Abstract
Objectives
We examined the incidence trends of bladder and kidney cancers using a population-based cancer registration data.
Methods
Age-standardized incidence rates were analyzed using data from the Shanghai Cancer Registry during 1973 to 2005. Annual percentage changes and 95% confidence intervals were calculated to evaluate the incidence changes. Age-period-cohort analysis was further implemented to assess the contributions of age, period and cohort effects to the trends using the intrinsic estimator method.
Results
In total, 12,676 bladder and 5,811 kidney cancer patients were registered in urban Shanghai. The age-standardized rates of bladder cancer in males increased from 6.39 to 7.66 per 100,000, or 0.62% per year, whereas the rates in females increased from 1.95 to 2.09 per 100,000, or 0.33% per year. For kidney cancer, the age-standardized rates in males increased from 1.20 to 5.64 per 100,000, or 6.98% per year. Similarly in females, the rates increased from 0.85 to 3.33 per 100,000, or 5.93% per year. Age-period-cohort analysis showed increasing curves of age and period effects but generally decreasing cohort effects for bladder and kidney cancers.
Conclusions
Our results show increasing incidence trends of bladder and kidney cancers in Chinese men and women, especially for kidney cancer.
Introduction
Nowadays, urinary bladder and kidney cancers are the most frequent malignant tumors of the urinary tract, together making up about 5% of all the cancers worldwide. And it also represents the 11th and 14th most common cancers respectively in terms of estimated age-standardized incidence rates [1]. In China, there were an estimated 54,927 new cases and 21,024 deaths of bladder cancer, and 32,508 new cases and 10,675 deaths of cancers of kidney, renal pelvis and ureter in 2008, respectively [1].
Earlier studies conducted in Western countries indicated that both urinary bladder and kidney cancers showed the increasing incidence trends in past several decades [2-5]. However, there has been little information about incidence trends of these two cancers in the Asian population so far. Thus, in order to have comprehensive knowledge of incidence trends of urinary bladder and kidney cancers in China, we conducted a time trend analysis on these cancers using data from the population-based Shanghai Cancer Registry.
Materials and Methods
Cancer patient and general population data
The Shanghai Cancer Registry, an associate member of the International Agency for Research on Cancer (IARC), is one of the largest population-based Cancer Registries in developing world. The data which is collected, processed and reported systematically by using standard procedures has been published in consecutive volumes of the Cancer Incidence of Five Continents series by IARC. The study populations were residents of all the ten original urban districts of Shanghai, namely Huang Pu, Nan Shi, Lu Wan, Xu Hui, Chang Ning, Jing An, Pu Tuo, Zha Bei, Hong Kou and Yang Pu districts. Male and female populations in urban area were obtained from the Shanghai Municipal Bureau of Public Security at the end of each year during 1973 to 2005. The mid-year population by sex was estimated based upon the populations at the ends of two consecutive years provided by Shanghai Municipal Bureau of Public Security and used as estimate of the annual average population. Based on eight national or local city censuses, information on population of urban area by sex-age group for census years was obtained. The population in the sex-age group for the remaining years between two consecutive censuses were estimated via linear interpolation method [6].
According to the regulation issued by the Shanghai Municipal Bureau of Public Health, all medical facilities in Shanghai are responsible for notifying all newly diagnosed cancer cases to the Shanghai Cancer Registry. In this study, all newly-diagnosed incidence cases of cancers of bladder, kidney, renal pelvis and ureter from 1973 to 2005 were registered in the Shanghai Cancer Registry. The calendar periods of 1973–2001 and 2002–2005 were respectively collected by the Shanghai Cancer Institute and the Shanghai Center for Disease Control and Prevention. The codes for different urinary tract cancer sites were converted into 10th revision of the International Classification of Diseases (ICD-10), i.e. C64 for malignant neoplasm of kidney, except renal pelvis (hence forth referred to as “kidney cancer” for short), C65 for malignant neoplasm of renal pelvis, C66 for malignant neoplasm of ureter, C67 for malignant neoplasm of bladder and C68 for malignant neoplasm of other and unspecified urinary organs. More detail information on the Shanghai Cancer Registry could be obtained from our previously published papers and monograph [6-8].
The data quality was depend on the diagnostic criteria of histological verification (HV) which included histological detection and cytological or biochemical detection [9]. The proportions of patients diagnosed by surgery or medical imaging such as B ultrasound, X-ray, computed tomography were also calculated in our analysis. Moreover, the proportions of clinical deduction and death certificate (It included the death certificate notification (DCN) and death certificate only (DCO) cases before 1987; And it was only the DCO cases after 1987.) were also used to present the quality of our data (Table 1).
Table 1. Proportions (%) of diagnostic evidence for urinary bladder and kidney cancers in urban Shanghai (1973-2005).
