Abstract
The main objective of this review is to re-examine the type of information transmitted by the dorsal and ventral spinocerebellar tracts (DSCT and VSCT respectively) during rhythmic motor actions such as locomotion. Based on experiments in the 1960s and 1970s, the DSCT was viewed as a relay of peripheral sensory input to the cerebellum in general, and during rhythmic movements such as locomotion and scratch. In contrast, the VSCT was seen as conveying a copy of the output of spinal neuronal circuitry, including those circuits generating rhythmic motor activity (the spinal central pattern generator, CPG). Emerging anatomical and electrophysiological information on the putative subpopulations of DSCT and VSCT neurons suggest differentiated functions for some of the subpopulations. Multiple lines of evidence support the notion that sensory input is not the only source driving DSCT neurons and, overall, there is a greater similarity between DSCT and VSCT activity than previously acknowledged. Indeed the majority of DSCT cells can be driven by spinal CPGs for locomotion and scratch without phasic sensory input. It thus seems natural to propose the possibility that CPG input to some of these neurons may contribute to distinguishing sensory inputs that are a consequence of the active locomotion from those resulting from perturbations in the external world.
 |
Katinka Stecina obtained her Ph.D. from the University of Manitoba in 2006. She did postdoctoral work as a Marie Curie Intra-European Fellow with H. Hultborn and J.B. Nielsen in Copenhagen, Denmark from 2007–2009. She currently has a postdoctoral grant from the DSF and studies neuronal networks used for left-right coordination. Brent Fedirchuk obtained his Ph.D. from the University of Manitoba in 1994. He did postdoctoral work with H. Hultborn from 1993–1995 then started his laboratory at the University of Manitoba in 2000. His current research is directed at how the electrical properties of spinal neurons are altered in preparation for a motor output. H. Hultborn has obtained his Ph.D. from Goteborg University, Sweden then moved on to establish his research group on the neuronal control of movement and spinal motoneurons at the University of Copenhagen and led this group for the last several decades.
Introduction
The dorsal and ventral spinocerebellar tracts (DSCT and VSCT) are the two main lumbar components of spinocerebellar pathways projecting directly from the spinal cord to the cerebellum. The classical DSCT projects via the dorsolateral funiculus and the VSCT projects via the ventral funiculus at the sacral and lower lumbar levels (e.g. Grant, 1962; Matsushita & Ikeda, 1970; Grant et al. 1982; Xu & Grant, 1994, 2005). The axons of DSCT neurons ascend uncrossed (i.e. same side as soma), while VSCT axons cross the midline within a segment of the soma and ascend in the contralateral spinal white matter. It has been described that both spinocerebellar pathways terminate ipsilaterally in the cerebellar cortex with respect to the location of their cell bodies in the spinal cord, i.e. the VSCT cross again in the cerebellum. The axons originating from DSCT and VSCT cells generally enter the cerebellum via the inferior cerebellar peduncle and via the superior cerebellar peduncle, respectively as described (Oscarsson, 1965; Bloedel & Courville, 2011); however, there are exceptions and both peduncles carry some VSCT and DSCT axons (Grant & Xu, 1988). In addition to their anatomical segregation, the DSCT and VSCT tracts have also been thought to serve different functional roles in sensory motor integration. The DSCT neurons mainly relay sensory input from the periphery and they are less influenced by the activity of neurons in the central nervous system than the VSCT cells (Holmqvist et al. 1956; Lundberg & Oscarsson, 1962; Lundberg, 1971; Arshavsky et al. 1986; Bosco & Poppele, 2001; Bloedel & Courville, 2011). It has been commonly accepted that during rhythmic movements, such as locomotion, the function of the DSCT is to relay activity of hindlimb sensory afferents while the VSCT cells are less influenced by the peripheral input and are driven by the output of the circuits generating rhythmic motor activity, i.e. the spinal central pattern generator (CPG; Arshavsky et al. 1986; Bloedel & Courville, 2011). In the first part of this review, the lumbar subpopulations of both DSCT and VSCT cells will be summarized and in the second part, key observations about the activity of DSCT and VSCT cells will be discussed in relation to rhythmic motor output and their putative functions in sensory motor control.
Organization of lumbar subpopulations of neurons in feline spinocerebellar tracts
The functional subdivisions of the DSCT and VSCT cells based on afferent input will not be discussed here in detail as they are summarized in several excellent reviews (Oscarsson, 1965; Mann, 1973; Walmsley, 1991; Bosco & Poppele, 1993). It has become clear that the spinocerebellar tracts are comprised of more populations than were originally identified by their sensory inputs and ipsilateral or contralateral ascending projections in the spinal cord. In Fig. 1A a schematic overview of the location of spinocerebellar tract cell somas in the lumbar spinal cord is presented. This illustration is similar to previous summary figures (Matsushita, 1983; Bloedel & Courville, 2011), but here we provide more details on the putative subpopulations of the lumbar DSCT and VSCT neurons. The subpopulations of DSCT and VSCT cells presented here are based on multiple studies about anatomical identification of somas, studies of sensory inputs for some subpopulations, as well as studies of termination areas in the cerebellar cortex [illustrated schematically in Fig. 1B and C (Grant, 1962; Matsushita et al. 1979; Matsushita & Okado, 1981a,b; Grant et al. 1982; Matsushita & Hosoya, 1982; Matsushita, 1983; Grant & Xu, 1988; Xu & Grant, 1988, 1994, 2005)]. Note that, (1) no consideration of termination areas in cerebellar nuclei is made here as in, for example, Matsushita & Ikeda (1970), and (2) that the naming of the lumbar spinocerebellar tracts in publications of anatomical studies is often associated with the cell population giving rise to the axons (Matsushita et al. 1979; Matsushita, 1983) but in our scheme in Fig. 1A, the names contain VSCT and DSCT labels to indicate whether the neurons cross (VSCT) or not (DSCT) at the spinal level.
Figure 1. Putative subpopulations of feline dorsal and ventral spinocerebellar tract (DSCT and VSCT) cells in the lumbar region.
