Abstract
Background: Recent studies report independent associations between psoriasis, cardiovascular (CV) events and risk factors. Blood Myeloperoxidase (MPO) from activated myeloid cells is associated with CV risk mainly through lipid oxidation, induction of endothelial dysfunction and release of IL-12 from macrophages. Objectives: To elucidate associations between psoriasis and conventional CV risk factors. Methods: We performed a cross-sectional study of 100 psoriasis patients and 53 controls, group matched on age, gender and body mass index, to assess levels of MPO in serum, as well as immunohistochemical staining from psoriasis skin lesions, psoriasis uninvolved skin, and normal skin. Results: Although the groups did not differ on waist circumference, glucose, cholesterol, triglycerides, creatinine or personal history of CV events, psoriasis patients had significantly higher waist-to-hip ratios, blood pressures, proportion of current smokers, and lower high density lipoprotein level than controls. Serum MPO level was elevated 2.5 fold (P<0.001) in psoriasis patients, even after adjusting for the CV risk factors on which the groups differed. MPO did correlate with coronary artery calcification, carotid plaque, carotid intima media thickness and flow mediated dilation, but did not correlate with psoriasis severity. However, MPO was highly expressed in lesional psoriatic skin and colocalized predominantly with CD45+ CD11b+ leukocytes. CD11b+ cell density correlated with circulation MPO levels. Conclusion: Lesional skin CD11b+ leukocytes activated to generate MPO may contribute to serum levels of MPO. Lesional CD11b+ cell activity may be an alternative measure of disease burden to PASI that underlies the MPO biomarker for systemic inflammation related to Cardiovascular Disease.
Keywords: Myeloperoxidase, psoriasis, cardiovascular disease, immunofluorescence, immunohistochemistry
Introduction
Psoriasis is a chronic and recrudescent inflammatory disease which affects approximately 2-3% of the world’s population, including 125 million people worldwide and 7.5 million Americans [1]. The negative impact of psoriasis on health-related quality of life is comparable to those of other major medical and psychiatric diseases, such as myocardial infarction (MI), congestive heart failure (CHF), cancer, type 2 diabetes mellitus, hypertension and depression.
Psoriasis is associated with cardiovascular diseases (CVDs) as well as with established cardiovascular (CV) risk factors. Although initial studies indicated that psoriasis may be associated with an increased prevalence of CVDs, including MI, CHF and stroke, as well as CV-related mortality, Gelfand et al. found that psoriasis may confer an independent risk for MI, even after adjusting for established CV risk factors, especially in young patients with severe psoriasis [2]. Shared chronic systemic inflammatory processes may explain the association between psoriasis, CV risk factors and CVDs. The chronic inflammation characteristic of psoriasis is also central to the pathophysiology of atherosclerotic plaque initiation, progression and rupture which lead to acute thrombotic events including MI and stroke A number of other systemic inflammatory diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus and chronic gingivitis are also associated with increased risk of CVDs and CRP elevation, although the mechanism of this risk elevation is not elucidated [3,4]. Murine studies with psoriasiform animal models have also established that sustained skin-specific inflammation is associated with increased aortic root vascular inflammation and arterial thrombosis [5].
Myeloperoxidase (MPO) is a pro-oxidative and proinflammatory hemeprotein stored in azurophilic granules of leukocytes and secreted upon cellular activation following inflammation [6,7]. MPO catalyzes the conversion of chloride and hydrogen peroxide to hypochlorite—a strong reactive oxygen species (ROS Although produced by monocytes, macrophages, Kupffer cells and microglial cells, 95% of circulating MPO in healthy individuals derives from polymorphonuclear neutrophils.
Emerging evidence in humans consistently suggest that MPO may not merely be a marker for CVDs, but that MPO-induced oxidative stress and inflammation may actively contribute to the formation, progression and destabilization of atherosclerotic plaques which lead to acute coronary syndromes (ACS) [8-11].
In this cross-sectional study, we sought to explore associations between psoriasis, conventional CV risk factors, and the CV-biomarker MPO. We found elevated serum levels of MPO in psoriasis with and without recognizable atherosclerotic lesions, and that MPO is overexpressed in lesional skin by CD11b+ leukocytes, as compared to psoriatic non-lesional and non-psoriatic skin.
Materials and methods
Patients
One hundred psoriasis patients and 53 controls 18 years or older were recruited from the dermatology clinic at University Hospitals Case Medical Center (UHCMC), Cleveland, Ohio, for this cross-sectional study. Control patients did not have psoriasis or any other inflammatory skin disorders (e.g., acne, atopic dermatitis, contact dermatitis, bullous pemphigoid, pemphigus vulgaris, alopecia areata) or inflammatory disorders in general. All patients provided informed consent to participate in the UHCMC institutional review board-approved protocol before initiation of the study.
History and physical examination
Each study patient completed a detailed questionnaire including socio-demographic information, smoking history, allergies, current medications and treatments, lifetime treatments for psoriasis, personal and family medical history especially of psoriasis and psoriatic arthritis (PsA) and CVDs. The study investigator then reviewed this completed questionnaire in detail with the patient at the study visit.
