Abstract
We show that various types of rods and cones in the dark-adapted salamander retina are electrically coupled with linear and symmetrical junctional conductances Gj (40–223 pS) and a rank order: RodC–large single cone, rod–large single cone, rod–small single cone, rod–accessory double cone and rod–principal double cone. By systematically comparing the transjunctional current–voltage (Ij–Vj) relations and average Gj values of the five types of rod–cone pairs recorded at day and night times, our results suggest that the differences in Gj values among various types of rod–cone pairs are not caused by circadian differences, and the circadian-dependent changes in rod–cone coupling observed in the fish and rodent retinas are not present in the tiger salamander. In addition to rod–cone coupling, there is a sign-inverting, unidirectional rod→cone current IRC, and the IRC–VCone relations are linear, with a reversal potential near the chloride reversal potential ECl. IRC can be observed in rods and cones separated by at least 260 μm, and its waveform resembles that of the rod-elicited horizontal cell (HC) response IHC. A glutamate transporter-associated chloride channel blocker TBOA suppresses IRC but not IHC. These results suggest that IRC is largely mediated by HCs via a sign-inverting feedback chemical synapse associated with a chloride channel. IRC significantly reduced rod→cone coupling in the frequency range below 15 Hz, allowing better separation of rod and cone signals in the dark-adapted retina.
Key points
Five types of rods and cones in the dark-adapted salamander retina are electrically coupled with linear and symmetrical junctional conductances Gj of different average values.
The average Gj values of the five types of rod–cone pairs recorded at day and night times suggest that the the circadian-dependent changes in rod–cone coupling observed in the fish and rodent retinas are not present in the tiger salamander.
In addition to rod–cone coupling, there is a sign-inverting, unidirectional rod→cone current IRC, and the current–voltage (IRC–VCone) relations are linear, with a reversal potential near the chloride reversal potential ECl.
IRC can be observed in rods and cones separated by at least 260 μm, and its waveform resembles that of the rod-elicited horizontal cell (HC) response IHC; a glutamate transporter-associated chloride channel blocker TBOA suppresses IRC without affecting IHC.
These results suggest that IRC is largely mediated by HCs via a sign-inverting feedback chemical synapse associated with a chloride channel in cones.
Introduction
Vertebrate retinas contain two types of photoreceptors, rods and cones, with the former registering dim light signals and the latter encoding brighter images (Dowling, 1987). Photoreceptors interact with one another through electrical synapses (coupling), which mediate a sign-preserving, bi-directional signalling network (Baylor et al. 1971; Attwell et al. 1984; Zhang & Wu, 2005). In most vertebrates, photoreceptor pairs of the same kind, i.e. rod–rod and cone–cone pairs, are strongly coupled (homocellular coupling; Li & Devries, 2004; Hornstein et al. 2004; Zhang & Wu, 2005), whereas photoreceptors of different kinds, such as rod–cone pairs, are weakly coupled (heterocellular coupling; Schwartz, 1975; Attwell et al. 1984; Li et al. 2010). In the salamander retina, rods are strongly coupled with one another where cones are not, and rods and cones are weakly coupled (Attwell et al. 1984), but detailed dual voltage clamp studies have not been performed on rod–cone pairs, and thus it is uncertain whether coupling exists between rods and every type of cones, and whether electrical synapses between various types of rod–cone pairs exhibit the same biophysical properties.
Similar to many other vertebrates, six morphologically distinct types of photoreceptors have been identified in the tiger salamander retina: large and small rods, large and small single cones (lC and sC), and double cones composed of principal and accessory members (pC and aC) (Zhang & Wu, 2009). The large and small rods accounted for 98.6% and 1.4% of all rods, respectively, and thus only large rods (R) are considered in most physiological studies, including the present one. Among large rods, about 10% are strongly coupled with cones (rodC, RC; Wu & Yang, 1988). Large/small single cones, principal/accessory members of double cones, and s-cone opsin-positive single cones accounted for about 66.9%, 23% and 10.1% of the total cones, respectively (Zhang & Wu, 2009). It is of great interest to determine whether rods are coupled with each of the four types of cones, and whether these couplings are symmetrical, linear, and of different strengths. In this study, we performed dual voltage clamp experiments on various types of morphologically identified rod–cone pairs in the flat-mounted salamander retina, and determined the bi-directional current–voltage relations and junctional conductance.
In addition to the sign-preserving, bi-directional rod–cone coupling, a sign-inverting, unidirectional rod→cone transient signalling pathway has been reported (Attwell et al. 1983). Due to limitations in data pool sizes and the microelectrode techniques used in the previous study, it is unclear whether such a sign-inverting signal pathway exists between all types of rod–cone pairs, what synaptic mechanisms are involved, and how the sign-inverting and sign-preserving signals interact. In this study, we systematically characterized synaptic properties of the sign-inverting rod→cone pathway by dual voltage clamp recordings from rod–cone pairs of different separation distances, as well as from rod–HC pairs. These experiments provide new evidence suggesting that a rod→HC→cone sign-inverting chemical synaptic pathway exists between rods and most cones, which suppresses rod–cone coupling in the rod→cone direction in a frequency-dependent fashion. This unidirectional inhibitory pathway may significantly contribute to segregation of rod- and cone-mediated information channels in the visual system.
Methods
Preparations
Flat-mounted retinas and living retinal slices of the larval tiger salamander (Ambystoma tigrinum) were used. The animals were purchased from Charles E. Sullivan Co. (Nashville, TN, USA) or Kon's Scientific Co., Inc. (Germantown, WI, USA), kept in aerated aquaria and fed with brine shrimp or dry fish food. Experimental procedures conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research and the NIH Guide for the Use of Laboratory Animals, and they were approved by the Committee of Animal Research of Baylor College of Medicine. The salamanders were kept in a room with strict day–night cycles: light turned on at 6 am and off at 6 pm. The majority of the experiments were carried out in the daytime (11.00–15.00 h) and the night time experiments (see Table 1 below) were carried out between 23.00 and 02.00 h. All animals were dark adapted for at least 2 h before the experiments, and then anaesthetized in 2% MS-222. The animals were then quickly decapitated and eyes were enucleated. Procedures for preparing retinal slices and flat-mounted retina are described in previous publications (Werblin, 1978; Wu, 1987b), and retinal neurons, as well as electrodes above the retina, were visualized with a dual Nitemare infrared scope (BE Meyers, Redmond, WA, USA) and a Zeiss-Hoffman or a Zeiss-DIC microscope.
Table 1.
Gj and IRCM for different rod–cone pairs recorded at day and night times
| R–lC pairs | R–pC pairs | RC–lC pairs | R–aC pairs | R–sC pairs | |
|---|---|---|---|---|---|
| Gj(d) (pS) | 110 ± 24 (12) | 43 ± 12 (8) | 221 ± 48 (7) | 77 ± 20 (7) | 87 ± 17 (6) |
| Gj(n) (pS) | 118 ± 18 (5) | 50 ± 11 (3) | 206 ± 23 (3) | 69 ± 12 (2) | 91 ± 26 (4) |
| IRCM (d) (pA) | 14.1 ± 4.8 (12) | 13.6 ± 3.1 (8) | 12.3 ± 4.0 (10) | 0 (7) | 11.7 ± 2.7 (6) |
| IRCM (n) (pA) | 15.2 ± 4.4 (5) | 14.1 ± 4.4 (3) | 11.8 ± 3.6 (3) | 0 (2) | 10.9 ± 2.9 (4) |
Average (±SD, number of cell pairs in parenthesis) values of junctional conductance Gj and maximum transient rod→cone currents IRCM (maximum IRC at the offset of the −50 to −130 mV voltage step in the next-neighbouring rod, number of cell pairs in parenthesis) of rod–large single cone (R–lC) pairs, rod–principal member of a double cone (R–pC) pairs, rodC–large single cone (RC–lC) pairs, rod–accessory member of a double cone (R–aC) pairs and rod–small single cone (R–sC) pairs. Gj(d) and IRCM (d) were recorded at day time and Gj(n) and IRCM (n) were recorded at night time (see Methods section).
