Abstract
MicroRNA-200c (miR-200c) influences sensitivity to chemotherapy and radiotherapy in vitro. This study was designed to investigate the prognostic potential of serum miR-200c in patients with advanced esophageal squamous cancer (ESCC). The serum levels of miR-200c was assayed by quantitative RT-PCR in 157 healthy subjects and 157 patients with advanced ESCC who were treated with platinum-based chemotherapy. The serum levels of miR-200c in advanced ESCC patients was significantly increased compared with those in controls (P < 0.001). Serum miR-200c expression was significantly associated with TNM stage (P = 0.037) and treatment response (P = 0.021). Patients with high expression of serum miR-200c had a higher risk for death than those with low expression of serum miR-200c (adjusted hazard ratios = 1.665, 95% confidence intervals: 1.135-2.443, P = 0.009). In conclusion, serum miR-200c may serve as predictor of survival for advanced ESCC and provide information for personalized therapy in advanced ESCC.
Keywords: Esophageal squamous cancer, microRNA, miR-200c, platinum, chemotherapy
Introduction
Esophageal cancer is the 8th most common tumor in the world and the 6th leading cause of death from cancer, with a five-year survival rate of 25% to 30% [1,2]. In Asia, esophageal squamous cancer (ESCC) constitutes 90% of all esophageal cancer. Patients are usually already at advanced stage when diagnosed [3]. Recently, with the progress in clinical operation, radiotherapy, and chemotherapy, as well as the increased awareness of perioperative medicine, the prognosis for esophagus cancer has significantly improved [4] but still remains bleak. Moreover, ESCC has low sensitivity to chemotherapy. Both hematogenous metastasis and local infiltration are found at an early stage [5]. Therefore, the identification of biomarkers for cancer detection and prognosis is important for treatment decisions of ESCC patients.
MicroRNA (miRNA), which consists of 19 to 25 nucleotides, is a series of noncoding small RNA found in eukaryotes. A number of studies [2,6-8] have shown that miRNA is abnormally expressed in certain types of cancer and is significantly involved in cancer occurrence and development. In addition, highly stable, cell-free miRNA with different expression patterns are found in circulating in different pathological states, and thus circulation miRNA has high potential as a molecular biomarker for cancer [9-15].
Previous studies [16-18] have shown that the miR-200 family inhibits the epithelial-mesenchymal transition (EMT), which plays important roles in embryonic development, cancer, and other diseases [19]. The miR-200 family directly acts on the E-cad transcription inhibitors ZEB1, ZEB2 (a potent E-cadherin repressor), EMT activator, and TGF-β and thus regulates EMT-associated genes, such as E-cad and zonula occludens 3 (ZO-3), as well as plakophilin [20]. Dysregulation of miR-200c was found in many types of cancer, including ovarian, gastric, esophageal and colorectal cancers, and was correlated with the prognosis of these cancers [6,15,21-23]. Furthermore, the expression level of miRNA was found to influence sensitivity to chemotherapy or radiotherapy [23-26].
In the present study, we evaluated the expression levels of serum miR-200c in 157 advanced ESCC patients receiving platinum-based chemotherapy and investigated the relationship between serum miR-200c and clinical outcome of advanced ESCC patients.
Materials and methods
Patients
A total of 157 advanced ESCC patients were recruited from Taizhou People’s Hospital during July 2008 through March 2012. All patients were histologically confirmed and had no history of other cancer. Patients were given at least two cycles of platinum-containing chemotherapy, including cisplatin/5-FU, cisplatin/paclitaxel, cisplatin/docetaxel, oxaliplatin/paclitaxel, oxaliplatin/docetaxel and oxaliplatin/capecitabine. The response to treatment was evaluated based on the WHO standard [27], which classified the ESCC patients into four groups: complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). Blood samples were collected before patients were treated with any anti-cancer therapy, including chemotherapy. In addition, sera from a set of age- and sex-matched 157 healthy subjects were also collected from Taizhou People’s Hospital. All participates gave written informed consent before enrolling in the study, and the study was approved by the ethical committees of Taizhou People’s Hospital.
