Abstract
We are presenting the initial results of inverted internal limiting membrane (ILM) flap technique for large macular hole. Five eyes of five patients with large diameter macular hole (>700 μm) were selected. All patients underwent inverted ILM flap technique for macular hole. Anatomical closure and functional success were achieved in all patients. There was no loss of best-corrected visual acuity in any of the patients. Inverted ILM flap technique in macular hole surgery seems to have a better hole closure rates, especially in large diameter macular holes. Larger case series is required to assess the efficacy and safety of this technique.
Keywords: Inverted internal limiting membrane flap technique, macular hole, macular hole surgery
Full-thickness macular holes are a relatively common cause of a significant reduction in central visual acuity. In 1988, Gass proposed a classification system for idiopathic macular holes, as well as a new hypothesis for its pathogenesis, which emphasizes the role of the vitreo-macular tangential traction in the formation of macular holes.[1]
Macular hole surgery is a well-established method for the treatment of idiopathic macular holes. The rationale for surgery is the identification and treatment of these vitreo-retinal traction forces, either tangential, antero-posterior or both.
The procedure for macular hole surgery is pars plana vitrectomy, posterior vitreous removal, internal limiting membrane (ILM) peeling, filling of the vitreous cavity with a gas bubble and post-operative face-down positioning for 1 week.[2,3]
This is a conventional procedure in cases with small to moderate holes but in cases with large holes, the anatomical and functional success is difficult to achieve. The inverted ILM flap technique improves both functional and anatomical outcome following vitrectomy, for large macular holes.[4] This study was done to present the initial surgical results of this technique.
Materials and Methods
It was a prospective, non-randomized, interventional case-series study of patients with large macular holes. All patients in the study had idiopathic large macular holes as determined by bio microscopy and optical coherence tomography (OCT) imaging. Exclusion criteria were macular holes of < 700 μm and macular hole due to other causes. Demographic and clinical data were collected for all patients, including age, sex, best-corrected visual acuity (BCVA) and intraocular pressure. Slit-lamp bio microscopy of the anterior segment and fundus with +90 D was also done. The diagnosis of macular hole in all patients was confirmed using spectral-domain OCT. The minimum linear diameter (MLD) of the macular hole was measured. Institutional ethical committee approval was obtained. After taking informed consent, surgery was done in all the patients. The follow-up was done at day 1, day 7, 1 month, 2 months and 6 months. Final BCVA was recorded and complete ophthalmic evaluation with OCT imaging was done during all the visits.
Surgical technique
All patients underwent 23G three-port pars plana vitrectomy with posterior vitreous detachment induction using Triamcinolone acetonide. Brilliant Blue dye assisted ILM peeling was done and instead of completely removing the ILM, a remnant of ILM attached to the margins of the macular hole was left in place and inverted into the hole after trimming with cutter. Fluid air exchange was performed. All surgeries were performed by a single vitreo-retinal surgeon. The patients were advised to maintain prone position for 5 days.
Results
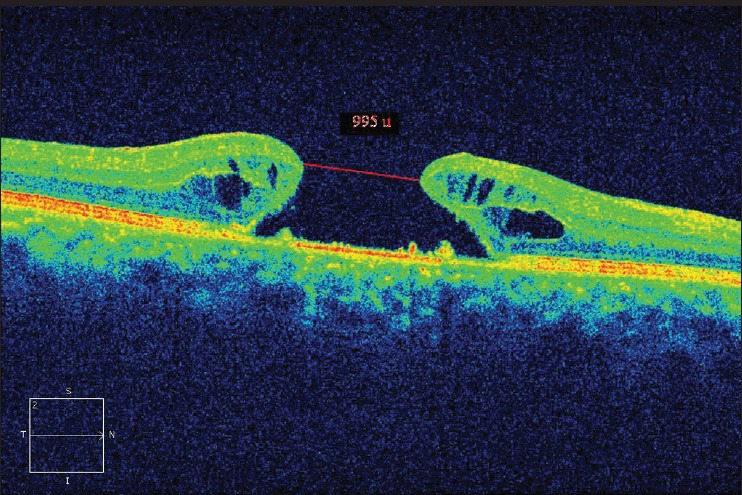
Demographic data and variables were analyzed [Table 1]. There were a total of five patients. The mean age of patients was 67.00 ± 5.41 years. All patients were women and pseudo-phakic. The mean minimal linear diameter of the macular holes was 811.4 μm (728-995 μm) [Figs 1, 3, 5]. Mean BVCA pre-operatively was 1.22 log-MAR units (1.10-1.22 log MAR units). Post-operatively, mean BCVA was 1.10 log MAR units (1.0-1.52 log MAR units). Improvement of visual acuity was registered in all patients (100%) [Figs. 2, 4, 6]. There were no intraoperative or post-operative complications. All the patients were followed up for a period of 6 months.