Also at the study visit, anthropometric measures obtained included height, weight, waist circumference (WC) measured at the level of the anterior superior iliac spines, and hip circumference measured at the level of the largest circumference around the buttocks.
Psoriasis patients were additionally evaluated by measuring their psoriasis area and severity index (PASI), body surface area (BSA) and physicians’ global assessment (PGA).
Serum analyses
A venous blood sample after at least an 8-hour fast was obtained from each patient at the study visit. Enzyme-linked immunosorbent assays (ELISAs) were used to determine serum levels of MPO (Kamiya Biomedical, Seattle, WA). LDL, HDL, total cholesterol, triglyceride, fasting blood glucose, creatinine and blood urea nitrogen (BUN) were quantified by the UHCMC clinical laboratory.
Skin biopsies
Optional 6 mm punch biopsies were obtained from psoriasis patients (lesional and non-lesional skin) and controls (non-psoriatic skin). These biopsies were embedded in tissue freezing medium (TFM) and stored in -80°C freezer until immunofluorescence or immunohistochemistry was performed.
Confocal immunofluorescent microscopy
8 μm tissue sections were cut and fixed as described previously [12]. Tissue sections were incubated with anti-human MPO (BD Biosciences, San Jose, CA) overnight at 4°C, anti-human CD45 (Abcam, Cambridge, MA) for 4 hours at room temperature, anti-human CD68, anti-human HAM56 (Dako, Glostrup, Denmark) for 2 hours at room temperature, anti-human CD11b conjugated to phycoerythrin (PE) (Abcam, Cambridge, MA) for 7 hours at 4°C or appropriate isotype controls. These antibodies were then stained with Alexa 488 for 7 hours at 4°C (Santa Cruz Biotechology, Inc., Santa Cruz, CA) or with Alexafluor 555- or 488-conjugated goat anti-mouse isotype-specific secondary antibodies (Invitrogen, Carlsbad, CA) when necessary. Hoechst 33253 was used as a nuclear marker in all fluorescent images. Images were acquired with a 25X Plan-Neofluoar, NA 0.8 Imm. Korr, Ph2 objective on a Zeiss LSM 510 META laser scanning confocal microscope (Carl Zeiss Microimaging, Jenna, Germany) located at the Neurosciences Imaging Center at Case Western Reserve University, Cleveland, Ohio.
Inmunohistochemistry
8 μm tissue sections were cut and fixed as described previously [13]. Blocked serial sections were incubated with anti-human MPO (BD Biosciences, San Jose, CA), anti-human Neutrophil elastase (Dako, Carpinteria, CA) anti-CD11b (BD Biosciences, San Jose, CA) or mouse isotype control (Dako, Carpinteria, CA) for 1 hour at room temperature. Sections were rinsed with PBS and incubated with biotin-labeled secondary antibody (Vector laboratories, Burlingame, CA) for 1 hour at room temperature. After washing, the sections were incubated for 30 minutes with the avidin-biotin peroxidase complex (Vector laboratories, Burlingame, CA). The sections were then rinsed and stained with 3,3-diaminobezidine (Vect- astin Kit, vector laboratories, Burlingame, CA) and counterstained with hematoxylin (Vector laboratories, Burlingame, CA).
Quantifying CD11b+ cells in psoriatic skin
Cd11b+ cells were divided by mm2 of papillary dermal skin and this was defined as the Cd11b index. In order to standardize the CD11b+ cell counting area between skin samples, the papillary dermal area used for counting was defined using the autofluorescence of elastin fibers at 488 nm. This area was then superimposed on the anti-CD11b+ immunohistochemistry color image, and the peroxidase-positive CD11b cells within the non-fluorescent area between the papillary dermis and the rete ridges was used for counting the CD11b positive events.
Statistical analyses
Means ± standard deviations were reported for normally distributed continuous variables. Medians with interquartile ranges were reported for non-normally distributed continuous variables. Counts with proportions were reported for categorical variables. Two-sample t-test, Wilcoxon test and Pearson chi-square test were applied to compare the group differences for the above three types of variables, respectively.
Univariable analyses were applied to assess the crude association of serum MPO level and psoriasis-specific measures and therapy use. Unadjusted means were reported for categorical variables. Relative changes in serum MPO level between groups or for every one unit increase were reported.
In the multivariable model, associations between serum MPO level and the presence of psoriasis in all subjects were evaluated, sequentially adjusting for age, gender, waist-to-hip ratio (WHR), current smoking status, systolic BP and serum HDL level in models 1 through 6.
The linear association between serum MPO and the CD11b index was estimated using the non-parametric Spearman’s Rho correlation coefficient. This was done for the entire sample, and also for the sample stratified by level of disease severity (mild, moderate, and severe) using the PASI scores. P-values <0.05 were considered statistically significant. Since the study is exploratory in nature, nominal p-values are reported, without correction for multiple uses of the data.