| Sites & Periods | No. of cases | Histological detection | Cytological or biochemical detection | Surgery or medical imaging | Clinical deduction | Death certificate a | Unknown |
|---|---|---|---|---|---|---|---|
| Bladder cancer | |||||||
| 1973-1975 | 651 | 45.78 | 0.00 | 17.51 | 4.92 | 31.80 | 0.00 |
| 1976-1978 | 732 | 54.37 | 0.41 | 13.25 | 2.05 | 29.92 | 0.00 |
| 1979-1981 | 786 | 61.20 | 0.64 | 11.20 | 3.82 | 23.16 | 0.00 |
| 1982-1984 | 875 | 61.94 | 1.49 | 8.80 | 4.46 | 23.31 | 0.00 |
| 1985-1987 | 1055 | 64.64 | 1.90 | 9.67 | 3.89 | 19.81 | 0.09 |
| 1988-1990 | 1105 | 72.58 | 1.09 | 17.56 | 4.34 | 4.34 | 0.09 |
| 1991-1993 | 1260 | 72.38 | 1.43 | 21.90 | 4.29 | 0.00 | 0.00 |
| 1994-1996 | 1258 | 74.96 | 0.64 | 21.14 | 3.18 | 0.00 | 0.08 |
| 1997-1999 | 1487 | 76.66 | 0.47 | 20.85 | 2.02 | 0.00 | 0.00 |
| 2000-2002 | 1595 | 77.62 | 2.07 | 17.68 | 1.44 | 0.88 | 0.31 |
| 2003-2005 | 1872 | 79.59 | 0.59 | 14.42 | 2.08 | 3.31 | 0.00 |
| Total | 12676 | 70.42 | 1.03 | 16.38 | 3.08 | 9.03 | 0.06 |
| Kidney cancer | |||||||
| 1973-1975 | 162 | 38.27 | 0.00 | 20.99 | 8.64 | 32.10 | 0.00 |
| 1976-1978 | 162 | 51.23 | 0.00 | 17.90 | 4.32 | 26.54 | 0.00 |
| 1979-1981 | 149 | 51.68 | 0.00 | 18.12 | 5.37 | 24.83 | 0.00 |
| 1982-1984 | 182 | 55.49 | 0.00 | 19.23 | 2.75 | 22.53 | 0.00 |
| 1985-1987 | 286 | 62.24 | 0.00 | 18.18 | 2.45 | 16.78 | 0.35 |
| 1988-1990 | 434 | 57.60 | 0.23 | 32.03 | 5.99 | 4.15 | 0.00 |
| 1991-1993 | 542 | 60.33 | 0.92 | 35.42 | 3.32 | 0.00 | 0.00 |
| 1994-1996 | 560 | 58.75 | 0.71 | 37.32 | 3.21 | 0.00 | 0.00 |
| 1997-1999 | 781 | 63.12 | 0.51 | 34.70 | 1.66 | 0.00 | 0.00 |
| 2000-2002 | 1098 | 69.40 | 1.18 | 27.50 | 1.09 | 0.73 | 0.09 |
| 2003-2005 | 1455 | 72.92 | 0.48 | 22.20 | 1.44 | 2.96 | 0.00 |
| Total | 5811 | 64.09 | 0.59 | 27.76 | 2.56 | 4.99 | 0.02 |
a The figure included the death certificate notification (DCN) and the death certificate only (DCO) cases before 1987; It was only the DCO cases after 1987.
Statistical analysis
In this study, 3-year age-adjusted incidence rates of urinary bladder and kidney cancers and calendar period from 1973-1975 to 2003-2005 were calculated by direct standardization with the World Standard Population adjusted by Doll R in 1966 [10]. The annual percentage change (APC) for incidence rates was used to examine the secular trends [11]. The natural logarithm of the rates were fitted by a regression line (y= α+βx+ɛ, where y = ln (rate) and x = calendar year). We used the weighted least squares method to estimate the parameter β and the weight is the inverse of the variance of ln (rate) which is approximately equal to the number of cases [11]. The APC was calculated as 100×(eβ- 1) with the 95% confidence interval calculated by the methods for population-based cancer statistics recommended by the National Cancer Institute (USA) [12].
An age-period-cohort model was further used to analyze bladder (ICD 10: C67) and kidney (ICD 10: C64) cancer incidences and provided a simultaneous assessment of the contribution of age, period and cohort effects on observed time trends. Ages included in the model were truncated and categorized into eleven 5-year age groups (30–34, 35–39 ... 80–84) since bladder and kidney cancers had few cases under the age of 30. Years of diagnosis were also truncated and divided into six 5-year calendar periods (1976–1980, 1981–1985 ... 2001–2005). As a result, sixteen 10-year overlapping birth cohorts were obtained by subtracting the 5-year age bands from the 5-year periods of diagnosis. In order to solve the problem of overlap, midpoints of each birth cohort bands were extracted to yield sixteen new groups (1896 , 1901 , 1906 , 1911 , 1916 , 1921 , 1926 , 1931 , 1936 , 1941 , 1946, 1951 , 1956 , 1961 , 1966 , 1971 ) .
We used a novel method to fit the age-period-cohort model—namely the intrinsic estimator (IE) method [13-15], which is aimed to deal with the non-identifiability problem [16,17]. The regression coefficients and its standard errors were computed using the STATA version 11 (STATA Corporation, College Station, TX, USA). For all the analysis in this study, two-sided P-values <0.05 were considered statistically significant.
Results
Between 1973 and 2005, there were 12,676 cases for urinary bladder cancer (ICD 10: C67), 5,811 cases for kidney cancer (ICD 10: C64), 631 cases for renal pelvis cancer (ICD 10: C65) and 493 cases for ureter cancer (ICD 10: C66) identified by the Shanghai Cancer Registry. During this period, the diagnosis of 70.42% patients was based on pathology and the proportion of death certificate cases was 9.03% for urinary bladder cancer. For kidney cancer, 64.09% of patients was diagnosed based on pathology and 4.99% as the death certificate cases. During the 33-year period, there was a sharp increase in the percentage of HV for these two cancers, while the death certificate cases experienced a drastic reduction (Table 1). The above messages indicated that our data was considerably reliable in this study.
