech during reading differs from free speaking (Van Lancker Sidtis et al., 2010) but is nevertheless affected by the disease (Van Lancker Sidtis et al., 2012). The intertrial interval randomly varied between 2 and 10 s (mean 6 s). Speech output was recorded with an MRI-compatible microphone (mr confon). The scanner background noise was filtered out from the recordings using Adobe Audition (San Jose, CA, USA) and behavioral data were screened for correct performance.
Fig. 1.
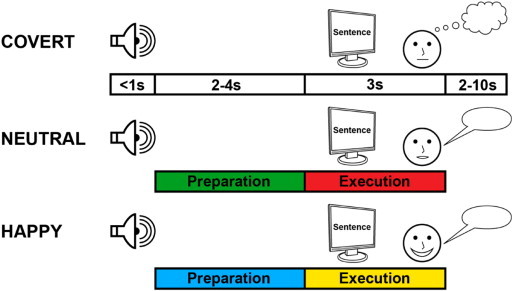
Study design. Participants performed a reading task during fMRI. An auditory cue indicated 2–4 s before sentence presentation whether the upcoming sentence should be read covertly with neutral inner intonation, overtly with neutral intonation, or overtly with happy intonation. Trials thus consisted of a preparation phase that allowed for a condition-specific setup of task-relevant networks and an execution phase during which speech processing occurred. We illustrate group comparisons for overt reading with neutral intonation > covert reading either in green (preparation phase) or in red (execution phase). Group differences for happy > neutral intonation of overt reading are illustrated in blue (preparation phase) and in yellow (execution phase).
2.6. fMRI image preprocessing
fMRI data were preprocessed and statistically analyzed with the standard parameters of SPM8. EPI volumes were spatially realigned (Friston et al., 1994), normalized to the standard EPI template of the Montreal Neurological Institute (MNI) (Friston et al., 1995) and resampled to an isotropic voxel size of 2 mm. Images were finally smoothed with an isotropic 8 mm full-width-at-half-maximum (FWHM) Gaussian kernel.
2.7. fMRI whole-brain analysis of task-related activity changes
Individual fMRI data were analyzed using the standard general linear model (GLM) for time-series in SPM8. Regressors capturing variance explained by the preparation and execution phases were entered separately for the three task conditions. This resulted in a linear model including seven conditions: Preparation for covert reading, preparation for overt reading with neutral intonation (neutral), preparation for overt reading with happy intonation (happy), three respective regressors for the execution phases, and a single regressor for presentation of the auditory cues to capture transient cue-related activations. All conditions of interest were convolved with a canonical hemodynamic response function (HRF). Regressors of no interest included six movement regressors containing the realignment parameters. Data were globally normalized and corrected for serial autocorrelations (AR1). The following contrasts were calculated on the individual first level: preparation for overt reading with neutral intonation > preparation for covert reading, preparation for happy > neutral overt reading, execution of overt reading with neutral intonation > execution of covert reading and execution of happy > neutral overt reading. By using covert reading as cognitive baseline for overt reading, we focus our analyses on sensorimotor aspects of speech and control for potential differences in linguistic processing (Kell et al., 2009). By contrasting happy with neutral intonation, we target affective and sensorimotor aspects of emotional prosody, as previously done in healthy participants (Pichon and Kell, 2013).
For the whole-brain analyses of group differences, the individual aforementioned contrast images were entered in t-tests. This was done because we were interested in interactions between condition and group (PD patients ON medication, PD patients OFF medication, and controls). As SPM ANOVAs do not allow for parallel definition of between and within group factors, we tested for differences between patients and controls (PD patients ON versus controls or PD patients OFF versus controls) by using between group comparisons (two sample t-tests). For comparing PD patients ON versus OFF medication we separately performed within group random-effects analyses (paired t-tests), as PD patients ON and OFF are dependent groups of identical individuals, however under different treatment conditions.
To restrict our search space for group differences to regions that responded to the conditions of interest, we created a mask covering the volume that responded to the highest hierarchical condition (happy intonation) corrected for activity related to the lowest hierarchical condition (covert reading). We thus additionally calculated the contrasts preparation for overt reading with happy intonation > preparation for covert reading and execution of overt reading with happy intonation > execution of covert reading on the first level. Two one sample t-tests were performed on these contrasts separately for the studied groups and suprathreshold voxels summed linearly using the imcalc tool in SPM (Fig. 2). A one sample t-test over all groups would have largely eliminated voxels that differed in activity between groups. The resulting activation map was thresholded at p < 0.05, family-wise error (FWE) corrected for multiple comparisons and used as mask for group analyses. To reduce Type I and Type II errors equally well, we report interactions between groups and trial-phase specific conditions at p < 0.005 (uncorrected) within the mask at p < 0.05, FWE corrected for multiple comparisons. We used this approach previously in a study on developmental stuttering (Kell et al., 2009).
Fig. 2.
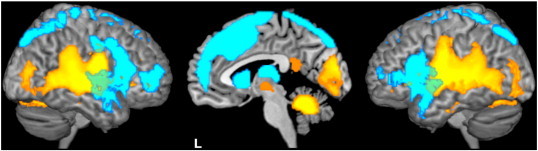
Brain activation during task preparation and execution. This mask has been created by summing linearly the one sample t-contrast maps of each group (20 control subjects, 20 PD patients, each ON and OFF medication) for overt reading with happy intonation > covert reading separately for preparation (depicted in blue) and execution (shown in yellow) and used as mask for group comparisons. All voxels are significant at p < 0.05 FWE corrected for multiple comparisons.
2.8. Effective connectivity analyses (psycho-physiological interactions)
Patients could differ from control participants not only in terms of task-related activity but also in functional interactions between brain regions. We thus additionally studied changes in functional connectivity that are induced by overt speaking between twelve regions of interest (ROIs) belonging to the speech network (separately for neutral and happy intonation and separately for preparation and execution), so called psycho-physiological interactions (PPI, effective connectivity) (Friston et al., 1997). Such context-dependent modulations of correlations between brain regions reflect measures of effective connectivity that are independent of task-related activity changes in the studied regions. This model-free measure has the advantage that more ROIs can be studied compared to dynamic causal modeling and that no strong sensory input needs to be pre-specified (which is a prerequisite to study preparatory effective connectivity). PPIs test whether temporal correlations between network nodes are modulated as a function of a psychological variable like overt > covert reading and happy > neutral intonation in our case. Please note that PPIs do not permit to infer directionality or causality (Friston et al., 1997; Gitelman et al., 2003). We selected the main left-hemisphere speech network nodes established in the literature: supplementary motor area (SMA) (− 14, 2, 64), inferior frontal gyrus (IFG) (− 44, 32, − 4), dorsal premotor cortex (dPMC) (− 40, 12, 44), auditory cortex (AC) (− 40, − 26, 12) and superior temporal sulcus (STS) (− 60, − 28, 2) (Hickok and Poeppel, 2004), together with the left caudate nucleus (CN) (− 10, 14, 6), and left putamen (PUT) (− 28, − 10, 8) as two subcortical structures involved in cognitive (CN) and motor processes (PUT) (Simonyan et al., 2013). Given their role in prosody production (Pichon and Kell, 2013), we additionally included the right superior temporal sulcus (STS) (50, − 36, 2), right dorsal (dstriatum) (16, 10, 2) and ventral striatum (vstriatum) (22, 8, − 6) but also studied connectivity changes with two cognitive control regions that showed anomalous activation patterns in our data, namely the left dorsolateral prefrontal cortex (DLPFC) (− 36, 30, 44) and left superior parietal lobule (SPL) (− 24, − 74, 50). The ROIs were centered on the local maxima of group activations within the anatomically defined regions.
For each seed region and task phase, separate PPIs were estimated. This was done by extracting the individual time courses of the first eigenvariate from a single voxel representing the individual local maximum of activation in a sphere with 5 mm radius centered around the peak of the group activation coordinate (see above). The time-series were corrected for amplitude changes induced by conditions of interest such that the connectivity analyses were performed solely on the residuals of the individual models. The deconvolved time series were then multiplied with volume-wise values reflecting whether the condition of interest was ongoing or not. This produced a PPI regressor that again was convolved with the canonical HRF before regressing it voxel-wise over the entire brain.
The PPI design matrix contained three regressors for every subject and seed region: one that reflected the physiological variable (adjusted time course), the psychological variable (task regressor contrasted against baseline) and a PPI regressor together with six realignment parameter regressors. We estimated four PPIs per seed region: One for preparation for overt reading with neutral intonation > covert reading, one for the execution of overt reading with neutral intonation > covert reading and two respective PPIs for overt reading with happy intonation > covert reading. The use of similar baselines for comparison of PPIs is critical (McLaren et al., 2012). For PPI analyses of preparation and execution of overt reading with neutral intonation > covert reading, two sample t-tests were used to compare task-related modulation of effective connectivity of a given seed region with all other ROIs (defined by 5 mm spheres centered on the local maxima of group activations, ROI-to-ROI analyses: CON vs. OFF and CON vs. ON (between group analyses) and OFF vs. ON (within group analysis)).
For connectivity pattern differences of affective prosody we estimated a 3-way ANOVA separately for preparation and execution crossing the following factors: subject (40 levels), group (2 levels), and condition (2 levels: overt reading with happy intonation > covert reading and overt reading with neutral intonation > covert reading). As SPM flexible factorial designs currently do not allow for parallel definition of between and within group factors, we performed separate analyses for each group comparison (CON vs. OFF, CON vs. ON and OFF vs. ON) each for preparation and execution of happy > neutral intonation.
