Abstract
Laser scanning photostimulation was used to map the distribution of the synaptic input zones (sites that give local synaptic inputs) for dorsal horn laminae III–IV neurons, in parasagittal and transverse slices of the rat lumbar spinal cord, and examine how these inputs differed for neurons of different morphologies. All neurons received local excitatory and inhibitory synaptic inputs from within laminae III–IV, while a subset of neurons also received excitatory input from the superficial laminae, especially lamina IIi, as well as the II/III border region. Two anatomical properties were found to be predictive of the dorsoventral position of a neuron's input zone relative to its soma: (1) both excitatory and inhibitory input zones were more dorsal for neurons with longer dorsal dendrites, and (2) excitatory, but not inhibitory, input zones were more dorsal (relative to the soma) for more ventral neurons, with the transition between the dorsal input zones of laminae III–IV neurons and the ventral input zones of lamina II neurons occurring at the II/III border. The observed morphophysiological correlations support the idea that interlaminar connectivity is mediated via translaminar dendritic extensions and that, more generally, local connectivity within the dorsal horn is governed by rules relating the position of a neuron's soma and dendrites to the position of the local presynaptic neurons from which it receives inputs, which are specific to the axis and direction (dorsal vs. ventral), whether the input is excitatory or inhibitory, and the laminar position of the postsynaptic neuron.
Key points
Axons of sensory neurons that detect painful and non-painful stimulation of body tissues project centrally to the dorsal horn of the spinal cord, where they are partially segregated in the superficial and deep laminae, respectively.
Interneuronal connections between superficial and deep laminae could potentially modulate sensory transmission and contribute to alterations that occur under conditions of pain hypersensitivity.
This study used a localized stimulation technique (laser scanning photostimulation) for high-resolution mapping of local interneuronal synaptic connections to laminae III–IV neurons, combined with intracellular staining for morphological analysis, in an in vitro‘slice’ preparation of the rat lumbar spinal cord.
Synaptic input from superficial laminae (I–II) was received by laminae III–IV neurons with long dorsal dendrites, supporting the idea that interlaminar connectivity is mediated via translaminar dendritic extensions and, more generally, that local connectivity is governed by rules that are specific to the laminar position and morphology of the postsynaptic neuron.
Introduction
Different classes of nociceptive and non-nociceptive primary afferent neurons innervating peripheral tissues show some degree of segregation in their regions of central termination within the spinal dorsal horn. Laminae I and outer II receive projections from slowly conducting myelinated (Aδ) and unmyelinated (C) nociceptive neurons, while laminae III–IV receive projections primarily from low-threshold mechanoreceptive neurons with large myelinated, rapidly conducting (Aβ) axons (Light & Perl, 1979; Brown et al. 1980, 1981; Sugiura et al. 1986). Dorsal horn neurons of different response classes show a laminar distribution that partly matches the laminar distribution of primary afferent terminations, but that also shows evidence of additional convergent input from distinct primary afferent classes (Willis & Coggeshall, 2004). In laminae III–IV, a subset of neurons responds to both non-noxious and noxious intensities of cutaneous mechanical stimulation (Brown & Franz, 1969; Sedivec et al. 1983; Kamogawa & Bennett, 1986), suggesting a possible central convergence of nociceptive and non-nociceptive primary afferent input onto these neurons. Laminae III–IV neurons could potentially receive nociceptive input via direct primary afferent contacts on dorsal dendrites that extend into laminae I–II (Todd, 1989; Naim et al. 1997), but responses to noxious stimuli are also observed in neurons that lack such dendrites (Maxwell et al. 1983; Bennett et al. 1984; Ma et al. 1996). An additional potential route for central convergence of afferent input is via local interconnections between dorsal horn neurons. The dorsal horn contains a complex system of intrinsic circuitry that could potentially mediate intermodality crosstalk between neuronal classes with distinct primary afferent input. We recently demonstrated the presence of inputs from laminae III–IV to a subset of laminae I–II neurons, using laser scanning photostimulation (LSPS), an electrophysiological method for mapping the location of local sites that are connected to a single postsynaptic neuron (Kato et al. 2009; Kosugi et al. 2013). The method of paired recording has also been used previously to examine interneuronal connectivity in the dorsal horn (Lu & Perl, 2003, 2005; Schneider, 2008; Santos et al. 2009; Luz et al. 2010; Zheng et al. 2010; Zhang & Schneider, 2011), although such studies have mostly not explored interlaminar connectivity. The limitations of microelectrode sampling make it difficult to get an overview of patterns of connectivity across neuronal populations and laminar regions with this approach, whereas LSPS can provide population-wide spatial information that is not possible with paired recordings. In addition, the photostimulus used for synaptic mapping does not activate axons of passage (Katz & Dalva, 1994), and so is more suited for studying local circuitry than more recent optogenetic methods for long-range circuitry mapping that activate both cell bodies and axons (Petreanu et al. 2007; Wang & Zylka, 2009; Anderson et al. 2010; Kiritani et al. 2012). In the present study, we used LSPS to map the distribution of intra- and translaminar local synaptic input to laminae III–IV neurons, and to identify dendritic determinants of local excitatory and inhibitory input patterns.
Methods
All experimental procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. Methods for spinal cord slice preparation, whole-cell recording, and laser scanning photostimulation were as described in Kato et al. (2009). Kato et al. (2007) discusses various methodological issues, including the evidence that the glutamate uncaging stimulus does not activate axons of passage, and that the synaptically evoked responses are mostly monosynaptic (also see Katz & Dalva, 1994). Kato et al. (2007, 2009) also give evidence on the spatial resolution of the synaptic mapping and the discrimination between direct and synaptic responses.
Briefly, parasagittal or transverse slices of 250 μm thickness were prepared from the enlargement of the lumbar spinal cord of urethane-anaesthetized rats (1.2–1.5 g kg−1 i.p.) at the postnatal age of 35–65 days (mostly 35–50 days). The rats were killed by exsanguination immediately after spinal cord removal. The Krebs solution in the recording chamber contained (in mm): NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25, and glucose 11. It also contained 100 μm 4-methoxy-7-nitroindolinyl caged l-glutamate (MNI caged l-glutamate; Tocris, St Ellisville, MO, USA). The patch pipettes (6–8 MΩ) were filled with a solution containing (in mm): potassium gluconate 136, KCl 5, CaCl2 0.5, MgCl2 2, EGTA 5, Hepes 5, and Mg-ATP 5 for recording EPSCs in the voltage-clamp mode; Cs2SO4 110, TEA-Cl 5, CaCl2 0.5, MgCl2 2, EGTA 5, Hepes 5 and Mg-ATP 5, for recording inhibitory outward currents in the voltage-clamp mode. Laminar regions were visualized by infrared differential interference contrast (IR-DIC) microscopy under ×10 and ×40 objectives. Blind whole-cell recordings were obtained from neurons at a depth of 50–100 μm in the slice. In some experiments, following the synaptic mapping with LSPS (described below), neurons were tested for a response of a slow inward current to bath application of substance P (2 μm for 1 min). A fluorescent dye (Alexa 555 hydrazide, tris salt, 40 μm; Invitrogen) was added to the pipette solution in order to visualize the neuron during the recording periods. Neurobiotin (0.1%, Vector, Burlingame, CA, USA) was also added for further anatomical examination following histological processing. Slices were fixed in paraformaldehyde for 1–3 days, and dendritic drawings were made from photomicrographs of stained cells. Measurements of the distance of the neuron's soma below the lamina II/III border were made from the IR-DIC and fluorescent images taken of the live slice during the recording.
