Abstract
Androgen Receptor (AR) signaling is critically important during the development and progression of prostate cancer (PCa). The AR signaling is also important in the development of castrate resistant prostate cancer (CRPC) where AR is functional even after androgen deprivation therapy (ADT); however, little is known regarding the transcriptional and functional regulation of AR in PCa. Moreover, treatment options for primary PCa for preventing the occurrence of CRPC is limited; therefore, novel strategy for direct inactivation of AR is urgently needed. In this study, we found loss of miR-34a, which targets AR, in PCa tissue specimens, especially in patients with higher Gleason grade tumors, consistent with increased expression of AR. Forced over-expression of miR-34a in PCa cell lines led to decreased expression of AR and prostate specific antigen (PSA) as well as the expression of Notch-1, another important target of miR-34a. Most importantly, BR-DIM intervention in PCa patients prior to radical prostatectomy showed reexpression of miR-34a, which was consistent with decreased expression of AR, PSA and Notch-1 in PCa tissue specimens. Moreover, BR-DIM intervention led to nuclear exclusion both in PCa cell lines and in tumor tissues. PCa cells treated with BR-DIM and 5-aza-dC resulted in the demethylation of miR-34a promoter concomitant with inhibition of AR and PSA expression in LNCaP and C4-2B cells. These results suggest, for the first time, epigenetic silencing of miR-34a in PCa, which could be reversed by BR-DIM treatment and, thus BR-DIM could be useful for the inactivation of AR in the treatment of PCa.
Keywords: BR-DIM, miR-34a, androgen receptor (AR), PSA, methylation
In this article published in AJTR, a minor concern regarding the composite Figure 4 has been raised by a reader although such a minor concern has no impact on the overall findings and conclusions previously reported. The original composite Figure 4B consisted two panels: one panel showing AR, PSA, GAPDH expression in C4-2B cells treated with BR-DIM, and other panel showing AR, PSA, GAPDH expression in LNCaP cells treated with BR-DIM. The original Western blot data came from separate run, which was used to create the published composite figure but we failed to provide such information. Therefore, in order to clarify this point, a modified composite figure has now been created. We therefore request you to publish Erratum to reflect this change where the lanes are separated with demarcation showing by a black vertical line (please see the edited figure). The amended figure is included below. The authors express regrets for this confusion.
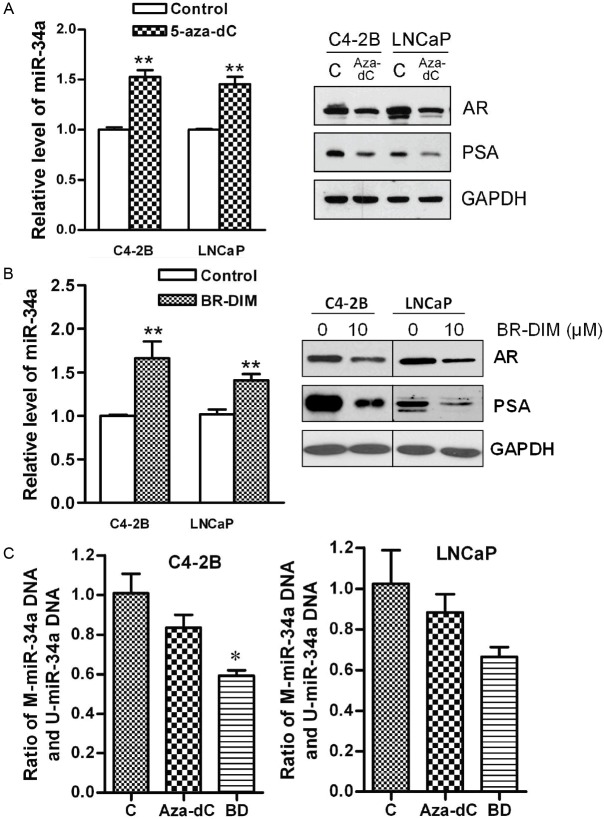
Figure 4.
BR-DIM regulated the expression of miR-34a, and the activity of AR pathway through demathylation. A: The miR-34a expression was up-regulated in cells treated with 5 μM of 5-aza-dC for 5 day compared with control cells (left panel) consistent with decreased expression of AR and PSA (right panel). B: The miR-34a expression was up-regulated in the cells treated with 6 μM of BR-DIM for 5 day compared with control cells (left panel) consistent with decreased expression of AR and PSA (right panel). C: C4-2B and LNCaP cells were treated with 5 μM 5-aza-dC or 6 μM BR-DIM for 5 days showing decreased methylation of miR-34a promoter. (C: control, aza-dC: 5-Aza-2'-deoxycytidine, BD: BR-DIM, *p<0.05, **p<0.01).