Recording and dye injection
Single and double-electrode whole-cell voltage clamp experiments were carried out with the Axon MultiClamp 700A amplifier connected to a DigiData 1200 interface and pClamp 9 software (Axon Instruments, Foster City, CA, USA). Patch pipettes were made on electrode pullers (p-87 Sutter Instruments, CA, USA) from 1.5 mm thin-walled borosilicate glass (Drummond, CA, USA). Estimates of the liquid junction potential at the tip of the patch electrode prior to seal formation normally vary from −9.2 to −9.6 mV. For simplicity, we corrected all holding potentials by 10 mV. For standard whole-cell patch clamp experiments, we used patch electrodes filled with internal solution containing the following (in mm): 106 potassium gluconate, 5 NaCl, 2 MgCl2, 0.5 CaCl2, 4 ATP, 0.3 GTP, 5 EGTA, 0.8 Lucifer Yellow and 5 Hepes buffered to pH 7.4 with KOH. In experiments that require ECl= 0 mV, we replace potassium gluconate in the pipette solution with KCl so that [Cl-] in both solutions was equal.
Light stimulation
The retinal slices and flat-mounted retinas were stimulated with two light beams that pass through narrow band (10 nm half-width) interference filters and through neutral density filters via a custom-made epi-illuminator system attached to the microscope. Apertures of various diameters and shapes can be focused on the retina by the microscope objective through the epi-illuminator system. Since we deliver an un-collimated stimulus light beam through an objective lens with large numerical aperture (Zeiss ×40/0.75 water), the incident light enters the retinal slice in many directions, and thus the effect of photoreceptor self-screening is minor (Field & Rieke, 2002). The intensity of unattenuated 500 nm light (log I= 0) is 2.05 × 107 photons μm−2 s−1 (calibrated with a radiometric detector, United Detector Technology, CA, USA).
Solutions
Retinal slices or flat-mounted retinas were placed at room temperature (20–23°C) in a recording chamber that was superfused continuously with oxygenated Ringer solution containing (in mm) 108 NaCl, 2.5 KCl, 1.2 MgCl2, 2 CaCl2, 5 glucose and 5 Hepes, adjusted to pH 7.6. All pharmacological agents (Sigma, St Louis, MO, USA) were dissolved in Ringer solution and pH was re-adjusted afterwards.
Cell morphology and immunocytochemistry
Cell morphology was visualized in living retinal slices and flatmounts through the use of Lucifer Yellow fluorescence with a confocal microscope (Zeiss 510). Images were acquired by using a ×40 water immersion objective (n.a. = 0.75), the 458 nm excitation line of an argon laser, and a long pass 505 nm emission filter. Consecutive optical sections were stacked into a single image using the Zeiss LSM-PC software and the stacked images were further processed in Adobe Photoshop 6.0 to improve the contrast. For immunocytochemistry, retinas were fixed in 4% paraformaldehye in phosphate-buffered saline (PBS; pH 7.4) for 30–60 min at room temperature, and then extensively rinsed with PBS. Whole-mount retinal tissue was blocked with 3% donkey serum in PBS with 0.5% Triton X-100 and 0.1% sodium azide from 2 h to overnight to reduce non-specific labelling. The tissue was then incubated in primary antibody in the presence of 1% donkey serum/PBS with 0.5% Triton X-100/0.1% sodium azide for 3–10 days at 4°C. Antibodies against Calbindin and recoverin were obtained from Chemicon International (Temecula, CA, USA) and used at a dilution of 1:1000. Secondary antibodies were donkey conjugated CY3 (Jackson ImmunoResearch, West Grove, PA, USA) and Alexa 488 (Molecular Probes), used at a dilution of 1:100.
Results
Sign preserving rod–cone coupling and the sign-inverting, transient rod→cone signal
We performed dual voltage clamp recordings from 106 rod–cone pairs in dark-adapted flat-mounted salamander retina under visual control with infrared illumination. Rods and cones were initially identified by their outer segment morphology in the flat-mounted retina and subsequently confirmed by Lucifer Yellow fluorescence with a confocal microscope (see Methods). Figure 1 shows an example illustrating synaptic interactions between a rod and a next-neighbouring large single cone. Panels A and C show simultaneous light-evoked current responses of the rod and the cone to a 500 nm, −3.0, 0.5 s light step, and panel B is the Lucifer Yellow fluorescent image of the rod and cone outer segments in the retinal flatmount. Panel Da shows current responses of the rod (IRod, held near its dark potential at −35 mV) to voltage clamp steps in the cone (VCone) from −50 mV to various voltages (−130 to +10 mV), and panel Db shows current responses of the cone (ICone, held near its dark potential at −35 mV) to the same set of voltage clamp steps in the rod (VRod). Voltage clamp steps in the driver cell (cone in Da and rod in Db) generated sustained transjunctional currents (Ij, green arrows) in the follower cell (rod in Da and cone in Db). Transjunctional currents in both directions exhibit nearly identical waveforms and amplitudes, indicating that Ij is bi-directional and symmetrical. Moreover, depolarizing voltage steps in driver cells elicited outward currents in themselves and inward Ij in follower cells, whereas hyperpolarizing voltage steps in driver cells elicited inward currents in themselves and outward Ij in follower cells, suggesting that Ij is sign-preserving. Therefore, similar to dual voltage clamp records from electrically coupled rod pairs (Zhang & Wu, 2005), Ij represents current flows between the rod and the cone through the gap junction channels. Statistics and rod/cone-subtype variations of Ij will be described later (Fig. 2).
Figure 1. Synaptic interactions between a rod and a next-neighboring cone.
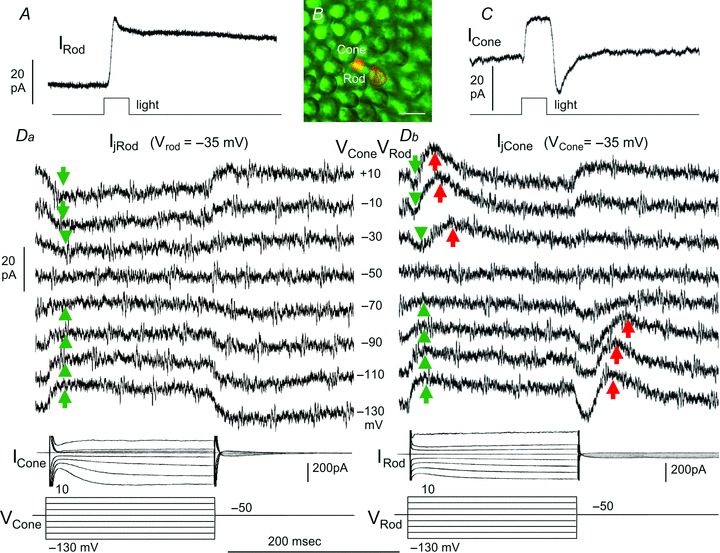
A and C, simultaneous light-evoked current responses of the rod and the cone to a 500 nm, −3.0, 0.5-s light step; B, Lucifer Yellow fluorescent image of the rod and cone outer segments in the retinal flatmount. Calibration bar: 20 μm. Da, current responses of the rod (IRod, held near its dark potential at −35 mV) to voltage clamp steps in the cone (VCone) from −50 mV to various voltages (−130 to +10 mV, middle column in D), ICone traces in Da are current responses of the cone to the voltage steps in itself. Db, current responses of the cone (ICone, held near its dark potential at −35 mV) to the same set of voltage clamp steps in the rod (VRod), IRod traces in Db are current responses of the rod to the voltage steps in itself. Green arrows: sustained, bi-directional transjunctional currents (Ij); red arrows: transient rod→cone currents (IRC).
Figure 2. Synaptic interactions between rods and cones.