The photostimulation apparatus (Prairie Technologies, Middleton, WI, USA) employed a continuous argon ion (UV) laser, as described in Kato et al. (2009). The laser beam was coupled to a fibre optic and transmitted through the ×40 water immersion objective (NA 0.80) of an Olympus BX51WI microscope, to deliver 5 mW at the specimen. The microscope was mounted on an x–y motorized stage (Scientifica, UK), while the tissue was mounted independently on a fixed platform. The stimulus site was changed by moving the entire microscope, so the laser beam was always centred within the microscope objective and the microscope field, and the optical properties of the stimulus were identical for all stimulus sites.
A flash duration of 3 ms and caged glutamate concentration of 100 μm were used. During synaptic mapping, photostimulation was delivered at 0.5–0.67 Hz. Dorsoventral, mediolateral and rostrocaudal spacing in both parasagittal and transverse slices was 50 μm. Sites were stimulated in an order determined by a non-nearest neighbour algorithm to avoid desensitization. Excitatory and inhibitory synaptic responses to photostimulation were recorded in separate experiments, under voltage clamp. Excitatory (inward) and inhibitory (outward) currents were recorded at holding potentials of −70 and 0 mV, respectively. Synaptic events as well as direct responses were detected with the aid of MiniAnalysis software (Synaptosoft, Fort Lee, NJ, USA) and confirmed by visual inspection. Peak amplitude and time of onset were determined for each event. Responses were classified as either direct or synaptic based on a latency criterion determined and validated in Kato et al. (2007, 2009): 0–6 ms for direct responses, 6–106 ms for synaptic responses. The amplitude of each event was measured from the current level that was present at the onset of that event, not from the pre-stimulus baseline; if one event started during the decay phase of a preceding event, the second event's amplitude was measured from its starting point in the preceding event's decay phase. Consequently, the presence of direct responses at a given stimulus site does not add to the measured amplitude of the synaptic responses evoked at that site. Synaptic response amplitude for each stimulation site was measured as the sum of the peak amplitudes of all of the synaptic events whose onset occurred within the time window of 6–106 ms, relative to stimulus onset (Fig. 1D; Kato et al. 2007). Spontaneous activity was measured during the 100 ms pre-stimulus interval of each stimulus trial. A moving average of spontaneous activity was then calculated from the pre-stimulus activity over 100 stimulus trials. This moving average of spontaneous activity was then subtracted from the activity measured in the response time window to obtain the net response in each trial.
Figure 1. Examples of synaptic input zone maps and photostimulation (PS)-evoked responses of neurons in laminae III–IV, from parasagittal slices.
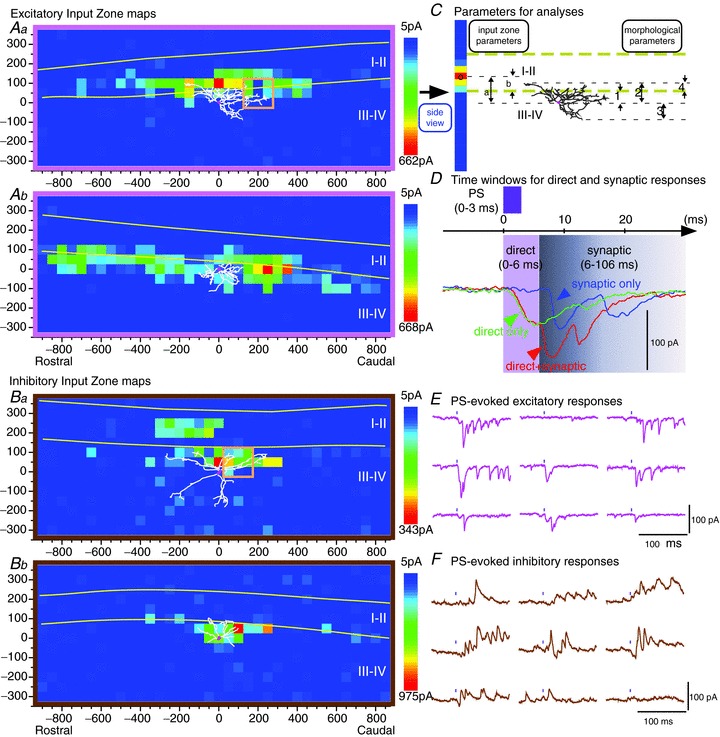
A, individual maps of excitatory input zones. The map in Aa exhibits a local peak or ‘hot spot’ that is contained within laminae I–II, and so is considered evidence of an input from neuron(s) within laminae I–II. The input zone map in Ab does not have local peaks that are clearly contained within laminae I–II, and so is not considered to constitute clear evidence of an input from laminae I–II neurons; although it extends into laminae I–II, this could be the result of input from presynaptic neurons whose somata are located ventral to the II/III border. Neurons with or without a ‘distinct’ excitatory input (i.e. a local peak in their input zone) from laminae I–II were grouped separately for the averaged maps in Fig. 2. B, individual maps of inhibitory input zones for two neurons. The neuron in Ba is one of the few neurons that received an inhibitory input from laminae I–II. C, diagram of parameters used in analyses. The 3-dimensional map in Aa (x, y, amplitude) is reduced to a single-column 2-dimensional map showing the distribution in the dorsoventral axis, by averaging across all rostrocaudal positions for each dorsoventral position (‘side view’, as in Fig. 1 of Anderson et al. 2010). (Note that a straightening procedure is done on the raw data prior to this averaging to make the laminar borders perfectly horizontal (see Methods). This single-axis distribution is then further reduced to a single position by calculating the weighted mean of the distribution along that axis (marked on the ‘side view’ in this example by a black open circle). This value of weighted mean was then used in subsequent correlation analyses between input zone parameters and morphological parameters. Input zone parameters: dorsoventral position (weighted mean) of the input zone relative to the soma (a) or relative to the lamina II/III border (b). Morphological parameters: 1, distance of soma below II/III border; 2, dorsal, and 3, ventral dendritic extent (relative to soma); 4, dorsal dendritic extent relative to II/III border. D, illustration of time windows for direct and synaptic responses (0–6 ms for direct responses, 6–106 ms for synaptic responses, as determined and validated in Kato et al. 2007, 2009). The amplitude of each event is measured from the level that is present at the onset of that event, not from the pre-stimulus baseline, i.e. it is not summed with the decaying component of a preceding event. Consequently, the presence of a direct response does not add to the measured amplitude of subsequent synaptic responses. E and F, examples of excitatory and inhibitory responses (inward and outward currents) evoked by photostimulation at each of the 9 sites enclosed in the square orange boxes in Aa and Ba, respectively. The black mark above each trace shows the time of the photostimulus. E shows examples of both direct and synaptic responses, but only the synaptic responses are included in the synaptic maps (according to the latency criteria illustrated in D). (These traces show that the responses evoked by the brief 3 ms photostimulus can be fairly prolonged, lasting many tens of milliseconds, as described in our previous studies (Kato et al. 2007). Nonetheless, our previous results, as well as those of previous investigators who have used LSPS, are consistent with the conclusion that the synaptically evoked responses are essentially all monosynaptic, as discussed in Kato et al. (2007, 2009). Their prolonged duration can be attributed to the slow time course of the photostimulation-evoked depolarization, sometimes resulting in the firing of more than one action potential, in the presynaptic neurons (Kato et al. 2007).)
A colour-coded contour map was made for each neuron showing the amplitude of the synaptic response evoked from each stimulation site in the photostimulation scanning grid (Fig. 1A and B). We refer to these as synaptic input maps. When combining individual maps to make population averages, the response amplitudes of individual neurons were normalized based on peak response amplitude in order to equalize their contribution to the averaged map. For construction of averaged maps, individual maps were aligned dorsoventrally either by soma location or by laminar borders. For averaging of parasagittal maps, as in our previous studies (Kato et al. 2009), a straightening procedure was done on the individual maps prior to combining them for averaging, in order to make the laminar borders straight and perfectly horizontal. This procedure involves measuring the dorsoventral position of the lamina II/III border at each rostrocaudal position, and then shifting the dorsoventral coordinates of each column of stimulus sites dorsally or ventrally so that the dorsoventral coordinate of the lamina II/III border has the same value at all rostrocaudal positions. As part of this straightening procedure, the dorsoventral dimension of each individual map is adjusted so that the dorsoventral width of laminae I–II is standardized to a uniform value at all rostrocaudal positions for all of the individual maps. A straightening procedure was used for the transverse maps that was similar to that used for the parasagittal maps, but it included one extra step prior to the straightening. Since the superficial laminae are curved in the transverse plane, each map was rotated clockwise or counterclockwise if necessary to make the superficial laminae horizontal at the neuron's position. The map rotation was done with custom software in Matlab (The MathWorks, Natick, MA, USA). The map straightening was done with custom software in LabView (National Instruments, Austin, TX, USA).