Statistical significance was set at a level or p value of 0.05. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Study patient characteristics
The clinical characteristics of psoriasis patients versus controls are presented in Table 1. Comparable to previous studies, the mean age of our psoriasis patients was 46.3 [14]. Although patient characteristics such as age, gender, race and BMI were similar between psoriasis and control subjects, psoriasis patients had significantly higher waist-to-hip ratios (WHRs), systolic and diastolic BPs, proportions of current cigarette smokers, percentages of those with first- or second-degree relatives afflicted by stroke(s); and lower serum HDL levels when compared to controls. The proportions of subjects using conventional CV-protective medications, including aspirin, statins and angiotensin-converting enzyme (ACE) inhibitors, did not differ significantly between psoriasis patients and controls.
Table 1.
Study patient clinical characteristics
| Controls (n=53) | Psoriasis (n=100) | p-value | |
|---|---|---|---|
| Mean ± sd or N (%) | Mean ± sd or N (%) | ||
| Demographics | |||
| *Age (years) | 45.1±18.0 | 46.3±16.2 | 0.596 |
| *Male gender | 26 (49.1%) | 52 (52.0%) | 0.729 |
| Other Race (vs. white race) | 8 (15.1%) | 13 (13.0%) | 0.720 |
| Conventional cardiovascular risk factors | |||
| *BMI (kg/m2) | 29.1±6.4 | 30.1±7.4 | 0.532 |
| WHR | 0.90±0.07 | 0.93±0.08 | 0.022 |
| WC (cm) | 39.0±5.8 | 40.1±6.5 | 0.350 |
| Fasting blood glucose (mg/dL) | 91.8±21.2 | 95.2±29.3 | 0.836 |
| Systolic BP (mmHg) | 119.4±15.5 | 128.1±16.8 | 0.002 |
| Diastolic BP (mmHg) | 73.6±8.5 | 79.5±9.7 | <0.001 |
| LDL (mg/dL) | 110.3±35.1 | 119.5±36.2 | 0.164 |
| HDL (mg/dL) | 55.3±13.3 | 51.1±14.7 | 0.046 |
| Total cholesterol (mg/dL) | 189.1±44.6 | 196.3±45.4 | 0.397 |
| Triglyceride (mg/dL) | 121.5±109.0 | 125.4±82.8 | 0.368 |
| Creatinine (mg/dL) | 1.0±0.3 | 1.0±0.2 | 0.857 |
| BUN (mg/dL) | 14.8±4.9 | 13.8±4.7 | 0.234 |
| Current cigarette smoker | 12 (22.6%) | 38 (38.0%) | 0.034 |
| Heart attack, stents, angioplasty, bypass surgery (personal) | 2 (3.8%) | 3 (3.0%) | 1.000 |
| Stroke (personal) | 0 (0%) | 0 (0%) | 1.000 |
| Premature heart attack (family) | 20 (37.7%) | 38 (38.0%) | 0.974 |
| Stroke (family) | 14 (26.4%) | 44 (44.0%) | 0.033 |
| Current medication use | |||
| Aspirin | 6 (11.3%) | 13 (13.0%) | 0.760 |
| Statins | 9 (17.0%) | 16 (16.0%) | 0.876 |
| ACE inhibitors | 4 (7.6%) | 14 (14.0%) | 0.239 |
Age, gender and BMI were group matched.
ACE inhibitors: angiotensin-converting enzyme inhibitors; BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; HDL: high density lipoprotein; LDL: low density lipoprotein; N: count; sd: standard deviation; WC: waist circumference; WHR: waist-to-hip ratio.
Table 2 elucidates psoriasis-specific measures in our patient population. Since psoriasis severity measures were not normally distributed, PASI, BSA and PGA are reported in medians and interquartile (25th-75th percentile) ranges, as 8.0 (3.6, 15.5), 6.0 (2.0, 16.5) and 2.0 (2.0, 3.0), respectively. Consistent with previous reports, a majority (90%) of our psoriatics were afflicted with plaque psoriasis, while 14% had concurrent PsA [14,15].
Table 2.
Psoriasis-specific measures in psoriasis patients only
| Psoriasis (n=100) | |
|---|---|
| Median (25th percentile, 75th percentile) or N (%) | |
| Psoriasis measures | |
| PASI | 8.0 (3.6, 15.5) |
| BSA | 6.0 (2.0, 16.5) |
| PGA | 2.0 (2.0, 3.0) |
| Plaque psoriasis | 90.0 (90.0%) |
| Concurrent PsA | 14.0 (14.0%) |
| Age when psoriasis was first diagnosed by a board-certified dermatologist (years) | 23.5 (17.0, 40.0) |
| Psoriasis duration (years) | 17.3 (9.0, 24.0) |
| Systemic, light or biologic treatment over lifetime | |
| None | 28.0 (28.0%) |
| 1 treatment | 23.0 (23.0%) |
| >1 treatments | 49.0 (49.0%) |
| Current systemic, light or biologic treatment | |
| No treatment | 55.0 (55.0%) |
| Treatment | 45.0 (45.0%) |
BSA: body surface area; N: count; PASI: psoriasis area and severity index; PGA: physicians’ global assessment; PsA: psoriatic arthritis.