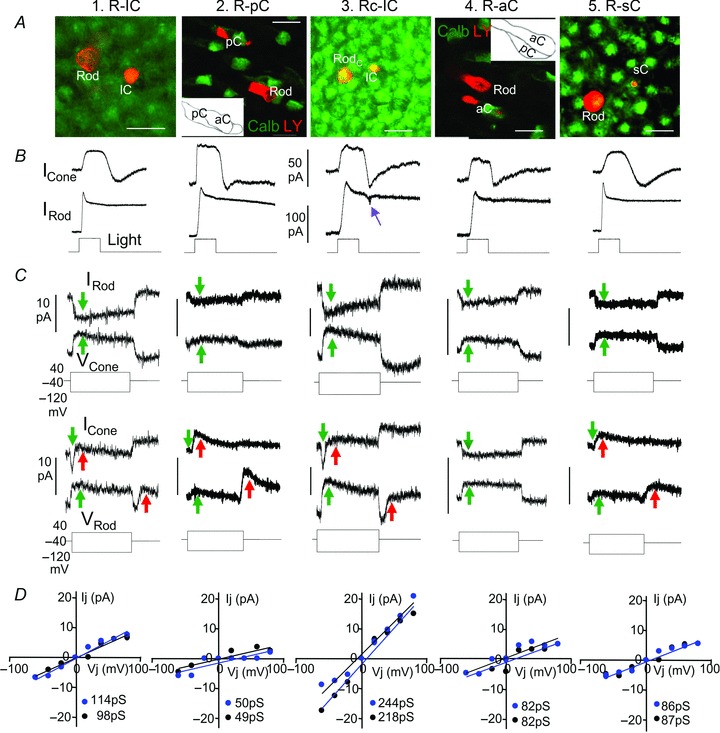
Lucifer Yellow-filled cell images in flat-mounted salamander retinas (A, calibration bars: 20 μm), simultaneous light-evoked current responses (B), bi-directional current responses of follower cells to positive and negative voltage clamp steps to driver cells (C), and Ij–V relations (D, blue, rod→cone; black, cone→rod) of (from left to right) a rod–large single cone (R–lC) pair, a rod–principal member of a double cone (R–pC) pair, a rodC–large single cone (RC–lC) pair, a rod–accessory member of a double cone (R–aC) pair and a rod–small single cone (R–sC) pair. Green arrows in C mark the sign-preserving, bi-directional sustained Ij, and red arrows mark the sign-inverting, rod→cone transient currents IRC in cones at positive voltage step onsets and negative voltage step offsets in rods. The purple arrow in B points to the transient OFF current notch characteristic of rodC light response. Red labels in A are Lucifer Yellow (LY) images of the rod–cone pairs, and green labels in the third and fifth panels are Calbindin-immunoreactive aCs in dark fields (photoreceptors were slightly flattened for better viewing of the pCs and aCs whose contours are shown in the insets), whereas green labels in other panels are recoverin staining of cones (heavier) and rods. The slopes (Gj) of each Ij–Vj relation in panel D are given in picosiemens (pS) at lower right corner of each plot.
In addition to Ij, a delayed transient outward current is seen in the cone at the onset of depolarizing voltage clamp steps and at the offset of hyperpolarizing voltage clamp steps in the rod (red arrows in panel Db). However, no such current response is observed in rods to voltage clamp steps in cones (panel Da). We named this transient, unidirectional rod→cone current IRC. Since IRC is outward at the cone dark resting potential in response to the rod depolarizing voltage onset and hyperpolarizing voltage offset, it is a sign-inverting signal, and thus it cannot be mediated by gap junction channels between rods and cones. Statistics and rod/cone-subtype variations of IRC will be described below.
Figure 2 and Table 1 summarize interactions between different types of rod–cone pairs with statistics of the junctional conductance of the rod–cone pairs and unidirectional transient rod→cone signals. Figure 2 shows interactions between five types of rod–cone pairs recorded in the daytime. Shown in panel A are Lucifer Yellow-filled cell images in flat-mounted salamander retinas of (from left to right) a rod–large single cone (R–lC) pair, a rod–principal member of a double cone (R–pC) pair, a rodC–large single cone (RC–lC) pair, a rod–accessory member of a double cone (R–aC) pair and a rod–small single cone (R–sC) pair. Red labels are Lucifer Yellow (LY) images of the rods and cones, and green labels in the second and fourth panels are Calbindin immunoreactive aCs in dark fields (photoreceptors were slightly flattened for better viewing of the pCs and aCs whose contours are shown in the insets), whereas green labels in other panels are recoverin staining of cones (heavier) and rods. Shown in panel B are simultaneous light-evoked current responses of the rod–cone pairs, and panel C shows bi-directional current responses of follower cells to positive and negative voltage clamp steps in driver cells. The purple arrow points to the transient OFF current notch characteristic of rodC (which are strongly coupled with cones and thus exhibit a mixed rod/cone response waveform with an OFF ‘notch’ current response; Wu & Yang, 1988; Wu, 1988), green arrows mark transjunctional currents Ij and red arrows mark the sign-inverting, rod→cone transient currents IRC in cones (note that IRC was not observed in the rod–aC pair). Panel D shows current–voltage relations of the peak transjunctional currents (Ij) vs. transjunctional voltage (Vj=Vf–Vd, where Vf and Vd are voltages of the follower and driver cells, respectively) of the five rod–cone pairs in both directions (black symbols, cone→rod; blue symbols, rod→cone). Transjunctional currents in most rod–cone pairs are sustained (with nearly constant amplitudes during the voltage step), except for some R–lC and RC–lC pairs that show small sags (possibly due to small contribution of currents from nearby unclamped rods that contain Ih hyperpolarization-activated current, (Attwell & Wilson, 1980; Barrow & Wu, 2009). To minimize the Ih contribution to Ij, we plot the peak transjunctional currents in panel D. These plots demonstrate that each of the five pairs of rods and cones is linearly, reciprocally and symmetrically coupled, and the junctional conductances (Gj, determined by the slope of the Ij–Vj relation) are given in each plot. We performed these experiments on, in total, 12 R–lC pairs, 8 R–pC pairs, 7 RC–lC pairs, 7 R–aC pairs and 6 R–sC pairs, the Ij–Vj relations are all linear and symmetrical and the average (± SD) values of Gj are listed in Table 1. It is evident from Table 1 that the relative strengths of rod–cone coupling, as indicated by the average Gj values, are (from strong to weak) rodC–large single cone, rod–large single cone, rod–small-single cone, rod–accessory double cone and rod–principal double cone. We also determined the strength of IRC by measuring the amplitudes of the transient outward current in the cone at the offset of the −40 to −120 mV voltage step in the next-neighbouring rod (we defined this current as IRCM because it is usually the maximum IRC). The average IRCM values of four types of rod–cone pairs are quite consistent, ranging from 11.7 pA to 14.1 pA, with the exception that no IRC was seen in all seven rod–accessory double cone pairs.
Since it has been suggested that rod–cone coupling in certain fish and rodent retinas is stronger at night than in the daytime (Ribelayga et al. 2008), we repeated the experiments performed in the daytime (described above) on five R–lC pairs, three R–pC pairs, three RC–lC pairs, two R–aC pairs and four R–sC pairs at night (see Methods section), and the average (± SD) values of Gj and IRCM (Gj(n) and IRCM(n)) are also listed in Table 1. All Ij–Vj relations obtained at night are linear and symmetrical (not shown). It is evident from Table 1 that Gj and IRCM values within each type of rod–cone pair do not exhibit any statistically significant differences between the two sets of experiments (daytime vs. night-time). This indicates that the circadian-dependent changes in rod–cone coupling observed in the fish and rodent are not present in the tiger salamander. Moreover, the strength of the sign-inverting, rod→cone transient current IRC does not change with the circadian clock.
The transient rod→cone IRC is mediated by a sign-inverting chemical synaptic pathway as it exhibits a chloride-dependent reversal potential
We next studied the transient, unidirectional rod→cone signal by first examining the current–cone voltage (IRC–VCone) relations and IRC's ion dependence. Figure 3Aa shows an example of such experiments in a rod–pC (principal double cone) pair in normal Ringer and internal solutions (ECl=−60 mV, see Methods section). Figure 3Ab illustrates experiments on another rod–pC pair in equimolar chloride in the Ringer and internal solutions (ECl= 0 mV). It is evident that IRC at both positive rod voltage (−40 to + 40 mV) onset and negative rod voltage (−40 to −120 mV) offset reverse near −50 mV (ERC) in normal solutions and reverse near −10 mV in equimolar chloride solutions. The current–cone voltage (IRC–VC) relations for both rod–pC pairs (Fig. 3B) are approximately linear, with a slopes of 280, 380, 130 and 270 pS for the onset and offset responses in normal and equimolar solutions, respectively. We repeated these experiments in 16 rod–cone pairs in normal solutions and 10 rod–cone pairs in equimolar chloride solutions, and the ERC histograms are given in Fig. 3C. These results suggest that the sign-inverting, transient IRCs are mediated by a chemical synapse (because of the existence of a reversal potential) associated with a conductance increase in the cone membrane (positive slope of the IRC–VC relation). Moreover, IRC is chloride dependent, as ERC moves with ECl and its average values are near ECl (about 10 mV short from ECl; see Discussion below). Since ECl of salamander cones has been shown to be near −45 mV (Thoreson & Bryson, 2004), and the cones’ dark membrane potential is about −35 to −40 mV (Wu & Yang, 1988; Wu, 1991), the rod→cone IRC associated with a chloride conductance increase should result in a transient cone hyperpolarization at both positive rod voltage onset and negative rod voltage offset under physiological (not voltage clamp) conditions (Attwell et al. 1983).