Incidence trends
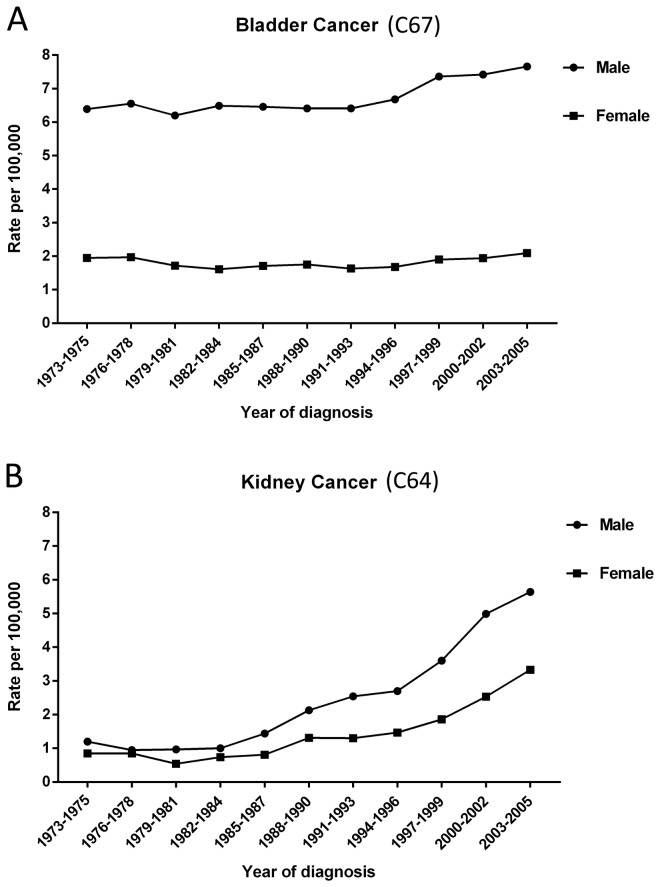
The age-standardized incidence rates of bladder cancer were around 7.66 per 100,000 for male and 2.09 per 100,000 for female during 2003 to 2005. During this 33-year period, the overall age-standardized incidence rates had increased annually by 0.62% (P <0.05) and 0.33% (P >0.05) for male and female respectively (Figure 1). The age-standardized incidence rates of kidney cancer during 2003 to 2005 were 5.64 per 100,000 for male and 3.33 per 100,000 for female which exceeded the rate of bladder cancer among females. During the years of 1973 to 2005, kidney cancer had a most dramatically increase with an APC of 6.98% (P <0.0001) for male and 5.93% (P <0.0001) for female (Figure 1). Besides, age-standardized incidence rates for cancers of kidney, renal pelvis and ureter (ICD 10: C64-66) together showed dramatically increase for both genders (Table 2).
Figure 1. Trends in incidence rates of cancers of bladder and kidney in urban Shanghai, 1973-2005.
Table 2. Incidence trends for cancers of bladder, kidney, renal pelvis and ureter by gender in urban Shanghai (1973-2005).
|
1973-1975
|
2003-2005
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Cases | Rate a | Cases | Rate a | Percent Change b (%) | APC c | P value d | 95% CI |
| Bladder (C67) | ||||||||
| Male | 470 | 6.39 | 1421 | 7.66 | 19.87 | 0.62 | 0.0017 | (0.25, 1.00) |
| Female | 181 | 1.95 | 451 | 2.09 | 7.18 | 0.33 | 0.1958 | (-0.18,0.85) |
| Kidney (C64) | ||||||||
| Male | 91 | 1.20 | 919 | 5.64 | 370.00 | 6.98 | <0.0001 | (6.28, 7.69) |
| Female | 71 | 0.85 | 536 | 3.33 | 291.76 | 5.93 | <0.0001 | (5.04, 6.83) |
| Kidney, renal pelvis and ureter (C64-66) e | ||||||||
| Male | 109 | 1.41 | 1044 | 6.35 | 350.35 | 6.43 | <0.0001 | (5.82, 7.04) |
| Female | 77 | 0.92 | 640 | 3.79 | 311.96 | 5.94 | <0.0001 | (5.14, 6.75) |
APC, annual percentage change; ICD-10, International Classification of Diseases, Tenth Revision; CI, confidence interval;
a Age-standardized rate to the World Standard Population
b Percent change between 1973 and 2005 was calculated by the age-standardized rate (world population)
c APC was calculated based on age-standardized (world population, per 100,000) incidence rate
d P value is calculated for the APCd
e The category kidney, renal pelvis and ureter (C64-66) is based on the list of the cancer sites from the cancer dictionary available in the CLOBOCAN database (IARC).
Age-period-cohort analysis
This IE method was used to disentangle the relative contributions of chronological age, historical period, and birth cohort on the incidences of urinary bladder and kidney cancers in urban Shanghai from 1975 to 2005. Age-period-cohort analysis showed apparently increasing trends of age and period effects but generally decreasing cohort effects for bladder and kidney cancers.
For bladder cancer, the age was an important factor contributing to the incidence trend. With it increased from 30 to 84 years old, the relative risk of bladder cancer showed a remarkable rise for both male and female. The risk in the aged 80-84 group in male was about five times higher than that for all ages combined. For female, it was about four times higher compared with all ages combined (Table 3). When we considered period effects for bladder cancer, the curve of relative risk also increased dramatically after the relatively mild rise before 1996. Moreover, the results indicated that for both genders the risks of bladder cancer incidence were more than doubled in 2001–2005 as compared to 1976–1980. It was calculated as the difference in the coefficients for two periods of 2001–2005 and 1976–1980, which is e0.444 to−0.348=2.21 in male and e0.503 to −0.253 =2.13 in female (Table 3). For the birth cohort effect, the most notable peak occurred in 1906 for both genders and the relative risks were both about two times higher than all birth cohorts combined by gender. Although the incidence risk of birth cohort showed a general decline for both genders, there still existed some differences between male and female. For men who born in 1956 and 1971, it showed two slight rising waves by further examining the curve of cohort effects. However, for women, this general decline (certain increase occurred in the first three cohorts) ceased until the latest birth cohort of 1971 (Table 3).