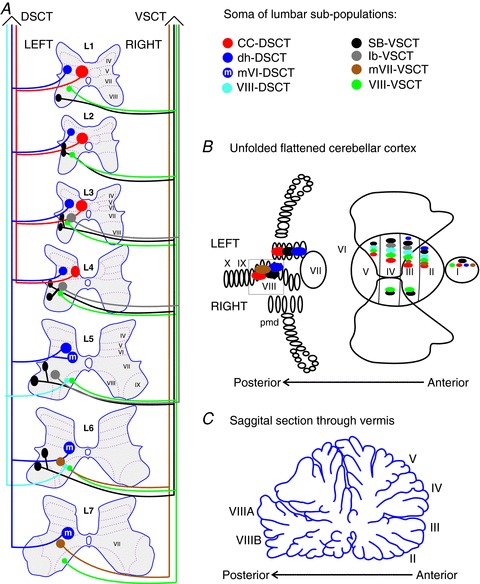
A, location of the somas of feline spinocerebellar tract cells (coloured regions) between the first and seventh lumbar spinal segments is illustrated schematically. Dotted lines and Roman numerals indicate an approximate outline of the Rexed laminae for reference. The colours indicate the various subpopulations; DSCT: Clarke's column (CC-DSCT, red), dorsal horn (dh-DSCT, dark blue), medial lamina VI DSCT (mVI-DSCT, dark blue with an ‘m’), lamina VIII (VIII -DSCT, light blue); and VSCT: spinal border cells (sb-VSCT, black), dorsolateral column (dl-VSCT, grey), medial lamina VII (mVII-VSCT, brown) and lamina VIII (VIII-VSCT, green). B, cerebellar projection areas of spinocerebellar tract neurons. The unfolded and flattened cerebellar surface as in Grant (1962) is labelled with Roman numerals to indicate regions according to Larsell's defined regions (Larsell, 1953). C, a saggital section through the vermis and the approximate location of the indicated Larsell's lobules for reference.
Subpopulations of dorsal spinocerebellar tract cells
The Clarke's column cells (CC-DSCT) are an anatomically segregated subpopulation of spinocerebellar tract cells. The recognition of large synapses between afferents and CC-DSCT cells (Szentagothai & Albert, 1955) followed by the recording of monosynaptic EPSPs in identified DSCT cells upon stimulation of sensory afferents were major milestones in the early understanding of the function of this tract (Mann, 1973). These large synapses appear to occur only on CC-DSCT even though dorsal horn DSCT cells (dh-DSCT) also receive strong excitatory input from the same nerves when higher threshold (e.g. group II) afferents are activated (Edgley & Jankowska, 1988). Stimulation of lower and then higher threshold afferents is used as a way to differentiate between dh-DSCT cells from the CC-DSCT cells together with stimulation of the medial longitudinal fasciculus (MLF). The dh-DSCT cells have no, or only weak excitation evoked from the MLF (Hammar et al. 2011). The dh-DSCT subpopulation in Fig. 1A includes the so-called ‘marginal neurons’ and ‘lamina V neurons’ and the ‘medial lamina V neurons of L5–L6’ of previous classification schemes (Matsushita, 19831979; Matsushita & Ikeda, 1980; Matsushita & Hosoya, 1982). This subpopulation also includes DSCT cells described in lamina V ranging from the eighth cervical to the sixth lumbar segments (Aoyama et al. 1973; Tapper et al. 1975; Matsushita et al. 1979) and cells extending to lamina IV–VI within the lumbar segments (Grant & Xu, 1988; Xu & Grant, 1988). Even though the lamina V-DSCT cells are often perceived as ‘the cutaneous subdivision’ of the DSCT (Tapper et al. 1975) they, and the dh-DSCT, probably receive input from other sensory receptors as has been described for cells in midlumbar segments (Edgley & Gallimore, 1988; Edgley & Jankowska, 1988). The projection sites in the cerebellar cortex of both CC-DSCT and dh-DSCT are ipsilateral with respect to their soma in the anterior lobe, paramedian lobule and posterior vermis as shown in Fig. 1B (Matsushita & Ikeda, 1980; Matsushita & Okado, 1981b; Matsushita & Hosoya, 1982; Grant & Xu, 1988; Xu & Grant, 1988).
The DSCT cells with somas in medial lamina VI (mVI-DSCT) were first described anatomically in the L5 and L6 lumbar segments (Matsushita et al. 1979) but probably extend to L7 (Grant et al. 1982). Some cells in these regions have projections to the posterior lobules of the cerebellum with axons using both peduncles for entry (Xu & Grant, 1988). The mVI-DSCT may include those cells in the lower lumbar regions that have prominent excitatory and/or inhibitory input from the spinocervical tract (Kahlat & Djouhri, 2012). The DSCT cells mainly in lamina VIII of the L5 and L6 segments (VIII-DSCT) were categorized as a separate subpopulation because they enter the cerebellum via the superior cerebellar peduncle unlike other DSCT cells and they project only to the anterior lobe (Xu & Grant, 1988).
Subpopulations of ventral spinocerebellar tract cells
Intracellular studies of VSCT cells described them to largely populate the L3 to the L6 segments (Burke et al. 1971), but they may extend as far rostral as the L1 and as caudal as the L7 segments (Matsushita et al. 1979). These cells were originally grouped together (Cooper & Sherrington, 1940; Eccles et al. 1961; Burke et al. 1971), but there are distinctive features allowing at least four subpopulations to be identified. The VSCT cells located at the lateral edge of the ventral grey matter are called ‘spinal border cells’ (SB-VSCT) in Fig. 1A. The SB-VSCT cells correspond to neurons of the ‘spinal border cells’ and cells of the ‘lateral lumbar nucleus’ according to the grouping by Matsushita and colleagues (1979) and neurons of the ‘dorsolateral nucleus’ and of the ‘ventrolateral nucleus’ according to the groups of Grant and colleagues (1982).
Another subpopulation in Fig. 1A includes cells with marked excitatory postsynaptic potentials from group Ib muscle afferents, hence called ‘Ib-VSCT’ (Jankowska et al. 2010; Shakya Shrestha et al. 2012). These cells correspond to the ‘ventromedial nucleus’ of Grant and Xu (1982) and the most medial region of spinal border cells defined by Matsushita (1983). Monosynaptic excitatory postsynaptic potentials are evoked mainly from group Ib fibres while inhibitory postsynaptic potentials are evoked from multiple sources in VSCT cells (Eccles et al. 1961; Oscarsson, 1965). The differentiation between SB-VSCT and Ib-VSCT cells can be done by identifying the source and relative size (or lack) of excitatory input from hindlimb afferents (Jankowska et al. 2010; Hammar et al. 2011). The SB-VSCT cells have excitatory input from group Ia muscle afferents and inhibitory input from group Ia and high threshold muscle afferents while the Ib-VSCT cells have excitation primarily from group Ib afferents and inhibition from the others. Both of these subpopulations receive monosynaptic input from the MLF (Hammar et al. 2011). The Ib-VSCT cells are labelled in Fig. 1A to be primarily located between the L3 and L5 segments around the middle of lamina VII based on the rationale that near this region (just medial to the dorsolateral nucleus) there has been a group of cells described to project only to the anterior lobe (Grant & Xu, 1988; Xu & Grant, 1988). It is possible that SB-VSCT cells project to both the anterior and posterior regions while Ib-VSCT cells project only to the anterior lobe.