Figure 3. Chloride dependence of IRC.
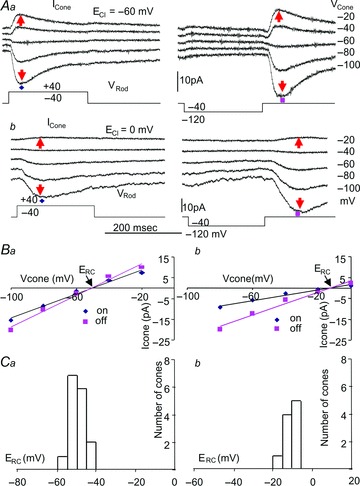
Aa, current responses of a pC (principal double cone) at various holding potentials to positive (left) and negative (right) voltage clamp steps in a next-neighbouring rod in normal Ringer and internal solutions (ECl=−60 mV). Ab, the same set of experiments on another next-neighbouring rod–pC pair in the Ringer and internal solutions with equimolar chloride (ECl= 0 mV). B, current–cone voltage (IRC–VC) relations of the two rod–pC pairs in A for IRC in normal Ringer (a) and in equimolar chloride (b) solutions. The reversal potentials (ERC) of IRC elicited by both positive (blue diamonds) and negative (pink rectangles) rod voltage steps are near −50 mV in normal solutions and near −10 mV in equimolar chloride solutions. C, ERC histograms of 16 rod–cone pairs in normal solutions and 10 rod–cone pairs in equimolar chloride solutions.
The transient, sign-inverting rod→cone IRC is mainly mediated by the rod→HC→cone synaptic pathway
There are at least two possible synaptic pathways that may mediate the unidirectional, sign-inverting IRC: the direct rod→cone chemical synapses made by rod teleodendrites on cone pedicles (Lasansky, 1973) and the rod→horizontal cell→cone feedback synaptic pathway (Baylor et al. 1971; Attwell et al. 1983). In order to investigate these two possibilities, we performed dual voltage clamp experiments on rod–cone pairs separated by different distances. Figure 4 shows the current responses of a cone to a negative voltage step (−40 to −100 mV) in a rod about 20 μm (a), 95 μm (b) and 260 μm (c) away from the cone. In the next-neighbouring rod–cone pair (a), the rod voltage step elicited both the sustained, sign-preserving Ij (green arrows) and the transient, sign-inverting IRC (red arrows). In the cones 95 and 260 μm away, the rod voltage step generates no Ij, but still generates IRC of smaller amplitude (b and c). We carried out similar studies on a total of 21 rod–cone pairs separated by various distances in 15 flatmount retinas. In each retina, we had at least one pair of next-neighbouring rod–cone pairs (separated by about 20 μm), and we used the IRC amplitudes as the normalization factor (or maximum IRC: IRCM) for the IRC values of rod–cone pairs separated by longer distances in the same retina. Figure 4C is the scatter plot of the normalized IRC–distance relation for 15 next-neighbouring rod–cone pairs (with IRCM, red circles) and 21 rod–cone pairs separated by various distances. These results suggest that IRC is unlikely to be completely mediated by direct rod→cone synaptic contacts, because rod and cone teleodendrites in the salamander retina extend laterally no more than 40 μm (Lasansky, 1973; Zhang & Wu, 2009), and voltage clamp signal from a rod to the coupled rod network can only be seen two to three rods (20–30 μm) away (Attwell & Wilson, 1980). Our observation that IRC (though of smaller amplitude) is transmitted from rods to cones 95–260 μm away suggests that certain interneurons with long lateral processes, such as the horizontal cells (HCs), are involved. It has been shown that the dendritic field diameters the salamander A- and B-type HCs (HCAs and HCBs) are 180 ± 20 μm and 105 ± 23 μm, and, due to homocellular coupling, their receptive field diameters are >500 μm and >1,000 μm, respectively (Zhang et al. 2006a,b). Therefore it is possible that the transient rod→cone IRC is mediated by these HCs (though most likely HCBs; see Discussion), as they are capable of carrying signals over at least hundreds of micrometres laterally in the outer retina. The observation that IRC is larger in closer rod–cone pairs opens the possibility that a synergistic short-range synaptic pathway, such as the direct rod→cone chemical synapses made by rod teleodendrites on cone pedicles (Lasansky, 1973), is also involved (see Discussion).
Figure 4. IRC between rods and cones of different separations.
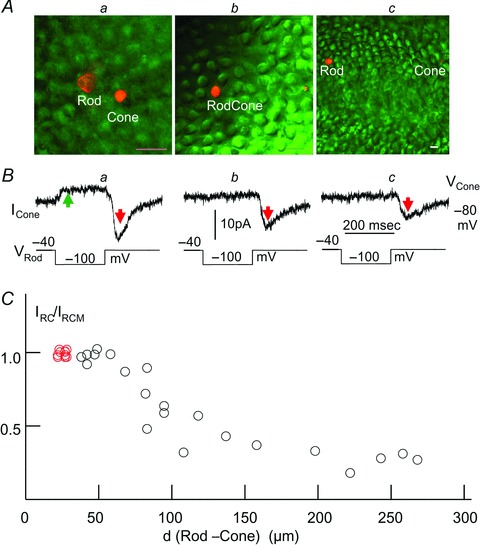
A, Lucifer Yellow fluorescent image of the rod and cone outer segments (calibration bars: 20 μm) separated by about 20 μm (a), 95 μm (b) and 260 μm (c) in the retinal flatmounts. B, current responses of the cones held at −80 mV to a negative voltage step (−40 to −100 mV) in the rods (same 3 rod–cone pairs in Aa–c). The rod voltage step elicited both the sustained, sign-preserving Ij (green arrow) and the transient, sign-inverting IRC (red arrows), shown in a, and elicited IRC of smaller amplitude but no Ij, shown b and c. C, scatter plot of the normalized IRC–distance (IRC/IRCMvs. d (rod–cone)) relation for 15 next-neighbouring rod–cone pairs (with IRCM, red circles) and 21 rod–cone pairs (18 R–lCs, 2 R–pCs and 1 RC–lC) separated by various distances.
To investigate the possible involvement of the rod→HC→cone pathway, we examined whether rod voltage steps that generate IRC in cones elicit similar current responses in HCs. Figure 5b shows current responses of a cone (ICone, held near its dark potential at −30 mV, panel A) and current responses of a HC (IHC, held near its dark potential at −20 mV, panel B) to the same set of voltage clamp steps in rods (VRod). Lucifer Yellow fluorescent images of the rod–HC pair in the living retinal slice and the rod–cone pair in the flat-mounted retina are shown in Fig. 5a. Previous studies have revealed that there are two types of HCs in the tiger salamander retina: the axon-less, narrow-field HCAs which exhibit cone-dominated light responses, and the axon-bearing, wide-field HCBs which exhibit rod-dominated light responses (Zhang et al. 2006a). In all rod–HC pairs in this study, we analysed the light response characteristics of each HC (not shown), and found that almost all rod–HCB pairs give rise to substantial IHC, whereas rod–HCA pairs give no or very small IHC. Therefore all rod–HC pairs described in this and following figures are rod–HCB pairs (see Discussion). It is evident from Fig. 5b that each rod voltage step evokes a transient IHC (blue arrows) of the same waveform and time course as the transient IRC it evokes in the cone (red arrows), except that the two currents are of opposite polarity (IHC is inward and IRC is outward). Sustained voltage steps in the rod generate transient current responses in HCs because voltage steps in a rod result in current flow to adjacent rods via rod–rod coupling, and Ih in the unclamped adjacent rods shapes the sustained current flow into transient voltage changes at the voltage step onset and offset (Attwell & Wilson, 1980; Barrow & Wu, 2009). Since a HC sums up synaptic inputs from many rods in its receptive field (all except one are unclamped and exhibit transient responses), its responses to sustained voltage steps in the clamped rod are transient.
Figure 5. Response waveforms and time course of IRC and IHC.
