Abstract
Body ‘ownership’ defines which things belong to us and can be manipulated by signals from cutaneous or muscle receptors. Whether signals from muscle proprioceptors on their own influence perceived ownership is unknown. We used finger-joint movement to induce illusory ownership of an artificial finger without vision. We coupled the subject's index finger to an artificial finger 12 cm above it. The experimenter held the subject's other index finger and thumb on the artificial finger and passively moved them congruently or incongruently for 3 min with the index finger and the grasping index finger and thumb intact or anaesthetised. When intact, congruent movement (19 subjects) reduced perceived vertical distance between index fingers to 1.0 (0.0, 2.0) cm [median (IQR)] from 3.0 (3.0, 4.0) cm with incongruent movement (P < 0.01). Simply grasping the artificial finger reduced perceived spacing between the grasping and test index fingers from 6.0 (5.0, 9.0) cm to 3.0 (3.0, 6.0) cm (P < 0.01), a new grasp illusion. Digital anaesthesia eliminated this grasp effect, after which congruent movement still reduced the perceived spacing between the index fingers to 1.0 (0.0, 2.75) cm compared to 4.0 (3.25, 6.0) cm with incongruent movement (P < 0.001). Subjects more strongly agreed that they were holding their own finger after congruent but not incongruent movement (P < 0.01). We propose that the brain generates possible scenarios and tests them against available sensory information. This process can function without vision or motor commands, and with only one channel of somatic information.
Key points
The brain keeps a representation of which things are part of our body. This sense of ownership is easily manipulated using brushing of the skin or movement of a limb to create an illusion of ownership over an inanimate object, such as a rubber hand.
We induced a sense of ownership of an artificial finger using movement of the index finger without vision of the hands. As cutaneous receptors had been anaesthetised, this illusion depended on proprioceptive signals from muscle receptors.
In addition, we found a new grasp illusion in which perceived distance between the index fingers decreases when subjects hold an artificial finger.
These results increase understanding of how the brain generates our body representation and may help in understanding diseases in which the sense of ownership is disrupted.
Introduction
Your hand is yours, not someone else's. While this may seem a truism, several lines of evidence indicate that the sense of body ownership, the feeling that your body belongs to you, is continuously updated and can be modified by sensory manipulation and disease (e.g. Boesebeck & Ebner, 2004; Moseley et al. 2008; Feinberg et al. 2010; review Proske & Gandevia, 2012). A landmark study by Botvinick and Cohen (1998) demonstrated the malleability of body ownership by manipulation of cutaneous signals to induce an illusion of ownership over an artificial hand. This phenomenon, referred to as the ‘rubber hand illusion’, is produced by lightly brushing a visible rubber hand and the subject's hidden hand synchronously. After a few minutes of brushing, subjects report that they feel the stimulus at the physical space occupied by the rubber hand rather than on their own hand. Subjects also report that the rubber hand feels like it is their hand.
The rubber hand illusion depends on the presence of spatially and temporally correlated sensory signals from vision, touch and proprioception, and this congruent information may have a role in continually updating our sense of body ownership (e.g. Botvinick & Cohen, 1998; Ehrsson et al. 2005; Shimada et al. 2009). While the original study by Botvinick and Cohen (1998) involved visual feedback and touch, the rubber hand illusion and its variants have been produced using visual feedback and proprioceptive signals from skin, joint and muscle receptors (Dummer et al. 2009), visual feedback and proprioceptive signals from muscle receptors alone (Walsh et al. 2011), as well as combined cutaneous and proprioceptive signals without visual feedback (Ehrsson et al. 2005; Petkova et al. 2012). Functional magnetic resonance imaging has provided some support for the role of multisensory integration in the self-attribution of body parts. Specifically, the rubber hand illusion is associated with increased activity in the ventral premotor cortex and parietocerebellar regions; activity in these brain regions is thought to reflect the neural processing of multisensory signals (e.g. Ehrsson et al. 2004, 2005). Despite strong evidence that correlated multisensory inputs can lead to a sense of ownership over an artificial body part, it is not known whether multiple channels of sensory information (e.g. skin plus muscle) are required for the illusion. Can the sense of body ownership be influenced by a single sensory channel, in this case, muscle receptors?
