Abstract
A subpopulation of dorsal root ganglion (DRG) neurons are intimately attached in pairs and separated solely by thin satellite glial cell membrane septa. Stimulation of one neuron leads to transglial activation of its pair by a bi-, purinergic/glutamatergic synaptic pathway, a transmission mechanism that we term sandwich synapse (SS) transmission. Release of ATP from the stimulated neuron can be attributed to a classical mechanism involving Ca2+ entry via voltage-gated calcium channels (CaV) but via an unknown channel type. Specific blockers and toxins ruled out CaV1, 2.1 and 2.2. Transmission was, however, blocked by a moderate depolarization (−50 mV) or low-concentration Ni2+ (0.1 mm). Transmission persisted using a voltage pulse to −40 mV from a holding potential of −80 mV, confirming the involvement of a low voltage-activated channel type and limiting the candidate channel type to either CaV3.2 or a subpopulation of inactivation- and Ni2+-sensitive CaV2.3 channels. Resistance of the neuron calcium current and SS transmission to SNX482 argue against the latter. Hence, we conclude that inter-somatic transmission at the DRG SS is gated by CaV3.2 type calcium channels. The use of CaV3 family channels to gate transmission has important implications for the biological function of the DRG SS as information transfer would be predicted to occur not only in response to action potentials but also to sub-threshold membrane voltage oscillations. Thus, the SS synapse may serve as a homeostatic signalling mechanism between select neurons in the DRG and could play a role in abnormal sensation such as neuropathic pain.
Key points
Sensory neurons in dorsal root ganglia (DRG) lack direct inter-somatic synaptic contacts but a subpopulation can communicate with their immediate neighbours via transglial, neuron–glial cell–neuron ‘sandwich synapses’.
We used gently dissociated chick DRG to explore the properties and identity of the voltage sensitive calcium channel responsible for gating transmitter (ATP) release at the neuron-to-glial cell synapse.
A combined pharmacological and biophysical characterization identified the T type, CaV3.2 calcium channel.
The low voltage-activated and inactivation-sensitive properties of CaV3.2 suggest that sandwich synapse transmission is gated not only by action potentials but also by sub-threshold membrane depolarizations.
CaV3.2 modulating agents are of interest as anaesthetics, raising the possibility that sandwich synapse transmission plays a role in the aetiology of DRG-derived abnormal sensation and pain.
Introduction
The dorsal root ganglion (DRG) houses the neuronal somata (NS) that serve the peripheral somatic sensory system. These NS are isolated by satellite glial cell (SGC) sheathes and extensive investigations have failed to detect significant inter-somatal synaptic contacts, either via axonal terminals or direct NS–NS contact (Shinder et al. 1998, 1999). DRG NS are, however, capable of calcium (Ca2+)-gated transmitter release in the form of activity-dependent secretion of ATP, which can activate their ensheathing SGCs via purinergic ligand-gated receptors (Zhang et al. 2007; Rozanski et al. 2013a,b).
A significant number of NS are ensheathed by a common SGC capsule and are separated from their neighbours by the membrane sheet from a single intervening glial cell (Pannese et al. 1991; Shinder et al. 1998, 1999; Rozanski et al. 2012). We recently isolated these NS pairs and tested for interneuronal communication by double voltage clamp (Rozanski et al. 2012). Stimulation of one neuron (designated NScis) with a rapid train of impulse-like depolarizing pulses led to a delayed, long-lasting and noisy response in its passive synaptic partner (NStrans). We also noted that a later second stimulus train invariably evoked a facilitated response. Further analysis has generated a reasonably complete description of the transmission pathway. Thus, ATP is released from NScis by a voltage-gated calcium channel (CaV)-dependent mechanism, presumably at classical presynaptic-like release sites, and activates P2Y2 receptors on the glial cell, triggering Ca2+ release from intracellular stores (Rozanski et al. 2013a). Elevated glial cytoplasmic Ca2+ gates glutamate release (by an unknown mechanism) to activate N-methyl-d-aspartate (NMDA) receptors on the bordering neuron (Rozanski et al. 2013b) to complete the transmission pathway. We have termed this novel neuron–glial cell–neuron transmission structure a sandwich synapse (SS) and hypothesize that it could represent a common mechanism of transmission in the nervous system (Rozanski et al. 2012, 2013a, b). To further understand its signalling pathway and to elucidate clues as to the functional role of SS transmission in the DRG, we set out to identify the calcium channel type that gates neurotransmitter secretion from one NS, using the response in the target neuron as a monitor for Ca2+-evoked transmitter release.