For the purposes of analysis, the 3-dimensional representation of the data contained in the synaptic input maps (x, y and amplitude) was reduced to a 2-dimensional plot of response amplitude along a single axis (Fig. 2D and E). For example, in a rostrocaudal plot (Fig. 2E), each point represents the sum of the response amplitudes across all dorsoventral positions, for a given rostrocaudal position. The plot representing the distribution along a single axis was further reduced to a single value of mean position along that axis, by calculating the weighted mean, or the mean value of all of the stimulus sites weighted by the amplitude at each site. These values of the mean position of the input zone for each axis were then used in correlation analyses to investigate relationships between synaptic input zones and soma-dendritic parameters. Statistical comparisons were done by ANOVA, paired or unpaired t tests, or χ2 test. Measurements are reported as mean ± SD.
Figure 2.
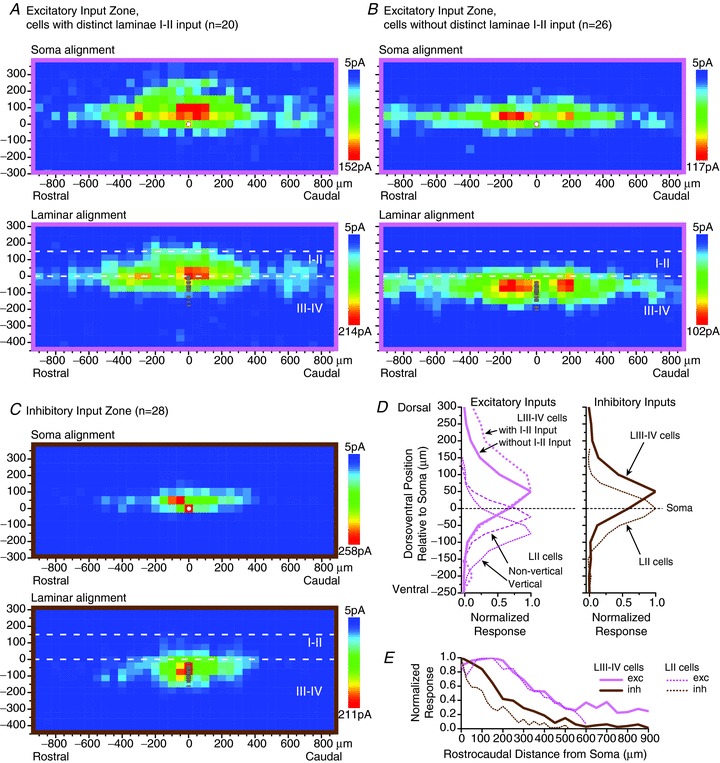
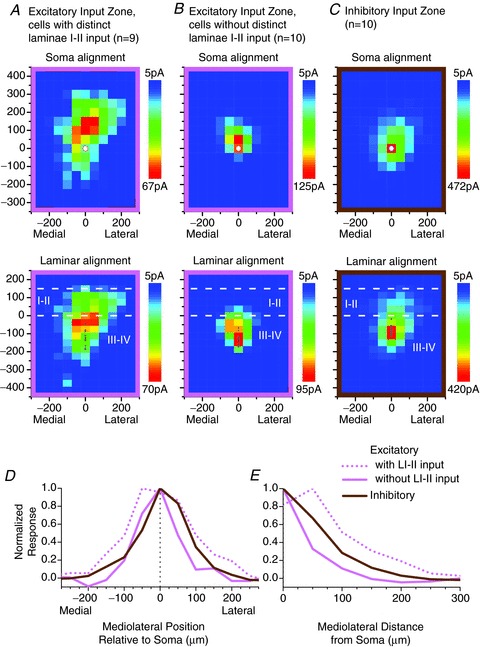
A–C, averaged parasagittal maps of excitatory (A and B) and inhibitory (C) synaptic input zones for laminae III–IV neurons. For the excitatory input maps, the neuronal sample was divided into neurons with and without a distinct excitatory input zone within laminae I–II (A and B, respectively). For each neuronal sample, separate averaged maps are shown in which the individual maps were aligned dorsoventrally either by soma position or by laminar borders (upper and lower maps, respectively, in A–C). The soma position of each of the neurons in the sample is marked by individual circles in the laminar-aligned maps, and is marked by a single white circle in the soma-aligned maps. D and E, plots of the dorsoventral (D) and rostrocaudal (E) distribution of the synaptic input zones relative to the neuron's soma for the laminae III–IV neurons, shown alongside the same plots for lamina II neurons from data of Kato et al. (2009). In D, separate plots are shown for the excitatory inputs to lamina II vertical and non-vertical cells (islet, central, radial and unclassified cells). (Note that ‘exc’ and ‘inh’ in E refer to the sign of the synaptic inputs, not the identity of the postsynaptic neurons.)
The spatial resolution of the synaptic mapping method used in the present study was investigated in previous studies (Kato et al. 2007, 2009). These findings on the spatial resolution are essential for interpreting the synaptic input maps, and particularly for making conclusions about the laminar location of the presynaptic neurons that contribute to the input zones contained in the maps, so they will be summarized briefly here. Since the evoking of synaptic responses by photostimulation requires the firing of action potentials in presynaptic neurons, the spatial resolution of the synaptic mapping is determined by the size of the area surrounding the stimulus from which neurons can be activated to threshold. This was examined by recording from single neurons in current clamp mode while mapping the locations of stimulus sites that are effective for evoking action potentials. Such photostimulation-evoked ‘action potential maps’ were obtained for a sample of both superficial and deep laminae dorsal horn neurons (Kato et al. 2007; also see additional ‘direct response’ maps in the supplemental data of Kato et al. 2009). The results showed that neurons could be activated to threshold only from sites overlying their soma or proximal dendrites, and that the maximal responses were almost always evoked from sites on or immediately surrounding the soma. This finding has specific relevance to the examination of synaptic inputs to laminae III–IV neurons from laminae I–II in the present study: the criterion for evidence that a neuron received input from laminae I–II was that the map of its synaptic input zone had a local peak within laminae I–II, since such a peak is likely to indicate the approximate location of the somata of the presynaptic neurons, whereas an input zone that has a peak within laminae III–IV but extends slightly across the laminar border into I–II could result from presynaptic neuron(s) whose somata are located slightly ventral to the lamina II/III border with proximal dendrites that enter laminae I–II (see criterion for grouping of neurons with and without a distinct laminae I–II input in Results).
Results
Whole-cell recordings were obtained from neurons in dorsal horn laminae III–IV in both parasagittal and transverse slices made from the enlargement of the lumbar spinal cord. Resting membrane potentials were −62.3 ± 5.9 mV. Input resistances were 318 ± 203 MΩ in parasagittal slices and 456 ± 349 MΩ in transverse slices. Membrane capacitances were 91 ± 446 pF in parasagittal slices and 65 ± 21 pF in transverse slices.