The neurons in medial lamina VII (mVII-VSCT) located in the L6, L7 and more caudal segments comprise a third VSCT subpopulation in our scheme. They project to the anterior lobe and to lobule VIII without projections to the paramedian lobule (Matsushita et al. 1979; Matsushita & Ikeda, 1980; Matsushita & Okado, 1981b; Matsushita & Hosoya, 1982; Xu & Grant, 1988) and enter the cerebellum through both the inferior and superior peduncles (Xu & Grant, 1988). The fourth subpopulation depicted in Fig. 1A are the scattered neurons in lamina VIII from the cervical to the lower lumbar (and even more caudal) segments with crossing projections in the spinal cord (Matsushita et al. 1979). These lamina VIII cells (VIII-VSCT) enter the cerebellum via the inferior cerebellar peduncle (Xu & Grant, 1988). The distinction between Ib-, mVII- and VIII-VSCT cells is purely anatomical at this point, and further studies need to establish more features of the mVII- and VIII-VSCT subpopulations.
The DSCT and VSCT subpopulations presented in Fig. 1 is admittedly a simplification to aid the reader in understanding the organization of these tracts. Our main goal here is to emphasize that future electrophysiological studies need to take into consideration the subpopulation to which the examined spinocerebellar tract cells may belong to further our understanding on the role of these systems. In the next part of the review, we will refer to some of the above discussed subpopulations while discussing the activity profile of cells comprising the DSCT and VSCT tracts in the absence and during rhythmic motor activity.
Activation of spinocerebellar tract neurons in the absence of central motor activity
DSCT cells are thought to be activated mainly by sensory afferents rather than by descending projections or spinal interneurons (INs) while VSCT cells are thought to be strongly activated by and convey information on the activity of spinal IN circuits (Lundberg & Weight, 1971; Bloedel & Courville, 2011). Morphological investigations of the excitatory (glutamatergic) synapses in apposition with identified DSCT and VSCT cells can contribute important information in this relation. Myelinated primary afferents express vesicular glutamatergic transporter-VGLUT1, while spinal excitatory INs and most descending tracts express VGlut2 (Varoqui et al. 2002; Todd et al. 2003; Alvarez et al. 2004; Du Beau et al. 2012). It has recently been demonstrated that few terminals containing VGlut1, but a high number of terminals containing VGlut2 are in apposition of SB-VSCT cells, while there is a more even distribution of VGlut1 and -2 terminals on other VSCT cells like those in the dorsolateral VSCT subpopulation (Shrestha et al. 2012). In contrast, VGlut1 is strongly dominating for the CC-DSCT cells, and somewhat less strongly in the case of the dh-DSCT cells (Shrestha et al. 2012). These observations on the proportion of VGlut1 or VGlut2 excitatory synaptic contacts support the idea that CC-DSCT cells are influenced more by peripheral afferent input than by information from spinal INs while VSCT cells receive most of their excitatory input from spinal INs and descending tracts.
Afferent input has been thought to underlie the background activity (i.e. tonic firing not linked to a stimulus) of CC-DSCT cells. The early recognition of a small subliminal fringe in the CC-DSCT pool (Holmqvist et al. 1956) and weak post-tetanic potentiation were the primary reasons for proposing a ‘one-to-one transmission’ from afferents to CC-DSCT cells. However, background activity also persists after partial removal of sensory input. The background firing frequency of DSCT cells still ranged from 3 to 14 Hz in preparations with the lumbar dorsal root sectioned or with denervated ipsilateral hindlimb nerves (Holmqvist et al. 1956; Pyatigorskii, 1970). Background activity of about 20 Hz has been described by chronic recordings of DSCT cells in awake cats, which is reduced to about 12 Hz during active sleep (Soja et al. 1995, 1996). In anaesthetized cats 34% of the examined CC-DSCT cells had background activity (Zytnicki et al. 1995) while in decerebrate preparations we found background activity in 40% of the DSCT cells without differentiating between the subpopulations (Fedirchuk et al. 2013). The background firing rates and percentage of active cells are probably dependent on the type and dose of anaesthesia (Soja et al. 2002) and/or level of decerebration. It is also possible that background activity may be in part driven by afferent inputs arising from sources other than the hindlimbs. Afferents entering the lower lumbar and sacral regions project to more rostral CC-DSCT cells (Szentagothai & Albert, 1955; Lundberg & Oscarsson, 1960), and input from the chest wall during respiratory movements drives thoracic and possibly lumbar CC-DSCT cells (Hirai et al. 1988).
Background activity may also be related to intrinsic membrane properties of DSCT cells in addition to the afferent drive. In CC-DSCT neurons a train of stimuli to the dorsal roots sometimes resulted in a tonic activity, which lasted for several minutes after the onset of the stimulus train (Holmqvist et al. 1956). Such long-lasting bursts of action potentials and background activity might be sustained by voltage-sensitive conductances in the cell membrane. In motoneurons even though they have no spontaneous activity at rest, the activation of voltage-dependent non-inactivating persistent inward currents (some Ca2+ and Na+ channels) are known to cause long-lasting activity in response to short-lasting synaptic inputs (Crone et al. 1988; Hounsgaard et al. 1988). These currents could certainly maintain a tonic firing without a tonic synaptic excitation. The Cav1.3 channel contributes strongly to these persistent inward currents and it is activated at rather hyperpolarized membrane potentials (Xu & Lipscombe, 2001). The CC-DSCT cells label strongly for this channel (Zhang et al. 2008, 2012). Therefore, these intrinsic properties may contribute significantly to the spontaneous activity in CC-DSCT cells even in the absence of ongoing synaptic excitation. In unanaesthetized and decerebrate preparations, a similar proportion of VSCT and DSCT cells was found to have tonic background activity (Fedirchuk et al. 2013) but in previous studies background activity of VSCT cells has not been discussed to the same extent as that of DSCT cells. More comparative studies are necessary to determine whether intrinsic membrane properties contribute more to the activity of the CC-DSCT cells than the firing of other subpopulations, and the conditions under which these properties are expressed (i.e. at rest or during locomotion, or both).