Table 3. Estimated regression coefficients and standard errors for bladder cancer (C67) incidence rate by gender.
| Intercept | s.e. | Age groups | Effect | s.e. | Periods | Effect | s.e. | Cohorts | Effect | s.e. |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| -9.077 | 0.035 | 30-34 | -2.379 | 0.150 | 1976-1980 | -0.348 | 0.041 | 1896 | 0.700 | 0.161 |
| 35-39 | -1.705 | 0.104 | 1981-1985 | -0.252 | 0.035 | 1901 | 0.711 | 0.093 | ||
| 40-44 | -1.107 | 0.085 | 1986-1990 | -0.135 | 0.030 | 1906 | 0.689 | 0.069 | ||
| 45-49 | -0.708 | 0.072 | 1991-1995 | 0.027 | 0.029 | 1911 | 0.622 | 0.057 | ||
| 50-54 | -0.173 | 0.062 | 1996-2000 | 0.263 | 0.030 | 1916 | 0.493 | 0.051 | ||
| 55-59 | 0.188 | 0.054 | 2001-2005 | 0.444 | 0.032 | 1921 | 0.408 | 0.046 | ||
| 60-64 | 0.611 | 0.044 | 1926 | 0.252 | 0.049 | |||||
| 65-69 | 0.979 | 0.037 | 1931 | 0.043 | 0.055 | |||||
| 70-74 | 1.255 | 0.035 | 1936 | -0.194 | 0.065 | |||||
| 75-79 | 1.438 | 0.039 | 1941 | -0.396 | 0.082 | |||||
| 80-84 | 1.601 | 0.048 | 1946 | -0.492 | 0.089 | |||||
| 1951 | -0.429 | 0.088 | ||||||||
| 1956 | -0.339 | 0.091 | ||||||||
| 1961 | -0.841 | 0.133 | ||||||||
| 1966 | -0.742 | 0.204 | ||||||||
| 1971 | -0.485 | 0.396 | ||||||||
| Female | ||||||||||
| -10.359 | 0.052 | 30-34 | -2.111 | 0.224 | 1976-1980 | -0.253 | 0.061 | 1896 | 0.340 | 0.233 |
| 35-39 | -1.456 | 0.156 | 1981-1985 | -0.267 | 0.053 | 1901 | 0.557 | 0.135 | ||
| 40-44 | -1.290 | 0.147 | 1986-1990 | -0.158 | 0.046 | 1906 | 0.778 | 0.097 | ||
| 45-49 | -0.514 | 0.110 | 1991-1995 | -0.055 | 0.046 | 1911 | 0.674 | 0.083 | ||
| 50-54 | -0.138 | 0.096 | 1996-2000 | 0.229 | 0.046 | 1916 | 0.666 | 0.073 | ||
| 55-59 | 0.164 | 0.083 | 2001-2005 | 0.503 | 0.049 | 1921 | 0.416 | 0.069 | ||
| 60-64 | 0.530 | 0.068 | 1926 | 0.221 | 0.074 | |||||
| 65-69 | 0.944 | 0.056 | 1931 | 0.051 | 0.082 | |||||
| 70-74 | 1.166 | 0.053 | 1936 | -0.072 | 0.097 | |||||
| 75-79 | 1.366 | 0.057 | 1941 | -0.346 | 0.127 | |||||
| 80-84 | 1.339 | 0.070 | 1946 | -0.329 | 0.135 | |||||
| 1951 | -0.379 | 0.137 | ||||||||
| 1956 | -0.581 | 0.151 | ||||||||
| 1961 | -0.601 | 0.195 | ||||||||
| 1966 | -0.852 | 0.309 | ||||||||
| 1971 | -0.541 | 0.581 |
For kidney cancer, the risk continued to rise rapidly until 70-74 years old group for both male and female, with a slight decline appearing afterwards. For age effects, the highest risk of kidney cancer incidence were more than doubled for male and almost 90% higher for female in comparison with all ages combined (calculated as e0.840 =2.32 in male and e0.629 =1.88 in female) (Table 4). For period effect of kidney cancer, a dramatically increase occurred after the group of 1981-1985 for both genders, indicating that there may be common risk pattern for both genders after the group of 1981-1985. The increased risk from 1976-1980 to 2001-2005 was more than nine fold for male and nearly sevenfold for female (calculated as the difference in the coefficients for two periods of 2001–2005 and 1976–1980, which is e 1.204 to -1.049 = 9.52 in male, and e 1.100 to -0.798 = 6.67 in female) (Table 4). Different from the age and period effects, the cohort effect for kidney cancer showed more complex changes between generations to generations. If we simply took 1936 birth cohort as the cut-off point, we would find that the incidence risk for the subsequent generations after 1936 was much lower than those who were born before 1936. Furthermore, for male, there was a marked peak occurring in the generation born in 1906 and the relative risk nearly doubled the risk of all birth cohorts combined. The birth cohort effect for female showed a general decline before 1961 but an apparent rise afterward which was distinct from male. Besides, we could also find dramatic fluctuations in the first several cohorts and a small peak around 1946 for female (Table 4). Additionally, more details about age, period and cohort effects for bladder and kidney cancers could be obtained in Figure S1.