Venous blood samples from each participate were collected in sterile tubes without anticoagulant. After leaving the tube in an upright position at room temperature for 30 min to 2 h, the samples were centrifuged at 2,000 g for 15 min. The supernatant was then centrifuged at 12,000 g for 10 min at 4°C to completely remove cellular components. Sera were then aliquoted and stored at -80°C for further use.
Quantitative RT-PCR (qRT-PCR)
RNA was isolated from 0.4 mL of serum using the mirVana PARIS Kit (Ambion, TX, USA) following the manufacturer’s instructions. Synthetic celmiR-39 was added to each sample to surveillance sample-to-sample variation in the RNA isolation [9]. RNA samples were suspended in 50 μL of RNase-free water, and the concentration of RNA samples was quantified by NanoDrop ND-1000 (Nanodrop, USA).
The One Step PrimeScript® miRNA cDNA Synthesis Kit (Takara, Dalian, China) was used to generate miRNA first-strand complementary DNA in a final volume of 20 μL. The reaction mixture consisted of the following: 10 μL of 2 × miRNA Reaction Buffer Mix, 2 μL of 0.1% BSA, 2 μL of miRNA PrimeScript® RT Enzyme Mix, and 1 μL of RNA. The reaction conditions were 37°C of incubation for 2 h, followed by 85°C for 5 s. The qRT-PCR assays were carried out in triplicate using SYBR® Premix Ex TaqTM II Kit (Takara, Dalian, China) on a 7500 Realtime PCR system (Applied Biosystems, USA) with the following conditions: 95°C for 10 s, followed by 40 cycles at 95°C for 5 s, and 60°C for 20 s. Amplification reaction was performed in a final volume of 20 μL containing 2 μL of the cDNA, 1 × Master Mix, 1 μL of each primer. Relative expression of miR200c was normalized to cel-mir-39, and the fold change was calculated by the equation 2-ΔΔCt [28].
Statistical analysis
All statistical analysis were performed using SPSS v20.0 software (SPSS, Inc., Chicago, IL). The expression level of serum miR-200c was compared using Wilcoxon nonparametric test. All patients were divided into two groups of high and low expression according to the median expression level of serum miR-200C. Chi-square test or Fisher’s exact test were performed to determine the relationship between the serum miR-200c and clinical pathological parameters. Kaplan–Meier and log-rank analyses were carried out to evaluate the effect of serum miR200c on ESCC survival. Hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) were with Cox regression models. P values < 0.05 were considered statistically significant.
Results
The expression levels of serum miR-200c
The qPCR was used to determine the expression levels of serum miR-200c in 157 advanced ESCC patients and healthy controls. We found that the levels of miR-200c in ESCC patients were significantly higher than those in controls (P < 0.001) (Figure 1A). In addition, patients with stage IV ESCC had significantly higher levels of miR-200c than those with stage III ESCC (P = 0.019) (Figure 1B).
Figure 1.
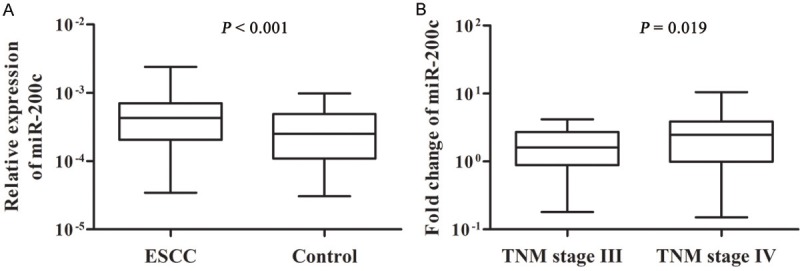
The serum levels of miR-200c in advanced ESCC patients and healthy controls. A: Comparison of miR-200c expression in 157 ESCC patients and 157 healthy controls. B: Comparison of miR-200c expression levels between TNM stage III and TNM stage IV.