Activation of spinocerebellar tract neurons during rhythmic motor activity
During rhythmic motor actions both DSCT and VSCT cells were found to be active; however, in early reports, DSCT activity ceased (Arshavskii et al. 1972a,b; Arshavsky et al. 1972) while rhythmic activity of VSCT cells persisted following partial removal of sensory input by deafferentation (Arshavskii et al. 1972a; Arshavsky et al. 1978, 1986). The activity of spinocerebellar cells has been investigated not only during ‘real’ locomotion but also during ‘fictive’ locomotion (Orsal et al. 1988) and scratching (Arshavsky et al. 1978). Fictive motor activity refers to the motor output monitored by electroneurograms from hindlimb nerves in decerebrate animals paralyzed by pharmacological blockade of neuromuscular junctions. The fictive motor output closely resembles the output during the real behaviour, but there is no actual movement and therefore no rhythmic sensory feedback. In this state, the rhythmic activity of spinocerebellar tract cells would be driven by inputs from the central neuronal networks involved in the generation of rhythmic motor output. The VSCT but not the DSCT cells were reported to be active during fictive scratch (Arshavsky et al. 1978). Work from our laboratory on the activity of VSCT and DSCT cells during fictive locomotion and scratch reported that during fictive locomotion 70% (57 of 81) and during fictive scratch 69% (20 of 29) of the DSCT neurons were phasically active as illustrated in Fig. 2 (Fedirchuk et al. 2013). The activity of the two cells presented in Fig. 2A and B were recorded in precollicular–postmamillary decerebrated preparations in which fictive locomotion was evoked by electrical stimulation (100 μA, 20 Hz) of the mesencephalic locomotor region (Fig. 2A) and fictive scratch was evoked by mechanically stimulating the skin over the left ear following application of 0.1% bicucculine on to the first cervical dorsal root on the left side (Fig. 2B). Extracellular recordings of action potentials from identified spinocerebellar tract cells were related to cyclic activity (after normalizing and averaging the cycles) and about the same proportion of DSCT cells were active during the flexor and extensor phases of locomotion or scratch. There were also some cells with activity spanning across the two phases. When possible, extracellular recordings were followed by an attempt to penetrate the identified cell to obtain intracellular records during another bout of motor activity. Note that in Fig. 2, the extracellular and intracellular data are overlaid for the sake of simplicity. After the action potential generating Na+ channels had been inactivated by injection of a depolarizing current, the intracellular recordings revealed rhythmic changes of the membrane potential. Intracellular recordings from some of the DSCT units revealed changes in the membrane potentials similar to locomotor or scratch drive potentials originally described for lumbar motoneurons during fictive locomotion (Shefchyk & Jordan, 1985) and during fictive scratch (Perreault, 2002).
Figure 2. Rhythmic activity of dorsal spinocerebellar tract (DSCT) neurons during fictive locomotion and scratch.
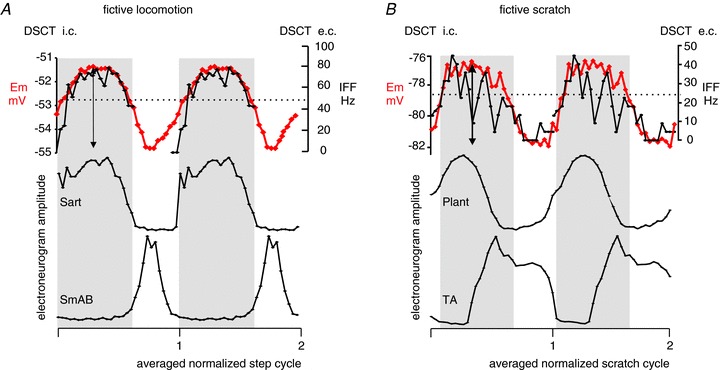
Overlay of averaged intracellular microelectrode recordings (i.c. thick red trace; membrane potential, Em) and the averaged instantaneous firing frequency plots (e.c., thin black trace, instantaneous firing frequency, IFF) outside two identified DSCT cells (one in A and one in B) recorded during fictive locomotion (A) and fictive scratch (B). The averages were based on the normalized fictive step cycle (A, 12 cycles for e.c. and eight cycles for i.c.) or scratch cycle (B, 25 for e.c. and seven for i.c.) after dividing the total cycle durations into 30 bins in which the amplitude of the instantaneous firing frequency (IFF, in Hz on the right axis) and the membrane potential (Em, in mV on the left axis) was measured. Dotted lines indicate the membrane potential before the evoked motor activity. Locomotor and scratch drive potentials (depolarization within grey boxes) had a profile that was similar to the IFF. The amplitude of the total change in the (double-headed arrows) was 3.5 mV in A and 5 mV in B. The electroneurogram amplitude traces are of rectified and filtered records after cycle-based averaging from the bout when intracellular records were collected. Activity in the hindlimb nerves innervating the sartorius (Sart), semimembranosus-anterior biceps (SmAB), plantaris (Plant) and tibialis anterior (TA) muscles is shown (the relative amplitudes are without calibration). Sart and TA are active during the flexion phase while SmAB and Plant are active during the extension phase of fictive locomotion and scratch. The soma of the DSCT cell illustrated in A was located in caudal L1 at a depth of 2.2 mm from the spinal cord surface, and in B it was in mid L4 at a depth of 2.1 mm.
In several animals both DSCT and VSCT cells displayed locomotor drive potentials during rhythmic activity (Fedirchuk et al. 2013). The cells examined had somas between L1 and L5 spinal segments and there was no relation between the phasic activity during fictive locomotion or scratch and the type of excitatory afferent input to a DSCT neuron. It is noteworthy that DSCT neurons active during the extension phase of fictive locomotion could be activated by group I afferents from either extensors or flexors. These findings were contrary to our expectations based on previous reports (Arshavsky et al. 1986). The apparent discrepancy between the studies in the 1960s and our recent report may be due to a sampling bias, as 11 DSCT cells were tested by Arshavsky and colleagues (Arshavskii 1972a) versus 81 cells by Fedirchuk et al. (2013) during fictive locomotion. In addition, the type of anaesthesia and level of decerebration could contribute to the differences. None the less, strong evidence for rhythmic input to several DSCT cells during two types of patterned activity has been presented in our study (Fedirchuk et al. 2013). In future studies it will be imperative to identify to which subpopulation the recorded DCST cell belongs, as it probably correlates with rhythmic activity (with input from the spinal CPG network).