Table 4. Estimated regression coefficients and standard errors for kidney cancer (C64) incidence rate by gender.
| Intercept | s.e. | Age groups | Effect | s.e. | Periods | Effect | s.e. | Cohorts | Effect | s.e. |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| -10.073 | 0.068 | 30-34 | -2.118 | 0.211 | 1976-1980 | -1.049 | 0.095 | 1896 | 0.274 | 0.691 |
| 35-39 | -1.475 | 0.142 | 1981-1985 | -0.996 | 0.088 | 1901 | 0.563 | 0.336 | ||
| 40-44 | -0.696 | 0.108 | 1986-1990 | -0.148 | 0.060 | 1906 | 0.643 | 0.218 | ||
| 45-49 | -0.146 | 0.090 | 1991-1995 | 0.270 | 0.052 | 1911 | 0.425 | 0.177 | ||
| 50-54 | 0.269 | 0.081 | 1996-2000 | 0.720 | 0.052 | 1916 | 0.328 | 0.147 | ||
| 55-59 | 0.510 | 0.077 | 2001-2005 | 1.204 | 0.057 | 1921 | 0.245 | 0.122 | ||
| 60-64 | 0.595 | 0.073 | 1926 | 0.173 | 0.109 | |||||
| 65-69 | 0.786 | 0.071 | 1931 | -0.096 | 0.101 | |||||
| 70-74 | 0.840 | 0.078 | 1936 | -0.291 | 0.099 | |||||
| 75-79 | 0.731 | 0.094 | 1941 | -0.335 | 0.103 | |||||
| 80-84 | 0.704 | 0.117 | 1946 | -0.347 | 0.100 | |||||
| 1951 | -0.159 | 0.093 | ||||||||
| 1956 | -0.358 | 0.100 | ||||||||
| 1961 | -0.295 | 0.123 | ||||||||
| 1966 | -0.365 | 0.197 | ||||||||
| 1971 | -0.404 | 0.407 | ||||||||
| Female | ||||||||||
| -10.592 | 0.063 | 30-34 | -1.316 | 0.187 | 1976-1980 | -0.798 | 0.098 | 1896 | -0.079 | 0.628 |
| 35-39 | -1.112 | 0.149 | 1981-1985 | -0.759 | 0.092 | 1901 | 0.452 | 0.306 | ||
| 40-44 | -0.746 | 0.129 | 1986-1990 | -0.233 | 0.070 | 1906 | 0.383 | 0.225 | ||
| 45-49 | -0.143 | 0.104 | 1991-1995 | 0.099 | 0.062 | 1911 | 0.103 | 0.193 | ||
| 50-54 | 0.163 | 0.095 | 1996-2000 | 0.591 | 0.057 | 1916 | 0.390 | 0.144 | ||
| 55-59 | 0.313 | 0.089 | 2001-2005 | 1.100 | 0.059 | 1921 | 0.287 | 0.119 | ||
| 60-64 | 0.516 | 0.081 | 1926 | 0.255 | 0.108 | |||||
| 65-69 | 0.456 | 0.081 | 1931 | 0.149 | 0.101 | |||||
| 70-74 | 0.629 | 0.083 | 1936 | -0.161 | 0.107 | |||||
| 75-79 | 0.649 | 0.096 | 1941 | -0.198 | 0.115 | |||||
| 80-84 | 0.590 | 0.119 | 1946 | -0.129 | 0.112 | |||||
| 1951 | -0.314 | 0.112 | ||||||||
| 1956 | -0.462 | 0.116 | ||||||||
| 1961 | -0.444 | 0.142 | ||||||||
| 1966 | -0.206 | 0.187 | ||||||||
| 1971 | -0.025 | 0.310 |
Discussion
The present study included 12,676 and 5,811 incidence cases of bladder and kidney cancer recorded in the population-based Shanghai Cancer Registry from 1973 through 2005. There was a relatively mild increase in age-standardized incidence rate of bladder cancer, but a dramatic rise for kidney cancer. From the age-period-cohort analysis, results indicated increasing curves of age and period effects but generally decreasing cohort effects both for bladder and kidney cancers.
For urinary bladder cancer, most Western countries showed much higher incidence rates compared with us. In 2008, the age-standardized incidence rates (age-adjusted to the world-standard population) in USA for all races were 21.1 per 100,000 in men and 5.8 per 100,000 in women which were nearly fourfold than that of both male and female in China [1]. Similarly in Europe, the age-standardized incidence rates (age-adjusted to the world-standard population) were threefold and twofold than that of male and female respectively in China [1]. In USA, the incidence trend in male showed a significant increase during 1975 to 1987 (APC=1.00%) and became stable afterwards. For female, this mild rise (APC=0.20%) was continuing until 2003 [18]. This mild increase in female in USA was quite similar with the trend of female in Shanghai during 1973 to 2005 (APC=0.38%).
The incidence rate of kidney cancer showed a regional disparity and race difference. Between 1973-1997 and 1988-1992, kidney cancer experienced global increase among men and women in all regions and ethnic groups, with a few exceptions, mostly in Scandinavian countries [19] In 1988-1992, kidney cancer incidence rates (age-adjusted to the world-standard population) were highest in Bas-Rhin (France) (14.5 per 100,000 in men and 6.9 per 100,000 in women) and lowest in Bombay, India (1.8 and 0.8, respectively) [19]. In the United States, the Asian and Pacific Islander showed the lowest rates (4.7 per 100,000 in men and 2.2 per 100,000 in women) than other races such as White, Black, American Indian/Alaska Native and Hispanic during 1998-2002 [20]. The incidence rates for kidney cancer rose consistently over time, with the increases more rapid among blacks than whites and a shift from a predominance among whites to blacks [20]. Although the incidence rate of kidney cancer in urban Shanghai was lower than that in some Western countries historically, the rates increased more sharply in Shanghai during the past decades [19].