Table 1.
Demographic and clinical profile of patients
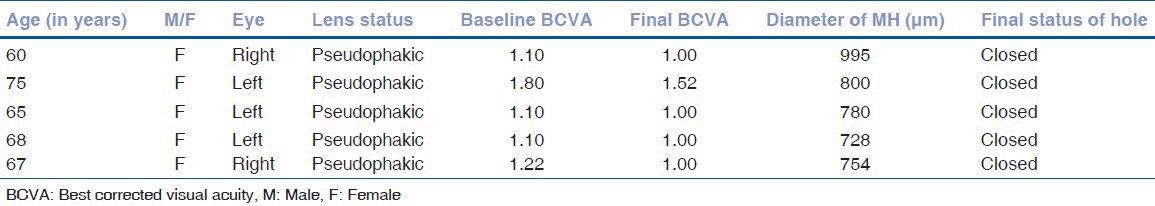
Figure 1.
Pre-operative optical coherence tomography of patient one
Figure 3.
Pre-operative optical coherence tomography of patient three
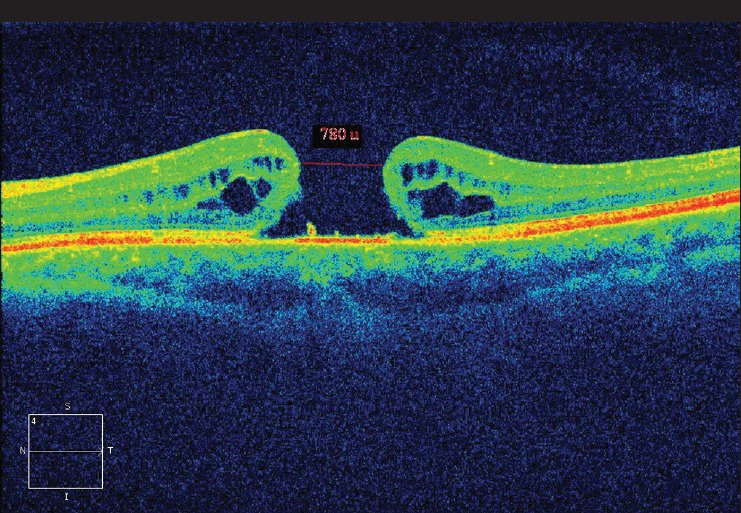
Figure 5.
Pre-operative fundus photo of patient one
Figure 2.
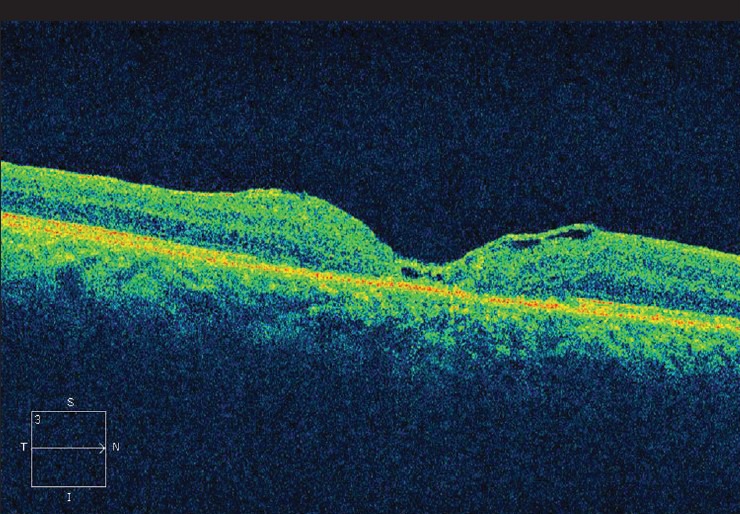
Post-operative optical coherence tomography of patient one
Figure 4.
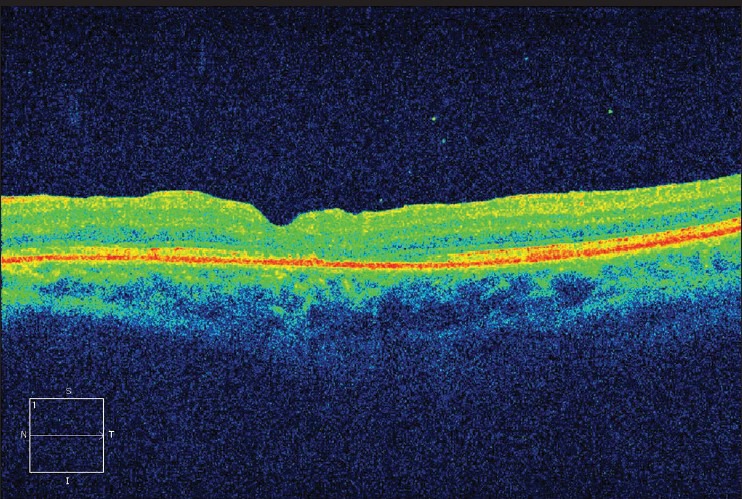
Post-operative optical coherence tomography of patient three
Figure 6.