Congruence of sensory stimuli is the other factor that has been identified as crucial for the generation of the rubber hand illusion. Many studies have shown that asynchrony between the stimuli applied to the rubber hand and the subject's real hand reduces the vividness of the illusion or even abolishes it (Armel & Ramachandran, 2003; Ehrsson et al. 2004, 2005; Shimada et al. 2009; Walsh et al. 2011). The importance of temporal congruence of sensory information on the sense of body ownership and the rubber hand illusion is further supported by the correlation between activity in parts of the cerebellum and the vividness of the illusion (Ehrsson et al. 2005). These cerebellar areas compute the relationship between sensory signals and between sensory and motor signals, including their timing (Miall et al. 1993; Ito, 2000; Miall & Reckess, 2002; Ohyama et al. 2003). It is unclear whether appropriately timed signals from muscle receptors, in the absence of other sensory signals, can generate a sense of ownership.
This study aimed to determine whether proprioceptive signals from muscle receptors could influence the sense of body ownership on their own. To answer this question it was necessary to eliminate visual feedback and non-muscle-based proprioceptive feedback (i.e. joint and cutaneous receptors). We modified the method we recently devised to induce a ‘plastic finger illusion’ to eliminate visual feedback (Walsh et al. 2011). The finger was used because it is feasible to block the digital nerves to remove all input from local cutaneous and joint receptors. As the muscles that move the fingers are located in the hand and forearm, muscle receptors that signal finger movements remain intact. In studying these questions, we found a new illusion in which sensory signals from skin receptors of the digits grasping the artificial finger, in the absence of movement, cause subjects to perceive their index fingers closer together. Digital nerve block of the grasping index finger and thumb confirmed that skin receptors were responsible for this grasp effect, and ensured our subsequent movement-based experiment focused solely on muscle receptors. In the current experiment subjects passively grasped an artificial finger located 12 cm above the index finger of their other hand. The artificial finger and the subject's index finger were moved congruently or incongruently, with the digital nerves of the index finger intact or blocked. We hypothesised that congruent movement would induce an illusion of ownership over the artificial finger during the digital nerve block when muscle receptors were the sole source of sensory information about the index finger. Furthermore, we hypothesised subjects would perceive their index fingers to be closer together during congruent movement compared to incongruent movement. Because perceived ownership and changes in proprioception can be dissociated (e.g. Rohde et al. 2011), we also assessed subjects’ response to a statement about finger ownership. Some preliminary results were presented as an abstract at IUPS (Gandevia et al. 2013).
Methods
Nineteen subjects participated in the main part of the study (12 male, mean age 36 years, range 24–57 years). Of these subjects, 10 (seven male, mean age 39 years, range 26–57) also performed a grasp experiment without vision and nine (five male, mean age 33 years, range 24–53) responded to a statement on perceived ownership of the artificial finger. Ten additional subjects (five male, mean age 28, range 23–35) participated in an experiment to validate our measure of perceived spacing. Subjects gave written informed consent. The experimental procedures were in accordance with the Declaration of Helsinki and approved by the University of New South Wales Human Research Ethics Committee. Subjects were informed about the experimental procedures but were unaware of the experimental hypothesis.
Experimental set-up
The subject sat at a table and put both hands into a box, one at time, right hand first. The box covered both of the subject's hands and the experimental apparatus so that the subject could not see their hands or the apparatus. The right forearm and hand rested semi-pronated on the lower of two tables that were inside the box (see Fig. 1A). The distal and middle segments of the right index finger (referred to as the ‘test’ index finger) were wrapped in a piece of neoprene and placed snugly in a pipe attached to a rotating shaft via a mechanical coupling. The top of the shaft was attached to an artificial index finger from a right prosthetic hand. This finger was made from and filled with silicone, and a narrow rigid plastic cylinder (diameter 0.5 cm, length 3 cm) ran down the inside of the finger like a bone. The shaft that connected the subject's test index finger to the artificial finger was co-linear with the proximal interphalangeal joint of both fingers. When the shaft's coupling was locked, movement of either the subject's test index finger or the artificial finger was reproduced in the other: movement between the two fingers was congruent. When the coupling was disengaged, the artificial finger and the subject's test index finger could move independently: movement between the two fingers was incongruent.
Figure 1. Experimental set-up and measurement validation.