We report group differences in effective connectivity between the twelve studied ROIs using small volume correction (SVC) within the same spheres that served as search volumes for the individual maxima, thresholded at p < 0.05, family-wise error (FWE) corrected for multiple comparisons within the search volumes in Tables 4, 6, 9 and 10 and illustrate them (Fig. 3, 5, 7 and 8) by depicting abstract (non-spherical) markers of those brain regions that show group differences in effective connectivity. Decreased effective connectivity in PD patients is illustrated in bluish gray, while increases in effective connectivity are illustrated in the color that corresponds to the condition and trial phase of interest (Fig. 1).
Table 4.
Group differences in effective connectivity during cognitive preparation for overt vs. covert reading with neutral intonation.
| Anatomical region | BA | MNI-coordinates (x, y, z) | T-value | p-value (SVC corr.) |
|---|---|---|---|---|
| Hypo-connectivity in PD patients | ||||
| PD OFF < control | ||||
| L dorsolateral prefrontal cortex | ||||
| L caudate nucleus | – | − 10, 22, − 4 | 3.85 | 0.003 |
| L inferior frontal gyrus | ||||
| L dorsolateral PFC | 9 | − 42, 28, 38 | 4.16 | 0.001 |
| L supplementary motor area | ||||
| L dorsolateral PFC | 9 | − 38, 28, 38 | 3.23 | 0.011 |
| PD ON < control | ||||
| L dorsolateral prefrontal cortex | ||||
| L inferior frontal gyrus | 47 | − 32, 46, − 6 | 3.75 | 0.003 |
| L caudate nucleus | – | − 8, 22, − 6 | 3.37 | 0.008 |
| L inferior frontal gyrus | ||||
| L dorsolateral PFC | 8, 9 | − 40, 30, 40 | 3.57 | 0.005 |
| L supplementary motor area | ||||
| L inferior frontal gyrus | 47 | − 36, 46, − 6 | 3.40 | 0.008 |
| Hyper-connectivity in PD patients | ||||
| PD OFF > control | ||||
| L caudate nucleus | ||||
| L putamen | – | − 22, 0, 4 | 3.47 | 0.006 |
| PD OFF > PD ON | ||||
| L caudate nucleus | ||||
| L putamen | – | − 26, 0, 2 | 2.85 | 0.025 |
| PD ON > control | ||||
| L dorsal premotor cortex | ||||
| L suppl. motor area | 6 | − 14, 2, 62 | 3.90 | 0.002 |
Seed regions are left-justified and target regions are tabulated.
Table 6.
Group differences in effective connectivity during overt vs. covert reading with neutral intonation (execution phase).
| Anatomical region | BA | MNI-coordinates (x, y, z) | T-value | p-value (SVC corr.) |
|---|---|---|---|---|
| Hypo-connectivity in PD patients | ||||
| PD OFF < control | ||||
| L auditory cortex | ||||
| L caudate nucleus | – | − 16, 0, 20 | 2.93 | 0.020 |
| L dorsal premotor cortex | ||||
| L caudate nucleus | – | − 16, − 2, 20 | 2.83 | 0.028 |
| PD ON < control | ||||
| L auditory cortex | ||||
| L dorsal premotor cortex | 6 | − 28, − 10, 58 | 3.15 | 0.014 |
| Hyper-connectivity in PD patients | ||||
| PD OFF > control | ||||
| L dorsolateral prefrontal cortex | ||||
| L putamen | – | − 22, 0, 4 | 3.89 | 0.002 |
| L dorsal premotor cortex | ||||
| L inferior frontal gyrus | 45 | − 38, 26, 12 | 3.48 | 0.007 |
| L superior temporal sulcus | ||||
| L inferior frontal gyrus | 45, 46 | − 34, 30, 14 | 3.16 | 0.012 |
| L dorsolateral PFC | 8 | − 38, 34, 38 | 3.03 | 0.017 |
| PD OFF > PD ON | ||||
| L dorsolateral prefrontal cortex | ||||
| L putamen | – | − 26, 8, − 2 | 2.94 | 0.022 |
| L inferior frontal gyrus | ||||
| L dorsal premotor cortex | 6 | − 46, 20, 44 | 4.11 | 0.001 |
| L superior temporal sulcus | ||||
| L inferior frontal gyrus | 44 | − 58, 14, 6 | 2.94 | 0.021 |
| L dorsolateral PFC | 9 | − 42, 34, 28 | 2.73 | 0.034 |
Seed regions are left-justified and target regions are tabulated.
Table 9.
Group differences in effective connectivity during cognitive preparation for affective vs. neutral prosody.
| Anatomical region | BA | MNI-coordinates (x, y, z) | T-value | p-value (SVC corr.) |
|---|---|---|---|---|
| Hypo-connectivity in PD patients | ||||
| PD OFF < control | ||||
| L superior parietal lobule | ||||
| L inferior frontal gyrus | 44, 45 | − 46, 16, 4 | 3.52 | 0.004 |
| R ventral striatum | ||||
| R dorsal striatuma | – | 14, 14, − 4 | 2.99 | 0.016 |
| PD ON < control | ||||
| L superior parietal lobule | ||||
| L inferior frontal gyrus | 45 | − 34, 18, 6 | 3.36 | 0.007 |
| Hyper-connectivity in PD patients | ||||
| PD OFF > control | ||||
| L supplementary motor area | ||||
| L dorsolateral PFC | 9 | − 40, 26, 38 | 3.26 | 0.008 |
| PD ON > control | ||||
| L dorsolateral prefrontal cortex | ||||
| L inferior frontal gyrus | 47 | − 48, 28, − 2 | 2.82 | 0.024 |
| L caudate nucleus | – | − 6, 18, − 4 | 2.90 | 0.011 |
| L supplementary motor area | ||||
| L dorsolateral PFC | 9 | − 40, 24, 38 | 3.64 | 0.003 |
Seed regions are left-justified and target regions are tabulated.
Not illustrated.
Table 10.
Group differences in effective connectivity during execution of affective vs. neutral prosody.
| Anatomical region | BA | MNI-coordinates (x, y, z) | T-value | p-value (SVC corr.) |
|---|---|---|---|---|
| Hypo-connectivity in PD patients | ||||
| PD OFF < control | ||||
| R superior temporal sulcus | ||||
| R dorsal striatum | – | 12, 10, 6 | 3.48 | 0.005 |
| Hyper-connectivity in PD patients | ||||
| PD OFF > control | ||||
| L superior parietal lobule | ||||
| Suppl. motor area | 6 | 2, 18, 64 | 2.88 | 0.021 |
| PD ON > control | ||||
| L superior parietal lobule | ||||
| Suppl. motor area | 6 | 0, 22, 60 | 3.55 | 0.004 |
Seed regions are left-justified and target regions are tabulated.
Fig. 3.
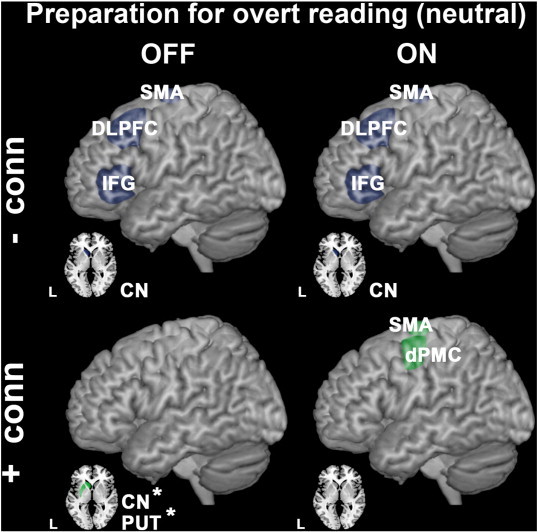
Group differences in effective connectivity during cognitive preparation for overt vs. covert reading with neutral intonation. Upper panels show brain regions between which effective connectivity was reduced in PD patients compared to controls (hypo-connectivity is shown in bluish gray). ROIs that exhibited increased effective connectivity between them (PD patients > controls) are illustrated in the lower panels (hyper-connectivity is shown in green). Comparisons between controls and PD patients OFF medication are illustrated on the left side, with PD ON medication on the right side. Levodopa-responsive anomalies in effective connectivity are asterisked (*). All group differences were significant at p < 0.05, FWE corrected after SVC.
Fig. 5.
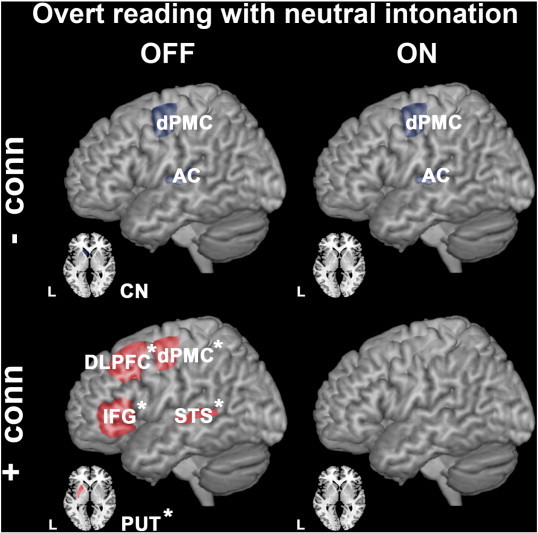
Group differences in effective connectivity during overt vs. covert reading with neutral intonation (execution phase). Upper panels illustrate brain regions between which effective connectivity was reduced in PD patients compared to controls (hypo-connectivity is shown in bluish gray). ROIs that exhibited increased effective connectivity between them (PD patients > controls) are illustrated in the lower panels (hyper-connectivity depicted in red). Comparisons between controls and PD patients OFF medication are illustrated on the left side, with PD ON medication on the right side. Levodopa-responsive anomalies in effective connectivity are asterisked (*). All group differences were significant at p < 0.05, FWE corrected after SVC.