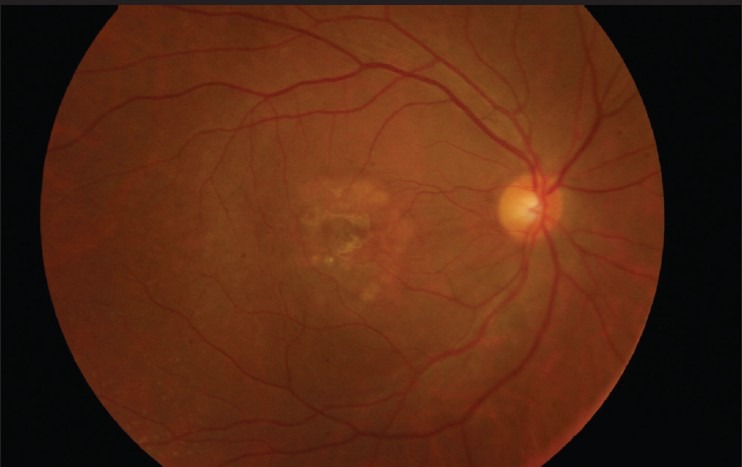
Post-operative fundus photo of patient one
Discussion
The improvement in technique and development of finer instrumentation in vitreo-retinal surgery has significantly improved the surgical outcome of macular holes in terms of anatomical and functional success. The Vitrectomy for Treatment of Macular Hole Study Group showed a clear benefit in closure rates and final visual acuity with surgery versus observation for stage III and IV macular hole.[5]
The single most reliable factor affecting the surgical outcome following surgery is the size of the hole. A number of studies have established that the MLD of the hole is closely related to the rate of anatomic success. Also, it has been shown that the most favorable outcomes for visual recovery were associated with better initial visual acuity.
Among all the different techniques for macular hole surgery, the one with the most positive effect on final outcome is vitrectomy with ILM peeling, in order to release tangential forces acting on the macular hole (MH). In addition to promoting hole closure, peeling of the ILM also reduces the probability of its reopening.[6,7] Dye assisted technique is safe and useful in visualizing the ILM, leading to the performance of successful peeling of ILM with minimal damage to the retina. Brilliant blue selectively stains the ILM and can be safely used for staining the ILM.[8] The peeled-off ILM contains Müller cell fragments which can induce gliosis and helping in closure of macular hole. Thus, if a segment of peeled-off ILM is left attached, it may provoke gliosis both inside the retina and on the surface of the ILM. The ILM also may be a scaffold for tissue proliferation. Michalewska et al. observed closure of the macular hole by a thin membrane with an appearance consistent with the ILM.[4]
Our study found that the closure rate of macular hole following this technique was 100% and the functional outcome were also better. But, a larger study group and longer follow-up period is required to further evaluate this method.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–39. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 2.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–9. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 3.Benson WE, Cruickshanks KC, Fong DS, Williams GA, Bloome MA, Frambach DA, et al. Surgical management of macular holes: A report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:1328–35. doi: 10.1016/s0161-6420(01)00731-x. [DOI] [PubMed] [Google Scholar]
- 4.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–25. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Freeman WR, Azen SP, Kim JW, el-Haig W, Mishell DR, 3rd, Bailey I. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch Ophthalmol. 1997;115:11–2. doi: 10.1001/archopht.1997.01100150013002. [DOI] [PubMed] [Google Scholar]
- 6.Yamanishi S, Emi K, Motokura M, Oshima Y, Nakayama M, Watanabe M. Visual outcome of macular hole surgery with internal limiting membrane peeling. Nihon Ganka Gakkai Zasshi. 2001;105:788–93. [PubMed] [Google Scholar]
- 7.Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology. 1999;106:1392–7. doi: 10.1016/S0161-6420(99)00730-7. [DOI] [PubMed] [Google Scholar]
- 8.Enaida H, Hisatomi T, Hata Y, Ueno A, Goto Y, Yamada T, et al. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26:631–6. doi: 10.1097/01.iae.0000236469.71443.aa. [DOI] [PubMed] [Google Scholar]