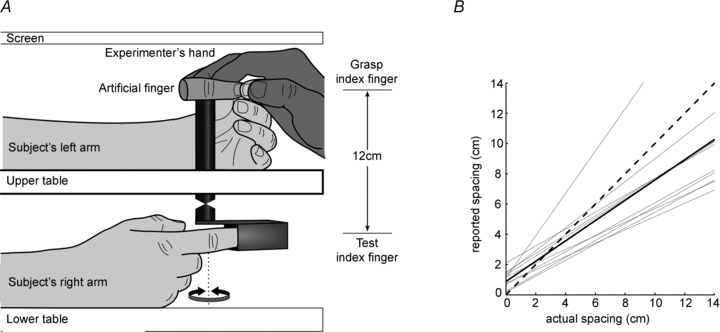
A, subjects had their right hand resting on the lower table and their left hand on the upper table, both screened from view. The right (test) index finger was held in a pipe connected to a shaft on which an artificial prosthetic finger was mounted, 12 cm separated the subject's test index and the artificial finger. The dotted line indicates the axis of rotation of the shaft, which was co-linear with the proximal interphalangeal joint of the artificial finger and the subject's test finger. A coupling on the shaft allowed movement between the artificial finger and the subject's test finger to be either congruent or incongruent. The experimenter held the subject's left (grasping) index finger and thumb in a passive pinch grip on the artificial finger. B, validation of the measure of perceived vertical spacing used in both experiments. Grey lines show regression fits to 10 subjects. The black line shows the group mean regression line and the dashed line is the line of identity. Subjects underestimated the distance between their fingers when the spacing between left and right index fingers was greater than ∼3 cm. None the less, the perceived spacing between subject's index fingers and the actual spacing was linearly related. R2 values for individual subjects varied from 0.96 to 0.99.
The subject's left forearm and hand rested on the upper table with their left index finger and thumb (referred to as the ‘grasping’ index finger and thumb) oriented to grasp the distal segment of the artificial finger. For all trials (excluding the non-grasping trials), the subject kept their hands relaxed and the experimenter held the subject's left index finger and thumb so that they were grasping the distal segment of the artificial finger. We call this a ‘passive grasp’ because the subject, with the help of the experimenter, is grasping the artificial finger with a passive left hand. A towel covered the table and the subject's shoulders and arms to remove all visual feedback. To ensure that the illusion reported by the subject was induced by muscle receptors from the test index finger rather than cutaneous or joint information from the same finger or the grasping index finger and thumb, the digital nerves of the test index finger and grasping index finger and thumb were blocked for some experimental trials (see ‘Digital nerve block’ below).
Experimental procedures
This experiment tested whether proprioceptive input from muscle receptors in the test index finger was sufficient to induce an illusion of body ownership over an artificial finger. To answer this question, sensory feedback and congruence of the movement between the subject's test index finger and the artificial finger were manipulated in four experimental trials. During the first trial, the subject's test index finger and grasping index finger and thumb were intact and the coupling on the rotating shaft was locked so that movements of the test and artificial fingers were directly coupled (i.e. congruent). The subject was instructed to keep their eyes closed and to keep both hands relaxed. Next, the subject's grasping index finger and thumb that were passively holding the artificial finger were moved by the experimenter to produce flexion–extension movements about the proximal interphalangeal joint for a period of 3 min. The velocity and amplitude of these movements were varied pseudo-randomly by the experimenter with all movements occurring at ∼5–20 ° s−1 between 10° and 40° of flexion at the proximal interphalangeal joint. Care was taken to ensure that movement in the test finger occurred only at this joint. In the grasping hand, movement of the metacarpophalangeal joint was common and small movements of the wrist occurred sometimes. The second experimental trial was similar to the first, but the coupling on the rotating shaft was unlocked. The experimenter produced movements of the test index finger and the artificial finger that were similar in velocity and amplitude to the movements in the congruent condition, except they were incongruent. The third and fourth trials were the same as the first and second trials, except that they were performed after the digital nerves of the test index finger and the grasping index finger and thumb were blocked with local anaesthetic (see below). Nine subjects were tested during a single experimental session with the intact trials performed first and another 10 subjects were tested over 2 days, with the blocked trials performed on the first day. For subjects doing the intact trials first, approximately 30 min separated the intact trials from the blocked trials, during which time the digital nerve blocks were applied. When subjects did the blocked trials first, we waited 24 h to perform the intact trials to ensure full recovery from the anaesthetic. The order of the congruent and incongruent trials was randomised.
The subject reported the perceived vertical spacing between their test index finger from their grasping index finger following congruent and incongruent movement trials when these digits were intact and when they were blocked. The subject was presented with an A3 sheet of paper (297 mm × 420 mm) in landscape orientation on which was printed a series of 21 vertical lines in the shape of error bars ranging in height from 0 to 20 cm in 1 cm steps. Two horizontally staggered lines, one thicker than the other, were used for 0 cm. The tops of each error bar were aligned vertically with one another, and each error bar was identified by a letter ranging from A to U. Ten of these sheets were used with the 21 error bars in random order so that the order on each page differed and no identifying letter ever corresponded to the same error bar. For each evaluation, a randomly selected sheet was presented at the subject's eye level, 60 cm from their forehead. The subject did not see a given sheet more than once. The subject was asked ‘which line represents the vertical distance between the tip of your left index finger and the tip of your right index finger?’ When set-up for the experiment, the subject's test index finger was 12 cm below their grasping index finger (Fig. 1A). The measure of the perceived vertical distance between the index fingers is new and so we performed a separate validation experiment (see below).