Fig. 7.
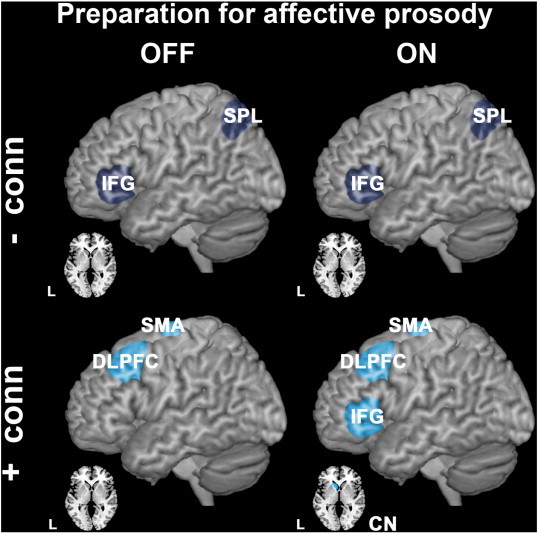
Group differences in effective connectivity during cognitive preparation for affective vs. neutral prosody. Upper panels show brain regions between which effective connectivity was reduced in PD patients compared to controls (hypo-connectivity is shown in bluish gray). ROIs that exhibited increased effective connectivity between them (PD patients > controls) are illustrated in the lower panels (hyper-connectivity is shown in blue). Comparisons between controls and PD patients OFF medication are illustrated on the left side, with PD ON medication on the right side. All group differences were significant at p < 0.05, FWE corrected after SVC.
Fig. 8.
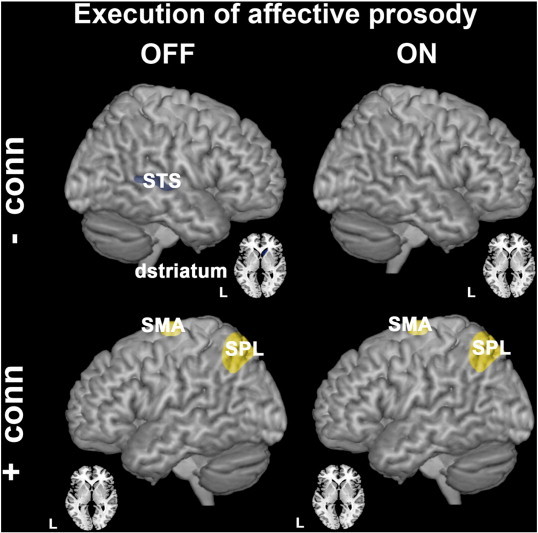
Group differences in effective connectivity during execution of affective vs. neutral prosody. Upper panels illustrate brain regions between which effective connectivity was reduced in PD patients compared to controls (hypo-connectivity is shown in bluish gray). ROIs that exhibited increased effective connectivity between them (PD patients > controls) are illustrated in the lower panels (hyper-connectivity depicted in yellow). Comparisons between controls and PD patients OFF medication are illustrated on the left side, with PD ON medication on the right side. All group differences were significant at p < 0.05, FWE corrected after SVC.
For all imaging results we identified the Brodmann areas corresponding to the MNI coordinates of activation by using the probability maps from the anatomy toolbox for SPM (Eickhoff et al., 2005) and the Tailarach daemon, the stereotactic atlas of the human brain (Lancaster et al., 2000; Talairach and Tournoux, 1988).
3. Results
The present study investigated the differences in voxel-wise brain activity and effective connectivity between selected ROIs during preparation for and execution of overt speaking (with neutral or happy intonation, respectively) in early PD patients without any overt speech or voice difficulties at the time of testing (ON and OFF medication) compared to healthy controls.
3.1. Behavioral results of overt reading
Due to the inclusion of only early stage PD patients (prior to Parkinsonian speech symptom onset), neutrally intonated speech did not significantly differ between PD patients and controls or between PD patients OFF and ON medication regarding all parameters of acoustic contrastivity (Inline Supplementary Table S1).
Inline Supplementary Table S1.
Table S1.
Acoustic speech analyses at the time of scanning.
| Condition | PD patients ON |
PD patients OFF |
Control participants |
Interaction Condition × group (PD vs. CON) |
Interaction Condition × group (ON vs. OFF) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | F-value (p-value) | F-value (p-value) | ||
| Intensity (dB) | Neutral prosody | 70.2 | 0.82 | 70.4 | 0.91 | 69.7 | 0.70 | 1.2 (0.304) | 0.3 (0.857) |
| Untrained happy prosody | 74.0 | 0.98 | 75.0 | 1.09 | 72.2 | 1.08 | |||
| Trained happy prosody | 82.7 | 0.38 | 83.2 | 0.62 | 83.0 | 0.59 | |||
| Intensity variation (dB) | Neutral prosody | 11.4 | 0.31 | 11.9 | 0.34 | 11.9 | 0.25 | 0.5 (0.712) | 1.1 (0.344) |
| Untrained happy prosody | 12.8 | 0.44 | 13.4 | 0.36 | 12.7 | 0.41 | |||
| Trained happy prosody | 13.6 | 0.78 | 13.7 | 0.36 | 13.5 | 0.26 | |||
| Spectral range (dB) | Neutral prosody | 84.4 | 0.71 | 83.6 | 0.87 | 83.5 | 0.76 | 0.5 (0.671) | 0.6 (0.614) |
| Untrained happy prosody | 87.4 | 0.95 | 88.4 | 1.00 | 86.6 | 1.25 | |||
| Trained happy prosody | 92.5 | 0.13 | 92.7 | 0.17 | 92.7 | 0.13 | |||
| Spectral change (dB/ms) | Neutral prosody | 12.8 | 1.12 | 13.0 | 1.45 | 10.7 | 1.00 | 1.4 (0.247) | 0.3 (0.838) |
| Untrained happy prosody | 15.6 | 1.20 | 16.7 | 1.50 | 13.4 | 1.30 | |||
| Trained happy prosody | 34.1 | 0.57 | 34.4 | 0.78 | 34.7 | 0.61 | |||
| Fundamental frequency variation (Hz) | Neutral prosody | 24.3 | 2.42 | 25.8 | 3.03 | 29.4 | 2.68 | 1.4 (0.244) | 1.2 (0.319) |
| Untrained happy prosody | 35.5 | 3.68 | 42.5 | 3.85 | 42.2 | 4.58 | |||
| Trained happy prosody | 53.3 | 3.38 | 52.7 | 4.02 | 59.0 | 2.87 | |||
| Reading time (s) | Neutral prosody | 22.4 | 0.46 | 22.8 | 0.47 | 23.2 | 0.55 | 0.9 (0.458) | 0.5 (0.710) |
| Untrained happy prosody | 22.7 | 0.54 | 22.2 | 0.46 | 23.5 | 0.55 | |||
| Trained happy prosody | 17.7 | 0.52 | 17.3 | 0.57 | 18.0 | 0.55 | |||
SEM = standard error of the mean; CON = controls.
Due to the inclusion of only early stage PD patients (prior to Parkinsonian speech symptom onset), neutrally intonated speech did not significantly differ between PD patients and controls or between PD patients OFF and ON medication regarding all parameters of acoustic contrastivity (Inline Supplementary Table S1).
Consistent with these findings of acoustic speech analyses, speech recordings were also perceptually indistinguishable and did not reveal any significant differences in perceptual ratings (Inline Supplementary Table S2a and b). Inter-rater reliability was high (Pearson correlation between ratings r = 0.7; p < 0.05).
Inline Supplementary Table S2.
Table S2.