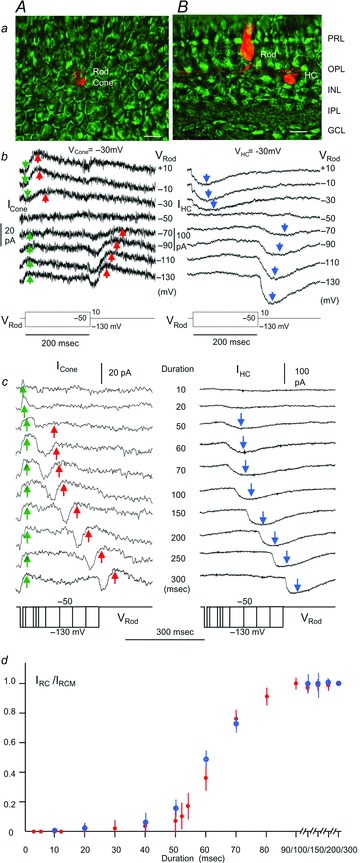
A, rod–large single cone pair; B, rod–HCB pair. Panel a, Lucifer Yellow fluorescent images of a rod–cone pair in the flat-mounted retina (A) and a rod–HC pair in the living retinal slice (B). Calibration bars, 20 μm. Panel b, current responses of the cone (ICone, held near its dark potential at −30 mV; A) and current responses of the HC (IHC, held near its dark potential at −20 mV; B) to the same set of voltage clamp steps in rods (VRod, −130 to +10 mV). Blue arrows, IHC; green arrows, Ij; red arrows, IRC. Panel c, current responses of a large single cone cone (ICone, held near its dark potential at −30 mV; A), and current responses of a HCB (IHC, held near its dark potential at −20 mV; B) to the same set of negative voltage clamp steps (−50 to −130 mV) of various durations (10 –300 ms) in rods. Red arrows, IRC; blue arrows, IHC. IRC is accompanied by a sustained Ij (green arrows) whereas IHC is not. The red and blue arrows mark the same time points of each IRC and IHC response pairs, showing that IRC and IHC peak approximately at the same times. Panel d, normalized, average (error bars =±SD, N = 11 for rod–cone pairs and 6 for rod–HCB pairs) amplitude–duration plots of IRC (red filled circles) and IHC (blue open circles).
In order to further establish that IRC and IHC are generated in the same manner, we examined how rod voltage step durations affect these two currents. Figure 5c shows IRC (red arrows; A) and IHC (blue arrows; B) elicited by an identical set of negative rod voltage steps of various durations. The cone is a large single cone (based on its outer segment morphology, not shown) and the HC is a B-type HC (based on its rod-dominated light responses, not shown). Note that IRC is accompanied by a sustained Ij (green arrows) whereas IHC is not. Both IRC and IHC are transient (IRC is outward and IHC is inward) currents after the offset of the negative rod voltage step, and they start to appear when the step duration is about 20–50 ms and saturate when the step duration is about 100 ms. The red and blue arrows mark the same time points of each IRC and IHC response pairs, showing that IRC and IHC peak approximately at the same times. The normalized amplitude–duration plots of IRC and IHC are shown in Fig. 5d, illustrating that IRC and IHC generated by rod voltage steps of various durations correlate very well. Taken together with the observation that IRC can be elicited between rod–cone pairs over 250 μm apart (Fig. 4), and IRC and IHC exhibit the same waveform and time courses by positive and negative rod voltage steps (Fig. 5b and c), we postulate that the transient rod→cone IRC is at least partially mediated by the transient rod→HC IHC via a sign-inverting HC→cone feedback synapse.
Suppression of the rod→HC and rod→cone synaptic signals
To further investigate the notion that the transient rod→cone IRC is mediated at least partially by the HC→cone feedback synapses, we studied actions of synaptic suppressing agents that have been proposed to affect the feedback synapse on IRC. These agents include the hemichannel blocker carbenoxolone (Kamermans et al. 2001; Kamermans & Fahrenfort, 2004), low extracellular pH (Hirasawa & Kaneko, 2003; Vessey et al. 2005) and the GABA receptor antagonist picrotoxin (PTX; Murakami et al. 1982; Wu, 1991). Additionally, we examined the effects of TBOA, a blocker of the glutamate transporter-associated chloride channels found in cones and certain types of bipolar cells (Picaud et al. 1995; Grant & Dowling, 1995; Wong et al. 2005). Since IRC may go through HCs, we needed to examine the effects of each synaptic suppressing agent on both the rod→HC IHC and the rod→cone IRC. If an agent does not block IHC but suppresses IRC, then it should be a blocker for the HC→cone feedback synapse. However, if an agent suppresses both IHC and IRC, it may or may not be a blocker of the HC→cone feedback synapse. It is important to note that if the rod→cone IRC is mediated through HCs, then any agent that blocks the rod→HC IHC should also block the rod→cone IRC. Figure 6A–C shows that application of 100 μm carbenoxolone, 100 μm DNQX (an AMPA/kainate receptor blocker that suppresses rod/cone–HC synapses; Yang & Wu, 1991) and Ringer solution of pH = 6.0 suppress both IHC and IRC. We also performed experiments on four rod–HC pairs and five rod–cone pairs in Ringer solution containing 10–20 mm Hepes, and the results (not shown) were very similar to those obtained in pH = 6.0 or DNQX. These results indicate that it is inconclusive whether hemichannels, AMPA/kainate receptors or extracellular pH are involved in the salamander HC→cone feedback synapses, although they support the idea that the rod→cone IRC is mediated through HCs (i.e. whenever the rod→HC IHC is suppressed, the rod→cone IRC is absent). PTX at 100 μm exerts little action on IHC and IRC (Fig. 6D), suggesting that GABA is not involved in either the rod→HC or HC→cone synapses. On the other hand, 100 μm TBOA exerts little action on IHC, but completely suppress IRC (Fig. 6E), suggesting that glutamate transporter-mediated chloride channels are involved in the HC→cone feedback synapse, but not in the rod→HC synapse. A requirement of such glutamate transporter-mediated HC→cone feedback synapse is that HCs must release glutamate under certain conditions, and a previous study from fish HCs suggest just that (Schutte & Schlemermeyer, 1993). An alternative (but not mutually exclusive) explanation of the TBOA result is that glutamate transporter-mediated chloride channels are involved in the direct rod→cone, sign-inverting chemical synapse (Lasansky, 1973).
Figure 6. Effects of synaptic suppressing agents on IRC and IHC.
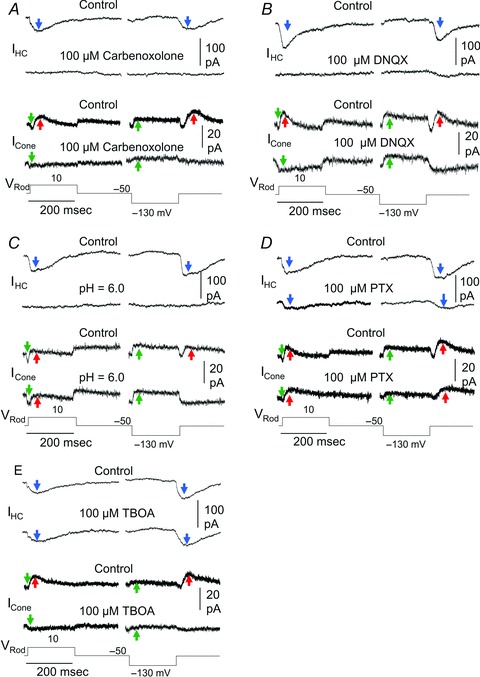
Effects of 100 μm carbenoxolone (A), 100 μm DNQX (B), Ringer solution of pH = 6.0 (C), 100 μm PTX (D) and 100 μm TBOA (E) on IHC and IRC. In each panel, the two upper traces are current responses of a HC to positive (−50 to 10 mV, left) and negative (−50 to −130 mV, right) rod voltage steps in normal Ringer solution (control), and the second traces are the same responses in the presence of synaptic blocking agents. The third and fourth traces are current responses from cones to the same set of rod voltage steps and blocking agents. All rod–HC pairs were recorded from retinal slices and all rod–cone pairs are from next-neighbouring cells in the flatmount retinas. Similar results were obtained from 5/5, 6/5, 4/6, 4/4 and 5/7 rod–HC/rod–cone pairs for the five experiments in panels A, B, C, D and E, respectively, of Fig. 6. Blue arrows, IHC; green arrows, Ij; red arrows, IRC.