In preliminary studies using the same apparatus as described above, we noticed that subjects experienced a reduction in perceived spacing between their index fingers after the experimenter closed their grasping index finger and thumb on the artificial finger, but before any movements had been made. This grasp effect was large and we considered that it might conceal the true size of the movement illusion. In the first 10 subjects, perceived index finger spacing was also evaluated when the subject was not grasping and then grasping the artificial finger; this was done before intact and blocked movement trials. This allowed us to determine whether removing skin receptor input from the grasping index finger and thumb was responsible for the grasp effect. In the last nine subjects, a baseline of perceived index finger spacing with full vision, to account for the effect of hiding subjects’ hands from view, was obtained at the end of the experiment. Perceived index finger spacing was measured with the digits intact and full vision of their grasping hand and the artificial finger.
Subject interviews and perceived ownership
A short structured interview consisting of six questions about the subject's perceptions and experience during the experiment was conducted after the trials with the digital nerve blocked. These interviews were recorded, transcribed and collated. After the first 10 subjects, we noted that subjects were not volunteering information about their perception of ownership over the artificial finger in the structured interview. Hence, we had the last nine subjects respond to the following ownership statement ‘I feel that I am holding my right index finger with my left hand’ after each experimental condition. This statement was based on one previously used to assess perceived ownership (Botvinick & Cohen, 1998; Walsh et al. 2011). The statement was rated on a seven-point Likert scale that contained the following options: ‘strongly agree’, ‘agree’, ‘somewhat agree’, ‘neither agree or disagree’, ‘somewhat disagree’, ‘disagree’ and ‘strongly disagree’. For statistical analysis and reporting of results the seven-point scale was converted to an ordinal scale from −3 (‘strongly disagree’) to +3 (‘strongly agree’). The experimenter read the statement while holding up a small chart that displayed the Likert scale and the subject responded verbally.
Digital nerve block
To block the digital nerves of the test index finger and grasping index finger and thumb, a total of 3–4 ml of 1% lignocaine without adrenaline was injected into the medial and lateral side of the digits about 1 cm distal to the metacarpophalangeal joint. A piece of tape was placed around the finger just distal to the metacarpophalangeal joint to impede the venous return from the finger and thus prolong the block. The block was clinically complete in 5–10 min, with loss of all light touch sensation. Light touch was tested intermittently to ensure that the block remained complete during the experimental trials. After the experiment, the tape was removed and digital sensation recovered within a few hours.
Validation of the measure of perceived index finger spacing
Our measure of perceived vertical spacing between the index fingers is new. We developed this measure because it was simple for subjects to understand and we could administer it simply and quickly. It was important to establish that the perceived spacing between index fingers reported by subjects was related to the actual spacing between their index fingers. To assess this, 10 subjects reported the perceived vertical spacing between their left and right index fingers using the same method used for the non-grasping trials in the main experiment (see above). However, vertical spacing between the index fingers was varied from 0 cm to 14 cm in 2 cm increments by changing the height of the grasping hand, with each spacing presented four times. The order of spacings was randomised and then adjusted to reduce the influence of very different previous trials.
Data and statistical analysis
Preliminary analysis confirmed that data were not normally distributed, and thus all values are presented as median (interquartile range) and non-parametric statistical tests were used. Wilcoxon paired-sample tests were used for all comparisons of congruent versus incongruent movement for the movement-induced illusions, and the grasp and no grasp conditions. For the validation of the measure of perceived index finger spacing, linear regression was performed on each subject's data and the slopes and intercepts from those regressions were used to determine a group mean line. All tests were carried out using SPSS (version 21; SPSS Inc., Chicago, IL, USA) with α= 0.05.
Results
We investigated if signals from muscle receptors alone could produce an illusion of ownership over an artificial finger. Subjects indicated the perceived vertical spacing between their left and right index finger. This measurement of perceived spacing was made before and after the artificial finger was passively grasped, and after the index finger and thumb holding the artificial finger were moved for 3 min. This movement could be congruent or incongruent with the subject's test index finger, and was done both with the hands intact and when the digital nerves of the test index finger and grasp index finger and thumb were blocked.
Validation and baseline measure of perceived index finger spacing
In the absence of vision, subjects were able to detect and report changes in vertical index finger spacing. The relation between perceived spacing and actual spacing was linear (R2 > 0.96, P < 0.001 for all subjects, Fig. 1B). For nine of 10 subjects in the validation experiment the fitted line had a slope less than unity. Thus, most subjects underestimated the distance between their index fingers. In the main experiments, spacing between index fingers was 12 cm. These results show that subjects perceive this spacing as 8.0 (6.6, 9.7) cm [median (IQR)], which is significantly less than the veridical spacing of 12 cm (P < 0.001).