Parasagittal maps
Maps of excitatory and inhibitory synaptic input zones were obtained for a sample of laminae III–IV neurons, in parasagittal slices of the lumbar enlargement of the rat spinal cord (Fig. 1A and B). For examining correlations between input zone and morphological parameters, the dorsoventral position of input zones and dendrites was defined in two ways: (1) position relative to the neuron's soma, or (2) laminar or ‘absolute’ position in the dorsal horn, relative to the lamina II/III border (Fig. 1C). For these correlations, input zone position was calculated as the weighted mean of the distribution along each axis (see Methods, ‘side view’ in Fig. 1C).
Excitatory synaptic input to laminae III–IV neurons was found from both laminae III–IV and laminae I–II. Most laminae III–IV neurons received substantial excitatory synaptic input from within laminae III–IV, while a subset of laminae III–IV neurons exhibited a distinct excitatory synaptic input zone within laminae I–II (Fig. 1Aa), usually in combination with input from laminae III–IV. In order to better illustrate the distribution of the I–II input, the subset of neurons with a distinct input from laminae I–II were grouped separately for averaging (Fig. 2A and B). The criterion for including neurons in this group was that the map of the excitatory input zone showed a distinct peak within laminae I–II, and not merely an extension across the II/III border of an input zone that had its peak within laminae III–IV (e.g. Fig. 1Ab), as the latter could result from presynaptic neuron(s) whose somata were located slightly ventral to the lamina II/III border, whereas a distinct peak within laminae I–II is evidence of presynaptic neurons with somata within laminae I–II (see comment on spatial resolution of the synaptic mapping in Methods). In contrast to excitatory synaptic input, inhibitory synaptic input arose almost entirely from within laminae III–IV, with only 3 of the 28 neurons in the sample exhibiting a distinct inhibitory input zone within laminae I–II (e.g. Fig. 1Ba). Consequently it was decided to combine the entire sample of inhibitory synaptic input maps as a single map for the purpose of averaging (Fig. 2C).
Both the excitatory and inhibitory input zones had their greatest dorsoventral distribution at the level of and dorsal to the neuron's soma (Fig. 2D). This contrasted with our previous findings for lamina II neurons, in which inhibitory input zones were at about the same level as the soma, and excitatory input zones were predominantly ventral to the soma (previous data for lamina II neurons from Kato et al. 2009, also shown in Fig. 2D for comparison). For the laminae III–IV population as a whole, the distribution of the excitatory input zones tended to extend further dorsally, relative to the soma, than the inhibitory input zones, and consequently the population as a whole received much more excitatory than inhibitory input from laminae I–II. Overall, the dorsal displacement relative to the soma of the excitatory input zones was greater than that of the inhibitory input zones (68 ± 49 μm vs. 45 ± 24 μm, P < 0.05, for calculation of dorsoventral weighted mean of the synaptic input zone relative to the soma).
The soma locations of the neuronal sample were distributed over a dorsoventral distance of about 200 μm below the lamina II/III border (see grey circles in laminar alignment maps of Fig. 2A–C; Fig. 3Aa and B). As might be expected, more ventral neurons tended to have more ventral input zones, but for the excitatory input zones this relationship was weakened by an opposing tendency for more ventral neurons to have input zones that were displaced further dorsally relative to the soma (correlation between position of input zone relative to soma and distance of the soma below the II/III border: r= 0.61, slope = 0.6, P < 0.0001; Fig. 3Aa). This relationship is illustrated schematically in Fig. 3Ab. This correlation with soma position was exhibited only by the excitatory input zones, not the inhibitory input zones (r= 0.25, P= 0.2; Fig. 3Aa; note that these correlation analyses only included the laminae III–IV neurons, not the lamina II neurons). The position of the inhibitory input zones relative to the soma showed relatively little variation across the population (with the exception of two neurons with relatively dorsal input zones; Fig. 3Aa). As a consequence, the laminar (absolute) position of the inhibitory input zones was strongly correlated with soma position (r= 0.74, P < 0.001), whereas this correlation with soma position was weaker for the excitatory input zones (r= 0.41, P < 0.01; Fig. 3B).
Figure 3. Dimensions of synaptic input zones and dendritic fields of laminae III–IV neurons, all from parasagittal slices except F, from transverse slices.
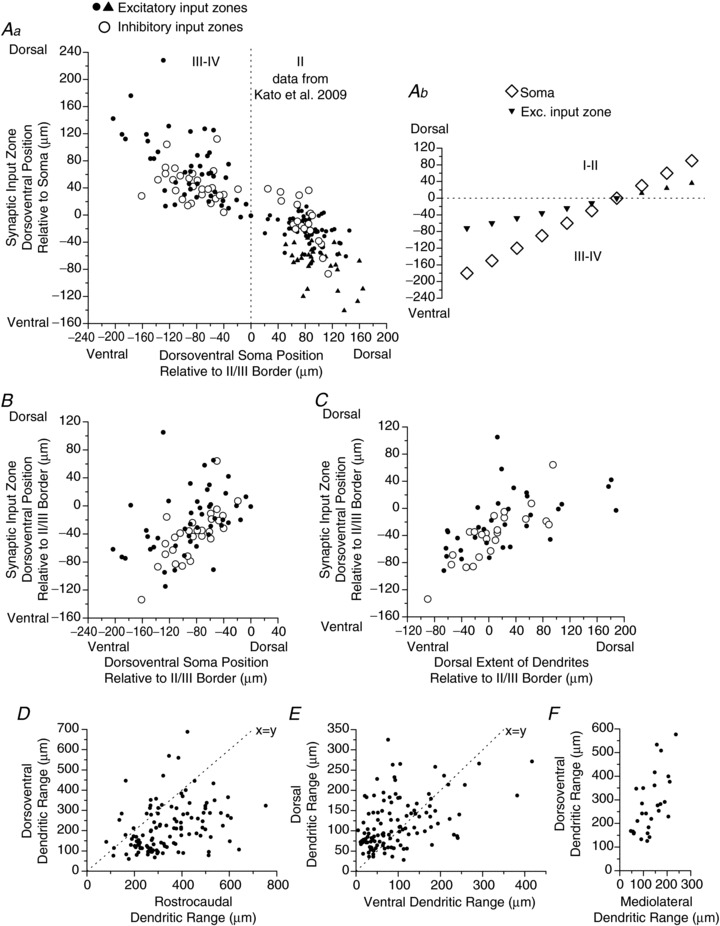
A–C, relationships between position of synaptic input zones and soma-dendritic anatomy. In Aa, B and C, open and filled symbols represent inhibitory and excitatory synaptic input zones, respectively. Aa and B, plots of dorsoventral position of the synaptic input zone relative to either the soma (Aa) or the lamina II/III border (B) vs. the dorsoventral position of the soma relative to the lamina II/III border. In Aa, data are also plotted for lamina II neurons obtained in Kato et al. 2009. In the lamina II data, excitatory input zones of vertical cells are represented by filled triangles. Ab is a schematic diagram of the relationship shown in Aa, illustrating how the mean position of the excitatory input zone (filled triangles) relative to the soma (open diamonds) shifts with increasing soma distance above or below the laminae II/III border. The variation in input zone position with dorsal dendritic length is not illustrated in this diagram. Correlations/regressions: Aa (laminae III–IV neurons only): excitatory, r= 0.61, slope = 0.60, P < 0.0001; inhibitory, r= 0.25, P= 0.20. B, excitatory, r= 0.41, P < 0.01; inhibitory, r= 0.74, P < 0.001). C, dorsoventral position of synaptic input zone relative to the lamina II/III border vs. dorsalmost extent of dendritic field relative to the lamina II/III border. Correlations: excitatory, r= 0.55, P= 0.001; inhibitory, r= 0.78, P < 0.0001. Values for synaptic input zone position in A–C are the weighted mean of the distribution, along the dorsoventral axis. Weighted mean of the distribution is calculated as the average dorsoventral position of the sites in the input zone map, where the average is weighted by the amplitude at each site. D–F, dendritic dimensions for the laminae III–IV sample, including additional neurons not represented in A–C for which dendritic staining was obtained but synaptic mapping was not successful. Dotted lines in D and E are x=y equality lines, not regression lines. All data are from parasagittal slices except in F, which are from transverse slices.