We first tested for the involvement of CaV1 family, CaV2.1 and CaV2.2 channel types using established toxin or drug blockers. CaV2.3 and CaV3 family channels were differentiated by biophysical and pharmacological profiling.
Methods
Preparations
Experiments were carried out on L3–5 lumbar DRG dissected from day 14 or 15 chick embryos. All experiments were terminal and animals were killed using approved procedures.
Cell preparation methods
The standard ‘dispase’ dissociation and incubation method has been described, originally for chick ciliary ganglia (Stanley & Goping, 1991; Stanley, 1991, 1993; Sun & Stanley, 1994; Li et al. 2004) and also DRG (Chan & Stanley, 2003). After incubation, the ganglia were washed with warmed and incubator-equilibrated MEM and were then gently separated by trituration through a standard 200 μl plastic pipette tip.
Electrophysiology and drug treatments
Whole-cell patch clamp current recordings were carried out on dissociated NS that formed SS contacts using standard methods as described (Chan & Stanley, 2003; Chan et al. 2007) and as adapted for simultaneous, two-electrode recording (Gentile & Stanley, 2005). The solutions and data acquisition were as previously described (Rozanski et al. 2012, 2013a, b). Drugs were added to the bath before or during experiments. Current–voltage and stimulus trial current traces were filtered at approximately 1 kHz and digitized at 50 and 200 μs per point, respectively. ω-Agatoxin IIIA (ω-Aga-IIIA) was purified to homogeneity by reversed-phase high-performance liquid chromatography as described previously (Mintz et al. 1991). Aliquots were Speed-Vac concentrated to dryness, taken up in distilled water as stock solutions, and added to the bathing medium to achieve a final concentration of 50 nm for block of high voltage activated (HVA) Ca2+ channel currents.
Quantification of data and statistical analysis
Experiments with high baseline noise or tonic activity (>2.5 pC), evidence of patch seal breakdown, or noise that was obviously unrelated to the recording were omitted from analyses. For SS transmission recordings, the midpoint of a quiet region of the trace was used to set the zero current level and the stimulus-evoked current response was integrated over a 1 min period following the end of the stimulation train. Charge transfer values are reported in pC (mean ± SEM). Facilitation analysis was as previously described (Rozanski et al. 2012, 2013b). Experimental data values were compared to control values reported previously (Rozanski et al. 2012). Differences between means were tested using a Student's t-test (OriginPro 8.5.1; OriginLab Corp., Northampton, MA, USA) and P < 0.05 was considered significant. Current–voltage relations were obtained from recordings (Clampfit 10.2; MDS Analytical Technologies, Sunnyvale, CA, USA) and expressed as current density (pA pF−1) or as a proportion of the peak current from each experiment, as indicated.
Results
Block of soma–soma transmission by cadmium
We previously showed that under double voltage clamp, stimulation of one neuron, NScis, with a train (100 Hz for 5 s) of short depolarizing pulses (2 ms, +40 mV) to trigger action potential-like inward Ca2+ tail currents resulted in a delayed and prolonged noisy inward current in the target partner neuron, NStrans, in a large majority of SS contacts (Rozanski et al. 2012). To test if voltage-sensitive calcium channels were involved in the transmission pathway we added extracellular Cd2+ (0.2 mm) to the bath. This blocked SS transmission and eliminated inward Ca2+ currents (ICa) in NScis (Rozanski et al. 2012), consistent with the hypothesis that SS transmission is initiated by excitation–secretion coupling via gating of CaV channels. Involvement of Ca2+ influx into NScis was also indicated by block of the SS transmission response in NStrans the fast Ca2+ scavenger BAPTA (Fig. 1A) without inhibiting NScis ICa (Fig. 1B). Thus, charge transfer in NStrans after stimulation of NScis with BAPTA (10 mm) present in the patch electrode solution was significantly different from controls (1.5 ± 21 pA, n= 3 and 240.7 ± 42.9 pA, n= 40; Pt test < 0.01, respectively). Our objective was to identify the CaV type responsible for excitation–secretion coupling at the DRG SS by modifying the NScis ICa while monitoring transglial transmission in NStrans.
Figure 1. Block of SS transmission by a rapid Ca2+ scavenger.