Putative spinal and supraspinal sources of input to spinocerebellar tract neurons during fictive motor behaviours
On the basis of combined anatomical and electrophysiological investigations in anaesthetized cats, an in-depth discussion on the putative neuronal connectivity between INs mediating synaptic input from afferents and supraspinal sources has been presented by Jankowska and colleagues (Shakya Shrestha et al. 2012). Here we provide a brief summary of putative input to spinocerebellar tract cells from spinal INs and a few supraspinal projections in relation to rhythmic motor activity.
In anaesthetized preparations, evidence for excitatory input from INs to DSCT cells is scanty compared to that in VSCT cells (Mann, 1973). Excitation can be evoked in dh-DSCT cells by intraspinal stimulation within motor nuclei innervating the hamstrings and ankle dorsiflexors while the same stimulation evokes no excitation in CC-DSCT cells (Hammar et al. 2011). Thus premotor excitatory (and inhibitory) INs contact dh-DSCT cells but not CC-DSCT cells. The phase-related excitation of many DSCT cells was evident during fictive locomotion and scratch as can be seen in Fig. 2 by the depolarization of the membrane potential with respect to the ‘resting level’ (dotted lines) measured before the start of rhythmic motor activity (about 1.5 mV depolarization in the illustrated cells). Based on extracellular recordings, it was estimated that 24 of 33 DSCT and 13 of 14 VSCT cells with tonic activity had increased firing frequency during fictive locomotion, i.e. could have received excitatory input or been disinhibited during rhythmic motor activity. Intermediate zone INs with predominant group Ib afferent input are rhythmically active during fictive locomotion (Angel et al. 2005) and they may contact DSCT cells based on two key observations. One is that electrical stimulation of muscle afferents evokes disynaptic excitation in DSCT cells during fictive locomotion (Fedirchuk and Hultborn unpublished observations) and the other is that intracellularly labelled excitatory intermediate zone INs have been found to project to lamina V–VI (Bannatyne et al. 2009). Commissural intermediate zone excitatory INs may control only VSCT but not DSCT cells, as the projection areas of reconstructed commissural cells were mostly in lamina VIII–IX (Stecina et al. 2008; Bannatyne et al. 2009).
The membrane potential changes in Fig. 2 illustrate about 2 mV hyperpolarization during rhythmic activity compared to the ‘resting level’. Inhibitory IN populations that project to spinocerebellar tract cells include premotor INs such as reciprocal Ia inhibitory INs (Lindstrom & Schomburg, 1973), non-reciprocal Ib inhibitory INs (Lundberg & Weight, 1971; Jankowska et al. 2010) and group II inhibitory INs (Jankowska et al. 2010). No disynaptic inhibitory inputs from reciprocal Ia inhibitory INs or Renshaw cells were found in DSCT cells (Lindstrom & Takata, 1977; Hongo et al. 1983b) but all of the above listed populations of inhibitory INs contact VSCT cells – Renshaw cells (Gustafsson & Lindstrom, 1973); Ib inhibitory INs (Hongo et al. 1983a,b); and II inhibitory INs (Jankowska & Puczynska, 2008). The Ib inhibitory INs are thought to be inhibited during fictive locomotion (McCrea et al. 1995) and fictive scratch (Perreault et al. 1999) while the Ia inhibitory (Geertsen et al. 2011) and mid-lumbar intermediate zone group II inhibitory INs (Shefchyk et al. 1990; Stecina, 2006) are rhythmically active during both motor tasks. It is therefore plausible that during rhythmic motor activity, Ib/II inhibitory INs relay phasic inhibitory input to the DSCT cells, while Ia inhibitory INs are responsible for rhythmic hyperpolarization of VSCT cells. It has to be noted here that multiple lines of evidence suggests that the non-reciprocal Ib inhibitory and group II inhibitory INs may fall into the same functional subdivision of inhibitory INs (Jankowska & Edgley, 2010). Both inhibitory and excitatory group Ib/II intermediate zone INs that project ipsilaterally and receive input from the pyramidal tracts have been found to project to laminae VII–IX (Stecina et al. 2008) and thus they might exert relatively stronger control over VSCT than DSCT cells. In contrast, the bilaterally projecting inhibitory Ib/II intermediate zone INs have been found to project to laminae IV–VI (Bannatyne et al. 2009), implicating them more in the control of DSCT rather than VSCT cells. Therefore VSCT cells maybe more likely to receive inhibitory input from both Ia and Ib/II inhibitory INs located on the same side of the spinal cord while DSCT cells may be inhibited during rhythmic motor activity by Ib/II inhibitory INs projecting from the contralateral side. Another distinctive feature supporting this hypothesis is the fact that inhibition of dh-DSCT cells is seldom evoked monosynaptically by stimulation in motor nuclei while inhibition of VSCT cells in the same preparation is monosynaptic and it is partly evoked by pre-motor inhibitory INs (Krutki et al. 2011).
Does the central pattern generator input to dorsal spinocerebellar tract serve as an efference copy?
The work by Bosco, Poppele and colleagues (as reviewed in Bosco & Poppele, 2001) has demonstrated that even individual DSCT cells may carry complex proprioceptive information on whole limb kinematics during passive limb movements. With a convergence of this sensory information and an input from the spinal interneuronal network generating such movements (an efference copy) it seems natural to propose the possibility that these neurons (or some subdivision of the DSCT population) may have the ability to distinguish sensory inputs that are a consequence of the active locomotion (reafference) from those resulting from perturbations in the external world (exafference) (von Holst & Mittelstaedt, 1950; see also discussion in Hantman & Jessell, 2010). The original concept was formulated by Hermann von Helmholtz (1867) as he described the observation that the visual world seems to ‘move’ if the eye ball is passively moved, while it is perceived to stay still when actively moved by the eye muscles. He argued that an efference copy is generated by the motor system and used to determine the expected re-afferent sensory input caused by the movement, thus achieving perceptual stability. Most of the research on neuronal (network) mechanisms underlying the efference copy and its use in sensory perception has been performed in insects and other invertebrate preparations (see e.g. Crapse & Sommer, 2008). Important studies have also been performed using single unit recordings from cortical and spinal neurons in primates (Wurtz & Sommer, 2004; Sommer & Wurtz, 2008; Alstermark & Isa, 2012), and fMRI in humans (e.g. Blakemore et al. 1998; Christensen et al. 2007). We would argue, that further investigations, particularly on the CPG input to DSCT cells and their role in the gating of sensory afferent input, would enable a more detailed and mechanistic understanding of how an efference copy is generated and used in vertebrates. However, such an aim would need additional experimental work, including a comparison of the firing pattern of classified subdivisions of DSCT cells during fictive locomotion with unperturbed and perturbed real locomotion. This should be possible to achieve experimentally as Bosco et al. (2006) already has compared the firing patterns of individual DSCT neurons during passive (locomotor-like) hindlimb movements with actual locomotion in decerebrate cats. The comparison of DSCT activity during treadmill walking and passive step-like movements revealed that about half of the DSCT cell population investigated had different responses during these two conditions (Bosco et al. 2006). However, that study focused on possible differences in the afferent input in the two situations, thus mainly keeping the discussion to a sensory context. In some earlier work on passive limb movement that was similar to slow walking albeit in anaesthetized preparations it was found that both stimulation of serotonergic neurons in the brainstem (Bosco et al. 2003) as well as the stimulation of peripheral nerves (Bosco & Poppele, 2003) can alter the phasic responses of DSCT cells. Poppele and colleagues were the first to argue that DSCT activity during rhythmic movements cannot be explained simply based on limb kinematics. The relative weighting of sensory input from the various receptors affecting DSCT cells may account for some of the differences found in their activity during active and passive stepping. However, based on our recent findings (Fedirchuk et al. 2013) we feel that the most important question is now to define what is under the control of the CPG. Thus the novel idea proposed here is that the input from the CPG provides the means by which DSCT cells along with VSCT cells contribute to the differentiation between exafference and reafference signals in the cerebellum. A full identification of the recorded neurons to ascribe them as belonging to a specific subgroup (as discussed above) would be needed in future studies of this kind, as it is probable that DSCT subpopulations serve different functions.