Frequency-dependent suppression of rod→cone coupling signal by IRC
We next studied how the sign-preserving synaptic signal Ij (via rod–cone coupling) and sign-inverting rod→cone IRC interact and affect rod and cone outputs. We have shown that depolarizing voltage clamp steps in a rod elicited a sustained outward Ij and a transient inward IRCduring the voltage step. Hyperpolarizing rod voltage steps elicited a sustained Ijduring the voltage step and a transient IRC in cones after the voltage step offset in adjacent cones (e.g. Fig. 1 and 4), and thus Ij and IRC elicited by these voltage steps do not overlap (interact) much. Such current separation allowed us to characterize the physiological and pharmacological properties of Ij and IRC individually (Figs 1–6). However, in addition to producing non-interacting Ij and IRC, these voltage steps differ from light-evoked voltage responses of rods and cones in several ways: (1) Rods and cones never depolarize above −30 mV to light stimuli, except for OFF overshoot responses in light-adapted cones and rodCs (Wu & Yang, 1988); (2) the rise time of rod and cone responses to dim light is much slower than that of the hyperpolarizing voltage step; (3) the recovery time course of dark-adapted rods is much slower than the offset of the hyperpolarizing voltage clamp steps (which generate the large transient IRC). In order to determine how Ij and IRC interact while rods and cones undergo physiological voltage changes, we examined Ij and IRC elicited by sinusoidal hyperpolarizing voltage changes of various frequencies. Figure 7A shows the current responses of a rod to sinusoidal hyperpolarizing voltage changes (under voltage clamp from −40 to −80 mV, top traces) of 0.5 Hz (a), 2 Hz (b) and 16 Hz (c) in a next-neighbouring large single cone, and the Fig. 7B shows current responses of the large single cone to the same sinusoidal voltage changes in the rod (sixth trace from the top, reverse the driver/follower cell order). Figure 7C shows current responses from the same large single cone to the sinusoidal rod voltage changes (as in Fig. 7B) recorded in the presence of 100 μm TBOA. The 0.5 and 2 Hz sinusoidal voltage changes elicited substantially smaller currents in the rod→cone direction (Fig. 7B) than the cone→rod direction (Fig. 7A), and the 16 Hz sinusoidal voltage changes elicited currents of the same steady-state amplitude in the two directions. When the sign-inverting IRC was blocked by TBOA (Fig. 7C), the sinusoidal voltage-elicited currents in the rod→cone direction (Fig. 7B) became almost identical to that in the cone→rod direction (Fig. 7A). We carried out these experiments in nine other rod–cone pairs with sinusoidal voltage changes at frequencies between 0.5 and 32 Hz, and the average frequency-dependent suppression of the rod→cone coupling signal (expressed as peak-to-peak current response in the rod→cone direction vs. that in the cone→rod direction, or vs. that in the presence TBOA), is illustrated in Fig. 7F.
Figure 7. Rod, cone and HC responses to sinusoidal voltage changes.
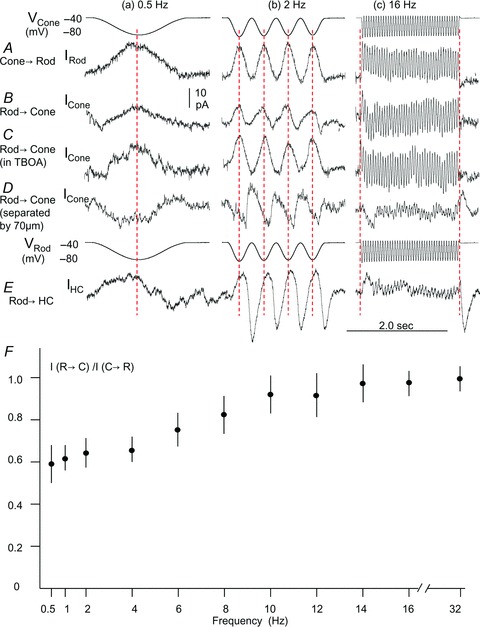
A, current responses of a rod to sinusoidal hyperpolarizing voltage changes (under voltage clamp from −40 to −80 mV, top traces) of 0.5 Hz (a), 2 Hz (b) and 16 Hz (c) in a next-neighbouring large single cone. B, current responses of the large single cone to the same sinusoidal voltage changes in the rod (sixth row traces from the top, reverse the driver/follower cell order). C, current responses from the same large single cone to the sinusoidal rod voltage changes (as in B) recorded in the presence of 100 μm TBOA. D, sinusoidal voltage-elicited IRC recorded from a large single cone that was about 70 μm from the rod. E, current responses of a horizontal cell (IHC) to the same set of sinusoidal rod voltage changes. F, average (error bars =±SD, N = 9, except that N = 7 for the 1, 10 and 14 Hz points) frequency–response relations between rods and cones. Each data point represents the ratio of the current generated by a sinusoidal voltage change of a given frequency in the rod→cone direction/current generated in cone→rod direction (I(rod→cone)/I(cone→rod)). Five of the 9 rod–cone pairs were normalized against the average of I(cone→rod) and I(rod→cone) in the presence of TBOA.
Figure 7D shows sinusoidal voltage-elicited IRC as they were recorded from a large single cone that was about 70 μm from the rod (Ij is negligibly small and IRC is about the same amplitude as that recorded from the next-neighbouring cone, verified by Ij and IRC in the cone to voltage clamp steps in the rod – similar to the protocol of Fig. 1, 2 and 4, not shown). It is evident that IRC elicited by 0.5 Hz rod voltage is inward, which is of opposite polarity to the Ij (third row traces). IRC elicited by 2 Hz rod voltage exhibits a sinusoidal response that is out of phase with the sinusoidal Ij (see red vertical dashed lines). These explain why the current responses elicited by the 0.5 and 2 Hz sinusoidal voltages are substantially smaller in the rod→cone direction than in the cone→rod direction. Our results in Fig. 7 show that such unidirectional reduction of the rod→cone coupling signal occurs in the frequency range below 15 Hz. At higher frequencies (e.g. 16 Hz, Fig. 7C), the steady-state IRC became negligible because the duration of each hyperpolarizing sinusoidal period (1/32 = 30 ms) was too short to generate the OFF IRC (see Fig. 5).
Figure 7E shows sinusoidal voltage-elicited IHC recorded from a horizontal cell. It is evident that IHC elicited by 0.5, 2 and 16 Hz rod voltage are in phase, of similar waveform and opposite polarity to the IRC (Fig. 7D), consistent again with the notion that IRC is mediated by IHC via a sign-inverting feedback synapse.
Discussion
Electrical coupling between rods and various types of cones
We have shown in this study that rods and cones in the tiger salamander retina are electrically coupled and the average junctional conductances between various types of adjacent rod/cone pairs illustrate a coupling strength order (strong to weak): rodC–large single cone, rod–large single cone, rod–small-single cone, rod–accessory double cone and rod–principal double cone. Our dual voltage clamp records suggest that the electrical synapses between all rod–cone pairs are linear and symmetrical. This resembles the linear and symmetrical electrical synapses between rods (Zhang & Wu, 2005) and between various types of cones (Li & Devries, 2004; Hornstein et al. 2004). The average junctional conductance of typical rod–cone pairs ranges from 40 to 103 pS, except for rodCs which are more strongly coupled with cones (mostly large single cones (Wu & Yang, 1988), with an average Gj over 200 pS). These Gj values are similar to the average Gj (121 pS) of rod–cone pairs recently reported in the ground squirrel (Li et al. 2010). The junctional conductance of adjacent rod–rod pairs in the salamander ranges from 500 to 1000 pS (Zhang & Wu, 2005), and between cone–cone pairs in the primate and ground squirrel ranges from 200 to 1200 pS (Li & Devries, 2004; Hornstein et al. 2004). These results support the notion that heterocellular coupling (e.g. rod–cone) is substantially weaker than homocellular (rod–rod or cone–cone) coupling (Wu, 2010). A schematic diagram of electrical coupling between various types of photoreceptors is given in Fig. 8. It should be noted that because of strong rod–rod coupling, indirect currents through nearby rods in the network may contribute to the transjunctional currents measured from the rod–cone pairs (e.g. rod→rod→cone or cone→rod→rod). In this study, we defined Ij, Vj and Gj from measurements made in the flat-mounted salamander retina, which include direct and indirect currents in the coupled photoreceptor network. Quantitative modelling of the photoreceptor network is needed to determine the exact contributions of direct/indirect current flows between rods and cones.
Figure 8. Summary of rod, cone and HC synaptic interactions.
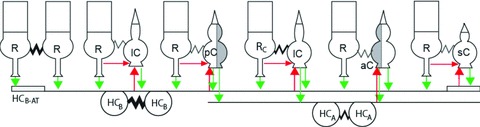
Schematic diagram of synaptic interactions among six types photoreceptors: rod (R), rodC (RC), large single cone (lC), principal member of a double cone (pC), accessory member of a double cone (aC, grey portion of the double cone), and small single cone (sC), as well as the A- and B-type horizontal cells and B-type HC axon terminals (HCA, HCB and HCB-AT). Zigzags, electrical synapses (thicker lines indicate stronger coupling); green arrows, glutamatergic synapses; large red arrows, HC→cone feedback synapses; small red arrows, possible direct rod→cone chemical synapses.