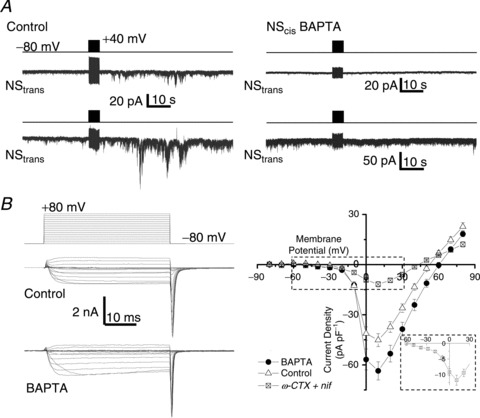
A, two paired NS were patch clamped simultaneously and both were held at −80 mV. A stimulus train (2 ms pulse to +40 mV at 100 Hz for 5 s, as indicated by upper protocol trace for each trial) was delivered to one neuron, designated NScis (not shown but as in Rozanski et al. 2012) with (right panel) or without (left panel) intracellular BAPTA while recording from its passive neuron pair, NStrans. B, Ca2+ current traces recorded from NScis evoked by a family of depolarizing voltage pulses, as indicated in the left upper panel, in the absence (left middle panel) and presence (left lower panel) of intracellular BAPTA (10 mm). The right panel plots steady state (mean amplitude of the last approximately 10 ms of the depolarizing pulse) current amplitudes against the pulse voltage for control (n= 14) and BAPTA (n= 15) neurons. The mean current-to-voltage relationship is also shown for NScis neurons in the presence of nifedipine (nif) and ω-conotoxin GIVA (ωCTX; both 2 μm, n= 11; current traces are shown in Fig. 2A). The inset is an expanded view of the boxed region and shows an LVA current shoulder.
Soma–soma transmission does not require CaV1, CaV2.1 or CaV2.2 channels
By analogy with chemical transmission at typical synapses, we expected that the neuronal release apparatus would be gated by a standard HVA CaV such as CaV1, CaV2.1 or CaV2.2. As shown previously in chick DRG neurons (Chan & Stanley, 2003), somatic ICa was markedly reduced by a cocktail of nifedipine (2 μm) and ω-conotoxin GIVA (2 μm; Fig. 2A; current to voltage relation Fig. 1B right panel) to block CaV1.x and CaV2.2, respectively. However, addition of nifedipine (n= 4, Fig. 2B) or ω-conotoxin GVIA (n= 4, Fig. 2C) to the bath had no obvious effect on SS transmission, either singly or as cocktails (Fig. 2D). Likewise, no effect was observed with a blocker for CaV2.1, ω-agatoxin IVA (0.2 μM; data not shown). Charge transfer in the 1 min period following the end of the stimulus train was not significantly different in the presence of nifedipine and ω-conotoxin GVIA compared to controls (282.5 ± 87.8 pA, n= 7 and 240.7 ± 42.9 pA, n= 40; Pt test > 0.1, respectively). Inter-trial facilitation, expressed as a percentage change in charge transfer, normalizing to the mean response to the first trial, in the presence of nifedipine and ω-conotoxin GVIA was also not significantly different from controls (250 ± 59%, n= 7 and 292 ± 64%, n= 13; Pt test > 0.1, respectively). These results suggest that the identity of the NScis CaV must be either the one remaining HVA type, CaV2.3, or a member of the CaV3 family of low voltage-activated (LVA) channels. For simplicity we term the Ca2+ current that remains in the presence of a nifedipine/ω-agatoxin IVA/ω-conotoxin GVIA cocktail, and is responsible for SS transmission, the ‘block-resistant ICa’.
Figure 2. CaV1 and CaV2.2.
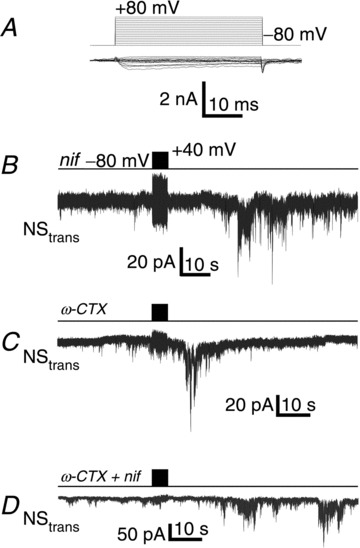
A, current trace family for voltage pulses as in Fig. 1B (left panel) in the presence of nifedipine (2 μm) and ω-conotoxin GVIA (2 μm). Mean steady state current amplitudes are plotted against voltage in Fig. 1B (right panel). B, current trace recorded from NStrans while stimulating NScis in the presence of nifedipine (nif) (2 μm) in the bath. C, as in B but with ω-conotoxin GVIA (ω-CTX) (2 μm). D, as in B but with both ω-conotoxin GVIA and nifedipine (ω-CTX + nif).