Perceptual speech analyses at the time of scanning.
| Condition | PD patients ON |
PD patients OFF |
Control participants |
Interaction Condition × group (PD vs. CON) |
Interaction Condition × group (ON vs. OFF) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | F-value (p-value) | F-value (p-value) | ||
| Table 2a | |||||||||
| Loudness (rater 1) | Neutral prosody | 3.0 | 0.05 | 3.0 | 0.05 | 2.7 | 0.13 | 2.6 (0.052) | 0.3 (0.801) |
| Untrained happy prosody | 3.7 | 0.11 | 3.8 | 0.10 | 3.4 | 0.13 | |||
| Trained happy prosody | 3.1 | 0.05 | 2.9 | 0.05 | 3.1 | 0.10 | |||
| Loudness (rater 2) | Neutral prosody | 3.0 | 0.00 | 3.0 | 0.00 | 3.0 | 0.05 | 0.5 (0.677) | 0.0 (1.000) |
| Untrained happy prosody | 3.0 | 0.07 | 3.0 | 0.00 | 3.0 | 0.00 | |||
| Trained happy prosody | 3.0 | 0.00 | 3.0 | 0.00 | 3.0 | 0.00 | |||
| Pitch (rater 1) | Neutral prosody | 3.5 | 0.15 | 3.6 | 0.15 | 3.4 | 0.20 | 0.6 (0.644) | 1.1 (0.367) |
| Untrained happy prosody | 3.3 | 0.19 | 3.2 | 0.19 | 3.3 | 0.13 | |||
| Trained happy prosody | 3.5 | 0.14 | 3.3 | 0.10 | 3.8 | 0.12 | |||
| Pitch (rater 2) | Neutral prosody | 3.7 | 0.11 | 3.8 | 0.10 | 3.9 | 0.08 | 0.6 (0.644) | 0.5 (0.686) |
| Untrained happy prosody | 3.8 | 0.10 | 3.7 | 0.11 | 3.7 | 0.10 | |||
| Trained happy prosody | 3.8 | 0.09 | 3.7 | 0.15 | 3.9 | 0.07 | |||
| Voice quality (rater 1) | Neutral prosody | 3.6 | 0.08 | 3.7 | 0.07 | 3.5 | 0.05 | 1.0 (0.389) | 0.3 (0.803) |
| Untrained happy prosody | 3.5 | 0.09 | 3.5 | 0.10 | 3.6 | 0.07 | |||
| Trained happy prosody | 3.7 | 0.07 | 3.6 | 0.07 | 3.8 | 0.07 | |||
| Voice quality (rater 2) | Neutral prosody | 3.8 | 0.06 | 3.8 | 0.05 | 3.8 | 0.06 | 0.0 (0.990) | 0.7 (0.568) |
| Untrained happy prosody | 3.8 | 0.06 | 3.8 | 0.06 | 3.8 | 0.06 | |||
| Trained happy prosody | 3.8 | 0.06 | 3.8 | 0.07 | 3.8 | 0.05 | |||
| Table 2b | |||||||||
| Comprehensibility (rater 1) | Neutral prosody | 4.0 | 0.00 | 4.0 | 0.00 | 4.0 | 0.05 | 1.6 (0.211) | 0.0 (1.000) |
| Untrained happy prosody | 4.0 | 0.00 | 4.0 | 0.00 | 4.0 | 0.00 | |||
| Trained happy prosody | 4.0 | 0.00 | 4.0 | 0.00 | 4.0 | 0.00 | |||
| Comprehensibility (rater 2) | Neutral prosody | 4.0 | 0.00 | 4.0 | 0.00 | 4.0 | 0.05 | 0.8 (0.461) | 0.8 (0.466) |
| Untrained happy prosody | 4.0 | 0.00 | 4.0 | 0.05 | 4.0 | 0.00 | |||
| Trained happy prosody | 4.0 | 0.00 | 4.0 | 0.00 | 4.0 | 0.00 | |||
| Speech tempo (rater 1) | Neutral prosody | 3.7 | 0.13 | 3.8 | 0.09 | 3.7 | 0.13 | 0.4 (0.650) | 0.3 (0.729) |
| Untrained happy prosody | 3.9 | 0.07 | 4.0 | 0.05 | 3.9 | 0.07 | |||
| Trained happy prosody | 3.9 | 0.08 | 4.0 | 0.00 | 4.0 | 0.05 | |||
| Speech tempo (rater 2) | Neutral prosody | 4.0 | 0.00 | 4.0 | 0.00 | 4.0 | 0.05 | 0.4 (0.685) | 0.4 (0.686) |
| Untrained happy prosody | 4.0 | 0.00 | 4.0 | 0.05 | 4.0 | 0.00 | |||
| Trained happy prosody | 4.0 | 0.07 | 4.0 | 0.00 | 4.0 | 0.00 | |||
SEM = standard error of the mean; CON = controls.
Consistent with these findings of acoustic speech analyses, speech recordings were also perceptually indistinguishable and did not reveal any significant differences in perceptual ratings (Inline Supplementary Table S2a and b). Inter-rater reliability was high (Pearson correlation between ratings r = 0.7; p < 0.05).
These early PD patients, still unaffected by overt speech symptoms, did not yet show speech initiation deficits or worse performance on executive testing, both OFF or ON medication, compared to the matched control participants (Inline Supplementary Table S3). No behavioral differences were observed between ON or OFF medicated state.
Inline Supplementary Table S3.
Table S3.
Speech initiation and executive function analyses.
| Instruction delay (s) | PD patients ON Reaction time (s) |
PD patients OFF Reaction time (s) |
Control participants Reaction time (s) |
Interaction Task × group (PD vs. CON) |
Interaction Task × group (ON vs. OFF) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | F-value (p-value) | F-value (p-value) | ||
| Reading task with neutral intonation | 0.33 | 1.01 | 0.01 | 1.03 | 0.01 | 1.01 | 0.02 | 0.3 (0.770) | 0.5 (0.585) |
| 0.67 | 0.91 | 0.01 | 0.92 | 0.01 | 0.92 | 0.01 | |||
| 1.00 | 0.88 | 0.01 | 0.91 | 0.01 | 0.91 | 0.02 | |||
| Reading task with happy intonation | 0.33 | 0.98 | 0.01 | 1.02 | 0.01 | 0.97 | 0.01 | 0.1 (0.893) | 0.0 (0.971) |
| 0.67 | 0.91 | 0.01 | 0.93 | 0.01 | 0.91 | 0.01 | |||
| 1.00 | 0.90 | 0.01 | 0.93 | 0.01 | 0.91 | 0.01 | |||
| Executive task with button press | 0.28 | 0.89 | 0.03 | 0.94 | 0.02 | 0.78 | 0.01 | 0.2 (0.880) | 0.1 (0.946) |
| 0.38 | 0.86 | 0.02 | 0.88 | 0.02 | 0.75 | 0.01 | |||
| 0.60 | 0.77 | 0.02 | 0.80 | 0.02 | 0.67 | 0.01 | |||
| 0.93 | 0.72 | 0.02 | 0.71 | 0.01 | 0.67 | 0.01 | |||
SEM = standard error of the mean; CON = controls.
These early PD patients, still unaffected by overt speech symptoms, did not yet show speech initiation deficits or worse performance on executive testing, both OFF or ON medication, compared to the matched control participants (Inline Supplementary Table S3). No differences were observed between ON or OFF medicated state.
Those PD patients who were re-tested behaviorally after two years showed a decrease in speech intensity (p = 0.033, Cohen's d 2.4) and spectral change (p < 0.001, Cohen's d 3.4), driven by the trained affective prosody condition (Inline Supplementary Table S4) with a concomitant decrease in perceptual loudness (p = 0.001, Cohen's d 1.7), driven by the untrained affective prosody condition (Inline Supplementary Table S5). These results confirm that our patient sample was indeed pre-symptomatic regarding speech symptoms at the time of scanning.
Inline Supplementary Table S4.
Table S4.
Comparison of acoustic speech analyses after two years.
| Condition | PD patients ON at the time of scanning (test 1) |
PD patients ON after two years (test 2) |
Interaction Condition × group (ON (test 1) vs. ON (test 2)) |
|||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | F-value (p-value) | ||
| Intensity (dB) | Neutral prosody | 71.0 | 0.76 | 72.0 | 1.08 | 3.6 (0.033) |
| Untrained happy prosody | 74.6 | 1.01 | 72.8 | 1.22 | ||
| Trained happy prosody | 82.7 | 0.43 | 79.3 | 1.14 | ||
| Intensity variation (dB) | Neutral prosody | 11.6 | 0.36 | 11.2 | 0.44 | 2.5 (0.094) |
| Untrained happy prosody | 13.1 | 0.45 | 11.2 | 0.63 | ||
| Trained happy prosody | 13.7 | 0.25 | 13.7 | 0.50 | ||
| Spectral range (dB) | Neutral prosody | 85.2 | 0.69 | 85.4 | 1.33 | 1.8 (0.175) |
| Untrained happy prosody | 88.0 | 0.97 | 87.1 | 1.18 | ||
| Trained happy prosody | 92.5 | 0.16 | 90.9 | 0.79 | ||
| Spectral change (dB/ms) | Neutral prosody | 12.2 | 1.46 | 13.2 | 0.83 | 10.7 (0.000) |
| Untrained happy prosody | 15.2 | 1.24 | 14.1 | 1.39 | ||
| Trained happy prosody | 34.1 | 0.73 | 25.3 | 1.56 | ||
| Fundamental frequency variation (Hz) | Neutral prosody | 26.4 | 2.93 | 30.5 | 3.03 | 0.2 (0.840) |
| Untrained happy prosody | 38.1 | 4.28 | 39.3 | 3.76 | ||
| Trained happy prosody | 52.8 | 4.31 | 52.6 | 5.01 | ||
| Speaking duration (s) | Neutral prosody | 22.1 | 0.55 | 22.1 | 0.71 | 0.9 (0.404) |
| Untrained happy prosody | 22.4 | 0.75 | 22.7 | 1.78 | ||
| Trained happy prosody | 17.7 | 0.67 | 20.1 | 1.23 | ||
SEM = standard error of the mean; CON = controls.
Inline Supplementary Table S5.
Table S5.