Association of miR-200c expression with clinical features of NSCLC patients
We further evaluated the relationships of miR-200c expression with clinicopathological characteristics. Serum miR-200c was weakly but significantly associated with TNM stage (P = 0.03) (Table 1). No other association between miR-200c expression and clinical characteristics was observed.
Table 1.
Association of serum miR-200c expression with clinicopathologic parameters
| Clinical features | miR-200c | P value | |
|---|---|---|---|
|
| |||
| High | Low | ||
| Age (years) | |||
| > 60 | 36 | 44 | 0.265 |
| ≤ 60 | 42 | 35 | |
| Sex | |||
| male | 60 | 55 | 0.368 |
| female | 18 | 24 | |
| Family history of ESCC | |||
| Yes | 23 | 29 | 0.397 |
| No | 55 | 50 | |
| Pathologic type | |||
| medullary | 27 | 30 | 0.828 |
| ulcerative | 36 | 34 | |
| fungating | 12 | 10 | |
| others | 3 | 5 | |
| Histologic grade | |||
| 1 | 3 | 6 | 0.624 |
| 2 | 45 | 45 | |
| 3 | 28 | 28 | |
| Tumor size (cm) | |||
| > 4 | 41 | 52 | 0.121 |
| ≤ 4 | 31 | 22 | |
| Drinking status | |||
| never drinker | 17 | 29 | 0.442 |
| former drinker | 3 | 3 | |
| current drinker | 13 | 12 | |
| TNM stage | |||
| III | 36 | 50 | 0.037 |
| IV | 42 | 29 | |
Association of serum miR-200c expression with outcome of platinum-based chemotherapy
Complete response was observed in 2 (1.3%) and partial response in 33 (21.0%) patients, with an overall response rate of 22.3%. Significantly higher response rate was associated with low miR-200c expression compared with high miR-200c expression (30.4% versus 14.1%, P = 0.021). This result remained significant even after adjusted for age, sex, TNM stage, tumor size, pathologic type, histology and drinking status (P = 0.048).
The median overall survival was 20.0 months (95% CI: 15.2-24.8) for all ESCC patients. Univariate analysis revealed that patients with low miR-200c expression had a statistically significantly reduction in risk of death compared with those with high miR-200c expression, which related to a survival advantage of 12.0 months [26.0 months (95% CI: 19.7-32.4) versus 14.0 months (95% CI: 11.0-17.0), HR = 0.533, P = 0.001] (Figure 2). This indicates that ESCC patients with low miR-200c expression may gain the greatest benefit in terms of prolonging survival when receiving platinum-based chemotherapy. In addition, TNM stage (HR = 1.663, 95% CI: 1.144-2.420, P = 0.008) and clinical response (HR = 2.102, 95% CI: 1.266-3.491, P = 0.004) were significantly associated with overall survival of advanced ESCC patients.
Figure 2.
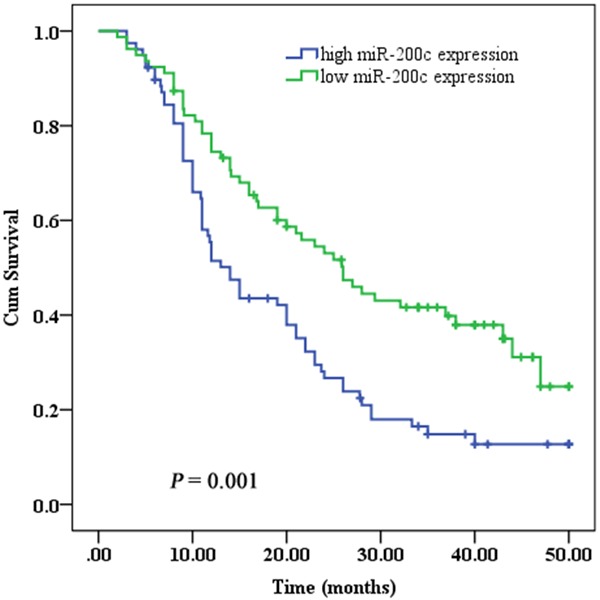
Kaplan–Meier curves of overall survival rates of advanced ESCC patients treated with platinum-based chemotherapy according to miR-200c expression.