It has been suggested that photoreceptor coupling in vertebrates is mediated by gap junction channels made of homologous connexin36 (Deans et al. 2002; Zhang & Wu, 2004). This is consistent with the linear and symmetrical Ij–Vj relations of the salamander rod–cone pairs described in this report, the salamander rod–rod pairs (Zhang & Wu, 2005), the primate cone–cone pairs (Hornstein et al. 2004) and the ground squirrel cone–cone and rod–cone pairs (Li & Devries, 2004; Li et al. 2010). In the tiger salamander retina, the gap junction plaques between two rods are found to contain about 10 times more channels than that between a rod and a cone (Zhang & Wu, 2004), and the ratio is consistent with the relative junctional conductance values of the rod–rod and rod–cone pairs presented in this and the previous study (Zhang & Wu, 2005).
It has been shown that rod–cone coupling can be modulated by light adaptation (Yang & Wu, 1989) and circadian clock (Ribelayga et al. 2008). Since all experiments in this study were carried out in fully dark-adapted salamander retinas, differences in Gj values among various types of rod–cone pairs are unlikely to be caused by varying degrees of light adaptation. Moreover, our systematic comparison of the Ij–Vj relations and average Gj values of the five types of rod–cone pairs obtained at day and night times (Table 1) clearly suggest that the differences in Gj values among various types of rod–cone pairs are not mediated by the circadian clock, and the circadian-dependent changes in rod–cone coupling observed in the fish retina are not present in the tiger salamander. It is possible that circadian-associated modulation of gap junction conductance was not readily detectable in our study because intracellular machinery required for regulation was washed away in the whole cell recording configuration. However, bi-directional current injection experiments with dual microelectrodes in salamander rod–cone pairs at day and night times did not show circadian-dependent changes in rod–cone coupling (S. M. Wu, unpublished results). Another possible explanation is that the circadian-associated changes of rod signals in cones (Ribelayga et al. 2008) are partially caused by circadian-dependent changes in cone photocurrents (such as response sensitivity and waveform, recorded with suction electrodes), not necessarily caused by modulation of gap junction conductance between rod and cones. Further experiments are needed to clarify this issue.
The transient, sign-inverting rod→cone pathway exists in most cones, and it is mainly mediated by the HC→cone feedback synapse through a TBOA-sensitive chloride conductance
We found that a sign-inverting, rod→cone unidirectional IRC in all cones, except for the accessory members of double cones (see Fig. 2 and Table 1). Since IRC is observable between rod–cone pairs more than 250 μm apart, we propose that this sign-inverting pathway is at least partially mediated by HCs, as HCs are the only cells in the outer retina having processes that extend laterally for such distance. This hypothesis is further supported by our results showing that voltage steps in rods elicit transient responses in HCs (IHC) of the same shape, delay and duration dependence as the IRC in cones (Fig. 5 and 6). IHC is inward (causing depolarization in HCs) and IRC is outward (causing hyperpolarization in cones), consistent with the finding that the HC→cone feedback synapse is sign inverting (Baylor et al. 1971).
Among synaptic blockers/modulators that have been implicated to block the HC→cone feedback synapses, we found that TBOA suppresses IRC without affecting IHC, suggesting that glutamate transporter-mediated chloride channels are involved in the HC→cone feedback synapse, but not in the rod→HC synapse. A requirement of such glutamate transporter-mediated HC→cone feedback synapse is that HCs must release glutamate under certain conditions, and a previous study from fish HCs suggests just that (Schutte & Schlemermeyer, 1993).
Figure 3 suggests that IRC is associated with a chloride-dependent cone conductance increase (positive slopes of IRC–VCone relations in Fig. 2B), as the reversal potential of IRC (ERC) correlates closely with ECl. The observation that ERC is about 10 mV short of ECl suggests that other ion channel(s), whose equilibrium potential lies near the resting potential of the cones, may be involved (Wong et al. 2005). The chloride-dependent ERC in conjunction with the TBOA block support the assertion that IRC is mediated by glutamate transporter-associated chloride channels in cones (Picaud et al. 1995).
Although our results suggest that IRC is likely to be mediated by the rod→HC→cone feedback synaptic pathway, it is not clear whether IRC and the depolarizing surround responses in cones (Skrzypek & Werblin, 1983) share the same synaptic mechanism. The transient outward IRC, accompanied by a conductance increase (Fig. 3), is the postsynaptic response to a transient HC depolarization (inward current, Fig. 5). It has been shown in the turtle retina that the depolarizing surround response (elicited by a light annulus-induced HC hyperpolarization) is also associated with a conductance increase (Gerschenfeld & Piccolino, 1980). Therefore, if both events are mediated by the HC→cone feedback synapse, the underlying mechanisms must be different, consistent with our pharmacological results indicating that IRC is not sensitive to the same synaptic blockers/modulators as the surround-induced feedback signals in cones (Verweij et al. 2003; Kamermans & Fahrenfort, 2004). It is possible that the HC→cone feedback synaptic signals are mediated by multiple mechanisms, each of which becomes dominant under different physiological conditions and stimulating signals.
Although both A-and B-type HCs have wide enough receptive fields to carry the rod→cone IRC signals, our results indicate that IRC is absent in rod–aC pairs (Fig. 2). It has been shown that B-type HC dendrites in the salamander retina contact principal double cone and large single cone pedicles, whereas A-type HC dendrites contact mostly pedicles of accessory double cones (Zhang & Wu, 2009). Therefore the two types of HCs may make feedback synapses with different types of cones, assuming that such feedback synapses are located near the immunolabelled HC contact areas in the cone pedicles. Results in this study (e.g. Fig. 5) suggest that rod voltage steps only elicit IHC in HCBs, not in HCAs (consistent with our previous findings that HCB light responses are rod dominated and HCA responses are cone dominated; Zhang et al. 2006a), and if accessory double cones receive feedback signals primarily from HCAs, then it is not surprising that IRC is absent in these cones. However, this does not exclude the possibility that the TBOA-sensitive chloride conductance and/or other feedback mechanisms exist between HCA and accessory double cones. A schematic diagram of the feedback synapses from HCA and HCB to various types of cone is given in Fig. 8.
Although our results are consistent with the idea that the sign-inverting rod→cone unidirectional IRC is mainly mediated by the HCs (mostly likely the B-type HCs), we cannot rule out the possible involvement of another anatomically established synaptic pathway. In Fig. 3, we found that the amplitude of IRC becomes smaller as the distance between the recorded rod and cone increases. This may reflect electrotonic decay of passive signal transmission in HC processes. Alternatively, it may suggest that a shorter-range pathway, such as the direct rod→cone synapse reported in an anatomical study (Lasansky, 1973), also contributes to IRC.
Frequency-dependent sign-preserving and sign-inverting signal interaction between rods and cones
We show in Fig. 7F that IRC serves as a unidirectional high-pass filter for the rod→cone coupling signals. Under dark-adapted conditions, dim 500 nm lights evoke voltage responses of 0.5–5 Hz (rise/decay time of 100–1000 ms) in rods, and elicit no cone responses (Wu, 1987a; Wu & Yang, 1992). IRC suppresses VRod-induced rod→cone Ij in this frequency range, minimizes the rod response spread into cones, and ensures that dim light signals are restricted in the rod-driven postsynaptic pathways (Wu et al. 2001). As light becomes brighter, the rise time of rod response becomes faster (20–50 ms or 10–25 Hz) but the recovery time becomes much slower (voltage tails, 1–10 s or 0.5–0.05 Hz; Wu & Yang, 1992). Therefore, the suppressive rod→cone IRC action is weak at the response onset but strong during the response recovery. Consequently, the effect of rod→cone coupling is more pronounced at the light onset than at the response recovery phase. This may partially explain why cones do not exhibit voltage tails after the offset of bright lights even though they are electrically coupled with rods that display large and slow-recovering voltage tails (Wu and Yang, 1992). The suppression of slow voltage tails in cones allows fast OFF responses in cone-driven signals in higher-order cells that are critical for motion detection and fast-changing objects in the visual system (Raninen & Rovamo, 1986; Sjostrand, 2003). Moreover, it has been shown that steady, dim background light enhances flicker responses in cones, a psychophysical phenomenon known as suppressive rod–cone interaction (Eysteinsson & Frumkes, 1989; Horiguchi et al. 1991). Since cone responses are partially mediated by rod photocurrents via coupling, the rod→cone IRC described in this study may contribute to the cone response suppression by reducing rod photocurrent flows into cones.