Effect of nickel on SS transmission
We tested the effect of Ni2+ (0.1 mm) both on naïve neuron pairs and by adding the ion in the interval between two stimulus trials. SS transmission was not observed in the pre-treated SS (Fig. 3A; 84.9 ± 42.8 pA, n= 3 and 240.7 ± 42.9 pA, n= 40; Pt test < 0.05, respectively; control data from Rozanski et al. 2012). The reliable pronounced facilitation observed following the second of two stimulus trials (Rozanski et al. 2012) can serve as a useful assay for transmission blockers (Rozanski et al. 2012, 2013a). This facilitation was virtually eliminated when Ni2+ was added after the first trial (control: 292 ± 64%, n= 13; Ni2+ 34.2 ± 6.9%, n=3; Pt test < 0.01, respectively; control data from Rozanski et al. 2012). The effect of Ni2+ was specific, as we only observed a small reduction in the NS HVA Ca2+ current (31.2 ± 7.0%; Fig. 3B–D) suggesting that the divalent blocks a specific CaV type associated with the transmitter release mechanism. Subtypes of both CaV3 and CaV2.3 channels have been reported to be sensitive to Ni2+. The observed reductions in ICa amplitude for both LVA and HVA current in our data would be consistent with this suggestion. Further analysis was directed to compare and contrast the role of these two channel types.
Figure 3. SS transmission is blocked by Ni2+.
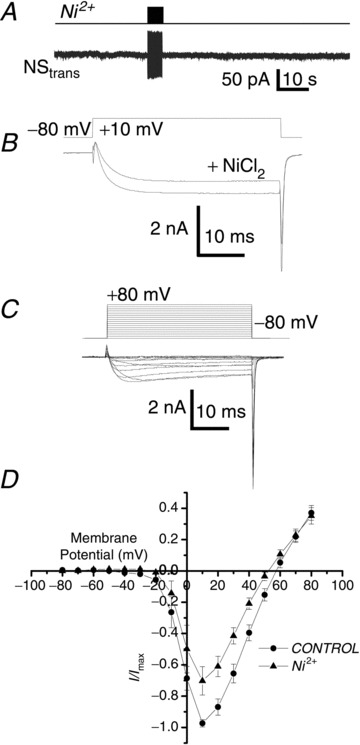
A, recording from NStrans during a stimulus train protocol to NScis (upper panel) as in Fig. 1A but in the presence of extracellular Ni2+ (0.1 mm). B, addition of Ni2+ to the bath resulted in a moderate reduction in the inward current evoked by a depolarization beyond HVA calcium channel threshold (+10 mV). C, family of Ca2+ currents evoked by pulse depolarizations in the presence of extracellular Ni2+. D, relationship of steady-state Ca2+ current amplitude to voltage plotted for control NScis recordings and in the presence of Ni2+ (n= 8).
Effect of membrane depolarization
Both CaV3 and CaV2.3 channels inactivate at relatively hyperpolarized membrane potentials and we used this as an independent test of the findings with Ni2+. We characterized the inactivation profile of the block-resistant ICa by steady-state inactivation analysis. The inactivation profile was fit by the sum of two Boltzman distributions (Fig. 4A), demonstrating both hyperpolarized and depolarized-inactivating CaV fractions. The latter can be attributed to an inactivation-resistant R type channel but identifying the former, hyperpolarized-inactivating current was more complex. The inactivation profile of LVA current alone was tested using a similar protocol but with test steps to −40 mV (Fig. 4B, left panel). This could be fit with a single Boltzmann relation with a V0.5 of ∼−53 mV. We next tested if SS transmission would persist when NScis was held at −50 mV holding potential–at which point most of the inactivation-sensitive current was blocked, based on the inactivation relationship in Fig. 4B (right panel). Transmission across the SS was markedly suppressed (39.2 ± 15.9 pA, n= 5 and 240.7 ± 42.9 pA, n= 40; Pt test < 0.01; Fig. 4C). We therefore concluded that the CaV type that gates ATP release is sensitive to voltage-dependent inactivation. These results were consistent with the findings with Ni2+ and the involvement of CaV3 or CaV2.3 channel types.
Figure 4. SS transmission is blocked by NScis membrane depolarization.