To obtain a baseline measure of perceived spacing, we evaluated perceived index finger spacing when subjects were able to see their grasping index finger and thumb and the artificial finger. This was done in the last nine subjects who participated in the main experiment. Under these conditions, subjects reported a median perceived spacing of 8.5 (8.0, 10.0) cm, which was also significantly less than 12 cm (P < 0.001).
Perceived index finger spacing
In the absence of vision and movement, passively holding the artificial finger with the intact grasping index finger and thumb reduced perceived spacing of the index fingers from 6.0 (5.0, 9.0) cm to 3.0 (3.0, 6.0) cm (P= 0.007; Fig. 2A). However, after blocking the digital nerves of the grasping index finger and thumb as well as the test index finger, grasping the artificial finger did not significantly reduce perceived index finger spacing (no grasp 8.0 (6.0, 10.0) cm, grasp 6.0 (4.0, 8.0) cm, P= 0.065; Fig. 2B). This result confirmed that the grasp effect was influenced by cutaneous inputs from the grasping index finger and thumb.
Figure 2. The median (10 subjects) effect of passively grasping the artificial finger on perceived vertical spacing between the index fingers.
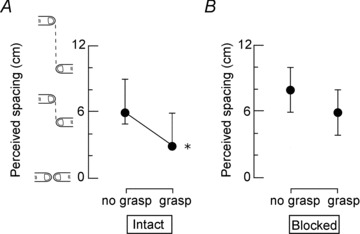
Results of the grasp effect when (A) the test index finger and the grasping index finger and thumb were intact, and (B) the digital nerves of these digits were blocked. Perceived index finger spacing was reduced significantly when subjects passively grasped the artificial finger and all digits were intact (P= 0.007). When the test index finger and grasping index finger and thumb were blocked, a passive grasp of the artificial finger did not significantly change perceived index finger spacing (P= 0.065). Results are shown as median ± interquartile range.
When the test index finger and grasping index finger and thumb were intact, perceived spacing between the index fingers was smaller after congruent movement [1.0 (0.0, 2.0) cm] compared to after incongruent movement [3.0 (3.0, 4.0) cm, P= 0.007, Fig. 3]. This corresponded to a reduction in perceived spacing between the incongruent and congruent conditions in 16 subjects, an increase in perceived spacing in two subjects and no change in one subject. After the digital nerves were blocked, the perceived spacing was 1.0 (0.0, 2.75) cm following congruent movement, which was significantly smaller than the perceived spacing following incongruent movement (4.0 (3.25, 6.0) cm; P < 0.001]. This corresponded to a reduction in perceived spacing between the incongruent to congruent conditions in 17 subjects and an increase in perceived spacing in two subjects.
Figure 3. The median (19 subjects) effect of movement congruence and blocking the digital nerves of the grasping and test digits on perceived vertical spacing between the index fingers.
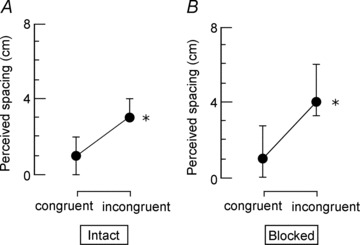
A, perceived vertical spacing between the index fingers when the hands are intact for congruent and incongruent movement trials. A significantly smaller median perceived spacing was reported after congruent movement compared to incongruent movement (P= 0.007). B, results for the same conditions after the digital nerves of the test index finger and the grasping index finger and thumb were blocked with local anaesthetic. Again, there is a significantly smaller perceived spacing after congruent movement compared to incongruent movement (P < 0.001). Results are shown as median ± interquartile range.
Subject interviews and perceived ownership
The first 10 subjects who participated in the main experiment received a structured interview about their experience after the session with the digital nerve block. When responses were compared, two common experiences emerged. The first was a feeling that the intact grasping index finger and thumb were holding, touching or aligned with their own test index finger, rather than the artificial finger (40% subjects). The second was a feeling that the blocked digits felt heavy (50% subjects).