Summary and concluding remarks
As reviewed above, it has been known since the first studies on feline DSCT neurons that they receive strong excitatory inputs from segmental afferents, and only minor inputs from descending pathways. This led to the dogma that the DSCT acted as an ascending relay of proprioceptive (and partly exteroceptive) sensory information. This idea seemed to be reinforced by the work of Arshavsky and colleagues (Arshavsky et al. 1972, 1978, 1986) suggesting that the phasic locomotor discharge of DSCT cells was only linked to the afferent feedback. The VSCT cells have always been considered to be more influenced by the activity of the spinal interneurons. The group of Arshavsky, and colleagues (Arshavskii et al. 1972a; Arshavsky et al. 1986) interpreted the discharge of the VSCT cells as reflecting an input ‘directly’ from the spinal CPG while Lundberg (1971) focused on results suggesting that the VSCT seemed to assess the transmission across last-order INs and that the excitation from descending and spinal motor centres would be a part of this process. Our recent finding (Fedirchuk et al. 2013) of a rhythmic central input directly activating DSCT cells, and gating the transmission of sensory information being conveyed through the DSCT represents a shift in our thinking of the spinocerebellar pathways. No doubt cerebellum needs information on all these aspects to facilitate compensation for unexpected perturbations during rhythmic motor activity. The segmental location of the DSCT and VSCT somas within the lumbar spinal cord and type of synaptic input has not yet proved to be a predictor of their rhythmic activity during patterned motor output (Fedirchuk et al. 2013), but more precise linking of subpopulations with rhythmic activity is required to clarify this issue. As a final conclusion, the information relayed by DSCT and VSCT cells is not as different as it has been previously considered.
Acknowledgments
The authors wish to thank Maria Setterbom for preparation of the figures. B. Fedirchuk was a Medical Research Council of Canada Postdoctoral Fellowship recipient, K. Stecina was supported by the EU FP6 MarieCurie Actions Intra-European Fellowship and the Danish Agency for Science Technology and Innovation.
Glossary
- CC
Clarke's column cell
- CPG
central pattern generator
- dh
dorsal horn
- DSCT
dorsal spinocerebellar tract
- Em
membrane potential
- IN
interneuron
- L
lumbar
- MLF
medial longitudinal fasciculus
- Pl
plantaris
- SB
spinal border cell
- SmAB
semimembranosus and anterior biceps
- VGlut1/2
vesicular glutamate transporter type1/2
- VSCT
ventral spinocerebellar tract
References
- Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci. 2012;35:559–578. doi: 10.1146/annurev-neuro-062111-150527. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Angel MJ, Jankowska E, McCrea DA. Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat. J Physiol. 2005;563:597–610. doi: 10.1113/jphysiol.2004.076034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama M, Hongo T, Kudo N. An uncrossed ascending tract originating from below Clarke's column and conveying group I impulses from the hindlimb muscles in the cat. Brain Res. 1973;62:237–241. doi: 10.1016/0006-8993(73)90634-3. [DOI] [PubMed] [Google Scholar]
- Arshavskii II, Berkinblit MB, Gel’fand IM, Orlovskii GN, Fukson OI. Activity of ventral spino-cerebellar tract neurons during locomotion of cats with deafferented hindlimbs. Biofizika. 1972a;17:1112–1118. (translated in Biophysics 1120:1762–1764) [PubMed] [Google Scholar]
- Arshavskii II, Berkinblit MV, Gel’fand IM, Orlovskii GN, Fukson OI. Activity of neurons of the dorsal spinocerebellar tract during locomotion. Biofizika. 1972b;17:487–494. (translated in Biophysics 417:508–514) [PubMed] [Google Scholar]
- Arshavsky YI, Berkinblit MB, Fukson OI, Gelfand IM, Orlovsky GN. Recordings of neurons of the dorsal spinocerebellar tract during evoked locomotion. Brain Res. 1972;43:272–275. doi: 10.1016/0006-8993(72)90295-8. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res. 1978;151:493–506. doi: 10.1016/0006-8993(78)91082-x. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovskii GN. Cerebellum and Rhythmical Movements. Berlin: Springer-Verlag; 1986. [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol. 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci. 1998;1:635–640. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Bloedel JR, Courville J. Cerebellar Afferent Systems Compr Physiol. The Handbook of Physiology. Wiley Online Library; 2011. pp. 735–829. [Google Scholar]
- Bosco G, Poppele RE. Broad directional tuning in spinal projections to the cerebellum. J Neurophysiol. 1993;70:863–866. doi: 10.1152/jn.1993.70.2.863. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 2001;81:539–568. doi: 10.1152/physrev.2001.81.2.539. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. Modulation of dorsal spinocerebellar responses to limb movement. II. Effect of sensory input. J Neurophysiol. 2003;90:3372–3383. doi: 10.1152/jn.00204.2003. [DOI] [PubMed] [Google Scholar]
- Bosco G, Rankin A, Poppele RE. Modulation of dorsal spinocerebellar responses to limb movement. I. Effect of serotonin. J Neurophysiol. 2003;90:3361–3371. doi: 10.1152/jn.00203.2003. [DOI] [PubMed] [Google Scholar]
- Bosco G, Eian J, Poppele RE. Phase-specific sensory representations in spinocerebellar activity during stepping: evidence for a hybrid kinematic/kinetic framework. Exp Brain Res. 2006;175:83–96. doi: 10.1007/s00221-006-0530-7. [DOI] [PubMed] [Google Scholar]
- Burke R, Lundberg A, Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res. 1971;12:283–294. doi: 10.1007/BF00237921. [DOI] [PubMed] [Google Scholar]
- Christensen MS, Lundbye-Jensen J, Geertsen SS, Petersen TH, Paulson OB, Nielsen JB. Premotor cortex modulates somatosensory cortex during voluntary movements without proprioceptive feedback. Nat Neurosci. 2007;10:417–419. doi: 10.1038/nn1873. [DOI] [PubMed] [Google Scholar]