Comparison of perceptual speech analyses after two years.
| Condition | PD patients ON at the time of scanning (test 1) |
PD patients ON after two years (test 2) |
Interaction Condition × group (ON (test1) vs. ON (test2)) |
|||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | F-value (p-value) | ||
| Loudness (rater 1) | Neutral prosody | 2.9 | 0.08 | 2.7 | 0.14 | 8.4 (0.001) |
| Untrained happy prosody | 3.5 | 0.15 | 2.7 | 0.14 | ||
| Trained happy prosody | 3.0 | 0.01 | 3.0 | 0.00 | ||
| Pitch (rater 1) | Neutral prosody | 3.3 | 0.22 | 2.8 | 0.24 | 1.9 (0.121) |
| Untrained happy prosody | 3.2 | 0.27 | 3.4 | 0.23 | ||
| Trained happy prosody | 3.6 | 0.19 | 3.6 | 0.15 | ||
| Voice quality (rater 1) | Neutral prosody | 3.6 | 0.12 | 3.1 | 0.14 | 1.7 (0.150) |
| Untrained happy prosody | 3.5 | 0.12 | 3.4 | 0.13 | ||
| Trained happy prosody | 3.7 | 0.10 | 3.6 | 0.09 | ||
| Comprehensibility (rater 1) | Neutral prosody | 4.0 | 0.00 | 4.0 | 0.00 | 1.1 (0.342) |
| Untrained happy prosody | 4.0 | 0.00 | 3.9 | 0.08 | ||
| Trained happy prosody | 4.0 | 0.00 | 4.0 | 0.00 | ||
| Speech tempo (rater 1) | Neutral prosody | 3.7 | 0.19 | 3.5 | 0.19 | 1.3 (0.276) |
| Untrained happy prosody | 3.9 | 0.08 | 3.7 | 0.19 | ||
| Trained happy prosody | 3.8 | 0.13 | 3.8 | 0.13 | ||
SEM = standard error of the mean; CON = controls.
Those PD patients who were re-tested behaviorally after two years showed a decrease in speech intensity (p = 0.033, Cohen's d 2.4) and spectral change (p < 0.001, Cohen's d 3.4), driven by the trained affective prosody condition (Inline Supplementary Table S4) with a concomitant decrease in perceptual loudness (p = 0.001, Cohen's d 1.7), driven by the untrained affective prosody condition (Inline Supplementary Table S5). These results confirm that our patient sample was indeed pre-symptomatic regarding speech symptoms at the time of scanning.
Inline Supplementary Table S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.016.
Inline Supplementary Table S2 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.016.
Inline Supplementary Table S3 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.016.
Inline Supplementary Table S4 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.016.
Inline Supplementary Table S5 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.016.
3.2. fMRI results of overt reading with neutral intonation
We first present activity and connectivity results during cognitive preparation (preparation phase) before detailing effects of ongoing articulation (execution phase).
3.2.1. Brain activity during cognitive preparation for overt reading with neutral intonation
All participants pre-activated the bilateral caudate nuclei, mesial and inferior lateral prefrontal areas together with anterior insula during cognitive preparation prior to motor preparation (see also Fig. 2) without any significant group differences.
3.2.2. Effective connectivity during cognitive preparation for overt reading with neutral intonation
Despite the absence of significant brain activity differences between groups, effective connectivity during cognitive preparation for overt reading differed between PD patients and controls.
Relative to healthy controls, PD patients showed a hypo-connectivity between the left CN and the DLPFC, IFG and supplementary motor area (SMA) during cognitive preparation, independent of dopaminergic state (Fig. 3, left and right upper panels; Table 4).
Increases in effective connectivity compared to controls were observed during preparation for overt speech: Only PD patients OFF medication exhibited subcortical hyper-connectivity between the CN and motor PUT (Fig. 3, left lower panel; Table 4). This subcortical hyper-connectivity was levodopa-responsive (Fig. 3, left lower panel asterisked; Table 4). PD patients ON medication instead showed stronger connectivity between left dPMC and SMA compared to controls (Fig. 3, right lower panel; Table 4).
3.2.3. Brain activity during overt reading (execution phase)
The general activation pattern of overt reading is illustrated in Fig. 2 and shows widespread bilateral perisylvian, basal ganglia and cerebellar involvement for all groups. PD patients over-activated left prefrontal regions involved in feedforward processing and executive control: The left dorsal premotor cortex (dPMC) and IFG were more strongly recruited when overt speech was produced compared to healthy controls (Fig. 4, left and right panels; Table 5).
Fig. 4.
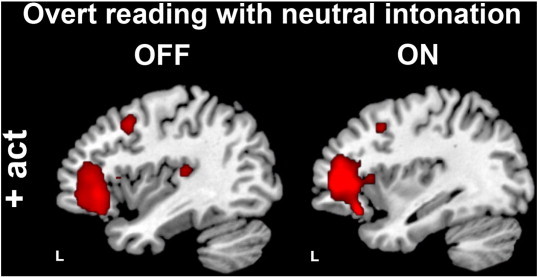
Group differences in brain activity during overt vs. covert reading with neutral intonation (execution phase). Compared to controls, PD patients over-activated the left inferior frontal gyrus, dorsal premotor cortex, and auditory cortex (the latter significant only for the OFF state, depicted in the left, in the ON state this group difference was just below threshold but not significantly different from the OFF state). All group differences were significant at p < 0.005, uncorrected, masked inclusively with the task-relevant network at p < 0.05, FWE corrected.
Table 5.
Group differences in brain activity during overt vs. covert reading with neutral intonation (execution phase).
| Anatomical region | BA | MNI-coordinates (x, y, z) | T-value | p-value |
|---|---|---|---|---|
| Hyper-activity in PD patients | ||||
| PD OFF > control | ||||
| L inferior frontal gyrus | 47 | − 42, 32, − 4 | 5.18 | 0.000 |
| L dorsal premotor cortex | 6 | − 40, 12, 44 | 4.18 | 0.000 |
| L auditory cortex | 41, 42 | − 40, − 26, 12 | 3.53 | 0.001 |
| PD ON > control | ||||
| L inferior frontal gyrus | 45 | − 36, 44, 6 | 5.14 | 0.000 |
| L dorsal premotor cortex | 6 | − 38, 12, 44 | 3.77 | 0.000 |
| L auditory cortex | 41, 42 | − 38, − 28, 8 | 2.64 | 0.006a |
Subthreshold.
In turn, we observed a decreased relative suppression of auditory cortex (AC) activity during processing of external auditory feedback (hearing one own's voice) in PD patients compared to controls with no significant differences between OFF and ON (p = 0.575) (Fig. 4, left and right panels; Table 5). The decrease of relative auditory cortex suppression correlated positively with the recruitment of left IFG (r = 0.83, p = 0.001).
There were no significant levodopa effects on brain activation during overt reading.
3.2.4. Effective connectivity during overt reading (execution phase)
Pathological hypo-connectivity in PD was observed between the left dPMC and the auditory feedback processing left AC (Fig. 5, left and right upper panels; Table 6). In PD OFF state, these regions were also less functionally connected with the left caudate nucleus (CN) compared to controls (Fig. 5, left upper panel; Table 6).
As during preparation for overt speech, we found compensatory increases in effective connectivity also during execution of overt speech: An increase in effective connectivity compared to controls was observed only in PD patients OFF medication between motor putamen (PUT), the left dPMC, IFG, dorsolateral prefrontal cortex (DLPFC) and superior temporal sulcus (STS) (Fig. 5, left lower panel; Table 6). Because this hyper-connectivity between the motor and executive control system and the sensory speech representations in STS was levodopa-responsive (Fig. 5, left lower panel asterisked and right lower panel; Table 6) and behavior was similar in ON and OFF state, it likely reflects compensatory efforts.
3.3. fMRI results of generation of affective prosody
3.3.1. Activity differences for affective prosody preparation and production
Activity maps revealed inverted activation profiles during preparation and execution of overt reading with happy versus neutral intonation: During preparation for affective compared to neutral prosody, PD patients over-activated the left DLPFC (Fig. 6, left lower panel; Table 7). This region was then hypo-activated during prosody production in PD relative to controls (Fig. 6, right upper panel; Table 8).
Fig. 6.
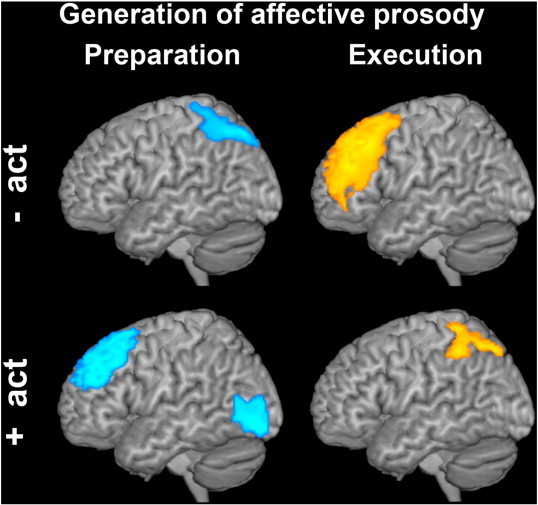
Group differences in brain activity during generation of affective vs. neutral prosody (preparation and execution phase). Only the comparison between PD patients OFF medication and controls is illustrated, as the contrast of PD patients ON state vs. controls revealed the same results. Compared to controls, PD patients hypo-activated the left superior parietal lobule and over-activated the left dorsolateral prefrontal cortex during preparation for affective prosody (preparation for affective prosody is shown in blue, on the left). During prosody production an opposite pattern was observed (execution of affective prosody is illustrated in yellow, on the right). Upper panels: hypo-activations, lower panels: hyper-activations in PD patients vs. controls. All group differences were significant at p < 0.005, uncorrected, masked inclusively with the task-relevant network at p < 0.05, FWE corrected.