In the subsequent nine subjects in whom we measured perceived ownership, responses to the statement ‘I feel that I am holding my right index finger with my left hand’ were modified by the movement stimulus and digital anaesthesia. With the digits intact there was a shift in the median response from −2.0 (−3.0, −2.0) after incongruent movement to −1.0 (−1.5, 1.0) after congruent movement (Fig. 4; P= 0.008). This was a consistent change for all the subjects and represents a change from ‘disagree’ to ‘somewhat disagree’. After incongruent movement, all subjects disagreed with the statement that it felt that they were holding their right index with their left hand. In contrast, four of the nine subjects agreed with the statement after congruent movement. When the digital nerves of the test index finger and the grasping index finger and thumb were blocked, the median response after incongruent movement was −2.0 (−3.0, −1.5), which was significantly less than the median response after congruent movement [2.0 (1.0, 2.0), P= 0.004]. This represents a change in median response from ‘disagree’ to ‘agree’. All but one subject responded with some agreement to the statement following congruent movement, but all subjects disagreed after incongruent movement. Simply grasping the artificial finger was not enough to induce perceived ownership over the artificial finger. When subjects answered the survey after grasping the artificial finger without vision, only one subject gave a response on the agree side of the Likert scale.
Figure 4. The median (nine subjects) effect of congruent movement and blocking the digital nerves of the grasping and test fingers on perceived ownership over the artificial finger.
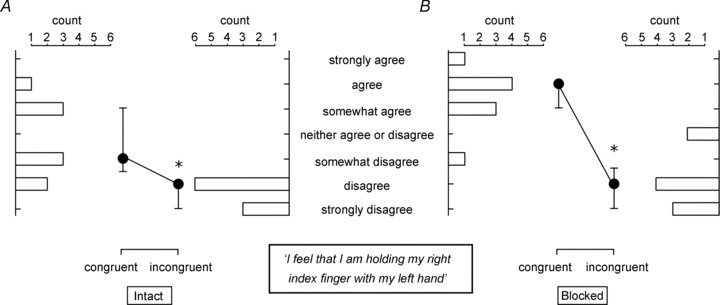
Filled circles show median ± interquartile range responses to the statement ‘I feel that I am holding my right index finger with my left hand’. Horizontal bars show the number of subjects that selected that particular response for the congruent and incongruent conditions. A, with the hands intact, median responses were significantly higher after congruent movement versus incongruent movement (P= 0.008); however, the median response after congruent movement was still on the side of disagreement. B, following digital nerve block the difference between median responses for incongruent and congruent movement was larger (P= 0.004) and all but one subject had some level of agreement with the statement after congruent movement.
Discussion
This study produced two novel findings about proprioception and body ownership. First, the sense of body ownership and perception of the index fingers can be manipulated using only signals from muscle receptors. Second, we described a new grasp illusion, which is induced when subjects grasp passively an artificial finger placed between their index finger and thumb. The discussion will consider the reduction in perceived spacing between index fingers after congruent movement as well as how these results and the new grasp effect provide insight into proprioception and the sense of body ownership.
Muscle receptor input and movement congruence
We have shown previously that, with visual feedback, muscle receptors signalling finger movement congruent with the motion of an artificial finger contribute to the sense of body ownership (Walsh et al. 2011). We now show that signals generated from muscle receptors can influence body ownership in the absence of other sensory signals. In the current experiment, the grasping index finger and thumb were used to move an artificial finger that could be coupled or uncoupled to the test index finger. Movement of the test index finger occurred at the proximal interphalangeal joint. Compared to incongruent movement, congruent movement more than halved the perceived spacing between the index fingers. The key experimental condition was when the digital nerves were blocked. This allowed us to determine whether input from muscle receptors signalling movement of the test index finger could influence the sense of body ownership without signals from skin and joint receptors from this finger and the grasping index finger and thumb. Cutaneous receptors proximal to the metacarpophalangeal joint can be activated during finger movements (Edin & Abbs, 1991), but we believe our experimental procedures ensured minimal contribution from these receptors to sensing finger movements in the test index finger. Given that the movement stimuli were passive, tendon organs were also unlikely to have been activated (e.g. Houk & Henneman, 1967; Burke et al. 1978; al-Falahe et al. 1990). Muscle spindles were therefore the main receptors contributing to the illusions in this experiment. Furthermore, our stimulus was movement, so movement-sensitive primary muscle spindle afferents will have been the main contributors to the induced illusions (e.g. Matthews, 1972).