- Cooper S, Sherrington CS. Grower's tract and spinal border cells. Brain. 1940;63:123–134. [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Beau A, Shakya Shrestha S, Bannatyne BA, Jalicy SM, Linnen S, Maxwell DJ. Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience. 2012;227:67–79. doi: 10.1016/j.neuroscience.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Hubbard JI, Oscarsson O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol. 1961;158:486–516. doi: 10.1113/jphysiol.1961.sp006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Gallimore CM. The morphology and projections of dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988;397:99–111. doi: 10.1113/jphysiol.1988.sp016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988;397:81–97. doi: 10.1113/jphysiol.1988.sp016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Stecina K, Kristensen KK, Zhang M, Meehan CF, Bennett DJ, Hultborn H. Rhythmic activity of feline dorsal and ventral spinocerebellar tract neurons during fictive motor actions. J Neurophysiol. 2013;109:375–388. doi: 10.1152/jn.00649.2012. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, Stecina K, Meehan CF, Nielsen JB, Hultborn H. Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. J Physiol. 2011;589:119–134. doi: 10.1113/jphysiol.2010.199125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. Spinal course and somatotopically localized termination of the spinocerebellar tracts. An experimental study in the cat. Acta Physiol Scand Suppl. 1962;56:1–61. [PubMed] [Google Scholar]
- Grant G, Xu Q. Routes of entry into the cerebellum of spinocerebellar axons from the lower part of the spinal cord. An experimental anatomical study in the cat. Exp Brain Res. 1988;72:543–561. doi: 10.1007/BF00250600. [DOI] [PubMed] [Google Scholar]
- Grant G, Wiksten B, Berkley KJ, Aldskogius H. The location of cerebellar-projecting neurons within the lumbosacral spinal cord in the cat. An anatomical study with HRP and retrograde chromatolysis. J Comp Neurol. 1982;204:336–348. doi: 10.1002/cne.902040405. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Lindstrom S. Recurrent control from motor axon collaterals of Ia inhibitory pathways to ventral spinocerebellar tract neurones. Acta Physiol Scand. 1973;89:457–481. doi: 10.1111/j.1748-1716.1973.tb05541.x. [DOI] [PubMed] [Google Scholar]
- Hammar I, Krutki P, Drzymala-Celichowska H, Nilsson E, Jankowska E. A trans-spinal loop between neurones in the reticular formation and in the cerebellum. J Physiol. 2011;589:653–665. doi: 10.1113/jphysiol.2010.201178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci. 2010;13:1233–1239. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Helmholtz H. Handbuch der Physiologischen Optik. Leipzig: Leopold Voss; 1867. [Google Scholar]
- Hirai N, Nakashima H, Tanaka Y. Activity of dorsal spinocerebellar tract neurones in the thoracic spinal cord in relation to respiratory movement. Brain Res. 1988;475:385–388. doi: 10.1016/0006-8993(88)90631-2. [DOI] [PubMed] [Google Scholar]
- Holmqvist B, Lundberg A, Oscarsson O. Functional organization of the dorsal spino-cerebellar tract in the cat. V. Further experiments on convergence of excitatory and inhibitory actions. Acta Physiol Scand. 1956;38:76–90. doi: 10.1111/j.1748-1716.1957.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol. 1983a;342:145–159. doi: 10.1113/jphysiol.1983.sp014844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983b;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J. Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci. 2010;32:881–893. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Puczynska A. Interneuronal activity in reflex pathways from group II muscle afferents is monitored by dorsal spinocerebellar tract neurons in the cat. J Neurosci. 2008;28:3615–3622. doi: 10.1523/JNEUROSCI.0466-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Hammar I. Collateral actions of premotor interneurons on ventral spinocerebellar tract neurons in the cat. J Neurophysiol. 2010;104:1872–1883. doi: 10.1152/jn.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlat K, Djouhri L. Differential input to dorsal horn dorsal spinocerebellar tract neurons in mid- and low-lumbar segments from upper cervical spinal cord in the cat. Neurosci Res. 2012;72:227–235. doi: 10.1016/j.neures.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Larsell O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953;99:135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Lindstrom S, Schomburg ED. Recurrent inhibition from motor axon collaterals of ventral spinocerebellar tract neurones. Acta Physiol Scand. 1973;88:505–515. doi: 10.1111/j.1748-1716.1973.tb05479.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom S, Takata M. Lack of recurrent depression from motor axon collaterals of IaIPSPs in dorsal spinocerebeller tract neurones. Brain Res. 1977;129:158–161. doi: 10.1016/0006-8993(77)90979-9. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12:317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Oscarsson O. Functional organization of the dorsal spino-cerebellar tract in the cat. VII. Identification of units by antidromic activation from the cerebellar cortex with recognition of five functional subdivisions. Acta Physiol Scand. 1960;50:356–374. doi: 10.1111/j.1748-1716.1960.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Oscarsson O. Two ascending spinal pathways in the ventral part of the cord. Acta Physiol Scand. 1962;54:270–286. doi: 10.1111/j.1748-1716.1962.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12:295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- Mann MD. Clarke's column and the dorsal spinocerebellar tract: a review. Brain Behav Evol. 1973;7:34–83. doi: 10.1159/000124397. [DOI] [PubMed] [Google Scholar]
- Matsushita M. Anatomical organization of the spinocerebellar system, as studied by the HRP method. Acta Morphol Hung. 1983;31:73–86. [PubMed] [Google Scholar]