Figure 4 gives a display of the relationship between dorsoventral position of the soma, dendrites and input zones, for each of the individual neurons in the sample obtained in parasagittal slices. Each column in the display shows a single-axis dorsoventral map of the input zone for a single neuron, along with the soma position and the dorsal and ventral dendritic range for that neuron (see ‘side view’ in Fig. 1C, modelled after the display used in Fig. 1 of Anderson et al. 2010). Examination of correlations between the input zone and the dendritic field found that the most relevant dendritic parameter was the dorsal extent (‘range’) of the dendrites. There was a significant correlation between the dorsal dendritic range and the dorsoventral position of the input zone relative to the soma, for both the excitatory and inhibitory input zones (excitatory, r= 0.47, P < 0.01; inhibitory, r= 0.68, P < 0.01).
Figure 4. Single-axis dorsoventral representation of the synaptic input maps, for each of the neurons in the parasagittal sample.
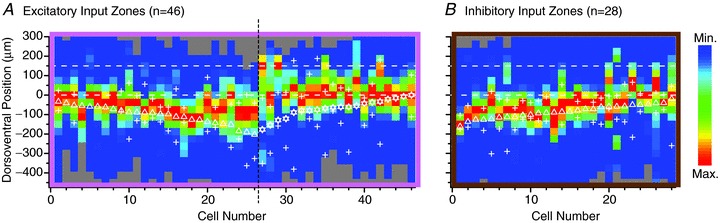
The rostrocaudal axis is not represented. Each column is the map for a single neuron, in which the original 2-dimensional parasagittal map was reduced to a single axis by summing across all rostrocaudal positions (as illustrated in ‘side view’ in Fig. 1C, modelled after Fig. 1 of Anderson et al. 2010). The dashed lines represent the borders of laminae I–II. A and B show excitatory and inhibitory input zones, respectively. In A, the neurons with and without distinct excitatory input from laminae I–II are arranged on the right and left side, respectively (cell numbers 27–46 and 1–26, respectively). The dorsoventral soma position of each neuron is marked by a star for the neurons with I–II input, and by a triangle for the other neurons. In each group, neurons are arranged in order of dorsoventral soma position. For each neuron, the dorsalmost and ventralmost extent of the dendritic field is marked by a + (only for those cells with good dendritic staining). The amplitude of the colour-coded scale was normalized across neurons.
The laminar distribution of the synaptic input zone was most strongly correlated with the laminar position of the dorsalmost dendrites. In other words, neurons whose dendrites extended to more dorsal positions in the dorsal horn also had synaptic input zones that extended further dorsally in the dorsal horn (Fig. 3C; excitatory, r= 0.53, P < 0.01; inhibitory, r= 0.78, P < 0.001). The laminar distribution of the synaptic input zone was thus related to a combination of the dorsoventral position of the neuron's soma and the neuron's dorsal dendritic length (which together determine the dorsalmost laminar extent of the dendrites).
Compared to dorsal dendritic length, ventral dendritic length had only a relatively weak correlation with the mean position of the inhibitory input zone (r= 0.48, P < 0.05), and no significant correlation with the mean position of the excitatory input zone (P= 0.11). However, the weighted mean calculation of the synaptic input zone is dominated by the inputs that are dorsal to the soma, because the input zones tend to extend further dorsal than ventral to the soma, and because the amplitude of the dorsal inputs is much greater than that of the ventral inputs, particularly for the excitatory inputs (Figs 2D and 4A). In order to focus specifically on the ventral extent of the input zone to investigate a possible relationship to ventral dendritic extent, a separate calculation was made of the ventral weighted mean of the input zones, by excluding from the calculation the sites dorsal to the soma. Ventral dendritic range was significantly correlated with the ventral weighted mean of the excitatory input zones (r= 0.52, P < 0.01), but not the inhibitory input zones (P= 0.17). (A separate calculation of the dorsal weighted mean of the input zones gave nearly identical results in the correlation analyses as the dorsoventral weighted mean, since the latter quantity is primarily influenced by the dorsal sites.) Thus, there is a correlation between the extent of the excitatory input zones and the extent of the dendrites in both the dorsal and ventral direction, although the amplitude of the ventral inputs is extremely low. To further illustrate the relationship between dorsal dendritic range and dorsoventral distribution of the input zone, maps of averaged excitatory input zones were made for the neurons with the shortest (Fig. 5A) and longest (Fig. 5B) dorsal dendritic range in the sample (<80 μm or >190 μm).
Figure 5.
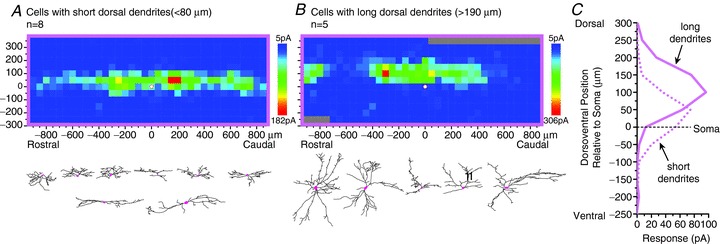
A–C, comparison of excitatory input zones, in parasagittal slices, of neurons with very short (A, <80 μm) or very long (B, >190 μm) dorsal dendritic extent, to illustrate that the neurons with longer dorsal dendrites tend to receive excitatory input from regions further dorsal to their soma. These cutoffs were chosen to include the neurons with the most extreme values in the population. Ventral dendritic extent and rostrocaudal dendritic extent are not correlated with the distribution of synaptic inputs (see text).
In contrast, no correlation was found between dendritic range and the distribution of the synaptic input zone in the rostrocaudal axis. Similar to previous findings for lamina II neurons, excitatory input zones were relatively widespread rostrocaudally, while inhibitory input zones were more restricted (Fig. 2A–C and E; 1133 ± 591 μm vs. 669 ± 409 μm, P < 0.001). This pattern was independent of whether the rostrocaudal dendritic range was relatively long or short. The relationship between the dendritic range and the distribution of the input zone in the dorsoventral axis described above was independent of the rostrocaudal dendritic range or the shape of the dendritic field in that it was dependent only on the absolute dendritic range in the dorsoventral axis, not on the ratio between the dorsoventral and rostrocaudal dendritic range.
An attempt was made to discern the presence of morphological subgroups within the population, in order to investigate possible relationships between dendritic morphology and input zones beyond the relationship to the dorsoventral dendritic range described above. Unlike in lamina II, no general morphological classification scheme has been developed for neurons in laminae III–IV, and the majority of the neurons did not fall into readily identifiable discrete morphological classes. The two groups of neurons that were distinguished by whether there was a clear excitatory input zone in laminae I–II (Fig. 2A, B and D) were both heterogeneous in their dendritic morphology, with no significant differences in any dendritic dimensions. The plots in Fig. 3D–F show the dendritic dimensions for a sample of laminae III–IV neurons, including both the neurons for which synaptic mapping was done as well as additional neurons that had good dendritic staining but for which the synaptic input maps were not obtained. The rostrocaudal dendritic range was greater than the dorsoventral dendritic range in the great majority of the neurons (84%, 99/118; mean range, rostrocaudal, 340 ± 130 μm; dorsoventral, 214 ± 117 μm; P < 0.0001, paired t test). There was a smaller but significant bias in the dorsal vs. the ventral direction, where 66% (78/118) of the neurons had a larger dorsal dendritic range (dorsal vs. ventral: 117 ± 62 μm vs. 96 ± 75 μm, P= 0.002, paired t test). There were significant correlations between rostrocaudal vs. dorsoventral dendritic range (r= 0.35, P < 0.0001), dorsal vs. ventral dendritic range (r= 0.44, P < 0.0001), and dorsoventral vs. mediolateral dendritic range (r= 0.62, P < 0.001, data from transverse slices). Neurons of different dendritic morphologies appeared to be present at all dorsoventral positions across laminae III–IV, with no apparent relationship between dendritic morphology and dorsoventral distribution, as determined from plots of the various dendritic dimensions, or the ratios of the dimensions vs. distance of the soma below the lamina II/III border (not illustrated).