Generating a sense of body ownership from a single sensory channel
The sense of body ownership is thought to be a centrally stored representation and it needs to be generated from sensory information. Body ownership is usually manipulated experimentally with a stimulus that provides congruent information to the brain via two or more sensory channels. This is typically done using the sense of touch and vision (e.g. Botvinick & Cohen, 1998; Ehrsson et al. 2007; Tsakiris et al. 2007) but it can be done using muscle receptors and vision (Walsh et al. 2011). It may be thought that vision is required and that the illusion results from the dominance of vision over other channels (e.g. Izumizaki et al. 2010). However, there are now three studies, two by others (Ehrsson et al. 2005; Petkova et al. 2012) and the present study, showing that vision is not required to manipulate the sense of body ownership. Uniquely, our results demonstrate that muscle receptors alone can influence the sense of body ownership as indicated by the results of the measure of perceived ownership (Fig. 4). Although sensory information was coming only from muscle receptors, it was coming from multiple sites, that is, both hands. Congruence between these two sources reduced perceived index finger spacing and induced an illusion of ownership over the artificial finger. To experience an illusion of ownership over the artificial finger, subjects required information from their grasping hand that was consistent with it moving, along with information from the test index finger that it was being moved. Cutaneous and joint receptors about the wrist may have detected small movements of the grasping hand despite our attempts to eliminate wrist movement. However, these skin and joint receptors in the wrist could not signal grasp-related events, they could only signal wrist movement. Furthermore, while receptors from muscles that move the grasp index finger and thumb remained intact, these receptors do not encode object contact or finger apposition during a grasping task (Dimitriou & Edin, 2008). It is notable that all the changes in perceived spacing between the fingers occur despite some differences in the absolute angle of the elbows and shoulders that should indicate that the hands are not aligned.
How does the brain determine if information from multiple sites is more consistent with one scenario or another? Is my left hand holding my right index finger or something else? Prediction-based models, which have been influential in the field of motor control, are one way sensory information could be combined to determine if actions are self-generated or externally generated (Blakemore et al. 1998; Shergill et al. 2003; Bays et al. 2006; review Proske & Gandevia, 2012). These models are not directly applicable to our study because subjects did not generate motor commands. An alternative is that the brain generates internal sensory representations of possible scenarios and verifies them for consistency with incoming sensory information. For the present experiment, one such scenario could be ‘I am moving my finger’. If the grasping index finger and thumb were moving the test index finger, motion of these digits would be congruent and the proprioceptive information would confirm this. Our experiments created a situation where information from muscle receptors was consistent with the grasping hand moving the test index finger, even though this did not physically occur. The brain appears to use the congruent movement information from the left and right sides of the body to determine the most probable scenario. Such a ‘scenario-testing’ model is attractive because it applies to many sensory situations, including the attribution of actions to self or other, normally described by prediction-based models. It is also consistent with many illusions in the visual system (Gregory, 2009).
In addition to considering the effect of congruent muscle receptor signals on perceived index finger spacing, we assessed how these congruent signals influenced subjects’ perceived ownership of the artificial finger. Responses to the ownership statement ‘It seems that I am holding my right index finger with my left hand’ indicate that the illusion of ownership following congruent movement was more vivid when the digital nerves were blocked compared to when they were intact. With the digital nerves intact, the median response to the ownership statement was ‘disagree’ after incongruent movement and ‘somewhat disagree’ after congruent movement. However, with the digital nerves blocked the median response to the ownership statement changed from ‘disagree’ after incongruent movement to ‘agree’ after congruent movement. This difference in the degree of perceived ownership between the intact and blocked conditions is probably due to touch information from the intact grasping index finger and thumb not being consistent with the subject holding or moving their own finger. This inconsistent self-touch information may be responsible for the lack of ownership after congruent movement. However, when self-touch information was eliminated by the digital nerve block, eight of nine subjects reported some level of perceived ownership over the artificial finger after congruent movement.
Finger grasp illusion
The novel grasp illusion is important because it rapidly reduced the perceived finger spacing between the index fingers compared to when the artificial finger was not grasped. The grasp effect occurred within seconds of passively grasping the artificial finger, although we did not quantify the time course in this study. This contrasts with the rubber hand illusion, which can take minutes to induce (e.g. Botvinick & Cohen, 1998; Ehrsson et al. 2005). There was a significant grasp effect when both hands were intact, but not when the digital nerves of the grasping index finger and thumb were blocked (Fig. 2). This illusion requires information from only one sensory channel (touch) and only one intact sensory site, the grasping hand, as the illusion can arise when just the test finger is blocked (unpublished observation). The scenario-testing approach proposed above can also explain this grasp effect. Grasping the test index finger would produce the same touch signals as grasping the artificial finger. In addition, the artificial finger feels like a real finger and it is not often that we grasp things that feel like a finger but are not a finger. Thus, the sensory signals arising from the grasping hand are consistent with a scenario in which the grasping hand is holding a finger. Furthermore, the broader sensory information is consistent with the subject grasping a finger as both of their hands are inside the same box in an orientation that makes holding their own finger a possibility. We predicted that cutaneous input from the grasping index finger and thumb was crucial to this grasp effect, which was confirmed when these digits were blocked and the difference in perceived index finger spacing between the no grasp and grasp conditions was no longer significant.