- Matsushita M, Hosoya Y. Spinocerebellar projections to lobules III to V of the anterior lobe in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1982;208:127–143. doi: 10.1002/cne.902080203. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Spinal projections to the cerebellar nuclei in the cat. Exp Brain Res. 1970;10:501–511. doi: 10.1007/BF00234266. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Spinocerebellar projections to the vermis of the posterior lobe and the paramedian lobule in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1980;192:143–162. doi: 10.1002/cne.901920110. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Okado N. Cells of origin of brainstem afferents to lobules I and II of the cerebellar anterior lobe in the cat. Neuroscience. 1981a;6:2393–2405. doi: 10.1016/0306-4522(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Okado N. Spinocerebellar projections to lobules I and II of the anterior lobe in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1981b;197:411–424. doi: 10.1002/cne.901970305. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hosoya Y, Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;184:81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsal D, Perret C, Cabelguen JM. Comparison between ventral spinocerebellar and rubrospinal activities during locomotion in the cat. Behav Brain Res. 1988;28:159–162. doi: 10.1016/0166-4328(88)90092-7. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of the spino- and cuneocerebellar tracts. Physiol Rev. 1965;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- Perreault M-C. Motoneurons have different membrane resistance during fictive scratching and weight support. J Neurosci. 2002;22:8259–8265. doi: 10.1523/JNEUROSCI.22-18-08259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault MC, Enriquez-Denton M, Hultborn H. Proprioceptive control of extensor activity during fictive scratching and weight support compared to fictive locomotion. J Neurosci. 1999;19:10966–10976. doi: 10.1523/JNEUROSCI.19-24-10966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatigorskii BY. Background activity of neurons of the dorsal spinocerebellar tract. Neirofiziologiya. 1970;2:26–34. [Google Scholar]
- Shakya Shrestha S, Bannatyne BA, Jankowska E, Hammar I, Nilsson E, Maxwell DJ. Inhibitory inputs to four types of spinocerebellar tract neurons in the cat spinal cord. Neuroscience. 2012;226:253–269. doi: 10.1016/j.neuroscience.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, Jordan L. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, McCrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Exp Brain Res. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Bannatyne BA, Jankowska E, Hammar I, Nilsson E, Maxwell DJ. Excitatory inputs to four types of spinocerebellar tract neurons in the cat and the rat thoraco-lumbar spinal cord. J Physiol. 2012;590:1737–1755. doi: 10.1113/jphysiol.2011.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja PJ, Fragoso MC, Cairns BE, Oka JI. Dorsal spinocerebellar tract neuronal activity in the intact chronic cat. J Neurosci Methods. 1995;60:227–239. doi: 10.1016/0165-0270(95)00023-n. [DOI] [PubMed] [Google Scholar]
- Soja PJ, Fragoso MC, Cairns BE, Jia WG. Dorsal spinocerebellar tract neurons in the chronic intact cat during wakefulness and sleep: analysis of spontaneous spike activity. J Neurosci. 1996;16:1260–1272. doi: 10.1523/JNEUROSCI.16-03-01260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja PJ, Taepavarapruk N, Pang W, Cairns BE, McErlane SA, Fragoso MC. Transmission through the dorsal spinocerebellar and spinoreticular tracts: wakefulness versus thiopental anesthesia. Anesthesiology. 2002;97:1178–1188. doi: 10.1097/00000542-200211000-00023. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K. 2006. p. 167. Preferential suppression of transmission and candidate neurones mediating reflex actions from muscle group II afferents during fictive motor activity-Electronic Thesis and Dissertations Collections (public access URL) Ph.D. thesis in the Department of Physiology. Manitoba, Winnipeg.
- Stecina K, Jankowska E, Cabaj A, Pettersson LG, Bannatyne BA, Maxwell DJ. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol. 2008;586:557–574. doi: 10.1113/jphysiol.2007.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentagothai J, Albert A. The synaptology of Clarke's column. Acta Morphol Acad Sci Hung. 1955;5:43–51. [PubMed] [Google Scholar]
- Tapper DN, Mann MD, Brown PB, Cogdell B. Cells of origin of the cutaneous subdivision of the dorsal spinocerebellar tract. Brain Res. 1975;85:59–63. doi: 10.1016/0006-8993(75)91005-7. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Selected Papers of Erich von Holst: The Behavioural Physiology of Animals and Man. London: Methuen; 1950. The reafference principle: Interaction between the central nervous system and the periphery; pp. 39–73. [Google Scholar]
- Walmsley B. Central synaptic transmission: studies at the connection between primary afferent fibres and dorsal spinocerebellar tract (DSCT) neurones in Clarke's column of the spinal cord. Prog Neurobiol. 1991;36:391–423. doi: 10.1016/0301-0082(91)90017-u. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA. Identifying corollary discharges for movement in the primate brain. Prog Brain Res. 2004;144:47–60. doi: 10.1016/S0079-6123(03)14403-2. [DOI] [PubMed] [Google Scholar]
- Xu Q, Grant G. Collateral projections of neurons from the lower part of the spinal cord to anterior and posterior cerebellar termination areas. A retrograde fluorescent double labeling study in the cat. Exp Brain Res. 1988;72:562–576. doi: 10.1007/BF00250601. [DOI] [PubMed] [Google Scholar]
- Xu Q, Grant G. Course of spinocerebellar axons in the ventral and lateral funiculi of the spinal cord with projections to the anterior lobe: an experimental anatomical study in the cat with retrograde tracing techniques. J Comp Neurol. 1994;345:288–302. doi: 10.1002/cne.903450210. [DOI] [PubMed] [Google Scholar]
- Xu Q, Grant G. Course of spinocerebellar axons in the ventral and lateral funiculi of the spinal cord with projections to the posterior cerebellar termination area: an experimental anatomical study in the cat, using a retrograde tracing technique. Exp Brain Res. 2005;162:250–256. doi: 10.1007/s00221-004-2132-6. [DOI] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Hultborn H, Sukiasyan N. The distribution of calcium channel Cav1.3 in the central nervous system and its functions in relation to motor control. In: Figgins MR, editor. Calcium Channels: properties, functions and regulation. Hauppauge, NY, USA: Nova Science Publishers; 2012. pp. 1–48. [Google Scholar]
- Zytnicki D, Lafleur J, Kouchtir N, Perrier JF. Heterogeneity of contraction-induced effects in neurons of the cat dorsal spinocerebellar tract. J Physiol. 1995;487:761–772. doi: 10.1113/jphysiol.1995.sp020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Moller M, Broman J, Sukiasyan N, Wienecke J, Hultborn H. Expression of calcium channel CaV1.3 in cat spinal cord: light and electron microscopic immunohistochemical study. J Comp Neurol. 2008;507:1109–1127. doi: 10.1002/cne.21595. [DOI] [PubMed] [Google Scholar]