A subgroup of neurons had fairly long dorsal dendrites and relatively short ventral dendrites, whereas a small number of neurons had relatively long dendrites in both the dorsal and ventral direction (examples of both are shown in Fig. 5B). The pattern of long ventral dendrites with minimal dorsal dendritic extension, similar to the large vertical cells of lamina II, was generally not found (Fig. 3E). A substantial number of neurons exhibited a relatively restricted dendritic field in both the dorsoventral and rostrocaudal axes, some of which were somewhat similar to the radial cells of lamina II (Fig. 3D; e.g. the first three neurons in the top row in Fig. 5A). A small number of neurons exhibited a rostrocaudally elongated, dorsoventrally flattened dendritic field reminiscent of the islet cells of lamina II (e.g. first neuron in second row of Fig. 5A). However, the majority of neurons did not fall into discrete, readily distinguishable categories, and attempts to discern differences in the input zones by constructing averaged maps for different putative morphological classes were not fruitful, beyond the relationship described above for dorsal dendritic range (Fig. 5). As noted above, there was no correlation between dimensions of dendrites and input zones in the rostrocaudal axis, and averaged synaptic input maps of neurons with long vs. short rostrocaudal dendritic ranges showed no differences (not illustrated).
Bath application of substance P (2 μm for 1 min) evoked a slow inward current of at least 20 pA in 14/31 neurons (45%) that were tested following mapping of their excitatory synaptic input zone, in parasagittal slices. The substance P-responsive and unresponsive groups were both morphologically heterogeneous. Both groups were distributed throughout the dorsoventral extent of laminae III–IV, with no apparent difference in dorosventral distribution. Neurons with either long or short dorsal dendrites were represented in both groups, with no apparent difference. No relationships were found between substance P response and any parameters of dendritic dimensions or synaptic input zone distributions, except that substance P responses were of higher amplitude in neurons with a relatively large rostrocaudal dendritic range (>400 μm). Substance P response amplitude in neurons with rostrocaudal range greater or less than 400 μm was 43 ± 33 vs. 17 ± 18 pA (P < 0.05, t test; excluding substance P-unresponsive neurons: 58 ± 22 vs. 34 ± 13 pA, P < 0.05, t test). Substance P responses of 25 pA or greater were present in 6/8 neurons greater than 400 μm vs. 4/15 neurons less than 400 μm in rostrocaudal dendritic range (P < 0.05, χ2 test; note that some neurons were not included due to incomplete anatomical data).
Transverse maps
The dorsoventral distribution of input zones observed in the transverse maps was roughly similar to that found in the parasagittal maps (Fig. 6A–C), except that there was a somewhat larger inhibitory input from laminae I–II in the transverse sample. The plots in Fig. 6D and E show the mediolateral distribution of the excitatory and inhibitory input zones, represented as either mediolateral position (D) or mediolateral distance (E; i.e. distance = absolute value of position). The excitatory input zones of the neurons with laminae I–II input have a broader mediolateral distribution than the excitatory input zones of the neurons without laminae I–II input, or the inhibitory input zones, as determined by a comparison of the weighted mean of the mediolateral distance curves in Fig. 6E (70 ± 39 μm vs. 24 ± 18 μm vs. 39 ± 19 μm, respectively; P < 0.01, ANOVA). The weighted mean of the mediolateral distance was correlated with the mediolateral dendritic range for the excitatory input zones (r= 0.56, P < 0.05) but not the inhibitory input zones (r= 0.20, P= 0.66). Neurons with excitatory laminae I–II input had a greater mediolateral dendritic range than neurons without excitatory laminae I–II input (174 ± 43 μm vs. 121 ± 35 μm; P < 0.05, t test). In addition, in the transverse sample, neurons with excitatory laminae I–II input had a greater dorsoventral dendritic range than neurons without excitatory laminae I–II input (380 ± 156 μm vs. 227 ± 77 μm; P < 0.05, t test), although this parameter was not significantly different in the parasagittal sample.
Figure 6.
A–C, averaged transverse maps of excitatory (A and B) and inhibitory (C) synaptic input zones for laminae III–IV neurons. Details as in Fig. 2A–C. D and E, plots of the mediolateral distribution of the input zones, shown as mediolateral position (D) or mediolateral distance (E) relative to the soma. Mediolateral distance is the absolute value of mediolateral position.
Discussion
The present study used laser scanning photostimulation to map the distribution of the synaptic input zones, or sites that give local synaptic inputs, to neurons in dorsal horn laminae III–IV, and examine how these inputs differed for neurons of different morphological properties across the population. All neurons received substantial local excitatory and inhibitory synaptic inputs from within laminae III–IV, while a subset of neurons also received excitatory input from the superficial laminae (I–II). Laminae III–IV neurons tended to receive their greatest excitatory synaptic input from positions dorsal to their soma, in contrast to neurons in lamina II, which we found previously tend to receive excitatory input from positions ventral to their soma (Kato et al. 2009; Kosugi et al. 2013). Two anatomical properties were found to be predictive of the dorsoventral position of the excitatory input zone relative to the soma: (1) input zones were more dorsal for neurons with longer dorsal dendrites, and (2) input zones were more dorsal (relative to the soma) for more ventral neurons (neurons further below the lamina II/III border). This latter relationship meant that there was a smooth transition between the dorsally positioned input zones of the laminae III–IV neurons and the ventrally positioned input zones of the lamina II neurons (Fig. 3Aa and b), with the transition occurring at the II/III border (i.e. neurons near the border had excitatory input zones at about the same dorsoventral level as their soma). Thus, this relationship between the input zone position and soma position seems to encompass the entire population of neurons across lamina II and laminae III–IV, which suggests that there may be common principles governing the organization of local inputs to neurons in these two regions. A diagram of this relationship (Fig. 3Aa) gives the impression of the input zone being pulled between two positional influences, the neuron's soma and the lamina II/III border. This results in a preferential input from the II/III border region, relative to other laminar positions, when averaged across neurons at all dorsoventral positions. This can be seen in the averaged maps of the excitatory input zone for the laminae III–IV population, especially in the group with laminae I–II input, and also to some degree for the lamina II population, especially the vertical cells (Kato et al. 2009; Kosugi et al. 2013). This input may be of significance in relation to the population of PKCγ-expressing neurons, which are present at highest density in a band that encompasses the II/III border region as well as the innermost part of lamina II. The PKCγ neurons have been identified as a predominantly excitatory population with heterogeneous dendritic morphology, which is activated by non-noxious mechanical stimuli and has been implicated in the generation of inflammatory and neuropathic pain hypersensitivity (Malmberg et al. 1997; Polgár et al. 1999; Martin et al. 1999, 2001; Miraucourt et al. 2007; Neumann et al. 2008). Possibly this population is an important source of excitatory input to neurons across widespread regions of the dorsal horn, including populations in both superficial and deep laminae. PKCγ-positive axonal boutons, like the somas, are concentrated in IIi and the II/III border region, but neurons outside this region could receive input from these axons via dorsally or ventrally extending dendrites.