Conclusion
In summary, a single sensory channel can modify the sense of body ownership. When information from a single sensory channel arises from distinct anatomical sites, congruence of sensory signals is crucial to generate a sense of body ownership. When the information arises from a single anatomical site, it is compared to and subsequently updates an internal body representation generated from broader sensory information. We propose that the brain compares the sensory information it receives for consistency with possible scenarios and accepts the scenario that best matches the sensory information. These results expand our understanding of proprioception and body representation and should provide new insight into clinical conditions in which they are disrupted.
Additional information
Competing interests
None declared.
Author contributions
All authors contributed to all parts of this study. M. Héroux and L. Walsh contributed equally. All authors approved the final version of the manuscript. All experiments were performed at Neuroscience Research Australia, Sydney, Australia.
Funding
This work was funded by the National Health and Medical Research Council (of Australia).
References
- al-Falahe NA, Nagaoka M, Vallbo ÅB. Response profiles of human muscle afferents during active finger movements. Brain. 1990;113:325–346. doi: 10.1093/brain/113.2.325. [DOI] [PubMed] [Google Scholar]
- Armel KC, Ramachandran VS. Projecting sensations to external objects: evidence from skin conductance response. Proc Biol Sci. 2003;270:1499–1506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Flanagan JR, Wolpert DM. Attenuation of self-generated tactile sensations is predictive, not postdictive. PLoS Biol. 2006;4:e28. doi: 10.1371/journal.pbio.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci. 1998;1:635–640. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Boesebeck F, Ebner A. Paroxysmal alien limb phenomena due to epileptic seizures and electrical cortical stimulation. Neurology. 2004;63:1725–1727. doi: 10.1212/01.wnl.0000143064.81746.e9. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Discharges in human muscle receptor afferents during block grasping. J Neurosci. 2008;28:12632–12642. doi: 10.1523/JNEUROSCI.3357-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer T, Picot-Annand A, Neal T, Moore C. Movement and the rubber hand illusion. Perception. 2009;38:271–280. doi: 10.1068/p5921. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Holmes NP, Passingham RE. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J Neurosci. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Wiech K, Weiskopf N, Dolan RJ, Passingham RE. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc Natl Acad Sci U S A. 2007;104:9828–9833. doi: 10.1073/pnas.0610011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg TE, Venneri A, Simone AM, Fan Y, Northoff G. The neuroanatomy of asomatognosia and somatoparaphrenia. J Neurol Neurosurg Psychiatry. 2010;81:276–281. doi: 10.1136/jnnp.2009.188946. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Walsh LD, Butler AA, Héroux ME. An illusion of grasping your own finger. Int Congr Physiol Sci. 2013;37:PCD149. [Google Scholar]
- Gregory R. Seeing Through Illusions. Oxford University Press; 2009. [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967;30:466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- Izumizaki M, Tsuge M, Akai L, Proske U, Homma I. The illusion of changed position and movement from vibrating one arm is altered by vision or movement of the other arm. J Physiol. 2010;588:2789–2800. doi: 10.1113/jphysiol.2010.192336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and their Central Actions. London: Arnold; 1972. [Google Scholar]
- Miall RC, Reckess GZ. The cerebellum and the timing of coordinated eye and hand tracking. Brain Cogn. 2002;48:212–226. doi: 10.1006/brcg.2001.1314. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a Smith predictor. J Mot Behav. 1993;25:203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Olthof N, Venema A, Don S, Wijers M, Gallace A, Spence C. Psychologically induced cooling of a specific body part caused by the illusory ownership of an artificial counterpart. Proc Natl Acad Sci U S A. 2008;105:13169–13173. doi: 10.1073/pnas.0803768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Zetterberg H, Ehrsson HH. Rubber hands feel touch, but not in blind individuals. PLoS One. 2012;7:e35912. doi: 10.1371/journal.pone.0035912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: Their roles in signalling body shape, body position and movements, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Rohde M, Di Luca M, Ernst MO. The Rubber Hand Illusion: feeling of ownership and proprioceptive drift do not go hand in hand. PLoS One. 2011;6:e21659. doi: 10.1371/journal.pone.0021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: The neuroscience of force escalation. Science. 2003;301:187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- Shimada S, Fukuda K, Hiraki K. Rubber hand illusion under delayed visual feedback. PLoS One. 2009;4:e6185. doi: 10.1371/journal.pone.0006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Moseley GL, Taylor JL, Gandevia SC. Proprioceptive signals contribute to the sense of body ownership. J Physiol. 2011;589:3009–3021. doi: 10.1113/jphysiol.2011.204941. [DOI] [PMC free article] [PubMed] [Google Scholar]