Table 7.
Group differences in brain activity during cognitive preparation for affective vs. neutral prosody.
| Anatomical region | BA | MNI-coordinates (x, y, z) | T-value | p-value |
|---|---|---|---|---|
| Hypo-activity in PD patients | ||||
| PD OFF < control | ||||
| L superior parietal lobule | 7 | − 24, − 74, 52 | 4.93 | 0.000 |
| PD ON < control | ||||
| L superior parietal lobule | 7 | − 24, − 74, 52 | 5.39 | 0.000 |
| Hyper-activity in PD patients | ||||
| PD OFF > control | ||||
| L dorsolateral PFC | 8, 9 | − 34, 30, 44 | 4.11 | 0.000 |
| L middle occipital gyrus | 19 | − 38, − 90, 6 | 3.21 | 0.000 |
| PD ON > control | ||||
| L dorsolateral PFC | 8, 9 | − 36, 30, 44 | 2.87 | 0.002 |
| L inferior occipital gyrus | 19 | − 36, − 82, − 6 | 4.08 | 0.000 |
Table 8.
Group differences in brain activity during execution of affective vs. neutral prosody.
| Anatomical region | BA | MNI-coordinates (x, y, z) | T-value | p-value |
|---|---|---|---|---|
| Hypo-activity in PD patients | ||||
| PD OFF < control | ||||
| L dorsolateral PFC | 8, 9 | − 36, 30, 44 | 5.45 | 0.000 |
| PD ON < control | ||||
| L dorsolateral PFC | 8, 9 | − 34, 32, 44 | 3.20 | 0.001 |
| Hyper-activity in PD patients | ||||
| PD OFF > control | ||||
| L superior parietal lobule | 7 | − 26, − 70, 52 | 4.03 | 0.003 |
| PD ON > control | ||||
| L superior parietal lobule | 7 | − 24, − 74, 50 | 4.42 | 0.001 |
An opposite activation pattern was observed in the left superior parietal lobule (SPL) (Fig. 6, left upper and right lower panels; Tables 7 and 8): This region was hypo-activated during preparation in PD patients but more strongly involved during actual prosody production. Additionally, during preparation for affective prosody PD patients showed increased activation in the left occipito-temporal junction. No effect of medication on prosody-related brain activity was found.
3.3.2. Effective connectivity during preparation for and execution of affective prosody
The hypo-activated left SPL was less functionally connected to the left IFG during preparation for affective prosody in PD patients (ON and OFF medication) relative to controls (Fig. 7, left and right upper panels; Table 9). During actual affective prosody production, the relatively over-activated left SPL was then hyper-connected to the left SMA (Fig. 8, left and right lower panels; Table 10).
In contrast, the aforementioned preparatory over-recruitment of the left DLPFC in PD patients was also accompanied by preparatory striato-prefrontal hyper-connectivity between the left DLPFC, SMA, IFG, and CN (Fig. 7, left and right lower panels; Table 9).
In addition, we found reduced preparatory effective connectivity between the right limbic ventral striatum (vstriatum: 22, 8, − 6) and sensorimotor dorsal striatum (dstriatum: 14, 14, − 4; p = 0.016 SVC corr.) in PD patients OFF medication (not illustrated; Table 9) when preparing for affective prosody. During actual production of happy intonation (execution phase), the right STS connected less efficiently to the right sensorimotor dorsal striatum in PD patients OFF medication compared to controls (Fig. 8, left and right upper panels; Table 10).
4. Discussion
Voice and speech symptoms in PD may be a consequence of different functional anomalies that we detected prior to symptom onset in early PD patients. First, hypophonia may result from hampered and thus diminished motor drive or ‘energization’ on the basis of striato-prefrontal hypo-connectivity in PD together with dysfunctional self-monitoring mechanisms. Second, the reduced monitoring of one's own utterances (external auditory feedback) could additionally affect the sharpness of speech motor representations and result in dysarthria in the long term, when the disease progresses. Third, disturbed modulation of speech routines by affective state may impair generation of affective prosody on the basis of a pathological interplay between limbic and sensorimotor parts of the striatum. Fortunately, PD patients show compensation that can serve as model for future therapies.
4.1. Diminished ‘energization’ underlies voice symptoms
Although all participants activated prefrontal brain regions and caudate nuclei without any significant group differences during the preparatory setup of the speech network, pathological hypo-connectivity between the CN and prefrontal cortices (IFG, DLPFC, SMA) was observed in PD patients independent of dopaminergic medication. Because this anomaly was not levodopa-responsive, decreased striato-prefrontal effective connectivity during cognitive preparation in PD may point to direct consequences of subcortical pathology. As pathoanatomical disease markers are mostly restricted to subcortical regions in early PD (Braak and Braak, 2000), our data suggest that basal ganglia pathology could induce hypophonia by affecting cortico-basal loops (Alexander and Crutcher, 1990). Direct cortico-striatal fiber projections between the DLPFC and the ipsilateral CN (Goldman and Nauta, 2004) suggest that the CN plays an important role in subcortical modulation of prefrontal function. The prefrontal cortex serves at least two different functions during preparation of a given task: First, medial prefrontal cortices mediate the drive for action, a function that has been termed ‘energization’ (Kouneiher et al., 2009; Stuss and Alexander, 2007) and that is impaired in akinetic mutism. Second, lateral prefrontal cortices code task rules and thus contribute in recruiting brain regions for orchestrated processing in a functional network (Dosenbach et al., 2006; Sakai and Passingham, 2006). The observed pathological hypo-connectivity between medial and lateral PFC and the CN may thus be interpreted as dysfunctional cognitive preparation for the motor act of articulation due to subcortical dopamine deficiency. Importantly, motor preparation and planning could only take place during the execution phase of trials when linguistic material was presented. Thus, our data suggest that hypophonia could be a consequence of hampered and thus diminished ‘energization’ and/or task coding, which is a non-motor deficit. It is tempting to speculate that initiation deficits as part of hypokinesia in general could at least partly be explained by reduced ‘energization’ in the cognitive domain on the basis of reduced striato-prefrontal connectivity. Yet, given that hypophonia is not levodopa-responsive, while general hypokinesia usually is, diminished ‘energization’ may only partly account for hypophonia. Muscle stiffness may also contribute to the generation of this symptom. Together, these factors may induce hypophonic speech with the typical flat prosodic profile.
Our patient sample did not show significant speech initiation deficits, which suggests compensatory mechanisms. Indeed, we found levodopa-responsive increases in effective connectivity during cognitive preparation for overt speech between associative CN and motor PUT (Simonyan et al., 2013) in PD OFF state. In contrast to previous studies (Maillet et al., 2012), after dopamine intake, PD patients showed stronger connectivity between dPMC and SMA compared to controls suggesting that once dopamine levels are restored, a different compensatory mechanism involving dorsal premotor cortices becomes functional. Strikingly, recruitment of the SMA has previously been related specifically with levodopa-induced improvement of general hypokinetic (Haslinger et al., 2001; Wu et al., 2011) and voice symptoms (Liotti et al., 2003) in PD.
We thus propose that striato-prefrontal hypo-connectivity during cognitive preparation for action may eventually result in motor initiation deficits but is compensated in early PD by subcortical plasticity. On medication, medial and lateral dorsal premotor cortices hyper-connect and contribute to compensation. Given that the former is involved in self-generated movement while the latter is more related to externally guided action (Cunnington et al., 2002; Jahanshahi et al., 1995), this particular cortical compensatory mechanism could potentially explain the efficacy of external cueing in PD. Providing external cues is particularly relevant in PD speech therapy, where dysfunctional self-monitoring mechanisms have been described both regarding phonematic processing (Mollaei et al., 2013) and scaling of speech intensity (Ho et al., 1999, 2000). Importantly, one aspect of the well-described LSVT is the focus on increasing loudness by increasing speech effort and automatizing it (Fox et al., 2002). We speculate that the therapeutic instruction results in a re-calibration of motor drive which in turn normalizes behavior and cortical functional anomalies in symptomatic PD patients (Liotti et al., 2003).
4.2. Reduced monitoring of external auditory feedback in PD
Compared to healthy controls, PD patients prior to speech symptom onset over-activated prefrontal regions involved in feedforward processing (Hickok and Poeppel, 2007; Perrier, 2012) and executive control (Kouneiher et al., 2009) (as previously demonstrated by Liotti et al. (2003) and Pinto et al. (2004) for symptomatic patients) and showed diminished physiological auditory cortex suppression during overt speaking independent of their dopaminergic state (ON or OFF treatment). Physiologically, AC activity is relatively suppressed during speech production or vocalization to increase sensitivity towards changes in external auditory feedback (Chang et al., 2012; Eliades and Wang, 2008). This suppression likely results from an interaction between motor and auditory cortices, potentially in form of a corollary discharge or efference copy of the feedforward motor command. The hypo-connectivity between auditory and dorsal premotor cortices supports the notion that PD patients monitor less their own external auditory feedback as previously suggested on the behavioral level (Ho et al., 2000; Mollaei et al., 2013). This could be a direct consequence of subcortical pathology given that the left AC is functionally coupled to the nigro-striatal dopaminergic system during speech production (Simonyan et al., 2013). Dysfunctional self-monitoring of speech intensity is a necessary pathophysiological component leading to voice symptoms, because if PD patients spontaneously detected their hypophonia (which is not the case on the behavioral level) they could correct their reduced motor drive.