Among the strongest morphophysiological correlations found was that between the absolute (laminar) positions of the synaptic input zone and the dorsalmost dendritic extension (i.e. neurons whose dendrites extended to more dorsal sites also received input from more dorsal sites). Such a relationship could be explained if the presynaptic neurons preferentially contacted postsynaptic targets that are at a similar dorsoventral level to their own soma (also see Discussion of Kosugi et al. 2013). This is generally consistent with the existing anatomical information on local projections: neurons in both lamina II, as well as laminae III–IV, have been described that have ventrally projecting axons with collaterals and arborizations deeper than their soma, but such arborizations tend to be relatively sparse compared to those described for neurons that have axonal arborizations at the same level as their cell body (Light & Kavookjian, 1988; Light & Willcockson, 1999; Eckert et al. 2003; Maxwell et al. 2007; Schneider, 1992). For the laminae III–IV neurons, this difference in density of arborization was quantified by Schneider (1992): neurons with axonal arborizations at the level of their soma had about five times as many axonal boutons as those whose axons projected to deeper levels. Neurons in deeper laminae also appear to have their densest local axonal arborizations at a similar level to their soma (Strassman et al. 1994).
More generally, the present results expand on the idea proposed in Kosugi et al. (2013) that local connectivity within the dorsal horn is governed by specific rules relating the dorsoventral position of a neuron's soma and dendrites to the dorsoventral position of the local presynaptic neurons from which it receives input, which depend on the direction (dorsal vs. ventral), the neuronal population, and whether the input is excitatory or inhibitory. It appears that the dorsoventral level to which a neuron sends its dendrites is strongly related to whether it will receive excitatory input from interneurons at that level, but only for a specific subset of those levels, which is specific to the neuronal population. The most striking example of this selectivity is the present finding for laminae III–IV neurons that the position of the excitatory input zone is correlated with the dendritic extent, but only in the dorsal, not the ventral direction. Local input is almost entirely from regions dorsal to their soma, and the length of the ventral dendrites has minimal influence on the dorsoventral distribution of the input zones. Conversely, lamina II neurons receive excitatory input primarily from levels ventral to their soma (Kato et al. 2009; Kosugi et al. 2013), and the ventral extent of the input zone appears to be correlated with the ventral extent of their dendrites (although, in the case of lamina II neurons, this apparent selectivity could be attributed to the fact that the population as a whole has relatively little dorsal dendritic extension; in contrast, laminae III–IV neurons commonly have dendrites that extend both dorsally and ventrally). Neurons in both lamina II and laminae III–IV tend to receive inhibitory inputs from dorsoventral levels near that of their soma (or slightly dorsal to the soma, in the case of laminae III–IV neurons). This could be explained if both excitatory and inhibitory interneurons preferentially contact postsynaptic targets that are at a similar dorsoventral level to their own soma, but only a subset of those potential targets, and that excitatory interneurons tend to target more distal dendritic sites compared to inhibitory interneurons that target more perisomatic and proximal dendritic sites (Fig. 7D).
Figure 7. Summary diagrams: neurons with long dendrites are critical for interlaminar connectivity within the dorsal horn.
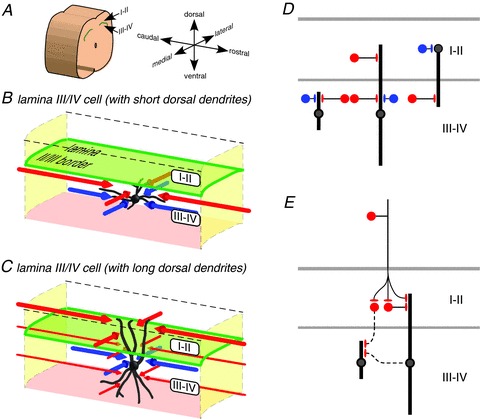
A–C, 3-dimensional schematic representation of excitatory and inhibitory input zones of neurons in laminae III–IV with short (B) or long (C) dorsal dendrites. The red and blue lines with arrowheads represent the relative dimensions of the excitatory and inhibitory synaptic input zones, respectively (i.e. the zones that contain the somas of neurons that are presynaptic to the illustrated postsynaptic neuron). Laminae III–IV neurons receive their greatest local excitatory and inhibitory input from regions dorsal to the level of the soma. Neurons with long dorsal dendrites receive excitatory input from more dorsal levels, extending into laminae I–II. The laminae III–IV neurons often have both dorsal and ventral dendrites, but are strongly biased towards receiving local excitatory input from sites dorsal to their somas. Excitatory input zones extend longer distances rostrocaudally than inhibitory input zones. Both excitatory and inhibitory input zones are relatively restricted mediolaterally. D, diagram of the dorsoventral organization of local excitatory and inhibitory synaptic inputs to laminae III–IV neurons with short or long dorsoventral dendrites, and lamina II neurons with long ventral dendrites (based on lamina II neuron data from Kato et al. 2009; Kosugi et al. 2013). Excitatory and inhibitory neurons have red and blue somas, respectively. Dendrites are represented as thick vertical lines, and only their dorsoventral dimension is represented. Whereas laminae III–IV neurons receive both excitatory and inhibitory inputs predominantly from positions dorsal to their somas, lamina II neurons receive inhibitory inputs from about the same level as their somas, and excitatory inputs from positions ventral to their somas; lamina II neurons with long ventral dendrites can receive excitatory input from laminae III–IV. Thus, translaminar input is received by neurons with long dorsal or ventral dendrites, in both lamina II and laminae III–IV. E, diagram to illustrate two possible polysynaptic routes (dotted lines) by which primary afferent input to the superficial laminae could reach laminae III–IV neurons with short dorsal dendrites (see Discussion). Although they are not shown as red in this diagram, laminae III–IV neurons with long dorsal dendrites (C, D and E) and lamina II neurons with long ventral dendrites (D) have been shown to be excitatory by Powell & Todd (1992) and Yasaka et al. (2010), respectively.
The present results further suggest that the rules governing the relationship between the dendritic extent and the position of the input zone are specific to each axis. The correlation between these parameters is not found in the rostrocaudal axis, for either laminae III–IV or lamina II neurons, but does appear to be present in the mediolateral axis, for laminae III–IV neurons as well as lamina I neurons (Kato et al. 2009; Kosugi et al. 2013). An additional level of specificity is that these mediolateral correlations are present only for the excitatory, not the inhibitory, input zones. A further finding of the present study was that the excitatory input zones of laminae III–IV neurons are much wider rostrocaudally than the inhibitory input zones, as was also found previously for neurons in the superficial laminae (Kato et al. 2007, 2009; Kosugi et al. 2013), which suggests that local circuitry is not sufficient to generate the large inhibitory receptive fields commonly found for dorsal horn neurons (cf. Discussion in Kato et al. 2011).
Some laminae III–IV neurons, in addition to responding to non-noxious mechanical stimulation, can also be activated, with greater response amplitude, by noxious stimulus intensities (Brown & Franz, 1969; Sedivec et al. 1983; Kamogawa & Bennett, 1986). It has been argued that these nociceptive responses might in some cases result from spatial summation of input from low-threshold mechanoreceptors, rather than an input from primary afferent nociceptors (Giesler & Cliffer, 1985). Nonetheless, there is evidence that some laminae III–IV neurons do receive monosynaptic contacts from substance P-containing, presumably nociceptive, primary afferents, which have been correlated in some cases with the presence of nociceptive responses (Naim et al. 1997; De Koninck et al. 1992; Ma et al. 1996). Nociceptive responses have been found not only in neurons with dendrites that extended into the superficial laminae, but also in neurons that lack such dorsal dendritic extensions, and which therefore presumably could not receive direct nociceptor input (Maxwell et al. 1983; Bennett et al. 1984; Ma et al. 1996). Although the present results indicate that such neurons do not tend to receive local interneuronal input from the superficial laminae, a possible alternative route by which they could receive polysynaptic nociceptor input would be from other laminae III–IV neurons that do possess dorsal dendritic extensions into the superficial laminae (Fig. 7E), as neurons with such dendritic extensions are known to be excitatory (Powell & Todd, 1992).
Acknowledgments
None.
Glossary
- LSPS
laser scanning photostimulation
Additional information
Competing interests
The authors declare no competing financial interest.