Therapeutically, 28 (28%) of our psoriasis patients had never tried systemic, light or biologic therapies and 55 (55%) were currently not on these psoriasis therapies (Table 2).
Elevated serum MPO in psoriasis
Psoriasis patients had significantly elevated serum MPO levels (2,827 ng/mL) when compared to controls (1,140 ng/mL relative change 2.48 [95% CI: 1.81, 3.38], p<0.001, Table 3). This difference was only minimally attenuated by adjusting for gender, WHR, current smoking status, BP and HDL (Table 4). Of interest, serum MPO levels did not correlate with measures of psoriasis severity (i.e., PASI, BSA, PGA, psoriasis duration, number of lifetime psoriasis therapies and concurrent PsA, Table 3).
Table 3.
Univariable linear regression analyses of serum MPO levels (ng/mL)
| Unadjusted mean (95% CI) or N | Unadjusted relative change in MPO between groups or for every 1 unit increase in variable (95% CI) | p-value | |
|---|---|---|---|
| Psoriasis patients (N=100) | |||
| Psoriasis measures | |||
| Psoriasis | Control: N=53 | 2.48 (1.81, 3.38) | <0.001 |
| 1,140.9 ng/ml (886.9, 1,467.7) | |||
| Psoriasis: N=100 | |||
| 2,827.6 ng/ml (2,353.8, 3,396.7) | |||
| PASI | 1.00 (0.99, 1.02) | 0.924 | |
| BSA | 1.00 (0.99, 1.01) | 0.831 | |
| PGA | 1.08 (0.89, 1.30) | 0.445 | |
| Psoriasis duration | 0.99 (0.98, 1.00) | 0.395 | |
| # of systemic, light or biologic therapy over lifetime | 1.05 (0.96, 1.15) | 0.248 | |
| Among Concurrent PsA | No: N=86 | 1.17 (0.70, 1.99) | 0.553 |
| 2,765.1 ng/ml (2,270.3, 3,367.8) | |||
| Yes: N=14 | |||
| 3,243.6 ng/ml (1,989.7, 5,287.7) | |||
BSA: body surface area; CI: confidence interval; MPO: myeloperoxidase; N: count; PASI: psoriasis area and severity index; PGA: physicians’ global assessment; PsA: psoriatic arthritis.
Table 4.
Multivariable linear regression analyses of serum MPO levels (ng/mL)
| Exposures of interest | Adjusted Mean (95% CI) or N | Adjusted relative change in MPO between groups (95% CI) | p-value |
|---|---|---|---|
| Psoriasis and control patients (N=153) | |||
| Presence of psoriasis | |||
| *Model 1 | Control: 1,130.4 (880.9, 1,450.5) | 2.50 (1.84, 3.41) | <0.001 |
| Psoriasis: 2,829.1 (2,359.8, 3,391.8) | |||
| Model 2 | Control: 1,146.5 (856.4, 1,534.7) | 2.50 (1.84, 3.40) | <0.001 |
| Psoriasis: 2,866.7 (2,277.5, 3,608.3) | |||
| Model 3 | Control: 1,133.9 (845.7, 1,520.4) | 2.45 (1.79, 3.36) | <0.001 |
| Psoriasis: 2,779.4 (2,167.1, 3,564.8) | |||
| Model 4 | Control: 1,076.7 (800.0, 1,449.1) | 2.34 (1.71, 3.21) | <0.001 |
| Psoriasis: 2,519.9 (1,918.9, 3,309.3) | |||
| Model 5 | Control: 1,090.3 (810.6, 1,466.6) | 2.24 (1.62, 3.09) | <0.001 |
| Psoriasis: 2,440.9 (1,851.4, 3,218.3) | |||
| Model 6 | Control: 1,095.0 (816.5, 1,468.4) | 2.22 (1.61, 3.06) | <0.001 |
| Psoriasis: 2,432.1 (1,849.8, 3,197.8) | |||
Model 1: MPO = exposure + age.
Model 2: Model 1 + gender. Model 3: Model 2 + waist-to-hip ratio. Model 4: Model 3 + current smoker. Model 5: Model 4 + systolic blood pressure. Model 6: Model 5 + HDL. CI: confidence interval; HDL: high density lipoprotein; MPO: myeloperoxidase; N: count.
Biomarker analysis
In addition to MPO, additional serum biomarkers relevant to systemic inflammation, CVD risk and metabolism were also evaluated: IL-15, VEGF, leptin, hsCRP and resistin. Among these, MPO, resistin and hsCRP were significantly elevated in psoriasis patients relative to controls. However, only MPO remained significant by multivariate analyses after adjusting for relevant cardiovascular confounding factors including age, gender, W/H ratio, smoking, BP and HDL (Supplementary Tables 1, 2, 3, 4 and 5).