By the age-period-cohort analysis, we found that the age effects for bladder and kidney cancers showed different increasing curves. Incidence risk of bladder cancer showed a moderate but linear increase up to age of 55 years old, with a greater increase thereafter. However, the age effect of kidney cancer rose sharply before 60 years old but showed a generally mild change afterwards. Moreover, the highest incidence risk occurred around 70-74 years old group for kidney cancer but 80-84 years old group for bladder cancer. Bladder and kidney cancers increased at different rates with age. It is possible that certain molecular and physiological changes specific to each cancer type might be affecting these disparate rates of change. Of note, kidney cancer may occur and dramatically rise at a relatively early age in comparison with bladder cancer. Thus, for future surveillance and screening on kidney cancer, more attentions should be paid to individuals after 35 years old. By controlling the age and birth cohort effects, the contribution of period effect can be better elucidated. Breakthroughs in medical technology and diagnostic techniques might be the main factor which influences period effect of certain cancer [21]. Since 1980s, with more advanced medical technology and facilities introduced to Shanghai, increased number of cancer patients had been detected. The wide use of cystoscopy might lead to increased examinations for urinary bladder cancer which enhanced the chance of early diagnosis. It had also shown that early detection of kidney and bladder cancers had risen with gradually increased use of imaging procedures during the study periods, such as ultrasonography, computed tomography, and magnetic resonance imaging [22,23]. These advanced diagnostic techniques, to some extent, contributed to the increased period effects on bladder and kidney cancers. Furthermore, although bladder and kidney cancers both showed increasing trends during 1976 to 2005, the relative risk of kidney cancer increased more sharply than that of bladder cancer.
Additionally, period effect subsumes a complex set of historical events including public health efforts and certain environmental risk factors which affect incidence of all society members [24]. Cigarette smoking, as well as passive smoking, which has possibilities to influence those of all ages, has consistently been observed to be a common risk factor for bladder and kidney cancers [25]. In 1984, China had approximately 250 million smokers, with a prevalence of 61% in men and 7% in women [26]. Since then, smoking prevalence in China became much higher among men, up to 63% in 1996 and 66% in 2002. Of the nonsmoker (mostly women and children) in 1996, 53.5% reported passive smoke exposure [27]. The number of smokers had approximately increased to 350 million in 2002 [28]. An estimated 72% of Chinese individuals over the age of 15 years had been exposed to tobacco, including those exposed to second-hand smoke [27]. Lack of powerful tobacco control activities as well as other effective and comprehensive public health efforts might partly explain the continuously rising period effects of bladder and kidney cancers in Shanghai.
Cohort effect reflects variations in incidence across groups of memberships born in the same year or years. It is well known that the significance of early life exposures in explaining the susceptibility to certain cancer and incidence later in the adulthood. The sharply declining trends of cohort effects for bladder and kidney cancers both ceased and began generally leveling off around 1940s. The most notable peak occurred in the birth cohort of 1906 for bladder and kidney cancers. In the early period of the twentieth century in China, the poorly living conditions and chronic malnutrition might be early life exposures to later development of bladder and kidney cancers for the specific birth cohort. Moreover, some unknown exposures earlier in life, such as exposure to cigarette smoking, childhood obesity and physical inactivity, may partly explain other slight changes of birth cohort effects for these two cancers in Shanghai [29-32]. In addition, the observed cohort effect may have bias due to miss-caught cases (before 1973) in oldest birth cohort.
This study also possessed some strengths and limitations. Firstly, we acknowledged that several limitations might exist in our study. One unavoidable limitation in this study is the potential information bias by under-diagnosis/reporting (lack of diagnostic capabilities) in the early years, which might somewhat contribute to the described rise in incidence, especially for kidney cancer, during the past 33 years. It is a question and a limitation for any cancer registry if they did a time trend analysis of incidence because of development of diagnosis and medical techniques. For example, in the last 2 decades, the use of abdominal imaging has also increased in the United States, leading to more renal cancers being detected at local or regional stages of disease [33]. Since the incidence of cancer presenting at a distant stage has not declined in the United States [33], this is thought to be a real increase, not only due to changes in the way the disease is diagnosed [34]. However, we failed to find any publications or resources for identifying the exact contributions of the information bias on the cancer trends. Moreover, increasing exposures to potential risk factors could also affect incidence trend of these two cancers in Shanghai, such as tobacco consumption, obesity and hypertension, etc. [35,36] which may contribute to the observed rise in our study. It is also possible that the trend toward a more westernized diet in Shanghai plays a role, since high consumption of meat and fat has been linked to kidney cancer risk [19]. In addition, the incidence trends of these two cancers in urban Shanghai from 1973 to 2005 were also in consistent with numerous studies on these cancers in Western populations. Therefore, we do think that this kind of rise in incidence may be caused by the combined effects of aging of population, improved medical techniques and the increasing exposures to certain risk factors. Furthermore, the natural characteristic of age-period-cohort analysis is still descriptive, not analytic research. As lacking further knowledge about the comprehensive effects of the risk factors of bladder and kidney cancers in urban Shanghai, explicit explanations of some results from age-period-cohort analysis were still challenging. This descriptive study could only try to offer some clues about risk factors of these cancers which were reflected by the effects of age, period and birth cohort illustrated in our study. However, it is the first time to do incidence trend analysis of urinary bladder and kidney cancers during past 33-years using the population-based registration data in China, as well as in other Asian countries. It could not only provide the upcoming studies with some basic data about urinary bladder and kidney cancers in Shanghai, but guide our future work for deeply explorations of the risk factors for these two cancers. Our present study also supplies an opportunity to make a comparison between Eastern and Western countries on bladder and kidney cancer incidences. And most importantly, it could guide the local health officials to make current efforts on prevention and surveillance of bladder and kidney cancers. Of note, we should pay more attention to the fast rise of kidney cancer incidence, especially for the middle-aged and high risk individuals with some potential risks. Besides, the novel IE method which enabled us to explore the time trends of the incidence rates without relying on any priors could provide more accurate estimators than some traditional methods [13].