Author contributions
The experiments were performed in the laboratory of A.M.S. G.K. and A.M.S. designed the experiments and drafted the paper. G.K. collected the data. G.K., M.K., M.M. and A.M.S. contributed to the analysis and interpretation of the data. All authors approved the final version of the manuscript.
Funding
This work was supported by NIH grant R01 NS057454 to A.M.S.
References
- Anderson CT, Sheets PL, Kiritani T, Shepherd GM. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci. 2010;13:739–744. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Nishikawa N, Lu GW, Hoffert MJ, Dubner R. The morphology of dorsal column postsynaptic spinomedullary neurons in the cat. J Comp Neurol. 1984;224:568–578. doi: 10.1002/cne.902240406. [DOI] [PubMed] [Google Scholar]
- Brown AG, Franz DN. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7:231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE, Noble R. Projections from Pacinian corpuscles and rapidly adapting mechanoreceptors of glabrous skin to the cat's spinal cord. J Physiol. 1980;307:385–400. doi: 10.1113/jphysiol.1980.sp013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE, Rose PK, Snow PJ. Spinal cord collaterals from axons of type II slowly adapting units in the cat. J Physiol. 1981;316:469–480. doi: 10.1113/jphysiol.1981.sp013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Ribeiro-da-Silva A, Henry JL, Cuello AC. Spinal neurons exhibiting a specific nociceptive response receive abundant substance P-containing synaptic contacts. Proc Natl Acad Sci U S A. 1992;89:5073–5077. doi: 10.1073/pnas.89.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert WA, III, McNaughton KK, Light AR. Morphology and axonal arborization of rat spinal inner lamina II neurons hyperpoarized by μ-opioid-selective agonists. J Comp Neurol. 2003;458:240–256. doi: 10.1002/cne.10587. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Jr, Cliffer KD. Postsynaptic dorsal column pathway of the rat. II. Evidence against an important role in nociception. Brain Res. 1985;326:347–356. doi: 10.1016/0006-8993(85)90044-7. [DOI] [PubMed] [Google Scholar]
- Kamogawa H, Bennett GJ. Dorsal column postsynaptic neurons in the cat are excited by myelinated nociceptors. Brain Res. 1986;364:386–390. doi: 10.1016/0006-8993(86)90853-x. [DOI] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Ji RR, Strassman AM. Differential wiring of local excitatory and inhibitory synaptic inputs to islet cells in rat spinal lamina II demonstrated by laser scanning photostimulation. J Physiol. 2007;580:815–833. doi: 10.1113/jphysiol.2007.128314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci. 2009;29:5088–5099. doi: 10.1523/JNEUROSCI.6175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kosugi M, Mizuno M, Strassman AM. Separate inhibitory and excitatory components underlying receptive field organization in superficial medullary dorsal horn neurons. J Neurosci. 2011;31:17300–17305. doi: 10.1523/JNEUROSCI.4474-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Dalva MB. Scanning laser photostimulation: a new approach for analyzing brain circuits. J Neurosci Methods. 1994;54:205–218. doi: 10.1016/0165-0270(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci. 2012;32:4992–5001. doi: 10.1523/JNEUROSCI.4759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi M, Kato G, Lukashov S, Pendse G, Puskar Z, Kozsurek M, Strassman AM. Subpopulation-specific patterns of intrinsic connectivity in mouse superficial dorsal horn as revealed by laser scanning photostimulation. J Physiol. 2013;591:1935–1949. doi: 10.1113/jphysiol.2012.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III-V) J Comp Neurol. 1988;267:172–189. doi: 10.1002/cne.902670203. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light AR, Willcockson HH. Spinal laminae I-II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. J Neurophysiol. 1999;82:3316–3326. doi: 10.1152/jn.1999.82.6.3316. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fibre input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II) J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz LL, Szucs P, Pinho R, Safronov BV. Monosynaptic excitatory inputs to spinal lamina I anterolateral-tract-projecting neurons from neighbouring lamina I neurons. J Physiol. 2010;588:4489–4505. doi: 10.1113/jphysiol.2010.197012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Ribeiro-Da-Silva A, De Koninck Y, Radhakrishnan V, Henry JL, Cuello AC. Quantitative analysis of substance P-immunoreactive boutons on physiologically characterized dorsal horn neurons in the cat lumbar spinal cord. J Comp Neurol. 1996;376:45–64. doi: 10.1002/(SICI)1096-9861(19961202)376:1<45::AID-CNE3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Liu H, Wang H, Malmberg AB, Basbaum AI. Inflammation-induced up-regulation of protein kinase Cγ immunoreactivity in rat spinal cord correlates with enhanced nociceptive processing. Neuroscience. 1999;88:1267–1274. doi: 10.1016/s0306-4522(98)00314-5. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Malmberg AB, Basbaum AI. PKCγ contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J Neurosci. 2001;21:5321–5327. doi: 10.1523/JNEUROSCI.21-14-05321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DJ, Bell MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory interneurons in superficial laminae of the rat dorsal horn. J Physiol. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DJ, Fyffe RE, Rethelyi M. Morphological properties of physiologically characterized lamina III neurones in the cat spinal cord. Neuroscience. 1983;10:1–22. doi: 10.1016/0306-4522(83)90076-3. [DOI] [PubMed] [Google Scholar]
- Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS One. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim M, Spike RC, Watt C, Shehab SA, Todd AJ. Cells in laminae III and IV of the rat spinal cord that possess the neurokinin-1 receptor and have dorsally directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. J Neurosci. 1997;17:5536–5548. doi: 10.1523/JNEUROSCI.17-14-05536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCγ interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Polgár E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Powell JJ, Todd AJ. Light and electron microscope study of GABA-immunoreactive neurones in lamina III of rat spinal cord. J Comp Neurol. 1992;315:125–136. doi: 10.1002/cne.903150202. [DOI] [PubMed] [Google Scholar]
- Santos SFA, Luz LL, Szucs P, Lima D, Derkach VA, Safronov BV. Transmission efficacy and plasticity in glutamatergic synapses formed by excitatory interneurons of the substantia gelatinosa in the rat spinal cord. PLoS One. 2009;4:e0847. doi: 10.1371/journal.pone.0008047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP. Functional properties and axon terminations of interneurons in laminae III-V of the mammalian spinal dorsal horn in vitro. J Neurophysiol. 1992;68:1746–1759. doi: 10.1152/jn.1992.68.5.1746. [DOI] [PubMed] [Google Scholar]
- Schneider SP. Local circuit connections between hamster laminae III and IV dorsal horn neurons. J Neurophysiol. 2008;99:1306–1318. doi: 10.1152/jn.00962.2007. [DOI] [PubMed] [Google Scholar]
- Sedivec MJ, Ovelmen-Levitt J, Karp R, Mendell LM. Increase in nociceptive input to spinocervical tract neurons following chronic partial deafferentation. J Neurosci. 1983;3:1511–1519. doi: 10.1523/JNEUROSCI.03-07-01511.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Potrebic S, Maciewicz RJ. Anatomical properties of brainstem trigeminal neurons that respond to electrical stimulation of dural blood vessels. J Comp Neurol. 1994;346:349–365. doi: 10.1002/cne.903460304. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Cells in laminae III and IV of rat spinal dorsal horn receive monosynaptic primary afferent input in lamina II. J Comp Neurol. 1989;289:676–686. doi: 10.1002/cne.902890411. [DOI] [PubMed] [Google Scholar]
- Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 3rd edn. New York: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Schneider SP. Short-term modulation at synapses between neurons in laminae II-V of the rodent spinal dorsal horn. J Neurophysiol. 2011;105:2920–2930. doi: 10.1152/jn.00684.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol. 2010;588:2065–2075. doi: 10.1113/jphysiol.2010.188052. [DOI] [PMC free article] [PubMed] [Google Scholar]