Skin MPO analyses
In circulation, neutrophils (SSC>400) appear to be the major producers of MPO, although we did not observe a statistically different percentage of MPO-producing cells (Supplementary Figure 1) in psoriasis versus healthy controls. Although monocytes (SSC<400) appear to be minor producers of MPO in circulation, they expressed MPO in lesional psoriatic skin (Figure 1A) and mainly localized to the dermo-epidermal junction and papillary dermis. This was in contrast to the minimal or non-detectable MPO expression in non-lesional psoriatic (Figure 1B) and non-psoriatic skin (Figure 1C) (n>7 patients). MPO+ cells co-localized with cells expressing CD45, a leukocyte-specific marker (Figure 2). However, MPO and the neutrophil-specific marker neutrophil elastase had distinct expression patterns, with MPO being present in much greater quantities in the dermo-epidermal junction and papillary dermis in sequential sections, indicating that most MPO+ cells were negative for neutrophil elastase, (Figure 3). The tissue macrophage-specific marker CD68 colocalized with a substantial subset of MPO+ cells (Figure 4C), and HAM colocalized with a minority of MPO+ cells (Figure 4F). Cells expressing CD11b, a marker of myeloid lineage, colocalized strongly with MPO positive cells (Figure 5), indicating MPO expression across a spectrum of myeloid cells in lesions. Taken together, the expression phenotype of MPO+ cells in psoriatic skin was that of more immature tissue macrophages with overlap into some mature macrophages, (CD45+, CD11b+, neutrophil elastaseneg, CD68+/- HAM56+/-).
Figure 1.
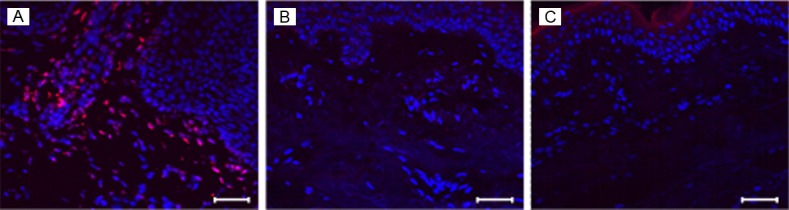
MPO is highly expressed in lesional psoriatic skin. 8 μm sections of frozen human skin biopsies were labeled with MPO antibody (red) and the nuclear stain Hoechst 33253 (blue). MPO was detectable by confocal microscopy in lesional psoriatic skin (A) primarily in the dermo-epidermal junction and papillary dermis, and was minimal in non-lesional psoriatic skin (B) and non-psoriatic skin (C). Scale bar = 50 μm. Representative image of n>7 patients.
Figure 2.

MPO positive cells co-localize with CD45+ cells within lesional psoriatic skin. 8 μm sections of frozen human skin biopsies were labeled with MPO antibody (red, A), CD45 antibody (green, B) and the nuclear stain Hoechst 33253 (blue). MPO was detectable by confocal microscopy mainly in the dermo-epidermal junction and papillary dermis, co-localized with CD45+ lymphocytes (white arrows, C). Scale bar = 50 μm. Representative image of n>7 patients.
Figure 3.
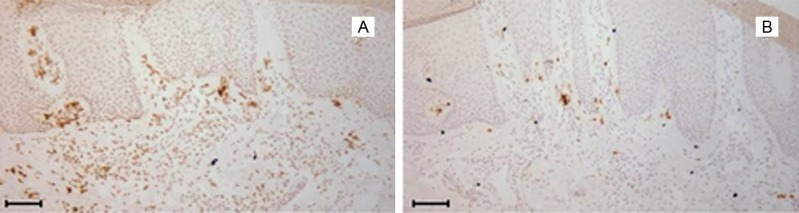
Neutrophil elastase and MPO expression patterns are distinct in serial tissue sections from lesional psoriatic skin. 8 μm serial sections of frozen human skin biopsies were labeled with either MPO (A) or neutrophil elastase (B) and counterstained with hematoxylin. Scale bar = 100 μm. Representative image of n>3 patients.
Figure 4.
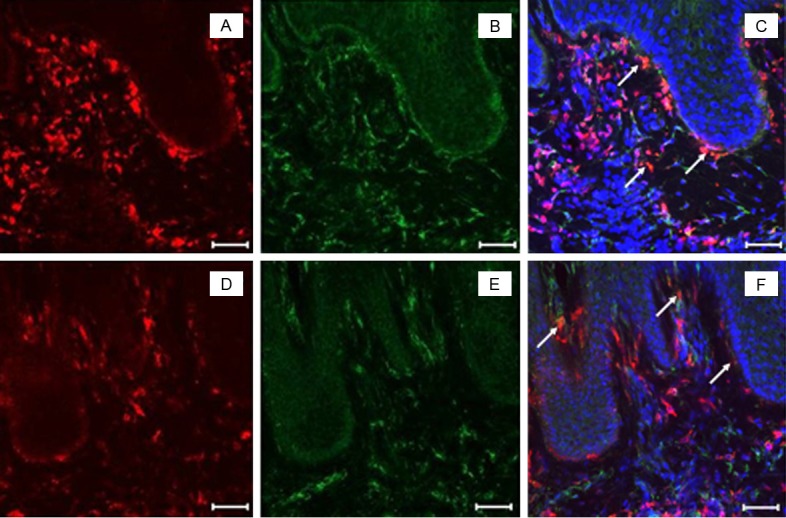
A subset of MPO+ cells in lesional psoriatic skin express mature macrophage-specific markers CD68 and HAM56. 8 μm sections of frozen human skin biopsies were labeled with MPO antibody (red, A and D), CD68 (green, B) or HAM56 (green, E) and the nuclear stain Hoechst 33253 (blue) in lesional psoriatic skin. Double labeling was detected by confocal microscopy. Cells that co-localized for MPO and either CD68 (C) or Ham56 (F) are indicated (white arrows). Scale bar = 50 μm. Representative image of n>3 patients.