Reduced use of external auditory feedback could also ultimately lead to speech symptoms: External auditory feedback may additionally inform a so called internal model that is thought to map phonematic speech motor representations onto representations of their sensory targets (Hickok et al., 2011). This internal model has been acquired during language learning but is also later throughout life constantly adapting to changes that otherwise would affect speech comprehensibility (Shiller et al., 2010). Such a mapping of sensory consequences of articulatory gestures critically depends on neurons with extremely narrow tuning curves (Engel and Wang, 2011). In PD, increased neural noise (Frank, 2005; Nicola et al., 2004) could render auditory-motor interactions ineffective that depend on precise mapping between finely tuned neuronal populations. We propose that as a consequence of reduced monitoring of external auditory feedback the internal speech motor representations are insufficiently updated, asking for additional executive control to maintain speech normal in early stages of the disease. This interpretation is supported by our finding that those PD patients who monitor auditory feedback less activate motor representations more strongly.
While the functional anomalies in terms of activation profiles were not levodopa-responsive, we observed a pronounced increase in effective connectivity between motor PUT, left prefrontal cortex (DLPFC, dPMC, and IFG), and the left STS in PD patients OFF medication which normalized after levodopa administration. The STS is thought to represent sensory speech representations while motor speech representations are found in opercular parts of the IFG. The dPMC plays a role in sensorimotor mapping and all regions are thought to contribute to the proposed internal model of speech processing (Hickok and Poeppel, 2007; Hickok et al., 2011). Given that patients produced normal speech both ON and OFF levodopa, the observed hyper-connectivity in regions associated with the internal speech model could point to increased mapping effort in internal sensorimotor loops in the OFF state. Levodopa administration seems to reduce the need for such compensation, possibly due to reduced neural noise in the system.
Taken together, our data suggest a reduced use of external auditory feedback that together with the diminished drive to act results in hypophonia but could also induce dysarthria on the basis of a successive deterioration of phonematic speech motor representations. However, even PD patients in later stages of their disease are able to achieve normal speaking if a therapist provides a useful external model or assists in increasing loudness (Fox et al., 2002). This suggests that although the auditory self-monitoring mechanisms are not fully operational, PD patients can use feedback given by a therapist to modulate speech behavior (Fox et al., 2002). This likely involves plasticity in cortico-basal loops. Once the prefrontal cortices are neuropathologically involved in the progressive disease, the therapeutic effects may vanish and speech intelligibility may deteriorate to incomprehensible levels due to fading speech motor representations or insufficient executive control.
4.3. ‘Artificial’ affective prosody as potential therapeutic tool
The included PD patients who did not yet experience overt speech symptoms produced normal affective prosody characterized on the acoustic level by stronger modulation of speech melody and increased intensity compared to neutral speech. Yet, these were the parameters that were first affected 2 years after inclusion in the study suggesting that affective prosody entails high demands on modulation of speech melody. Given that we trained all participants to produce happy intonation despite the neutral nature of the sentences, this condition can be interpreted as depending on an external model given by the experimenter. Indeed, PD patients in early stages of their disease are able to achieve normal affective prosody when they are requested to imitate emotional speech (Möbes et al., 2008).
We have previously shown that preparation for affective prosody generation relates to a functional interplay between ventral and dorsal striatum together with increased effective connectivity between striatum and a cortical network involved in autobiographical memory (Pichon and Kell, 2013). PD patients OFF medication showed diminished functional coupling between right ventral and dorsal striatum suggesting diminished modulation of speech plans by affect. Yet, affective prosody in our experiment was ‘artificial’ and not authentic and depended largely on an external therapeutic model. Nevertheless, healthy controls seemed to have better access to limbic information compared to early PD patients.
Patients also showed a functional anomaly on the cortical level: They involved prefrontal cortices more strongly than controls during cognitive preparation while physiological parietal pre-activation was delayed into the execution phase together with a similarly altered connectivity profile. Hence, we speculate that this could reflect different executive control of model learning in PD patients compared to controls. Recent neuroimaging studies revealed that parietal cortices contribute to sensorimotor adaptation for speech (Shum et al., 2011) and monitor features of produced speech (Carota et al., 2010). In addition, over-activations of lateral premotor and inferior parietal cortices during motor movements have been identified as compensatory mechanism for reduced striato-mesial-prefrontal activation in PD (Sabatini et al., 2000; Samuel et al., 1997). As described above, this exact striato-prefrontal connectivity (CN, SMA, IFG, and DLPFC) is pathologically decreased during cognitive preparation for neutral speech in PD patients (ON and OFF medication). Thus, training speech melody and loudness with an external model and therapeutic feedback (as instantiated for happy reading in our experiment) partly remedied this functional anomaly. The LSVT program focuses on training loudness but effects generalize to improvements of speech modulation (prosody), articulation, and even facial expression (Fox et al., 2002). Interestingly, the extension of this specific therapeutic approach into physiotherapy, the so-called LSVT LOUD and LSVT BIG (Fox et al., 2012) proves that increasing the amplitude of speech and non-speech movements by external therapeutic models is efficient in reducing hypokinesia in general. In these therapy programs, patients learn to overcome their hesitation to move and speak in a way they often describe as exaggerated. Consequently, patients initially largely depend on external feedback given by the therapist before they automatize the newly learned motor routines. This adequately circumvents the here shown dysfunctional auditory self-monitoring systems of PD patients.
Please note that signs of reduced monitoring of external auditory feedback (as described for neutral intonation above) were also observed for generation of affective prosody: The right STS that has previously been shown to be sensitive to acoustic features of prosodic modulations in perceptual (Wiethoff et al., 2008) and speech production studies (Pichon and Kell, 2013) was less functionally coupled to the right dorsal striatum during execution of affective prosody in PD patients OFF medication.
4.4. Limitations
In our study some limitations occur. Given that we did not conduct a longitudinal study, we cannot answer the question how the anomalies detected in the included early PD patients develop once the disease progresses and compensatory mechanisms may fail. Another limitation was the use of a reading task in our study as compared to free speech. Reading clearly differs from free speaking but is nevertheless affected in PD (Van Lancker Sidtis et al., 2012). Yet, because we were interested in cognitive task preparation, we were not able to study free speech. Reading is also an easier task than sentence repetition in the noisy scanner conditions.
We used PPI analyses to assess effective connectivity changes. While this robust measure has important advantages over model-based connectivity analyses (see Material and methods), it does not allow for directionality and causality statements of the described brain networks (Friston et al., 1997). A further step would be the use of dynamic causal modeling to search for differences in causality and directionality in a more selected set of brain regions. Finally, connectivity analyses so far are restricted to a certain number of regions of interest so that we cannot rule out other changes in effective connectivity beyond the studied regions.
4.5. Conclusion
Taken together, our data show that PD patients' hypophonia could arise from a diminished drive to act resulting from a pathological functional interplay between the striatum and prefrontal cortices that is accompanied by dysfunctional self-monitoring mechanisms. Given that PD patients monitor less the sensory outcome of their utterances even before they become symptomatic, faded speech motor representations may be a cause rather than a consequence of hypokinetic dysarthria. While these symptoms may be partly remedied by levodopa-induced facilitation of cortical compensatory mechanisms involving dorsal premotor cortices, behavioral therapies more efficiently improve speech by complementing the fading internal model by newly learned speech routines on the basis of external models and feedback given by the therapist.
Role of the funding source
This work was supported by an Innovation grant to CAK from the Medical Faculty of Goethe University. All authors had full access to the data of the study as well as final responsibility for the decision to submit for publication.