Acknowledgments
We thank Roy Jacoby, Cameron Cowan and David Simons for critically reading this manuscript.
Glossary
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- GABA
γ aminobutyric acid
- HC
horizontal cell
- I–V
current–voltage relations
- PTX
picrotoxin
- TBOA
dl-threo-β-benzyloxyasparate.
Additional information
Competing interests
All three authors have no competing interests.
Author contributions
Conception and design of the experiments and collection, analysis and interpretation of data: F.G., J.-J.P. and S.M.W. Drafting the article or revising it critically for important intellectual content: S.M.W. All authors approved the final version of the manuscript.
Funding
This work was supported by grants from NIH (EY004446 and EY019908), NIH Vision Core (EY 02520), the Retina Research Foundation (Houston), and the Research to Prevent Blindness, Inc.
References
- Attwell D, Werblin FS, Wilson M, Wu SM. A sign-reversing pathway from rods to double and single cones in the retina of the tiger salamander. J Physiol. 1983;336:313–333. doi: 10.1113/jphysiol.1983.sp014583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Wilson M, Wu SM. A quantitative analysis of interactions between photoreceptors in the salamander (Ambystoma) retina. J Physiol. 1984;352:703–737. doi: 10.1113/jphysiol.1984.sp015318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow AJ, Wu SM. Low-conductance HCN1 ion channels augment the frequency response of rod and cone photoreceptors. J Neurosci. 2009;29:5841–5853. doi: 10.1523/JNEUROSCI.5746-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG, O’Bryan PM. Lateral interaction between vertebrate photoreceptors. Vision Res. 1971;11:1195–1196. doi: 10.1016/0042-6989(71)90134-9. [DOI] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The Retina, an Approachable Part of the Brain. Cambridge, MA, USA: Harvard University Press; 1987. [Google Scholar]
- Eysteinsson T, Frumkes TE. Physiological and pharmacological analysis of suppressive rod–cone interaction in Necturus retina [corrected] J Neurophysiol. 1989;61:866–877. doi: 10.1152/jn.1989.61.4.866. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35:733–747. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM, Piccolino M. Sustained feedback effects of L-horizontal cells on turtle cones. Proc R Soc Lond B Biol Sci. 1980;206:465–480. doi: 10.1098/rspb.1980.0008. [DOI] [PubMed] [Google Scholar]
- Grant GB, Dowling JE. A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. J Neurosci. 1995;15:3852–3862. doi: 10.1523/JNEUROSCI.15-05-03852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M, Eysteinsson T, Arden GB. Temporal and spatial properties of suppressive rod–cone interaction. Invest Ophthalmol Vis Sci. 1991;32:575–581. [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Schnapf JL. Electrical coupling between red and green cones in primate retina. Nat Neurosci. 2004;7:745–750. doi: 10.1038/nn1274. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol. 2004;14:531–541. doi: 10.1016/j.conb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Organization of outer synaptic layer in the retina of larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265:471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Li W, Chen S, Devries SH. A fast rod photoreceptor signalling pathway in the mammalian retina. Nat Neurosci. 2010;13:414–416. doi: 10.1038/nn.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Devries SH. Separate blue and green cone network in the mammalian retina. Nat Neurosci. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shimoda Y, Nakatani K, Miyachi E, Watanabe S. GABA-mediated negative feedback from horizontal cells to cones in the carp retina. Jpn J Physiol. 1982;32:911–926. doi: 10.2170/jjphysiol.32.911. [DOI] [PubMed] [Google Scholar]
- Picaud SA, Larsson HP, Grant GB, Lecar H, Werblin FS. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol. 1995;74:1760–1771. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- Raninen A, Rovamo J. Perimetry of critical flicker frequency in human rod and cone vision. Vision Res. 1986;26:1249–1255. doi: 10.1016/0042-6989(86)90105-7. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod–cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte M, Schlemermeyer E. Depolarization elicits, while hyperpolarization blocks uptake of endogenous glutamate by retinal horizontal cells of the turtle. Cell Tissue Res. 1993;274:553–558. doi: 10.1007/BF00314553. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Rod–rod interaction in the retina of the turtle. J Physiol. 1975;246:617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrand FS. Color vision at low light intensity, dark adaptation, Purkinje shift, critical flicker frequency and the deterioration of vision at low illumination. Neurophysiology at the nanometer range of neural structure. J Submicrosc Cytol Pathol. 2003;35:117–127. [PubMed] [Google Scholar]
- Skrzypek J, Werblin F. Lateral interactions in absence of feedback to cones. J Neurophysiol. 1983;49:1007–1016. doi: 10.1152/jn.1983.49.4.1007. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ. Chloride equilibrium potential in salamander cones. BMC Neurosci. 2004;5:53:53. doi: 10.1186/1471-2202-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:1049–1057. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da SN, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Cohen ED, Dowling JE. Retinal bipolar cell input mechanisms in giant danio. II. Patch-clamp analysis of on bipolar cells. J Neurophysiol. 2005;93:94–107. doi: 10.1152/jn.00270.2004. [DOI] [PubMed] [Google Scholar]
- Wu SM. Light-dependent synaptic delay between photoreceptors and horizontal cells in the tiger salamander retina. Vision Res. 1987a;27:363–367. doi: 10.1016/0042-6989(87)90085-x. [DOI] [PubMed] [Google Scholar]
- Wu SM. Synaptic connections between neurons in living slices of the larval tiger salamander retina. J Neurosci Methods. 1987b;20:139–149. doi: 10.1016/0165-0270(87)90046-x. [DOI] [PubMed] [Google Scholar]
- Wu SM. The off-overshoot responses of photoreceptors and horizontal cells in the light-adapted retinas of the tiger salamander. Exp Eye Res. 1988;47:261–268. doi: 10.1016/0014-4835(88)90009-7. [DOI] [PubMed] [Google Scholar]
- Wu SM. Input–output relations of the feedback synapse between horizontal cells and cones in the tiger salamander retina. J Neurophysiol. 1991;65:1197–1206. doi: 10.1152/jn.1991.65.5.1197. [DOI] [PubMed] [Google Scholar]
- Wu SM. Synaptic organization of the vertebrate retina: general principles and species-specific variations: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2010;51:1263–1274. doi: 10.1167/iovs.09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Gao F, Maple BR. Integration and segregation of visual signals by bipolar cells in the tiger salamander retina. Prog Brain Res. 2001;131:125–143. doi: 10.1016/s0079-6123(01)31012-9. [DOI] [PubMed] [Google Scholar]
- Wu SM, Yang XL. Electrical coupling between rods and cones in the tiger salamander retina. Proc Natl Acad Sci U S A. 1988;85:275–278. doi: 10.1073/pnas.85.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Yang XL. Modulation of synaptic gain by light. Proc Natl Acad Sci U S A. 1992;89:11755–11758. doi: 10.1073/pnas.89.24.11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Modulation of rod–cone coupling by light. Science. 1989;244:352–354. doi: 10.1126/science.2711185. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Coexistence and function of glutamate receptor subtypes in the horizontal cells of the tiger salamander retina. Vis Neurosci. 1991;7:377–382. doi: 10.1017/s0952523800004867. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Zhang J, Wu SM. Electrical coupling, receptive fields, and relative rod/cone inputs of horizontal cells in the tiger salamander retina. J Comp Neurol. 2006a;499:422–431. doi: 10.1002/cne.21117. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Connexin35/36 gap junction proteins are expressed in photoreceptors of the tiger salamander retina. J Comp Neurol. 2004;470:1–12. doi: 10.1002/cne.10967. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Physiological properties of rod photoreceptor electrical coupling in the tiger salamander retina. J Physiol. 2005;564:849–862. doi: 10.1113/jphysiol.2005.082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Immunocytochemical analysis of photoreceptors in the tiger salamander retina. Vision Res. 2009;49:64–73. doi: 10.1016/j.visres.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang AJ, Wu SM. Immunocytochemical analysis of GABA-positive and calretinin-positive horizontal cells in the tiger salamander retina. J Comp Neurol. 2006b;499:432–441. doi: 10.1002/cne.21116. [DOI] [PubMed] [Google Scholar]