Multivariate analysis based on the Cox proportional hazards regression model was fitted using the significant prognostic factors determined by the univariate analysis. Multivariate survival analysis identified TNM stage (P = 0.042), serum miR-200c (P = 0.009) and clinical response (P = 0.013) as independent factors (Table 2). Patients with high miR-200c expression had a 1.665-fold (95% CI: 1.135-2.443) increased risk of death compared with those with low miR-200c expression.
Table 2.
Univariate and multivariate Cox regression analysis of overall survival in a discovery cohort of 164 cases
| Features | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years), > 60 vs ≤ 60 | 1.266 (0.969-1.655) | 0.083 | ||
| Sex, male vs female | 0.834 (0.547-1.273) | 0.401 | ||
| Family history of ESCC, no vs yes | 0.818 (0.557-1.201) | 0.304 | ||
| Pathologic type, medullary vs others | 1.094 (0.883-1.357) | 0.412 | ||
| Histologic grade, 1 + 2 vs 3 | 1.149 (0.837-1.576) | 0.39 | ||
| Tumor size (cm), > 4 vs ≤ 4 | 0.786 (0.519-1.190) | 0.256 | ||
| Drinking, never/current/former | 1.060 (0.796 to 1.413) | 0.69 | ||
| TNM stage, IV vs III | 1.663 (1.144-2.420) | 0.008 | 1.485 (1.015-2.173) | 0.042 |
| miR-200c, low vs high | 1.877 (1.289-2.732) | 0.001 | 1.665 (1.135-2.443) | 0.009 |
| Clinical response, SD + PD vs CR + PR | 2.102 (1.266-3.491) | 0.004 | 1.912 (1.146-3.190) | 0.013 |
Discussion
Physicians frequently face a dilemma in the medical practice that for the first time, cancer patients are resistant to both chemotherapy and radiotherapy, and thus have to consider changing the therapeutic regimen. This causes delay in cancer treatment and results in poor prognosis. Therefore, biomarker-guided personalized therapies may be key to improving the curative effect of anti-cancer therapy and reduce the psychological stresses of patients and doctors.
It is sometimes difficult to obtain cancer tissue specimens, especially in patients with late-stage disease. However, it is easy to obtain blood samples, and thus circulation molecules are more attractive biomarkers for the diagnosis and prognosis of cancer. Recently, researchers have shed some light on the noncoding regions of the genome. As a crucial factor affecting the occurrence and development of cancer, miRNAs are likely to be new direction for the breakthrough in cancer diagnosis and therapy [9,29]. Many studies have demonstrated that miRNAs with highly stability in circulation hold great promise as a new class of biomarkers for cancer detection and prognosis [9-15].
The role of miR-200c in cancer is complicated. miR-200c can function as context-dependent oncogene or tumor suppressor, even in the same type of cancer. Prislei et al. [21] found that when HuR was expressed primarily in the nucleus of ovarian cancer cells, overexpression of miR-200c inhibited TUBB3 expression, which resulted in better prognosis. By contrast, when HuR was expressed in the cytoplasm of ovarian cancer cells, miR-200c enhanced TUBB3 expression, which resulted in poor prognosis [21]. In addition, miR-200c was upregulated in ESCC [23], but downregulated in esophageal adenocarcinoma [30]. Furthermore, ESCC patients with overexpression of miR-200c had poor prognosis [23]. In the present study, we also found that serum miR-200c was upregulated, and high expression of serum miR-200c was associated with worse survival time, which was consistent previous study [31]. These results indicated that the majority of circulating miR-200c may be cancer-derived.