Figure 5.

Lesional psoriatic MPO+ cells express CD11b. 8 μm sections of frozen human skin biopsies were labeled with MPO antibody (green, A), CD11b (red, B) and the nuclear stain Hoechst 33253 (blue) in lesional psoriatic skin. Double staining was detected by confocal microscopy and localized primarily to the dermo-epidermal junction and papillary dermis. Co-localized (MPO+ CD11b+) cells are indicated (white arrows, C). Scale bar = 50 μm. Representative image of n>9 patients.
Correlation among skin CD11b+ cells and serum MPO levels
Given the abundance of CD11b+ MPO+ cells in psoriatic lesions, the lack of an alteration in MPO+ circulating monocytes and the lack of correlation between serum MPO and PASI measures, we asked whether variation in lesional CD11b+ myeloid cells correlated with serum MPO levels.
Cutaneous CD11b+ cells were counted in the papillary dermis defined by autofluorescence of elastin. The number of CD11b+ cells divided by the papillary dermis area was calculated and constituted the CD11b+ density index. The CD11b+ density index was 282±29 cells/mm2. There was a substantial variation in density among patients however, a correlation between CD11b+ mean index and psoriasis patients within the upper quartile of severity (PASI>15) did reach statistical significance (Figure 6, r=0.714, p=0.047).
Figure 6.
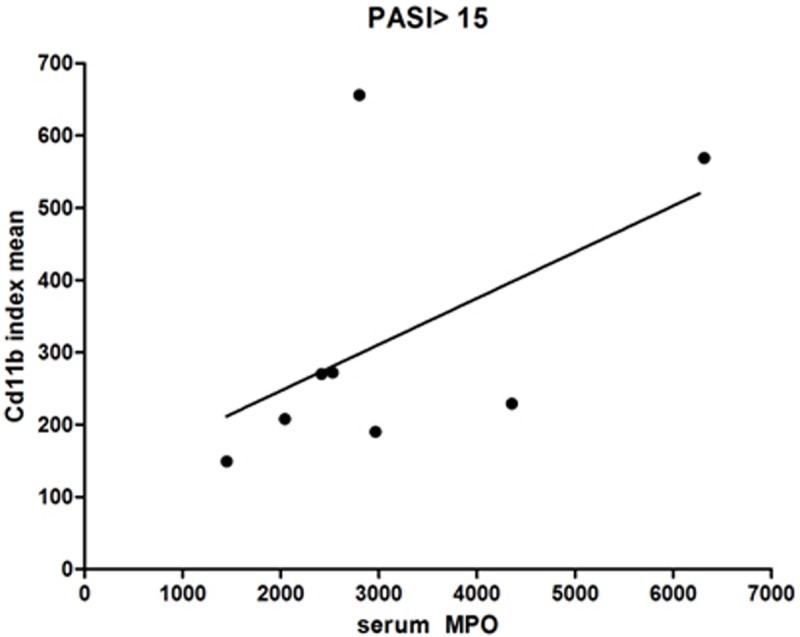
Serum MPO correlates with increased CD11b+ cells within high PASI patients. 8 μm sections of frozen lesional psoriatic skin of patients with PASI>15 and above were labeled and counted for CD11b+ cells and the MPO serum levels were found to correlate with the CD11b index (CD11b+ cells divided by μ2 ) using a non-parametric Spearman correlation (n=8).
Discussion
CV risk factors in psoriasis patients
Consistent with previous reports, our cross-sectional study demonstrated psoriasis patients to be at increased CV risk with significantly higher WHRs than controls even after group matching on the basis of BMI, (Table 1).
Previous studies have noted that while patients with more severe psoriasis exhibit more ubiquitous lipid abnormalities (i.e., higher total cholesterol, triglycerides), while patients with milder psoriasis may have lower HDL levels as the only detectable lipid abnormality when compared to controls [16,17]. These findings are consistent with the results of our study: Our psoriasis patients were predominantly mild in severity as occurs in the general psoriatic population, and our psoriasis patients only had significantly lower serum HDL levels than controls (Table 1).
While none of our study subjects have had a stroke, we did find that psoriasis patients were more likely than controls to have first- and second-degree family members with history of one or more strokes (Table 1), which may predispose them to cerebrovascular and other CV events.
Interestingly, we discovered that despite having more conventional CV risk factors than controls, the proportion of psoriasis patients currently taking CV-protective medications, including aspirin, statins and ACE inhibitors were not significantly different from controls (Table 1).