In summary, our study showed mildly increasing incidence trends in urinary bladder cancer but sharply increased incidence rate in kidney cancer in Shanghai, China. Aging of population, advancing diagnostic techniques and exposing to some unknown risks may together contribute to the increasing incidence trends of bladder and kidney cancers. Further studies should aim at exploring those potential risk or protective factors and reducing the incidence rates of these cancers.
Supporting Information
Age, period and cohort effects for bladder (C67) and kidney (C64) cancers by gender. Note: The reference group for the cohort coefficients is the mean influence of all cohorts combined, and the reference groups for the period and age coefficients are the mean influence of all periods and ages combined, respectively. For example, the value of 0.711 for the 1901 birth cohort of male indicates that membership in this cohort nearly doubled the risk (e0.711= 2.04) of incidence of bladder cancer compared to all cohorts combined, which is independent of period and age effects.
(TIF)
Acknowledgments
The authors would also like to acknowledge the staffs from the Shanghai Cancer Registry for their contributions on the collection, management and processing of incidence data.
Funding Statement
This study was supported by the fund of Shanghai Municipal Health Bureau Key Disciplines and Specialties Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; ; 2010. Available: http://globocan.iarc.fr , Accessed 10 October 2012 [Google Scholar]
- 2. Sun M, Thuret R, Abdollah F, Lughezzani G et al. (2011) Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol 59: 135-141. doi: 10.1016/j.eururo.2010.10.029. PubMed: 21035250. [DOI] [PubMed] [Google Scholar]
- 3. Ondrusova M, Ondrus D, Muzik J, Hunakova L, Hes O et al. (2011) Trends in the kidney cancer incidence and mortality in the Slovak and Czech Republics in 1980-2005 - in the context of an international comparison. Neoplasma 58: 165-171. doi: 10.4149/neo_2011_02_165. PubMed: 21288054. [DOI] [PubMed] [Google Scholar]
- 4. Zheng T, Holford TR, Chen Y, Ma JZ, Mayne ST et al. (1996) Time trend and age-period-cohort effect on incidence of bladder cancer in Connecticut, 1935-1992. Int J Cancer 68: 172-176. doi: 10.1002/(SICI)1097-0215(19961009)68:2. PubMed: 8900423. [DOI] [PubMed] [Google Scholar]
- 5. Kiemeney LA, Coebergh JW, Koper NP, van der Heijden LH, Pauwels RP et al. (1994) Bladder cancer incidence and survival in the south-eastern part of The Netherlands, 1975-1989. Eur J Cancer 30A: 1134-1137. PubMed: 7654445. [DOI] [PubMed] [Google Scholar]
- 6. Gao YT, Lu W (2007) Cancer Incidence, Mortality and Survival Rates in Urban Shanghai (1973-2000). Shanghai, China: Second Military Medical University Press. [Google Scholar]
- 7. Gao S, Yang WS, Bray F, Va P, Zhang W et al. (2012) Declining rates of hepatocellular carcinoma in urban Shanghai: incidence trends in 1976-2005. Eur J Epidemiol 27: 39-46. doi: 10.1007/s10654-011-9636-8. PubMed: 22160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu QJ, Vogtmann E, Zhang W, Xie L, Yang WS et al. (2010) Cancer incidence among adolescents and young adults in urban Shanghai, 1973-2005. PLOS ONE 7: e42607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray F, Parkin DM (2009) Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer 45: 747-755. doi: 10.1016/j.ejca.2008.11.032. PubMed: 19117750. [DOI] [PubMed] [Google Scholar]
- 10. Doll R, Payne P, Waterhouse J, eds. (1966) Cancer Incidence in Five Continents: a Technical Report. Berlin: Springer-Verlag; (for UICC) [Google Scholar]
- 11. Kim HJ, Fay MP, Feuer EJ, Midthune DN (2000) Permutation test for joinpoint regression with applications to cancer rates. Statist. Med. 19: 335-51 (correction: 2001; 20: 655) [DOI] [PubMed] [Google Scholar]
- 12. Kleinbaum Kupper, Muller (1988) Applied Regression Analysis and Other Multivariable Methods. PWS-Kent, Boston, Mass., 2nd edition. [Google Scholar]
- 13. Yang Y, Fu W, Land KC (2004) A methodological comparison of age-period-cohort models: The intrinsic estimator and conventional generalized linear models. Sociol Methodol 34: 75-110. doi: 10.1111/j.0081-1750.2004.00148.x. [DOI] [Google Scholar]
- 14. Yang Y, Schulhofer-Wohl S, Fu W, Land KC (2008) The intrinsic estimator for age-period-cohort analysis: What it is and how to use it. Am J Sociol 113: 1697-1736. doi: 10.1086/587154. [DOI] [Google Scholar]
- 15. Fu W, Land KC, Yang Y (2011) On the intrinsic estimator and constrained estimators in age-period-cohort models. Sociol Methods Res 40: 453-466. doi: 10.1177/0049124111415355. [DOI] [Google Scholar]
- 16. Holford TR (1983) The estimation of age, period and cohort effects for vital rates. Biometrics 39: 311-324. doi: 10.2307/2531004. PubMed: 6626659. [DOI] [PubMed] [Google Scholar]
- 17. Holford TR (1991) Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health 12: 425-457. doi: 10.1146/annurev.pu.12.050191.002233. PubMed: 2049144. [DOI] [PubMed] [Google Scholar]