Previous studies have shown a correlation between miR-200c expression and resistance to anticancer drugs [23-26]. The underlying resistance mechanisms have also been investigated. miR-200c direct suppresses TUBB3 expression, which is associated with resistance to microtubule-binding chemotherapeutic agents in many cancers [21,25]. PI3K/Akt signaling pathways are disrupted in many types of cancer, including ESCC [32], which affect their sensitivity to chemotherapy [33]. Furthermore, serum miR-200c was associated with clinical outcome of ESCC patients receiving neoadjuvant chemotherapy [31]. In this study, we also found an association of serum miR-200c expression with clinical outcome of advanced ESCC patients receiving platinum-based chemotherapy. Advanced ESCC patients with high miR-200c expression had poor response to chemotherapy. This result partly reflected the overexpression of miR-200c in advanced ESCC tissues.
In summary, this study identified significant association between circulating miR-200c and treatment response and prognosis of advanced ESCC patients receiving platinum-based chemotherapy. miR-200c is a potential biomarker in evaluating the efficacy of chemotherapy schemes for advanced ESCC patients.
Acknowledgements
This work was supported by the 12th Five-Year Plan Key Project of Science and Technology, China (grant No. 2013ZX10002007), the Shanghai Committee of Science and Technology, China (grant No. 13440701500), and the Jiangsu Province Science and Technology Support Program, China (grant No. BE2012729).
Disclosure of conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Enzinger PC, Mayer RJ. Medical progress - Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Zhang T, Wang Q, Zhao D, Cui Y, Cao B, Guo L, Lu SH. The oncogenetic role of microRNA-31 as a potential biomarker in oesophageal squamous cell carcinoma. Clin Sci (Lond) 2011;121:437–447. doi: 10.1042/CS20110207. [DOI] [PubMed] [Google Scholar]
- 3.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 4.Barber TW, Duong CP, Leong T, Bressel M, Drummond EG, Hicks RJ. 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med. 2012;53:864–871. doi: 10.2967/jnumed.111.101568. [DOI] [PubMed] [Google Scholar]
- 5.Tachimori Y, Kanamori N, Uemura N, Hokamura N, Igaki H, Kato H. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2009;137:49–54. doi: 10.1016/j.jtcvs.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Zhang Y, Wang J, Chen J, Yang C, Cai K, Wang X, Shi F, Dou J. MicroRNA-200c overexpression inhibits tumorigenicity and metastasis of CD117+CD44+ ovarian cancer stem cells by regulating epithelial-mesenchymal transition. J Ovarian Res. 2013;6:50. doi: 10.1186/1757-2215-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirajima S, Komatsu S, Ichikawa D, Takeshita H, Konishi H, Shiozaki A, Morimura R, Tsujiura M, Nagata H, Kawaguchi T, Arita T, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Clinical impact of circulating miR-18a in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2013;108:1822–1829. doi: 10.1038/bjc.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R, Liao J, Yang M, Shi Y, Peng Y, Wang Y, Pan E, Guo W, Pu Y, Yin L. Circulating miR-155 expression in plasma: a potential biomarker for early diagnosis of esophageal cancer in humans. J Toxicol Environ Health A. 2012;75:1154–1162. doi: 10.1080/15287394.2012.699856. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Jiang L, Sun C, Guo L, Lin M, Huang J, Zhu L. Decreased circulating miR-375: A potential biomarker for patients with non-small-cell lung cancer. Gene. 2014;534:60–65. doi: 10.1016/j.gene.2013.10.024. [PubMed] [Google Scholar]