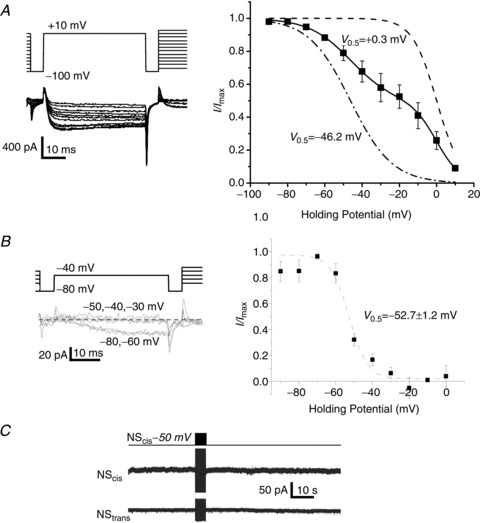
A, steady-state inactivation analysis of NScis HVA and LVA calcium current in the presence of nifedipine (2 μm) and ω-conotoxin GIVA (2 μm). Left panel: the currents were evoked by a voltage step to +10 mV after briefly returning the membrane potential to −100 mV prior to a step increment of +10 mV for 5 s. Right panel: plot of steady-state current amplitude against the holding potential normalized to maximum current (n= 4). The plot exhibited two sigmoidal curves and was fit by the sum of two Boltzmann relations with V0.5 values as indicated. The individual fitted Boltzmann relations were normalized to maximum current and plotted for display. B, as in A, but with a test pulse to –40 mV to examine LVA Ca2+ current alone. The normalized data were fit by a single Boltzmann relation with the indicated V0.5 value (n= 3). C, NStrans recording during a stimulus train to NScis, as in Fig. 1A but with NScis held at –50 mV to inactivate LVA current (see B).
Distinguishing between CaV3 and CaV2.3 channel types
The block-resistant Ca2+ current fraction is difficult to resolve with respect to channel type primarily because CaV2.3 can exhibit a broad range of properties that overlap those of CaV3 channels. For example, Ni2+ still blocked a large fraction of the block-resistant ICa (data not shown) when the membrane potential was held at −50 mV to inactivate the LVA current. This current fraction is probably due to a Ni2+-sensitive HVA CaV2.3 channel subtype but this was not explored further. Lacking a selective and reliable blocker for either channel type we used a combination of activation properties and sensitivity to two agents that block CaV2.3 currents, ω-AgaIIIA and SNX482, to differentiate between these possibilities.
At a holding potential of −80 mV, block-resistant ICa exhibited both HVA and LVA current components, with the latter observed as a small shoulder on the current-to-voltage (I–V) relationship between −50 and −20 mV (Fig. 1B inset; Fig. 5A and B). We have established that the DRG neuron LVA current includes CaV3.2 channels, based on single channel analysis and the detection of CaV3.2 mRNA (Weber et al. 2010 and Supplemental Data), providing evidence for the presence of the sole Ni2+-sensitive member of the CaV3 family. We could not presume that these channels were responsible for ATP secretion, however, due to the overlapping activation/inactivation properties. Thus, although most of our evidence was consistent with the hypothesis that SS transmission is gated by CaV3.2, gating by a subpopulation of CaV2.3 remained a viable alternative possibility.
Figure 5. Role of LVA channels in SS transmission.
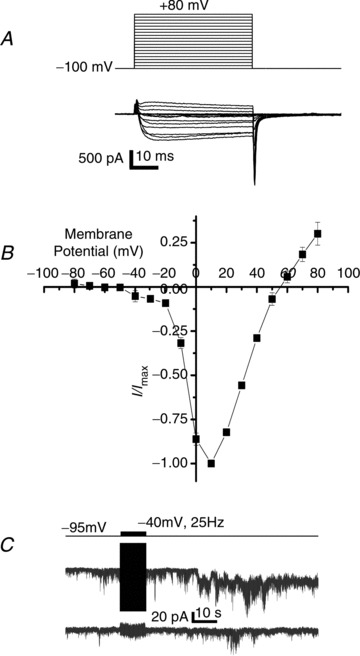
A, current traces (bottom panel) evoked in NScis by depolarizing step potentials (top panel) in the presence of nifedipine (2 μm) and ω-conotoxin GIVA (2 μm). B, current to voltage plot for n= 4 recordings as in A. C, trace recorded from NStrans held at −80 mV during a 25 Hz, −40 mV amplitude and 20 ms duration pulse train delivered to NScis (upper protocol trace).
We reasoned that if CaV3 are responsible for gating transmitter release then it should be possible to observe transmission with pulses that evoke CaV3 current but are sub-threshold for HVA channels. For this experiment we held NScis at −95 mV and pulses were given to −40 mV, below the CaV2.3 channel threshold (see Discussion). Since CaV3 channels have a slow activation time constant at this negative pulse potential (Randall & Tsien, 1997) we also increased the duration of each pulse to 20 ms. Robust transmission was observed with this protocol (Fig. 5C), establishing a key parameter in the identification of the responsible calcium channel type.