Acknowledgments
We thank Rüdiger Hilker, Ute Rockel, and Jan-Suk Kang for help with patient recruitment, the Frankfurt Senior Citizens University for support with control participant recruitment, Sriramya Somasundaram and Lisa Schulz for perceptually judging all speech recordings, Marion Behrens for neuropsychological testing, Swann Pichon for helpful ideas, and Frederic von Wegner for help with analyzing the acoustic speech dataset.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Aronson A.E. Thieme; 1990. Clinical Voice Disorders: An Interdisciplinary Approach. [Google Scholar]
- Benke T., Bosch S., Andree B. A study of emotional processing in Parkinson's disease. Brain Cogn. 1998;38(1):36–52. doi: 10.1006/brcg.1998.1013. [DOI] [PubMed] [Google Scholar]
- Berardelli A. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124(11):2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Boersma P., Weenik D. Institute of Phonetic Sciences of the University of Amsterdam, Report 132. 1996. PRAAT, a system for doing phonetics by computer, version 3.4. [Google Scholar]
- Braak H., Braak E. Pathoanatomy of Parkinson's disease. J. Neurol. 2000;247(2):II3–II10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- Caekebeke J.F. The interpretation of dysprosody in patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 1991;54(2):145–148. doi: 10.1136/jnnp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancelliere A.E.B., Kertesz A. Lesion localization in acquired deficits of emotional expression and comprehension. Brain Cogn. 1990;13(2):133–147. doi: 10.1016/0278-2626(90)90046-q. [DOI] [PubMed] [Google Scholar]
- Canter G.J. Speech characteristics of patients with Parkinson's disease: I. Intensity, pitch, and duration. J. Speech Hear. Disord. 1963;28(3):221. doi: 10.1044/jshd.2803.221. [DOI] [PubMed] [Google Scholar]
- Carota F. Neural dynamics of the intention to speak. Cereb. Cortex. 2010;20(8):1891–1897. doi: 10.1093/cercor/bhp255. [DOI] [PubMed] [Google Scholar]
- Chang E.F. Human cortical sensorimotor network underlying feedback control of vocal pitch. Proc. Natl. Acad. Sci. 2012;110(7):2653–2658. doi: 10.1073/pnas.1216827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax B.J. The dopamine transporter: immunochemical characterization and localization in brain. J. Neurosci. 1995;15(3):1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L.S. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158 Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48(9):2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Cunnington R. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15(2):373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- de Frias C.M. Influence of COMT gene polymorphism on fMRI-assessed sustained and transient activity during a working memory task. J. Cogn. Neurosci. 2010;22(7):1614–1622. doi: 10.1162/jocn.2009.21318. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J.C. Variation in dopamine genes influences responsivity of the human reward system. Proc. Natl. Acad. Sci. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eliades S.J., Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453(7198):1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Engel T.A., Wang X.-J. Same or different? A neural circuit mechanism of similarity-based pattern match decision making. J. Neurosci. 2011;31(19):6982–6996. doi: 10.1523/JNEUROSCI.6150-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Elton R.L., Committee U.D. Unified Parkinson's disease rating scale. Recent Dev. Park. Dis. 1987;2:153–163. [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox C.M. Current perspectives on the Lee Silverman Voice Treatment (LSVT) for individuals with idiopathic Parkinson disease. Am. J. Speech-Lang. Pathol. 2002;11(2):111–123. [Google Scholar]
- Fox C. LSVT LOUD and LSVT BIG: behavioral treatment programs for speech and body movement in Parkinson disease. Park. Dis. 2012 doi: 10.1155/2012/391946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.J. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J. Cogn. Neurosci. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- Friston K.J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995;3(3):165–189. [Google Scholar]
- Friston K.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M. The role of the dopamine transporter DAT1 genotype on the neural correlates of cognitive flexibility. Eur. J. Neurosci. 2010;31(4):754–760. doi: 10.1111/j.1460-9568.2010.07102.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S.S., Tourville J.A., Guenther F.H. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J. Speech Lang. Hear. Res. 2008;51(5):1183. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman D.R. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19(1):200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goldman P.S., Nauta W.J.H. An intricately patterned prefronto-caudate projection in the rhesus monkey. J. Comp. Neurol. 2004;171(3):369–385. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- Haslinger B. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124(3):558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hickok G., Houde J., Rong F. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron. 2011;69(3):407–422. doi: 10.1016/j.neuron.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker R. Disease progression continues in patients with advanced Parkinson's disease and effective subthalamic nucleus stimulation. J. Neurol. Neurosurg. Psychiatry. 2005;76(9):1217–1221. doi: 10.1136/jnnp.2004.057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.K. Speech volume regulation in Parkinson's disease: effects of implicit cues and explicit instructions. Neuropsychologia. 1999;37(13):1453–1460. doi: 10.1016/s0028-3932(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Ho A.K., Bradshaw J.L., Iansek R. Volume perception in Parkinsonian speech. Mov. Disord. 2000;15(6):1125–1131. doi: 10.1002/1531-8257(200011)15:6<1125::aid-mds1010>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55(3):181. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M. Self-initiated versus externally triggered movements. Brain. 1995;118(4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Kalbe E. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int. J. Geriatr. Psychiatry. 2004;19(2):136–143. doi: 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- Karoum F., Chrapusta S.J., Egan M.F. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J. Neurochem. 1994;63(3):972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kell C.A. How the brain repairs stuttering. Brain. 2009;132(10):2747–2760. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Kell C.A. Lateralization of speech production starts in sensory cortices — a possible sensory origin of cerebral left dominance for speech. Cereb. Cortex. 2011;21(4):932–937. doi: 10.1093/cercor/bhq167. [DOI] [PubMed] [Google Scholar]
- Klostermann F. Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2008;79(5):522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- Kouneiher F., Charron S., Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat. Neurosci. 2009;12(7):939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M. Hypophonia in Parkinson's disease neural correlates of voice treatment revealed by PET. Neurology. 2003;60(3):432–440. doi: 10.1212/wnl.60.3.432. [DOI] [PubMed] [Google Scholar]
- Logemann J.A. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J. Speech Hear. Disord. 1978;43(1):47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- Maillet A. Levodopa effects on hand and speech movements in patients with Parkinson's disease: a fMRI study. PloS One. 2012;7(10):e46541. doi: 10.1371/journal.pone.0046541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M. Catechol-O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116(1):127–138. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M. Catechol-O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003;28(8):1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- McLaren D.G. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möbes J. Emotional speech in Parkinson's disease. Mov. Disord. 2008;23(6):824–829. doi: 10.1002/mds.21940. [DOI] [PubMed] [Google Scholar]
- Mollaei F., Shiller D.M., Gracco V.L. Sensorimotor adaptation of speech in Parkinson's disease. Mov. Disord. 2013 doi: 10.1002/mds.25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola S.M., Hopf F.W., Hjelmstad G.O. Contrast enhancement: a physiological effect of striatal dopamine? Cell Tissue Res. 2004;318(1):93–106. doi: 10.1007/s00441-004-0929-z. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perrier P. Gesture planning integrating knowledge of the motor plant's dynamics: a literature review from motor control and speech motor control. Speech Plann. Dyn. 2012;191–238 [Google Scholar]
- Pichon S., Kell C.A. Affective and sensorimotor components of emotional prosody generation. J. Neurosci. 2013;33(4):1640–1650. doi: 10.1523/JNEUROSCI.3530-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S. Subthalamic nucleus stimulation and dysarthria in Parkinson's disease: a PET study. Brain. 2004;127(3):602–615. doi: 10.1093/brain/awh074. [DOI] [PubMed] [Google Scholar]
- Preibisch C. Event-related fMRI for the suppression of speech-associated artifacts in stuttering. Neuroimage. 2003;19(3):1076–1084. doi: 10.1016/s1053-8119(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Ramig L.O., Fox C., Sapir S. Parkinson's disease: speech and voice disorders and their treatment with the Lee Silverman Voice Treatment. Semin. Speech Lang. 2004;25(2):169–180. doi: 10.1055/s-2004-825653. [DOI] [PubMed] [Google Scholar]
- Rascol O. Limitations of current Parkinson's disease therapy. Ann. Neurol. 2003;53(S3):S3–S15. doi: 10.1002/ana.10513. [DOI] [PubMed] [Google Scholar]
- Reuter M. The modulatory influence of the functional COMT Val158Met polymorphism on lexical decisions and semantic priming. Front. Hum. Neurosci. 2009;3 doi: 10.3389/neuro.09.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romito L.M., Albanese A. Dopaminergic therapy and subthalamic stimulation in Parkinson's disease: a review of 5-year reports. J. Neurol. 2010;257:298–304. doi: 10.1007/s00415-010-5720-3. [DOI] [PubMed] [Google Scholar]
- Rosen K.M. Parametric quantitative acoustic analysis of conversation produced by speakers with dysarthria and healthy speakers. J. Speech Lang. Hear. Res. 2006;49(2):395–411. doi: 10.1044/1092-4388(2006/031). [DOI] [PubMed] [Google Scholar]
- Sabatini U. Cortical motor reorganization in akinetic patients with Parkinson's disease. A functional MRI study. Brain. 2000;123(2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Sakai K., Passingham R.E. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J. Neurosci. 2006;26(4):1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain. 1997;120(6):963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Sano A. A 40-nucleotide repeat polymorphism in the human dopamine transporter gene. Hum. Genet. 1993;91(4):405–406. doi: 10.1007/BF00217369. [DOI] [PubMed] [Google Scholar]
- Sesack S.R. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiller D.M., Gracco V.L., Rvachew S. Auditory–motor learning during speech production in 9–11-year-old children. PloS One. 2010;5(9):e12975. doi: 10.1371/journal.pone.0012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum M. Sensorimotor integration for speech motor learning involves the inferior parietal cortex. Eur. J. Neurosci. 2011;34(11):1817–1822. doi: 10.1111/j.1460-9568.2011.07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K., Herscovitch P., Horwitz B. Speech-induced striatal dopamine release is left lateralized and coupled to functional striatal circuits in healthy humans: a combined PET, fMRI and DTI study. Neuroimage. 2013;70:21–32. doi: 10.1016/j.neuroimage.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinder-Meredith Speech Characteristics Rating Scale. 2009. http://wwwspeech-language-therapycom/speech/pdf/scrpdf
- Stuss D.T., Alexander M.P. Is there a dysexecutive syndrome? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007;362(1481):901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; New York: 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Van Lancker Sidtis D. Voice and fluency changes as a function of speech task and deep brain stimulation. J. Speech Lang. Hear. Res. 2010;53(5):1167–1177. doi: 10.1044/1092-4388(2010/09-0154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lancker Sidtis D., Cameron K., Sidtis J.J. Dramatic effects of speech task on motor and linguistic planning in severely dysfluent Parkinsonian speech. Clin. Linguist. Phon. 2012;26(8):695–711. doi: 10.3109/02699206.2012.696307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoff S. Cerebral processing of emotional prosody-influence of acoustic parameters and arousal. Neuroimage. 2008;39(2):885–893. doi: 10.1016/j.neuroimage.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Wu T. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage. 2011;55(1):204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- Yesavage J.A. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