- 12.Lin Q, Chen T, Lin Q, Lin G, Lin J, Chen G, Guo L. Serum miR-19a expression correlates with worse prognosis of patients with non-small cell lung cancer. J Surg Oncol. 2013;107:767–771. doi: 10.1002/jso.23312. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Si Q, Xiao S, Xie Q, Lin J, Wang C, Chen L, Chen Q, Wang L. Prognostic significance of serum miR-17-5p in lung cancer. Med Oncol. 2013;30:353. doi: 10.1007/s12032-012-0353-2. [DOI] [PubMed] [Google Scholar]
- 14.Liu N, Cui RX, Sun Y, Guo R, Mao YP, Tang LL, Jiang W, Liu X, Cheng YK, He QM, Cho WC, Liu LZ, Li L, Ma J. A four-miRNA signature identified from genome-wide serum miRNA profiling predicts survival in patients with nasopharyngeal carcinoma. Int J Cancer. 2013 doi: 10.1002/ijc.28468. doi: 10.1002/ijc.28468. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Toiyama Y, Hur K, Tanaka K, Inoue Y, Kusunoki M, Boland CR, Goel A. Serum miR-200c Is a Novel Prognostic and Metastasis-Predictive Biomarker in Patients With Colorectal Cancer. Ann Surg. 2013 doi: 10.1097/SLA.0b013e3182a6909d. doi: 10.1097/SLA.0b013e3182a6909d. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, VandenBoom TG 2nd, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Hurteau GJ, Carlson JA, Roos E, Brock GJ. Stable expression of miR-200c alone is sufficient to regulate TCF8 (ZEB1) and restore E-cadherin expression. Cell Cycle. 2009;8:2064–2069. doi: 10.4161/cc.8.13.8883. [DOI] [PubMed] [Google Scholar]
- 21.Prislei S, Martinelli E, Mariani M, Raspaglio G, Sieber S, Ferrandina G, Shahabi S, Scambia G, Ferlini C. MiR-200c and HuR in ovarian cancer. BMC Cancer. 2013;13:72. doi: 10.1186/1471-2407-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Diaz P, Lorenzo-Patino MJ, Haz M, Santamarina I, Blanco M, Fernandez-Tajes J, Quindos M, Carral A, Figueroa A, Anton-Aparicio LM, Calvo L. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. doi: 10.1186/1479-5876-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamano R, Miyata H, Yamasaki M, Kurokawa Y, Hara J, Moon JH, Nakajima K, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Overexpression of miR-200c Induces Chemoresistance in Esophageal Cancers Mediated Through Activation of the Akt Signaling Pathway. Clin Cancer Res. 2011;17:3029–3038. doi: 10.1158/1078-0432.CCR-10-2532. [DOI] [PubMed] [Google Scholar]
- 24.Cochrane DR, Howe EN, Spoelstra NS, Richer JK. Loss of miR-200c: A Marker of Aggressiveness and Chemoresistance in Female Reproductive Cancers. J Oncol. 2010;2010:821717. doi: 10.1155/2010/821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009;8:1055–1066. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi L, Zhang S, Wu H, Zhang L, Dai X, Hu J, Xue J, Liu T, Liang Y, Wu G. MiR-200c Increases the Radiosensitivity of Non-Small-Cell Lung Cancer Cell Line A549 by Targeting VEGF-VEGFR2 Pathway. PLoS One. 2013;8:e78344. doi: 10.1371/journal.pone.0078344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 30.Smith CM, Watson DI, Leong MP, Mayne GC, Michael MZ, Wijnhoven BP, Hussey DJ. miR-200 family expression is downregulated upon neoplastic progression of Barrett’s esophagus. World J Gastroenterol. 2011;17:1036–1044. doi: 10.3748/wjg.v17.i8.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka K, Miyata H, Yamasaki M, Sugimura K, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M, Doki Y. Circulating miR-200c Levels Significantly Predict Response to Chemotherapy and Prognosis of Patients Undergoing Neoadjuvant Chemotherapy for Esophageal Cancer. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-3093-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Burris HA 3rd. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol. 2013;71:829–842. doi: 10.1007/s00280-012-2043-3. [DOI] [PubMed] [Google Scholar]