Distinguishing R- and T-type currents requires a multi-drug profile analysis as currently there are no antagonists that reliably block one or other type. The spider toxin ω-AgaIIIA blocks a number of HVA currents, including R-type (CaV2.3), without effects on T-type (CaV3) currents (Mintz et al. 1991) and caused a substantial reduction in the NS Ca2+ current (Fig. 6A and B). It did not, however, block transmission through the SS as assessed by the facilitation test: the response to a second stimulus trial given after addition of ω-AgaIIIA (50 nm) to the bath exhibited similar current enhancement as in controls (196 ± 69%, n= 5 and 292 ± 64%, n= 13; Pt test > 0.1, respectively, control data from Rozanski et al. 2012). Thus, we failed to block transmission with a toxin that is known to inhibit at least a part of the CaV2.3 channel population. However, an LVA/Ni2+-sensitive CaV2.3 channel subtype (Tottene et al. 2000) could yet be involved. To test this possibility we took advantage of previous findings that the CaV2.3-selective antagonist SNX482 blocks Ni2+-sensitive, LVA CaV2.3 channels (Newcomb et al. 1998; Tottene et al. 2000). SNX482 neither inhibited the DRG Ca2+ current (Fig. 7A and B) nor blocked transmission across the SS (facilitation test: 311 ± 130%, n= 4 and 292 ± 64%, n= 13, respectively; Pt test > 0.1; control data from Rozanski et al. 2012), arguing that an LVA and Ni2+-sensitive CaV2.3 subtype does not play a significant role at this synapse. Thus, the simplest interpretation of all of our findings is that CaV3.2 channels control transmitter release from the stimulated neuron at the SS.
Figure 6. ω-AgaIIIA effect on NS Ca2+ current.
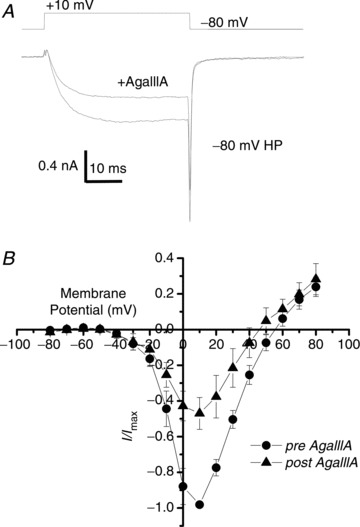
A, addition of ω-AgaIIIA to the bath (final concentration 50 nm) resulted in substantial reduction of inward current evoked by a depolarization beyond the HVA calcium channel threshold (+10 mV). B, relationship of steady-state Ca2+ current amplitude to voltage plotted for control NScis recordings and in the presence of ω-AgaIIIA.
Figure 7. SNX482 effect on NS ICa.
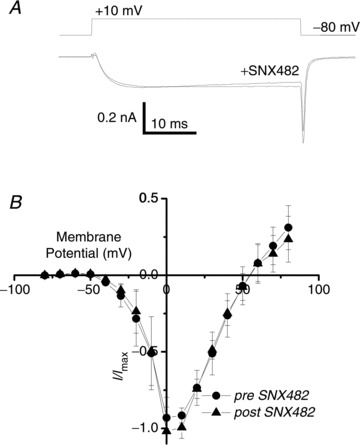
A, addition of SNX482 to the bath (final concentration 100 nm) did not inhibit the inward current evoked by a depolarization to +10 mV. B, relationship of steady-state Ca2+ current amplitude to voltage plotted for control NScis recordings and in the presence of SNX482.
Discussion
Our main findings are that chemical transmission between the two neurons of the neuron–glial cell–neuron SS is gated by LVA and inactivation-sensitive voltage-gated calcium channels, identified as CaV3.2 type. Although other types of calcium channels are present on these neurons that carry far more net inward Ca2+ current, these appear to contribute little, if anything, to excitation–secretion coupling at the SS.
The involvement of CaV channels in the gating of transmitter release from the stimulated neuron at the DRG SS synapse was first indicated by sensitivity to block with low concentrations of extracellular Cd2+ (Rozanski et al. 2012). This was further supported by the inhibition of transmission observed with an intracellular Ca2+ scavenger (Rozanski et al. 2013a; and this study). Based on these observations and studies by others we attribute ATP secretion from the SS neuron to classical presynaptic transmitter release sites located on the glial aspect of the NS. We anticipated that these would be gated by HVA CaV, probably (for chick) CaV2.2 but with CaV2.1 or a member of the CaV1 family as alternatives. However, this was ruled out by persistence of transmission in the presence of established selective blockers. This conclusion was strengthened by three additional findings: transmission was blocked by a low concentration of extracellular Ni2+ or by a moderate depolarization of the holding potential (−50 mV), and it persisted using voltage pulses that were sub-threshold for HVA channel types, ruling out the possibility that the blockers were for some reason inactive at this location.