MPO and psoriasis
Serum MPO and psoriasis
In this cross-sectional study we demonstrate a significantly elevated serum MPO level in psoriasis patients when compared to controls, even after controlling for traditional CV risk factors (Tables 3, 4). Although the difference in serum MPO levels between psoriasis patients and controls remained highly significant after adjusting for traditional CV risk factors, it was minimally attenuated by WHR, current smoking status and BP (Table 4). This is consistent with previous studies, which have noted that abdominal visceral fat, cigarette smoking, and hypertension are associated with elevated systemic MPO levels or increased oxidative stress [18-22].
Skin MPO and psoriasis
In this study we detected elevated MPO expression in lesional psoriatic skin, as compared to non-lesional psoriatic skin and non-psoriatic skin which expressed minimal or non-detectable MPO (Figure 1). We found MPO-expressing cells to be predominantly located in the dermo-epidermal junction and papillary dermis of lesional psoriatic skin, where there are abundant blood vessels from which leukocytes may enter skin tissue via diapedesis (Figure 1). MPO+ cells co-localized with cells expressing CD45, a leukocyte-specific marker, indicating that MPO is most likely produced by leukocytes (Figure 2).
Importantly, distinct expression patterns of MPO and neutrophil elastase could be explained by the following hypothesis: Most psoriatic lesions are chronic, and the acute-inflammatory neutrophils should only be present in considerable quantities when the lesions first develop, while the chronic-inflammatory macrophages are expected to predominate after that. This thought is substantiated by a previous study which reported that neutrophils aggregated in the stratum corneum mainly in acute psoriatic lesions [23]. Moreover, in chronic atherosclerotic lesions removed during vascular surgery, MPO co-localized primarily with lesional macrophages without significant involvement of neutrophils [24]. The authors concluded that the source of MPO may differ between stable chronic atherosclerotic disease and acute plaque disruption, rupture and thrombosis.
In our study, cells positive for CD68 and HAM56, two markers of mature tissue macrophages, did not represent the majority of MPO+ cells in lesional psoriatic skin (Figure 4). However, we discovered that cells expressing CD11b, a marker of blood monocytes and immature macrophages at the point when they first enter tissue, represented a major source of MPO+ cells (Figure 5).
Hence, we show that in patients with psoriasis, a chronic inflammatory disease, monocytes and macrophages may indeed be the predominant source of MPO. This is distinct from the situation in more acute conditions where neutrophil is the main source of MPO. It may be that as blood monocytes enter psoriatic lesional skin, they transiently express MPO, accounting for the co-localization of MPO with CD11b+ blood monocytes and immature macrophages, but as they mature into CD68+ and HAM56+-expressing macrophages, MPO production wanes.
Serum MPO and psoriasis severity
Despite MPO elevations in psoriatic blood and lesional skin, we found that serum MPO levels did not correlate with psoriasis severity as evaluated by PASI, BSA and PGA. All three measures are assessments of psoriatic skin involvement in terms of skin area involved and/or erythema, induration and scaling, and do not account for occurrences within the blood of psoriasis patients or the chronicity of psoriatic skin lesions. Therefore, if MPO is predominantly produced by monocytes and immature macrophages when they first enter psoriatic lesions, serum MPO level would not be expected to associate with these measures.
Interestingly, serum MPO levels also did not associate with the duration for which patients have been diagnosed with psoriasis, the number of lifetime systemic, light or biological treatments patients have tried, and the concurrent diagnosis of PsA. However, a correlation between the serum MPO levels and CD11b+ cells in skin appeared within patients with PASI over 15 only (Figure 6).
Our study had a number of strengths. Experienced practicing dermatologists (NJK, KDC) diagnosed patients with psoriasis. This is a significant improvement over database studies where misclassification may be an important confounder. A 2006 study showed that the agreement between self-reported and dermatologists’ diagnoses of psoriasis was only moderate [25]. Also, we excluded patients with other inflammatory skin disorders from the controls, so as not to weaken differences between psoriasis and controls due to shared inflammatory mechanisms. Furthermore, we group matched on age, gender and BMI, to eliminate the effects of these well-established CV risk factors in assessing novel associations.
Several limitations to our study should be considered. We used a cross-sectional design which can only evaluate associations, not causations. Hence, further prospective studies are needed to evaluate temporal and causal relationships. Also, it is possible that biases and unmeasured or unknown confounders could explain some of the observed associations. However, selection and information biases are unlikely to explain our findings given that psoriasis patients and controls were recruited from the same well-defined source population and information was collected in the same manner for both groups, respectively. Furthermore, patients seeking dermatological care for psoriasis or other skin diseases may be more likely to engage in healthier lifestyles and health maintenance.