- 18. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N et al. (2013) SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD. pp. 1975-2010 Available: http://seer.cancer.gov/csr/1975_2010/. Accessed 16 July 2013 [Google Scholar]
- 19. Mathew A, Devesa SS, Fraumeni JF, Chow WH (2002) Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev 11: 171-178. doi: 10.1097/00008469-200204000-00010. PubMed: 11984136. [DOI] [PubMed] [Google Scholar]
- 20. Chow WH, Dong LM, Devesa SS (2010) Epidemiology and risk factors for kidney cancer. Nat. Rev Urol 7: 245-257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarone RE, Chu KC, Gaudette LA (1997) Birth cohort and calendar period trends in breast cancer mortality in the United States and Canada. J Natl Cancer Inst 89: 251-256. doi: 10.1093/jnci/89.3.251. PubMed: 9017006. [DOI] [PubMed] [Google Scholar]
- 22. Barentsz JO, Ruijs SH, Strijk SP (1993) The role of MR imaging in carcinoma of the urinary bladder. AJR Am J Roentgenol 160: 937-947. doi: 10.2214/ajr.160.5.8470608. PubMed: 8470608. [DOI] [PubMed] [Google Scholar]
- 23. Bretheau D, Lechevallier E, Eghazarian C, Grisoni V, Coulange C (1995) Prognostic significance of incidental renal cell carcinoma. Eur Urol 27: 319-323. PubMed: 7656910. [DOI] [PubMed] [Google Scholar]
- 24. Frost WH (1995) The age selection of mortality from tuberculosis in successive decades, 1939. Am J Epidemiol 141: 4-9. PubMed: 7801964. [DOI] [PubMed] [Google Scholar]
- 25. Scélo G, Brennan P (2007) The epidemiology of bladder and kidney cancer. Nat Clin Pract Urol 4: 205-217. doi: 10.1038/ncpuro0760. PubMed: 17415353. [DOI] [PubMed] [Google Scholar]
- 26. Zhang J, Ou JX, Bai CX (2011) Tobacco smoking in China: prevalence, disease burden, challenges and future strategies. Respirology 16: 1165-1172. doi: 10.1111/j.1440-1843.2011.02062.x. PubMed: 21910781. [DOI] [PubMed] [Google Scholar]
- 27. Yang G, Fan L, Tan J, Qi G, Zhang Y et al. (1999) Smoking in China: findings of the 1996 National Prevalence Survey. JAMA 282: 1247-1253. doi: 10.1001/jama.282.13.1247. PubMed: 10517427. [DOI] [PubMed] [Google Scholar]
- 28. Yang GH, Ma JM, Liu N, Zhou LN (2005) Smoking and passive smoking in Chinese, 2002.Zhonghua Liu Xing Bing Xue Za Zhi 26: 77-83 [PubMed]
- 29. Tao L, Xiang YB, Wang R, Nelson HH, Gao YT et al. (2010) Environmental tobacco smoke in relation to bladder cancer risk--the Shanghai bladder cancer study [corrected]. Cancer Epidemiol Biomarkers Prev 19: 3087-3095. doi: 10.1158/1055-9965.EPI-10-0823. PubMed: 21056942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leiba A, Kark JD, Afek A, Levi Z, Barchana M et al. (2012) Overweight in adolescence is related to increased risk of future urothelial cancer. Obesity (Silver Spring) 20: 2445-2450. doi: 10.1038/oby.2012.83. PubMed: 22510956. [DOI] [PubMed] [Google Scholar]
- 31. Gray L, Lee IM, Sesso HD, Batty GD (2012) Association of body mass index in early adulthood and middle age with future site-specific cancer mortality: the Harvard Alumni Health Study. Ann Oncol 23: 754-759. doi: 10.1093/annonc/mdr270. PubMed: 21677311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tavani A, Zucchetto A, Dal Maso L, Montella M, Ramazzotti V et al. (2007) Lifetime physical activity and the risk of renal cell cancer. Int J Cancer 120: 1977-1980. doi: 10.1002/ijc.22438. PubMed: 17266025. [DOI] [PubMed] [Google Scholar]
- 33. Hock LM, Lynch J, Balaji KC (2002) Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: An analysis of surveillance, epidemiology, and end results program data. J Urol 167: 57-60. doi: 10.1016/S0022-5347(05)65382-7. PubMed: 11743275. [DOI] [PubMed] [Google Scholar]
- 34. Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, et al. (2007) SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD. pp. 193-202 [Google Scholar]
- 35. Liu L, Ikeda K, Chen M, Yin W, Mizushima S et al. (2004) Obesity, emerging risk in China: trend of increasing prevalence of obesity and its association with hypertension and hypercholesterolaemia among the Chinese. Clin Exp Pharmacol Physiol 31 Suppl 2: S8-10. doi: 10.1111/j.1440-1681.2004.03954.x. PubMed: 15649295. [DOI] [PubMed] [Google Scholar]
- 36. Song Y, Wang HJ, Ma J, Wang Z (2013) Secular trends of obesity prevalence in urban Chinese children from 1985 to 2010: gender disparity. PLOS ONE 8: e53069. doi: 10.1371/journal.pone.0053069. PubMed: 23308137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Age, period and cohort effects for bladder (C67) and kidney (C64) cancers by gender. Note: The reference group for the cohort coefficients is the mean influence of all cohorts combined, and the reference groups for the period and age coefficients are the mean influence of all periods and ages combined, respectively. For example, the value of 0.711 for the 1901 birth cohort of male indicates that membership in this cohort nearly doubled the risk (e0.711= 2.04) of incidence of bladder cancer compared to all cohorts combined, which is independent of period and age effects.
(TIF)