Our findings implicated an LVA CaV type and left only two viable candidates: a Ni2+-sensitive CaV2.3 subtype or CaV3.2, the sole Ni2+-sensitive member of the CaV3 family. Interestingly, the CaV2.3 gene has not been confirmed in the chick genome, but the protein is predicted (XP_422255.3). As far as we are aware, our study represents the first report of the corresponding ‘R type’ Ca2+ current in chick: we can be confident that at least a fraction of the HVA, ‘block-resistant’ (in the presence of CaV1.x, 2.1 and 2.2 antagonists) Ca2+ current is R type. We believe this because it exhibits several key characteristics that include a relatively hyperpolarized range of inactivation, sensitivity to both Ni2+ and ω-AgaIIIA together with a considerable heterogeneity in biophysical properties. Definitive identification will require a molecular approach, but our interest here is solely whether CaV2.3 is involved in transmitter release at the SS. If so, based on our experimental observations, the CaV2.3 subtype would have to be blocked by a moderate depolarization of the NScis holding potential, and hence inactivation sensitive, blocked by a low concentration of Ni2+ and activated at a relatively hyperpolarized voltage range. Fortunately, this could be tested as this set of characteristics describes a CaV2.3 subtype that is sensitive to SNX482 (Tottene et al. 2000). Although the possibility remains that we are dealing with a novel CaV2.3 channel type, insensitivity of the residual, block-resistant ICa and SS transmission to SNX482 argues against gating of transmitter release by a CaV2.3 subtype, at least based on current knowledge.
The simplest interpretation of our results is therefore that transmitter release from NScis is gated by the Ni2+-sensitive CaV3.2 channel (Lee et al. 1999), which contains the αH pore-forming subunit. It is well established that CaV3 channels exist in chick DRG neurons – indeed, LVA current (Carbone & Lux, 1984) and single T-type (CaV3 family) calcium channel currents (Nowycky et al. 1985) were originally characterized using this preparation and we have shown, at least on the basis of mRNA levels that CaV3.2 is by far the predominant subtype in the chick DRG (Weber et al. 2010, Supplemental Data).
Previous reports have presented evidence for evoked release of ATP from DRG NS (Zhang et al. 2007) but the specific mechanism remains elusive. CaV gated release implies standard vesicular release but synaptic vesicle (SV) clusters remain to be identified at SS contacts (Pannese et al. 1991; Rozanski et al. 2012). However, due to the vast NS–NS contact area, active zone-like structures could be hard to demonstrate and may take a dedicated serial section-electron microscopy study. Furthermore, putative active zones could be dispersed and consist of just a very few SVs at each contact.
Action potential-gated transmitter release from presynaptic nerve terminals is almost exclusively controlled by members of the CaV2 family of channels, but CaV1 family channels can trigger SV fusion, in particular at presynaptic contacts gated by tonic depolarization, such as hair cells and retinal ganglion cells. SV discharge by CaV3 family channels is rare but was first reported at the ocular rod receptors (Pan et al. 2001) and more recently at neuronal contacts (see Carbone et al. 2006; Weiss et al. 2012). The obvious functional implication of CaV3-type channels is that secretion can be gated by depolarizations that are sub-threshold for action potentials. This is particularly interesting for the DRG, as spontaneous sub-threshold voltage oscillations, or waves, have been reported in somata, which could conceivably activate the SS. Thus, these contacts may serve a role in homeostasis, modulating neuronal excitability or biological state, independently of action potential input via the axon.
Neural and glial activities in the DRG have been implicated as factors in the aetiology of pain with CaV3 channel blockers, specifically CaV3.2 (Bourinet et al. 2005), of interest as analgesics. The finding that these channels mediate transmitter release for DRG SS transmission provides an interesting and novel candidate mechanism in pain-suppression research.
Acknowledgments
None.
Glossary
- CaV
voltage-gated calcium channel
- DRG
dorsal root ganglion
- HVA
high voltage activated
- LVA
low voltage activated
- NS
neuronal somata
- NScis
stimulated SS neuronal somata
- NStrans
passive SS neuronal somata
- SGC
satellite glial cell
- SS
sandwich synapse
- SV
synaptic vesicle
Additional information
Competing interests
The authors have no competing interests.
Author contributions
G.M.R. and A.R.N. carried out the recordings, analysed the data and contributed to the writing of the manuscript. M.A. purified the toxin and critiqued the manuscript. E.F.S. conceived and supervised the project, wrote the manuscript and is responsible for the work. All the authors have read and approved the final version.
Funding
This project was supported by a Canadian Institute of Health Research award (MOP-86643) and a Canada Research Chair to E.F.S., and Ontario Graduate Scholarships to G.M.R. and A.R.N.