In conclusion, inflammatory MPO serum levels are significantly higher in psoriasis patients compared to controls, even after controlling for conventional CV risk factors, and did not correlate with psoriasis severity measured by PASI, BSA, PGA. MPO is expressed in significantly higher quantities in psoriatic lesional skin than psoriatic non-lesional and non-psoriatic skin, and predominantly localizes to the dermo-epidermal junction and papillary dermis. MPO+ cells co-localize mostly with CD11b+ monocytes and immature tissue macrophages which are instrumental in the development of atherosclerotic and psoriatic lesions. Hence, MPO may be an important mediator between psoriasis and CVDs, and may be used to predict CV risk so that high risk psoriatic populations may be more aggressively managed. MPO may also be therapeutically targeted to prevent CVDs in psoriasis patients.
Acknowledgements
We would like to thank Dr. Ward for her useful comments in editing this manuscript. This study was funded by the Murdough Family Center for Psoriasis, Department of Dermatology, University Hospitals Case Medical Center, Cleveland, Ohio and the National Institutes of Health (P30AR39750, P50AR05508, KDC).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.NPF. About psoriasis: statistics. 2009. National Psoriasis Foundation. [Google Scholar]
- 2.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 3.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 4.Rhew EY, Ramsey-Goldman R. Premature atherosclerotic disease in systemic lupus erythematosus--role of inflammatory mechanisms. Autoimmun Rev. 2006;5:101–5. doi: 10.1016/j.autrev.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Gao H, Loyd CM, Fu W, Diaconu D, Liu S, Cooper KD, McCormick TS, Simon DI, Ward NL. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067–75. doi: 10.1038/jid.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 7.Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465–7. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- 8.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacobs JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–30. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellani LW, Chang JJ, Wang X, Lusis AJ, Reynolds WF. Transgenic mice express human MPO -463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in -463G males. J Lipid Res. 2006;47:1366–77. doi: 10.1194/jlr.M600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–91. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–6. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toichi E, Torres G, McCormick TS, Chang T, Mascelli MA, Kauffman CL, Aria N, Gottlieb AB, Everitt DE, Frederick B, Pendley CE, Cooper KD. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177:4917–26. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 14.Patel V, Horn EJ, Lobosco SJ, Fox KM, Stevens SR, Lebwohl M. Psoriasis treatment patterns: results of a cross-sectional survey of dermatologists. J Am Acad Dermatol. 2008;58:964–9. doi: 10.1016/j.jaad.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Lebwohl M. Psoriasis. Lancet. 2003;361:1197–204. doi: 10.1016/S0140-6736(03)12954-6. [DOI] [PubMed] [Google Scholar]
- 16.Reynoso-von Drateln C, Martinez-Abundis E, Balcazar-Muñoz BR, Bustos-Saldaña R, González-Ortiz M. Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. J Am Acad Dermatol. 2003;48:882–5. doi: 10.1067/mjd.2003.446. [DOI] [PubMed] [Google Scholar]
- 17.Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta. 2001;303:33–9. doi: 10.1016/s0009-8981(00)00358-2. [DOI] [PubMed] [Google Scholar]
- 18.Andelid K, Bake B, Rak S, Linden A, Rosengren A, Ekberg-Jansson A. Myeloperoxidase as a marker of increasing systemic inflammation in smokers without severe airway symptoms. Respir Med. 2007;101:888–95. doi: 10.1016/j.rmed.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 19.de la Sierra A, Larrousse M. Endothelial dysfunction is associated with increased levels of biomarkers in essential hypertension. J Hum Hypertens. 2009;24:373–9. doi: 10.1038/jhh.2009.91. [DOI] [PubMed] [Google Scholar]
- 20.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–7. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 21.Rebolledo OR, Marra CA, Raschia A, Rodriguez S, Gagliardino JJ. Abdominal adipose tissue: early metabolic dysfunction associated to insulin resistance and oxidative stress induced by an unbalanced diet. Horm Metab Res. 2008;40:794–800. doi: 10.1055/s-2008-1081502. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph TK, Rudolph V, Baldus S. Contribution of myeloperoxidase to smoking-dependent vascular inflammation. Proc Am Thorac Soc. 2008;5:820–3. doi: 10.1513/pats.200807-063TH. [DOI] [PubMed] [Google Scholar]
- 23.Kapuscinska R, Wysocka J, Niczyporuk W, Ratomski K. Cytofluorimetric assay for evaluation of CD16 receptor expression and myeloperoxidase (MPO) activity of neutrophils in patients with psoriasis vulgaris treated with PUVA. Wiad Lek. 2004;57:599–602. [PubMed] [Google Scholar]
- 24.Babaev VR, Bobryshev YV, Sukhova GK, Kasantseva IA. Monocyte/macrophage accumulation and smooth muscle cell phenotypes in early atherosclerotic lesions of human aorta. Atherosclerosis. 1993;100:237–48. doi: 10.1016/0021-9150(93)90210-l. [DOI] [PubMed] [Google Scholar]
- 25.Jagou M, Bastuji-Garin S, Bourdon-Lanoy E, Penso-Assathiany D, Roujeau JC. Poor agreement between self-reported and dermatologists’ diagnoses for five common dermatoses. Br J Dermatol. 2006;155:1006–12. doi: 10.1111/j.1365-2133.2006.07402.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