References
- Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005;24:315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Giancippoli A, Marcantoni A, Guido D, Carabelli V. A new role for T-type channels in fast “low-threshold” exocytosis. Cell Calcium. 2006;40:147–154. doi: 10.1016/j.ceca.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones 1. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Chan AW, Khanna R, Li Q, Stanley EF. Munc18: a presynaptic transmitter release site N type (CaV2.2) calcium channel interacting protein. Channels. 2007;1:11–20. [PubMed] [Google Scholar]
- Chan AW, Stanley EF. Slow inhibition of N-type calcium channels with GTP gamma S reflects the basal G protein-GDP turnover rate. Pflugers Arch. 2003;446:183–188. doi: 10.1007/s00424-003-1030-2. [DOI] [PubMed] [Google Scholar]
- Gentile L, Stanley EF. A unified model of presynaptic release site gating by calcium channel domains. Eur J Neurosci. 2005;21:278–282. doi: 10.1111/j.1460-9568.2004.03841.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. BJ. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, Gαo, and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, Adams ME, Bean BP. Inhibition of N- and L-type Ca2+ channels by the spider venom toxin ω-Aga-IIIA. Proc Natl Acad Sci U S A. 1991;88:6628–6631. doi: 10.1073/pnas.88.15.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, Loo JA, Dooley DJ, Nadasdi L, Tsien RW, Lemos J, Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Pan ZH, Hu HJ, Perring P, Andrade R. T-type Ca2+ channels mediate neurotransmitter release in retinal bipolar cells. Neuron. 2001;32:89–98. doi: 10.1016/s0896-6273(01)00454-8. [DOI] [PubMed] [Google Scholar]
- Pannese E, Ledda M, Arcidiacono G, Rigamonti L. Clusters of nerve cell bodies enclosed within a common connective tissue envelope in the spinal ganglia of the lizard and rat. Cell Tissue Res. 1991;264:209–214. doi: 10.1007/BF00313957. [DOI] [PubMed] [Google Scholar]
- Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Rozanski GM, Kim H, Li Q, Wong FK, Stanley EF. Slow chemical transmission between dorsal root ganglion neuron somata. Eur J Neurosci. 2012;36:3614–3621. doi: 10.1111/j.1460-9568.2012.08233.x. [DOI] [PubMed] [Google Scholar]
- Rozanski GM, Li Q, Kim H, Stanley EF. Purinergic transmission and transglial signalling between neuron somata in the dorsal root ganglion. Eur J Neurosci. 2013a;37:359–365. doi: 10.1111/ejn.12082. [DOI] [PubMed] [Google Scholar]
- Rozanski GM, Li Q, Stanley EF. Transglial transmission at the dorsal root ganglion sandwich synapse: glial cell to postsynaptic neuron communication. Eur J Neurosci. 2013b;37:1221–1228. doi: 10.1111/ejn.12132. [DOI] [PubMed] [Google Scholar]
- Shinder V, Amir R, Devor M. Cross-excitation in dorsal root ganglia does not depend on close cell-to-cell apposition. Neuroreport. 1998;9:3997–4000. doi: 10.1097/00001756-199812210-00002. [DOI] [PubMed] [Google Scholar]
- Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28:743–761. doi: 10.1023/a:1007090105840. [DOI] [PubMed] [Google Scholar]
- Stanley EF. Single calcium channels on a cholinergic presynaptic nerve terminal. Neuron. 1991;7:585–591. doi: 10.1016/0896-6273(91)90371-6. [DOI] [PubMed] [Google Scholar]
- Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- Stanley EF, Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991;11:985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XP, Stanley EF. Characterization of an ATP receptor on a cholinergic presynaptic nerve terminal. Soc. Neurosci. Abstracts. 1994;20:1120. [Google Scholar]
- Tottene A, Volsen S, Pietrobon D. α1E subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AM, Wong FK, Tufford AR, Schlichter LC, Matveev V, Stanley EF. N-type Ca2+ channels carry the largest current: implications for nanodomains and transmitter release. Nat Neurosci. 2010;13:1348–1350. doi: 10.1038/nn.2657. [DOI] [PubMed] [Google Scholar]
- Weiss N, Hameed S, Fernandez-Fernandez JM, Fablet K, Karmazinova M, Poillot C, Proft J, Chen L, Bidaud I, Monteil A, Huc-Brandt S, Lacinova L, Lory P, Zamponi GW, De WM. A Cav3.2/syntaxin-1A signalling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem. 2012;287:2810–2818. doi: 10.1074/jbc.M111.290882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]


